Abstract
Studies of RecBCD–Chi interactions in Escherichia coli have served as a model to understand recombination events in bacteria. However, the existence of similar interactions has not been demonstrated in bacteria unrelated to E. coli. We developed an in vivo model to examine components of dsDNA break repair in various microorganisms. Here, we identify the major exonuclease in Lactococcus lactis, a Gram-positive organism evolutionarily distant from E. coli, and provide evidence for exonuclease–Chi interactions. Insertional mutants of L. lactis, screened as exonuclease-deficient, affected a single locus and resulted in UV sensitivity and recombination deficiency. The cloned lactococcal genes (called rexAB) restored UV resistance, recombination proficiency, and the capacity to degrade linear DNA, to an E. coli recBCD mutant. In this context, DNA degradation is specifically blocked by the putative lactococcal Chi site (5′-GCGCGTG-3′), but not by the E. coli Chi (5′-GCTGGTGG-3′) site. RexAB-mediated recombination was shown to be stimulated ≈27-fold by lactococcal Chi. Our results reveal that RexAB fulfills the biological roles of RecBCD and indicate that its activity is modulated by a short DNA sequence. We speculate that exonuclease/recombinase enzymes whose activities are modulated by short DNA sequences are widespread among bacteria.
The DNA repair machinery of Escherichia coli includes RecBCD, a powerful, double-stranded (ds) DNA exonuclease. It degrades dsDNA ends that may be produced, for example, by breaks at the replication fork (1, 2). Exonucleolytic activity of RecBCD is modulated by the sequence 5′-GCTGGTGG-3′ (3), called Chi (referred to as ChiEc), when properly oriented on a dsDNA linear substrate. The exonuclease appears to stall at ChiEc, and the enzyme continues on the DNA as a helicase, with modified exonuclease activity (4). Single-stranded DNA is generated, and recombination is stimulated downstream (with respect to the direction of DNA degradation) of ChiEc (see ref. 5 for review).
Despite its important role in E. coli, the extent to which an exonuclease–Chi interaction is involved in DNA repair in other organisms is unknown. In vitro studies suggest that RecBCD–Chi interactions are conserved in enterobacteria closely related to E. coli (6). Exonuclease analogs of RecBCD have been identified by activity and/or sequence homology in less closely related organisms, including Haemophilus influenzae, Bacillus subtilis, and Mycobacterium tuberculosis (refs. 7–10, EMBL/GenBank/DDBJ database). The B. subtilis exonuclease, AddAB, which has been studied extensively (11–13), shares little sequence similarity with RecBCD (mainly in ATP-binding motifs and helicase motifs present in RecB and AddA). However, it is functionally similar to RecBCD, because it has exonuclease and helicase activity, and is necessary for transduction and transformation (11). To date, exonuclease–Chi interactions have not been shown in these or any other organisms distant from E. coli.
We previously reported the sequence of the putative Chi site (5′-GCGCGTG-3′, referred to as ChiLl, and/or its complement) of Lactococcus lactis, a Gram-positive organism unrelated to E. coli (14). The sequence assignment was based on the capacity of ChiLl to modulate exonuclease activity on a linear dsDNA substrate provided by rolling circle (RC) plasmids (15). This finding strongly suggested that a RecBCD analog exists. Here, we confirm the existence of a RecBCD analog in L. lactis and show that it interacts specifically with the lactococcal ChiLl site to attenuate exonuclease activity and stimulate recombination. Our results argue for the existence of couples like RecBCD and ChiEc in a broad spectrum of microorganisms.
MATERIALS AND METHODS
Bacterial Strains and Plasmid Constructions.
Plasmid pRC1-nuc was constructed as follows: plasmid pBS∷nuc (16), digested with PstI and filled in, was fused to PvuII-linearized pVS41 (17). The resulting plasmid was cut by SmaI-EcoRV and religated to eliminate the pBS (pBluescript) replicon, resulting in a pVS41 replicon fused to nuc. To construct plasmid pRexAB, the small HincII-BamHI fragment of pBR322 was first cloned into HincII-BamHI-treated pGB2 (low-copy-number plasmid; ref. 18) to generate the vector. An FspI-ClaI L. lactis chromosomal DNA fragment containing rexB (recovered from the cloned junction of transposon insertion 1; see Fig. 2) was inserted in the SmaI-ClaI cut vector. The resulting plasmid was cut by HincII and ClaI, and a BsrGI (fill-in)-ClaI L. lactis chromosomal DNA fragment (recovered from the cloned junction of transposon insertion 2; Fig. 2) containing rexA was inserted. This construct contains the whole rexAB operon. To construct pGB2:PAragam, which expresses the bacteriophage λ gam gene (19) under control of an arabinose-inducible promoter, a blunted BamHI-SalI fragment containing gam was cloned into SmaI and SalI cut vector pBAD18 (20). The expression cassette, present on a ClaI-HindIII fragment, was recloned in AccI-HindIII sites of pGB2. Strains and plasmids used directly in experiments are presented in Tables 1 and 2, respectively.
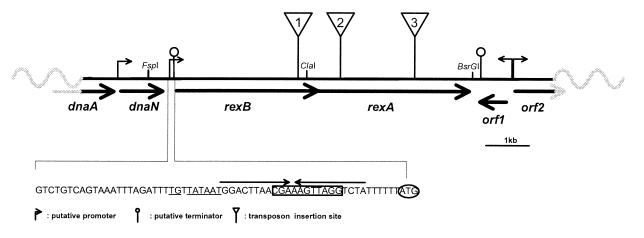
Figure 2.
Organization of the exonuclease genes. rexA and rexB are organized in tandem, and their ORFs overlap by 5 bp. Putative promoters, terminators, and transposon insertion sites are shown. Bold arrows indicate ORFs. Sequence of the rexB upstream region is shown. Underlined nucleotides, putative extended −10 promoter; boxed letters, putative Shine–Dalgarno sequence; arrows above nucleotides indicate a potential hairpin; start codon is circled. Restriction sites used in cloning are shown.
Table 1.
Strains used in this study
| Strain | Description | Source |
|---|---|---|
| L. lactis | ||
| MG1363 | Derivative of natural isolate ML3 cured of plasmids and prophages | 21 |
| VEL 1122 | recA derivative of MG1363, Tetr | 22 |
| CL56-5 | Eryr marker chromosomally integrated in MG1363 | P. Le Bourgeois |
| REX2 | Stable exonuclease-defective ISS1insertional MG1363 mutant (insertion “2” in Fig. 2) | This work |
| E. coli | ||
| AB1157 | thr-1 leu-6 proA2 his-4 thi-1 argE3 lacYI galK2 ara-14 xyl-5 mtl-1 tsx-33 supE44 rspL31 kdgK51 | A. J. Clark |
| JC5519 | As AB1157 but recB21recC22 | A. J. Clark |
| BW6175 | HfrPK3, PO137, argE86::Tn10 | 23 |
| V186 | Δ(thyA-argA)232 IN(rrnD-rrnE)1 | 24 |
| V182 | As V186 plus pDWS2 | 24 |
| TG1 | F′ traD36 lacIqΔ(lacZ)M15 proA+B+/supE Δ(hsdM-mcrB) (rK−mK−McrB−) thi Δ(lac-proAB) |
Table 2.
Plasmids used in this study*
| Plasmids | Description | Reference |
|---|---|---|
| pGh9:ISS1 | Transposon delivery vector | 25 |
| pRC1-nuc | RC plasmid pVS41, containing nuc reporter gene | This work |
| pDWS2 | pBR322 plasmid containing cloned recBCD genes of E. coli | 24 |
| pRC2 | pVS41 PvuII joined to large EcoRV-PvuII fragment of pACYC184 and containing MCS linker cloned into PvuII site | This work |
| pRC2-Chi-† | Chi- linker cloned into BamHI-EcoRI sites of pRC2 | This work |
| pRexAB | pGB2 joined to FspI-BsrGI fragment, which carries the rexAB genes of L. lactis | This work |
| pΔB1a | pBR322 with a 111-bp (ScaI-PvuI) deletion in the bla gene | This work |
| pGB2:PAragam | gam expression controlled by arabinose-inducible promoter | This work |
Details on vectors and oligonucleotide sequences in Materials and Methods.
Chi- corresponds to either ChiLl
, ChiLl1, ChiLl2, ChiLl1-2 or ChiEc, as noted in text.
Media, Bacterial Transformation, and DNA Preparation.
E. coli strains were grown in Luria–Bertani (LB) broth; 0.01% thymidine was added for strain V186 and derivatives. L. lactis strains were grown at 30°C in M17 medium containing 0.5% glucose (M17-glu). Antibiotics were used as follows: 50 μg/ml streptomycin (Str), 125 μg/ml rifampicin (Rif), 5 μg/ml erythromycin (Ery) for L. lactis and 150 μg/ml for E. coli, 100 μg/ml ampicillin (Amp), 15 μg/ml kanamycin (Kn); 50 μg/ml spectinomycin (Spc), 10 μg/ml chloramphenicol (Cm) for E. coli and 3 μg/ml for L. lactis, and 10 μg/ml tetracycline (Tet). L. lactis strains were electrotransformed as described (26). E. coli strains were electrotransformed (27) or transformed by the Hanahan method (28). Whole-cell DNA minilysates were prepared as described (29).
Detection of High-Molecular-Weight Linear Plasmid Multimers (HMW).
HMW accumulation was detected on whole-cell lysates after agarose gel electrophoresis (15). Plasmid DNA, labeled by chemiluminescence by using the ECL system (Amersham), was used as probe. Southern blot hybridization was performed as recommended by kit suppliers.
DNA Sequencing.
An 11-kb fragment containing the putative lactococcal exonuclease genes was amplified from genomic DNA by long accurate PCR (30). A random library of AluI partial subfragments (500–1,500-bp size range) of the 11-kb segment was inserted into SmaI-linearized and dephosphorylated M13mp18. Single-stranded DNA was prepared (Beckman Biomek 1000 laboratory workstation; ref. 31). DNA sequencing was performed by using the Applied Biosystems PRISM sequencing kit on the Catalyst station. To complete the sequence, dye terminator method (protocol supplied by Applied Biosystems) was used. We used xbap and xnip programs (32) for fragment assembly and consensus sequence analyses, and blast algorithm (on the National Center for Biotechnology Information e-mail server) for homology searches.
Cloning of Oligonucleotide Linkers.
Six oligonucleotide pairs were synthesized (DNA synthesizer Oligo 1000, Beckman): MCS, 5′-CTGGAATTCGTCGACGGATCC-3′ and 3′-GACCTTAAGCAGCTGCCTAGG-5′; ChiLlm, 5′-AATTCCTGCAGCCGCGTGG-3′ and 3′-GGACGTCGGCGCACCCTAG-5′; ChiLl1, 5′-AATTCCTGCAGCACGCGCG-3′ and 3′-GGACGTCGTGCGCGCCTAG-5′; ChiLl2, 5′-AATTCCTGCAGGCGCGTGG-3′ and 3′-GGACGTCCGCGCACCCTAG-5′; ChiLl1–2, 5′-AATTCACGCGCTGCAGGCGCGTGG-3′ and 3′-GTGCGCGACGTCCGCGCACCCTAG5′; ChiEc2, 5′-AATTCCTGCAGGCTGGTGGG-3′ and 3′-GGACGTCCGACCACCCCTAG-5′. Chi sites are underlined; the base in bold creates a mutation in ChiLl, resulting in ChiLlm. ChiLl2, ChiLlm, and ChiEc2 are oriented for recognition by a RecBCD molecule entering through the tail of a σ-form RC plasmid replication intermediate formed during plus-strand replication. ChiLl1 is in the opposite orientation. ChiLl1–2 contains sites in both orientations. Linker sequences were verified by DNA sequencing after cloning into plasmid pRC2.
Transposition Mutagenesis and Mutant Screening Test.
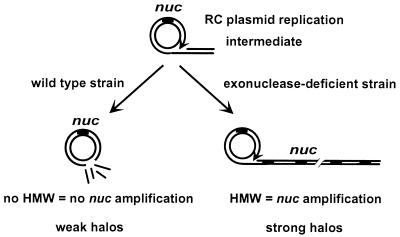
Plasmid pGh9:ISS1 was used to perform transposition mutagenesis (25) on strain MG1363 containing the HMW-reporter plasmid pRC1-nuc; potential exonuclease-defective mutants were screened (Fig. 1). pRC1-nuc encodes the staphylococcal nuclease (Nuc), a secreted enzyme (33). Because Nuc has no intracellular activity in L. lactis (Y. Le Loir and I. Poquet, this laboratory, unpublished results), its use does not affect intracellular DNA metabolism. Exonuclease-deficient mutants would accumulate HMW and therefore be detected as Nuc overproducers by plate tests by using an overlay containing Toluidine blue and denatured DNA (16). Accumulation of HMW in mutant candidates was verified by Southern hybridization by using pRC1-nuc DNA as probe.
Figure 1.
Screening system to isolate exonuclease-defective mutants of L. lactis. RC plasmid replication may generate a σ-shaped molecule with dsDNA tail (Upper; arrow indicates direction of replication). This tail may be degraded by exonuclease, thereby restoring a monomeric circle (Left Lower). Expression of the nuc reporter gene (present on pRC1-nuc) is low and results in weak halos around colonies. If the exonuclease is inactivated, the σ form is extended and HMW accumulates, amplifying the nuc gene. Amplification leads to overexpression of Nuc, which gives rise to strong halos of DNA digestion around colonies in plate tests (Right Lower).
A stable, exonuclease-defective ISS1-generated mutant, called REX2, was constructed by excision of the transposed vector (of transposition “2” in Fig. 2) as described (25); its structure was confirmed by Southern hybridization.
T4 gene 2 Test for Exonuclease Activity.
Bacteriophage T4 gene 2am mutant (T4g2; provided by W. Wackernagel, Univ. of Oldenburg, Germany) was used to examine exonuclease activity (34, 35). Plaque-forming units were measured in E. coli recB21recC22 strain JC5519 containing pRexAB, JC5519, or wild-type AB1157, as described (15) except that cultures and plates were incubated at 30°C. This temperature was used because RexAB exonuclease activity appears to be thermosensitive (note that optimal growth temperature of L. lactis is 30°C).
Conjugation Tests in L. lactis and E. coli.
Strr-Rifr derivatives of REX2 and of VEL1122 were isolated by first plating cells on Rif. Rifr clones were then plated on Str to select double Strr-Rifr mutants. Strr-Rifr strains were used as recipients in surface mating experiments (36, 53). Strain CL56–5 was the Hfr donor and IL1776 was the plasmid donor. CL56–5, a derivative of MG1363, contains a chromosomally integrated Eryr marker near the initiation site of chromosomal transfer (P. Le Bourgeois, Univ. Paul Sabatier, France). IL1776, an IL1403 derivative, contains plasmid pIL205, which confers Cm resistance; pIL205 is conjugative in the absence of homologous recombination (37). Mid-log phase donor and recipient cells were mixed at a ratio of 1:2, then 0.1 ml was spread on an M17-glu agar plate. After overnight incubation, cells were resuspended and dilutions were plated on M17-glu medium containing Ery, Cm, Ery-Str-Rif, or Cm-Str-Rif, as appropriate, or nonselective medium, for donor, recipient, and transconjugant cells. Control plates containing donor or recipient cells only were also plated. Conjugation frequency was determined as the number of transconjugants divided by the total number of recipients.
E. coli matings (38) were performed by using Hfr strain BW6175 as donor. Because the L. lactis exonuclease is thermosensitive, recipient strains were grown at 30°C. Matings were performed at 35°C for 30 min (pili are poorly formed at 30°C) and plated at either 35 or 30°C (conjugation efficiencies were similar at both temperatures).
Ultraviolet Light-Sensitivity Tests in L. lactis and in E. coli.
Tests (22) were performed on cells maintained at 30°C, except that irradiation was carried out with a Stratalinker 2400 UV source (Stratagene).
ChiLl-Stimulated Recombination Tests.
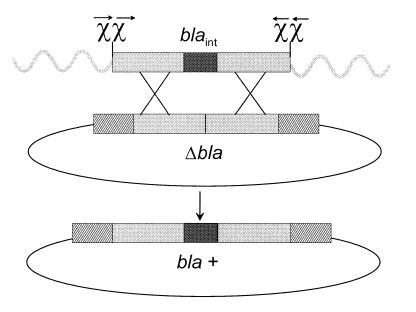
The strategy described by Dabert and Smith (39) to examine ChiEc-stimulated homologous recombination by gene conversion was modified to examine the effect of ChiLl. We constructed a deletion derivative of the β-lactamase gene (bla) of pBR322 so that a double crossover event would convert cells to Ampr. For this purpose, the ScaI-PvuI internal fragment of plasmid pBR322 (positions 3735 through 3846 of the pBR322 sequence) was deleted to give rise to pΔBla. Primers were designed to PCR-amplify a bla gene fragment (positions 3374 through 4214 on the pBR322 sequence) covering the DNA deleted from pΔBla, plus ≈360 bp additional bla gene DNA (see Fig. 4); primer tails immediately flanking bla gene DNA contained double ChiLl or ChiEc sites. Primer couples generating double ChiLl sites were: 5′-CAAATAGGTCTTATTGAAAATGATGGAGTACACGCGCTCACGCGCAATGTAGATAACTACGATACGGGAGGGC-3′ and 5′-CATTCAGCACAATGGTTGAATAATTACAAACACGCGCTCACGCGCTATCCGCTCATGAGACAATAACCCTG-3′. Primer couples generating double ChiEc sites were: 5′-CAAATAGGTCTTATTGAAAATGATGGAGTACCACCAGCTCCACCAGCATGTAGATAACTACGATACGGGAGGGC-3′ and 5′-CATTCAGCACAATGGTTGAATAATTACAAACCACCAGCTCCACCAGCTATCCGCTCATGAGACAATAACCCTG-3′. Chi site complements are in bold, and bla sequences are in italics. The resulting PCR fragments were cloned into the SmaI polylinker site of a pBS derivative deleted for the entire bla gene and replaced by a Knr marker (provided by P. Renault, Institut National de la Recherche Agronomique, France). The final linear fragment containing DNA internal to the bla gene flanked by Chi sites and surrounded by heterologous DNA was excised on a PvuII fragment, and its structure was confirmed by sequencing. Linear DNA samples were quantitated on agarose gels by using known quantities of marker DNA.
Figure 4.
Strategy to test ChiL1 effect on RexAB-mediated recombination. Linear transforming DNA contains an internal fragment of bla (blaint, solid rectangle) and has an additional 360 bp flanking homology with bla (grey rectangle). Double Chi sites on the linear fragments, (either ChiL1 or ChiEc) oriented for recognition to enhance recombination, are represented by χχ. Wavy lines represent heterologous dsDNA tails. Plasmid pΔBla bears an internal deletion of bla (Δbla), the target for gene conversion. Hatched rectangles represent ends of the bla gene not present on linear DNA. Double crossover homologous recombination would be required to convert cells to Ampr (bla+).
To determine the efficiency of gene conversion, electrocompetent cells were prepared. Known amounts of pACYC184 DNA were used to test competence. For electrotransformation of linear fragments, ≈200 ng DNA was used. Incubation after electrotransformation was performed at 35°C for 90 min. Colonies were counted after 48 hr at 35°C. Numbers of Ampr transformants obtained with ChiLl- or ChiEc-containing linear DNAs were compared for each strain. E. coli strain JC5519 recB21C22 containing pRexAB and pΔBla was used to test whether ChiLl stimulates RexAB-mediated homologous recombination. Electrocompetent recB21C22 containing pΔBla was used as negative recipient control, and TG1 containing pGB2:PAragam and pΔBla was used as positive control. Electrocompetent cells of this strain were prepared in LB containing 0.02% l-arabinose.
RESULTS
Isolation of Exonuclease-Defective Mutants of L. lactis.
A screening test was devised to isolate exonuclease-minus insertional mutants of L. lactis strain MG1363. RC plasmid DNA replication generates a linear dsDNA substrate that can be attacked by exonucleases (Fig. 1) (14, 15). If exonuclease activity is absent, a σ-form replication intermediate accumulates, giving rise to HMW. DNA amplification by HMW accumulation can be scored if a reporter gene is on the plasmid. An L. lactis MG1363 strain was constructed containing plasmid pRC1-nuc, which has a marker gene whose activity is detected by a plate overlay (Materials and Methods). pRC1-nuc, which lacks both ChiEc and ChiLl, is present only in monomeric form, and gives rise to weak halos. If HMW is generated through exonuclease inactivation, strong halos are produced because of marker gene amplification (Fig. 1). Insertion sequence delivery plasmid pGh9:ISS1 (25) was established in the strain to perform insertional mutagenesis.
From approximately 20,000 mutants screened for marker gene activity, ≈30 colonies produced large halos. Hybridization tests confirmed that three mutants accumulated HMW. Because our previous studies in E. coli indicated that HMW accumulates if the host strain is exonuclease-defective (15), we considered it likely that the three mutants interrupted a L. lactis gene encoding a major exonuclease.
Sequence Determination of Putative Exonuclease Genes.
DNA regions flanking the transposon insertion sites of the three mutants were recovered by cloning. Hybridization experiments revealed that the insertion sites were closely spaced (within ≈3 kb; Fig. 2), indicating that all three may be localized within a single genetic locus. Sequencing of the junctions distal to the transposon insertion sites delimited an 11-kb fragment that presumably encompasses the exonuclease gene(s). Long-range PCR was performed on genomic DNA extracted from the parental MG1363 strain to generated this 11-kb segment that was sequenced. Two ORFs of 1,100 and 1,174 aa were identified and found to share 23 and 34% overall identity with AddB and AddA of B. subtilis, respectively. AddAB is known to have several functions equivalent to those of RecBCD, including exonuclease, helicase, and recombinase activities (11, 12). These sequence similarities provided further evidence that the three mutants identified by the RC plasmid assay inactivate the major exonuclease of L. lactis. We tentatively assigned the name RexA and RexB (for Recombination exonuclease) to the identified ORFs encoded by rexA and rexB, respectively. The identification of a single genetic locus by our screening procedure confirms the use of RC plasmids as a biological probe of RecBCD-like exonuclease activity.
Sequence Analyses of the rexAB Operon.
The operon is localized near the dnaA homolog of L. lactis (Fig. 2) and is immediately adjacent to dnaN (encoding a DNA polymerase III subunit), thus placing rexAB close to the replication origin (ref. 40; A. Wind, Chr. Hansen, Denmark, personal communication). In other genomes, this position is occupied by recF (40). Like addAB of B. subtilis, rexAB genes are organized in tandem, with translational overlap, and are likely to be cotranscribed. The putative transcriptional initiation site of rexAB is characterized by an extended −10 promoter (41). In addition, a putative rho-independent transcriptional terminator of the upstream dnaN gene exists just downstream of the −10 promoter and overlaps the rexAB ribosome binding site (Fig. 2). This organization suggests that transcription of the exonuclease genes is affected by dnaN expression.
Biological Activities of an Exonuclease-Deficient L. lactis Strain.
REX2, a stable derivative of one of the insertional exonuclease mutants, showed UV light sensitivity intermediate between that of the parental MG1363 strain and the UV-sensitive recA mutant of MG1363 (ref. 22; Table 3). E. coli recBC (42) and B. subtilis addAB (12) mutants have similar phenotypes. Recombination capacity of REX2 was examined (Table 3) by using a lactococcal Hfr conjugation test. A Strr-Rifr derivative of REX2 was isolated and used as recipient. Compared with the parental strain recipient, conjugational transfer of a chromosomal marker was reduced ≈300-fold in REX2. Two other independently obtained Strr-Rifr REX2 derivatives gave comparable conjugation frequencies. As expected, transfer requires a recA-proficient recipient. Frequencies of plasmid conjugation, which does not require homologous recombination, were about the same in all recipients. These results indicate that the major exonuclease has a role in L. lactis repair and recombination that is equivalent to that reported in E. coli and B. subtilis.
Table 3.
Biological characteristics of the L. lactis REX2 mutant
| L. lactis strain | Relative survival and frequencies
|
||
|---|---|---|---|
| UV irradiation* | Conjugational recombination† | Plasmid conjugation‡ | |
| Wt (MG1363) | 1 | 1 | 1 |
| rexB (REX2) | 0.27 | 0.0034 | 0.97 |
| recA (VEL1122) | 0.02 | 0.00047 | 1.06 |
For each study, results are means of at least three experiments, and standard deviation <50% of mean values.
Cells were irradiated at 20 J/m2. Survival of wt strain was 0.11.
Recombination frequency of wt strain was 1.25 × 10−4 per viable recipient.
Plasmid pIL205 conjugational transfer in wt strain was 1.0 × 10−3.
Complementation of E. coli recBCD-Deficient Strains by the Cloned L. lactis Exonuclease Genes.
Plasmid pRexAB, carrying the two putative exonuclease subunit genes, was established in E. coli V186 ΔrecBCD or JC5519 recB21recC22 strains. Bacteriophage T4g2 DNA is sensitive to exonuclease degradation (34), and its plaque-forming ability provides a simple test to evaluate host exonuclease activity (35). A wild-type (wt) E. coli strain expressing RecBCD is resistant to T4g2 phage infection, whereas the recB21recC22 strain is sensitive (Table 4). When rexAB genes are present in the recB21recC22 strain, T4g2 multiplication is inhibited, thus showing that the lactococcal exonuclease is active in E. coli.
Table 4.
pRexAB complements a recBCD defect in E. coli
| E. coli strain [plasmid] | Relative values for
|
||
|---|---|---|---|
| pfu of T4g2* | Survival after UV irradiation† | Frequency of conjugational recombination‡ | |
| Wt | 0.0005 | 1 | 1 |
| recBCD | 0.0002 | 0.2 | 0.4 |
| [pRexAB] | |||
| recBCD | 1 | 0.0073 | 0.0038 |
For each study, results are means of at least three experiments, and standard deviation <50% of mean values.
Values given are relative to a plaque titer of 1010 pfu/ml on JC5519 (recBCD). Wt strain is AB1157.
Cells were irradiated at 25 J/m2. Survival of wt strain (V182) was 2.4 × 10−1. recBCD strain is V186.
Recombination frequency of wt strain (AB1157) was 1.6 × 10−3 per viable recipient. recBCD strain is JC5519.
UV sensitivity of the RecBCD-deficient strain was partially alleviated by the lactococcal exonuclease (Table 4), showing that the DNA repair defect can be complemented. Hfr crosses were performed to examine conjugational recombination efficiency in the presence of rexAB (Table 4). In the presence of pRexAB, recombination frequencies were within 2.5-fold of those measured in the wt recipient, and 100-fold higher than those in the recBCD-defective host (43). These results show that rexAB provides the functions necessary for efficient repair and recombination in E. coli.
Introduction of L. lactis Exonuclease Genes into a ΔrecBCD E. coli Strain Directs Chi Specificity to the Putative Lactococcal ChiLl Site.
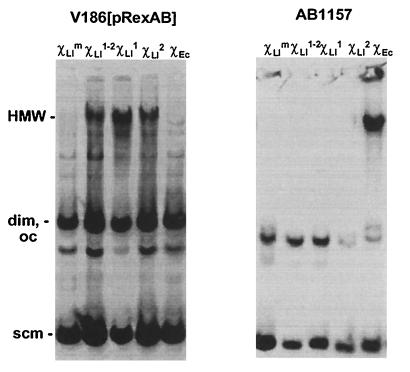
The accumulation of HMW is an accurate reflection of the presence of Chi on an RC plasmid (15). Plasmid pRC2 containing either ChiLl or ChiEc was established in an E. coli strain containing rexAB genes. pRC2 accumulated HMW when it contained ChiLl in either orientation but not when it contained ChiEc (Fig. 3). At present, we cannot explain the effect of ChiLl in both orientations (which was also observed in L. lactis; ref. 14). When the same plasmids were established in the wild-type E. coli strain, only plasmids containing ChiEc accumulated HMW (Fig. 3). These results show that RexAB exonuclease activity is modulated specifically by the putative ChiLl site. They also show that modulation of RexAB does not require other host-specific factors. These results lead us to propose that regulation of a major exonuclease by interaction with a specific DNA sequence is a feature conserved among diverse microorganisms.
Figure 3.
ChiLl is specifically recognized by RexAB. E. coli strains containing plasmid pRC2 with ChiLl or ChiEc were grown to mid-logarithmic phase, and whole-cell lysates were prepared. HMW was detected by Southern hybridization by using pRC2 as probe. Plasmids are: pRC2-ChiLlm (containing mutated ChiLl, χLlm lanes), pRC2-ChiLl1–2 (containing head-to-head copies of ChiLl, χLl1–2 lanes), pRC2-ChiLl1 (containing ChiLl in orientation 1, χLl1 lanes), pRC2-ChiLl2 (containing ChiLl in orientation 2, χLl2 lanes), pRC2-ChiEc2 (containing ChiEc in orientation 2, χEc lanes). Smear and bands below HMW in V186[pRexAB] strain appear to be secondary products of HMW accumulation.
RexAB-Mediated Recombination Is Stimulated by ChiLl in E. coli.
It was reported in E. coli that gene replacement with linear DNA fragments is stimulated by correctly oriented ChiEc sites in the DNA-flanking regions of homologies (39). To determine whether ChiLl stimulates RexAB-mediated recombination in an E. coli recB21recC22 strain, we modeled experiments on the published strategy. The target for gene replacement was on pΔBla. Restoration of bla on pΔBla by gene conversion with a linear molecule requires a double crossover event (Fig. 4). Constructions were made to produce linear fragments containing the internal region missing from pΔBla plus ≈360 bp of adjacent bla DNA. This DNA was flanked by sequences containing either double ChiLl or ChiEc sites on either side and heterologous DNA at the extremities (Fig. 4 and Materials and Methods). To first ensure that ChiLl- and ChiEc-containing fragments were potentially active for gene conversion, we used as recipient TG1 containing pGB2:PAragam and pΔBla; in this strain, expression of λ gam inhibits Chi recognition but allows Hfr recombination to occur (19). The two fragments transformed this strain with about equal efficiencies (Table 5). Similar results were obtained in a recBCsbcB recipient, which is recombination-competent but Chi activity-defective; however, electrocompetence of the strain was poor (data not shown). In contrast, nearly no transformants were obtained in the recB21recC22 recipient. We then tested for recombination in the presence of RexAB. E. coli recB21recC22 cells containing pRexAB and pΔBla were transformed with equivalent amounts of linear DNA containing either the ChiLl or ChiEc sites (Table 5). In this context, DNA fragments containing ChiLl gave ≈27-fold more transformants than those containing ChiEc. These results demonstrate that ChiLl is a crossover instigator that requires RexAB [hot-spot activity in the vicinity of Chi (44), remains to be shown]. We hypothesize that a strategy of DNA degradation and repair by an exonuclease and a short sequence that modulates its activity is widespread among bacteria.
Table 5.
ChiLl stimulates RexAB-mediated recombination
| E. coli strain (plasmids) | Transformation efficiency
|
No. Ampr transformants* (No. tfo)
|
ChiLl stimulation† | |
|---|---|---|---|---|
| pACYC184 (1 μg) | ChiLl‡ | ChiEc‡ | ||
| recB21C22 | 3 × 109 | 650 | 22 | 27† |
| (pΔB1a, pRexAB) | (7) | (7) | ||
| recB21C22 | 8 × 109 | 8 | 7 | 1 |
| (pΔB1a) | (2) | (2) | ||
| Wt | 1.5 × 1010 | 615 | 567 | 1† |
| (pGB2:PAragam, pΔB1a) | (4) | (4) | ||
Values represent total number of transformants from multiple experiments (number in parentheses).
ChiLl stimulation is the ratio of Ampr transformants obtained with linear DNA fragments containing ChiLl compared with ChiEc. The presented ratio was corrected by taking into account transformation efficiencies in the strain depleted for Chi activity (TG1 containing pGB2:PAragam and pΔB1a).
ChiLl and ChiEc correspond to double Chi sites present at ends of linear fragments used for gene conversion.
DISCUSSION
Conservation of Exonuclease–Chi Interactions in Bacteria.
Demonstration in E. coli of exonuclease–Chi activities have led to the assumption that a strategy for recombination and DNA repair involving modulation of exonucleolytic activity by specific DNA sequences is ubiquitous among bacteria and possibly in higher organisms (45, 46). Our demonstration of exonuclease–Chi interactions in L. lactis, a Gram-positive organism distant from E. coli, provides support for these assumptions for bacteria, despite little conservation of enzyme sequences. Furthermore, demonstration of RexAB-ChiLl-mediated DNA protection and recombination in a heterologous host suggests that these processes do not require other host-specific factors.
The biological test based on RC plasmids we developed to analyze the exonuclease and Chi in L. lactis appears to be specific for these two elements. It thus constitutes a simple test that could be used to study analogs in other organisms in which RC plasmids replicate (14).
Extent of Exonuclease Conservation.
The functional conservation of exonuclease–Chi interactions is surprising in view of the structural divergence observed between the known enzymes. Three-subunit exonucleases like RecBCD are present in E. coli and other Gram-negative bacteria and, curiously, in Gram-positive bacterium M. tuberculosis, whereas two-subunit exonucleases are present in L. lactis, B. subtilis, and possibly in Listeria monocytogenes. Significant homologies are found in just one subunit, corresponding to RecB, AddA, or RexA, which contains helicase motifs, an ATP-binding site, and two C-terminal end motifs (10, 13). Experiments with AddAB suggest that these motifs are generally required for exonuclease activity (13). In contrast, the RexB subunit is only 23% identical to AddB and bears no apparent similarity with the three-subunit enzyme (compared with 61% identity between L. lactis and B. subtilis RecA proteins; ref. 47). Interestingly, blast analyses reveal 28 and 23% identity over 100- and 200-aa stretches, respectively, between RexA and human RAD50, a protein involved in repair of dsDNA breaks in eukaryotes (48).
RexAB, unlike the three other sequenced exonucleases, has one (rather than two) putative ATP-binding site, in RexA, which is presumably the equivalent of the ATP-binding site in RecB and AddA. Mutational analyses of both RecBCD and AddAB indicate that the common ATP-binding site is critical for activities (49, 50), whereas the second ATP-binding site, which is present in RecD and AddB (10), but not in RexB, had little and no effect, respectively, on enzyme activities (12, 51). The lack of the second ATP-binding site in RexAB argues that it is not essential for activity.
RexAB-ChiLl Interactions in DNA Repair.
Our results show that RexAB and ChiLl conserve several biological properties originally found for RecBCD and ChiEc. ChiLl modulates RexAB-mediated DNA degradation. It is also a “crossover instigator” of RexAB-mediated homologous recombination. However, our test did not prove that ChiLl is a recombination “hot spot,” which originally characterized ChiEc in bacteriophage λ crosses (44). Experiments by Stahl and coworkers with λ showed that RecBC(D−)-mediated crossover events are localized at the phage extremity (52), suggesting that the hot spot of RecBCD-mediated recombination is the point at which linear dsDNA is no longer degraded. Although it remains to be proven, exonuclease attenuation and recombination–stimulation, demonstrated here for the RexAB-ChiLl couple, may be sufficient to produce the hot-spot effect of Chi.
Acknowledgments
We thank A. von Wright, Y. Le Loir, P. Langella, P. Le Bourgeois, and G. Smith for generous gifts of strains and plasmids, J.-J. Godon for the L. lactis conjugation protocol, and V. Biaudet for expert sequence analyses. S. Sourice provided invaluable assistance and discussion. P. Duwat, B. Michel, E. Maguin, F. Chedin, I. Poquet, and M. Gasson offered good council. We thank F. Stahl for his insights in telling a Chi-like site from the real thing.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: HMW, high-molecular-weight linear plasmid multimers; RC, rolling circle.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database [accession no. U76424 (rexAB)].
References
- 1.Michel B, Ehrlich S D, Uzest M. EMBO J. 1997;16:430–438. doi: 10.1093/emboj/16.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuzminov A. Mol Microbiol. 1995;16:373–384. doi: 10.1111/j.1365-2958.1995.tb02403.x. [DOI] [PubMed] [Google Scholar]
- 3.Smith G R, Kunes S M, Schultz D W, Taylor A, Triman K L. Cell. 1981;24:429–436. doi: 10.1016/0092-8674(81)90333-0. [DOI] [PubMed] [Google Scholar]
- 4.Dixon D A, Kowalczykowski S C. Cell. 1993;73:87–96. doi: 10.1016/0092-8674(93)90162-j. [DOI] [PubMed] [Google Scholar]
- 5.Myers R S, Stahl F W. Annu Rev Genet. 1994;28:49–70. doi: 10.1146/annurev.ge.28.120194.000405. [DOI] [PubMed] [Google Scholar]
- 6.McKittrick N H, Smith G R. J Mol Biol. 1989;210:485–495. doi: 10.1016/0022-2836(89)90125-3. [DOI] [PubMed] [Google Scholar]
- 7.de Vries J, Wackernagel W. J Gen Microbiol. 1992;138:31–38. doi: 10.1099/00221287-138-1-31. [DOI] [PubMed] [Google Scholar]
- 8.Fleischmann R D, Adams M D, White O, Clayton R A, Kirkness E F, et al. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 9.Wilcox N S, Smith H O. J Biol Chem. 1976;251:6127–6134. [PubMed] [Google Scholar]
- 10.Kooistra J, Venema G. J Bacteriol. 1991;173:3644–3655. doi: 10.1128/jb.173.12.3644-3655.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kooistra J, Haijema B J, Venema G. Mol Microbiol. 1993;7:915–923. doi: 10.1111/j.1365-2958.1993.tb01182.x. [DOI] [PubMed] [Google Scholar]
- 12.Haijema B J, Noback M, Hesseling A, Kooistra J, Venema G, Meima R. Mol Microbiol. 1996;21:989–999. doi: 10.1046/j.1365-2958.1996.601424.x. [DOI] [PubMed] [Google Scholar]
- 13.Haijema B, Venema G, Kooistra J. J Bacteriol. 1996;178:5086–5091. doi: 10.1128/jb.178.17.5086-5091.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biswas I, Maguin E, Ehrlich S D, Gruss A. Proc Natl Acad Sci USA. 1995;92:2244–2248. doi: 10.1073/pnas.92.6.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dabert P, Ehrlich S D, Gruss A. Proc Natl Acad Sci USA. 1992;89:12073–12077. doi: 10.1073/pnas.89.24.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le Loir Y, Gruss A, Ehrlich S D, Langella P. J Bacteriol. 1994;176:5135–5139. doi: 10.1128/jb.176.16.5135-5139.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Wright A, Saarela M. Plasmid. 1994;31:106–110. doi: 10.1006/plas.1994.1011. [DOI] [PubMed] [Google Scholar]
- 18.Churchward G, Belin D, Nagamine Y. Gene. 1984;31:165–171. doi: 10.1016/0378-1119(84)90207-5. [DOI] [PubMed] [Google Scholar]
- 19.Murphy K C. J Bacteriol. 1991;173:5808–5821. doi: 10.1128/jb.173.18.5808-5821.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guzman L-M, Belin D, Carson M J, Beckwith J. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gasson M. J Bacteriol. 1983;154:1–9. doi: 10.1128/jb.154.1.1-9.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duwat P, Ehrlich S D, Gruss A. Mol Microbiol. 1995;17:1121–1131. doi: 10.1111/j.1365-2958.1995.mmi_17061121.x. [DOI] [PubMed] [Google Scholar]
- 23.Wanner B L. J Mol Biol. 1986;1991:39–58. doi: 10.1016/0022-2836(86)90421-3. [DOI] [PubMed] [Google Scholar]
- 24.Amundsen S K, Neiman A M, Thibodeaux S M, Smith G R. Genetics. 1990;126:25–40. doi: 10.1093/genetics/126.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maguin E, Prevost H, Ehrlich S D, Gruss A. J Bacteriol. 1996;178:931–935. doi: 10.1128/jb.178.3.931-935.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holo H, Nes I F. Appl Environ Microbiol. 1989;55:3119–3123. doi: 10.1128/aem.55.12.3119-3123.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dower W J, Miller J F, Ragsdale C W. Nucleic Acids Res. 1988;16:6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanahan D. DNA Cloning: A Practical Approach. Vol. 1. Oxford: IRL; 1985. pp. 109–135. [Google Scholar]
- 29.Projan S J, Carleton S, Novick R P. Plasmid. 1983;9:182–190. doi: 10.1016/0147-619x(83)90019-7. [DOI] [PubMed] [Google Scholar]
- 30.Sorokin A, Lapidus A, Capuano V, Galleron N, Pujic P, Ehrlich S D. Genome Res. 1996;6:448–453. doi: 10.1101/gr.6.5.448. [DOI] [PubMed] [Google Scholar]
- 31.Azevedo V, Sorokin A, Ehrlich S D, Serror P. Mol Microbiol. 1993;10:397–405. doi: 10.1111/j.1365-2958.1993.tb02671.x. [DOI] [PubMed] [Google Scholar]
- 32.Dear S, Staden R. Nucleic Acids Res. 1991;19:3907–3911. doi: 10.1093/nar/19.14.3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shortle D. Gene. 1983;22:181–189. doi: 10.1016/0378-1119(83)90102-6. [DOI] [PubMed] [Google Scholar]
- 34.Lipinska B, Rao A S, Bolten B M, Balakrishnan R, Goldberg E B. J Bacteriol. 1989;171:488–497. doi: 10.1128/jb.171.1.488-497.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silverstein, J. L. & Goldberg, E. B. (1976) 72, 195–211. [DOI] [PubMed]
- 36.Langella P, Chopin A. FEMS Microbiol Lett. 1989;60:149–152. doi: 10.1016/0378-1097(89)90498-9. [DOI] [PubMed] [Google Scholar]
- 37.Langella P, Le Loir Y, Ehrlich S D, Gruss A. J Bacteriol. 1993;175:5806–5813. doi: 10.1128/jb.175.18.5806-5813.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller J H. A Short Course in Bacterial Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Lab. Press; 1992. [Google Scholar]
- 39.Dabert P, Smith G R. Genetics. 1997;145:877–889. doi: 10.1093/genetics/145.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salazar L, Fsihi H, de Rossi E, Riccardi G, Rios C, Cole S T, Takiff H E. Mol Microbiol. 1996;20:283–293. doi: 10.1111/j.1365-2958.1996.tb02617.x. [DOI] [PubMed] [Google Scholar]
- 41.Kumar A, Malloch R A, Fujita N, Smillie D A, Ishihama A, Hayward R S. J Mol Biol. 1993;232:406–418. doi: 10.1006/jmbi.1993.1400. [DOI] [PubMed] [Google Scholar]
- 42.Willetts N S, Clark A J. J Bacteriol. 1969;100:231–239. doi: 10.1128/jb.100.1.231-239.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clark A J. Annu Rev Genet. 1973;7:67–86. doi: 10.1146/annurev.ge.07.120173.000435. [DOI] [PubMed] [Google Scholar]
- 44.Lam S T, Stahl M M, McMilin K D, Stahl F W. Genetics. 1974;77:425–433. doi: 10.1093/genetics/77.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marshall B, Isidro G, Boavida M G. Gene. 1996;174:175–179. doi: 10.1016/0378-1119(96)00515-x. [DOI] [PubMed] [Google Scholar]
- 46.Aoki H, Kajino K, Arakawa Y, Hino O. Proc Natl Acad Sci USA. 1996;14:7300–7304. doi: 10.1073/pnas.93.14.7300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duwat P, Ehrlich S D, Gruss A. Appl Environ Microbiol. 1992;58:2674–2678. doi: 10.1128/aem.58.8.2674-2678.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gilbertson L A, Stahl F W. Proc Natl Acad Sci USA. 1994;91:11934–11937. doi: 10.1073/pnas.91.25.11934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hsieh S, Julin D A. Nucleic Acids Res. 1992;20:5647–5653. doi: 10.1093/nar/20.21.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haijema B J, Meima R, Kooistra J, Venema G. J Bacteriol. 1996;178:5130–5137. doi: 10.1128/jb.178.17.5130-5137.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Korangy F, Julin D A. J Biol Chem. 1992;267:1733–1740. [PubMed] [Google Scholar]
- 52.Thaler D S, Sampson E, Siddiqi I, Rosenberg S M, Thomason L C, Stahl F W, Stahl M M. Genome. 1989;31:53–67. doi: 10.1139/g89-013. [DOI] [PubMed] [Google Scholar]
- 53.Godon J J, Pillidge C J, Jury K, Shearman C A, Gasson M J. Dev Biol Stand. 1995;85:423–430. [PubMed] [Google Scholar]