Abstract
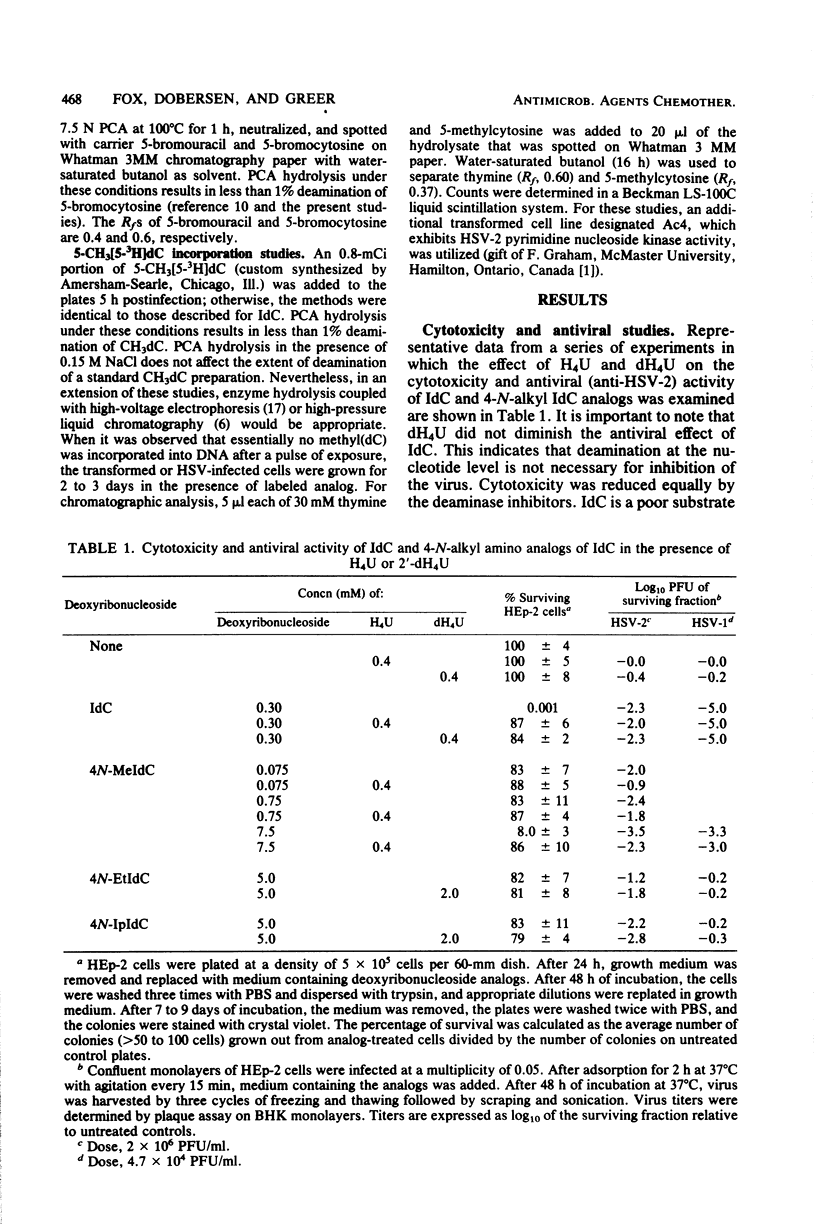
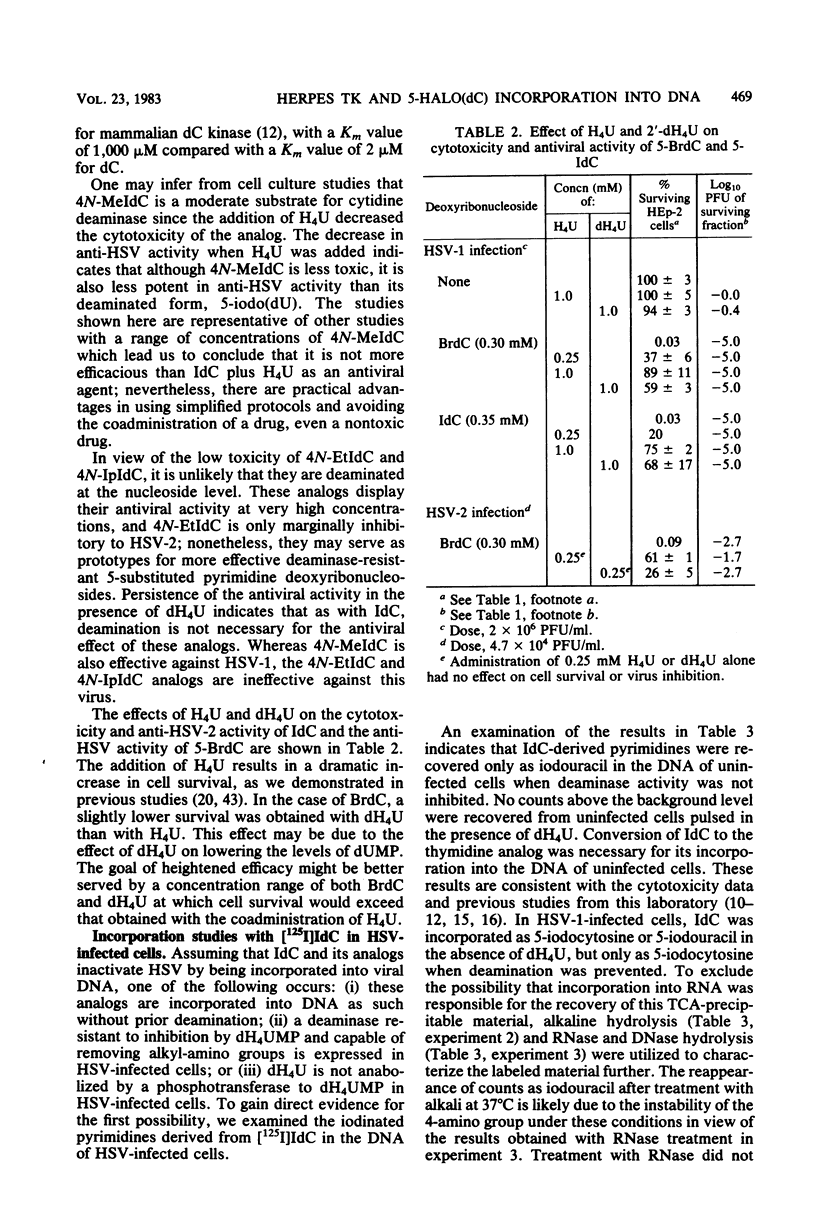
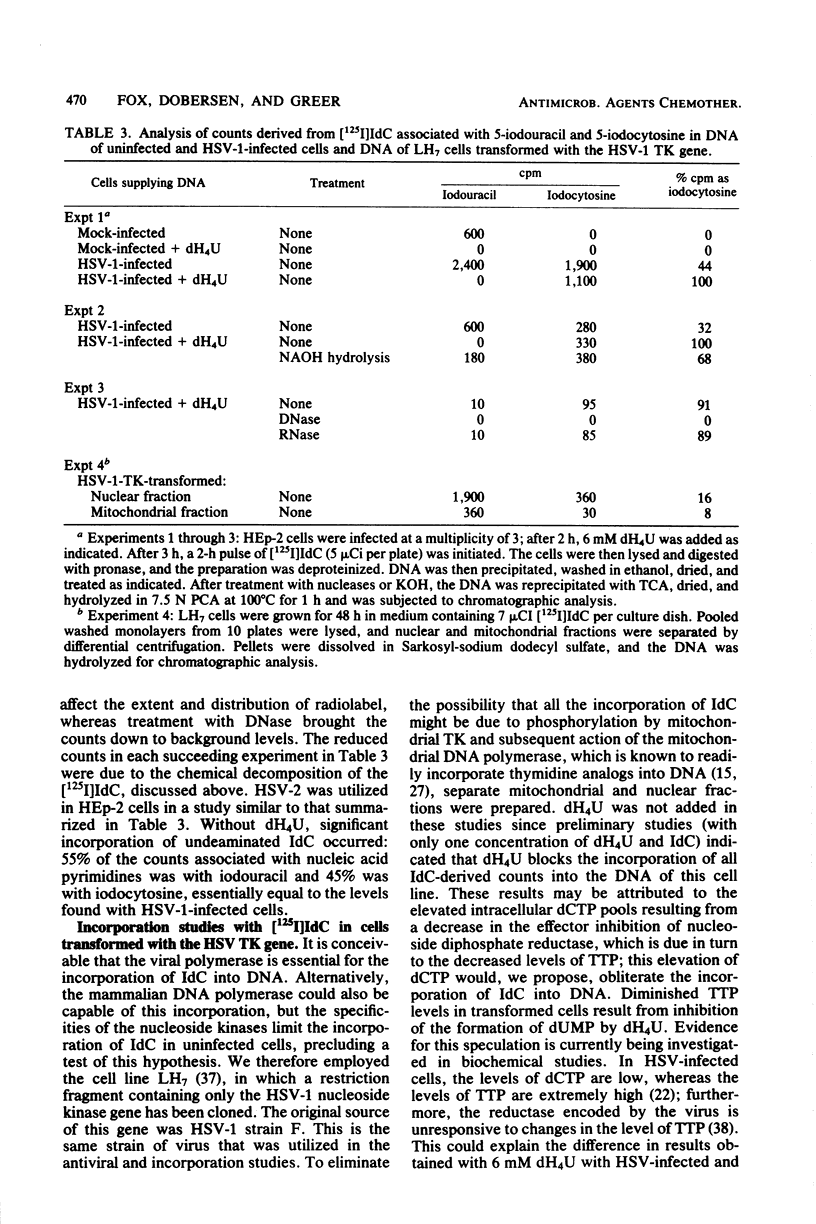
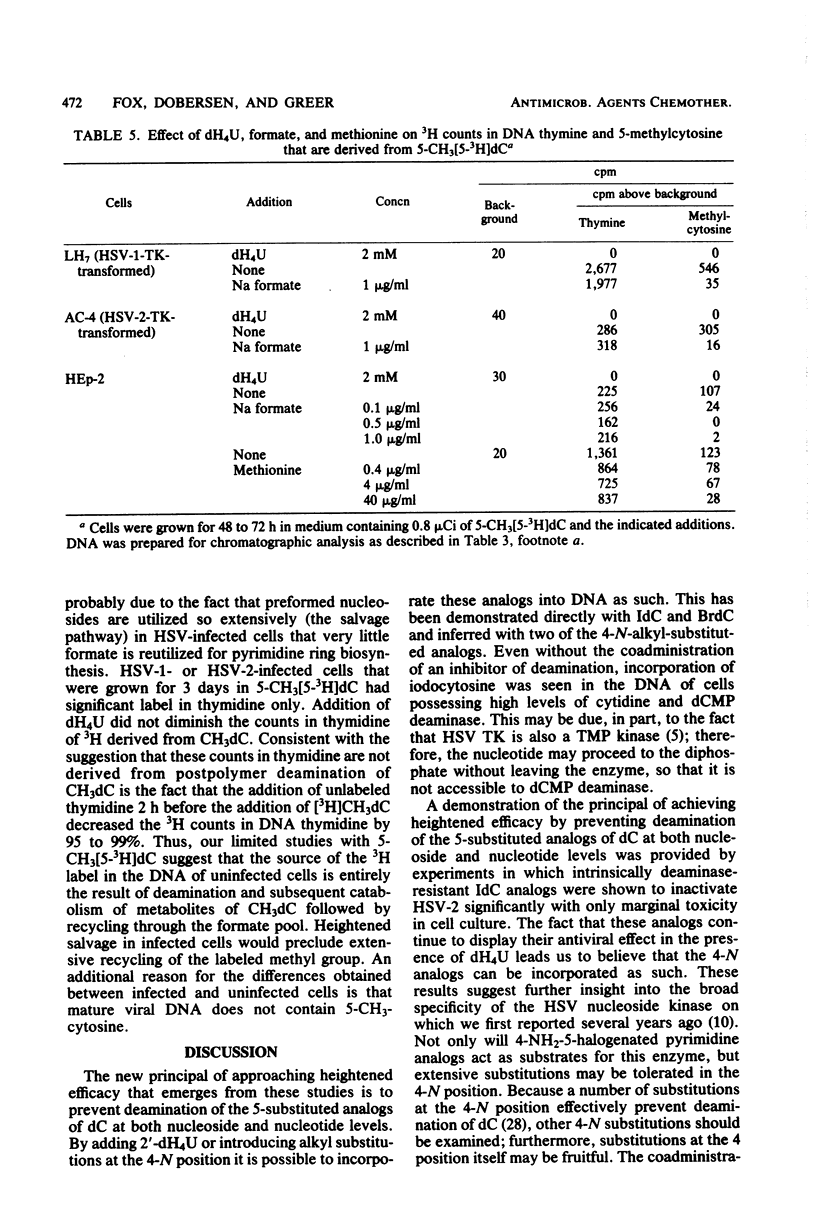
The incorporation into DNA of 5-bromocytosine and 5-iodocytosine, derived from their respective administered deoxyribonucleoside analogs, has been demonstrated in studies with cells infected with herpes simplex virus types 1 and 2 (HSV-1 and HSV-2) and in cells transformed with the thymidine kinase gene of HSV-1. No significant incorporation of iodocytosine or iodouracil occurred in the DNA of uninfected or nontransformed cells when the deaminating enzymes were inhibited, in accord with past studies in our laboratory with 5-bromodeoxycytidine and tetrahydrouridine. When 2′-deoxytetrahydrouridine, a potent inhibitor of cytidine deaminase and dCMP deaminase, was utilized, all the counts in DNA that were derived from [125I]iododeoxycytidine appeared as iodocytosine in HSV-infected cells. In the absence of a deaminase inhibitor, 32 to 45% of the counts associated with DNA pyrimidines appeared as iodocytosine, and 55 to 68% appeared as iodouracil in HSV-infected cells. Substantial incorporation of iodocytosine (16%) occurred in cells transformed with the HSV thymidine kinase gene, suggesting the importance of the specificity of cellular nucleoside kinases and the activity of the deaminases in presenting unmodified bases to an undiscriminating polymerase. Incorporation into DNA of bromocytosine derived from [3H]bromodeoxycytidine was demonstrated in HSV-2 infected cells; very little incorporation of bromocytosine compared with bromouracil could be demonstrated in these cells in the absence of inhibition of the deaminases (19% of the total counts associated with pyrimidines with deaminase inhibition and 1.5% without). Limited studies with 5-methyl[5-3H]deoxycytidine indicated essentially no (or very little) incorporation of this analog as such in the DNA of HSV-1- and HSV-2-infected and -transformed cells. This suggests an exclusion or repair mechanism preventing inappropriate methylcytosine incorporation in DNA. The addition of nucleoside and deoxyribonucleoside deaminase inhibitors, which leads to the incorporation of 5-halogenated analogs of deoxycytidine into DNA as such, does not impair their antiviral activity. We infer from studies with 4-N-alkyl (ethyl and isopropyl)-substituted analogs of iododeoxycytidine that they are incorporated as such into DNA without deamination and effectively inhibit the virus at concentrations that are marginally toxic. Among the several reasons presented for the heightened potential efficacy of analogs of deoxycytidine compared with those of deoxyuridine is that the former, as analogs of 5-methyldeoxycytidine, may impair viral replication by perturbing processes involving methylation and changes in the methylation of deoxycytidine in DNA which appear to be important for the process of HSV maturation. In addition, this capacity to perturb methylation may, in turn, be the key to their potential as agents affecting entry into or emergence from latency, a process in which dramatic changes in the postpolymer 5-methylation of deoxycytidine occur in the DNA of herpesviruses.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bacchetti S., Graham F. L. Transfer of the gene for thymidine kinase to thymidine kinase-deficient human cells by purified herpes simplex viral DNA. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1590–1594. doi: 10.1073/pnas.74.4.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessman M. J., Lehman I. R., Adler J., Zimmerman S. B., Simms E. S., Kornberg A. ENZYMATIC SYNTHESIS OF DEOXYRIBONUCLEIC ACID. III. THE INCORPORATION OF PYRIMIDINE AND PURINE ANALOGUES INTO DEOXYRIBONUCLEIC ACID. Proc Natl Acad Sci U S A. 1958 Jul 15;44(7):633–640. doi: 10.1073/pnas.44.7.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird A. P. Use of restriction enzymes to study eukaryotic DNA methylation: II. The symmetry of methylated sites supports semi-conservative copying of the methylation pattern. J Mol Biol. 1978 Jan 5;118(1):49–60. doi: 10.1016/0022-2836(78)90243-7. [DOI] [PubMed] [Google Scholar]
- CREASEY W. A. Studies on the metabolism of 5-iodo-2'-deoxycytidine in vitro. Purification of nucleoside deaminase from mouse kidney. J Biol Chem. 1963 May;238:1772–1776. [PubMed] [Google Scholar]
- Camiener G. W. Studies of the enzymatic deamination of ara-cytidine. V. inhibition in vitro and in vivo by tetrahydrouridine and other reduced pyrimidine nucleosides. Biochem Pharmacol. 1968 Sep;17(9):1981–1991. doi: 10.1016/0006-2952(68)90114-7. [DOI] [PubMed] [Google Scholar]
- Chen M. S., Prusoff W. H. Association of thymidylate kinase activity with pyrimidine deoxyribonucleoside kinase induced by herpes simplex virus. J Biol Chem. 1978 Mar 10;253(5):1325–1327. [PubMed] [Google Scholar]
- Christman J. K., Price P., Pedrinan L., Acs G. Correlation between hypomethylation of DNA and expression of globin genes in Friend erythroleukemia cells. Eur J Biochem. 1977 Nov 15;81(1):53–61. doi: 10.1111/j.1432-1033.1977.tb11926.x. [DOI] [PubMed] [Google Scholar]
- Christman J. K. Separation of major and minor deoxyribonucleoside monophosphates by reverse-phase high-performance liquid chromatography: a simple method applicable to quantitation of methylated nucleotides in DNA. Anal Biochem. 1982 Jan 1;119(1):38–48. doi: 10.1016/0003-2697(82)90662-5. [DOI] [PubMed] [Google Scholar]
- Clough D. W., Kunkel L. M., Davidson R. L. 5-Azacytidine-induced reactivation of a herpes simplex thymidine kinase gene. Science. 1982 Apr 2;216(4541):70–73. doi: 10.1126/science.6175023. [DOI] [PubMed] [Google Scholar]
- Constantinides P. G., Jones P. A., Gevers W. Functional striated muscle cells from non-myoblast precursors following 5-azacytidine treatment. Nature. 1977 May 26;267(5609):364–366. doi: 10.1038/267364a0. [DOI] [PubMed] [Google Scholar]
- Cooper G. M., Greer S. Phosphorylation of 5-halogenated deoxycytidine analogues by deoxycytidine kinase. Mol Pharmacol. 1973 Nov;9(6):704–710. [PubMed] [Google Scholar]
- Cooper G. M., Greer S. The effect of inhibition of cytidine deaminase by tetrahydrouridine on the utilization of deoxycytidine and 5-bromodeoxycytidine for deoxyribonucleic acid synthesis. Mol Pharmacol. 1973 Nov;9(6):698–703. [PubMed] [Google Scholar]
- Cooper G. M. Phosphorylation of 5-bromodeoxycytidine in cells infected with herpes simplex virus. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3788–3792. doi: 10.1073/pnas.70.12.3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E., Balzarini J., Descamps J., Huang G. F., Torrence P. F., Bergstrom D. E., Jones A. S., Serafinowski P., Verhelst G., Walker R. T. Antiviral, antimetabolic, and cytotoxic activities of 5-substituted 2'-deoxycytidines. Mol Pharmacol. 1982 Jan;21(1):217–223. [PubMed] [Google Scholar]
- Dobersen M. J., Greer S. Herpes simplex virus type 2 induced pyrimidine nucleoside kinase: enzymatic basis for the selective antiherpetic effect of 5-halogenated analogues of deoxycytidine. Biochemistry. 1978 Mar 7;17(5):920–928. doi: 10.1021/bi00598a028. [DOI] [PubMed] [Google Scholar]
- Dobersen M. J., Jerkofsky M., Greer S. Enzymatic basis for the selective inhibition of varicella-zoster virus by 5-halogenated analogues of deoxycytidine. J Virol. 1976 Nov;20(2):478–486. doi: 10.1128/jvi.20.2.478-486.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford J. P., Coca-Prados M., Hsu M. T. Enzymatic analysis of 5-methylcytosine content in eukaryotic DNA. Study of intracellular Simian Virus 40 DNA. J Biol Chem. 1980 Aug 25;255(16):7544–7547. [PubMed] [Google Scholar]
- Fox L. M., Mekras J. A., Bagwell C. B., Greer S. B. Capacity of deoxycytidine to selectively antagonize cytotoxicity of 5-halogenated analogs of deoxycytidine without loss of antiherpetic activity. Antimicrob Agents Chemother. 1982 Sep;22(3):431–441. doi: 10.1128/aac.22.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason M. K., Fraenkel-Conrat H. Biological consequences of incorporation of 5-fluorocytidine in the RNA of 5-fluorouracil-treated eukaryotic cells. Proc Natl Acad Sci U S A. 1976 May;73(5):1528–1531. doi: 10.1073/pnas.73.5.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer S., Schildkraut I., Zimmerman T., Kaufman H. 5-Halogenated analogs of deoxycytidine as selective inhibitors of the replication of herpes simplex viruses in cell culture and related studies of intracranial herpes simplex virus infections in mice. Ann N Y Acad Sci. 1975 Aug 8;255:359–365. doi: 10.1111/j.1749-6632.1975.tb29243.x. [DOI] [PubMed] [Google Scholar]
- Jackson R. C. The regulation of thymidylate biosynthesis in Novikoff hepatoma cells and the effects of amethopterin, 5-fluorodeoxyuridine, and 3-deazauridine. J Biol Chem. 1978 Oct 25;253(20):7440–7446. [PubMed] [Google Scholar]
- Jamieson A. T., Bjursell G. Deoxyribonucleoside triphosphate pools in herpes simplex type 1 infected cells. J Gen Virol. 1976 Apr;31(1):101–113. doi: 10.1099/0022-1317-31-1-101. [DOI] [PubMed] [Google Scholar]
- Jerkofsky M. A., Dobersen M. J., Greer S. Selective inhibition of the replication of varicella-zoster virus by 5-halogenated analogs of deoxycytidine. Ann N Y Acad Sci. 1977 Mar 4;284:389–395. doi: 10.1111/j.1749-6632.1977.tb21975.x. [DOI] [PubMed] [Google Scholar]
- Jerkofsky M., Dobersen M. J., Greer S. Inhibition of the replication of human cytomegalovirus by bromodeoxycytidine in the absence of detectable increases in bromodeoxycytidine kinase activity. Intervirology. 1980;14(5-6):233–238. doi: 10.1159/000149191. [DOI] [PubMed] [Google Scholar]
- Jones P. A., Taylor S. M. Cellular differentiation, cytidine analogs and DNA methylation. Cell. 1980 May;20(1):85–93. doi: 10.1016/0092-8674(80)90237-8. [DOI] [PubMed] [Google Scholar]
- Krenitsky T. A., Tuttle J. V., Koszalka G. W., Chen I. S., Beacham L. M., 3rd, Rideout J. L., Elion G. B. Deoxycytidine kinase from calf thymus. Substrate and inhibitor specificity. J Biol Chem. 1976 Jul 10;251(13):4055–4061. [PubMed] [Google Scholar]
- Lansman R. A., Clayton D. A. Selective nicking of mammalian mitochondrial DNA in vivo: photosensitization by incorporation of 5-bromodeoxyuridine. J Mol Biol. 1975 Dec 25;99(4):761–776. doi: 10.1016/s0022-2836(75)80183-5. [DOI] [PubMed] [Google Scholar]
- MALEY F. Nucleotide interconversions. VII. The effect of halogenated and N-methyl derivatives of deoxycytidylic acid on deoxycytidylate deaminase and thymidylate synthetase. Biochim Biophys Acta. 1962 Jul 9;61:135–138. doi: 10.1016/0926-6550(62)90040-3. [DOI] [PubMed] [Google Scholar]
- MALEY G. F., MALEY F. Nucleotide interconversions. X. Deoxyribo- and ribonucleoside 5'-phosphate synthesis via a phosphotransferase reaction in chick embryo extracts. Arch Biochem Biophys. 1963 May;101:342–349. doi: 10.1016/s0003-9861(63)80022-3. [DOI] [PubMed] [Google Scholar]
- Maley F., Maley G. F. Tetrahydrodeoxyuridylate: a potent inhibitor of deoxycytidylate deaminase. Arch Biochem Biophys. 1971 Jun;144(2):723–729. doi: 10.1016/0003-9861(71)90379-1. [DOI] [PubMed] [Google Scholar]
- Meuth M., Green H. Induction of a deoxycytidineless state in cultured mammalian cells by bromodeoxyuridine. Cell. 1974 Jun;2(2):109–112. doi: 10.1016/0092-8674(74)90099-3. [DOI] [PubMed] [Google Scholar]
- Nakayama K., Ruth J. L., Cheng Y. C. Differential effect of nucleoside analog triphosphates on ribonucleotide reductases from uninfected and herpes simplex virus-infected HeLa cells. J Virol. 1982 Jul;43(1):325–327. doi: 10.1128/jvi.43.1.325-327.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nass M. M. Mitochondrial DNA. I. Intramitochondrial distribution and structural relations of single- and double-length circular DNA. J Mol Biol. 1969 Jun 28;42(3):521–528. doi: 10.1016/0022-2836(69)90240-x. [DOI] [PubMed] [Google Scholar]
- Pater M. M., Pater A., di Mayorca G., Smiley J. R. Expression of herpesvirus thymidine kinase gene under control of early promoter of SV40. Virology. 1982 Mar;117(2):536–540. doi: 10.1016/0042-6822(82)90496-2. [DOI] [PubMed] [Google Scholar]
- Pellicer A., Wigler M., Axel R., Silverstein S. The transfer and stable integration of the HSV thymidine kinase gene into mouse cells. Cell. 1978 May;14(1):133–141. doi: 10.1016/0092-8674(78)90308-2. [DOI] [PubMed] [Google Scholar]
- Renis H. E. Comparison of cytotoxicity and antiviral activity of 1-beta-D-arabino-furanosyl-5-iodocytosine with related compounds. Cancer Res. 1970 Jan;30(1):189–194. [PubMed] [Google Scholar]
- Sager R., Kitchin R. Selective silencing of eukaryotic DNA. Science. 1975 Aug 8;189(4201):426–433. [PubMed] [Google Scholar]
- Salditt-Georgieff M., Jelinek W., Darnell J. E., Furuichi Y., Morgan M., Shatkin A. Methyl labeling of HeLa cell hnRNA: a comparison with mRNA. Cell. 1976 Feb;7(2):227–237. doi: 10.1016/0092-8674(76)90022-2. [DOI] [PubMed] [Google Scholar]
- Schildkraut I., Cooper G. M., Greer S. Selective inhibition of the replication of herpes simplex virus by 5-halogenated analogues of deoxycytidine. Mol Pharmacol. 1975 Mar;11(2):153–158. [PubMed] [Google Scholar]
- Summers W. C., Summers W. P. [125I]deoxycytidine used in a rapid, sensitive, and specific assay for herpes simplex virus type 1 thymidine kinase. J Virol. 1977 Oct;24(1):314–318. doi: 10.1128/jvi.24.1.314-318.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wataya Y., Santi D. V. Thymidylate synthetase catalyzed dehalogenation of 5-bromo-and 5-iodo-2'-deoxyuridylate. Biochem Biophys Res Commun. 1975 Nov 17;67(2):818–823. doi: 10.1016/0006-291x(75)90886-4. [DOI] [PubMed] [Google Scholar]


