Abstract
Catalase, an enzyme which detoxifies H2O2, may interfere with cardiac aging. To test this hypothesis, contractile and intracellular Ca2+ properties were evaluated in cardiomyocytes from young (3–4 mo) and old (26–28 mo) FVB and transgenic mice with cardiac overexpression of catalase. Contractile indices analyzed included peak shortening (PS), time-to-90% PS (TPS90), time-to-90% relengthening (TR90), half-width duration (HWD), maximal velocity of shortening/relengthening (± dL/dt) and intracellular Ca2+ levels or decay rate. Levels of advanced glycation endproduct (AGE), Na+/Ca2+ exchanger (NCX), sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA2a), phospholamban (PLB), myosin heavy chain (MHC), membrane Ca2+ and K+ channels were measured by western blot. Catalase transgene prolonged survival while did not alter myocyte function by itself. Aging depressed ± dL/dt, prolonged HWD, TR90 and intracellular Ca2+ decay without affecting other indices in FVB myocytes. Aged FVB myocytes exhibited a stepper decline in PS in response to elevated stimulus or a dampened rise in PS in response to elevated extracellular Ca2+ levels. Interestingly, aging-induced defects were nullified or significantly attenuated by catalase. AGE level was elevated by 5-fold in aged FVB compared with young FVB mice, which was reduced by catalase. Expression of SERCA2a, NCX and Kv1.2 K+ channel was significantly reduced although levels of PLB, L-type Ca2+ channel dihydropyridine receptor and β-MHC isozyme remained unchanged in aged FVB hearts. Catalase restored NCX and Kv1.2 K+ channel but not SERCA2a level in aged mice. In summary, our data suggested that catalase protects cardiomyocytes from aging-induced contractile defect possibly via improved intracellular Ca2+ handling.
Keywords: Catalase, cardiomyocytes, aging, shortening/relengthening, Ca2+ cycling protein
INTRODUCTION
Cardiac senescence, or cardiac aging, is referred to a dramatic decline in cardiac pump function and contractile reserve with advanced age. It is an irreversible biological process which contributes to high cardiovascular mortality (Lakatta et al. 1987; Lakatta 2000). Although occult diseases and sedentary life style may contribute to senescence-related changes in cardiovascular function, biological aging or advanced age itself has been demonstrated to be the single most independent risk factor for deteriorated cardiac morphology and function (Fleg et al. 1995; Lakatta et al. 1987; Lakatta 2000). Several rationales have been speculated for cardiac aging including prolonged action potential duration, myosin isozyme switch, impaired intracellular Ca2+ homeostasis, altered membrane structure and permeability and accumulation of reactive oxygen species (ROS), which may lead to abnormal cardiac contractile function (Kass et al. 2001; Lakatta 1999; Lakatta 2000). Although the precise link between advanced age and altered cardiac contractile function has not been fully elucidated, the “free radical theory of aging” seems to draw the most attention for development of cardiac aging (Yang et al. 2005). Data from our recent studies revealed that cardiac overexpression of heavy metal scavenging antioxidant metallothionein prolongs life span, alleviates aging-associated cardiac contractile dysfunction, insulin insensitivity and mitochondrial damage (Fang et al. 2005; Yang et al. 2006). To further study the role of enzymatic antioxidants on cardiac senescence, the present study was designed to examine the effect of catalase, an enzyme for H2O2 detoxification, on aging-elicited alteration in cardiomyocyte contractile function and intracellular Ca2+ cycling proteins. We hypothesized that catalase shifts redox (i.e., antioxidant/oxidant) balance to be pro-antioxidant and thus protects against oxidative stress-induced cardiac insult under senescence. To this end, a cardiac-specific catalase transgenic mouse line was employed in which catalase was constitutively over-expressed (~50 fold) in myocytes, distributing from sarcoplasmic reticulum (SR), nucleus to peroxisomes (Zhou and Kang 2000). Cardiomyocyte contractile and intracellular Ca2+ transient properties were examined at young (3–4 month) and old (26–28 month) ages. Protein abundance of major intracellular Ca2+ regulating proteins sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA2a), phospholamban (PLB), Na+/Ca2+ exchanger (NCX), membrane ion channels such as voltage-gated L-type Ca2+ channel dihydropyridine receptor (DHPR) and K+ channels, myosin heavy chain isozyme and advanced glycation endproduct (AGE), a protein post-translational modification product highly correlated with aging, were evaluated in hearts from young and old catalase transgenic and wild-type FVB mice.
MATERIALS AND METHODS
Cardiac catalase over-expression transgenic mice and Kaplan-Meier’s survival curve
All animal procedures used here were approved by the Animal Care and Use Committee at the University of Wyoming (Laramie, WY, USA). Production of cardiac-specific catalase transgenic mice was described in detail previously (Kang et al. 1996; Zhang et al. 2003). In brief, an 8-kb catalase transgene driven by a myosin heavy chain (MHC) promoter containing the entire coding sequences of the catalase cDNA was purified on a matrix of diatemaceous earth and filtered through a 0.22-μm filter. Approximately 100 copies of purified transgene insert were microinjected into one pronucleus of each one-cell mouse embryo of inbred strain FVB. The transgene transcription of catalase was controlled by mouse α-MHC gene. To identify transgenic founder mice, genomic DNA was isolated from 1-cm tail clips from 4-week-old mice. DNA was subjected to Southern and dot blot analyses, which were probed with a 550-bp SmaI/NotI fragment derived from the rat insulin II portion of the catalase transgene. This probe was hybridized to an 8000-bp EcoRI fragment of the transgene, consistent with the presence of a unique EcoRI site in the catalase transgene (600 bp upstream of the MHC transcription initiation site). Founder mice were bred with mice of the same strain and transgenic offspring were identified by a polymerase chain reaction (PCR) using a primer pair derived from the MHC promoter and rat catalase cDNA. Reverse: aat atc gtg ggt gac ctc aa; Forward: cag atg aag cag tgg aag ga (Zhang et al. 2003). Transgenic positive mice (heterozygotes) and their transgenic negative littermates (FVB) were used for experiments. Adult male FVB and catalase transgenic mice at young (3–4 month-old) and old (26–28 month-old) ages were used in this study unless otherwise stated. To configure the Kaplan-Meier survival curve, FVB and catalase mice of both genders were separated after weanling. All mice were housed (7–8 mixed mice per cage until they were genotyped at ~ 3 month of age) in a pathogen free environment in our animal facility within School of Pharmacy. Mice were maintained with a 12/12-light/dark cycle with free access to regular rodent chow and tap water. Mortality was recorded every three months (excluding unexpected death such as fighting).
Measurement of catalase enzyme activity
Frozen ventricular tissues were homogenized and sonicated on ice in a buffer containing 20 mM MOPS, 620 mM sucrose, 1 mM EDTA at pH 7.2. The homogenate was centrifuged at 13,000 × g for 30 min (4°C) and protein concentration in the extracted supernatant fraction was determined by the Bradford assay (Bradford 1976). Ice-cold catalase buffer (50 mM potassium phosphate monobasic, 50 mM sodium phosphate dibasic, pH 7.0) was added to extracted samples and H2O2 was used to initiate the reaction. Catalase activity value was calculated from the spectrophotometric reading at 240 nm with respect to appropriate concurrent blanks (Brown-Borg et al. 1999).
Isolation of mouse cardiomyocytes
Hearts were rapidly removed from anesthetized mice and mounted onto a temperature-controlled (37°C) Langendorff system. After perfusing with a modified Tyrode’s solution (Ca2+ free) for 2 min, the heart was digested with a Ca2+-free KHB buffer containing liberase blendzyme 4 (Hoffmann-La Roche Inc., Indianapolis, IN, USA) for 20 min. The modified Tyrode solution (pH 7.4) contained the following (in mM): NaCl 135, KCl 4.0, MgCl2 1.0, HEPES 10, NaH2PO4 0.33, glucose 10, butanedione monoxime 10, and the solution was gassed with 5% CO2-95% O2. The digested heart was then removed from the cannula and left ventricle was cut into small pieces in the modified Tyrode’s solution. Tissue pieces were gently agitated and pellet of cells was resuspended. Extracellular Ca2+ was added incrementally back to 1.20 mM over a period of 30 min. Isolated myocytes were used for experiments within 8 hours of isolation. Normally, a yield of at least 50–60% viable rod-shaped cardiomyocytes with clear sacromere striations was achieved. Isolation with low yield of myocytes (< 40%, usually as a result of over-digestion) was not used. Myocytes with obvious sarcolemmal blebs or spontaneous contractions were not chosen for study. Only rod-shaped myocytes with clear edges were selected for mechanical and intracellular Ca2+ studies (Duan et al. 2002).
Cell shortening/relengthening and response to various stimulus frequencies or extracellular Ca2+
Mechanical properties of ventricular myocytes were assessed using a SoftEdge MyoCam® system (IonOptix Corporation, Milton, MA, USA) (Duan et al. 2002). In brief, cells were placed in a Warner chamber mounted on the stage of an inverted microscope (Olympus, IX-70) and superfused (~1 ml/min at 25°C) with a buffer containing (in mM): 131 NaCl, 4 KCl, 1 CaCl2, 1 MgCl2, 10 glucose, 10 HEPES, at pH 7.4. The cells were field stimulated with supra-threshold voltage at a frequency of 0.5 Hz (unless otherwise stated), 3 msec duration, using a pair of platinum wires placed on opposite sides of the chamber connected to a FHC stimulator (Brunswick, NE, USA). The myocyte being studied was displayed on the computer monitor using an IonOptix MyoCam camera. An IonOptix SoftEdge software was used to capture changes in cell length during shortening and relengthening. Cell shortening and relengthening were assessed using the following indices: peak shortening (PS) - indicative of peak ventricular contractility, time-to- 90% PS (TPS90) - indicative of contraction duration, and time-to-90% relengthening (TR90) – represents cardiomyocyte relaxation duration, half-width duration (HWD) – duration between 50% shortening and 50% relengthening and an indicative of late contraction and early relaxation, maximal velocities of shortening (+ dL/dt) and relengthening (−dL/dt) - indicatives of maximal velocities of ventricular pressure rise/fall. In the case of altering stimulus frequency from 0.1 Hz to 5.0 Hz, the steady state contraction of myocyte was achieved (usually after the first 5–6 beats) before PS was recorded. The contractile response of myocytes to increasing concentrations of extracellular Ca2+ (0.5 to 3.0 mM) was performed after switching extracellular Ca2+ concentration to a different perfusion level for 5 min.
Intracellular Ca2+ transient measurement
Myocytes were loaded with fura-2/AM (0.5 μM) for 10 min and fluorescence measurements were recorded with a dual-excitation fluorescence photomultiplier system (Ionoptix). Myocytes were placed on an Olympus IX-70 inverted microscope and imaged through a Fluor × 40 oil objective. Cells were exposed to light emitted by a 75W lamp and passed through either a 360 or a 380 nm filter, while being stimulated to contract at 0.5 Hz. Fluorescence emissions were detected between 480–520 nm by a photomultiplier tube after first illuminating the cells at 360 nm for 0.5 sec then at 380 nm for the duration of the recording protocol (333 Hz sampling rate). The 360 nm excitation scan was repeated at the end of the protocol and qualitative changes in intracellular Ca2+ concentration were inferred from the ratio of fura-2 fluorescence intensity (FFI) at two wavelengths (360/380). Fluorescence decay time was measured as an indication of the intracellular Ca2+ clearing rate. Both single and bi-exponential curve fit programs were applied to calculate the intracellular Ca2+ decay constant (Duan et al. 2002).
Western blot analysis
Protein levels of SERCA2a, PLB, NCX, Kv1.2 potassium channel, voltage-gated L-type Ca2+ channel dihydropyridine receptor α subunit (DHPR-α) and the post-translational modification product AGE were examined by standard Western blot. Left ventricular tissues were homogenized and centrifuged at 1,000 × g for 10 min. The supernatants were then centrifuged at 70,000 × g for 30 min at 4°C. The 100,000-g pellets were cellular membrane fractions and were used for immunoblotting of SERCA2a, PLB, NCX, Kv1.2, DHPR-α and AGE. Membrane proteins (35 μg/lane) were separated on 10 – 15% SDS-polyacrylamide gels in a minigel apparatus (Mini-PROTEAN II, Bio-Rad, Hercules, CA, USA) and transferred to polyvinylidene difluoride membranes. The membrane was blocked with TBS-0.05% Tween 20 (TBS-T) with 5% nonfat dry milk for 60 min and was then incubated with monoclonal anti- SERCA2a (1:2,000, Affinity BioReagentTM, Golden, CO, USA), monoclonal anti-PLB (1:2,000, Abcam Inc, Cambridge, MA, USA), polyclonal anti-NCX (1:1,000, Swant, Bellinzona, Switzerland), monoclonal anti-Kv1.2 (1;1,000, Upstate Biotechnology, Lake Placid, NY, USA), polyclonal anti-DHPR-α (1:200, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), and anti-AGE monoclonal (1:1,000) (Trans Genic Inc., Kumamoto, Japan) in TBS-T with 5% BSA overnight at 4°C. The antigens were detected by the luminescence method (ECL Western blotting detection kit, Amersham) with peroxidase-linked anti-mouse (SERCA2a, PLB, Kv1.2), anti-rabbit (NCX) or anti-goat (DHPR-α) IgG (1:5,000 dilution). Quantification of band and AGE was determined using Quantity One density of SERCA2a, NCX, PLB, Kv1.2, DHPR-α software (Bio-Rad, version 4.4.0, build 36) after conversion of radiographic film to TIFF files (8-bit grayscale) using a Scanjet 7400c (Hewlett-Packard, Palo Alto, CA, USA) and reported in optical density per square millimeter. Densitometry was performed by outlining the bands with the volume rectangle tool initially set on the control band of interest (Zhang et al. 2003).
MHC isoform analysis by gel electrophoresis
Samples were prepared for gel electrophoresis according to method described by Blough and colleagues (Blough et al. 1996). Each sample (20–30 mg heart tissue) was placed in a microcentrifuge tube and 30 μl of sample buffer was added per mg of tissue. The sample was homogenized for ~10 sec, heated for 2 min at 95°C, chilled on ice for 5 min before being centrifuged. The supernatant was saved and diluted 1:10 with sample. Three μl of diluted samples was loaded for gel electrophoresis performed at 8°C in a PROTEAN II unit (Bio-Rad, Hercules, CA, USA). Stacking gels consisted of 4% acrylamide (acrylamide:bis = 50:1) and 5% glycerol (v/v, pH 8.8). Gels were run at a constant voltage of 200 V for 30 hr, fixed for a minimum of 2 hr in 5% glutaraldehyde before being silver-stained and scanned with a Bio-Rad GS-800 densitometer to determine the amount of MHC-β as a percentage of total MHC (Li et al. 2006).
Statistical analyses
Data were presented as Mean ± SEM. Log rank test was used for Kaplan-Meier survival comparison. Statistical significance (p < 0.05) for each variable was determined by analysis of variance (ANOVA) or t-test, where appropriate.
RESULTS
General characteristics of mice and the Kaplan-Meier survival curve
General features of young and old FVB and catalase transgene mice are shown in Table 1, transgenic catalase over-expression did not elicit any notable effect on body, heart weights and heart size (heart-to-body weight ratio) compared with those of age-matched FVB wild-type littermates. Older mice display heavier body and heart weights (although not heart size, i.e., heart weight normalized to body weight) compared with young counterparts, as expected. The fasting blood glucose levels were similar among all four mouse groups studied, excluding potential contribution of diabetes to aging-induced effects, if any. The Kaplan-Meier curve comparison depicts that catalase transgenic mice display significantly better survival rates than FVB mice in both genders. The median lifespan was 3 and 6 month longer in male (30 months) and female (33 months) catalase mice, respectively, than that of FVB mice (27 months in both genders). The survival curves of FVB and catalase mice begin to separate from each other after 15 months of age with catalase mice exhibiting a reduced mortality rate in both genders (Fig. 1). To verify cardiac over-expression of catalase, catalase enzyme activity assay was performed. Result shown in Fig. 2 depicts a nearly 6-fold increase in cardiac catalase activity in young transgenic mice compared with the wild-type FVB mice. Aging itself triggered a significant decline in cardiac catalase activity, which can easily be masked by cardiac over-expression of catalase.
Table 1.
General features of young (3–4 month-old) and old (26–28 month-old) wild-type FVB and catalase (CAT) transgenic mice.
| Mice group | FVB-Young | FVB-Old | CAT-Young | CAT-Old |
|---|---|---|---|---|
| Body weight (g) | 20.4 ± 0.5 | 30.5 ± 0.9* | 20.1 ± 0.5 | 31.1 ± 0.6* |
| Heart weight (mg) | 106 ± 4 | 169 ± 8* | 106 ± 3 | 160 ± 6* |
| Heart/Body weight (mg/g) | 5.19 ± 0.11 | 5.55 ± 0.23 | 5.26 ± 0.07 | 5.14 ± 0.14 |
| Fasting blood glucose (mM) | 5.54 ± 0.31 | 5.71 ± 0.35 | 5.62 ± 0.37 | 5.43 ± 0.35 |
Mean ± SEM, n = 19 – 24 mice per group,
p < 0.05 vs. FVB-young group.
Fig 1.
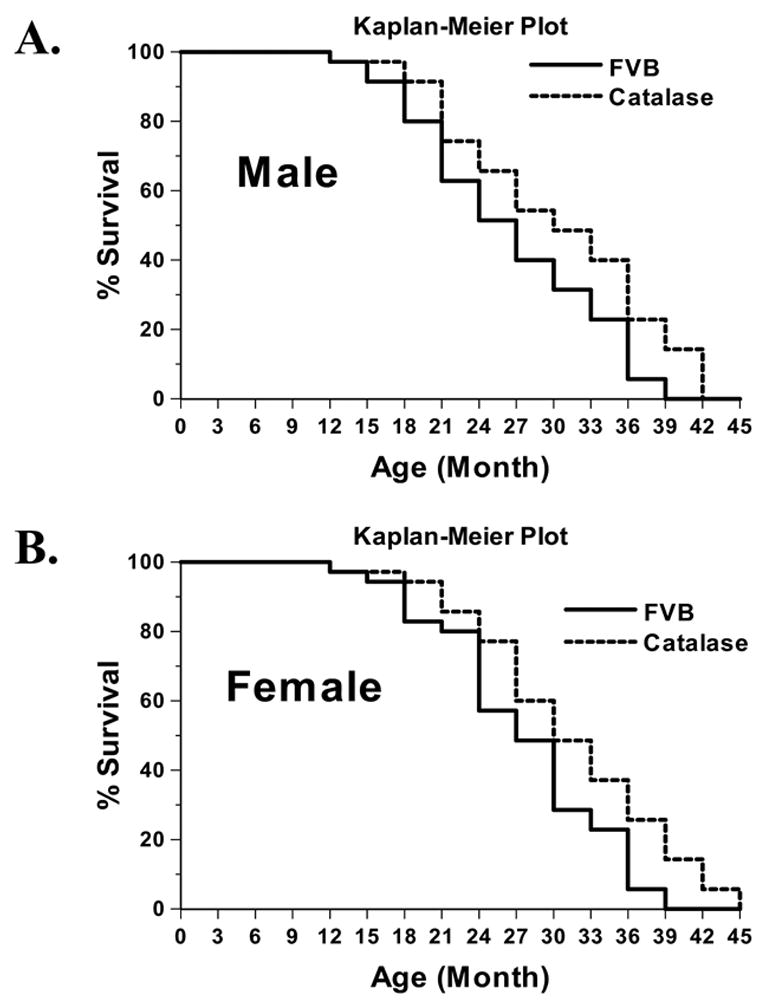
Cumulative survival curve (Kaplan-Meier survival plot) of male and female FVB and catalase mice. Survival rate of mice was checked every three months. The cumulative survival rate was plotted against the time course (n = 35 mice per group). Log rank test was performed to compare the FVB and catalase lines with a p value of 0.0117 and 0.0202 in male (panel A) and female (panel B) mice, respectively.
Fig. 2.
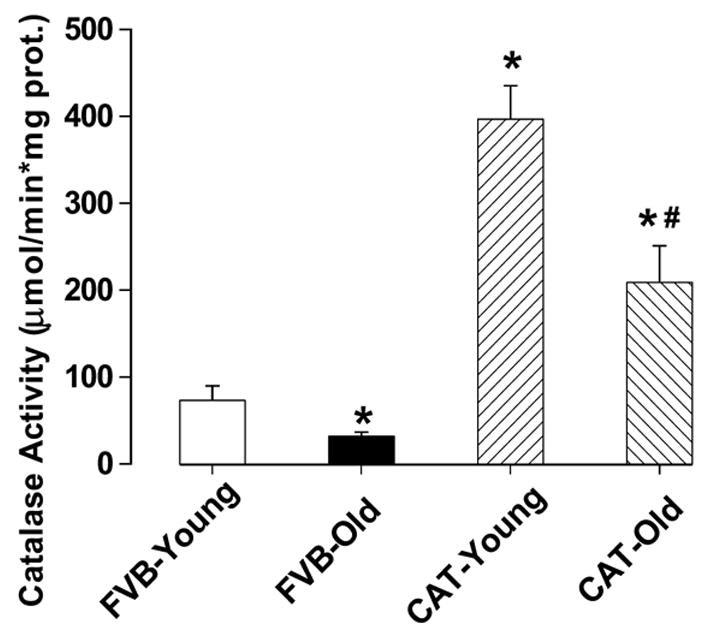
Cardiac catalase activity in male young and old FVB and catalase (CAT) mice. Mean ± SEM, n = 8–9 hearts per group, * p < 0.05 vs. FVB-young group, # p < 0.05 vs. FVB-old group.
Mechanical and intracellular Ca2+ properties of cardiomyocytes
Mechanical properties obtained under extracellular Ca2+ of 1.0 mM and a stimulus frequency of 0.5 Hz indicated that resting cell length and peak shortening (PS) amplitude were all similar in cardiomyocytes among young and old male FVB and catalase mice. However, myocytes from old FVB mice displayed significantly reduced maximal velocity of shortening/relengthening (± dL/dt) compared with myocytes from young FVB mice. The reduced ± dL/dt was associated with normal shortening duration (TPS90), prolonged relengthening duration (TR90) and half-width duration (HWD) in old FVB myocytes. Interestingly, aging-induced mechanical dysfunctions were not observed in old catalase transgenic mice, suggesting a beneficial role of the antioxidant catalase against aging-associated cardiac dysfunction. Catalase transgene itself did not affect mechanical contractile properties in young mouse group (Fig. 3). Our further study using the intracellular fluorescence dye fura-2 revealed reduced intracellular Ca2+ clearing rate (both single and bi-exponential curve fit), enhanced baseline intracellular Ca2+ levels associated with unchanged electrically-stimulated rise in intracellular Ca2+ in aged FVB cardiomyocytes, consistent with the observation of prolonged TR90 and normal PS in these myocytes. Similar to its effect on cell shortening, catalase abolished aging-induced prolongation in intracellular Ca2+ clearing rate and elevation in resting intracellular Ca2+ level in aged cardiomyocytes without affecting any indices tested in young cardiomyocytes (Fig. 4).
Fig 3.
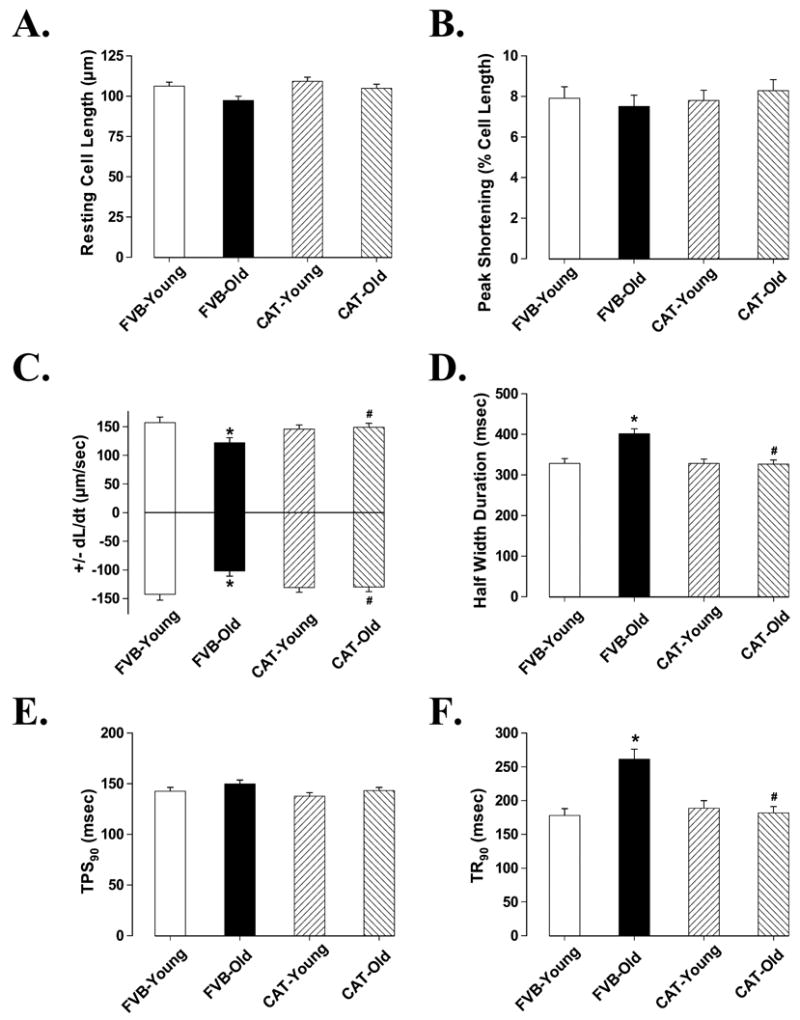
Contractile properties of cardiomyocytes from male young and old FVB and catalase (CAT) mice. A: Resting cell length; B: Peak shortening (PS, normalized to cell length); C: Maximal velocity of shortening (+ dL/dt) and relengthening (− dL/dt); D: Half width duration; E: Time-to-90% PS (TPS90); F: Time-to-90% relengthening (TR90). Mean ± SEM, n = 68 – 69 cells from 8 mice per group, * p < 0.05 vs. FVB-young group, # p < 0.05 vs. FVB-old group.
Fig 4.
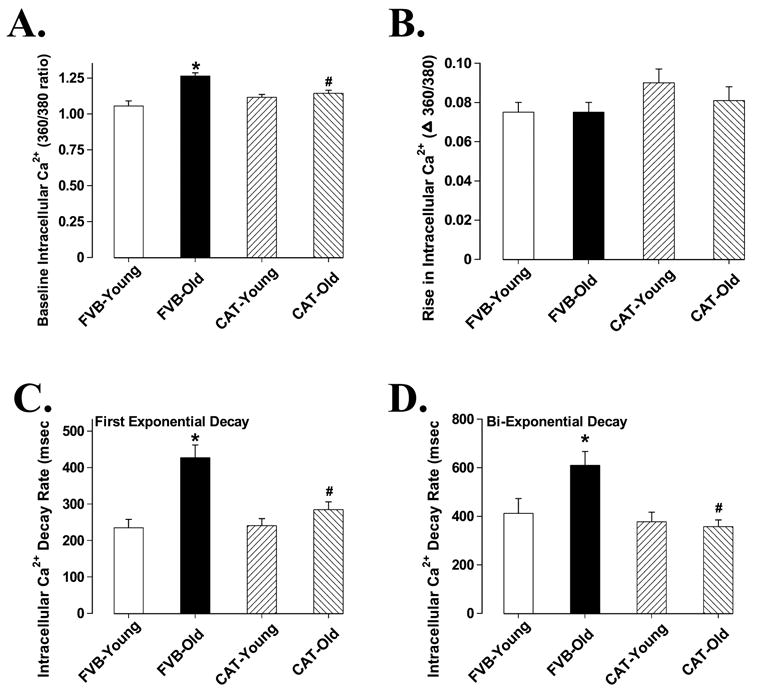
Intracellular Ca2+ properties of cardiomyocytes from male young and old FVB and catalase (CAT) mice. A: Baseline intracellular Ca2+ levels (360/380 ratio); B: Rise in intracellular Ca2+ levels in response to electrical stimulus (360/380 ratio); C: First exponential decay constant of intracellular Ca2+; and D: Bi-exponential decay constant of intracellular Ca2+. Mean ± SEM, n = 43 cells from 5 mice per group, * p < 0.05 vs. FVB-young group, # p < 0.05 vs. FVB-old group.
Effect of increasing stimulation frequency or extracellular Ca2+ concentration on myocyte shortening
Murine hearts normally contract at high frequencies, whereas our mechanical recording was conducted at 0.5 Hz. To evaluate the impact of aging on cardiac contractile function under higher frequencies, we raised stimulus frequency up to 5.0 Hz (300 beats/min) and recorded the steady-state peak shortening. Cells were initially stimulated to contract at 0.5 Hz for 5 min to ensure steady-state before commencing the frequency study. All the recordings were normalized to PS at 0.1 Hz of the same myocyte. Fig. 5A shows negative staircases in PS amplitude in cardiomyocytes from all four mouse groups when stimulus frequency was increased from 0.1 Hz to 0.5 Hz or higher. The percent decrease of PS from its value at 0.1 Hz was significantly greater in aged FVB myocytes than young myocytes at higher frequencies (0.5 – 5.0 Hz), suggesting reduced intracellular Ca2+ cycling or stress intolerance under advanced aging. Similar to its effect on myocyte contractile function, catalase transgene ablated high stimulating frequency-induced stepper reduction in PS among aged cardiomyocytes without affecting the response pattern in young cardiomyocytes. To examine the influence of extracellular Ca2+ levels on PS amplitude, as an estimate of the myofilament Ca2+ sensitivity, myocytes were first stimulated to contract at 0.5 Hz in a contractile buffer containing 0.5 mM CaCl2. After the steady-state PS had been recorded, the extracellular Ca2+ level was increased to 1.0, 2.0 and 3.0 mM. Fig.5B shows that increases in extracellular Ca2+ concentration from 0.5 mM to 3 mM resulted in a positive staircase in PS in myocytes from young FVB mice. Interestingly, the increase in PS in response to elevated extracellular Ca2+ concentration was significantly dampened in myocytes from aged FVB mice, suggesting a reduced Ca2+ sensitivity in aged cardiomyocytes. Catalase restored reduced Ca2+ sensitivity in aged cardiomyocytes without affecting the response in young cardiomyocytes.
Fig. 5.
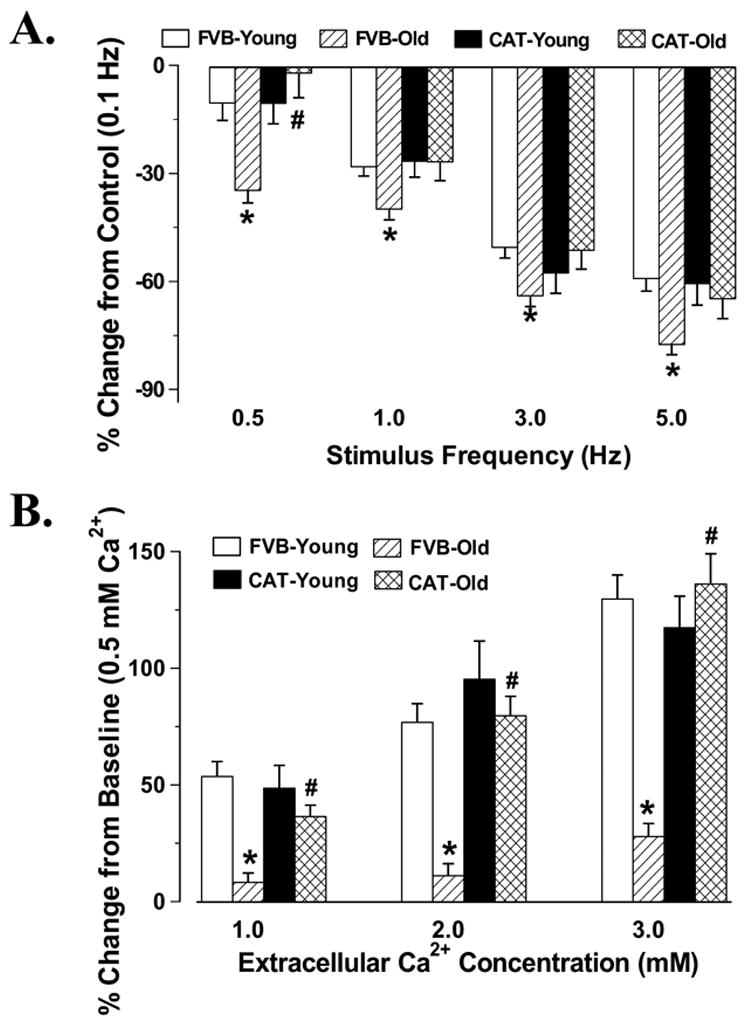
Peak shortening (PS) amplitude of cardiomyocytes from male young or old FVB and catalase (CAT) mice at different stimulus frequencies (Panel A with an extracellular Ca2+ concentration of 1.0 mM) or different extracellular Ca2+ concentrations (Panel B with a stimulus frequency of 0.5 Hz). PS was shown as % change from PS value at respective baseline (0.1 Hz for panel A and 0.5 mM Ca2+ for panel B) of the same cell. Mean ± SEM, n = 26 – 29 cells from 8 mice per group, * p < 0.05 vs. respective young group, # p < 0.05 vs. FVB-old group.
Expression of AGE and intracellular Ca2+ transport/regulatory proteins
Western blot analysis demonstrated a significant elevation in the levels of post-translational modification product AGE in aged FVB hearts which was nullified by catalase. Catalase itself significantly reduced the levels of AGE in young hearts (Fig. 6A). Cardiac contractile function is maintained by a cascade of intracellular Ca2+ regulatory/cycling proteins including SERCA, Na+-Ca2+ exchanger (NCX), phospholamban and membrane ion channels (Bers 2002). Aging has been demonstrated to affect the expression and function in some of these Ca2+ cycling proteins (Koban et al. 1998; Pavlovic et al. 2005; Schmidt et al. 2002; Xu and Narayanan 1998). Data obtained from our study indicated that aging down-regulated expression of SERCA2a, NCX and Kv1.2 potassium channel without any significant effect on expression of phospholamban and voltage-gated L-type Ca2+ channel dihydropyridine receptor α-subunit (DHPR-α). Catalase significantly alleviated aging-induced reduction in NCX and Kv1.2 potassium channel expression without affecting the level of SERCA2a. Protein expression of SERCA2a, NCX, phospholamban, Kv1.2 and DHPR-α was unaffected by catalase transgene in young mice (Fig. 6 and Fig. 7). It was indicated that expression of MHC isoforms is also closely associated with cardiac function phenotype (Olsson et al. 2004). We thus examined the pattern of MHC isozyme distribution in aged mouse heart. Result shown in Fig. 7A and Fig. 7B revealed similar distribution of β-MHC isozyme in young and old mouse hearts, which was not altered by catalase transgene.
Fig 6.
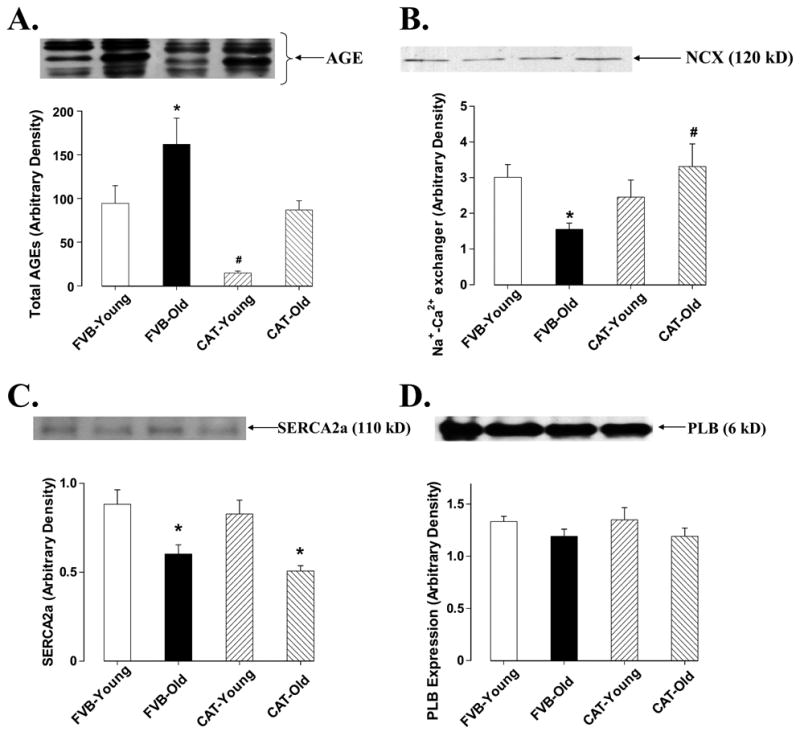
Western blot analysis of AGE, NCX, SERCA2a and PLB in male young or old FVB and catalase (CAT) transgenic mouse hearts. Insets: actual gel blots using specific anti-AGE, anti-NCX, aniti-SERCA2a and anti-PLB antibodies. Mean ± SEM, n = 9 – 13 samples per group, * p < 0.05 vs. FVB-young group, # p <0.05 vs. FVB-old group.
Fig 7.
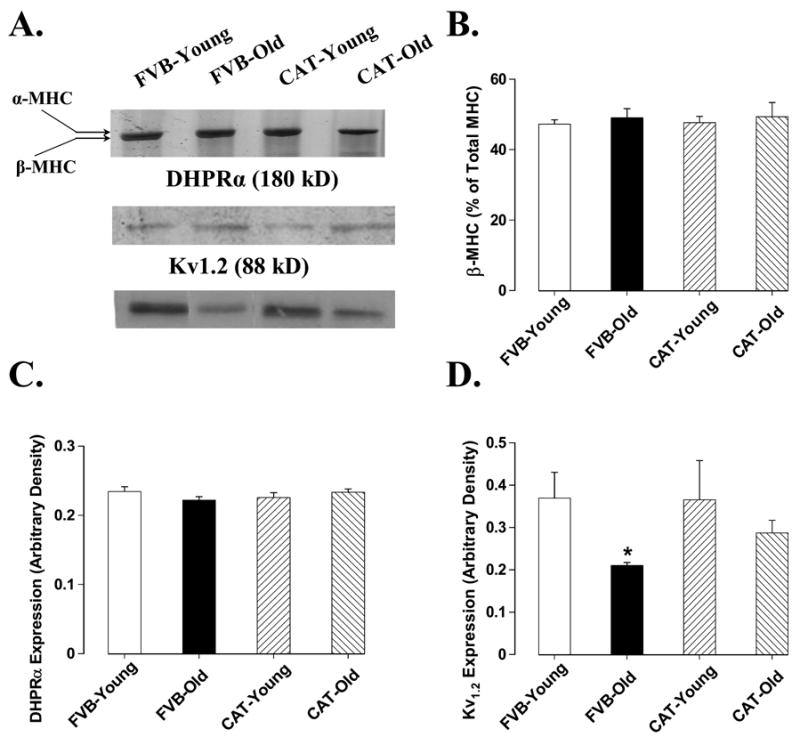
Western blot analysis of DHPR-α voltage-dependent Ca2+ channel, Kv1.2 potassium channel and myosin heavy chain (MHC) β-isozyme distribution in male young or old FVB and catalase (CAT) transgenic mouse hearts. Panel A: actual gel blots depicting protein expression using specific antibodies; Panel B-D: expression of DHPR-α, Kv1.2 and β-MHC. Mean ± SEM, n = 4 – 5 samples per group, * p < 0.05 vs. FVB-young group, # p <0.05 vs. FVB-old group.
DISCUSSION
Our study presented for the first time that cardiac-specific overexpression of catalase attenuated aging-induced cardiac contractile and intracellular Ca2+ handling abnormalities. Catalase transgene prolonged lifespan, ameliorates aging-induced decline in peak cardiac contractile capacity under increased stimulus frequency and elevated extracellular Ca2+ concentration. The catalase-induced cardiac protection against aging-induced cardiac defects may be underscored by alteration in AGE level and protein expression of certain Ca2+ regulatory proteins such as Na+-Ca2+ exchanger and potassium channel. These data supported the notion that ROS especially H2O2, the main enzymatic target of catalase, may play a significant role in the pathogenesis of aging-associated cardiac contractile defects. Since catalase transgene itself did not overtly alter cardiac contractile and intracellular Ca2+ properties in young mice, indicating that catalase may not be innately harmful to cardiac function even at the levels of 50-fold overexpression. Therefore, it may be considered as a beneficial route to delay the cardiac aging process and to minimize senescence-associated high cardiac mortality (Cutler 2005).
Mounting evidence from human and experimental animals has indicated compromised cardiac function in senescence (Fleg et al. 1995; Lakatta 1999; Li et al. 2005). Studies from various murine strains including C57BL/6 and FVB mice depicted diminished cardiac contractile function and enhanced susceptibility to injury such as ischemia-reperfusion, due to either aging-associated decrease in coronary circulation and collateral flow or aging-related decline in intrinsic myocardial contractile capacity (Azhar et al. 1999; Fang et al. 2005; Li et al. 2005; Willems et al. 2003; Yang et al. 2006). Results from our present study revealed reduced maximal velocity of contraction and relaxation, prolongation of relaxation and half-width duration in aged FVB cardiomyocytes, similar to previously published report in aged cardiomyocytes (Fang et al. 2005; Li et al. 2005; Yang et al. 2006). Our data demonstrated that catalase transgene attenuated aging-induced cardiac contractile dysfunction, prolonged intracellular Ca2+ clearing, reduced Na+-Ca2+ exchanger and Kv1.2 expression as well as AGE accumulation, indicating that facilitated intracellular Ca2+ cycling and reduced oxidative stress. Our observation of improved lifespan and cardiac contractile function in aged catalase mice is consistent with recent finding that median and maximum life spans were maximally increased by ~5 months and 5.5 months, respectively, in transgenic mice overexpressing human catalase localized to the peroxisome, the nucleus, or mitochondria (Schriner et al. 2005). The discrepancy in lifespan prolongation between our present study and the one done by Schriner and colleagues (Schriner et al. 2005) may be due to different sites of catalase overexpression. Nonetheless, results from these two studies are complimentary to each other. Transgenic mice with catalase overexpression localized to peroxisome, nucleus or mitochondria exhibit delayed cardiac pathology, reduced oxidative damage, H2O2 production and H2O2-induced aconitase inactivation as well as diminished onset of mitochondrial deletion (Schriner et al. 2005). These data was in support of our observation of improved cardiac contractile function, intracellular Ca2+ handling, contractile capacity in response to higher stimulus or extracellular Ca2+ and Ca2+ cycling/transport proteins in hearts from cardiac specific catalase over-expression mice.
Data from our current study depicted impaired intracellular Ca2+ handling especially prolonged intracellular Ca2+ clearance and elevated resting intracellular Ca2+ levels in aged cardiomyocytes. Cardiac intracellular Ca2+ homeostasis is maintained by a number of membrane ion channels and Ca2+ transport/regulatory proteins within cardiomyocytes (Bers 2002). SERCA and Na+-Ca2+ exchanger are considered the main machineries to remove Ca2+ from cytosolic space for relaxation to occur. However the relative contribution for intracellular Ca2+ extrusion by SERCA and Na+-Ca2+ exchanger are still controversial, and may vary among different species (Bers 2002; Hove-Madsen and Bers 1993; Li et al. 1998). Cannell estimated that Na+-Ca2+ exchanger accounts for ~75% of intracellular Ca2+ extrusion in cardiomyocytes (Cannell 1991). However, Bers suggested that Na+-Ca2+ exchanger contributes to less that 10% of intracellular Ca2+ removal while SERCA is responsible for 90% Ca2+ removal in mouse hearts (Bers 2002). Our data revealed remarkable down-regulation of both Na+-Ca2+ exchanger and SERCA2a but not phospholamban, an endogenous inhibitor of SERCA, in myocytes from aged wild-type FVB mice. The reduced SERCA2a and Na+-Ca2+ exchanger expression may account for, at least in part, impaired cardiomyocyte relaxation (prolonged relaxation and half-width duration), prolonged intracellular Ca2+ clearance and possibly intracellular Ca2+ overload in senescent myocytes. In addition, reduced Kv1.2 protein expression in aged FVB hearts also suggest possible contribution of potassium channel in prolonged repolarization phase of action potential and thus elongated relaxation duration. Unchanged DHPR-α and β-MHC seem to rule out contribution from L-type Ca2+ channel and myosin isozyme switch in cardiomyocyte dysfunction under the present experimental setting. Somewhat conflicting data have been seen for expression or function of cardiac Ca2+, K+ channels and MHC isozyme under senescence including augmented (Dibb et al. 2004; Schuyler and Yarbrough 1990), unchanged (Josephson et al. 2002; Krishnamurthy et al. 2004; Long et al. 1999) or depressed (Liu et al. 2000; Long et al. 1999; Ranki et al. 2002) Ca2+ or K+ channel expression as well as β-MHC distribution. Animal species, strains, gender and experimental conditions may play significant roles in these discrepant findings. Nonetheless, our data revealed that aging-induced reduction in Na+-Ca2+ exchanger and Kv1.2 channel but not SERCA2a was reconciled by the catalase transgene, suggesting possible roles of Na+-Ca2+ exchanger and Kv1.2 channel rather than SERCA2a in catalase-elicited beneficial effect on cardiomyocyte aging. In addition, our observation that catalase ameliorates aging-induced decline in peak cardiac contractile capacity or cardiomyocyte Ca2+ sensitivity under increased stimulus frequency and elevated extracellular Ca2+ concentration also depicts a role of enhanced SR reserve and myofilament Ca2+ sensitivity in the beneficial effect of catalase. Although direct link between antioxidant capacity and cardiac intracellular Ca2+ homeostasis is still somewhat vague, an ample of evidence has suggested impaired intracellular Ca2+ handling following enhanced oxidative stress. H2O2 has been demonstrated to impair SERCA function and SR Ca2+ uptake as well as reduce SR intracellular Ca2+ release through reaction with S-H groups of RyR (Zaidi and Michaelis 1999). H2O2 has also been suggested to alter Ca2+ release from mitochondria and induce DNA damage (Ramasarma 1990). By catalyzing conversion of H2O2 to oxygen and water, catalase is expected to shift redox balance towards antioxidant end, leading to increased myocardial antioxidant capacity, which may offset the detrimental effect of H2O2.
Experimental limitations: Although our current study has provided evidence that catalase may rescue aging-associated cardiomyocyte contractile dysfunction, caution should be taken to extrapolate data from isolated cardiomyocytes to whole heart. It is possible that advanced age affects the sensitivity of cardiomyocytes to the enzymatic insults during the isolation procedure whereas catalase protects the aged cardiomyocytes against the enzymatic insults. In addition, since the healthiest cardiomyocytes are usually chosen for mechanical recordings, therefore a small sub-population of catalase-expressing myocytes which exhibit functional improvement may not truly reflect the contractile state of the whole heart.
In conclusion, our study revealed that cardiac specific overexpression of catalase prolongs lifespan and rescues aging-induced contractile and intracellular Ca2+ dysfunction as well as reduced contractile capacity under high stimulus frequency or increased extracellular Ca2+ levels in cardiomyocytes possibly through improvement in intracellular Ca2+ regulatory protein or membrane ion channel, in addition to its known anti-apoptotic/anti-oxidant capacity. These data, in conjunction with prolonged life span in catalase over-expression mice, have shed some light on the clinical potential of catalase enzyme in aging-associated cardiac dysfunction.
Acknowledgments
The authors would like to thank Faye L. Lopez, Bonnie Zhao, Nicholas S. Aberle II, Karissa LaCour, Kelly Guo and Cindy Fang for their assistance in data acquisition and analysis. Founder catalase transgenic mice were obtained from Dr. Paul N. Epstein at the University of Louisville, Louisville, KY. This work was supported in part by National Institute of Health grants to JR (NIA: 1R03 AG21324-01 and NIAAA: 1R15 AA13575-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Azhar G, Gao W, Liu L, Wei JY. Ischemia-reperfusion in the adult mouse heart influence of age. Exp Gerontol. 1999;34:699–714. doi: 10.1016/s0531-5565(99)00031-5. [DOI] [PubMed] [Google Scholar]
- Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- Blough ER, Rennie ER, Zhang F, Reiser PJ. Enhanced electrophoretic separation and resolution of myosin heavy chains in mammalian and avian skeletal muscles. Anal Biochem. 1996;233:31–35. doi: 10.1006/abio.1996.0003. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM, Bode AM, Bartke A. Antioxidative mechanisms and plasma growth hormone levels: potential relationship in the aging process. Endocrine. 1999;11:41–48. doi: 10.1385/ENDO:11:1:41. [DOI] [PubMed] [Google Scholar]
- Cannell MB. Contribution of sodium-calcium exchange to calcium regulation in cardiac muscle. Ann N Y Acad Sci. 1991;639:428–443. doi: 10.1111/j.1749-6632.1991.tb17330.x. [DOI] [PubMed] [Google Scholar]
- Cutler RG. Oxidative stress and aging: catalase is a longevity determinant enzyme. Rejuvenation Res. 2005;8:138–140. doi: 10.1089/rej.2005.8.138. [DOI] [PubMed] [Google Scholar]
- Dibb KM, Rueckschloss U, Eisner DA, Isenberg G, Trafford AW. Mechanisms underlying enhanced cardiac excitation contraction coupling observed in the senescent sheep myocardium. J Mol Cell Cardiol. 2004;37:1171–1181. doi: 10.1016/j.yjmcc.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Duan J, McFadden GE, Borgerding AJ, Norby FL, Ren BH, Ye G, Epstein PN, Ren J. Overexpression of alcohol dehydrogenase exacerbates ethanol-induced contractile defect in cardiac myocytes. Am J Physiol Heart Circ Physiol. 2002;282:H1216–H1222. doi: 10.1152/ajpheart.00780.2001. [DOI] [PubMed] [Google Scholar]
- Fang CX, Dong F, Ren BH, Epstein PN, Ren J. Metallothionein alleviates cardiac contractile dysfunction induced by insulin resistance: role of Akt phosphorylation, PTB1B, PPARgamma and c-Jun. Diabetologia. 2005;48:2412–2421. doi: 10.1007/s00125-005-1940-y. [DOI] [PubMed] [Google Scholar]
- Fleg JL, Shapiro EP, O’Connor F, Taube J, Goldberg AP, Lakatta EG. Left ventricular diastolic filling performance in older male athletes. JAMA. 1995;273:1371–1375. [PubMed] [Google Scholar]
- Hove-Madsen L, Bers DM. Sarcoplasmic reticulum Ca2+ uptake and thapsigargin sensitivity in permeabilized rabbit and rat ventricular myocytes. Circ Res. 1993;73:820–828. doi: 10.1161/01.res.73.5.820. [DOI] [PubMed] [Google Scholar]
- Josephson IR, Guia A, Stern MD, Lakatta EG. Alterations in properties of L- type Ca channels in aging rat heart. J Mol Cell Cardiol. 2002;34:297–308. doi: 10.1006/jmcc.2001.1512. [DOI] [PubMed] [Google Scholar]
- Kang YJ, Chen Y, Epstein PN. Suppression of doxorubicin cardiotoxicity by overexpression of catalase in the heart of transgenic mice. J Biol Chem. 1996;271:12610–12616. doi: 10.1074/jbc.271.21.12610. [DOI] [PubMed] [Google Scholar]
- Kass DA, Shapiro EP, Kawaguchi M, Capriotti AR, Scuteri A, deGroof RC, Lakatta EG. Improved arterial compliance by a novel advanced glycation end-product crosslink breaker. Circulation. 2001;104:1464–1470. doi: 10.1161/hc3801.097806. [DOI] [PubMed] [Google Scholar]
- Koban MU, Moorman AF, Holtz J, Yacoub MH, Boheler KR. Expressional analysis of the cardiac Na-Ca exchanger in rat development and senescence. Cardiovasc Res. 1998;37:405–423. doi: 10.1016/s0008-6363(97)00276-9. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy G, Patberg KW, Obreztchikova MN, Rybin AV, Rosen MR. Developmental evolution of the delayed rectifier current IKs in canine heart appears dependent on the beta subunit minK. Heart Rhythm. 2004;1:704–711. doi: 10.1016/j.hrthm.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Lakatta EG. Cardiovascular aging research: the next horizons. J Am Geriatr Soc. 1999;47:613–625. doi: 10.1111/j.1532-5415.1999.tb02579.x. [DOI] [PubMed] [Google Scholar]
- Lakatta EG. Cardiovascular aging in health. Clin Geriatr Med. 2000;16:419–444. doi: 10.1016/s0749-0690(05)70021-5. [DOI] [PubMed] [Google Scholar]
- Lakatta EG, Mitchell JH, Pomerance A, Rowe GG. Human aging: changes in structure and function. J Am Coll Cardiol. 1987;10:42A–47A. doi: 10.1016/s0735-1097(87)80447-3. [DOI] [PubMed] [Google Scholar]
- Li L, Chu G, Kranias EG, Bers DM. Cardiac myocyte calcium transport in phospholamban knockout mouse: relaxation and endogenous CaMKII effects. Am J Physiol. 1998;274:H1335–H1347. doi: 10.1152/ajpheart.1998.274.4.H1335. [DOI] [PubMed] [Google Scholar]
- Li SY, Du M, Dolence EK, Fang CX, Mayer GE, Ceylan-Isik AF, LaCour KH, Yang X, Wilbert CJ, Sreejayan N, Ren J. Aging induces cardiac diastolic dysfunction, oxidative stress, accumulation of advanced glycation endproducts and protein modification. Aging Cell. 2005;4:57–64. doi: 10.1111/j.1474-9728.2005.00146.x. [DOI] [PubMed] [Google Scholar]
- Li SY, Yang X, Ceylan-Isik AF, Du M, Sreejayan N, Ren J. Cardiac contractile dysfunction in Lep/Lep obesity is accompanied by NADPH oxidase activation, oxidative modification of sarco(endo)plasmic reticulum Ca(2+)-ATPase and myosin heavy chain isozyme switch. Diabetologia. 2006;49:1434–1446. doi: 10.1007/s00125-006-0229-0. [DOI] [PubMed] [Google Scholar]
- Liu SJ, Wyeth RP, Melchert RB, Kennedy RH. Aging-associated changes in whole cell K(+) and L-type Ca(2+) currents in rat ventricular myocytes. Am J Physiol Heart Circ Physiol. 2000;279:H889–H900. doi: 10.1152/ajpheart.2000.279.3.H889. [DOI] [PubMed] [Google Scholar]
- Long X, Boluyt MO, O’Neill L, Zheng JS, Wu G, Nitta YK, Crow MT, Lakatta EG. Myocardial retinoid X receptor, thyroid hormone receptor, and myosin heavy chain gene expression in the rat during adult aging. J Gerontol A Biol Sci Med Sci. 1999;54:B23–B27. doi: 10.1093/gerona/54.1.b23. [DOI] [PubMed] [Google Scholar]
- Olsson MC, Palmer BM, Stauffer BL, Leinwand LA, Moore RL. Morphological and functional alterations in ventricular myocytes from male transgenic mice with hypertrophic cardiomyopathy. Circ Res. 2004;94:201–207. doi: 10.1161/01.RES.0000111521.40760.18. [DOI] [PubMed] [Google Scholar]
- Pavlovic M, Schaller A, Pfammatter JP, Carrel T, Berdat P, Gallati S. Age-dependent suppression of SERCA2a mRNA in pediatric atrial myocardium. Biochem Biophys Res Commun. 2005;326:344–348. doi: 10.1016/j.bbrc.2004.11.040. [DOI] [PubMed] [Google Scholar]
- Ramasarma T. H2O2 has a role in cellular regulation. Indian J Biochem Biophys. 1990;27:269–274. [PubMed] [Google Scholar]
- Ranki HJ, Crawford RM, Budas GR, Jovanovic A. Ageing is associated with a decrease in the number of sarcolemmal ATP-sensitive K+ channels in a gender-dependent manner. Mech Ageing Dev. 2002;123:695–705. doi: 10.1016/s0047-6374(01)00415-8. [DOI] [PubMed] [Google Scholar]
- Schmidt AG, Zhai J, Carr AN, Gerst MJ, Lorenz JN, Pollesello P, Annila A, Hoit BD, Kranias EG. Structural and functional implications of the phospholamban hinge domain: impaired SR Ca2+ uptake as a primary cause of heart failure. Cardiovasc Res. 2002;56:248–259. doi: 10.1016/s0008-6363(02)00541-2. [DOI] [PubMed] [Google Scholar]
- Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, Wallace DC, Rabinovitch PS. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- Schuyler GT, Yarbrough LR. Comparison of myosin and creatine kinase isoforms in left ventricles of young and senescent Fischer 344 rats after treatment with triiodothyronine. Mech Ageing Dev. 1990;56:39–48. doi: 10.1016/0047-6374(90)90113-t. [DOI] [PubMed] [Google Scholar]
- Willems L, Garnham B, Headrick JP. Aging-related changes in myocardial purine metabolism and ischemic tolerance. Exp Gerontol. 2003;38:1169–1177. doi: 10.1016/j.exger.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Xu A, Narayanan N. Effects of aging on sarcoplasmic reticulum Ca2+-cycling proteins and their phosphorylation in rat myocardium. Am J Physiol. 1998;275:H2087–H2094. doi: 10.1152/ajpheart.1998.275.6.H2087. [DOI] [PubMed] [Google Scholar]
- Yang X, Doser TA, Fang CX, Nunn JM, Janardhanan R, Zhu MJ, Sreejayan N, Quinn MT, Ren J. Metallothionein prolongs survival and antagonizes senescence-associated cardiomyocyte diastolic dysfunction: Role of oxidative stress. FASEB J. 2006 doi: 10.1096/fj.05-5288fje. in press. [DOI] [PubMed] [Google Scholar]
- Yang X, Sreejayan N, Ren J. Views from within and beyond: narratives of cardiac contractile dysfunction under senescence. Endocrine. 2005;26:127–137. doi: 10.1385/ENDO:26:2:127. [DOI] [PubMed] [Google Scholar]
- Zaidi A, Michaelis ML. Effects of reactive oxygen species on brain synaptic plasma membrane Ca(2+)-ATPase. Free Radic Biol Med. 1999;27:810–821. doi: 10.1016/s0891-5849(99)00128-8. [DOI] [PubMed] [Google Scholar]
- Zhang X, Klein AL, Alberle NS, Norby FL, Ren BH, Duan J, Ren J. Cardiac-specific overexpression of catalase rescues ventricular myocytes from ethanol-induced cardiac contractile defect. J Mol Cell Cardiol. 2003;35:645–652. doi: 10.1016/s0022-2828(03)00080-4. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Kang YJ. Cellular and subcellular localization of catalase in the heart of transgenic mice. J Histochem Cytochem. 2000;48:585–594. doi: 10.1177/002215540004800502. [DOI] [PubMed] [Google Scholar]


