Abstract
Wnt signaling through frizzled (Fz) receptors plays key roles in just about every developmental system that has been studied. Several Wnt-Fz signaling pathways have been identified including the Wnt/planar cell polarity (PCP) pathway. PCP signaling is crucial for many developmental processes that require major cytoskeletal rearrangements. Downstream of Fz, PCP signaling is thought to involve the GTPases, Rho, Rac and Cdc42 and regulation of the JNK cascade. Here we report on the localization of these GTPases and JNK in the lens and assess their involvement in the cytoskeletal reorganization that is a key element of FGF-induced lens fiber cell differentiation.
Keywords: Wnt, Frizzled, lens development, lens fiber differentiation, lens epithelium, small GTPases, microtubules
Introduction
Wnt signaling is implicated in many developmental systems as a key regulator of a wide variety of processes including proliferation, differentiation, survival, apoptosis and migration. However, it is only relatively recently that Wnt signaling has been investigated in the context of lens development. Various studies have shown that Wnts and their Frizzled (Fz) receptors are expressed throughout mammalian lens development (1, 2, 3, 4). For example, in situ hybridisation has shown that Wnts, 5a, 5b, 7a, 7b, 8a and 8b all have very similar patterns of expression and have been detected at early stages of lens morphogenesis in the lens placode, pit and vesicle. Later when the epithelial cells and fiber cells differentiate from the anterior and posterior lens vesicle cells respectively, to form the polarized lens structure, Wnt mRNA expression is maintained in the epithelium and in the early stages of fiber differentiation. The lens grows by proliferation of epithelial cells and differentiation of their progeny into secondary fibers at the lens equator. This process involves major reorganization of the cytoskeleton as the cells elongate into millimeter long fibers. During early stages of their differentiation, Wnts continue to be strongly expressed in these elongating fiber cells (3). All ten Fzs show similar patterns of mRNA expression (to each other and to the Wnts) during lens morphogenesis and differentiation. Immunolocalization has also shown that Fz proteins are localized in the epithelium and the fibers. Fz proteins are present throughout the fibers and are particularly strongly expressed towards the basal ends of cells at the lens equator where lens epithelial cells migrate posteriorly and begin their elongation and differentiation into fibers (4). This is consistent with studies that have implicated the Wnt/β-catenin pathway in regulating aspects of the fiber differentiation process including crystallin gene expression (5). This latter study also reported that not all Wnt-induced responses could be accounted for by canonical Wnt/β-catenin signaling and suggested that non-canonical signaling might have a role, particularly in the morphological aspects of fiber differentiation.
After engaging with Fz receptors and activating Dishevelled (Dvl), in addition to canonical signaling, Wnts can also signal through other distinct pathways. These are commonly referred to as non-canonical and include planar cell polarity (PCP) and Ca2+ signaling pathways (6). The PCP pathway has been the focus of much attention recently mainly because it has been duly recognised as a critical regulator of many major morphogenetic processes. Although PCP signaling is not as well understood as the intensively studied canonical pathway it is thought that downstream of Dvl the small GTPases, Rho, Rac and Cdc42 are activated and this leads to regulation of the JNK cascade (6, 7, 8, 9). Interest in these small GTPases and the JNK cascade has generally been in relation to their roles in organizing the cytoskeleton and regulating changes in cell shape and behaviour (10). This is also true in the lens where previous studies have detected small GTPases (11), and shown that their inhibition mediates changes in cytoskeletal organisation in cultured human lens epithelial cells (12, 13). Similarly in vivo studies with transgenic mice show that lens-specific overexpression of the GTPase inhibitor, C3-exozyme, leads to abnormal cytoskeletal organisation and impaired fiber elongation (14, 15). However, whilst GTPases and JNK can be activated by many different stimuli, there is now a general consensus that they are downstream of Fz activation of Dvl and through this pathway they are critical for regulating cell polarity and morphogenetic movements in vertebrates (7, 8). The fact that this pathway shares components with those involved in the phenomenon of PCP in Drosophila has led to it being generally referred to as the Wnt/PCP pathway (8). Unlike the Wnt canonical signaling pathway, which can exploit β-catenin reporter models such as TOPgal mice (16), there are no transcriptional reporters for the PCP pathway and characterization of signaling through this pathway generally relies on localization of, and assessment of activity of, its signaling components in various experimental models.
Thus, to explore the possible involvement of Wnt/PCP signaling in regulating cell migration and/or elongation of cells at the lens equator, this study set out to examine the distribution patterns of the small GTPases, RhoA, Rac1 and Cdc42. Localization of activated JNK was also assessed by utilising a phospho-specific antibody. As the processes of migration and fiber differentiation can be induced in lens epithelial explants by FGF, this model system was then used to further assess the involvement of Wnt/PCP signaling in regulating these processes. Results from these studies provide a framework for discussion of the mechanisms that may be involved in regulating the complex cytoskeletal dynamics that drive directed cell migration and fiber elongation in the lens.
Methods
All animal procedures were performed in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research and the animal care guidelines published by the Institute for Laboratory Animal Research (Guide for the Care and Use of Laboratory Animals). All studies were approved by the Institutional Ethics Committee of the University of Sydney.
Histology
Whole eyes were collected from postnatal day 1 (P1) FVB/N mice. All tissues were fixed in 10% neutral buffered formalin and processed for paraffin embedding. Once embedded, 5–7 μm thick sections were cut for subsequent immunofluorescence studies.
Lens epithelial explant cultures
P10 Wistar rats were sacrificed by CO2 asphyxiation followed by cervical dislocation. Eyeballs were removed with curved scissors and placed in pre-warmed 37°C M199 medium with Earle’s salts (Trace Scientific, NSW, Australia), supplemented with 0.1% BSA, 0.5 mM L-glutamine, 100 IU/ml penicillin, 100 μg/ml streptomycin and 2.5 μg/ml Amphostat (all from Trace Scientific). Using a dissecting microscope (Leica, NSW, Australia), lenses were dissected from other ocular tissues and transferred to a culture dish with fresh medium.
Lens epithelial explants were prepared by tearing the anterior lens capsule (with adherent epithelial cells) so that 4–5 flaps were stripped back to the lens equator (Fig. 1). The fiber mass was then gently separated from the capsule and discarded. Using the tips of fine forceps, the capsule with adherent epithelial cells uppermost, was flattened and pinned out on the bottom of the culture dish. In these explants, the flaps of anterior capsule extended out from the cell-free posterior capsule (Fig. 1). Thus, the cells in the peripheral regions of the epithelium had a cell-free posterior capsule for migration and differentiation into fibers. After explantation, the culture medium was replaced with 1ml of fresh, equilibrated M199. 150 ng/ml of FGF2 (R&D systems, MN, USA) was added to some dishes to induce cell migration/elongation. Explants were cultured at 37°C in 5% CO2 for one to three days.
Figure 1.
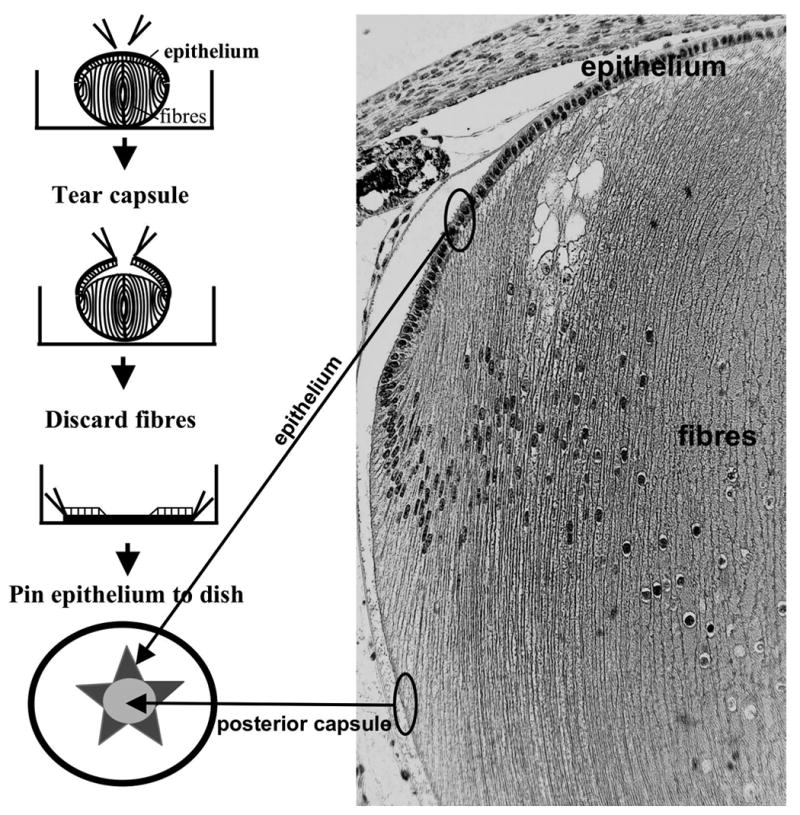
Preparation of lens epithelial explants. Using fine forceps the lens capsule at the anterior pole (with adherent epithelial cells) was torn so that 4–5 flaps could be stripped back to the lens equator. The fiber mass was then gently separated from the capsule and discarded. Using the tips of the forceps, the capsule, with adherent epithelial cells uppermost, was flattened and pinned out on the bottom of the culture dish. In these explants, the flaps of anterior capsule extended out from the cell-free posterior capsule. Thus, the cells in the peripheral regions of the epithelium had a cell-free posterior capsule for migration and differentiation into fibers. The sagittal section of the P21 rat lens indicates the origin of the cellular and non-cellular components of the explant.
Immunofluorescence
Paraffin sections and lens epithelial explants were used for immunohistochemistry. Explants were fixed in HistoChoice (Amresco, OH, USA) for 5 minutes or 2% paraformaldehyde for 20 minutes. Explants were also treated with 0.1% Tween 20/PBS for 5 minutes. Both paraffin sections and explants were blocked with goat or rabbit serum in 0.1% BSA for 30 minutes. The primary antibodies used in this study were against Fz, Dvl, RhoA, Rac1, Cdc42, phospho-PAK, phospho-JNK, (all from Santa Cruz, CA, USA), α-tubulin, acetylated-tubulin (Sigma, MO, USA), total β-catenin, active (Upstate, MA, USA), N-cadherin (Zymed, CA, USA) and pericentrin (Abcam, Cambridge, UK). The specificity of all the antibodies used in this study was checked by Western blotting on a mouse lens homogenate and in all cases a band of appropriate molecular weight was detected (data not shown). Primary antibodies were diluted to approximately 4 μg/ml with 3% serum in 0.1% BSA/PBS. Primary antibody was applied and explants or sections were incubated at 4ºC overnight. For double-labeling, some tissues were incubated with a second primary antibody. Protein reactivity was visualised with Alexa-conjugated secondary antibodies. For controls, the primary antibody was replaced by non-immune serum. Phalloidin-FITC (Sigma) was used to bind to F-actin at room temperature for 1 hour and the cell nuclei of tissues were labelled using propidium iodide (diluted 1:10000) (Molecular Probes, CA, USA). Fluorescence was visualised using a Zeiss LSM-5 confocal microscope (Zeiss, Jena, Germany).
Results
Localization of small GTPases in the lens
Immunohistochemical analysis of the small GTPases, RhoA, Rac1 and Cdc42 were conducted on sagittal sections of mouse lenses at P1. All were localized in the lens but each had its own distinct pattern of expression.
RhoA
Reactivity for RhoA was present in the cytoplasm of both epithelial cells and fibers (Figure 2A, 2A1, 2A2). RhoA protein was clearly present in all cells throughout the epithelium but in the fibers it was predominantly present in the outer cortical region (Figure 2A, 2A1). Reactivity was particularly noticeable at the basal ends of the cells at the lens equator as well as the ends of the elongating fibers as they extend to the sutures, both anteriorly and posteriorly (Figure 2A, 2A2, arrows).
Figure 2.
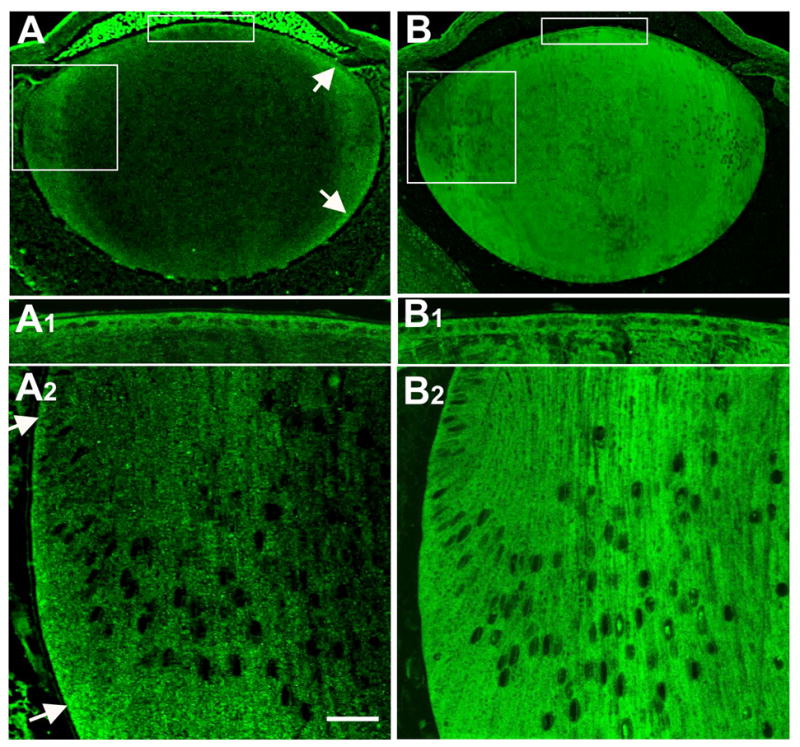
Localization of RhoA and Rac1. RhoA and Rac1 were immunolocalized in sagittal sections of mouse lens at P1 (A, B). The uppermost boxed region includes the epithelium and this is shown in more detail in A1 and B1. The lower boxed region includes the lens equator and cortex and this is shown in more detail in A2 and B2. RhoA reactivity was present in the cytoplasm of lens epithelial cells and fibers (A1, A2). In the fiber mass, RhoA was predominantly distributed in the outer cortical region (A). Reactivity was particularly prominent at the basal ends of the cells at the lens equator as well as the ends of the elongating fibers as they extended towards the sutures, both anteriorly and posteriorly (A, A2, arrows). Reactivity for Rac1 was present throughout the epithelium and the fibers (B, B1, B2). It was localized in the cytoplasm of both epithelial cells and fiber cells and appeared uniformly distributed throughout the lens. Note that the bright fluorescence in the anterior chamber in A is an artifact. Scale bar: (A, B), 133 μm; (A1, A2, B1, B2), 25 μm.
Rac1
Reactivity for Rac1 was present in the cytoplasm of both epithelial cells and fibers (Figure 2B, 2B1, 2B2). Unlike RhoA, the reactivity for Rac1 appeared to present uniformly throughout the lens fiber mass in both nuclear and cortical regions (compare Figure 2A with Figure 2B). Some fiber cell nuclei also showed reactivity for Rac1 (figure 2B2).
Cdc42
Reactivity for Cdc42 was present in the cytoplasm of both epithelial cells and fibers (Figure 3A, 3A1-A3). This was evident throughout the epithelium and the fibers. At the lens equator it was also noted that Cdc42 reactivity was particularly strong at the basal ends of the cells (Figure 3A, 3A2, 3A3, arrows). This basal reactivity in cortical fibers extended all the way to the posterior pole of the lens (Figure 3A, arrows).
Figure 3.
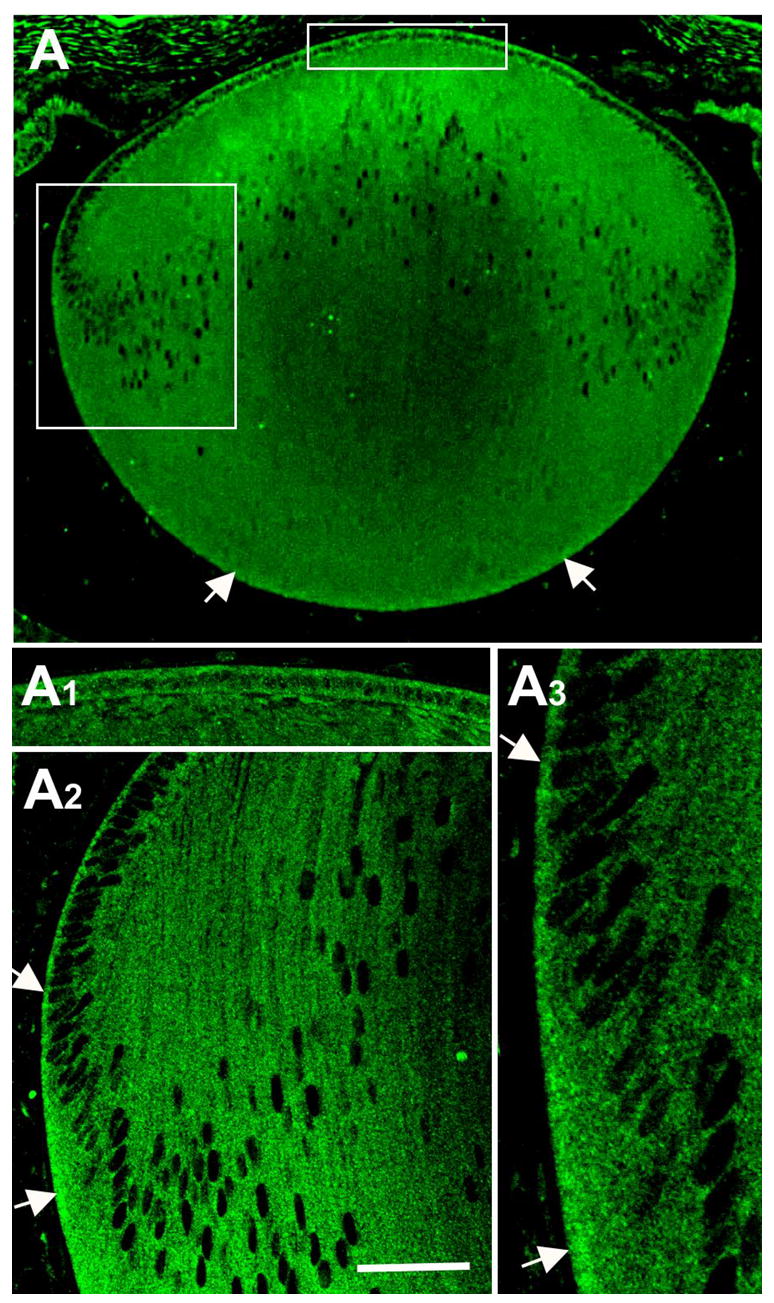
Localisation of Cdc42. Cdc42 was immunolocalized in sagittal sections of mouse lens at P1 (A). The uppermost boxed region includes the epithelium and this is shown in more detail in A1. The lower boxed region includes the equator and cortex and this is shown in more detail in A2 and A3. Reactivity for Cdc42 was present throughout the epithelium and the fibers (A, A1–3). At the lens equator reactivity was particularly strong at the basal ends of the cells (A2, A3, arrows). Basal reactivity in cortical fibers extended to the posterior pole of the lens (A, arrows). Scale bar: (A), 133 μm; (A1, A2), 25 μm; (A3), 12 μm.
JNK activity in the lens
The availability of an antibody that recognised the phosphorylated form of JNK (p-JNK), allowed us to assess the functional status of the JNK cascade. Only weak cytoplasmic reactivity was present in the central epithelial cells but closer to the lens equator, reactivity was clearly stronger and tended to be basally situated (Figure 4A, B, upper large arrow). Nuclear reactivity was also detectable throughout the epithelium but became markedly stronger below the lens equator coinciding with pronounced cell migration/elongation (Figure 4A, A1, B, B1 asterisks). Below the lens equator, distinct baso-lateral reactivity was evident in most cells (Figure 4B, lower large arrow). As cell elongation progressed and the basal ends of the fibers progressively moved posteriorly towards the posterior sutures, the baso-lateral reactivity was maintained. It was also clear that this extended along the margins of cortical fibers (Figure 4B, small arrows). Some reactivity was evident at the interface between fibers and epithelial cells (arrowhead); however, it was not clear if this originated from the apical region of epithelial or fiber cells, or possibly both.
Figure 4.
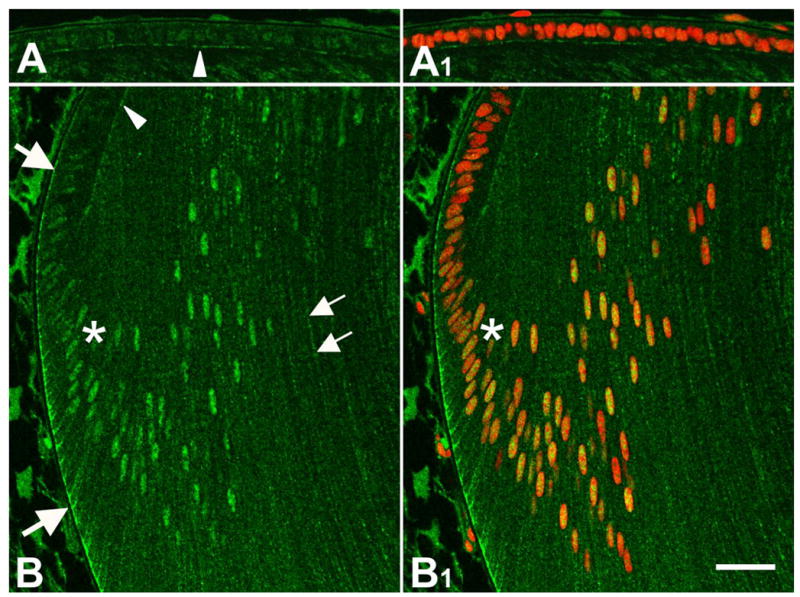
Localization of p-JNK. p-JNK was immunolocalized in sagittal sections of mouse lens at P1 (A, A1, B, B1). Propidium iodide (red) was used to counterstain nuclei (A1, B1). Only weak cytoplasmic p-JNK reactivity was present in the central epithelial cells and this was predominantly localized in their nuclei (A, A1). At the lens equator, p-JNK cytoplasmic reactivity was clearly stronger and tended to be basally situated (B, upper arrow). Below the equator, at about the point where the cells elongated into fibers, nuclear reactivity for p-JNK became distinctly stronger (B, B1, asterisk). Below the equator, distinct baso-lateral reactivity also became very prominent as the cells elongated into fibers (B, lower arrow). p-JNK reactivity extended along the margins of cortical fibers (B, small arrows). Some reactivity was evident at the interface between fibers and epithelial cells (arrowhead). Scale bar: (A, A1, B, B1), 25 μm.
FGF-induced migration and elongation: organization of the cytoskeleton
Numerous studies have shown that lens epithelial cells can be induced to undergo many fiber-specific changes when exposed to FGF in explant cultures (17). To further investigate the expression of Wnt-Fz PCP signaling molecules during epithelial cell migration and differentiation into fibers, a lens epithelial explant culture system was applied. Explants (see Methods, Figure 1) were set up so that equatorial lens epithelial cells could migrate/elongate on the exposed posterior capsule.
One day after explantation, cell processes were evident, particularly in the cells at the leading edge of the epithelial sheet (Figure 5A, B). Although processes were present in both control and FGF-treated explants, they tended to be more extensive in the latter (Figure 5A, B). After three days, cells in FGF-treated explants had moved across the capsule towards the centre of the explant (location of the posterior pole in vivo; see diagram in Figure 1). Cells at the leading edge showed extensive cell processes and in most cases these were aligned towards the centre of the explant (Figure 5D). In the absence of added FGF (controls), after three days culture, only a few cell processes extended from cells at the leading edge and overall there was little evidence of migratory activity (Figure 5C).
Figure 5.
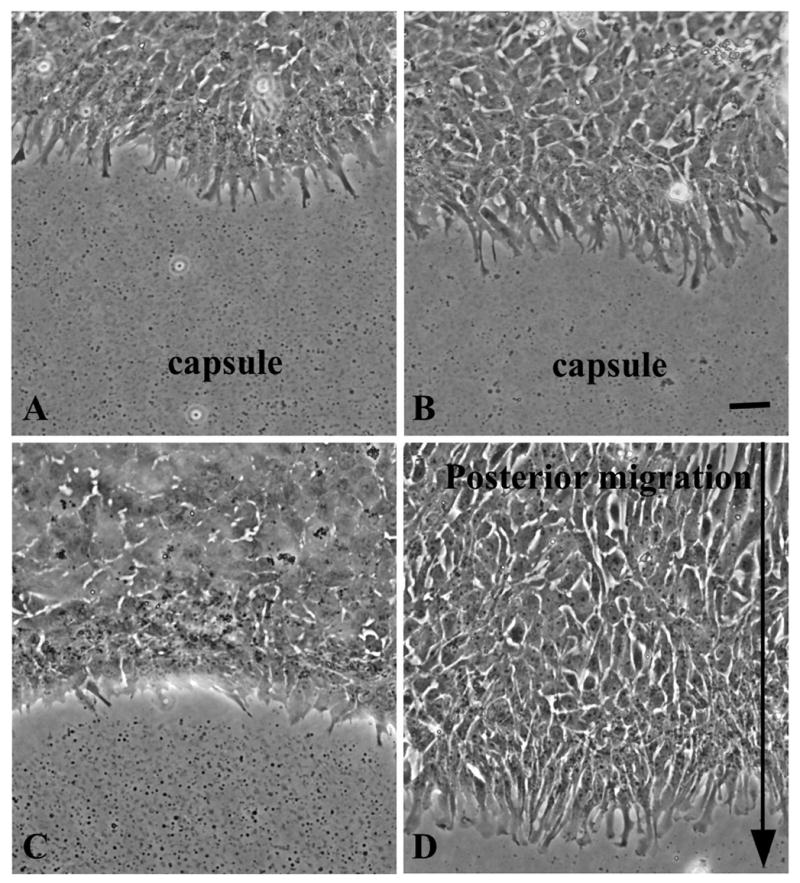
FGF-induced lens epithelial cell migration/elongation. Lens epithelial explants from rats at P10 were set up as shown in Figure 1 and cultured without (A, C, controls) or with 150 ng/ml FGF2 (B, D). After 1 day of culture, equatorial lens epithelial cells in both control and FGF-treated explants exhibited extensive cell processes, particularly in the cells at the leading edge (A, B). After 3 days prominent cell processes were still evident in FGF-treated explants (D). These were often aligned and pointing in the same direction i.e. towards the centre of the explant. The arrow shows direction of movement of the epithelial sheet as well as alignment of cell processes (D). Scale bar: 20 μm
F-actin and β-catenin
To assess the influence of FGF on microfilament dynamics, explants were probed for F-actin. β-catenin was also localized as it plays a key role in linking the actin cytoskeleton with cell adhesion complexes at the cell membrane (18). In FGF-treated explants, F-actin and β-catenin were localized throughout the cells and were particularly abundant in the cell processes (Figure 6B, D). At the leading edge, these processes were clearly aligned as they extended along the posterior capsule and pointed towards the centre of the explant. At their ends the processes exhibited both filopodia-like and lamellipodia-like protrusions. In controls, although some cell processes with F-actin and β-catenin were evident, these were not as extensive or aligned as in FGF-treated explants (Figure 6A, C). Also in controls, cells back from the leading edge often showed that localization of F-actin and β-catenin was maintained at the cell margins and highlighted the cobblestone-like packing of cells in this region (Figure 6A, C, arrows). In other studies of freshly prepared lens epithelial whole mounts we have shown that β-catenin and E-cadherin are characteristically localised at the cell margins (data not shown).
Figure 6.
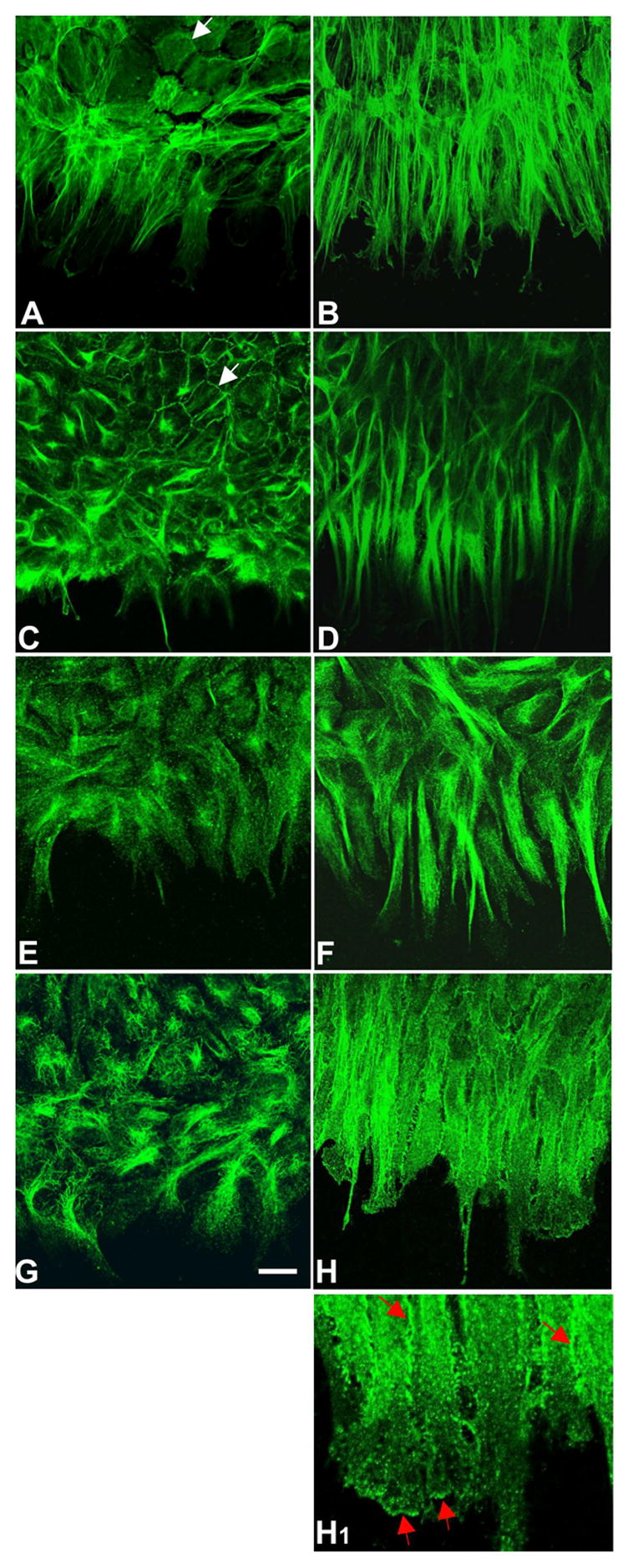
Immunolocalization of F-actin, β-catenin and α-tubulin. Lens epithelial explants were cultured without (A, C, E, G, controls) or with 150 ng/ml FGF2 (B, D, F, H, H1) for 3 days and immunolocalization carried out for F-actin (A, B), β-catenin (C, D), α-tubulin (E, F) and acetylated α-tubulin (G, H, H1). In FGF-treated explants, the F-actin, β-catenin and α-tubulin showed similar strong reactivity in the extensive cell processes at the leading edge (B, D, F). In controls, reactivity for F-actin, β-catenin and α-tubulin was evident but was not as prominent as in FGF-treated explants (A, C, E). Back from the leading edge, F-actin and β-catenin could be seen outlining the cobblestone-like packing of many cells (A, C, arrows). Stronger reactivity for acetylated α-tubulin at cell margins as well as at the ends of the cell processes, indicated that stabilized microtubules predominated in these regions (H, H1 red arrows). In contrast, in controls the acetylated α-tubulin was present in fine filamentous form and showed no clear directional orientation (G). Scale bar: (AH), 20μm; (H1), 8μm.
An antibody specific for the unphosphorylated (active) form of β-catenin showed similar, although weaker, cytoplasmic reactivity as for total β-catenin in FGF-treated and control explants. In addition, many nuclei, particularly in the elongated cells in FGF-treated explants, showed distinct reactivity for unphosphorylated β-catenin (data not shown). This is consistent with other reports that Wnt/β-catenin signaling has a role in FGF-induced fiber differentiation (5).
α-tubulin
To assess the influence of FGF on microtubule dynamics, explants were probed for α-tubulin. The acetylated form of α-tubulin was also localized as this has been reported to accumulate preferentially in stabilized microtubules (19). Reactivity for α-tubulin was prominent in FGF-treated explants. It was particularly strong in the cell processes and clearly showed their uniform alignment towards the explant centre (Figure 6F). Acetylated α-tubulin was similarly abundant in FGF-treated explants but appeared more evenly distributed throughout the full width of the cell processes. Stronger reactivity for acetylated α-tubulin at cell margins as well as in lamellipodia and filopodia of the cell processes indicated that stabilized microtubules predominated in these regions (Figure 6H, H1 red arrows). In contrast, in controls the α-tubulin was present in fine filamentous form and showed no clear directional orientation (Figure 6E). This was particularly evident after localization of acetylated α-tubulin (Figure 6G).
FGF-induced migration and elongation: expression/activation of small GTPases and JNK
RhoA
This small GTPase showed strong reactivity in the cells at the leading edge of the FGF-treated explants. The most notable feature was the presence of very strong patches of reactivity. These were distributed throughout the processes as well as being around the nuclei (Figure 7B, B1). Reactivity was also detected in some nuclei. In controls, RhoA reactivity was much weaker than in the FGF-treated cells and tended to be more diffuse (Figure 7A, A1). Occasionally a strong patch was present in a cellular process in controls but these were clearly not as prominent as in FGF-treated explants (Figure 7A, A1 and 7B, B1, arrows).
Figure 7.
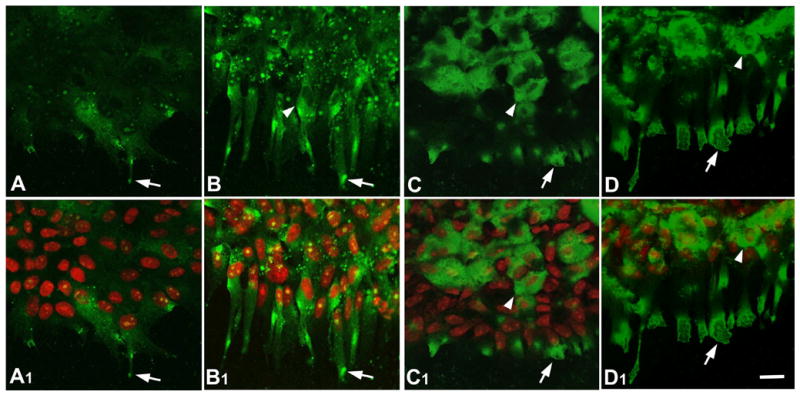
Localization of RhoA and Rac1. Lens epithelial explants were cultured without (A, A1, C, C1, controls) or with 150 ng/ml FGF2 (B, B1, D, D1) for 3 days and immunolocalization carried out for RhoA (A, A1, B, B1) and Rac1 (C, C1, D, D1). Propidium iodide (red) was used to counterstain nuclei (A1, B1, C1, D1). In controls, a few patches of RhoA (A, A1, arrow) and Rac1 (C, C1, arrow) reactivity were strongly present in cellular processes extending from the cell sheet. Some cells, back from the leading edge, also showed strong reactivity for Rac1 throughout their cytoplasm (C, C1, arrowhead). In FGF-treated explants, strong patches of RhoA reactivity were present in the cytoplasm of the abundant cell processes (arrow) as well as being around the nuclei (arrowhead) in B and B1. In FGF-treated explants, Rac1 was particularly strong at the ends of the abundant cell processes (D, D1, arrow). Back from the leading edge, Rac1 reactivity was also strong throughout the cytoplasm of many cells (D, D1, arrowhead). Scale bar: 20μm.
Rac1
Prominent reactivity for Rac1 was evident in cells at the leading edge of FGF-treated explants. This was most prominent around their nuclei and at the ends of their cell processes (Figure 7D, D1, arrows). Controls showed similar prominent Rac1 reactivity around nuclei and cell processes (Figure 7C, C1, arrows); however, in the cell processes this was not as strong or extensive as in FGF-treated explants.
Cdc42 and Pak
Fine particulate reactivity for Cdc42 was present throughout the cytoplasm of most cells in FGF-treated explants. However, reactivity was most distinct in aligned cell processes of the cells at the leading edge of the cell sheet (Figure 8B). In addition, there was a ‘hot spot’ for Cdc42 next to the nucleus and facing the direction of migration (Figure 8B, arrows). In controls, reactivity for Cdc42 was also evident, particularly around the cell nuclei. However, it was notable that there was no comparable nuclear ‘hot spot’ nor was the reactivity aligned in any particular direction as in FGF-treated explants (Figure 8A).
Figure 8.
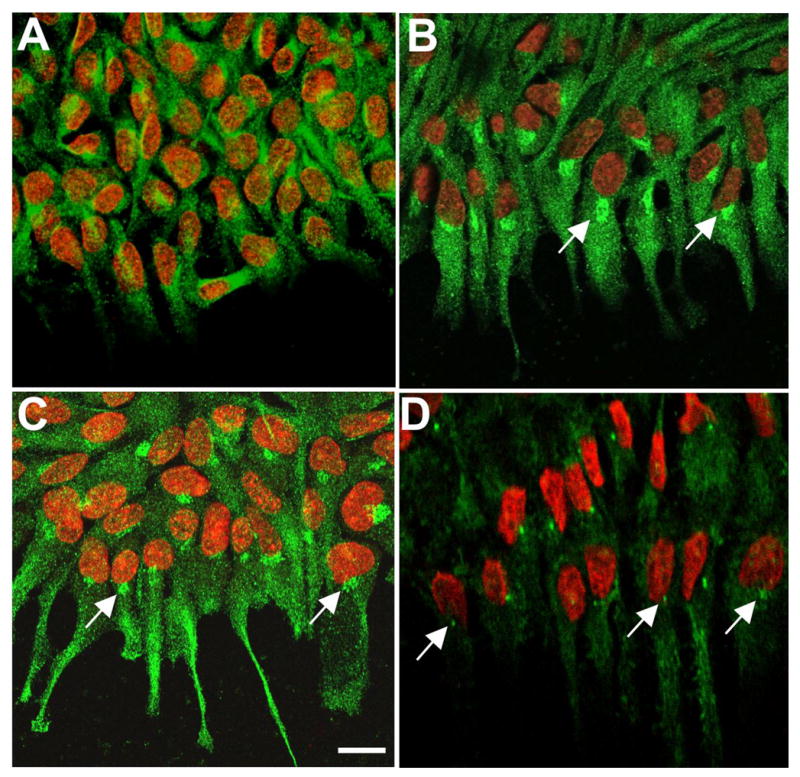
Localization of Cdc42, p-Pak and pericentrin. Lens epithelial explants were cultured without (A, controls) or with 150 ng/ml FGF2 (B, C, D) for 3 days and immunolocalization carried out for Cdc42 (A, B), p-Pak (C) and pericentrin (D). Propidium iodide (red) was used to counterstain nuclei (A, B, C, D). In controls, fine particulate Cdc42 reactivity was present in the cytoplasm of most cells (A). In FGF-treated explants, fine particulate Cdc42 reactivity was primarily in the aligned cell processes at the leading edge of the cell sheet (B). In most cells, the strongest Cdc42 reactivity was localized in a ‘hot spot’ next to the nucleus. In most cases this was facing the direction of migration (B, arrows). In FGF-treated explants, p-Pak reactivity was evident in the aligned cell processes and also very distinct in a ‘hot spot’ next to the nucleus at leading edge (C). In FGF-treated explants, a spot of pericentrin reactivity was also often present next to the nucleus and facing the direction of migration (D). Scale bar: 20μm.
Cdc42 is known to activate Pak at the leading edge of migrating cells with subsequent localization of Rac to this region (20). The availability of an antibody specific for activated (phosphorylated) Pak provided an opportunity to visualise the sites of Cdc42 activity. In FGF-treated explants phospho-Pak (p-Pak) reactivity was evident in the aligned cell processes and was very distinct in a ‘hot spot’ on the side of the nucleus that faced the cell’s leading edge (Figure 8C, arrows). Although there were some similarities between p-Pak and Cdc42 localisation, notably the presence of a juxta-nuclear ‘hot spot’, Cdc42 tended to be more uniformly distributed in the processes than p-Pak. Using an antibody to pericentrin, a key component of centrosomes, a juxta-nuclear spot of reactivity was also evident in many cells in FGF-treated explants. When groups of cells had aligned processes, the pericentrin-reactive spot was often on the side of the nucleus in line with the processes at the leading edge (Figure 8D, arrows). However, although similarly situated, the pericentrin-reactive spot was smaller that the ‘hot-spot’ of Cdc42/p-Pak reactivity. In control explants, pericentrin-reactive spots showed no alignment (not shown)
JNK
Particulate reactivity for p-JNK was evident throughout the cytoplasm and cell processes of cells at the leading edge of FGF-treated explants, indicating that the JNK signaling cascade was activated in these cells (Figure 9B, B1, arrows). Reactivity was also distinctly evident in the nuclei of many of these cells (Figure 9B, B1, arrowheads). In controls, particulate p-JNK reactivity was present in both cytoplasm and nuclei but was much weaker that in FGF-treated explants (Figure 9A, A1).
Figure 9.
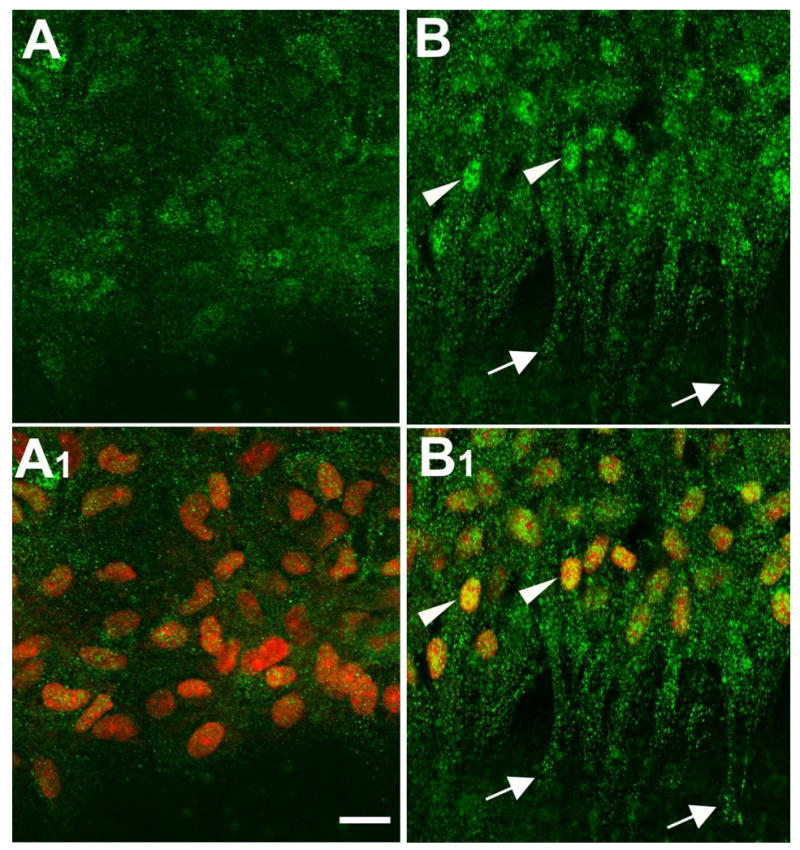
Localization of p-JNK. Lens epithelial explants were cultured without (A, A1, controls) or with 150 ng/ml FGF2 for 3 days (B, B1) and immunolocalization carried out for p-JNK. Propidium iodide (red) was used to counterstain nuclei (A1, B1). In controls, weak particulate p-JNK reactivity was detected in both cytoplasm and nuclei of cells at, and near, the leading edge (A, A1). In FGF-treated explants, particulate reactivity for p-JNK was more strongly detected throughout the cytoplasm of cells at the leading edge and was distinct in the nuclei of many of these cells (B, B1). Scale bar: 20μm.
N-cadherin and initiation of cell-cell adhesion in FGF-treated explants
During the transition of epithelial cells into fibers, N-cadherin replaces E-cadherin as the main cell-cell adhesion molecule. In the lens it is not known specifically how fiber cells become aligned and form adhesion complexes along their considerable length. However, it is known from studies on other systems that the small GTPases, Cdc42 and Rac can have an important role in enhancing the formation of cadherin-related adhesion junctions through reorganisation of the cytoskeleton (21). To examine this in FGF-treated explants we co-localized Cdc42 and N-cadherin. Cdc42 showed a similar pattern of localization as described earlier. Reactivity for Cdc42 clearly defined the cytoplasm of the cell processes at the leading edge (Figure 10A). N-cadherin reactivity was primarily present at the cell margins and appeared as spots or short broken lines that extended along the length of the cell processes (Figure 10B, C, arrows). They tended to be most distinct where two cells were in close association. This was reminiscent of the formation of short ‘spot- or line-like’ junctions that have been reported to be a critical step in the development of mature adhesion junctions (22).
Figure 10.
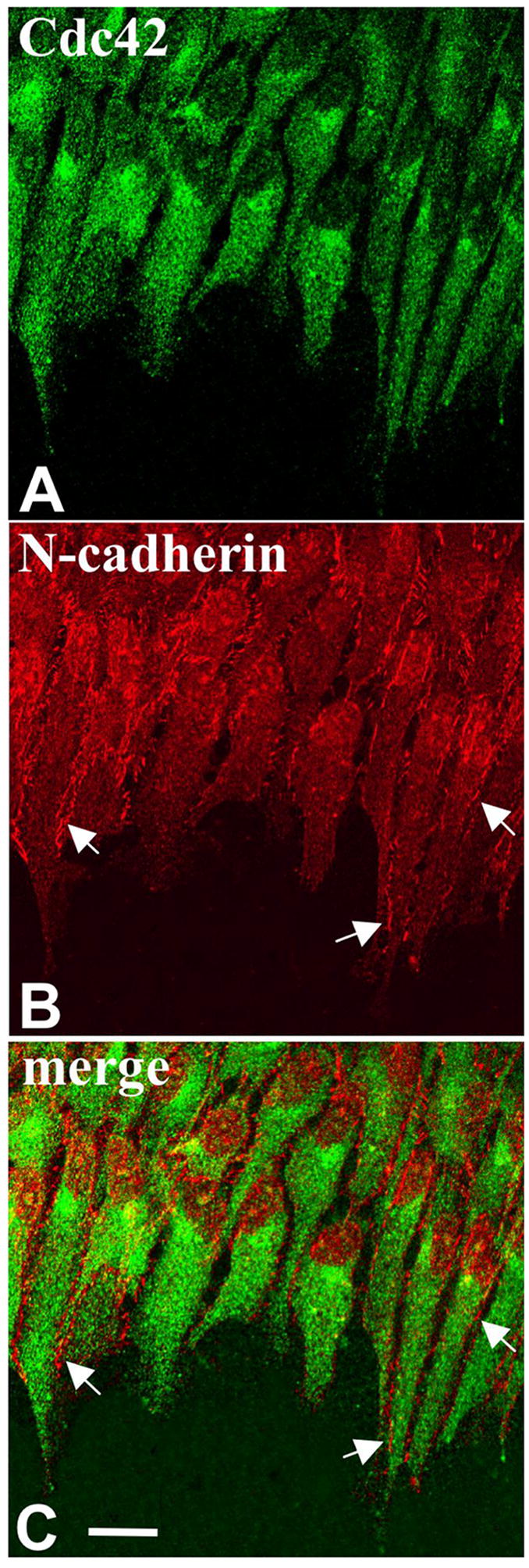
Co-localization of Cdc42 and N-cadherin. Lens epithelial explants were cultured with 150 ng/ml FGF2 (A, B, C) for 3 days and immunolocalization carried out for Cdc42 (A) and N-cadherin (B). Propidium iodide (red) was used to counterstain nuclei (C). Cdc42 shows a similar pattern as described in Figure 8. Note that as before the 'hot spot' was oriented on the side of the nucleus facing the direction as the processes (A). N-cadherin reactivity was primarily present at the cell margins and appeared as spots or short broken lines along the length of the cell processes (B, arrows). It was most distinct at the cell margin between adjacent cells. Cdc42 and N-cadherin images are merged in C. Scale bar: 20μm.
Fz, Dvl are also prominent in the FGF-induced processes
The key components of the Wnt/PCP signaling pathway, Fz and Dvl, were also shown to be present in the cells in FGF-treated explants. Both showed similar patterns of fine particulate reactivity throughout the cells and this clearly defined the aligned cell processes that extended from the cells at the leading edge of the cell sheet (Figure 11A, B).
Figure 11.
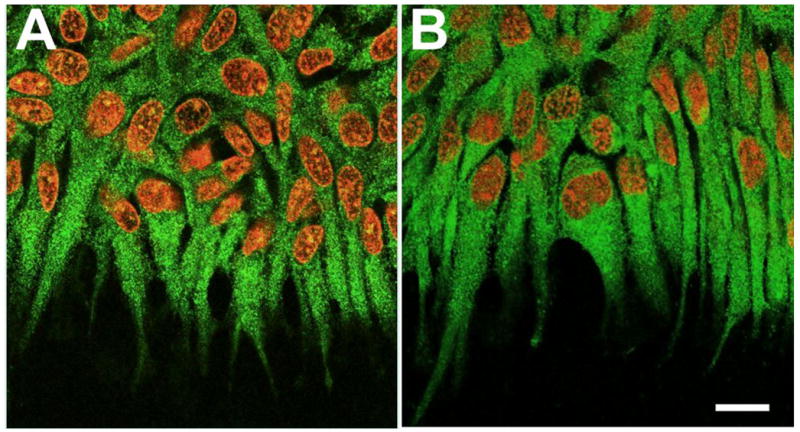
Localization of Fz and Dvl. Lens epithelial explants were cultured with 150 ng/ml FGF2 (A, B) for 3 days and immunolocalization carried out for Fz (A) and Dvl (B). The explants were counterstained with propidium iodide to show the nuclei (red). In FGF-treated explants, fine particulate Fz reactivity was detected throughout the cells. It was distinct in the aligned cell processes at the leading edge of the cell sheet (A). Dvl showed a similar pattern of localization with prominent reactivity in the aligned cell processes (B). Scale bar: 20μm.
Discussion
Localization of GTPases and JNK in the lens
This study investigated the expression of the small GTPases, RhoA, Rac1 and Cdc42, as well as other molecules that could potentially be involved in Wnt/PCP signaling in the lens. All the GTPases were shown to be present in both epithelial and fiber cells, although in some cases noticeably stronger expression was detected at the lens equator and during fiber cell differentiation and maturation. This was the case for Cdc42 and RhoA, the former being prominent at the basal ends of the equatorial lens cells and the latter being strongest throughout the equatorial/cortical fibers.
The detection of Cdc42 throughout the lens is consistent with its known role, in association with Rac and Rho, in microfilament assembly in general (10, 20), and in the lens in particular (13). In cultured human lens epithelial cells, activation of Rho and Rac by various growth factors results in the formation of actin stress fibers, membrane ruffles and focal adhesions, and these events can be inhibited by specific Rho GTPase inhibitors, such as C3-exozyme and the Rho kinase inhibitor Y27632. Similarly, transfection of cultured human lens cells with dominant negative forms of Cdc42 and RhoA results in inhibition of filipodia and stress fiber formation, respectively (12). Since microfilament regulation is an important function in both the epithelium and fibers, ubiquitous expression of Cdc42, as shown in the current study, would be expected. However, the stronger reactivity for Cdc42 that was detected at the basal pole of cells at the lens equator and in the elongating/migrating fibers may reflect another important function of Cdc42 that relates to assembly of polarised microtubules. In this role localized activation of Cdc42 is critical for the assembly of the polarised microtubule cytoskeleton and involves activation of a Par6/aPKC complex. This in turn causes inactivation of GSK3 and subsequent accumulation of APC on the plus ends of the microtubules specifically at the leading edge of the cell (10). These APC-labelled microtubules are then incorporated at the membrane so that they are aligned perpendicular to the direction of migration. Such a role for Cdc42 in the lens is consistent with its strong localization at the basal ends of the elongating fiber cells at the lens equator as this is where the plus ends of the microtubules are located (23).
The localization of RhoA in elongating and cortical fibers up to the point where they form the lens sutures is interesting given its known role in regulating cell migration and elongation. Similarly, a previous study of postnatal mice reported RhoB, being primarily localized to the outer cortical fibers (24). As fiber cells elongate they migrate along the lens capsule posteriorly, and along the apical surface of the lens epithelial sheet, anteriorly. This migration and concomitant cell elongation continues to where fibers from opposing parts of the lens meet and form sutures (25). As Rho is known to regulate formation of the actin-myosin filaments that generate contractile force in migrating cells as well as allowing the addition of actin monomers (26), its localisation pattern is consistent with such a role in promoting migration/elongation in the lens.
The strong baso-lateral localization of p-JNK is also consistent with this region being a major site of organization of cytoskeletal components as the JNK cascade is well known for roles in regulating cell migration and cytoskeleton rearrangement (27). Cdc42, which also has strong baso-lateral localization, has also been shown to activate the JNK cascade (28, 29, 30). Although many studies have focussed on the role of JNK in relation to actin organisation, important roles for JNK in regulating the dynamics and organisation of microtubules have also been identified (31, 32). In addition to the strong reactivity at the basal ends of the cells it was noted that p-JNK reactivity extended along the margins of cortical fibers (Figure 4A, B, small arrows). This localization may also be influenced by Cdc42 and Rac1 in these regions as cadherin-mediated cell-cell adhesions have been reported to enhance the activity of these Rho family members (33), and in turn, as noted above, this can activate JNK. Reactivity for p-JNK was also present in cell nuclei. This was present throughout the epithelium but clearly became stronger towards the equator and as the cells elongated into fibers. JNKs are well known for their ability to regulate transcription by phosphorylating a variety of nuclear substrates including c-Jun (34). Nuclear reactivity may relate to the reported ability of JNK to regulate proliferation in lens epithelial cells and, for example, Aquaporin 0 expression in lens fibers (35, 36).
FGF-treated explants: cytoskeletal rearrangements
The localization patterns shown by the small GTPases and p-JNK in the postnatal lens were generally consistent with what is known about their biological functions, particularly in relation to their roles in organisation and rearrangement of cytoskeletal components. The link between expression of these molecules and migration/fiber differentiation was explored further by studying FGF-treated lens epithelial explants.
The morphological and immunohistochemical analyses of epithelial cells in explant cultures clearly showed that FGF-promoted cell migration and the formation of extensive cell processes. These were often aligned and oriented in the direction of migration and this was invariably towards the centre of the explant. Controls also showed some cell processes but these were randomly orientated and extended in no particular direction. Localization of F-actin and α-tubulin showed that microfilaments and microtubules extended throughout the processes in FGF-treated explants and that they too were oriented in the same direction. Localization of these components also helped define filopodia-like and/or lamellipodia-like protrusions at the ends of the processes. At the cell margins, including the edges of lamellipodia, strong reactivity for acetylated α-tubulin was noted. As this form of α-tubulin accumulates in stable microtubules (19), this indicates that in the FGF-treated explants at the leading edges of migrating cells and at their margins where they are aligned with neighbouring cells, stable microtubules predominate. Other studies have shown that stable microtubules in migrating wound-edge fibroblasts were oriented towards the cell’s leading edge and it was proposed that these stable microtubules were involved in directing cellular organelles and other important cellular components to this region (37). Stable microtubules may have a similar role in the lens as organelles are known to accumulate at the migrating tips of young fiber cells and membrane proteins need to be directed to the cell margins (23).
FGF-treated explants: localization of GTPases and JNK
The small GTPases and JNK were all present in the migrating/elongating cells in FGF-treated explants and all exhibited distinct distribution patterns. Phospho-JNK was fairly evenly distributed throughout the cell cytoplasm and was also prominent in nuclei as was the case in the lens fibers in vivo. RhoA exhibited patchy distribution throughout the cells at the leading edge of explants. Rac1 tended to be strong around nuclei but consistently showed very strong reactivity at the leading edges of the cellular extensions. Cdc42 tended to be strongly reactive throughout the cell processes but in addition showed a reactive ‘hot spot’ just next to the nucleus and facing the direction of migration. Phospho-Pak, a Cdc42 effector molecule, showed a similar ‘hot spot’ next to the nucleus and was also strongly reactive in the cell processes though, unlike Cdc42, it tended to be stronger towards the leading edges of the cells. Such distribution patterns in lens explants are generally consistent with known roles of these small GTPases in cell migration. However, there were some interesting features of Cdc42 and Rac1 localization that deserve special comment. Other studies have shown that as cells migrate, Cdc42 activates Pak and this in turn has an important role in localising activated Rac to the front end of the cells where it regulates the formation of lamellipodia extensions. Cdc42 also has a role in the formation of filopodia extensions (10, 20). The localisation of Rac1 to the front end of the cell processes in FGF-treated explants is consistent with such a model. In addition, in some cell types at least, Cdc42 has a key role in controlling the polarity of both the microfilament and microtubule cytoskeletons. Cdc42 activity has been shown to be critical for realignment of the Golgi and centrosome to face the direction of migration (10, 20). In FGF-treated explants there was clear orientation of the Cdc42 (and p-Pak) ‘hot spot’ towards the direction of migration. In addition, pericentrin localisation showed that the centriole in aligned cells often faced the direction of migration. It will be important in future studies with FGF-treated lens explants to determine if this reflects a similar role, as in other cell types, in controlling the polarity of the cell cytoskeleton and subsequently directional cell migration (10, 20).
Taken together with the analysis of the F-actin and tubulin localization, these studies provide indications as to how lens cells might align with each other as they migrate and elongate into fiber cells. A current model for cell-cell adhesion involves the activation of Cdc42 by nectins and this results in the formation of filipodia and lamellipodia (22, 21). When cells meet through these protrusions, nectins recruit cadherins into micro-clusters known as ‘primordial spot-like junctions’ which can then fuse to form short ‘line-like junctions’ that develop into mature adhesion junctions (22). In this context it was interesting to see that in FGF-treated explants N-cadherin was localized in a ‘spot- and line-like’ manner between adjacent cells. Whilst the model described above has been developed from studies of nectin and E-cadherin interactions, it has been proposed that formation of N-cadherin mediated adhesions might also depend on a similar mechanism (22).
Thus the induction of changes in the cytoskeleton as visualised by localization of F-actin, α-tubulin and N-cadherin gives us some insights into the way the cells might form highly aligned fibers as they migrate posteriorly and anteriorly towards the lens sutures. In addition, the localization of key molecules involved in organization of the microfilaments and microtubules, indicates that they may have a role in organising the cytoskeleton during this process of directed migration and aligned elongation.
Are the GTPases and JNK activated by Wnt/Fz signaling?
Whilst it is well known that Rho, Rac, Cdc42 and JNK have important, and often specific, roles in organising the cytoskeleton, it is not always clear in any given situation if this is part of a Wnt/PCP signaling event. Roles for the small GTPases in convergent extension movements during vertebrate morphogenesis, one of the classical examples of non-canonical Wnt signaling and more specifically the PCP branch of this pathway, have been well documented. For example, over-expression of a dominant-negative form of Cdc42 in Xenopus embryos interferes with convergent extension in Xenopus (38). In other settings, activation of small GTPases and JNK, and downstream effects including involvement in organization of the cytoskeleton, appear to be elicited by a wide range of stimuli. For example, Rho can be activated by engagement with integrins, Rac can produce lamellipodia in response to PDGF and Cdc42 promotes filipodia formation in response to interleukin 1 (10). However, it is possible that in these different contexts, Fz activation may be required to mediate activation and/or localization of the small GTPases. Indeed, in the case of lens fiber cell differentiation in vitro, there is evidence that Wnt signaling, at least in relation to the β-catenin pathway, is downstream of an FGF trigger (5). A major question that now needs addressing, is whether Wnt/PCP signaling is also downstream of this FGF trigger and if this involves activation/localization of GTPases and JNK in the migrating/elongating cells at the lens equator. Unlike the canonical β-catenin pathway, which can be better identified by genetic and biochemical methods, the involvement of non-canonical Wnt-Fz pathways in any given situation is not so easily defined. In this study we show that key Wnt/PCP signalling molecules, Fz and Dvl, are present in the FGF-induced cell processes. However, in future studies on the lens, it will be important to test the link between Fz signaling and activation of the small GTPases and JNK. As there are a number of important Wnt-Fz regulatory molecules, one approach to address this question would be to manipulate expression of one of these regulators of Wnt-Fz signaling and to assess any downstream effects on the small GTPases and JNK.
Finally, it is also worth noting that other key PCP molecules including Fat, Four-jointed, Flamingo, Strabismus and Prickle, have all been detected in the rat lens by RT-PCR (Reinten, Stump, Lovicu and McAvoy, unpublished observations). In addition, Discs-large-1 and Scribble proteins have been localized in the mouse lens (39). In future studies on the mammalian lens a major challenge will be to determine if these PCP molecules have similar roles in asymmetric localization of Fz receptors and/or other key proteins involved in cell-cell and cell-matrix interactions, as has been elegantly shown in Drosophila eye and wing and other developmental systems (6, 40).
Footnotes
Supported by Sydney Foundation for Medical Research, NEI grant EY03177 (USA) and NHMRC (Australia).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Liu H, Mohamed O, Dufort D, Wallace VA. Characterization of Wnt signaling components and activation of the Wnt canonical pathway in the murine retina. Dev Dyn. 2003;227:323–34. doi: 10.1002/dvdy.10315. [DOI] [PubMed] [Google Scholar]
- 2.Stump RJ, Ang S, Chen Y, von Bahr T, Lovicu FJ, Pinson K, de Iongh RU, Yamaguchi TP, Sassoon DA, McAvoy JW. A role for Wnt/beta-catenin signaling in lens epithelial differentiation. Dev Biol. 2003;259:48–61. doi: 10.1016/s0012-1606(03)00179-9. [DOI] [PubMed] [Google Scholar]
- 3.Ang SJ, Stump RJ, Lovicu FJ, McAvoy JW. Spatial and temporal expression of Wnt and Dickkopf genes during murine lens development. Gene Expr Patterns. 2004;4:289–95. doi: 10.1016/j.modgep.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Stump RJ, Lovicu FJ, McAvoy JW. Expression of Frizzleds and secreted frizzled-related proteins (Sfrps) during mammalian lens development. Int J Dev Biol. 2004;48:867–77. doi: 10.1387/ijdb.041882yc. [DOI] [PubMed] [Google Scholar]
- 5.Lyu J, Joo CK. Wnt signaling enhances FGF2-triggered lens fiber cell differentiation. Development. 2004;131:1813–24. doi: 10.1242/dev.01060. [DOI] [PubMed] [Google Scholar]
- 6.Karner C, Wharton JKA, Carroll TJ. Planar cell polarity and vertebrate organogenesis. Seminars in Cell & Developmental Biology. 2006;17:194–203. doi: 10.1016/j.semcdb.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Habas R, Dawid IB, He X. Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes Dev. 2003;17:295–309. doi: 10.1101/gad.1022203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Habas R, He X. Activation of Rho and Rac by Wnt/frizzled signaling. Methods Enzymol. 2006;406:500–11. doi: 10.1016/S0076-6879(06)06038-1. [DOI] [PubMed] [Google Scholar]
- 9.Kohn AD, Moon RT. Wnt and calcium signaling: beta-catenin-independent pathways. Cell Calcium. 2005;38:439–46. doi: 10.1016/j.ceca.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 10.Hall A. Rho GTPases and the control of cell behaviour. Biochem Soc Trans. 2005;33:891–5. doi: 10.1042/BST20050891. [DOI] [PubMed] [Google Scholar]
- 11.Rao PV, Zigler JS, Jr, Garland D. Analysis of small GTP-binding proteins of the lens by GTP overlay assay reveals the presence of unique GTP-binding proteins associated with fiber cells. Exp Eye Res. 1997;64:219–27. doi: 10.1006/exer.1996.0197. [DOI] [PubMed] [Google Scholar]
- 12.Girao H, Pereira P, Ramalho J, Quinlan R, Prescott A. Cholesterol oxides mediated changes in cytoskeletal organisation involves Rho GTPases small star, filled. Exp Cell Res. 2003;291:502–13. doi: 10.1016/j.yexcr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Maddala R, Reddy VN, Epstein DL, Rao V. Growth factor induced activation of Rho and Rac GTPases and actin cytoskeletal reorganization in human lens epithelial cells. Mol Vis. 2003;9:329–36. [PubMed] [Google Scholar]
- 14.Rao V, Wawrousek E, Tamm ER, Zigler S., Jr Rho GTPase inactivation impairs lens growth and integrity. Lab Invest. 2002;82:231–9. doi: 10.1038/labinvest.3780415. [DOI] [PubMed] [Google Scholar]
- 15.Maddala R, Deng PF, Costello JM, Wawrousek EF, Zigler JS, Rao VP. Impaired cytoskeletal organization and membrane integrity in lens fibers of a Rho GTPase functional knockout transgenic mouse. Lab Invest. 2004;84:679–92. doi: 10.1038/labinvest.3700105. [DOI] [PubMed] [Google Scholar]
- 16.DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–68. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- 17.Lovicu FJ, McAvoy JW. Growth factor regulation of lens development. Dev Biol. 2005;280:1–14. doi: 10.1016/j.ydbio.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 18.Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–7. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palazzo A, Ackerman B, Gundersen GG. Cell biology: Tubulin acetylation and cell motility. Nature. 2003;421:230. doi: 10.1038/421230a. [DOI] [PubMed] [Google Scholar]
- 20.Cau J, Hall A. Cdc42 controls the polarity of the actin and microtubule cytoskeletons through two distinct signal transduction pathways. J Cell Sci. 2005;118:2579–87. doi: 10.1242/jcs.02385. [DOI] [PubMed] [Google Scholar]
- 21.Irie K, Shimizu K, Sakisaka T, Ikeda W, Takai Y. Roles and modes of action of nectins in cell-cell adhesion. Semin Cell Dev Biol. 2004;15:643–56. doi: 10.1016/j.semcdb.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Takai Y, Shimizu K, Ohtsuka T. The roles of cadherins and nectins in interneuronal synapse formation. Curr Opin Neurobiol. 2003;13:520–6. doi: 10.1016/j.conb.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 23.Lo WK, Wen XJ, Zhou CJ. Microtubule configuration and membranous vesicle transport in elongating fiber cells of the rat lens. Exp Eye Res. 2003;77:615–26. doi: 10.1016/s0014-4835(03)00176-3. [DOI] [PubMed] [Google Scholar]
- 24.Maddala R, Peng YW, Rao PV. Selective expression of the small GTPase RhoB in the early developing mouse lens. Dev Dyn. 2001;222:534–7. doi: 10.1002/dvdy.1202. [DOI] [PubMed] [Google Scholar]
- 25.Bassnett S, Missey H, Vucemilo I. Molecular architecture of the lens fiber cell basal membrane complex. J Cell Sci. 1999;112:2155–65. doi: 10.1242/jcs.112.13.2155. [DOI] [PubMed] [Google Scholar]
- 26.Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003;4:446–56. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- 27.Xia Y, Karin M. The control of cell motility and epithelial morphogenesis by Jun kinases. Trends Cell Biol. 2004;14:94–10128. doi: 10.1016/j.tcb.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 28.Coso OA, Chiariello M, Yu JC, Teramoto H, Crespo P, Xu N, Miki T, Gutkind JS. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell. 1995;81:1137–4629. doi: 10.1016/s0092-8674(05)80018-2. [DOI] [PubMed] [Google Scholar]
- 29.Honda T, Shimizu K, Kawakatsu T, Fukuhara A, Irie K, Nakamura T, Matsuda M, Takai Y. Cdc42 and Rac small G proteins activated by trans-interactions of nectins are involved in activation of c-Jun N-terminal kinase, but not in association of nectins and cadherin to form adherens junctions, in fibroblasts. Genes Cells. 2003;8:481–91. doi: 10.1046/j.1365-2443.2003.00649.x. [DOI] [PubMed] [Google Scholar]
- 30.Kam AY, Chan AS, Wong YH. Rac and Cdc42-dependent regulation of c-Jun N-terminal kinases by the delta-opioid receptor. J Neurochem. 2003;84:503–13. doi: 10.1046/j.1471-4159.2003.01535.x. [DOI] [PubMed] [Google Scholar]
- 31.Chang L, Jones Y, Ellisman MH, Goldstein LS, Karin M. JNK1 is required for maintenance of neuronal microtubules and controls phosphorylation of microtubule-associated proteins. Dev Cell. 2003;4:521–33. doi: 10.1016/s1534-5807(03)00094-7. [DOI] [PubMed] [Google Scholar]
- 32.Rosso SB, Sussman D, Wynshaw-Boris A, Salinas PC. Wnt signaling through Dishevelled, Rac and JNK regulates dendritic development. Nat Neurosci. 2005;8:34–42. doi: 10.1038/nn1374. [DOI] [PubMed] [Google Scholar]
- 33.Erasmus JC, Braga VM. Rho GTPase activation by cell-cell adhesion. Methods Enzymol. 2006;406:402–15. doi: 10.1016/S0076-6879(06)06029-0. [DOI] [PubMed] [Google Scholar]
- 34.Bogoyevitch MA, Boehm I, Oakley A, Ketterman AJ, Barr RK. Targeting the JNK MAPK cascade for inhibition: basic science and therapeutic potential. Biochim Biophys Acta. 2004;1697:89–101. doi: 10.1016/j.bbapap.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 35.Choi J, Park SY, Joo CK. Hepatocyte growth factor induces proliferation of lens epithelial cells through activation of ERK1/2 and JNK/SAPK. Invest Ophthalmol Vis Sci. 2004;45:2696–704. doi: 10.1167/iovs.03-1371. [DOI] [PubMed] [Google Scholar]
- 36.Golestaneh N, Fan J, Fariss RN, Lo WK, Zelenka PS, Chepelinsky AB. Lens major intrinsic protein (MIP)/aquaporin 0 expression in rat lens epithelia explants requires fibroblast growth factor-induced ERK and JNK signaling. J Biol Chem. 2004;279:31813–22. doi: 10.1074/jbc.M403473200. [DOI] [PubMed] [Google Scholar]
- 37.Palazzo AF, Joseph HL, Chen Y-J, Dujardin DL, Alberts AS, Pfister KK, Vallee RB, Gundersen GG. Cdc42, dynein, and dynactin regulate MTOC reorientation independent of Rho-regulated microtubule stabilization. Current Biology. 2001;11:1536–41. doi: 10.1016/s0960-9822(01)00475-4. [DOI] [PubMed] [Google Scholar]
- 38.Myers DC, Sepich DS, Solnica-Krezel L. Convergence and extension in vertebrate gastrulae: cell movements according to or in search of identity? Trends Genet. 2002;18:447–55. doi: 10.1016/s0168-9525(02)02725-7. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen MM, Rivera C, Griep AE. Localization of PDZ domain containing proteins Discs Large-1 and Scribble in the mouse eye. Mol Vis. 2005;11:1183–99. [PubMed] [Google Scholar]
- 40.Montcouquiol M, Sans N, Huss D, Kach J, Dickman JD, Forge A, Rachel RA, Copeland NG, Jenkins NA, Bogani D, Murdoch J, Warchol ME, Wenthold RJ, Kelley MW. Asymmetric localization of Vangl2 and Fz3 indicate novel mechanisms for planar cell polarity in mammals. J Neurosci. 2006;26:5265–75. doi: 10.1523/JNEUROSCI.4680-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]


