Abstract
Purpose of review
The oculomotor periphery was classically regarded as a simple mechanism executing complex behaviors specified explicitly by neural commands. A competing view has emerged that many important aspects of ocular motility are properties of the extraocular muscles and their associated connective tissue pulleys. This review considers current concepts regarding aspects of ocular motility that are mechanically determined versus those that are specified explicitly as innervation.
Recent findings
While it was established several years ago that the rectus extraocular muscles have connective tissue pulleys, recent functional imaging and histology has suggested that the rectus pulley array constitutes an inner mechanism, analogous to a gimbal, that is rotated torsionally around the orbital axis by an outer mechanism driven by the oblique extraocular muscles. This arrangement may account mechanically for several commutative aspects of ocular motor control, including Listing’s Law, yet permits implementation of non-commutative motility. Recent human behavioral studies, as well as neurophysiology in monkeys, are consistent with implementation of Listing’s Law in the oculomotor periphery, rather than centrally.
Summary
Varied evidence now strongly supports the conclusion that Listing’s Law and other important ocular kinematics are mechanically determined. This finding implies more limited possibilities for neural adaptation to some ocular motor pathologies, but indicates possibilities for surgical treatments.
Keywords: connective tissue, extraocular muscles, eye movements, Listing’s Law, ocular counter-rolling, pulleys, saccades, vestibulo-ocular reflex
Introduction
Interpretation of abnormal ocular motility can be quite complex, since it is founded on an understanding of the anatomy of the extraocular muscles (EOMs), the orbital connective tissue apparatus, and on some general principles of motor innervation that ordinarily coordinate binocular movements. Perhaps surprisingly, our understanding of even fundamental gross anatomy of the EOMs has been significantly clarified in the late twentieth century.
Laminar structure of extraocular muscles
The oculorotary EOMs are bilaminar [1]. The global layer is located adjacent to the globe in rectus EOMs and in the central core of the oblique EOMs [2,3•]. In the rectus EOMs and the superior oblique muscle the global layer anteriorly becomes continuous with the terminal tendon that inserts on the sclera [4]. The orbital layer of each rectus EOM terminates posterior to the sclera, and its fibers insert on connective tissue pulleys [4,5•].
Gross structure of extraocular muscles
Rectus EOMs do not follow straight paths from origins to insertions. In eccentric gaze, rectus EOM paths are inflected in the anterior orbit. Joel M. Miller first conceived the modern concept of pulleys [6]. The so-called pulleys of Miller cause the anterior rectus EOM paths, and thus their pulling directions, to change with eye position. This is shown in the axial magnetic resonance images in Fig. 1, illustrating that the anterior inferior rectus path changes direction by half the horizontal gaze change. All rectus EOMs behave similarly.
Figure 1. Axial magnetic resonance images (2 mm thickness, T1-weighted) of a right orbit taken at the level of the lens, fovea, and optic nerve (top), and simultaneously in a more inferior image plane along the path of the inferior rectus muscle (bottom), in abduction (left) and adduction (right).
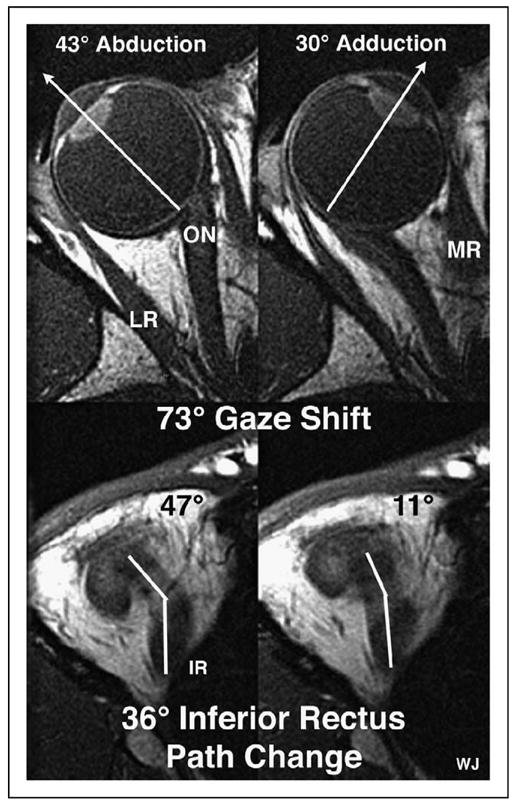
Note the two segments in the inferior rectus path, with an inflection corresponding to the location of the inferior rectus pulley. For this 73° horizontal gaze shift, there was a corresponding 36° shift in inferior rectus muscle path anterior to the inflection at its pulley. IR, inferior rectus muscle; LR, lateral rectus muscle; MR, medial rectus muscle; ON, optic nerve. Reproduced with permission from [13••].
Structural anatomy of pulleys
Pulleys thus act as mechanical origins of the rectus EOMs. Pulleys consist of rings of dense collagen encircling the EOM. Smooth muscle is present in pulley suspensions [7,8], particularly in a distribution called the inframedial peribulbar smooth muscle between the medial rectus and inferior rectus pulleys [9]. The overall structure of the orbital connective tissues is schematized in Fig. 2.
Figure 2. Diagram of EOMs and orbital connective tissues.
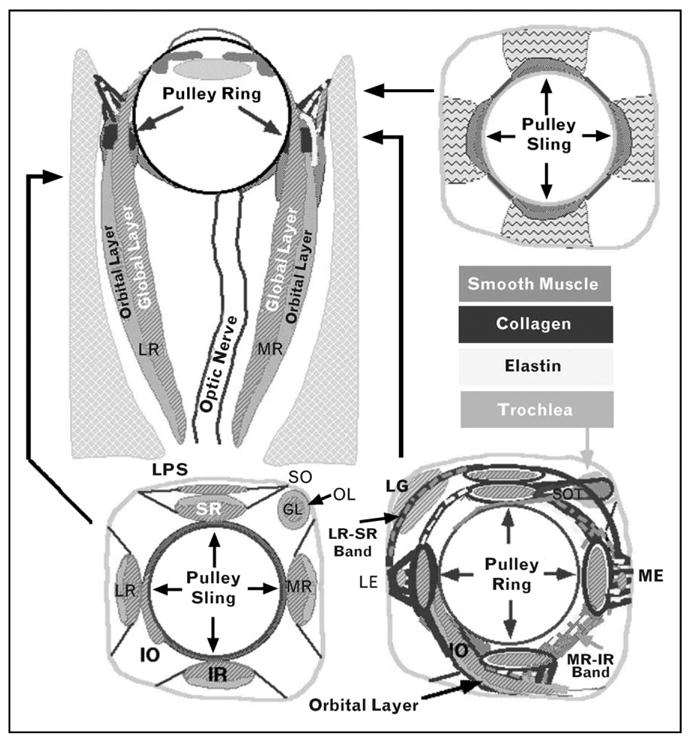
Coronal views are depicted at three levels indicated on the axial view. The functional pulleys are at the level depicted on the lower right. GL, global layer; IO, inferior oblique muscle; IR, inferior rectus muscle; LG, lacrimal gland; LE, lateral enthesis, the attachment of the pulley suspension to the orbital bone; LPS, levator palpebrae superioris muscle; LR, lateral rectus muscle; ME, medial enthesis, the attachment of the pulley suspension to the orbital bone; MR, medial rectus muscle; OL, orbital layer; SO, superior oblique muscle; SOT, superior oblique tendon; SR, superior rectus muscle.
The inferior rectus pulley is coupled to the inferior oblique pulley in a bond stiffened by a heavy elastin deposit at their point of crossing [9,10]. The orbital layer of the inferior oblique muscle inserts partly on the conjoined inferior oblique–inferior rectus pulleys, partly on the sheath of the inferior oblique muscle temporally and partly on the lateral rectus pulley.
Although the rigid trochlea of the superior oblique muscle been known since antiquity [11,12], its immobility is exceptional, and also unique is the superior oblique’s indirect orbital layer insertion via the superior oblique sheath on the superior rectus pulley [3•]. Net superior oblique pulling direction probably changes half as much as ocular duction despite an immobile pulley, because of the uniquely broad, thin insertion of the tendon as it wraps over the globe [13••].
Functional anatomy of pulleys
The insertion of each rectus EOM’s orbital layer on its pulley is arguably the main driving force that linearly moves that pulley posteriorly during contraction. Fibers on the orbital surface of each rectus EOM form bundles that extend up to 1 mm [5•] to insert into the dense encircling tissue [2,4]. Pulley locations and shifts during ocular rotation have been quantified from coronal magnetic resonance imaging (MRI) in secondary and tertiary gazes associated with inflections in EOM paths. All four rectus pulleys move anteroposteriorly in coordination with their scleral insertions, by the same amounts. The inferior oblique pulley moves anteroposteriorly by half as much as the inferior rectus insertion [10].
The 95% confidence intervals for the horizontal and vertical coordinates of normal rectus pulleys range over less than ± 0.6 mm [14]. Pulley stability in the coronal plane implies high stiffness of pulley suspensions. The anteroposterior mobility of the pulleys is accomplished by application of substantial force by the orbital layer of each EOM (Fig. 2).
Kinematics of pulleys
Sequential rotations are not mathematically commutative, so that final eye orientation depends on the order of rotations [15]. Ocular torsion is constrained (when the head is upright and immobile) by Listing’s Law: eye torsion in any gaze direction is that reached by a single rotation from primary eye position about an axis lying in a plane [16]. Listing’s Law is satisfied if the ocular rotational velocity axis shifts by half of the shift in ocular duction [17]. Conformity to this ‘half-angle rule’ makes the sequence of ocular rotations effectively commutative [18].
The active pulley hypothesis explains how rectus pulley positioning can implement the half-angle kinematics required by Listing’s Law [4,13••,19,20]. Each EOM imparts rotational velocity to the globe in a direction perpendicular to the path of its tendon as the tendon approaches the globe. In Fig. 3a and b it is seen that an horizontal rectus EOM’s pulling direction tilts posteriorly by half the angle of upward gaze if the pulley is located as far posterior to globe center as the insertion is anterior to globe center. If all rectus EOMs are similarly arranged, this configuration mechanically enforces Listing’s Law since all rectus forces acting on the globe exhibit half-angle kinematics.
Figure 3. Diagram of EOM and pulley behavior for half-angle kinematics conforming to Listing’s Law.
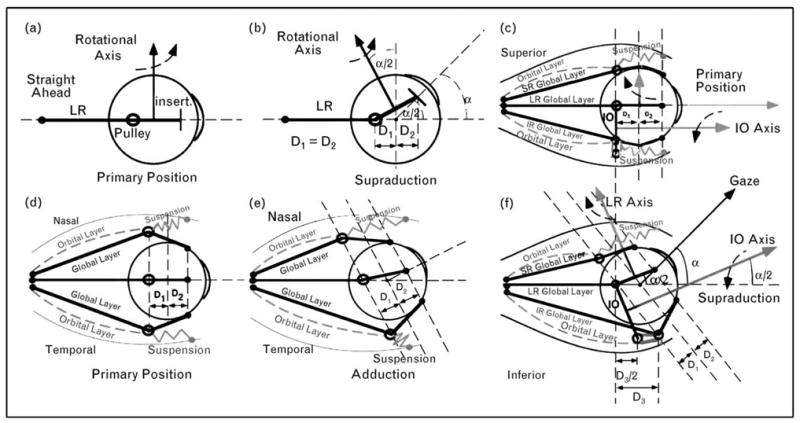
(a) Lateral view. For each rectus EOM, the rotational velocity axis is perpendicular to the segment from pulley to scleral insertion. The velocity axis for the lateral rectus is vertical in primary position. (b) Lateral view. In supraduction to angle α, the lateral rectus rotational velocity axis tilts posteriorly by angle α/2 if distance D1 from pulley to globe center is equal to distance D2 from globe center to insertion. (c) Lateral view. In primary position, the terminal segment of the inferior oblique muscle lies in the plane of the lateral rectus and inferior rectus pulleys into which the inferior oblique muscle’s orbital layer inserts. The inferior oblique rotational velocity axis is parallel to primary gaze direction. (d) Superior view of rectus EOMs and pulleys in primary position, corresponding to (a). (e) Superior view. In order to maintain D1=D2 in an oculocentric reference in adduction, the medial rectus pulley must shift in the orbit posteriorly, and the lateral rectus pulley anteriorly. This is proposed to be implemented by contractile changes in the orbital layers of these EOMs, working against elastic pulley suspensions. (f) Lateral view similar to (c). In supraduction to angle α, the inferior rectus pulley shifts anteriorly by distance D3, as required by the relationship shown in (e). The inferior oblique pulley shifts anteriorly by D3/2, shifting the inferior oblique rotational velocity axis superiorly by angle α/2. IO, inferior oblique muscle; IR, inferior rectus muscle; LR, lateral rectus muscle; MR, medial rectus muscle; SR, superior rectus muscle. Reproduced with permission from [13••].
It has been shown analytically that agonist–antagonist EOM pair alignment is possible only if pulley locations are not fixed in the orbit [21•]. Tertiary gazes such as adducted supraduction require anteroposterior shift of rectus pulleys in the orbit, maintaining constant relationship to the globe (Fig. 3d and e). The active pulley hypothesis proposes that these shifts are generated by contractile activity of the orbital layers of EOMs acting against pulley suspension elasticity [4,20,22,23]. Computational simulation suggests that forces required to implement anteroposterior pulley shifts are too great to be passive results of changes in tensions of the traversing EOMs [20]. Further, anteroposterior rectus pulley movements persist even after enucleation [24]. The inferior oblique pulley shifts anteroposteriorly by half of vertical ocular rotation [10], shifting its rotational axis similarly (Fig. 3d and f) [10].
Optimal stereopsis requires ocular torsion to align corresponding retinal meridia in convergence [25]. In central gaze, excyclotorsion occurs in convergence that violates Listing’s Law [26]. During asymmetrical convergence to a target aligned to one eye, extorsion occurs in both eyes [27]. MRI during convergence has demonstrated extorsional shift of most human rectus pulleys [28]. It is likely that all four rectus pulleys shifted extorsionally about 1.9°, and amount similar to globe extorsion under these conditions [29]. The rectus pulley array apparently rotates about the orbital axis in coordination with ocular torsion, changing the torsional pulling directions of all rectus EOMs without altering their half-angle dependence on horizontal and vertical eye position. If Listing’s Law were a property of the rectus pulleys then extorsion of the rectus pulley array would cause a parallel, torsional offset in Listing’s plane.
The high stiffness of the rectus pulley suspensions necessary to stabilize them against sideslip would severely limit passive torsional shifts, and always to less than ocular torsion [28]. An active mechanism has been suggested for torsional shifts of rectus pulleys in convergence. The inferior oblique muscle’s orbital layer inserts on the inferior rectus and lateral rectus pulleys [10]. Contraction of the inferior oblique orbital layer would directly extorsionally shift the lateral rectus and inferior rectus pulleys, and contractile inferior oblique muscle thickening has been demonstrated by MRI during convergence [28]. Inferior lateral rectus pulley shift could be coupled to lateral superior rectus pulley shift via the dense connective tissue band between them [30]. The superior oblique’s orbital layer inserts on the superior oblique sheath posterior to the trochlea, with both the tendon and sheath reflected at that rigid pulley [3•]. Anterior to the trochlea, the superior oblique sheath inserts on the superior rectus pulley. Although not directly demonstrated by MRI, relaxation of the superior oblique orbital layer during convergence is consistent with single-unit recordings in the monkey trochlear nucleus [31], and could contribute to extorsion of the pulley array. The inframedial peribulbar smooth muscle might also contribute to rectus pulley extorsion [9].
Current controversies
Surprisingly, even the anatomy of the EOMs and associated connective tissues remains controversial. One fundamental issue has, however, been resolved in the past year.
Existence of pulleys
Because of their distributed nature, some anatomic controversy persists regarding existence of the pulleys of Miller, with the alternative supposition being that the penetrations of the rectus EOMs through Tenon’s fascia are mechanically insignificant, or the archaic view that the connective tissues serve only to limit ocular rotations [32•]. The elasticity of the lateral rectus pulley suspension has been questioned based on manipulation of human orbits exenterated for cancer, suggesting inconsistency with large anteroposterior pulley shifts [32•]. However, the mechanical assessments were only semi-quantitative, and were limited by artifacts introduced by tissue removal, and failure to consider resting tissue lengths [33]. Histological evidence has previously been presented suggesting the presence of EOM pulleys in rodents [34], and Ruskell et al. [5•] have proposed that orbital layer insertion into connective tissue sleeves may be a general mammalian feature [5•]. Histological study in rat, including three-dimensional reconstruction, suggested insertion of the inferior rectus orbital layer on a pulley [35•].
Ruskell et al. [5•] studied isolated human and monkey rectus EOMs near their pulleys. They reported tendons leaving the orbital surface of EOMs to insert in sleeves or other surrounding connective tissues, and considered their results to confirm the observation of Demer et al. [4] that the orbital fibers of rectus EOMs separate from the global fibers and insert in the sheath, and that these fibers are unlikely to contribute significantly to ocular rotation [5•]. These investigators favored an interpretation, consistent with the coordinated control postulate ofÂthe active pulley hypothesis, that the sleeve tendon insertions coordinate the movement of the connective tissues against viscoelastic resistance of fascia [5•].
Dimitrova et al. [36] used electrically stimulated abducens motor units to evoke eye movements from central to secondary gaze positions in anesthetized cats and monkeys before and after removal of the lateral bone and adjacent orbital tissue. Although removal of the lateral rectus pulley predictably increased the amplitude and velocity of horizontal eye movements, there was no effect on vertical eye movements [36]. Dimitrova et al. [36] attributed increased horizontal eye movement to transmission of orbital layer force to the tendon, although they also noted that pulley removal would also reduce elastic loading of the pulley suspension as another mechanism. The experiment of Dimitrova et al. was not a test of the active pulley hypothesis, which would have required examination of tertiary gazes. However, this paper did emphasize probable transmission of orbital layer force to the global layer and thence to the insertional tendon. Interlaminar force coupling is probably important, but its magnitude is currently unknown.
Fixed pulleys?
Koene and Erklens [21•] recently published theoretical analysis of ocular rotations assuming fixed pulley locations substantially posterior and much closer to the orbital center than observed in MRI data [14,20]. These authors showed that the rotational velocity axes produced by agonist–antagonist rectus EOM pairs with these assumed pulleys are misaligned so that their co-contraction produces net torque, especially for more posteriorly located pulleys [21•]. This effect would be reduced for the observed actively shifting pulleys located much more anteriorly and peripherally in the orbit, and might not be very important. However, the analysis highlights the heretofore neglected geometric consequences of travel of anterior tendons of relaxing EOMs over the curved globe surface in gaze positions where the rectus pulleys do not enforce straight paths.
Mechanically determined kinematics of Listing’s Law
The past year has seen resolution of a fundamental controversy in ocular kinematics: the basis of Listing’s Law. Long regarded an organizing principle of ocular motility, Listing’s Law effectively reduces the rotational freedom of the eye from three (horizontal, vertical, and torsional) to only two (horizontal and vertical) degrees during visually guided eye movements [17]. Conformity with Listing’s Law can be demonstrated by expressing ocular rotational axes as mathematical ‘quaternions’, that, when plotted, lie in Listing’s plane [15]. Unlike one-dimensional velocity that is simply the time derivative of position, three-dimensional (3-D) eye velocity is a function both of eye position and its derivative. Each time derivative of each component of 3-D eye position is a coordinate velocity, but this differs from 3-D velocity in a way critical to neural programming of saccades [18,37–39]. The ocular rotational position axis will be constrained to a plane if, in the velocity domain, the ocular velocity axis changes by half the amount of eye position change. Since in most situations the eye begins in Listing’s plane, half-angle behavior constrains the eye to remain in the plane. However, if eye position were somehow to begin outside Listing’s plane at onset of an eye movement subsequently conforming to half-angle kinematics, eye position would remain in a plane parallel to but displaced from Listing’s plane.
The vestibulo-ocular reflex (VOR) violates Listing’s Law since the reflex must compensate for head rotation about arbitrary axes irrespective of eye position [40–42]. Controversy has arisen because the VOR does not violate Listing’s Law with the ideal zero-angle dependency, but has an axis dependency on eye position of approximately one-quarter [40–42]. Controversy exists regarding quarter-angle VOR behavior, with most studies reporting it [40–42,43••]. Others have reported that the monkey and human VOR have zero-angle dependency for some head-rotation axes [44], particularly when torsional VOR gain is high [45], or in monkey viewing a structured visual environment [46].
Earlier investigations of the temporal dynamics of VOR axis behavior yielded confusing findings. During manually delivered transient yaw head, the human VOR reportedly matched the head’s rotational axis for the first 47 ms following head-rotation onset, and thereafter in upward of central gaze to tend towards Listing’s Law behavior [41]. However, in down gaze, and during self-generated yaw rotation, the human VOR axis reportedly remained aligned with the head’s [41]. Others have reported that the human VOR during manually imposed yaw transients follows Listing’s Law partially at lower head velocities, while aligning with the head at higher velocities [47]. One study found time dependence of the VOR axis [48], suggested to be due to rectus pulley shifts [41]. That suggestion was recently evaluated by studies of the effect of vertical gaze on the human VOR axis in response to whole-body transient yaw at high acceleration [49,50•]. Under these conditions, the VOR exhibited quarter-angle dependency at all times [50•]. Such kinematics would be consistent with neural drive to a mechanical implementation of quarter-angle VOR kinematics, and a different mechanical specification of half-angle behavior during saccades.
The roles of neural and mechanical factors in ocular kinematics have been hotly debated. Prior to modern descriptions of orbital tissues, it seemed obvious that Listing’s Law was implemented neurally in pre-motor circuits [51–53,54••,55]. The active pulley hypothesis then proposed to account for Listing’s Law mechanically, but physiologic violations of Listing’s Law continued to suggest a role for central neural control [56]. A neural role in Listing’s Law appeared tenable given the observation of ocular extorsion and temporal tilting of Listing’s plane during convergence [57,58], associated with torsional repositioning of the rectus EOM pulley array [28] and alteration of trochlear motor neuron discharge in monkeys [31].
Crane et al. [43••] studied the transition between the angular VOR’s quarter-angle strategy and saccades’ half-angle behavior. These investigators used the yaw VOR to drive ocular torsion out of Listing’s plane, and then evoked a vertical saccade using a visual target. To return the saccade’s position domain axis to Listing’s plane would require that the saccade’s velocity axis violate the half-angle rule in the process of canceling the initial non-Listing torsion. If instead the saccade’s velocity axis conformed to the half-angle rule, the saccade would begin and end with non-Listing torsion induced by the VOR. Crane et al. [43••] found that saccades observed half-angle kinematics, and maintained any non-Listing initial torsion. This suggests that the half-angle velocity relationship is the fundamental principle underlying Listing’s Law, as would be expected from coordinated active positioning of the rectus pulleys. However, intra-saccadic torsion returning the eye to Listing’s plane has been observed after torsional optokinetic nystagmus had driven the eye out of Listing’s plane [59], a difference perhaps related to the entrainment of quick phases during nystagmus, and seemingly impossible with a purely mechanical system [59]. Reconciliation of these findings would require differences in neural control of visual saccades compared with vestibular quick phases, which is plausible [43••] given the ability of the vestibular system to drive saccades [60].
A recent study evaluated human EOMs during ocular counter-rolling, an otolith-mediated, static torsional VOR that can be evoked in an MRI scanner by lateral decubitus positioning [61••]. The coronal plane positions of the rectus EOMs shifted torsionally in the same direction as ocular torsion evoked by ocular counter-rolling. Torsion of the rectus pulley array was roughly half of the ocular torsion reported by others in the same lateral decubitus position. Torsional shift of the rectus pulley array half of ocular torsion would change rectus EOM pulling directions by one-quarter of ocular torsion (Fig. 4), ideal for quarter-angle kinematics of the VOR. Oblique EOMs exhibited cross-section changes consistent with their putative roles in torsional repositioning of rectus pulleys [61••]. This finding, considered in context of saccade kinematics during the VOR [43••], suggests that the array of rectus pulleys resembles a kind of ‘inner gimbal’ conforming to Listing’s half-angle kinematics for visually guided movements such as fixations and saccades, but which is rotated by the oblique EOMs to implement eye movements such as the VOR.
Figure 4. Diagrammatic representation of effects of head tilt on rectus pulley shifts in lateral (top) and frontal (bottom) views.
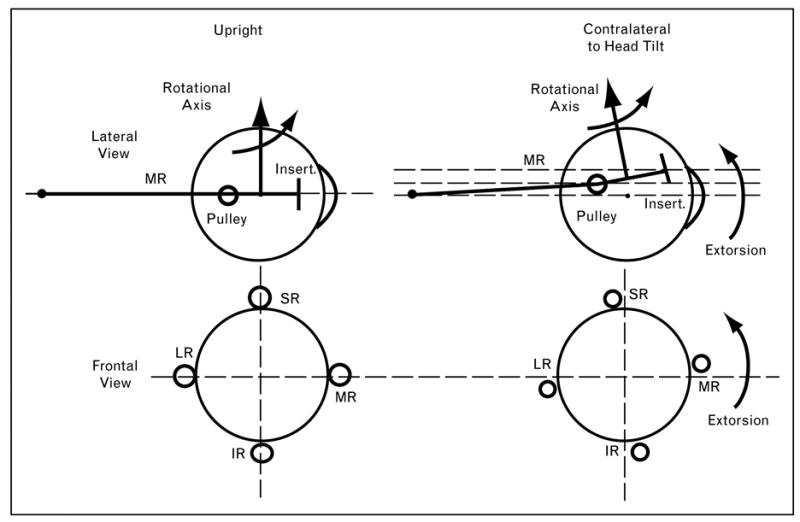
With the head upright, the inferior (IR), lateral (LR), medial (MR), and superior rectus (SR) pulleys are arrayed in frontal view as along the arms of a cross. The medial rectus passes through its pulley, represented as a ring, to its scleral insertion. The rotational velocity axis imparted by the medial rectus is perpendicular to the segment from the pulley to the insertion. The pulley array extorts during head tilt to the contralateral side. If during head tilt the medial rectus pulley shifts superiorly by half the distance the insertion shifts, then the rotational velocity axis imparted by the medial rectus would change by one-quarter of the angle of ocular torsion. Reproduced with permission from [61••].
Older recordings of trochlear motor neuron discharge suggest that ocular extorsion during convergence is neurally commanded [31]. If the ocular torsion specified by Listing’s Law were similarly commanded, torsional commands should be reflected in discharge patterns of neurons innervating the oblique and vertical rectus EOMs. Ghasia et al. [62••] recently recorded from motor neurons and nerve fibers innervating the vertical rectus and oblique EOMs in monkeys during smooth pursuit conforming to Listing’s Law. This study found no neural commands for Listing’s Law torsion in motor units innervating the cyclovertical EOMs, although there were small discharge variations with eye position possibly related to passive stretching of the oblique EOMs [37]. This strong evidence for a mechanical basis of Listing’s Law was also recently supported by the experiment of Klier et al. [63] in which electrical stimulation was delivered to the abducens nerve of behaving monkeys to evoke saccade-like movements. By varying starting vertical position, Klier et al. [63] demonstrated that the evoked eye movements had velocity axes that changed by half the angle of vertical gaze, thus conforming to Listing’s Law. Direct electrical stimulation of CN6 would appear to allow no possibility of downstream neural processing. The decisive conclusion from these two experiments is that Listing’s Law has a mechanical basis, and is not specified by the instantaneous neural commands. These two results were predicted by the active pulley hypothesis [4], while the neural theory of Listing’s Law predicted opposite results [54••]. However, the single unit recording experiment of Ghasia et al. [62••] uncovered a new mystery for which no explanation currently exists. Neurons driving cyclovertical EOMs not only did not command half-angle Listing’s torsion, but also did not command the quarter-angle torsion evident in the VOR. This observation indicates that quarter-angle VOR behavior is also mechanical, rather than neural. An early suggestion had been made than quarter-angle behavior could be implemented mechanically by retraction of rectus pulleys [4], but subsequent recognition that this idea would be unrealistic under many conditions [53] led to abandonment of the concept of pulley retraction [19,28]. Furthermore, uncoordinated anteroposterior shift in pulley location would be inconsistent with the recent experiments of Crane et al. [43••] demonstrating transition between quarter-angle VOR, and half-angle saccade behavior without measurable latency. The foregoing results seemingly require that quarter-angle VOR behavior be due to mechanical phenomena not previously considered. Since orbital microanatomy has now been thoroughly re-examined, it is unlikely that undiscovered structures such as additional pulleys or gross articulations can explain quarter-angle VOR behavior. One remaining possibility might be differential activation of subpopulations of EOM fibers and motor neurons, which are known to exist in superabundance relative to requirements of the conventionally recognized eye movements [64,65]. The rectus EOMs near their insertions are shaped as broad, thin straps [19]. Rectus EOMs could generate appreciable torsional actions if fiber tensions were unequally distributed across the broad tendon widths, a mechanism that might, at no additional latency, alter the transverse location of an EOM’s functional origin without shift in location of the pulley ring.
Implications for neural control
Some tentative conclusions can now be reached concerning neural control of eye movements generally, and some older data probably should be reinterpreted. The central neural signals correlated with all types of eye movement would be expected to reflect effects of torsional reconfiguration of rectus pulleys during the VOR. Recordings from burst neurons in monkeys appear compatible with torsional shift of rectus pulleys transverse to the EOM axes induced by static head tilt [66]. In monkeys the displacement plane for 3-D eye positions during pursuit and saccades shifts opposite to changes in head orientation [67], and such shifts may be dynamic during semicircular canal stimulation [68,69]. Hess and Angelaki [67] earlier suggested that shift in Listing’s plane is mediated by otolith input to the 3-D neural integrator, but the finding may be reconciled with the observation that lesion of the integrator in the rostral interstitial nucleus of the medial longitudinal fasciculus also abolishes shift in Listing’s plane associated with ocular counter-rolling [70] if the torsional shift of pulleys is mediated by the 3-D neural integrator.
In monkeys, preferred directions of saccadic superior colliculus neurons shift in the opposite direction, and by slightly more than half the amount, of static head tilt [71]. Frens et al. [71] concluded that the changes in the horizontal and vertical pulling directions of the EOMs during ocular counter-rolling are probably about two-thirds of ocular torsion.
Regardless of the ocular motor subsystem involved, torsional rectus pulley shifts during the VOR would preserve the advantage of apparent commutativity of the peripheral ocular motor apparatus for concurrent saccades and pursuit. This commutativity would be valuable even though higher-level sensorimotor transformations must account for 3-D geometrical effects of eye and head orientation [56,71–73]. Neural processing for the VOR must be generated in three dimensions, based on transduction of head motion in three degrees of freedom, and on 3-D eye orientation in the head.
Recent results do suggest that some low level visuo-motor processing may be simpler than previously believed. In saccade programming, it appears tenable to map visual retinal error onto corresponding zero-torsion motor error commands within Listing’s plane as modeled by the displacement-feedback model of Crawford and Guitton [74]. The displacement-feedback model, with a downstream mechanism for half-angle behavior, can simulate the visuo-motor transformations necessary for accurate and kinematically correct saccades within a reasonable oculomotor range, but had been rejected by Crawford and Guitton [74], who supposed that saccades from non-Listing torsional starting positions return to Listing’s plane. Crane et al.’s [43••] recent demonstration that such saccades maintain their initial non-Listing torsion suggests that the displacement-feedback model, lacking in a neural representation of Listing, is plausible for control of saccades to visual targets. In the context of realistic EOM pulleys, sensorimotor integration of saccades does not require explicit neural computation of ocular torsion. This simplification solves some complexity, but moves other kinematic issues to a higher level. When head movements are involved, neural consideration of torsion is geometrically unavoidable for target localization in space [72,74].
The weight of the evidence indicates that several aspects of ocular kinematics are primarily implemented by a complex mechanical articulation, rather than by complex neural commands to a simpler mechanical arrangement. This insight alters the interpretation of common situations, and offers hope of mechanical (that is, surgical) solutions to clinical disorders that might earlier have been believed to have neural origins. If the active pulley hypothesis is correct, oblique EOM function would not be critical for Listing’s Law [75], although oblique EOM tone might set initial orientation of Listing’s plane. This is supported by the finding in chronic superior oblique paralysis that Listing’s Law is observed, albeit with temporal tilting of Listing’s plane [76,77], and that this temporally tilted Listing’s plane is not changed by vergence [78•]. Orientation of Listing’s plane varies considerably both among individuals and between eyes of individuals, making it unlikely that absolute Listing’s plane orientation is very important to either vision or ocular motor control [50•]. Although oblique EOMs do not actively participate in generation of Listing’s Law, passive elastic tensions arising from stretching and relaxation of oblique EOMs would create torques violating Listing’s Law unless their innervations were adjusted to compensate [37]. Consequently, recordings of small changes in oblique EOM innervations during pursuit movements conforming to Listing’s Law [62••,79] do not negate a pulley contribution, and nor do dynamic violations of Listing’s Law during saccades in superior oblique palsy [76].
Conclusion
The anatomy of the ocular motor effector apparatus fundamentally differs from traditional teaching. The following encapsulates this author’s broad concept of the effector apparatus, greatly simplified here for heuristic purposes. Rather than consisting of mechanically simple EOMs rotating the globe under explicit neural control of every kinematic nuance, the system consists of a rather intricate mechanical arrangement comprised of a trampoline-like suspension supported by the rectus EOMs and their associated connective tissues, which in turn is circumferentially controlled by the oblique EOMs. The rectus EOMs and their pulleys constitute the inner suspension that implements kinematics in two dimensions, corresponding largely to the two-dimensional organization of the retina and subcortical visual system, and so conform mechanically to Listing’s Law without additional neural specification. The inner suspension has effectively commutative properties. Analogous to a gimbal arrangement, the outer suspension moves the inner with force from the oblique EOMs to generate torsion not conforming to Listing’s Law, so non-commutatively influencing the inner suspension. The extent to which neural adaptive processes can adjust or compensate for ocular kinematics that normally are mechanically determined is an important question, since the answer will inform us about the clinical significance of many ocular motor abnormalities, and the degree to which they may be amenable to surgical cure [80••].
Acknowledgments
Supported by US Public Health Service grants EY08313, EY00331, and DC005224. J.D. received an award from Research to Prevent Blindness and is Leonard Apt Professor of Ophthalmology. J.M. Miller provided helpful criticisms.
Abbreviations
- 3-D
three dimensional
- EOM
extraocular muscle
- MRI
magnetic resonance imaging
- VOR
vestibulo-ocular reflex
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 000–000).
- 1.Porter JD, Baker RS, Ragusa RJ, Brueckner JK. Extraocular muscles: basic and clinical aspects of structure and function. Surv Ophthalmol. 1995;39:451–484. doi: 10.1016/s0039-6257(05)80055-4. [DOI] [PubMed] [Google Scholar]
- 2.Oh SY, Poukens V, Demer JL. Quantitative analysis of rectus extraocular muscle layers in monkey and humans. Invest Ophthalmol Vis Sci. 2001;42:10–16. [PubMed] [Google Scholar]
- • 3.Kono R, Poukens V, Demer JL. Superior oblique muscle layers in monkeys and humans. Invest Ophthalmol Vis Sci. 2005;46:2790–2799. doi: 10.1167/iovs.04-1147. Demonstration of the fine histological structure of the superior oblique muscle, showing that the orbital layer inserts on the connective tissue system. [DOI] [PubMed] [Google Scholar]
- 4.Demer JL, Oh SY, Poukens V. Evidence for active control of rectus extraocular muscle pulleys. Invest Ophthalmol Vis Sci. 2000;41:1280–1290. [PubMed] [Google Scholar]
- • 5.Ruskell GL, Kjellevold Haugen IB, Bruenech JR, van der Werf F. Double insertions of extraocular rectus muscles in humans and the pulley theory. J Anat. 2005;206:295–306. doi: 10.1111/j.1469-7580.2005.00383.x. Histological examination of mainly sagittal sections of human and monkey rectus EOMs demonstrating orbital-layer insertions into connective tissue sleeves, and a lesser degree of global layer insertions into connective tissues. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller JM. Functional anatomy of normal human rectus muscles. Vision Res. 1989;29:223–240. doi: 10.1016/0042-6989(89)90126-0. [DOI] [PubMed] [Google Scholar]
- 7.Demer JL, Miller JM, Poukens V, et al. Evidence for fibromuscular pulleys of the recti extraocular muscles. Invest Ophthalmol Vis Sci. 1995;36:1125–1136. [PubMed] [Google Scholar]
- 8.Demer JL, Poukens V, Miller JM, Micevych P. Innervation of extraocular pulley smooth muscle in monkeys and humans. Invest Ophthalmol Vis Sci. 1997;38:1774–1785. [PubMed] [Google Scholar]
- 9.Miller JM, Demer JL, Poukens V, et al. Extraocular connective tissue architecture. J Vis. 2003;3:240–251. doi: 10.1167/3.3.5. [DOI] [PubMed] [Google Scholar]
- 10.Demer JL, Oh SY, Clark RA, Poukens V. Evidence for a pulley of the inferior oblique muscle. Invest Ophthalmol Vis Sci. 2003;44:3856–3865. doi: 10.1167/iovs.03-0160. [DOI] [PubMed] [Google Scholar]
- 11.Fink WH. Surgery of the vertical muscles of the eye. Springfield, IL: Thomas; 1962. pp. 37–121. [Google Scholar]
- 12.Helveston EM, Merriam WW, Ellis FD, et al. The trochlea: a study of the anatomy and physiology. Ophthalmology. 1992;80:124–133. doi: 10.1016/s0161-6420(82)34835-6. [DOI] [PubMed] [Google Scholar]
- •• 13.Demer JL. Pivotal role of orbital connective tissues in binocular alignment and strabismus. The Friedenwald lecture. Invest Ophthalmol Vis Sci. 2004;45:729–738. doi: 10.1167/iovs.03-0464. This is a lecture paper broadly reviewing the EOM pulley system, including kinematics and clinical implications for strabismus, detailed references, and some new data. This is a good overall recent summary of the active pulley hypothesis, but please note that the torsional reconfiguration of the rectus pulleys during ocular counter-rolling is probably only half as much as suggested in this reference. [DOI] [PubMed] [Google Scholar]
- 14.Clark RA, Miller JM, Demer JL. Three-dimensional location of human rectus pulleys by path inflections in secondary gaze positions. Invest Ophthalmol Vis Sci. 2000;41:3787–3797. [PubMed] [Google Scholar]
- 15.Haslwanter T. Mathematics of three-dimensional eye rotations. Vision Res. 1995;35:1727–1739. doi: 10.1016/0042-6989(94)00257-m. [DOI] [PubMed] [Google Scholar]
- 16.Ruete CGT. Ocular physiology. Strabismus. 1999;7:43–60. doi: 10.1076/stra.7.1.43.654. [DOI] [PubMed] [Google Scholar]
- 17.Tweed D, Vilis T. Geometric relations of eye position and velocity vectors during saccades. Vision Res. 1990;30:111–127. doi: 10.1016/0042-6989(90)90131-4. [DOI] [PubMed] [Google Scholar]
- 18.Quaia C, Optican LM. Commutative saccadic generator is sufficient to control a 3-D ocular plant with pulleys. J Neurophysiol. 1998;79:3197–3215. doi: 10.1152/jn.1998.79.6.3197. [DOI] [PubMed] [Google Scholar]
- 19.Demer JL. Anatomy of strabismus. In: Taylor D, Hoyt C, editors. Pediatric Ophthalmology and Strabismus. 3. London: Elsevier; 2005. pp. 849–861. [Google Scholar]
- 20.Kono R, Clark RA, Demer JL. Active pulleys: Magnetic resonance imaging of rectus muscle paths in tertiary gazes. Invest Ophthalmol Vis Sci. 2002;43:2179–2188. [PubMed] [Google Scholar]
- • 21.Koene AR, Erklens CJ. Properties of 3D rotations and their relation to eye movement control. Biol Cybern. 2004;90:410–417. doi: 10.1007/s00422-004-0477-3. This is a geometric analysis of the velocity axes imparted by EOMs in positions assumed by the authors. These positions are quite dissimilar from pulley positions determined empirically. [DOI] [PubMed] [Google Scholar]
- 22.Demer JL. Extraocular muscles. In: Jaeger EA, Tasman PR, editors. Duanes Clinical Ophthalmology. Vol. 1. Philadelphia: Lippincott; 2000. pp. 1–23. [Google Scholar]
- 23.Demer JL. The orbital pulley system: A revolution in concepts of orbital anatomy. Ann NY Acad Sci. 2002;956:17–32. doi: 10.1111/j.1749-6632.2002.tb02805.x. [DOI] [PubMed] [Google Scholar]
- 24.Detorakis ET, Engstrom RE, Straatsma BR, Demer JL. Functional anatomy of the anophthalmic socket: Insights from magnetic resonance imaging. Invest Ophthalmol Vis Sci. 2003;44:4307–4313. doi: 10.1167/iovs.03-0171. [DOI] [PubMed] [Google Scholar]
- 25.Schreiber K, Crawford JD, Fetter M, Tweed D. The motor side of depth vision. Nature. 2001;410:819–822. doi: 10.1038/35071081. [DOI] [PubMed] [Google Scholar]
- 26.Bruno P, van den Berg AV. Relative orientation of primary positions of the two eyes. Vision Res. 1997;37:935–947. doi: 10.1016/s0042-6989(96)00219-2. [DOI] [PubMed] [Google Scholar]
- 27.Steffen H, Walker MF, Zee DS. Rotation of Listing’s plane with convergence: Independence from eye position. Invest Ophthalmol Vis Sci. 2000;41:715–721. [PubMed] [Google Scholar]
- 28.Demer JL, Kono R, Wright W. Magnetic resonance imaging of human extraocular muscles in convergence. J Neurophysiol. 2003;89:2072–2085. doi: 10.1152/jn.00636.2002. [DOI] [PubMed] [Google Scholar]
- 29.Allen MJ, Carter JH. The torsional component of the near reflex. Am J Optom. 1967;44:343–349. [PubMed] [Google Scholar]
- 30.Kono R, Poukens V, Demer JL. Quantitative analysis of the structure of the human extraocular muscle pulley system. Invest Ophthalmol Vis Sci. 2002;43:2923–2932. [PubMed] [Google Scholar]
- 31.Mays LE, Zhang Y, Thorstad MH, Gamlin PD. Trochlear unit activity during ocular convergence. J Neurophysiol. 1991;65:1484–1491. doi: 10.1152/jn.1991.65.6.1484. [DOI] [PubMed] [Google Scholar]
- • 32.van den Bedem SPW, Schutte S, van der Helm FCT, Simonsz HJ. Mechanical properties and functional importance of pulley bands or ‘faisseaux tendineux’. Vis Res. 2005;45:2710–2714. doi: 10.1016/j.visres.2005.04.016. A challenge to the active pulley hypothesis, but based on semiquantitative observations of orbital specimens removed for cancer. The authors expressed doubt that the elastic properties of the pulley suspension are suitable for large shifts of the pulleys. [DOI] [PubMed] [Google Scholar]
- 33.Demer JL. Letter to Editor Regarding van den Bedem, Schutte, van der Helm, and Simonsz: Mechanical Properties and Functional Importance of Pulley Bands or ‘Faisseaux Tendineux. Vis Res. 2005 doi: 10.1016/j.visres.2005.10.010. in press. [DOI] [PubMed] [Google Scholar]
- 34.Khanna S, Porter JD. Evidence for rectus extraocular muscle pulleys in rodents. Invest Ophthalmol Vis Sci. 2001;42:1986–1992. [PubMed] [Google Scholar]
- • 35.Felder E, Bogdanovich S, Rubinstein NA, Khana TS. Structural details of rat extraocular muscles andd three-dimensional reconstruction of the rat inferior rectus muscle and muscle-pulley interface. Vis Res. 2005;45:1945–1955. doi: 10.1016/j.visres.2005.01.031. A histological study of the region of the inferior rectus pulley in rat, including a 3-D reconstruction of an isolated inferior rectus muscle and some of its associated connective tissues. Isolation of this specimen may have altered some connective tissue relationships to the EOM, but attachment of the pulley to the orbital layer was confirmed. [DOI] [PubMed] [Google Scholar]
- 36.Dimitrova DM, Shall MS, Goldberg SJ. Stimulation-evoked eye movements with and without the lateral rectus muscle pulley. J Neurophysiol. 2003;90:3809–3815. doi: 10.1152/jn.00622.2003. [DOI] [PubMed] [Google Scholar]
- 37.Quaia C, Optican LM. Dynamic eye plant models and the control of eye movements. Strabismus. 2003;11:17–31. doi: 10.1076/stra.11.1.17.14088. [DOI] [PubMed] [Google Scholar]
- 38.Raphan T. Modeling control of eye orientation in three dimensions. I Role of muscle pulleys in determining saccadic trajectory. J Neurophysiol. 1998;79:2653–2667. doi: 10.1152/jn.1998.79.5.2653. [DOI] [PubMed] [Google Scholar]
- 39.Raphan T. Modeling control of eye orientation in three dimensions. In: Fetter M, Haslwanter T, Misslisch H, Tweed D, editors. Three-dimensional Kinematics of Eye, Head, and Limb Movements. Amsterdam: Harwood; 1997. pp. 359–374. [Google Scholar]
- 40.Misslisch H, Tweed D, Fetter M, et al. Rotational kinematics of the human vestibuloocular reflex. III Listing’s law. J Neurophysiol. 1994;72:2490–2502. doi: 10.1152/jn.1994.72.5.2490. [DOI] [PubMed] [Google Scholar]
- 41.Thurtell MJ, Black RA, Halmagyi GM, et al. Vertical eye position-dependence of the human vestibuloocular reflex during passive and active yaw head rotations. J Neurophysiol. 1999;81:2415–2428. doi: 10.1152/jn.1999.81.5.2415. [DOI] [PubMed] [Google Scholar]
- 42.Crane BT, Tian J, Demer JL. Human angular vestibulo-ocular reflex initiation: relationship to Listing’s Law. Ann NY Acad Sci. 2005;1039:1–10. doi: 10.1196/annals.1325.004. [DOI] [PubMed] [Google Scholar]
- •• 43.Crane BT, Tian J, Demer JL. Kinematics of vertical saccades during the yaw vestibulo-ocular reflex in humans. Invest Ophthalmol Vis Sci. 2005;46:2800–2809. doi: 10.1167/iovs.05-0147. This study used binocular, 3-D scleral magnetic search coil recordings and transient, whole-body, high acceleration in yaw to study kinematics of interactions between the VOR and saccades in humans. The VOR was used to drive initial ocular torsion out of Listing’s plane, and vertical saccades were evoked visually from this position. While subsequent ocular torsion never returned to Listing’s plane, vertical saccades conformed closely to half-angle kinematics. This robust finding is contrary to earlier claims based on experiments conducted under less controlled conditions, and answers a longstanding objection to a mechanical basis of Listing’s Law. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crawford JD, Vilis T. Axes of eye rotation and Listing’s law during rotations of the head. J Neurophysiol. 1991;65:407–423. doi: 10.1152/jn.1991.65.3.407. [DOI] [PubMed] [Google Scholar]
- 45.Tweed D, Sievering D, Misslisch H, et al. Rotational kinematics of the human vestibuloocular reflex. I Gain matrices . J Neurophysiol. 1994;72:2467–2479. doi: 10.1152/jn.1994.72.5.2467. [DOI] [PubMed] [Google Scholar]
- 46.Misslisch H, Hess BJ. Three-dimensional vestibuloocular reflex of the monkey: Optimal retinal image stabilization versus Listing’s law. J Neurophysiol. 2000;83:3264–3276. doi: 10.1152/jn.2000.83.6.3264. [DOI] [PubMed] [Google Scholar]
- 47.Palla A, Straumann D, Obzina H. Eye-position dependence of three-dimensional ocular rotation axis orientation during head impulses in humans. Exp Brain Res. 1999;129:127–133. doi: 10.1007/s002210050943. [DOI] [PubMed] [Google Scholar]
- 48.Migliaccio AA, Cremer PD, Aw ST, et al. Vergence-mediated changes in the axis of eye rotation during the human vestibulo-ocular reflex can occur independent of eye position. Exp Brain Res. 2003;151:238–248. doi: 10.1007/s00221-003-1447-z. [DOI] [PubMed] [Google Scholar]
- 49.Crane BT, Tian JR, Demer JL. Temporal dynamics of ocular position dependence of the initial human vestibulo-ocular reflex. Invest Ophthalmol Vis Sci. 2005 doi: 10.1167/iovs.05-0172. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- • 50.Crane BT, Tian JR, Demer JL. Human angular vestibulo-ocular reflex initiation: relationship to Listing’s Law. Ann NY Acad Sci. 2005;1039:26–35. doi: 10.1196/annals.1325.004. This study employed transient, high acceleration in yaw to study the initial human VOR in 3-D, finding quarter-angle dependence on the VOR axis on vertical eye position without time dependence. [DOI] [PubMed] [Google Scholar]
- 51.Crawford JD, Vilis T. Symmetry of oculomotor burst neuron coordinates about Listing’s plane. J Neurophysiol. 1992;68:432–448. doi: 10.1152/jn.1992.68.2.432. [DOI] [PubMed] [Google Scholar]
- 52.Tweed D. Visual-motor optimization in binocular control. Vis Res. 1997;37:1939–1951. doi: 10.1016/s0042-6989(97)00002-3. [DOI] [PubMed] [Google Scholar]
- 53.Misslisch H, Tweed D. Neural and mechanical factors in eye control. J Neurophysiol. 2001;86:1877–1883. doi: 10.1152/jn.2001.86.4.1877. [DOI] [PubMed] [Google Scholar]
- •• 54.Angelaki DE, Hess BJ. Control of eye orientation: where does the brain’s role end and the muscle’s begin? Eur J Neurosci. 2004;19:1–10. doi: 10.1111/j.1460-9568.2004.03068.x. A good summary of the fundamental controversy regarding the neural versus mechanical implementation of Listing’s Law, advocating the position that most ocular kinematics are neurally encoded. [DOI] [PubMed] [Google Scholar]
- 55.Angelaki DE. Three-dimensional ocular kinematics during eccentric rotations: Evidence for functional rather than mechanical constraints. J Neurophysiol. 2003;89:2685–2696. doi: 10.1152/jn.01137.2002. [DOI] [PubMed] [Google Scholar]
- 56.Klier EM, Crawford JD. Human oculomotor system acounts for 3-D eye orientation in the visual-motor transformation for saccades. J Neurophysiol. 1998;80:2274–2294. doi: 10.1152/jn.1998.80.5.2274. [DOI] [PubMed] [Google Scholar]
- 57.Kapoula Z, Bernotas M, Haslwanter T. Listing’s plane rotation with convergence: role of disparity, accommodation, and depth perception. Exp Brain Res. 1999;126:175–186. doi: 10.1007/s002210050727. [DOI] [PubMed] [Google Scholar]
- 58.Mok D, Ro A, Cadera W, et al. Rotation of Listing’s plane during vergence. Vision Res. 1992;32:2055–2064. doi: 10.1016/0042-6989(92)90067-s. [DOI] [PubMed] [Google Scholar]
- 59.Lee C, Zee DS, Straumann D. Saccades from torsional offset positions back to Listing’s plane. J Neurophysiol. 2000;83:3141–3253. doi: 10.1152/jn.2000.83.6.3241. [DOI] [PubMed] [Google Scholar]
- 60.Tian JR, Crane BT, Demer JL. Vestibular catch-up saccades in labyrinthine deficiency. Exp Brain Res. 2000;131:448–457. doi: 10.1007/s002219900320. [DOI] [PubMed] [Google Scholar]
- •• 61.Demer JL, Clark RA. Magnetic resonance imaging of human extraocular muscles during static ocular counter-rolling. J Neurophysiol. 2005;94:3292–3302. doi: 10.1152/jn.01157.2004. Demonstration by MRI in humans of torsional reconfiguration of rectus EOM pulleys during ocular counter-rolling reviews the kinematic and neural implications. This is the first investigation of pulley behavior during any VOR. [DOI] [PubMed] [Google Scholar]
- •• 62.Ghasia FF, Angelaki DE. Do motoneurons encode the noncommutativity of ocular rotations? Neuron. 2005;47:281–293. doi: 10.1016/j.neuron.2005.05.031. A decisive, single-unit recording study in alert monkeys indicating absence of commands to cyclovertical EOMs to encode the torsion of Listing’s Law during visually guided eye movement. This supports the conclusion that Listing’s Law is mechanical. Perplexingly, no commands to cyclovertical EOMs were observed to encode the quarter-angle torsion during the VOR. [DOI] [PubMed] [Google Scholar]
- 63.Klier EM, Meng H, Angelaki DE. Abducens nerve/nucleus stimulation produces kinematically correct three-dimensional eye movements. Soc Neurosci Abstr. 2005:abstract 475.4. [Google Scholar]
- 64.Goldberg SJ, Wilson KE, Shall MS. Summation of extraocular motor unit tensions in the lateral rectus muscle of the cat. Muscle Nerve. 1997;20:1229–1235. doi: 10.1002/(sici)1097-4598(199710)20:10<1229::aid-mus4>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 65.Goldberg SJ, Meredith MA, Shall MS. Extraocular motor unit and whole-muscle responses in the lateral rectus muscle of the squirrel monkey. J Neurosci. 1998;18:10629–10639. doi: 10.1523/JNEUROSCI.18-24-10629.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Scherberger H, Cabungcal J-H, Hepp K, et al. Ocular counterroll modulates the preferred direction of saccade-related pontine burst neurons in the monkey. J Neurophysiol. 2001;86:935–949. doi: 10.1152/jn.2001.86.2.935. [DOI] [PubMed] [Google Scholar]
- 67.Hess BJM, Angelaki DE. Gravity modulates Listing’s plane orientation during both pursuit and saccades. J Neurophysiol. 2003;90:1340–1345. doi: 10.1152/jn.00167.2003. [DOI] [PubMed] [Google Scholar]
- 68.Hess BJM, Angelaki DE. Kinematic principles of primate rotational vestibulo-ocular reflex II. Gravity-dependent modulation of primary eye position. J Neurophysiol. 1997;78:2203–2216. doi: 10.1152/jn.1997.78.4.2203. [DOI] [PubMed] [Google Scholar]
- 69.Hess BJM, Angelaki DE. Kinematic principles of primate rotational vestibulo-ocular reflex. I Spatial organization of fast phase velocity axes. J Neurophysiol. 1997;78:2193–2202. doi: 10.1152/jn.1997.78.4.2193. [DOI] [PubMed] [Google Scholar]
- 70.Crawford JD, Tweed DB, Vilis T. Static ocular counterroll is implemented through the 3-D neural integrator. J Neurophysiol. 2003;90:2777–2784. doi: 10.1152/jn.00231.2003. [DOI] [PubMed] [Google Scholar]
- 71.Frens MA, Suzuki Y, Scherberger H, et al. The collicular code of saccade direction depends on the roll orientation of the head relative to gravity. Exp Brain Res. 1998;120:283–290. doi: 10.1007/s002210050402. [DOI] [PubMed] [Google Scholar]
- 72.Crawford JD, Martinez-Trujillo JC, Kleier EM. Neural control of three-dimensional eye and head movements. Curr Opin Neurosci. 2003;13:655–662. doi: 10.1016/j.conb.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 73.Van Opstal AJ, Hepp K, Hess BJ, et al. Two- rather than three-dimensional representation of saccades in monkey superior colliculus. Science. 1991;252:1313–1315. doi: 10.1126/science.1925545. [DOI] [PubMed] [Google Scholar]
- 74.Crawford JD, Guitton D. Visual-motor transformations required for accurate and kinematically correct saccades. J Neurophysiol. 1997;78:1447–1467. doi: 10.1152/jn.1997.78.3.1447. [DOI] [PubMed] [Google Scholar]
- 75.Porrill J, Warren PA, Dean P. A simple control laws generates Listing’s positions in a detailed model of the extraocular muscle system. Vis Res. 2000;40:3743–3758. doi: 10.1016/s0042-6989(00)00211-x. [DOI] [PubMed] [Google Scholar]
- 76.Wong AMF, Sharpe JA, Tweed D. Adaptive neural mechanism for Listing’s law revealed in patients with fourth nerve palsy. Invest Ophthalmol Vis Sci. 2002;43:1796–1803. [PubMed] [Google Scholar]
- 77.Straumann D, Steffen H, Landau K, et al. Primary position and Listing’s law in acquired and congenital trochlear nerve palsy. Invest Ophthalmol Vis Sci. 2003;44:4282–4292. doi: 10.1167/iovs.02-1181. [DOI] [PubMed] [Google Scholar]
- • 78.Migliaccio AA, Cremer PD, Sw ST, Halmagyi GM. Vergence-mediated changes in Listing’s plane do not occur in an eye with superior oblique palsy. Invest Ophthalmol Vis Sci. 2004;45:3043–3047. doi: 10.1167/iovs.04-0014. This study demonstrated in humans that the Listing’s planes of eyes with trochlear palsy did not tilt temporally with convergence in the normal manner, consistent with the idea that the superior oblique muscle is involved in the temporal tilting. [DOI] [PubMed] [Google Scholar]
- 79.Angelaki DE, Dickman DJ. Premotor neurons encode torsional eye velocity during smooth-pursuit eye movements. J Neurosci. 2003;23:2971–2979. doi: 10.1523/JNEUROSCI.23-07-02971.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •• 80.Demer JL. The anatomy of strabismus. In: Taylor D, Hoyt C, editors. Pediatric Ophthalmology and Strabismus. 3. London: Elsevier; 2005. pp. 849–861. This is a detailed, recent textbook chapter describing the functional anatomy of the orbital pulley system, with applications to strabismus and strabismus surgery. [Google Scholar]


