Abstract
Cotricotropin-releasing hormone (CRH) and related peptides are produced in skin that is dependent on species and anatomical location. Local peptide production is regulated by ultraviolet radiation (UVR), glucocorticoids and phase of the hair cycle. The skin also expresses the corresponding receptors (CRH-R1 and CRH-R2), with CRH-R1 being the major receptor in humans. CRH-R1 is expressed in epidermal and dermal compartments, and CRH-R2 predominantly in dermal structures. The gene coding for CRH-R1 generates multiple isoforms through a process modulated by UVR, cyclic adenosine monophosphate (cAMP) and phorbol 12-myristate 13-acetate. The phenotypic effects of CRH in human skin cells are largely mediated by CRH-R1alpha through increases in concentrations of cAMP, inositol triphosphate (IP3), or Ca2+ with subsequent activation of protein kinases A (PKA) and C (PKC) dependent pathways. CRH also modulates the activity of nuclear factor of kappa light polypeptide gene enhancer in B-cells (NF-kappaB), activator protein 1 (AP-1) and cAMP responsive element binding protein (CREB). The cellular functions affected by CRH depend on cell type and nutritional status and include modulation of differentiation program(s), proliferation, viability and immune activity. The accumulated evidence indicates that cutaneous CRH is also a component of a local structure organized similarly to the hypothalamo-pituitary-adrenal axis.
Keywords: Human Skin, Hormone, Corticotropin, CRH, CRH-R1, CRH-R2, Urocortin, Review
2. INTRODUCTION
CRH is the central trigger of HPA axis, and together with related peptides urocortin I–III also regulate behavioral, autonomic, endocrine, reproductive, cardiovascular, gastro-intestinal, metabolic and immune systemic functions (1–11). Other actions include local immunomodulatory (predominantly proinflammatory) effects (12–14), differing from a central immunosuppressive activity (through the HPA axis) (3). Of note, locally produced CRH can directly regulate steroid hormone production by adrenals and gonads (1, 2). Furthermore, CRH in the immune cells can induce production and release of proopiomelanocortin (POMC) derived adrenocorticotropin (ACTH) and beta-endorphin peptides.
In vertebrates these peptides interact with membrane-bound CRH-R1 and CRH-R2 (1, 2). Both receptor types belong to the group II subfamily of G protein-coupled receptors (GPCRs). CRH-R1 binds CRH and urocortin I with high affinity; it does not bind urocortin II (strescopin related protein). CRH-R2 shows preferential affinity for urocortin II, although it also binds CRH, however, with lower affinity than CRH-R1. Signal transduction through CRH-Rs is coupled to the activation of adenylate cyclase (AC), phospholipase C (PLC) and calcium channels (1, 2, 5, 6).
3. ALTERNATIVELY SPLICED CRH-RS ISOFORMS: AN OVERVIEW
In humans the gene coding for CRH-R1 generate at least 7 alternatively spliced CRH-R1 transcripts (1, 2, 6, 15, 16); it contains 14 exons with exon 6 being unique in that it is only present in CRH-R1beta (that contains all 14 exons). In the main functional isoform, CRH-R1alpha, exon 6 is spliced out; in other isoforms there are additional splicing sites, for example, CRH-R1c (exon 3 is spliced out), CRH-R1d (exons 13 is absent), CRH-R1e (exons 3, 4 are absent, which cause frame shift and early stop codon in exon 8), CRH-R1f (exon 12 is absent resulting in frame shift), CRH-R1g (exons 11, 27 bp of exon 10 and 28 bp of exon 12 are absent), and CRH-R1h (insertion of cryptic exon between exons 4 and 5 cause frame shift and early termination codon in exon 5). Alternatively spliced CRH-R1 isoforms have also been identified in the rat (17), mouse (15) and hamster (18).
As predicted from the analysis of human genomic DNA the CRH-R2 gene contains 15 exons (6), and generates at least 3 major isoforms (CRH-R2alpha, beta and gamma) (1, 2). Additional alternatively spliced forms of CRH-R2, as well as multiple promoters for the gene, have also been described (Figure 1) (19, 20).
Figure 1.

Schematic representation of mammalian CRH-R2 gene structure. A. Human CRH-R2 according to Slominski et al, 2000. (6). Arrows represent position of PCR primers. B. Human CRH-R2 according to Catalona et al, 2003. (19). Putative promoters sites are shown by * (19). C. Alternatively spliced CRH-R2 isoforms alpha, beta, gamma (6, 19) and the isoform from stomach (GenBank accession No. E12750; Patent: JP199707289-A). D. Predicted structure of mouse CRH-R2 gene. The structure of mouse gene has been obtained by comparing mouse genomic DNA with published mouse CRH-R2 cDNA and by comparing mouse genomic DNA with human CRH-R2 exons (Blast program at http://www.ncbi.nlm.nih.gov/blast). The black box represents mouse exon 3 corresponding to human exon 3 (65% homology). Murine exon 3 contains multiple stop codons and its insertion into the final CRH-R2 mRNA would not produce a functional CRH-R2 receptor. Thus, the equivalent of the human CRH-R2gamma isoform will not be expressed in the mouse. Exon 4 has 77% homology at the nucleotide level and 91.3% at the protein level with its human counterpart. Arrows represent position of PCR primers. (Reproduced with permission from Endocrine Society "20").
4. PREDICTED STRUCTURE AND FUNCTION OF ALTERNATIVELY SPLICED CRH-R1 ISOFORMS IN THE SKIN
4.1. Overview
In human skin, CRH-R1 is the major receptor, expressed in both epidermal and dermal compartments; CRH-R2 is detected predominantly in dermal structures (20). The protein structures predicted for the CRH-R1 isoforms are presented in figures 2 and 3. Amino acid sequences predicted are based on cDNA of full sequences of CRH-R1alpha (NM_004382), CRH-R1c (HSU16273), CRH-R1d (AF180301), and of partial sequences demonstrating alternative splicing of CRH-R1e, f, g and h (AF369651, AF369652, AF369653 and AF374231 respectively). The predicted protein structures indicate that CRH-R1alpha, beta, c, d, f and g represent membrane bound proteins, while CRH-R1e and h are soluble proteins. The above receptor isoforms are translatable in mammalian cell system (21).
Figure 2.

Predicted amino acid sequences of CHR-R1 isoforms. The amino acid sequences of CRH-R1 isoforms were predicted on the basis of their cDNA sequences (NM_004382; HSU16273; AF180301; AF369651; AF369652; AF369653 and AF374231), and aligned using Align Plus 5 (Clone Manager 7, Scientific & Educational Software, Cary, NC). Poorly aligned fragments caused by frame shifts in CRH-R1 e, f and h (shown in italic) were corrected to match with organization of exons. Exons 1, 3, 5, 7, 9, 11, 13 are underlined and arrows indicate the positions of introns. The localization of exon 6 is indicated by a space. Cryptic exon of CRH-R1h is shown in bold. The transmembrane regions of CRH-R1 isoforms (shown in blue) were detected with TMHMM server which uses a hidden Markov model to predict proteins’ transmembrane topology (111). The probable sequence of distorted TM region of CRH-R1d and CRHR1g (shown in red) was confirmed with HMMTOP transmembrane topology prediction server (112). Note that the predicted coding regions for both soluble isoforms of CRH-R1 (e and h) also contain additional open reading frames (orf) named CRH-R1e2 and CRH-R1h2, respectively. These code putative 7TM domains of CRH-R1 of unknown function (15). Signal peptide sequence 1–23 (green) is also shown as previously reported (27).
Figure 3.
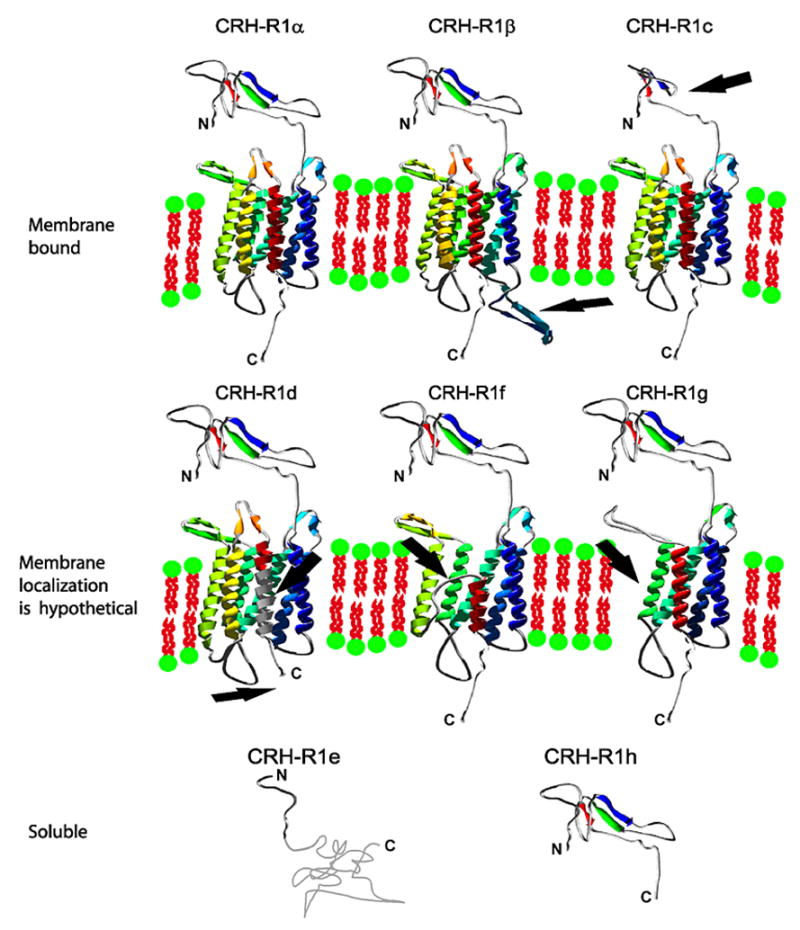
Models and localization of human CHR-R1 isoforms. Models of ECD1 and 7TM domains of human CRH-R1 isoforms were prepared separately based on the comparison of protein sequences (Figure 2). Arrows mark deleted or inserted regions of CRH-R1 isoforms caused by alternative splicing in comparison to CRH-R1alpha. Modeling was preformed with the Swiss Model server and Deep View/Swiss-PdbViewer (103) ECD1 of human CRH-R1alpha was modeled using solved structure of mouse CRH-R2 (1u34A.pdb) and protein sequence of human CRH-R1alpha (NM_004382). Then model of CRH-R1alpha was served as a template for modeling of CRH-R1c, e and h domains. CRH-R1 isoforms: beta, d, f and g possess identical protein sequence of ECD1 as CRH-R1alpha. 3D models of 7TM domains of human CHR-R1 isoforms were prepared with CRH-R1 model generated by G. Vriend as a template (CRFR_HUMAN.ent file was downloaded from www.gpcr.org server (113). Note that Vriend’s model was automatically created by WHAT IF software on based of the alpha carbon coordinates provided by J. Baldwin (114). CRH-R1 isoforms: alpha, beta, and c possess identical protein sequence of 7TM as CRH-R1alpha. The structure of whole receptor remains unknown and orientation of ECD1 to 7TM is arbitrary (model of ECD1 is placed above 7TM for each isoform except isoforms e and h, where 7TM domain is not present). CRH-R1e with frame shift, codes a fragment of 61 amino acids in length without homology to any known structure. This fragment is shown as a random chain. C-terminal ends followed by seventh transmembrane helix are shown as random fragments and were not modeled. Presented models of human CRH-R1 isoforms are also available in separated pdb files.
4.2. Membrane bound receptors
Extensive analysis of skin specimens and cutaneous cell lines has determined that CRH-R1alpha is the most prevalent isoform expressed in the skin (20). However, a protein of 45–47 kD (corresponding to the theoretical mass of unprocessed protein) has been detected only in selected skin cells (22). The most abundant and prevalent receptor forms are glycosylated that have high molecular weight (mw) (60–70 kD), although a 55 kD form has been found in normal melanocytes and in selected cancer and melanoma lines (22). Exposure of skin cells to CRH or related peptides results in the rapid activation of the CRH-R1alpha receptor, coupling to Galpha s followed by cAMP synthesis or coupling to Galpha q with activation of IP3 signaling (6, 22, 23), similarly to other systems (1, 2). Ligand activation of CRH-R1alpha in skin cells also induces Ca2+ influx (24); this requires opening of voltage-activated Ca2+ ion channels separate from the cyclic nucleotide-gated ion channels (25). Thus, in the same skin cell CRH-R1alpha can be coupled to different signal transduction pathways (22), in agreement with results obtained with COS cells transfected with CRH-R1alpha. In COS cells ligand binding to the receptor can be coupled to different G proteins (26) or lead to the transcriptional activation of differing cis-elements such as cAMP, calcium and serum response elements (CRE, CaRE, SRE, respectively) or AP-1 (21).
So far, expression of CRH-R1beta has not been detected in the skin. As regards the CRH-R1c isoform, this was detected only in selected abnormal skin cells (15). CRH-R1c has a deletion of the large fragment of extracellular domain 1 (ECD1) (Figure 3), responsible for proper ligand binding, affecting therefore ligand affinity and/or specificity.
The three splicing variants predominanting in the skin, CRH-R1 d, f, g have a partial deletion within the seven transmembrane domain (7TM) (figures 2, 3). All of them possess ECD1 (27) and two other extracellular coils 2 (EC2) and EC3 (28) (involved in substrate binding), but their stability inside the membrane, interactions with G proteins and thus ligand signal transduction may be impaired. Our detection of absent CRH or urocortin induced cAMP production in COS cells transiently transfected with isoforms f and g is consistent with this predicted structure (21). It is also possible that these isoforms may not reach cell surface being instead targeted for degradation.
As regards to CRH-R1d, the fragment 7TM domain is substituted by a sequence coded by exon 13 (Figure 2), but the predicted model (Figure 3) suggests an intramembrane location for the crucial residues responsible for Galpha s and Galpha q interactions. Deletion of exon 13 has been found to be devoid of an effect on CRH binding but decreases coupling to G-proteins and lowers (by 10 fold) cAMP stimulation (as compared to CRHR1alpha), and totally abolishes IP3 production (29). A recent study on the calcitonin receptor (also belonging to family B of GPCRs) revealed that expression of CTRdelta e13 isoform (structurally similar to the CRH-R1 variant d) significantly reduced surface expression of the calcitonin receptor C1a (homolog of CRH-R1alpha), and consequently reduced the cAMP response and Erk phosphorylation after ligand stimulation (30). Deletion of a fragment of the 7TM domain was also noted in a splicing variant of sheep CRHR1 lacking 133 nucleotides from C terminus. It was shown that the deletion did not affect binding of CRH physiological level, but it did impair Galpha s activation and cAMP accumulation.(31). Although the deletion in sheep CRH-R1 was larger then in CRH-R1d, the properties of the protein was similar to CRHR1d (29). The reported frame shift resembled CRHR1f, which lacks exon 11 with the following frame shift resulting in the absence of the transmembrane fragments: TM6 and TM7. Compared to CRHR1alpha, the isoform CRHR1g lacks exons 11, 27 bp of exon 10 and 28 bp of exon 12. This region codes TM5 and TM6 and the third intracellular loop (IC3). It has been shown that the deletion in this region abolishes the receptor’s ability to transfer signals to the Galpha s/AC/PKA pathway, which could result in a switch to the Galpha q/PLC/PKC/ERK1-2 pathway as already reported (32).
4.3. Soluble CRHR1 isoforms with full deletion of TM
Soluble forms of CRHR1 (27) and CRHR2 (33) containing only ECD1 domain are still able to bind ligands, but the constructs will not be able to interact with G-proteins to pass a signal through the cAMP or IP3 pathways due to absence of the whole 7TM domain. Because both isoforms possess the signal peptide allowing protein transport, but lack the 7TM region, it has been concluded that they are likely to be released from the cells. The CRHR1h, as natural model of the ECD1 domain, should however be able to bind ligands and so act as a scavenger for CRH and urocortin peptides. In accordance with this postulate, co-transfection of CRH-R1 h with CRH-R1alpha did modify CRH-R1alpha activity (21), suggesting a modulatory property of the soluble form of the receptor, a property well described in cytokine receptor family (34) and in the receptors for advanced glycation endproducts (RAGE) (reversion of symptoms in mouse models of atherosclerosis) (35).
CRH-R1e has a deletion of exons 3 and 4, which causes frame shift and a predicted C-terminal fragment without similarity to any known protein. The existence of binding properties for this isoform is questionable because of the deletion of most of the ECD1 domain (CRH-R1e sequence codes amino acids 1–40 of CRH-R1alpha containing signal peptide covering amino acids 1–23 (15)) (figures 2, 3). We speculate that this isoform could act as modulator of signaling of the membrane bound CRH-R1 isoform(s) or, that it could affect the isoforms mRNA levels. Splicing of CRH-R1 mRNA in favor of CRH-R1e isoform, could result in unstable mRNA or protein, which would then be degraded leading to lower membrane expression of full length receptor (futility cycle).
In summary, the functional assignments to any of the different CRH-R1 isoforms may represent specific endocrine responses to stress at the cellular level. This selective processing could provide a mechanistic explanation for the organ and cell type dependent variability in the phenotypic responses to CRH or related peptides, and would contribute towards better understanding of the general biology of alternative splicing.
5. CUTANEOUS CRH-R1 COUPLING TO SIGNAL TRANSDUCTION PATHWAYS
5.1. Second messengers
Studies of CRH-R1 activation in skin cells of the signal transduction pathways close to the cell membrane showed stimulation of three different second messengers: Ca2+, cAMP and IP3 (6, 12, 21–23, 25, 36–39). Thus, CRH and urocortin induce increases in intracellular Ca2+ concentration (in HaCaT keratinocytes, epidermal keratinocytes, melanocytes and melanoma cells) caused by a Ca2+ influx from the opening of voltage-activated Ca2+ ion channels (inhibited by EGTA, d-cis-diltiazem and verapamil) (25, 39). CRH-R1 effect on Ca2+ ion channels did not involve cyclic nucleotide-gated ion channels (no effect of Mg2+) (25). Increased Ca2+ levels were also detected at extremely low ligand concentrations (10−13 M). A very interesting finding was noted in HaCaT keratinocytes, where the Ca2+ flux induced by CRH occurred in the cytoplasm. The increase was steady, whereas that induced by urocortin was restricted to the cell nucleus and exhibited a remarkable oscillatory pattern, defining a unique intracellular signal transduction pathway (25). These results imply that different CRH-R1 signaling pathways co-exist in the same cell. CRH also stimulated IP3 production in all the tested skin cells (melanocytes, keratinocytes and fibroblasts) (22, 23). Taken together, the data (6, 7, 12, 22–25, 39) indicate that increases in intracellular Ca2+ concentrations are induced by two mechanisms: a) stimulation of IP3, which activates release of Ca2+ from intracellular stores; and b) direct coupling to voltage-activated Ca2+ ion channels, whose opening leads to rapid cytosolic Ca2+ influx. We also found that CRH induced the production of cAMP in skin cells expressing CRH-R1alpha. These included epidermal melanocytes, HaCaT keratinocytes, dermal fibroblasts and the majority of melanoma cells (22, 37, 38). However, cAMP accumulation was not detected after CRH-R1alpha activation in normal epidermal keratinocytes (22). In the case of normal human epidermal keratinocytes, the lack of CRH/urocortin-induced production of cAMP (22) together with lack of effect of PKA inhibitors on CRH induced keratinocyte differentiation program (23) indicates poor coupling of CRH-R1alpha to Gs in these cells. In SKMEL-188 melanoma cells, which only express the CRH-R1d isoform, the lack of coupling to cAMP with overt stimulation of Ca2+ flux suggests that the CRH-R1d signal transduction pathway is only coupled to either voltage-activated Ca2+ ion channels or PLC (12, 15, 22, 25, 39).
5.2. Transcriptional regulators
5.2.1. CREB
Classically, the activation of CRH-R1alpha is known to stimulate Gs protein with activation of AC, cAMP accumulation, PKA activation and CREB phosphorylation at Ser-133. Phosphorylated CREB activates CRE elements in respective gene promoters (6, 16, 40). Indeed, we found that CRH does stimulate CREB DNA binding activity in HaCaT cells (Figure 4), although this was not seen in normal adult epidermal keratinocytes.
Figure 4.
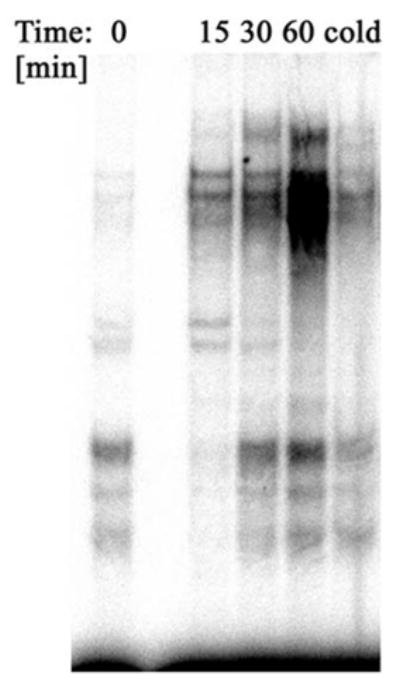
Corticotropin-releasing hormone (CRH) increases CREB binding activity in HaCaT cells. HaCaT cells were stimulated with 100 nM CRH in serum free Dulbecco’s modified Eagles’s medium (DMEM) for 0 (control), 15, 30 and 60 minutes. Nuclear extracts were subjected to electrophoretic mobility shift assay analysis using a 32P-labelled CREB oligonucleotide probe (5′-AGA GAT TGC CTG ACG TCA GAG AGC TAG -3′) as described (42, 53). Cold represents nuclear extract pre-incubated with 50X excess of cold oligonucleotide.
5.2.2. AP-1
CRH stimulates Gq protein that activates phospholipase C, leading to IP3 accumulation with subsequent PKC activation. This leads indirectly to stimulation of expression and accumulation of AP-1 transcription factors in the nucleus (6, 16, 40). Differentiation of the keratinocytes has been linked to activation of AP-1 family members such as JunB, JunD, Fra1 (41). We have tested in keratinocytes activation of AP-1 induced by CRH (23, 42), and found that CRH activated JunD, which is linked to the early steps of the keratinocyte differentiation program (23, 42).
5.2.3. NF-kappaB
NF-kappaB acts as a “switch-board” in the regulation of cell differentiation, cell viability and cytokine production. NF-kappaB activation is stimulated by many pathways that converge on inhibitor of NF-kappaB (Ikappa-B) kinases complex (43). Phosphorylation and subsequent degradation of Ikappa-B proteins allow for release and then translocation of NF-kappaB to the nucleus, where it can bind to transcription-regulatory sequences to activate specific genes. Keratinocytes are active players in the skin immune system releasing cytokines that initiate inflammation and affect the mode of immune response (Th1 versus Th2) (44–48). Peripheral CRH may exert predominantly a proinflammatory effect with selective increase in Th1-type responses in experimental models of inflammation (49). In agreement with this, we found that CRH stimulates secretion of interleukin 6 (IL-6) and IL-11 in immortalized HaCaT keratinocytes (50) and stimulates intercellular adhesion molecule 1 (ICAM-1) and human leukocyte antigen DR (HLA-DR) expression by normal human keratinocytes (51). We also found that CRH decreased the levels of IL-1beta in the supernatants from cultured HaCaT (50) and of interferon (IFN)-gamma-stimulated expression of ICAM-1 in HaCaT keratinocytes (Figure 5). CRH also down-regulates expression of IL-18 (a member of IL-1 family) in HaCaT keratinocytes (52). In an attempt to define the mechanisms underlying the above (contradictory) immunoregulatory effects we tested immortalized HaCaT keratinocytes and observed a dual effect of CRH (Figure 6) (12, 53). Thus, in stressed cells (defined as maintained in medium without growth factors), CRH either enhanced or diminished NF-kappaB activation depending on the length of incubation (Figure 6). Specifically, in mode 1 when cells are subjected to prolonged starvation (twelve hours) the addition of CRH upregulates NF- kappaB signaling pathway (probably to enhance cell survival) (Figure 7A). Under conditions when p50/p50 dimers were downregulated and p50/p65 dimers were activated, IL-1beta production was increased (Figure 7B). In mode II, under the stress of acute serum starvation (15–30 min) that activates the NF- kappaB pathway; the pathway was significantly attenuated by CRH (Figure 8). Specifically, CRH downregulated p50/p50 and p50/p65 dimers of NF- kappaB, diminished kappaB-driven CAT reporter gene activity and inhibited IkappaB-beta degradation (12, 53). Moreover, CRH inhibited the transcription of the NF-kappaB responsive genes, IL-2 and heat shock protein 90 (HSP-90) (12, 53). Therefore, we suggest that to counteract the sustained (prolonged starvation) or acute (rapid serum starvation) stress, CRH activates or inhibits, respectively, NF-kappaB in an attempt to stabilize internal homeostasis of HaCaT keratinocytes. This is in agreement with reports showing either stimulatory or inhibitory effects of CRH on immune cells (54, 55), and the dependence of CRH effect on NF- kappaB activity on experimental models (56, 57). Hence the apparently contradictory effects of CRH may simply depend on the prevailing environmental context (12, 58) through actions on NF-kappaB.
Figure 5.
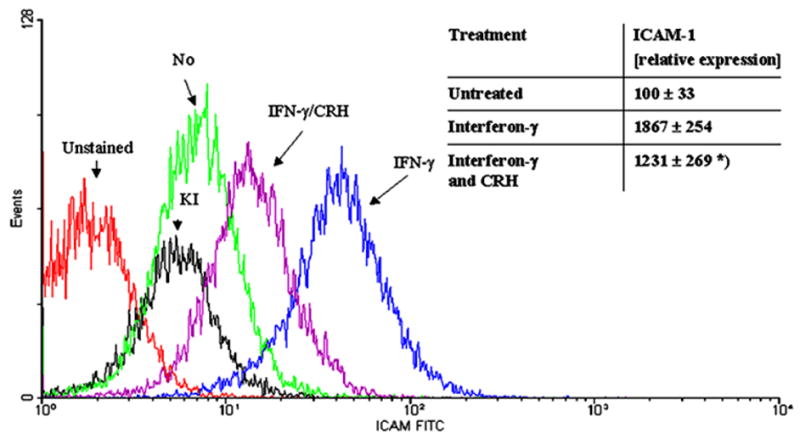
CRH decreases IFN-gamma-enhanced expression of ICAM-1 on HaCaT cells. Cells were stimulated for 48 hours in DMEM medium containing 1% serum. IFN-gamma (1000 U/ml) was added for 48 hours and CRH (100 nM) for 24 hours. Cells were stained with fluorescein isothiocyanate (FITC)-conjugated anti-ICAM-1 antibody (DAKO, Carpinteria, CA) and read with Epics Coulter cytometer. Histograms were created from listmode data using Winmdi 2.8 (freeware from Joseph Trotter, The Scripps Research Institute, La Jolla, CA, USA). Representative histograms: red – unstained cells, black – cells stained with isotype control antibody; remaining histograms show cells stained with FITC-conjugated anti-ICAM-1 antibody: green – not stimulated cells, blue – cells stimulated with IFN-gamma, violet - cells stimulated with IFN- gamma and CRH. The mean fluorescence intensities are recorded in arbitrary units. Data shown in the table are related to the mean of untreated control (100%) and presented as mean ± SD (n=4), *p<0.05.
Figure 6.

Effect of CRH on p50 antigen localization in HaCaT keratinocytes is dependent on growth conditions. Mode I: p50 antigen was localized to the cytoplasm in cells cultured for 12 hours either in serum containing medium (A) or serum free media (B). Treatment with CRH resulted in translocation of p50 to the nucleus (C). Mode II: treatment of cells with serum free media for 30 minutes resulted in translocation of p50 to the nucleus (D), while concomitant addition of CRH prevented this process (p50 remained in the cytoplasm) (E). Cells were fixed, permeabilized, stained with anti-p50 goat polyclonal IgG and FITC-conjugated donkey anti-goat IgG (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and photographed with a NIKON Eclipse E800 microscope. Images were processed with Lucia G (Laboratory Imaging, Ltd., Prague, Czech Republic) and Adobe Photoshop (San Jose, CA, USA) software. Magnification 40X. The results shown are representative of three independent experiments.
Figure 7.
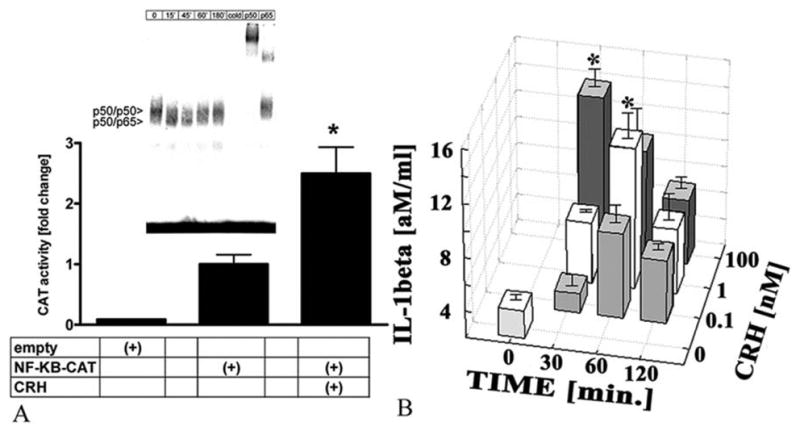
CRH modulates NF-kappaB activity in HaCaT keratinocytes pre-incubated in serum free medium for 12 hours prior to the experiments (Mode I). A. CRH increases transcription of NF-kappaB-dependent reporter construct. HaCaT cells were transiently transfected with pUX-CAT (empty) or pUX-CAT 3XHLAkappaB (NF-κB-CAT) vector (53), incubated for 12 hours in serum free media and then treated with 100 nM CRH (+CRH) or vehicle (0) for 30 minutes. The data represent the mean ± SEM of four assays. The differences are statistically significant (P<0.001). A, insert. CRH modulates NF-kappaB DNA binding in a time dependent fashion. Nuclear extracts were prepared from control cells (0) or cells treated with 100 nM CRH (+) for 0–180 minutes and subjected to EMSA with a 23P-labeled NF-kappaB probe (59). The CRH-modulated complexes are denoted by arrow-heads. Specific Rel proteins in DNA complexes in untreated cells were detected by supershift assay with p50 and p65 antisera. Cold represents extract incubated with a 50-fold excess of unlabeled NF-kappaB probe. B. CRH stimulates IL-1beta mRNA transcription in HaCaT cells preincubated for 12 hours in serum free medium (Mode I). The cells were treated with 1 or 100 nM CRH for 30–120 minutes. Control (0) represents untreated cells. Specific mRNAs were assayed by colorimetric Quantikine mRNA test (R&D Systems, Inc., Minneapolis, MN).
Figure 8.

CRH treatment of HaCaT keratinocytes attenuates NF-kappaB DNA activity in induced by immediate serum deprivation (Mode II). CRH inhibits NF-kappaB DNA binding. Nuclear extracts were prepared from HaCaT cells treated with serum free medium without (0) or with 100 nM CRH (CRH) and subjected to electrophoretic mobility shift assay with a 23P-labeled NF-kappaB probe (59). CRH-modulated complexes are marked with arrow-heads.
In normal epidermal keratinocytes CRH solely stimulates NF-kappaB activity (59), by inducing translocation of the NF-kappaB subunit p65 from the cytoplasm to the nucleus with expression of the kappaB-driven CAT reporter gene. NF-kappaB translocation was accompanied by degradation of IkappaB-alpha. Specificity of the CRH effects was documented by the use of CRH-R1 antagonists antalarmin and alpha-helical-CRH-[9-41](59). These findings are in agreement with reports showing CRH stimulated expression of cell surface adhesion molecules in normal keratinocytes (51), CRH-induced NF-kappaB activation in human primary epidermal keratinocytes is consistent with reported CRH-induced NF-kappaB activation in the immune system (56). At basal levels the triggering of the NF-kappaB pathway by CRH in normal keratinocytes further supports the concept of local epidermal regulatory circuits based on substances also found in the nervous system (6, 7, 12, 60). Because the skin is exposed to noxious environmental stimuli it must be able to differentiate among them and respond accordingly in a localized and timely manner.
The differences between CRH responses in HaCaT immortalized keratinocytes and normal epidermal keratinocytes can be best explained by either a molecular defect in HaCaT cells (their p53 gene is mutated and therefore not functional (61) or, by an extra coupling of the CRH-R1alpha signaling system to AC (22, 36), which is absent in normal keratinocytes (22).
Initial experiments with CRH in human epidermal melanocytes showed that it inhibited translocation of the subunit p65 (from cytoplasm to the nucleus), of NF-kappaB DNA binding activity, and of kappaB-driven luciferase activity (62). It is likely that in normal melanocytes, the inhibition of NF-kappaB activity may reflect indirect stimulation of downstream (immunosuppressor) elements of the HPA axis (62) (and see below), since POMC derived ACTH and alpha melanocyte stimulating hormone (alpha-MSH) are both known to inhibit NF-kappaB signaling (63, 64). This is in contrast to normal epidermal keratinocytes, where CRH directly stimulates NF-kappaB, probably because CRH-R1 signaling is uncoupled from cAMP (22) and does not affect POMC activity (37), favoring pro-inflammatory and Th1-skewed immune response. Nevertheless, when CRH-R1alpha is overexpressed in normal keratinocytes and co-transfected with POMC promoter its activity is stimulated upon CRH addition (Figure 9). Accordingly, in melanocytes, the CRH induced production of POMC effectors would counterbalance or abrogate the proinflammatory environment.
Figure 9.

Effect of CRH on the activity of POMC promoter in human epidermal adult keratinocytes overexpressing CRH-R1alpha. Human epidermal adult keratinocytes were transfected with luciferase reporter gene plasmid containing POMC promoter region (from −771 to −8 bp), transfection efficiency control plasmid (phRL-TK, Promega) and plasmid overexpressing CRH-R1alpha (21, 62). Cells were incubated with CRH for 24 hours (peptide added every 12 hours). Then cells were lysed and luciferase activity was measured as described previously. Data is presented as a ratio of POMC-promoter-specific signal divided by efficiency control signal (mean ± SEM, n=4). Treatment with CRH increased of POMC-promoter-driven signal. Incubation of cells with 10−5 antalarmin for 1 hour before addition of CRH to abrogate the signal.
6. CRH RELATED PEPTIDES IN THE SKIN
Human skin expresses the genes for CRH and urocortin I (urc I/urocortin) and produces the corresponding peptides as documented by reversed phase-high performance liquid chromatographic and liquid chromatography/mass spectrometry analyses (7, 24, 65–69). These are also produced by cells of epidermal and dermal origin (normal, immortalized and malignant keratinocytes and melanocytes, fibroblasts, sebocytes and mast cells CRH) (66, 67, 70, 71). Immunocytochemistry has further detected the peptides in epidermal, dermal and adnexal compartments under physiologic and pathologic conditions (6, 20, 51, 68, 72–74). We have also found expression of mRNA for urc II (strescopin-related peptide) in the human skin and in cultured human normal and malignant epidermal melanocytes and keratinocytes, normal dermal fibroblasts and subcutaneous adipose tissue (20), suggesting that major cutaneous compartments have the capability to produce the peptide for local use.
In contrast to human skin, production of the CRH peptide in mouse skin (although it varied in a hair cycle dependent fashion) was not accompanied by corresponding CRH gene expression (6, 24, 65, 75). This suggested an extracutaneous source for the CRH presumably, release from local nerve endings (6, 75). Mouse skin, however, does express the urc I gene with production of the corresponding peptide in hair cycle-dependent fashion, albeit in a pattern opposite to the expression of CRH (68). In relation to urc II its mRNA and peptide antigens were also detected in the mouse skin (20, 76), being highly expressed in epidermal, dermal, and adexal compartments, as well as in skeletal muscle (76).
In human skin CRH production is regulated by environmental and other factors, i.e., ultraviolet radiation and forskolin stimulate CRH production, while dexamethasone inhibits it (66, 67). Most recently we have produced new data documenting UVB stimulation of CRH production in epidermal melanocytes (Zbytek et al, in preparation). Thus, UVB stimulated gene transcription, with translation of the corresponding message, and secretion of CRH peptide into the extracellular environment. This effect was time and dose dependent (Zbytek et al, in preparation). In cultured dermal fibroblasts, UVB similarly stimulated CRH production and release, and increased POMC expression (Figure 10).
Figure 10.

UVB radiation stimulates CRH in human adult dermal fibroblasts. Fibroblasts were stimulated with UVB radiation, and after 24 hours RNA was extracted from cells and protein collected. A: CRH mRNA levels were measured with real-time RT-PCR based on Taqman chemistry (Applied Biosystems, assay Hs00174941_m1 for CRH and Hs99999901_s1 for 18SrRNA). Results are presented as mean ± SEM (n=3), **p<0.005. A, insert: CRH level was measured with ELISA (Phoenix Pharmaceuticals, Belmont, CA). Results are presented as mean ± SEM (n=3), *p<0.05. B: POMC mRNA levels were measured with the real-time RT-PCR based on Sybr Green chemistry (37). Bands representative of products of POMC and 18SrRNA PCR reactions are shown in the insert.
In conclusion, skin has the capability to produce CRH and the related peptides urc I and II; peptide production is species dependent and regulated by intrinsic factors (hair cycle in mice), and environmental agents (UVB in humans). The variety of skin cell populations producing the peptides and their pleiotropic effects requires high selectivity in the signal transduction pathways systems through, for example, differential expression of CRH-Rs and their isoforms.
7. PHENOTYPIC EFFECTS OF CRH AND UROCORTIN IN CULTURED SKIN CELLS
In rodent skin both CRH and urocortin can modify function of the skin immune system and activate mast cells (12, 13). CRH can also modify DNA synthesis in the dermal and epidermal compartments of cultured mouse skin depending on the phase of the hair growth cycle (24). These differential effects are apparently related to fluctuations in CRH-R1 and CRH-R2 expression during the hair growth cycle (20, 24, 65). In the mouse activation of CRH-R2 can stimulate hair growth; this effect has been claimed as proprietary (see patent from 2005 by Vale et al. US patent 20040034882).
Skin vasculature is a recognized target for CRH and urocortin peptides (6, 77–79), where, depending on the animal model used, CRH can induce local vascular dilatation (6, 77–79) and either inhibit or stimulate angiogenesis (80, 81). Interestingly, both CRH and urocortin demonstrated anti-edema effects (82–84). In the specific case of human skin, Clifton et al clearly have shown vasodilatory action of CRH, mediated by mechanisms that included both indirect effects, activation of mast cells or endothelial-dependent pathways, and a direct action on the blood vessel wall (77–79). These vascular effects of CRH appear to be dependent on the local level of estrogenization (77).
In human cultured normal and immortalized keratinocytes CRH and urocortin inhibited cell proliferation (23, 42, 51, 68), while stimulating the cell differentiation program (23, 42) through overlapping mechanisms (12, 23, 42), consistent with coupling of CRH-R1 to either IP3 or Ca2+ signaling (see above). Thus, the activation of CRH-R1 in keratinocytes leads to inhibition of cell proliferation through G0/1 arrest, which is accompanied by increased expression of inhibitor of cyclin dependent kinase p16 (Ink4a) protein (23, 42). This antiproliferative effect is attenuated by PKC inhibitors but not by inhibitors of PKA or mitogen activated protein (MAP) kinases (23). The cell cycle withdrawal was associated with the induction of keratinocyte differentiation, with CRH stimulating expression of cytokeratin 1 and involucrin, and inhibiting expression of cytokeratin 14 at both mRNA and protein levels (23, 42). CRH also increased cell granularity and size of the cells. We have concluded that activation of CRH-R1 induces non-random parallel activation of sequential signal transduction cascades governing both keratinocyte differentiation and inhibition of cell proliferation through G0/1 arrest. We also propose that this program is triggered by activation of CRH-R1, and that it includes induction of transduction pathway involving sequential activation of PLC, PKC, AP-1 and p16 (23).
In immortalized HaCaT keratinocytes, where CRH produces a dose dependent inhibition of proliferation; the shape of the inhibition curve is determined by the concentration of Ca2+ in the growth medium (36, 42). In these cells CRH action also pointed to inhibition of the G0/1 to S phase transition of the cell cycle. CRH increased AP-1 binding activity, cell granularity, cytokeratin 1 and involucrin expression, and inhibited cytokeratin 14 expression. These results are consistent with CRH induction of the differentiation program in normal epidermal keratinocytes. Thus, the overall CRH cutaneous actions promote protective functions for the epidermis, that appear to include the triggering or acceleration of the differentiation program.
CRH modulates keratinocytes immune functions through changes in expression of cell surface adhesion molecules (51) and in cytokine production (50). CRH-Rs are also expressed on sebaceous glands (6, 50) and their activation by CRH can stimulate steroidogenic and secretory activities of sebocytes (70). These phenotypic effects could be connected to modulation of intracellular concentrations of cAMP, IP3, Ca2+ or NF-kappaB activity (12, 68) (23–25, 39, 42), generally supporting a mixture of endocrine-related and non-endocrine related actions for CRH and related peptides in the skin.
CRH can affect cutaneous expression of growth factors/pleiotropic cytokines that can regulate proliferation, differentiation and immune interactions (4, 12, 22). In this regard we have found skin cell type dependent coupling to signal transduction pathways, whereas the effects on cell proliferation, were also dependent on cell type and nutrition conditions. Specifically, CRH stimulated dermal fibroblast proliferation, by increasing transition from G1/0 to the S phase, while in keratinocytes it inhibited cell proliferation. In both normal and immortalized melanocytes CRH showed the nutrition-related dichotomy effect inhibiting melanocyte proliferation in serum-containing medium, while enhancing DNA synthesis in serum free media. The latter effect was related to inhibition of early and late apoptosis. Thus, under the stress of starvation CRH acts on epidermal melanocytes as survival factor (anti-apoptotic) simultaneously, while inhibiting growth factor-induced cell proliferation. In conclusion, CRH and related peptides couple CRH-R1 to any of a series signal transduction pathways, to regulate cell viability and proliferation in cell type and growth condition dependent manners.
8. CRH/UROCORTIN – CRH-RS AXIS IN THE HUMAN HAIR FOLLICLE
8.1. Overview
Accumulating evidence from studies on human skin have revealed the expression of a neuroendocrine system in the human hair follicle, which included at least CRH, urocortin, and CRH receptors, the POMC peptides alpha-MSH, ACTH and beta-endorphin and their associated receptors: melanocortin receptor 1 (MC1-R), and mi-opiate receptor etc. (6, 7, 20, 24, 51, 70, 72–74, 85–95). These studies indicate that human hair follicles are also peripheral endocrine mini-organs that are both a source (including a metabolizing source) and a target of multiple neuro-steroido-hormones (4, 74, 91, 95–98). This endocrine capacity resides in the main cell populations of the hair follicle (keratinocytes, melanocytes and fibroblasts). The human hair follicle expresses the full capacity to respond to CRH with functional changes including hair fiber elongation, melanocyte proliferation and melanogenesis (20, 73, 74, 91, 92).
The first description of the simultaneous expression of CRH, CRH-R1 and POMC system in human hair follicles in situ was provided by Kono and colleagues (72), complementing and confirming previous studies on the in situ detection of the above elements (6, 24, 51, 68, 85, 89). Subsequent studies from different laboratories further confirmed non-random expression of CRH-R1 and CRH-R2 and their ligands in the human hair follicle (Figure 11) (20, 73, 74) (99). All these components were expressed at both the message and protein levels both in situ and in vitro in various hair follicle cell populations (figures 11 and 12). CRH-R1alpha was the predominant isoform in hair follicle keratinocytes and melanocytes and follicular dermal papilla fibroblasts suggesting that this receptor is the likely principal regulator of local CRH-dependent activities.
Figure 11.

Human hair follicles express CRH, Urocortin and CRH-R1 and CRH-R2. CRH and urocortin expression is detected in keratinocytes of the outer root sheath (ORS) and hair bulb pre-cortex, but only weakly in basal keratinocytes of the hair matrix (M). Only relatively undifferentiated melanocytes located in the peripheral and proximal matrix express CRH and urocortin. Hair follicle fibroblasts including follicular papilla (FP) cells and dermal sheath (DS) fibroblasts also expressed CRH and urocortin (a, b). A similar distribution of both CRH-R1 and CRH-R2 is observed, but the expression commonly exhibited a marked nuclear pattern, especially in ORS keratinocytes (c, d). Negative control incubated with an irrelevant primary antibody (e). Tissue source: 49 year old female.
Figure 12.

CRH, urocortin, CRH-R1 and CRH-R2 are expressed in hair follicle melanocytes, hair follicle keratinocyte and follicular papilla fibroblasts in vitro. CRH (a), urocortin (b), CRH-R1 (c), CRH-R2 (d) were variably expressed in cultured hair follicle melanocyte (HFM, female 58ys), hair follicle keratinocytes (HFK, male 59 yrs) and follicular papilla fibroblasts (FPF, female 53 yrs). Negative control HFM (e), HFK (f) and FPF (g) incubated with an irrelevant primary antibody.
The CRH-R2alpha gene (but not CRH-R2beta) was readily detected in hair follicle keratinocytes and follicular papilla fibroblasts, but not in cultured follicular melanocytes and dermal fibroblasts (20). Similarly, CRH-R2 protein was strikingly absent from epidermal keratinocytes in situ, but strongly expressed in multiple keratinocytes lineages of the human anagen hair follicle, including outer and inner root sheaths, hair bulb matrix, and the differentiating pre-cortex, as well the hair growth–controlling follicular papilla. A potential role for CRH/urocortin in hair cycling was inferred by the upregulation of CRH-R2 protein expression in those parts of the hair follicle close to, but not directly affected by, the apoptosis-driven regression characteristic of catagen (20). These cells included not only the most proximal keratinocytes, but also fibroblasts of the catagen follicular papilla and its collapsing capillary endothelium (20). Thus, the molecular and in situ data are in agreement, except for the detection of CRH-R2 protein (but not message) in epidermal and follicular melanocytes. This discrepancy may be explained in part by changes induced during the enforced melanocyte proliferation in vitro. However, we have more recently also detected CRH-R2 protein expression in cultured follicular melanocytes using both immunocytochemistry (Figure 12) and immunoblotting (99), which raises the alternative possibility that variation in melanocyte gene expression may reflect their heterogeneous differentiation status in the hair follicle in situ and in vitro (100, 101). The Paus laboratory reported a similar distribution of CRH and CRH-R1 in human scalp hair follicles both at the gene and protein levels (73, 74). Interestingly, the expression of CRH-R1 and CRH-R2 (and also of MC1-R and MC2-R) is upregulated after treatment of anagen hair follicles in ex vivo organ culture with CRH (74) that also upregulated the expression of POMC mRNA and the expression of ACTH and alpha-MSH peptides in the outer root sheath of the same hair follicles.
8.2. CRH/urocortin and hair follicle pigmentation
The POMC system is fully expressed in the human scalp hair follicle pigmentary unit and the behavior of follicular melanocytes in vitro is modulated via action at the MC1-R (by alpha-MSH and ACTH) and the mi-opiate receptor (by beta-endorphin) pathways (90, 91). Under our own cell culture conditions, beta-endorphin was as potent as the traditional melanocortins ACTH and alpha-MSH at inducing melanogenesis and dendricity in follicular melanocytes (90, 91). Because the supply of POMC may be regulated locally via CRH, we assessed the role of CRH, urocortin and receptor-selective CRH-related peptides in the human hair follicle and its pigmentary unit (91, 102). While CRH and urocortin were detected in the anagen hair follicle epithelial and mesenchymal subpopulations (7, 20) (99), these proteins were expressed at conspicuously low levels in the differentiated melanogenic zone of the anagen hair bulb. Melanocytes distributed in this region on the anagen hair follicle are highly differentiated (92, 100, 103).
However, like with the POMC system components, both CRH and urocortin, together with CRH-R1 and R2 are expressed in a minor population of poorly differentiated follicular melanocytes located in the most peripheral and proximal hair bulb and outer root sheath (73, 91), suggesting that follicular melanocytes may only be responsive during the early stages of melanocyte differentiation. While CRH, urocortin and CRH-R1 and R2 are detected on follicular papilla fibroblasts (Figure 12), urocortin alone is absent from cultured follicular keratinocytes (Figure 12), related, perhaps, to differentiation status in culture.
CRH and related peptides can also modulate follicular melanocyte phenotype in vitro (102) involving both CRH-R1 and/or CRH-R2 pathways. Using a modified urocortin peptide with reduced affinity for CRH-R1, signalling preferentially through CRH-R2 (104), we found that signalling via CRH-R1 is likely to be more important for stimulating melanogenesis, dendricity and cell proliferation in follicular melanocytes. Further support for these findings may be found in the stimulation of follicular melanogenesis in ex vivo cultured hair follicles incubated with CRH (73). However, experiments with CRH analogs that are active at both CRH-R1 and CRH-R2 suggest that both receptor systems are differentially active in these cells.
8.3. CRH/urocortin and hair follicle fiber growth
The hair bulb matrix contains one of the body’s most rapidly proliferative tissues and the hair fiber is the product of a differentiation program involving eight or more distinct keratinocyte lineages. In this regard CRH can significantly inhibit hair fiber elongation in organ cultured hair follicles (74, 102) (Figure 13) due to the inhibition of proliferation in the anagen hair bulb and corresponding premature precipitation into an apoptosis-driven catagen like state. However, treatment of human scalp hair follicles with urocortin does not inhibit hair fiber elongation and may even protect the hair follicle from entry in catagen (102) (Kauser et al unpublished data). Thus, like in the epidermis, CRH signalling via CRH-R1 leads to inhibition of keratinocyte proliferation with corresponding induction of keratinocyte differentiation, while preferential activation with CRH-R2-selective urocortin analogs may invoke the opposite effect facilitating continue hair fiber production.
Figure 13.

Photo-micrographs showing effect of CRH-related peptides on hair fiber elongation at day 0 (D0) and day 9 (D9) in ex vivo organ culture.
9. CRH ACTIVATES LOCAL EQUIVALENT OF HPA AXIS
Since CRH plays a central role in the response to systemic stress via the HPA axis, we proposed that a similar structural organization is preserved in the skin (105). Indeed, the human skin expresses molecular elements of that axis including POMC with processing to final ACTH peptide, MC2-R, CRH and functional CRH-R1, and the skin has also corticosteroidogenic activity (6, 7, 60, 106–108).
When normal epidermal melanocytes and dermal fibroblasts were incubated with CRH, a functional cascade was released that was structured hierarchically along the same algorithm as in the HPA axis: CRH activated CRH-R1 and stimulated cAMP accumulation, with increased POMC gene expression and production of ACTH (38). This effect was absent in keratinocytes. Melanocytes also responded to CRH and ACTH with enhanced production of cortisol and corticosterone, abolished by POMC gene silencing or by the potent CRH-R1 antagonist, antalarmin. Cortisol chemical identity was confirmed by liquid chromatography-mass spectrometry/mass spectrometry (38). Fibroblasts responded to CRH and ACTH with enhanced production of corticosterone but not cortisol, with ACTH being a more potent stimulator (37). Thus, the skin displays a CRH-led system organized similar to that operating at the systemic level, although in fibroblasts, it diverges in its distal step, where corticosterone, instead of cortisol, is the main product stimulated by ACTH (37). This pattern defines a fractal nature in the responses to stress with analogous activation sequences at the single cell and whole body levels.
Tests performed on isolated human hair follicles showed that CRH treatment upregulated the expression of POMC mRNA, of ACTH and alpha-MSH peptides, of CRH-R1 and CRH-R2 and also of MC1-R and MC2-R (74). CRH thus induces upregulation of the POMC, its cleavage into the melanocortin peptides, as well as stimulation of melanocortin receptor expression. Either CRH or ACTH stimulation of these hair follicles resulted in the enhanced expression of cortisol in keratinocytes of the outer root sheath of the human scalp hair follicle, and secretion into the media (74). Conversely, hydrocortisone treatment of isolated hair follicles was associated with a down-regulation of CRH-R1 in the hair follicle outer root sheath, mirroring its classical central feedback regulatory mechanisms (74).
10. PERSPECTIVE
While the biological significance of the cutaneous HPA axis (7, 60, 109) as whole remains to be determined (4), the isolated effects of each and every one of its elements is currently being uncovered (37, 38, 74). It already appears that some of them may become therapeutically important in the management of skin diseases (CRH and CRH-R isoforms), other have already proven their value in this area (corticosteroids). Selective targeting of CRH-R1 has been proposed for the treatment of skin hypeproliferative disorders and malignant tumors that include melanoma (patent WO0153777). An anti-melanoma effect for selective CRH-R1 agonists has already been observed in experimental models of melanoma (110). Moreover, the therapeutic use of alterantively spliced CRH-R1 isoforms has also been proposed (patent WO03024990)
Acknowledgments
Source of support: NIH Grant #1R01-AR047079
References
- 1.Grammatopoulos DK, Chrousos GP. Functional characteristics of CRH receptors and potential clinical applications of CRH-receptor antagonists. Trends Endocrinol Metab. 2002;13:436–44. doi: 10.1016/s1043-2760(02)00670-7. [DOI] [PubMed] [Google Scholar]
- 2.Hillhouse EW, Randeva H, Ladds G, Grammatopoulos D. Corticotropin-releasing hormone receptors. Biochem Soc Trans. 2002;30:428–32. doi: 10.1042/bst0300428. [DOI] [PubMed] [Google Scholar]
- 3.Chrousos GP. The Hypothalamic-Pituitary-Adrenal Axis and Immune-Mediated Inflammation. N Engl J Med. 1995;332:1351–1363. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- 4.Slominski A. Neuroendocrine system of the skin. Dermatology. 2005;211:199–208. doi: 10.1159/000087012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slominski A, Wortsman J, Linton E, Pisarchik A, Zbytek B. The skin as a model for the immunomodulatory effects of corticotropin-releasing hormone. In: Schaefer M, Stein C, editors. Mind over Matter - Regulation of Peripheral Inflammation by the CNS. Birkhaeuser Verlag; Basel, Boston, Berlin: 2003. [Google Scholar]
- 6.Slominski A, Wortsman J, Pisarchik A, Zbytek B, Linton EA, Mazurkiewicz JE, Wei ET. Cutaneous expression of corticotropin-releasing hormone (CRH), urocortin, and CRH receptors. Faseb J. 2001;15:1678–93. doi: 10.1096/fj.00-0850rev. [DOI] [PubMed] [Google Scholar]
- 7.Slominski A, Wortsman J, Luger T, Paus R, Solomon S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev. 2000;80:979–1020. doi: 10.1152/physrev.2000.80.3.979. [DOI] [PubMed] [Google Scholar]
- 8.Perrin MH, Vale WW. Corticotropin releasing factor receptors and their ligand family. Ann NY Acad Sci. 1999;885:312–328. doi: 10.1111/j.1749-6632.1999.tb08687.x. [DOI] [PubMed] [Google Scholar]
- 9.Spiess J, Rivier J, Rivier C, Vale W. Primary structure of corticotropin-releasing factor from ovine hypothalamus. Proc Natl Acad Sci U S A. 1981;78:6517–21. doi: 10.1073/pnas.78.10.6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–7. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 11.Aguilera G, Rabadan-Diehl C, Nikodemova M. Regulation of pituitary corticotropin releasing hormone receptors. Peptides. 2001;22:769–74. doi: 10.1016/s0196-9781(01)00390-4. [DOI] [PubMed] [Google Scholar]
- 12.Slominski A, Wortsman J, Linton E, Pisarchik A, Zbytek B. The skin as a model for the immunodulatory effects of corticotropin-releasing hormone. In: Schäfer M, Stein C, editors. Mind over Matter - Regulation of Peripheral Inflammation by the CNS. Birkhäuser Verlag; Basel: 2003. [Google Scholar]
- 13.Theoharides TC, Cochrane DE. Critical role of mast cells in inflammatory diseases and the effect of acute stress. J Neuroimmunol. 2004;146:1–12. doi: 10.1016/j.jneuroim.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 14.Karalis K, Sano H, Redwine J, Listwak S, Wilder RL, Chrousos GP. Autocrine or paracrine inflammatory actions of corticotropin-releasing hormone in vivo. Science. 1991;254:421–3. doi: 10.1126/science.1925600. [DOI] [PubMed] [Google Scholar]
- 15.Pisarchik A, Slominski AT. Alternative splicing of CRH-R1 receptors in human and mouse skin: identification of new variants and their differential expression. Faseb J. 2001;15:2754–6. doi: 10.1096/fj.01-0487fje. [DOI] [PubMed] [Google Scholar]
- 16.Eckart K, Jahn O, Radulovic J, Radulovic M, Blank T, Stiedl O, Brauns O, Tezval H, Zeyda T, Spiess J. Pharmacology and biology of corticotropin-releasing factor (CRF) receptors. Receptors Channels. 2002;8:163–77. [PubMed] [Google Scholar]
- 17.Tsai-Morris CH, Buczko E, Geng Y, Gamboa-Pinto A, Dufau ML. The genomic structure of the rat corticotropin releasing factor receptor. A member of the class II G protein-coupled receptors. J Biol Chem. 1996;271:14519–25. doi: 10.1074/jbc.271.24.14519. [DOI] [PubMed] [Google Scholar]
- 18.Pisarchik A, Slominski A. Corticotropin releasing factor receptor type 1: molecular cloning and investigation of alternative splicing in the hamster skin. J Invest Dermatol. 2002;118:1065–72. doi: 10.1046/j.1523-1747.2002.01770.x. [DOI] [PubMed] [Google Scholar]
- 19.Catalano RD, Kyriakou T, Chen J, Easton A, Hillhouse EW. Regulation of corticotropin-releasing hormone type 2 receptors by multiple promoters and alternative splicing: identification of multiple splice variants. Mol Endocrinol. 2003;17:395–410. doi: 10.1210/me.2002-0302. [DOI] [PubMed] [Google Scholar]
- 20.Slominski A, Pisarchik A, Tobin DJ, Mazurkiewicz JE, Wortsman J. Differential expression of a cutaneous corticotropin-releasing hormone system. Endocrinology. 2004;145:941–50. doi: 10.1210/en.2003-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pisarchik A, Slominski A. Molecular and functional characterization of novel CRFR1 isoforms from the skin. European Journal of Biochemistry. 2004;271:2821–2830. doi: 10.1111/j.1432-1033.2004.04216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slominski A, Zbytek B, Pisarchik A, Slominski RM, Wortsman J. CRH functions as a growth factor/cytokine in the skin. J Cell Physiol. 2005 doi: 10.1002/jcp.20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zbytek B, Slominski AT. Corticotropin-releasing hormone induces keratinocyte differentiation in the adult human epidermis. J Cell Physiol. 2005;203:118–26. doi: 10.1002/jcp.20209. [DOI] [PubMed] [Google Scholar]
- 24.Slominski AT, Botchkarev V, Choudhry M, Fazal N, Fechner K, Furkert J, Krause E, Roloff B, Sayeed M, Wei E, Zbytek B, Zipper J, Wortsman J, Paus R. Cutaneous expression of CRH and CRH-R. Is there a "skin stress response system?". Ann N Y Acad Sci. 1999;885:287–311. doi: 10.1111/j.1749-6632.1999.tb08686.x. [DOI] [PubMed] [Google Scholar]
- 25.Wiesner B, Roloff B, Fechner K, Slominski A. Intracellular calcium measurements of single human skin cells after stimulation with corticotropin-releasing factor and urocortin using confocal laser scanning microscopy. J Cell Sci. 2003;116:1261–8. doi: 10.1242/jcs.00301. [DOI] [PubMed] [Google Scholar]
- 26.Wietfeld D, Heinrich N, Furkert J, Fechner K, Beyermann M, Bienert M, Berger H. Regulation of the coupling to different G proteins of rat corticotropin-releasing factor receptor type 1 in human embryonic kidney 293 cells. J Biol Chem. 2004;279:38386–94. doi: 10.1074/jbc.M405335200. [DOI] [PubMed] [Google Scholar]
- 27.Perrin MH, Fischer WH, Kunitake KS, Craig AG, Koerber SC, Cervini LA, Rivier JE, Groppe JC, Greenwald J, Moller Nielsen S, Vale WW. Expression, purification, and characterization of a soluble form of the first extracellular domain of the human type 1 corticotropin releasing factor receptor. J Biol Chem. 2001;276:31528–34. doi: 10.1074/jbc.M101838200. [DOI] [PubMed] [Google Scholar]
- 28.Liaw CW, Grigoriadis DE, Lovenberg TW, De Souza EB, Maki RA. Localization of ligand-binding domains of human corticotropin-releasing factor receptor: a chimeric receptor approach. Mol Endocrinol. 1997;11:980–5. doi: 10.1210/mend.11.7.9946. [DOI] [PubMed] [Google Scholar]
- 29.Grammatopoulos DK, Dai Y, Randeva HS, Levine MA, Karteris E, Easton AJ, Hillhouse EW. A novel spliced variant of the type 1 corticotropin-releasing hormone receptor with a deletion in the seventh transmembrane domain present in the human pregnant term myometrium and fetal membranes. Mol Endocrinol. 1999;13:2189–202. doi: 10.1210/mend.13.12.0391. [DOI] [PubMed] [Google Scholar]
- 30.Seck T, Baron R, Horne WC. The alternatively spliced deltae13 transcript of the rabbit calcitonin receptor dimerizes with the C1a isoform and inhibits its surface expression. J Biol Chem. 2003;278:23085–93. doi: 10.1074/jbc.M211280200. [DOI] [PubMed] [Google Scholar]
- 31.Myers DA, Trinh JV, Myers TR. Structure and function of the ovine type 1 corticotropin releasing factor receptor (CRF1) and a carboxyl-terminal variant. Mol Cell Endocrinol. 1998;144:21–35. doi: 10.1016/s0303-7207(98)00157-9. [DOI] [PubMed] [Google Scholar]
- 32.Papadopoulou N, Chen J, Randeva HS, Levine MA, Hillhouse EW, Grammatopoulos DK. Protein kinase A-induced negative regulation of the corticotropin-releasing hormone R1alpha receptor-extracellularly regulated kinase signal transduction pathway: the critical role of Ser301 for signaling switch and selectivity. Mol Endocrinol. 2004;18:624–39. doi: 10.1210/me.2003-0365. [DOI] [PubMed] [Google Scholar]
- 33.Perrin MH, DiGruccio MR, Koerber SC, Rivier JE, Kunitake KS, Bain DL, Fischer WH, Vale WW. A soluble form of the first extracellular domain of mouse type 2beta corticotropin-releasing factor receptor reveals differential ligand specificity. J Biol Chem. 2003;278:15595–600. doi: 10.1074/jbc.M210476200. [DOI] [PubMed] [Google Scholar]
- 34.Levine SJ. Mechanisms of soluble cytokine receptor generation. J Immunol. 2004;173:5343–8. doi: 10.4049/jimmunol.173.9.5343. [DOI] [PubMed] [Google Scholar]
- 35.Yan SF, Ramasamy R, Naka Y, Schmidt AM. Glycation, inflammation, and RAGE: a scaffold for the macrovascular complications of diabetes and beyond. Circ Res. 2003;93:1159–69. doi: 10.1161/01.RES.0000103862.26506.3D. [DOI] [PubMed] [Google Scholar]
- 36.Slominski AT, Roloff B, Zbytek B, Wei ET, Fechner K, Curry J, Wortsman J. Corticotropin releasing hormone and related peptides can act as bioregulatory factors in human keratinocytes. In Vitro Cell Dev Biol Anim. 2000;36:211–6. doi: 10.1290/1071-2690(2000)036<0211:CRHARP>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 37.Slominski A, Zbytek B, Semak I, Sweatman T, Wortsman J. CRH stimulates POMC activity and corticosterone production in dermal fibroblasts. J Neuroimmunol. 2005;162:97–102. doi: 10.1016/j.jneuroim.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 38.Slominski A, Zbytek B, Szczesniewski A, Semak I, Kaminski J, Sweatman T, Wortsman J. CRH stimulation of corticosteroids production in melanocytes is mediated by ACTH. Am J Physiol Endocrinol Metab. 2005;288:E701–E706. doi: 10.1152/ajpendo.00519.2004. [DOI] [PubMed] [Google Scholar]
- 39.Fazal N, Slominski A, Choudhry MA, Wei ET, Sayeed MM. Effect of CRF and related peptides on calcium signaling in human and rodent melanoma cells. FEBS Letters. 1998;435:187–190. doi: 10.1016/s0014-5793(98)01067-9. [DOI] [PubMed] [Google Scholar]
- 40.Foreman JC, Johansen T. Textbook of Receptor Pharmacology. CRC Press; 2002. [Google Scholar]
- 41.Eckert RL, Crish JF, Efimova T, Dashti SR, Deucher A, Bone F, Adhikary G, Huang G, Gopalakrishnan R, Balasubramanian S. Regulation of involucrin gene expression. J Invest Dermatol. 2004;123:13–22. doi: 10.1111/j.0022-202X.2004.22723.x. [DOI] [PubMed] [Google Scholar]
- 42.Zbytek B, Pikula M, Slominski RM, Mysliwski A, Wei E, Wortsman J, Slominski AT. Corticotropin-releasing hormone triggers differentiation in HaCaT keratinocytes. Br J Dermatol. 2005;152:474–80. doi: 10.1111/J.1365-2133.2005.06217.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Q, Verma IM. NF-kB regulation in the immune system. Nature Reviews Immunology. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 44.Ullrich SE. Does exposure to UV radiation induce a shift to a Th-2-like immune reaction? Photochem Photobiol. 1996;64:254–8. doi: 10.1111/j.1751-1097.1996.tb02454.x. [DOI] [PubMed] [Google Scholar]
- 45.Barker JN, Mitra RS, Griffiths CE, Dixit VM, Nickoloff BJ. Keratinocytes as initiators of inflammation. Lancet. 1991;337:211–4. doi: 10.1016/0140-6736(91)92168-2. [DOI] [PubMed] [Google Scholar]
- 46.Kupper TS, Fuhlbrigge RC. Immune surveillance in the skin: mechanisms and clinical consequences. Nat Rev Immunol. 2004;4:211–22. doi: 10.1038/nri1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nickoloff BJ, Nestle FO. Recent insights into the immunopathogenesis of psoriasis provide new therapeutic opportunities. J Clin Invest. 2004;113:1664–75. doi: 10.1172/JCI22147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kondo S, Jimbow K. Dose-dependent induction of IL-12 but not IL-10 from human keratinocytes after exposure to ultraviolet light A. J Cell Physiol. 1998;177:493–8. doi: 10.1002/(SICI)1097-4652(199812)177:3<493::AID-JCP12>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 49.Benou C, Wang Y, Imitola J, VanVlerken L, Chandras C, Karalis KP, Khoury SJ. Corticotropin-releasing hormone contributes to the peripheral inflammatory response in experimental autoimmune encephalomyelitis. J Immunol. 2005;174:5407–13. doi: 10.4049/jimmunol.174.9.5407. [DOI] [PubMed] [Google Scholar]
- 50.Zbytek B, Mysliwski A, Slominski A, Wortsman J, Wei ET, Mysliwska J. Corticotropin-releasing hormone affects cytokine production in human HaCaT keratinocytes. Life Sci. 2002;70:1013–21. doi: 10.1016/s0024-3205(01)01476-x. [DOI] [PubMed] [Google Scholar]
- 51.Quevedo ME, Slominski A, Pinto W, Wei E, Wortsman J. Pleiotropic effects of corticotropin releasing hormone on normal human skin keratinocytes. In Vitro Cell Dev Biol Anim. 2001;37:50–4. doi: 10.1290/1071-2690(2001)037<0050:peocrh>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 52.Park HJ, Kim HJ, Lee JH, Lee JY, Cho BK, Kang JS, Kang H, Yang Y, Cho DH. Corticotropin-releasing hormone (CRH) downregulates interleukin-18 expression in human HaCaT keratinocytes by activation of p38 mitogen-activated protein kinase (MAPK) pathway. J Invest Dermatol. 2005;124:751–5. doi: 10.1111/j.0022-202X.2005.23656.x. [DOI] [PubMed] [Google Scholar]
- 53.Zbytek B, Pfeffer LM, Slominski AT. Corticotropin-releasing hormone inhibits nuclear factor-kappaB pathway in human HaCaT keratinocytes. J Invest Dermatol. 2003;121:1496–9. doi: 10.1111/j.1523-1747.2003.12612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hagan P, Poole S, Bristow AF. Immunosuppressive activity of corticotrophin-releasing factor. Inhibition of interleukin-1 and interleukin-6 production by human mononuclear cells. Biochem J. 1992;281(Pt 1):251–4. doi: 10.1042/bj2810251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leu SJ, Singh VK. Stimulation of interleukin-6 production by corticotropin-releasing factor. Cell Immunol. 1992;143:220–7. doi: 10.1016/0008-8749(92)90018-k. [DOI] [PubMed] [Google Scholar]
- 56.Zhao J, Karalis KP. Regulation of nuclear factor-kappaB by corticotropin-releasing hormone in mouse thymocytes. Mol Endocrinol. 2002;16:2561–70. doi: 10.1210/me.2001-0334. [DOI] [PubMed] [Google Scholar]
- 57.Lezoualc'h F, Engert S, Berning B, Behl C. Corticotropin-releasing hormone-mediated neuroprotection against oxidative stress is associated with the increased release of non-amyloidogenic amyloid beta precursor protein and with the suppression of nuclear factor-kappaB. Mol Endocrinol. 2000;14:147–59. doi: 10.1210/mend.14.1.0403. [DOI] [PubMed] [Google Scholar]
- 58.Paez Pereda M, Sauer J, Perez Castro C, Finkielman S, Stalla GK, Holsboer F, Arzt E. Corticotropin-releasing hormone differentially modulates the interleukin-1 system according to the level of monocyte activation by endotoxin. Endocrinology. 1995;136:5504–10. doi: 10.1210/endo.136.12.7588301. [DOI] [PubMed] [Google Scholar]
- 59.Zbytek B, Pfeffer LM, Slominski AT. Corticotropin-releasing hormone stimulates NF-kappaB in human epidermal keratinocytes. J Endocrinol. 2004;181:R1–7. doi: 10.1677/joe.0.181r001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Slominski A, Wortsman J. Neuroendocrinology of the skin. Endocrine Rev. 2000;21:457–487. doi: 10.1210/edrv.21.5.0410. [DOI] [PubMed] [Google Scholar]
- 61.Lehman TA, Modali R, Boukamp P, Stanek J, Bennett WP, Welsh JA, Metcalf RA, Stampfer MR, Fusenig N, Rogan EM, et al. p53 mutations in human immortalized epithelial cell lines. Carcinogenesis. 1993;14:833–9. doi: 10.1093/carcin/14.5.833. [DOI] [PubMed] [Google Scholar]
- 62.Zbytek B, Pfeffer L, Slominski A. CRH inhibits NF-kappaB in melanocyte via POMC signaling.in preparation. 2005 [Google Scholar]
- 63.Moustafa M, Szabo M, Ghanem GE, Morandini R, Kemp EH, MacNeil S, Haycock JW. Inhibition of tumor necrosis factor-alpha stimulated NFkappaB/p65 in human keratinocytes by alpha-melanocyte stimulating hormone and adrenocorticotropic hormone peptides. J Invest Dermatol. 2002;119:1244–53. doi: 10.1046/j.1523-1747.2002.19602.x. [DOI] [PubMed] [Google Scholar]
- 64.Haycock JW, Wagner M, Morandini R, Ghanem G, Rennie IG, Mac Neil S. Alpha-melanocyte-stimulating hormone inhibits NF-kappaB activation in human melanocytes and melanoma cells. J Invest Dermatol. 1999;113:560–6. doi: 10.1046/j.1523-1747.1999.00739.x. [DOI] [PubMed] [Google Scholar]
- 65.Roloff B, Fechner K, Slominski A, Furkert J, Botchkarev VA, Bulfone-Paus S, Zipper J, Krause E, Paus R. Hair cycle-dependent expression of corticotropin-releasing factor (CRF) and CRF receptors in murine skin. Faseb J. 1998;12:287–97. doi: 10.1096/fasebj.12.3.287. [DOI] [PubMed] [Google Scholar]
- 66.Slominski A, Ermak G, Mazurkiewicz JE, Baker J, Wortsman J. Characterization of corticotropin-releasing hormone (CRH) in human skin. J Clin Endocrinol Metab. 1998;83:1020–4. doi: 10.1210/jcem.83.3.4650. [DOI] [PubMed] [Google Scholar]
- 67.Slominski A, Baker J, Ermak G, Chakraborty A, Pawelek J. Ultraviolet B stimulates production of corticotropin releasing factor (CRF) by human melanocytes. FEBS Lett. 1996;399:175–6. doi: 10.1016/s0014-5793(96)01315-4. [DOI] [PubMed] [Google Scholar]
- 68.Slominski A, Roloff B, Curry J, Dahiya M, Szczesniewski A, Wortsman J. The skin produces urocortin. J Clin Endocrinol Metab. 2000;85:815–23. doi: 10.1210/jcem.85.2.6381. [DOI] [PubMed] [Google Scholar]
- 69.Slominski A, Szczesniewski A, Wortsman J. Liquid chromatography-mass spectrometry detection of corticotropin-releasing hormone and proopiomelanocortin-derived peptides in human skin. J Clin Endocrinol Metab. 2000;85:3582–8. doi: 10.1210/jcem.85.10.6863. [DOI] [PubMed] [Google Scholar]
- 70.Zouboulis CC, Seltmann H, Hiroi N, Chen W, Young M, Oeff M, Scherbaum WA, Orfanos CE, McCann SM, Bornstein SR. Corticotropin-releasing hormone: an autocrine hormone that promotes lipogenesis in human sebocytes. Proc Natl Acad Sci U S A. 2002;99:7148–53. doi: 10.1073/pnas.102180999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cao J, Papadopoulou N, Kempuraj D, Boucher WS, Sugimoto K, Cetrulo CL, Theoharides TC. Human Mast Cells Express Corticotropin-Releasing Hormone (CRH) Receptors and CRH Leads to Selective Secretion of Vascular Endothelial Growth Factor. J Immunol. 2005;174:7665–75. doi: 10.4049/jimmunol.174.12.7665. [DOI] [PubMed] [Google Scholar]
- 72.Kono M, Nagata H, Umemura S, Kawana S, Osamura RY. In situ expression of corticotropin-releasing hormone (CRH) and proopiomelanocortin (POMC) genes in human skin. Faseb J. 2001;15:2297–9. doi: 10.1096/fj.01-0254fje. [DOI] [PubMed] [Google Scholar]
- 73.Ito N, Ito T, Betterman A, Paus R. The human hair bulb is a source and target of CRH. J Invest Dermatol. 2004;122:235–7. doi: 10.1046/j.1523-1747.2003.22145.x. [DOI] [PubMed] [Google Scholar]
- 74.Ito N, Ito T, Kromminga A, Bettermann A, Takigawa M, Kees F, Straub RH, Paus R. Human hair follicles display a functional equivalent of the hypothalamic-pituitary-adrenal (HPA) axis and synthesize cortisol. Faseb J. 2005 doi: 10.1096/fj.04-1968fje. Published online June 9, 2005. [DOI] [PubMed] [Google Scholar]
- 75.Slominski A, Ermak G, Hwang J, Mazurkiewicz J, Corliss D, Eastman A. The expression of proopiomelanocortin (POMC) and of corticotropin releasing hormone receptor (CRH-R) genes in mouse skin. Biochim Biophys Acta. 1996;1289:247–51. doi: 10.1016/0304-4165(95)00159-x. [DOI] [PubMed] [Google Scholar]
- 76.Chen A, Blount A, Vaughan J, Brar B, Vale W. Urocortin II gene is highly expressed in mouse skin and skeletal muscle tissues: localization, basal expression in corticotropin-releasing factor receptor (CRFR) 1- and CRFR2-null mice, and regulation by glucocorticoids. Endocrinology. 2004;145:2445–57. doi: 10.1210/en.2003-1570. [DOI] [PubMed] [Google Scholar]
- 77.Clifton VL, Crompton R, Read MA, Gibson PG, Smith R, Wright IM. Microvascular effects of corticotropin-releasing hormone in human skin vary in relation to estrogen concentration during the menstrual cycle. J Endocrinol. 2005;186:69–76. doi: 10.1677/joe.1.06030. [DOI] [PubMed] [Google Scholar]
- 78.Clifton VL, Crompton R, Smith R, Wright IM. Microvascular effects of CRH in human skin vary in relation to gender. J Clin Endocrinol Metab. 2002;87:267–70. doi: 10.1210/jcem.87.1.8149. [DOI] [PubMed] [Google Scholar]
- 79.Crompton R, V, Clifton L, Bisits AT, Read MA, Smith R, Wright IM. Corticotropin-releasing hormone causes vasodilation in human skin via mast cell-dependent pathways. J Clin Endocrinol Metab. 2003;88:5427–32. doi: 10.1210/jc.2003-030377. [DOI] [PubMed] [Google Scholar]
- 80.Arbiser JL, Karalis K, Viswanathan A, Koike C, Anand-Apte B, Flynn E, Zetter B, Majzoub JA. Corticotropin-releasing hormone stimulates angiogenesis and epithelial tumor growth in the skin. J Invest Dermatol. 1999;113:838–42. doi: 10.1046/j.1523-1747.1999.00760.x. [DOI] [PubMed] [Google Scholar]
- 81.Tjuvajev J, Kolesnikov Y, Joshi R, Sherinski J, Koutcher L, Zhou Y, Matei C, Koutcher J, Kreek MJ, Blasberg R. Anti-neoplastic properties of human corticotropin releasing factor: involvement of the nitric oxide pathway. In Vivo. 1998;12:1–10. [PubMed] [Google Scholar]
- 82.Wei ET, Thomas HA. Correlation of neuroendocrine and anti-edema activities of alanine-corticotropin-releasing factor analogs. Eur J Pharmacol. 1994;263:319–21. doi: 10.1016/0014-2999(94)90729-3. [DOI] [PubMed] [Google Scholar]
- 83.Gjerde EA, Woie K, Wei ET, Reed RK. Corticotropin-releasing hormone inhibits lowering of interstitial pressure in rat trachea after neurogenic inflammation. Eur J Pharmacol. 1998;352:99–102. doi: 10.1016/s0014-2999(98)00403-8. [DOI] [PubMed] [Google Scholar]
- 84.Torpy DJ, Webster EL, Zachman EK, Aguilera G, Chrousos GP. Urocortin and inflammation: confounding effects of hypotension on measures of inflammation. Neuroimmunomodulation. 1999;6:182–6. doi: 10.1159/000026380. [DOI] [PubMed] [Google Scholar]
- 85.Slominski A, Wortsman J, Mazurkiewicz JE, Matsuoka L, Dietrich J, Lawrence K, Gorbani A, Paus R. Detection of proopiomelanocortin-derived antigens in normal and pathologic human skin. J Lab Clin Med. 1993;122:658–66. [PubMed] [Google Scholar]
- 86.Slominski A, Paus R, Wortsman J. On the potential role of proopiomelanocortin in skin physiology and pathology. Mol Cell Endocrinol. 1993;93:C1–C6. doi: 10.1016/0303-7207(93)90131-3. [DOI] [PubMed] [Google Scholar]
- 87.Katsarou-Katsari A, Singh LK, Theoharides TC. Alopecia areata and affected skin CRH receptor upregulation induced by acute emotional stress. Dermatology. 2001;203:157–61. doi: 10.1159/000051732. [DOI] [PubMed] [Google Scholar]
- 88.Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84:1155–228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- 89.Slominski A, Heasley D, Mazurkiewicz JE, Ermak G, Baker J, Carlson JA. Expression of proopiomelanocortin (POMC)-derived melanocyte-stimulating hormone (MSH) and adrenocorticotropic hormone (ACTH) peptides in skin of basal cell carcinoma patients. Hum Pathol. 1999;30:208–15. doi: 10.1016/s0046-8177(99)90278-2. [DOI] [PubMed] [Google Scholar]
- 90.Kauser S, Thody AJ, Schallreuter KU, Gummer CL, Tobin DJ. beta-Endorphin as a regulator of human hair follicle melanocyte biology. J Invest Dermatol. 2004;123:184–95. doi: 10.1111/j.0022-202X.2004.22724.x. [DOI] [PubMed] [Google Scholar]
- 91.Kauser S, Thody AJ, Schallreuter KU, Gummer CL, Tobin DJ. A fully functional proopiomelanocortin/melanocortin-1 receptor system regulates the differentiation of human scalp hair follicle melanocytes. Endocrinology. 2005;146:532–43. doi: 10.1210/en.2004-1145. [DOI] [PubMed] [Google Scholar]
- 92.Slominski A, Wortsman J, Plonka PM, Schallreuter KU, Paus R, Tobin DJ. Hair follicle pigmentation. J Invest Dermatol. 2005;124:13–21. doi: 10.1111/j.0022-202X.2004.23528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Paus R, V, Botchkarev A, Botchkareva NV, Mecklenburg L, Luger T, Slominski A. The skin POMC system (SPS). Leads and lessons from the hair follicle. Ann N Y Acad Sci. 1999;885:350–63. doi: 10.1111/j.1749-6632.1999.tb08690.x. [DOI] [PubMed] [Google Scholar]
- 94.Stander S, Bohm M, Brzoska T, Zimmer KP, Luger T, Metze D. Expression of melanocortin-1 receptor in normal, malformed and neoplastic skin glands and hair follicles. Exp Dermatol. 2002;11:42–51. doi: 10.1034/j.1600-0625.2002.110105.x. [DOI] [PubMed] [Google Scholar]
- 95.Bohm M, Eickelmann M, Li Z, Schneider SW, Oji V, Diederichs S, Barsh GS, Vogt A, Stieler K, Blume-Peytavi U, Luger TA. Detection of functionally active melanocortin receptors and evidence for an immunoregulatory activity of {alpha}-melanocyte-stimulating hormone in human dermal papilla cells. Endocrinology. 2005 doi: 10.1210/en.2005-0665. [DOI] [PubMed] [Google Scholar]
- 96.Zouboulis CC, Bohm M. Neuroendocrine regulation of sebocytes -- a pathogenetic link between stress and acne. Exp Dermatol. 2004;13(Suppl 4):31–5. doi: 10.1111/j.1600-0625.2004.00254.x. [DOI] [PubMed] [Google Scholar]
- 97.Zouboulis CC, Degitz K. Androgen action on human skin -- from basic research to clinical significance. Exp Dermatol. 2004;13(Suppl 4):5–10. doi: 10.1111/j.1600-0625.2004.00255.x. [DOI] [PubMed] [Google Scholar]
- 98.Arck PC, Slominski A, Theoharides TC, Peters EMJ, Paus R. Neurobiology of Stress: Skin takes center stage. J Invest Dermatol. 2005 doi: 10.1038/sj.jid.5700104. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kauser SA, Slominski A, Wei E, T, D Tobin J. Modulation of the human hair follicle pigmentary unit by CRH and urocotrin peptides. FASEB J. doi: 10.1096/fj.05-5257com. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tobin DJ. Biology of hair pigmentation. In: Forslind B, Lindberg M, editors. Hair, Nail and Skin. Marcel Dekker; New York: 2004. [Google Scholar]
- 101.Tobin DJ, Bystryn JC. Different populations of melanocytes are present in hair follicles and epidermis. Pigment Cell Res. 1996;9:304–10. doi: 10.1111/j.1600-0749.1996.tb00122.x. [DOI] [PubMed] [Google Scholar]
- 102.Kauser S, Slominski A, Wei E, Tobin DJ. Corticotropin-releasing hormone and related peptides stimulate follicular melanocyte behaviour in vitro and also modulate human hair fibre elongation in organ culture. Journal of the German Society of Dermatology. 2004;6:494. [Google Scholar]
- 103.Tobin DJ, Paus R. Graying: gerontobiology of the hair follicle pigmentary unit. Exp Gerontol. 2001;36:29–54. doi: 10.1016/s0531-5565(00)00210-2. [DOI] [PubMed] [Google Scholar]
- 104.Wei ET, Thomas HA, Christian HC, Buckingham JC, Kishimoto T. D-amino acid-substituted analogs of corticotropin-releasing hormone (CRH) and urocortin with selective agonist activity at CRH1 and CRH2beta receptors. Peptides. 1998;19:1183–90. doi: 10.1016/s0196-9781(98)00085-0. [DOI] [PubMed] [Google Scholar]
- 105.Slominski A, Mihm MC. Potential mechanism of skin response to stress. Int J Dermatol. 1996;35:849–51. doi: 10.1111/j.1365-4362.1996.tb05049.x. [DOI] [PubMed] [Google Scholar]
- 106.Slominski A, Zjawiony J, Wortsman J, Semak I, Stewart J, Pisarchik A, Sweatman T, Marcos J, Dunbar C, CTR A novel pathway for sequential transformation of 7-dehydrocholesterol and expression of the P450scc system in mammalian skin. Eur J Biochem. 2004;271:4178–88. doi: 10.1111/j.1432-1033.2004.04356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Slominski A, Gomez-Sanchez CE, Foecking MF, Wortsman J. Metabolism of progesterone to DOC, corticosterone and 18OHDOC in cultured human melanoma cells. FEBS Lett. 1999;455:364–6. doi: 10.1016/s0014-5793(99)00889-3. [DOI] [PubMed] [Google Scholar]
- 108.Slominski A, Ermak G, Mihm M. ACTH receptor, CYP11A1, CYP17 and CYP21A2 genes are expressed in skin. J Clin Endocrinol Metab. 1996;81:2746–9. doi: 10.1210/jcem.81.7.8675607. [DOI] [PubMed] [Google Scholar]
- 109.Slominski A, Mihm M. Potential mechanism of skin response to stress. Int J Dermatol. 1996;35:849–851. doi: 10.1111/j.1365-4362.1996.tb05049.x. [DOI] [PubMed] [Google Scholar]
- 110.Carlson KW, Nawy SS, Wei ET, Sadee W, Filov VA, Rezsova VV, Slominski A, Quillan JM. Inhibition of mouse melanoma cell proliferation by corticotropin-releasing hormone and its analogs. Anticancer Res. 2001;21:1173–9. [PubMed] [Google Scholar]
- 111.Krogh A, Larsson B, von Heijne G, Sonnhammer EL. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol. 2001;305:567–80. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- 112.Tusnady GE, Simon I. The HMMTOP transmembrane topology prediction server. Bioinformatics. 2001;17:849–50. doi: 10.1093/bioinformatics/17.9.849. [DOI] [PubMed] [Google Scholar]
- 113.Horn F, Bettler E, Oliveira L, Campagne F, Cohen FE, Vriend G. GPCRDB information system for G protein-coupled receptors. Nucleic Acids Res. 2003;31:294–7. doi: 10.1093/nar/gkg103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Baldwin JM, Schertler GF, Unger VM. An alpha-carbon template for the transmembrane helices in the rhodopsin family of G-protein-coupled receptors. J Mol Biol. 1997;272:144–64. doi: 10.1006/jmbi.1997.1240. [DOI] [PubMed] [Google Scholar]


