Abstract
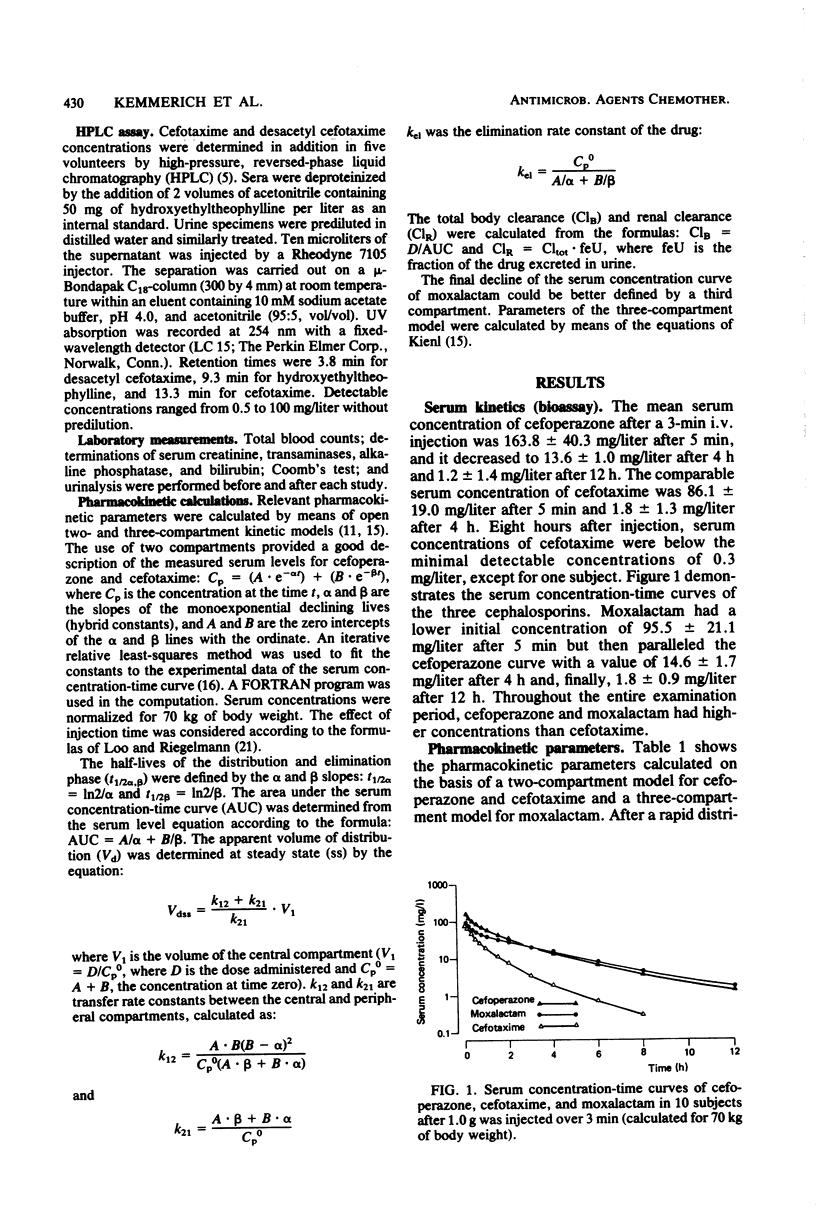
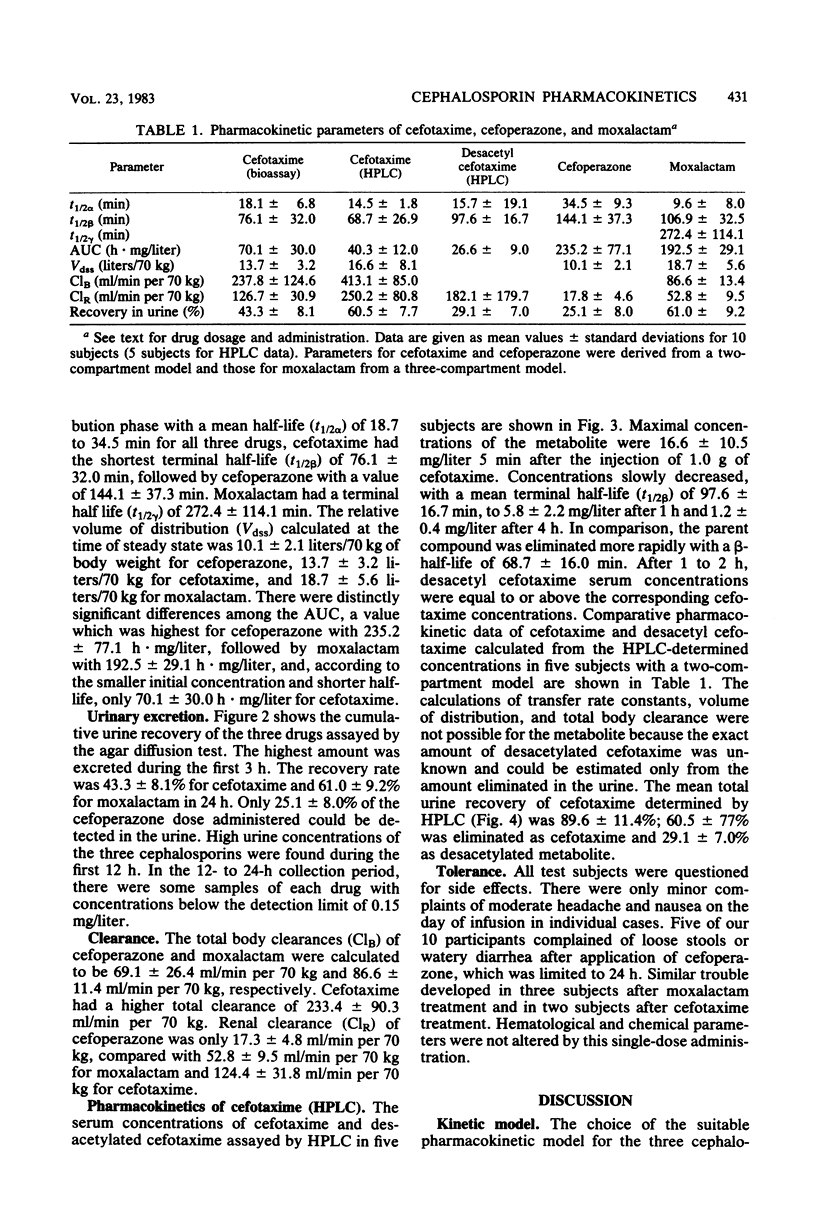
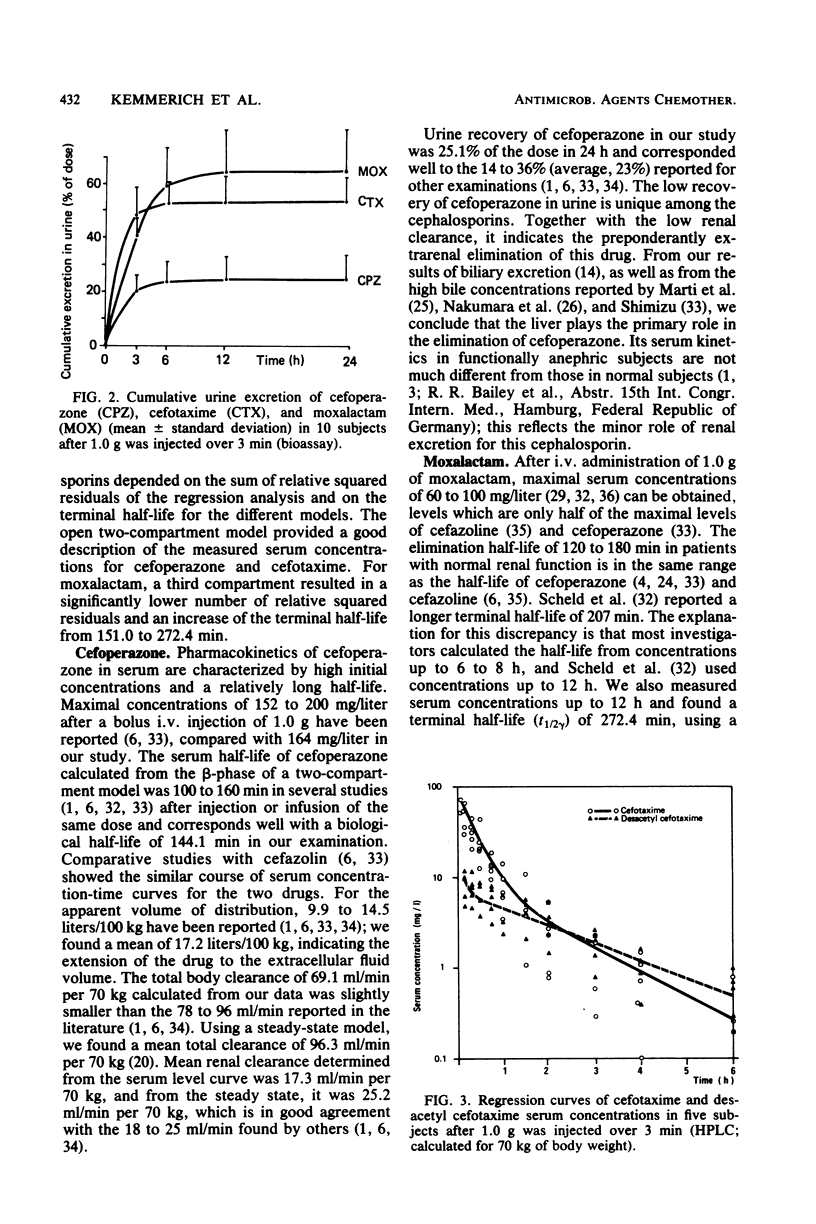
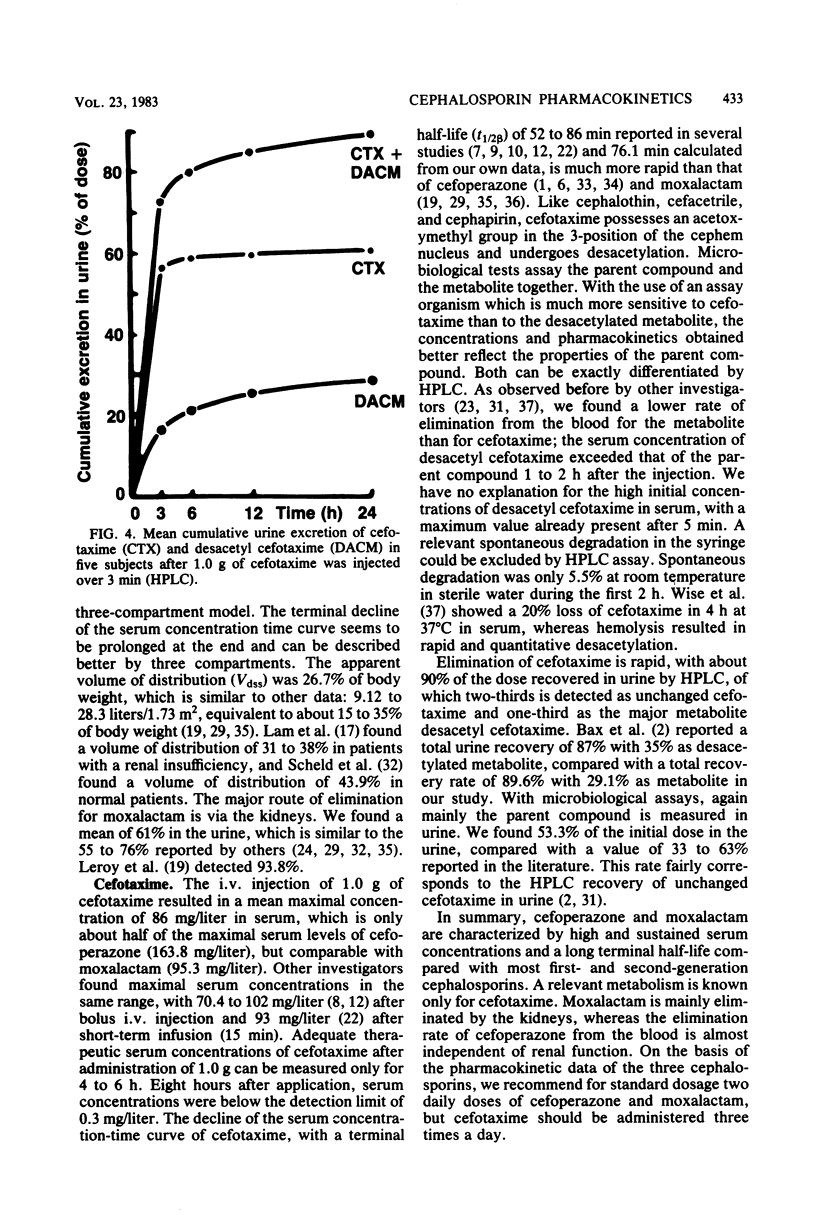
The pharmacokinetics of cefoperazone, cefotaxime, and moxalactam were compared in a cross-over randomized study in 10 healthy volunteers. Each subject received 1.0 g of the three drugs by bolus intravenous injection over 3 min. Serum and urine concentrations were assayed by a microbiological method and in addition by high-pressure liquid chromatography (HPLC) for cefotaxime in five subjects. Maximal concentrations in serum 5 min after the injection were 163 +/- 40.3 mg/liter for cefoperazone, 86.1 +/- 19.0 mg/liter for cefotaxime, and 95.5 +/- 21.1 mg/liter for moxalactam. After 12 h, 1.2 +/- 1.4 mg of cefoperazone per liter and 1.8 +/- 0.9 mg of moxalactam per liter could still be measured. Eight hours after the administration of cefotaxime, serum concentrations were below the detection limit of 0.3 mg/liter in most subjects. By HPLC analysis, the mean maximal concentration of desacetyl cefotaxime was 16.6 +/- 10.5 mg/liter 5 min after application; the metabolite exceeded the serum concentration of the parent compound after 1 to 2 h. Relevant pharmacokinetic parameters were calculated, using two- and three-compartment models. The terminal half-life was 144.1 +/- 37.3 min for cefoperazone, 76.1 +/- 32.0 min for cefotaxime, and 272.4 +/- 114.1 min for moxalactam. The apparent volume of distribution corresponded to the extracellular volume. Only 25.1 +/- 8% of cefoperazone could be detected in urine compared with 53.3 +/- 8.1% of cefotaxime and 61.0 +/- 9.2% of moxalactam in 24 h. A total of 89.6 +/- 11.4% of the cefotaxime dose could be recovered by HPLC in urine, 60.6 +/- 7.7% as cefotaxime and 29.1 +/- 7.0% as desacetyl cefotaxime.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balant L., Dayer P., Rudhardt M., Allaz A. F., Fabre J. Cefoperazone: pharmacokinetics in humans with normal and impaired renal function and pharmacokinetics in rats. Clin Ther. 1980;3(Spec Issue):50–59. [PubMed] [Google Scholar]
- Bolton W. K., Scheld W. M., Spyker D. A., Overby T. L., Sande M. A. Pharmacokinetics of moxalactam in subjects with various degrees of renal dysfunction. Antimicrob Agents Chemother. 1980 Dec;18(6):933–938. doi: 10.1128/aac.18.6.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton W. K., Scheld W. M., Spyker D. A., Sande M. A. Pharmacokinetics of cefoperazone in normal volunteers and subjects with renal insufficiency. Antimicrob Agents Chemother. 1981 May;19(5):821–825. doi: 10.1128/aac.19.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig W. A. Single-dose pharmacokinetics of cefoperazone following intravenous administration. Clin Ther. 1980;3(Spec Issue):46–49. [PubMed] [Google Scholar]
- Esmieu F., Guibert J., Rosenkilde H. C., Ho I., Le Go A. Pharmacokinetics of cefotaxime in normal human volunteers. J Antimicrob Chemother. 1980 Sep;6 (Suppl A):83–92. doi: 10.1093/jac/6.suppl_a.83. [DOI] [PubMed] [Google Scholar]
- Estey E. H., Weaver S. S., Ho D. H., Bodey G. P. Clinical pharmacology of moxalactam in patients with malignant disease. Antimicrob Agents Chemother. 1981 Apr;19(4):639–644. doi: 10.1128/aac.19.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillastre J. P., Leroy A., Humbert G., Godin M. Pharmacokinetics of cefotaxime in subjects with normal and impaired renal function. J Antimicrob Chemother. 1980 Sep;6 (Suppl A):103–111. doi: 10.1093/jac/6.suppl_a.103. [DOI] [PubMed] [Google Scholar]
- Fu K. P., Aswapokee P., Ho I., Matthijssen C., Neu H. C. Pharmacokinetics of cefotaxime. Antimicrob Agents Chemother. 1979 Nov;16(5):592–597. doi: 10.1128/aac.16.5.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt D. J., Koch-Weser J. Clinical pharmacokinetics (second of two parts). N Engl J Med. 1975 Nov 6;293(19):964–970. doi: 10.1056/NEJM197511062931905. [DOI] [PubMed] [Google Scholar]
- Kayser F. H. Microbiological studies on cefoperazone. Clin Ther. 1980;3(Spec Issue):24–33. [PubMed] [Google Scholar]
- Koeppe P., Hamann C. A program for non-linear regression analysis to be used on desk-top computers. Comput Programs Biomed. 1980 Dec;12(2-3):121–128. doi: 10.1016/0010-468x(80)90058-6. [DOI] [PubMed] [Google Scholar]
- Lam M., Manion C. V., Czerwinski A. W. Pharmacokinetics of moxalactam in patients with renal insufficiency. Antimicrob Agents Chemother. 1981 Mar;19(3):461–464. doi: 10.1128/aac.19.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang S. D., Edwards D. J., Durack D. T. Comparison of cefoperazone, cefotaxime, and moxalactam (LY127935) against aerobic gram-negative bacilli. Antimicrob Agents Chemother. 1980 Mar;17(3):488–493. doi: 10.1128/aac.17.3.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy A., Humbert G., Fillastre J. P. Pharmacokinetics of moxalactam in subjects with normal and impaired renal function. Antimicrob Agents Chemother. 1981 Jun;19(6):965–971. doi: 10.1128/aac.19.6.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lode H., Kemmerich B., Koeppe P., Belmega D., Jendroschek H. Comparative pharmacokinetics of cefoperazone and cefotaxime. Clin Ther. 1980;3(Spec Issue):80–88. [PubMed] [Google Scholar]
- Loo J. C., Riegelman S. Assessment of pharmacokinetic constants from postinfusion blood curves obtained after I.V. infusion. J Pharm Sci. 1970 Jan;59(1):53–55. doi: 10.1002/jps.2600590107. [DOI] [PubMed] [Google Scholar]
- Lüthy R., Blaser J., Bonetti A., Simmen H., Wise R., Siegenthaler W. Comparative multiple-dose pharmacokinetics of cefotaxime, moxalactam, and ceftazidime. Antimicrob Agents Chemother. 1981 Nov;20(5):567–575. doi: 10.1128/aac.20.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüthy R., Münch R., Blaser J., Bhend H., Siegenthaler W. Human pharmacology of cefotaxime (HR 756), a new cephalosporin. Antimicrob Agents Chemother. 1979 Aug;16(2):127–133. doi: 10.1128/aac.16.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maksymiuk A. W., LeBlanc B. M., Brown N. S., Ho D. H., Bodey G. P. Pharmacokinetics of cefoperazone in patients with neoplastic disease. Antimicrob Agents Chemother. 1981 Jun;19(6):1037–1041. doi: 10.1128/aac.19.6.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Hashimoto I., Sawada Y., Mikami J., Bekki E., Hirasawa S., Abe H., Watanabe Y. Cefoperazone concentrations in bile and gall bladder wall after intravenous administration. Antimicrob Agents Chemother. 1980 Dec;18(6):980–982. doi: 10.1128/aac.18.6.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Aswapokee N., Aswapokee P., Fu K. P. HR 756, a new cephalosporin active against gram-positive and gram-negative aerobic and anaerobic bacteria. Antimicrob Agents Chemother. 1979 Feb;15(2):273–281. doi: 10.1128/aac.15.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Fu K. P., Aswapokee N., Aswapokee P., Kung K. Comparative activity and beta-lactamase stability of cefoperazone, a piperazine cephalosporin. Antimicrob Agents Chemother. 1979 Aug;16(2):150–157. doi: 10.1128/aac.16.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons J. N., Romano J. M., Levison M. E. Pharmacology of a new 1-oxa-beta-lactam (LY127935) in normal volunteers. Antimicrob Agents Chemother. 1980 Feb;17(2):226–228. doi: 10.1128/aac.17.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves D. S., White L. O., Holt H. A., Bahari D., Bywater M. J., Bax R. P. Human metabolism of cefotaxime. J Antimicrob Chemother. 1980 Sep;6 (Suppl A):93–101. doi: 10.1093/jac/6.suppl_a.93. [DOI] [PubMed] [Google Scholar]
- Scheld W. M., Spyker D. A., Donowitz G. R., Bolton W. K., Sande M. A. Moxalactam and cefazolin: comparative pharmacokinetics in normal subjects. Antimicrob Agents Chemother. 1981 Apr;19(4):613–619. doi: 10.1128/aac.19.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu K. Cefoperazone: absorption, excretion, distribution, and metabolism. Clin Ther. 1980;3(Spec Issue):60–79. [PubMed] [Google Scholar]
- Srinivasan S., Francke E. L., Neu H. C. Comparative pharmacokinetics of cefoperazone and cefamandole. Antimicrob Agents Chemother. 1981 Feb;19(2):298–301. doi: 10.1128/aac.19.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S., Fu K. P., Neu H. C. Pharmacokinetics of moxalactam and cefazolin compared in normal volunteers. Antimicrob Agents Chemother. 1981 Feb;19(2):302–305. doi: 10.1128/aac.19.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R., Baker S., Livingston R. Comparison of cefotaxime and moxalactam pharmacokinetics and tissue levels. Antimicrob Agents Chemother. 1980 Sep;18(3):369–371. doi: 10.1128/aac.18.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R., Wright N., Wills P. J. Pharmacology of cefotaxime and its desacetyl metabolite in renal and hepatic disease. Antimicrob Agents Chemother. 1981 Apr;19(4):526–531. doi: 10.1128/aac.19.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]



