Abstract
Adolescence is a critical period for the initiation of drug use, starting with tobacco and alcohol and progressing to marijuana and other illicit drugs. These findings have led to the suggestion that tobacco and alcohol are ‘gateway’ drugs that sensitize maturing reward pathways to the effects of illicit substances such as cocaine. To test this hypothesis, we have examined whether low-dose nicotine pretreatment alters acquisition of cocaine self-administration in adolescents more than in adults. Male and female Sprague-Dawley rats, aged postnatal day (P) 28 or P86, were given two daily intravenous injections of nicotine (0.03 mg/kg/0.1 ml) or saline for four days. At P32 and P90, rats were placed in self-administration chambers and tested for acquisition of cocaine (0.2 or 0.5 mg/kg/inj) for five days. Data were collapsed across cocaine dose and sex since there was no significant effect of these variables. Adolescent rats pretreated with nicotine exhibited significantly greater cocaine-reinforced responding as compared to saline controls or adults (p < 0.01). This drug pretreatment effect did not generalize to all rewards, since nicotine did not increase responding for sucrose pellets in adolescents. These findings provide evidence that the adolescent brain is uniquely vulnerable to the effects of nicotine on subsequent drug reward.
Keywords: adolescence, cocaine, food reward, gateway theory, nicotine, self-administration
1. Introduction
Adolescence is a critical period for the onset of recreational drug use. Initiation of alcohol and tobacco use occurs during the early teens, and that of illicit substances shortly thereafter [17,28]. Epidemiological studies have characterized a progression of drug use from tobacco and alcohol to marijuana and other illicit drugs [28]. Those who initiate tobacco use in early adolescence show a pattern of heavier lifetime consumption and greater difficulty quitting than those who start as older adolescents or young adults [11,16]. Furthermore, individuals who smoke cigarettes before the age of 15 are estimated to be eighty times more likely to use illegal drugs than those who do not [11]. Such findings have led to the hypothesis that tobacco may serve as a ‘gateway’ to illegal drug use [29], although this concept has been disputed [35].
Animal studies have shown that early adolescence is a time of increased sensitivity to the rewarding and stimulant effects of nicotine [33]. When given a two bottle choice, early adolescent mice [postnatal day (P)23–45] were the only group to prefer nicotine solution over water [3]. In conditioned place preference (CPP) studies, periadolescent rats were shown to exhibit a highly significant preference for the nicotine-paired compartment whereas adult rats did not [6,47]. Rats of this age also quickly acquired self-administration for a nicotine/acetaldehyde mixture, a finding not seen in adults [7]. Recent studies have further shown that the negative effects of nicotine are experienced less in adolescence. Whereas adult rats display aversion for flavored solutions paired with nicotine, adolescent rats do not [49]. Somatic signs of nicotine withdrawal are also diminished in adolescent rats as compared to adults [38]. Thus, there is increasing evidence that the brief period of early adolescence is a developmental period in which rodents are particularly sensitive to the rewarding effects nicotine and are less sensitive to its aversive properties.
Animal studies have also shown that nicotine administration during adolescence causes unique long term changes [45], some of which may contribute to increased sensitivity to the effects of addictive drugs. Chronic nicotine treatment in adolescents, but not adults, produces persisting changes in nicotinic acetylcholine receptor (nAChR) subunit mRNA [4] and binding [1] in regions associated with reward. This differential age effect was also seen with AMPA glutamate receptors. Nicotine treatment during periadolescence in mice has been shown to result in downregulation of GluR2/3 subunits in the striatum, whereas nicotine treatment in adulthood had the opposite effect [2]. Chronic nicotine administration also causes increased dopamine transporter density and decreased in serotonin transporter binding in the striatum of periadolescent, but not adult, rats [20]. Taken together, these studies suggest that adolescent exposure to nicotine causes unique changes in brain reward circuitry.
Similar neural mechanisms, in particular activation of dopaminergic afferents to the nucleus accumbens (NAc), have been implicated in the addictive effects of nicotine and cocaine [24,30]. Furthermore, acetylcholine’s action on nAChRs regulates many aspects of adult dopaminergic activity, both at the midbrain cell bodies and in the striatum [22,50]. An emerging literature has indicated an important role of the cholinergic system in cocaine sensitization and reward. Cocaine self-administration induces an acute activation of cholinergic interneurons, which is highly correlated with behavioral performance [10], and a chronic increase in acetylcholine levels in the NAc shell [36]. Pharmacological and molecular studies have also shown that nAChR activation is essential for cocaine reward [34,51], and for the behavioral and neurochemical sensitization induced by repeated cocaine treatment [43,52].
Given the important role of nAChRs in the mechanisms of cocaine action, one might expect chronic nicotine treatment during adolescence to alter subsequent cocaine reward. There have been conflicting reports, however, of the consequences of adolescent nicotine exposure on the subsequent effects of cocaine. Whereas Kelley and Middaugh [31] reported that adolescent nicotine treatment reduced cocaine reward in CPP tests in adult mice, Collins and Izenwasser [19] found that brief periadolescent treatment with nicotine sensitized adolescent male rats to cocaine-induced locomotor activity. Furthermore, to date, there have been no reports on whether early adolescent nicotine treatment influences the sensitivity of adolescents to the rewarding effects of cocaine. In the present study, we have evaluated whether low-dose nicotine treatment alters subsequent acquisition of cocaine self-administration in adolescents but not adults. The treatment paradigm used was brief, intravenous administration of nicotine in order to model the influence of early smoking.
2. Methods
2.1. Animals
Male and female Sprague Dawley rats were obtained from Charles River at P17 or P74. Animals delivered at P17 were housed as cross-fostered litters of 10 pups until weaning (P21). Adolescent rats, after weaning, and adult rats were group housed in temperature (21°C) and humidity (50%) controlled rooms, on a 12-hour light-dark cycle (lights on at 0700) with food and water available ad libitum, except where noted. All animals were handled for 3 days prior to treatment to minimize stress effects. Only one animal per litter per experimental group was used. All tests were performed during the light part of the light-dark cycle. The animals used in this study were housed in an AAALAC-accredited vivarium maintained by UCI University Laboratory Animal Resources personnel. All experimental procedures were performed in compliance with NIH Guide for Care and Use of Laboratory Animals (NIH No 85-23, rev. 1985) and approved by the UCI Institutional Animal Care and Use Committee.
2.2. Catheter implant and surgical procedure
Prior to treatment, rats were surgically prepared with a chronic catheter implanted into the right external jugular vein, as described by Belluzzi et al. [7]. Animals were anesthetized with Equithesin (0.3 ml/100g) and a small area on both their back and lower neck was shaved. The catheter was passed subcutaneously from the animal’s back to the jugular vein where it was implanted. The cannula was flushed daily with sterile heparinized saline solution (0.6 ml of 1000 units/ml heparin in 30 ml saline) to maintain catheter patency. All animals were given 3 days to recover before beginning experiments.
2.3. Body weights and temporal food restriction
Experimental and control animals were weighed daily to assure that the developing rats maintained their normal growth curve. During cocaine self-administration, experimental animals were temporally food restricted. Each adolescent and adult rat received 15–20 g or 20–25 g, respectively, which is sufficient to maintain normal growth. Rats were fed 1 hr after each session and any remaining chow was removed 2 hr prior to the next test. This minor restriction in food availability provided motivation to explore the self-administration chamber.
2.4. Nicotine pretreatment
Two intravenous nicotine (0.03 mg/kg/0.1 ml) or saline injections, spaced one minute apart, were administered daily for 4 consecutive days during early adolescence (P28–31) or adulthood (P86–89). Dividing the daily 0.06 mg/kg drug dose into two injections was designed to reduce toxic effects and nicotinic receptor desensitization. The injection interval chosen is the standard time-out interval used in nicotine self-administration experiments [21].
2.5. Cocaine self-administration
Animals were placed into a self-administration chamber measuring 28 × 25 × 30 cm high equipped with two nose poke holes. The control of all experimental parameters and the collection of all data were done by Med Associates computer systems. Starting at P32 or P90, animals were tested for 5 days in daily 2-hour sessions to nose poke on a FR1 (fixed ratio 1) schedule to deliver a fixed i.v. dose of cocaine (200 or 500 μg/kg/injection). During each 1.1-sec infusion, a signal light above the reinforced hole briefly illuminated, after which the house light shut off for the 20-sec time out period. During this time the animal could not receive another reinforced response. To control for nonspecific activating effects of drugs, activity on a second (inactive) hole whose activation had no programmed consequences was recorded. A maximum of 100 infusions was allowed. At the end of the acquisition period, the implanted catheter was tested for patency with propofol, a rapid (5–10 sec) anesthetic. Data were discarded from any rat not demonstrating immediate anesthesia. The health of the animals was carefully monitored daily. Animals were temporally food restricted one day prior to, and throughout, the experiment to ensure exploration and facilitate self-administration. The animals were given enough food immediately after each session to ensure that their weight followed a normal growth curve.
2.6. Sucrose operant responding
In an apparatus similar to that used for self-administration, a separate group of animals was tested in daily 20-min sessions for responding for sucrose pellets. During each reinforced response, a signal light above the reinforced hole briefly illuminated and a 45 mg sucrose pellet dropped. To control for nonspecific activity, responses on a second (inactive) hole whose activation had no programmed consequences was recorded. Sucrose pellets not eaten at the end of the session were recorded. A maximum of 100 pellets were allowed. Animals were food restricted one day prior to, and throughout, the experiment. The animals were given enough food to maintain a normal growth curve.
2.7. Drugs
(−)-Nicotine di-(+)-tartrate was dissolved in saline and the pH adjusted to 7.2–7.4 with dilute NaOH. All nicotine doses were calculated as free base. Cocaine hydrochloride (NIDA) was dissolved in saline.
2.8. Statistical analysis
Data were analyzed initially for day 1 responding at 15 min time intervals across the two hour experimental period (Figure 1A). Data were analyzed by five-way ANOVA for Sex × Age × Pretreatment × Cocaine dose × Time, with repeated measures on Time. The significant main or interaction effects were further analyzed by one-way ANOVA with Dunnett’s-adjusted post hoc comparisons. Acquisition of self-administration across days was then analyzed for the first hour of testing since some rats reached the maximum allowed responses by this time point. For group differences in reinforced responding over days, daily 1-h self-injections for each group over the 5-day acquisition period were analyzed by five-way ANOVA for Sex × Age × Pretreatment × Cocaine dose × Day, with repeated measures on Day (Figure 1B). Since there was no significant effect of Cocaine dose or Sex, data were collapsed across these variables. The significant main or interaction effects were further analyzed by one-way ANOVA with Dunnett’s-adjusted post hoc comparisons. Criteria for self-administration were established as twelve reinforced responses over the two hour test session with a minimum 2:1 ratio of reinforced to non-reinforced responding [26]. The percentage of animals reaching criterion in each group on the first and fifth testing days were compared by χ2 analysis (Figure 1C). For total adolescent cocaine self-administration (Figure 2A), 5-day response totals were analyzed by three-way ANOVA for Sex × Pretreatment × Cocaine dose. Since there were no significant effects of Cocaine dose or Sex, data were collapsed across these variables. For sucrose operant responding (Figure 2B), 5-day response totals were analyzed by two-way ANOVA for Sex × Pretreatment, and no significant effect of Sex was found so data were collapsed across this variable. A one-way ANOVA for Pretreatment was run for both cocaine self-administration and sucrose operant responding. For comparisons of reinforced and non-reinforced responding, Bonferroni-adjusted post hoc t-tests were performed. Acquisition of sucrose operant responding over the 5-day acquisition period (Figure 2C) was analyzed by two-way ANOVA for Pretreatment × Day, with repeated measures on Day.
Figure 1. Effect of age and nicotine pretreatment on cocaine self-administration.
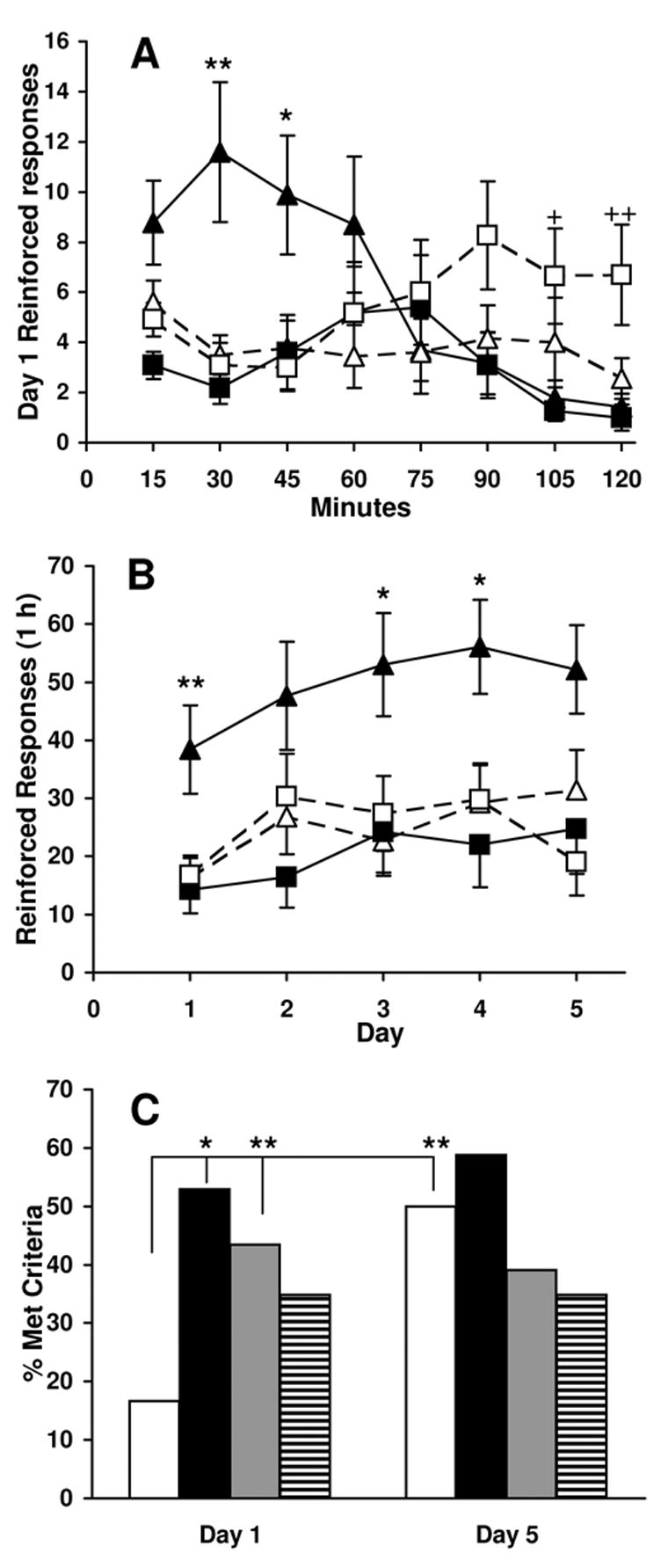
Adult and adolescent animals were given i.v. nicotine or saline daily for four days prior to cocaine self-administration tests. Figures show responses collapsed across sex and cocaine dose because there was no significant effect of these variables. (A) The mean (± SEM) day 1 reinforced responses for adult (square) and adolescent (triangle) rats pretreated with nicotine (closed) or saline (open) plotted in 15-min time bins for a 2-hr test session. Nicotine pretreated adolescent rats had greater reinforced responding than the other treatment groups at the beginning of the first cocaine self-administration session. Saline treated adult rats displayed increased responding in the last 30 minutes of the session. *p < 0.03, **p < 0.002 vs. other conditions; +p < 0.05, ++p < 0.02 vs. P32 nicotine. (B) The mean (± SEM) reinforced responses for adult (square) and adolescent (triangle) rats pretreated with nicotine (closed) or saline (open) in 1 hr are plotted daily. Nicotine-treated adolescent rats self-injected significantly more cocaine than the other groups. *p < 0.04, **p < 0.007 vs. other conditions. (C) The percentage of animals meeting criteria for acquisition in P32 saline (white), P32 nicotine (black), P90 saline (gray), and P90 nicotine (striped) groups on day 1 and day 5. Criteria were set as 12 reinforced responses over the 2 hr session with a minimum 2:1 ratio of reinforced to non-reinforced responding. Saline pretreated adolescent group had significantly fewer rats meet criteria on day 1 than nicotine treated adolescents and saline adult controls, and it was the only group to show a significant difference in acquisition on day 1 vs. day 5. *p < 0.02, **p < 0.01; adults, n = 23/group; adolescents n = 17–18/group.
Figure 2. Effect of nicotine pretreatment on adolescent responding for cocaine or sucrose.
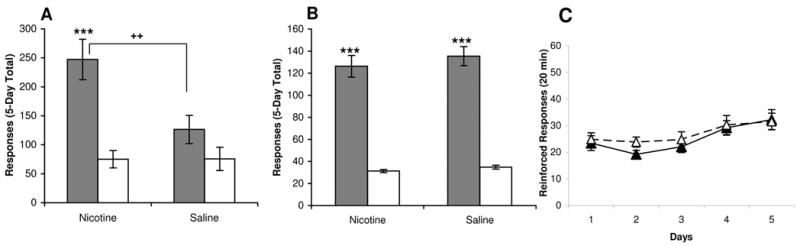
Figures show responses collapsed across sex and cocaine dose because there was no significant effect of these variables. (A) Nicotine pretreated adolescent rats had significantly higher 5-day total cocaine-reinforced responses (closed bars) than saline pretreated animals, ++ p < 0.01. In addition, nicotine treated animals showed significantly greater reinforced (closed) than nonreinforced (open) responding, *** p < 0.001; n = 17–18/group. (B) No effect of nicotine pretreatment was seen on adolescent animals responding for sucrose pellets in 20-min daily sessions. Both groups showed significantly higher reinforced (closed) responding than nonreinforced (open), *** p < 0.001; n = 19–20/group. (C) The mean (± SEM) reinforced responses for adolescent rats pretreated with nicotine (closed) or saline (open) in 20-min are plotted daily. There was no effect of nicotine pretreatment on daily reinforced responding for sucrose pellets, n = 19–20/ group.
3. Results
3.1. Acquisition of cocaine self-administration
Adolescent rats (P32) pretreated with nicotine quickly learned to self-administer cocaine compared to the other treatment groups (Figure 1A). Overall analysis of responding on day 1 showed a significant interaction of Age × Pretreatment [F(1,65) = 4.44, p < 0.05]. Since no significant effect of Sex [F(1,65) = 0.001, p = 0.97] or Cocaine dose [F(1,65) = 0.44, p = 0.51] was found, data were collapsed across these variables. Whereas reinforced responding in nicotine-pretreated adolescents was significantly elevated at 30 and 45 min as compared to other groups, this difference disappeared after the first hour of the test session. During the second hour of the initial test session, responding of saline-treated adults was significantly elevated as compared to other groups.
Since many nicotine-treated adolescent rats reached maximal responding within the first hour, 1-hr response totals were compared across all five test days (Figure 1B). Overall analysis yielded an effect of Age [F(1,65) = 7.64, p < 0.01], an Age × Pretreatment interaction [F(1,65) = 6.61, p = 0.01], and Day [F(4,260) = 4.83, p = 0.001]. Since no significant effect of Sex [F(1,65) = 1.72, p = 0.19] or Cocaine dose [F(1,65) = 0.87, p = 0.35] was found, data were collapsed across these variables. As shown in Figure 1b, increased responding of nicotine-pretreated adolescent rats, as compared to saline-treated adolescents and both adult groups, was still apparent, although not significant, on the final day of testing. This significantly increased responding of nicotine-pretreated adolescent rats as compared to other groups across the five test days was also seen when 2-hr response totals were analyzed (data not shown).
Criterion for acquisition of self-administration was defined as twelve reinforced responses over the two hour test period and a minimum 2:1 ratio of reinforced to non-reinforced responding. The percentage of animals in each group reaching this criterion on days 1 and 5 of testing is shown in Figure 1C. There was a significant difference in the percentage of saline- and nicotine-pretreated adolescents reaching criterion on day 1 (χ2 = 6.72, p<0.02); whereas 53% of nicotine-pretreated adolescents reached criterion, only 16% of saline-pretreated adolescents did. An age difference in saline-pretreated animals was also evident on day 1 of testing, with significantly more adults reaching criterion than adolescents (43 vs. 16%; χ2 = 6.90, p<0.01). In contrast to the other groups, there was significant acquisition of responding of saline-pretreated adolescents over the 5 day testing period (χ2 = 6.78, p<0.01). As a result, the differences in responding of saline-pretreated adolescents, as compared to nicotine-pretreated adolescents and saline-pretreated adults, had disappeared by day 5. There was no significant difference between adult pretreatment groups at either day 1 or day 5 of testing.
Temporal food restriction, nicotine pretreatment, and cocaine self-administration did not impede weight gain in adolescent rats, compared with age-matched, drug-naïve controls, or adult animals. Average body weights on the first day prior to nicotine pretreatment were 84.9 ± 1.5 and 89.0 ± 1.4 g for adolescent female and male rats, respectively, and final body weights on the last day of self-administration were 124.0 ± 2.1 and 138.5 ± 2.4 g. For matched controls, initial body weights were 84.3 ± 2.7 and 84.4 ± 1.8 for adolescent female and male groups, respectively, and final body weights were 124.5 ± 2.8 and 132.4 ± 2.5 g. Over the 9 day experimental period, there was a significant effect of Sex [F(1,77) = 9.16, p = 0.003] and Day [F(8,616) = 1424.09, p < 0.001], but no effect of Treatment [F(2,77) = 2.06, p = 0.13] or Sex × Treatment [F(2,77) = 0.92, p = 0.40] on weight gain. Average body weights on the first day prior to nicotine treatment were 240.2 ± 2.5 and 374.5 ± 5.8 g for adult female and male rats, respectively, and final body weights on the last day of self-administration were 248.9 ± 3.1 and 378.7 ± 5.5 g. Over the 9 day experimental period, there was a significant effect of Sex [F(1,46) = 280.83, p < 0.001] and Day [F(8,368) = 7.32, p = 0.001], but no effect of Treatment [F(1,46) = 0.16, p = 0.70] or Sex × Treatment [F(1,46) = 0.23, p = 0.63] on weight gain.
3.2. Comparison of cocaine and sucrose reward in adolescents
As shown in Figure 2, the enhancing effect of adolescent nicotine pretreatment on cocaine reward in adolescents does not generalize to responding for sucrose, a natural reinforcer. Figure 2A illustrates the effect of adolescent nicotine pretreatment, as compared to saline, on 5-day total cocaine reinforced and nonreinforced responses. Adolescent rats pretreated with nicotine showed significantly greater reinforced responses for cocaine than saline treated controls (p < 0.01). In addition, nicotine pretreated animals discriminated between the reward and nonreward nosepoke (p < 0.001), whereas saline pretreated adolescent animals did not (p = 0.12).
In order to determine whether enhanced responding in nicotine pretreated animals was specific to drug reward, male and female adolescent rats were tested for operant responding for sucrose pellets, a natural reward (Figure 2B). Analysis of five day total responding showed a significant effect of Reinforcement [F(1,35) = 230.9, p < 0.001], and no effect of Pretreatment [F(1,35) = 1.02, p = 0.32] or Sex [F(1,35) = 1.21, p = 0.28]. As shown in Figure 2B, both saline- and nicotine-pretreated adolescents acquired responding on this operant task and received equivalent rewards. Daily reinforced responding for sucrose, shown in Figure 2C, did not differ between the two treatment groups. Thus, low dose nicotine pretreatment in adolescent rats does not affect initial acquisition of sucrose operant responding.
4. Discussion
Epidemiological studies evaluating the ‘gateway’ theory in humans have been unable to control for environmental, genetic, and other complex factors that confound the analysis. Animal studies allow for a more controlled experiment and can identify the underlying mechanism for the progression of drug use. A number of recent findings in animals suggest that the adolescent brain is uniquely sensitive to the acute and chronic effects of nicotine [33,45]. We now show that exposure to nicotine during early adolescence enhances the reinforcing effects of cocaine. Intravenous administration of a low dose of nicotine for four days during early adolescence, but not adulthood, increased subsequent initial acquisition of cocaine self-administration. This enhancement of cocaine reward by prior nicotine exposure was evident in both males and females. However, the enhancing effect of nicotine pretreatment did not generalize to all operant conditioning, since responding for sucrose was unchanged.
In this study, we did not find an effect of cocaine dose on the acquisition of self-administration. Other studies with naive acquisition in both adolescents and adults show a similar lack of dose effect in this early phase [26,27]. However, a cocaine dose-effect in responding is not necessary to show enhanced sensitivity, especially since both doses used are considered to be low. In the literature, adult rats pretreated with psychostimulants have been shown to acquire cocaine self-administration more rapidly [27,42] and have higher rates of responding [40] than rats pretreated with saline. This decrease in latency to acquire self-administration has been interpreted to indicate that cocaine pre-exposure sensitizes the rats to the reinforcing effects of cocaine [41]. In our study, nicotine- pretreated adolescent animals showed a similar pattern of enhanced responding. This finding suggests that brief treatment with low doses of nicotine in early adolescence, but not adulthood, may cross-sensitize the brain to cocaine reward.
4.1 Methodological considerations
We have used a brief exposure period and a low dose of nicotine, given by intravenous injection, in order to better model early teenage smoking than the modes of chronic administration used in previous studies [1,3,19]. Intravenous administration of nicotine is less stressful than subcutaneous or intraperitoneal injections, gives equivalent amounts of drug as the animal matures, and more closely mimics the pharmacokinetics of smoking. The daily 60 μg/kg dose of nicotine used in this study is comparable to the amount of nicotine inhaled in 2–4 cigarettes [9]. Among smokers aged 12 to 17, the average number of cigarettes smoked per day is 5.2 [44].
Acquisition of cocaine reinforcement was measured using stringent conditions for self-administration. Rats did not receive prior response training and were not stressed by isolation housing. Although group housed animals were temporally food restricted in order to promote exploration, all animals maintained their normal growth curve compared to non-restricted controls. The observed nicotine-induced changes in initial acquisition of cocaine self-administration do not reflect a generalized change in appetitive responding, since no corresponding changes in food reinforced responding were observed. Although this study examined only the initial acquisition of cocaine self-administration, further experiments which test higher schedules of reinforcement may uncover additional changes in motivational drive.
4.2 Neural mechanisms
Both human and animal studies have shown adolescence to be a period of rapid flux in brain growth and maturation [39]. Corticolimbic systems and their monoamine projections, which comprise the motivational circuitry implicated in addiction, have not yet fully developed by adolescence [15]. In particular, the structural and neurochemical maturation of the prefrontal cortex is not complete [8,46]. Projections from the prefrontal cortex regulate activity of the nucleus accumbens and its dopaminergic afferents [13,14], which mediate the pleasurable effects of drugs of abuse. Exposure to nicotine during adolescence may alter the maturation of this system and result in a change in reward threshold, increasing the vulnerability for drug dependence. Such a mechanism would be consistent with our earlier findings [13,14], in which we have shown sensory cortex to be uniquely sensitive to the effects of nicotine during an earlier critical developmental period.
Alternatively, nicotine may act directly upon developing dopamine systems which continue to mature throughout adolescence [5]. Nicotinic receptors play a critical role in regulating the activity of dopaminergic afferents to striatum and modulating goal-directed behavior [22]. Adolescent treatment with nicotine during a sensitive period of striatal maturation may produce long-term changes in this circuitry. Support for this concept has been provided by recent studies showing long-term changes in striatal dopamine transporters and AMPA receptors following chronic adolescent nicotine exposure [2,20]. Further neurochemical studies will be required to show whether our brief intravenous nicotine exposure paradigm produces similar changes in striatum.
4.3 Clinical implications
Several epidemiological studies concur that adolescents are more susceptible than adults to nicotine dependence. A recent study showed that students who have tried a single cigarette by age 11 remain vulnerable to future smoking up to three years later [25]. Adolescents progress to nicotine dependence faster than adults, and this progression can occur even before daily smoking habits occur [16,18]. Together, these findings suggest that adolescents are vulnerable to persisting changes from even a brief exposure to nicotine. Recent animal studies have provided significant evidence in support of this conclusion [33].
In contrast to tobacco use, the risk of initiating cocaine use and becoming dependent is higher for young adults than for adolescents [37,48]. Such epidemiological findings suggest that adolescents may be less sensitive to the rewarding effects of cocaine than adults. Although recent animal studies have not supported this conclusion [12,26], these studies have involved the administration of cocaine over a period of several days. In our self-administration paradigm, in which nose-pokes are used instead of levers, a significant proportion of animals acquire self-administration on the first day of testing. We have shown an initial age difference in acquisition of cocaine self-administration in saline-pretreated controls, with a much smaller percentage of adolescents achieving criterion for responding than adults. Over the five day testing period, the younger animals learned to self-administer cocaine and achieved similar levels of responding as adults.
Nicotine pretreatment greatly enhanced initial acquisition of cocaine self-administration in adolescents, such that adolescent responding was greater than that of adults. Although this enhancement of responding was most apparent on the first day of testing, it was still evident on day 5. This nicotine enhancement of the initial rewarding effects of cocaine, if found in humans, could have profound effects on the trajectory of subsequent drug use. Clinical studies have shown that adolescents who display an increased ‘liking’ or ‘wanting’ during their first experience with cocaine are more likely to continue use of the drug [23,32]. Furthermore, those adolescents who had previously smoked were found to have higher initial ‘liking’ and ‘wanting’ scores and were more likely to become cocaine-dependent than non-smokers [32]. Our findings are consistent with these clinical studies, and provide support for the hypothesis that nicotine sensitizes central reward pathways in adolescence to the effects of illicit drugs such as cocaine.
Acknowledgments
This work was supported by PHS grant DA 19138 and a graduate fellowship, DA 07318 (S.C.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abreu-Villaca Y, Seidler FJ, Slotkin TA. Impact of adolescent nicotine exposure on adenylyl cyclase-mediated cell signaling: enzyme induction, neurotransmitter-specific effects, regional selectivities, and the role of withdrawal. Brain Res. 2003;988:164–72. doi: 10.1016/s0006-8993(03)03368-7. [DOI] [PubMed] [Google Scholar]
- 2.Adriani W, Granstrem O, Macri S, Izykenova G, Dambinova S, Laviola G. Behavioral and neurochemical vulnerability during adolescence in mice: studies with nicotine. Neuropsychopharmacology. 2004;29:869–78. doi: 10.1038/sj.npp.1300366. [DOI] [PubMed] [Google Scholar]
- 3.Adriani W, Macri S, Pacifici R, Laviola G. Peculiar vulnerability to nicotine oral self-administration in mice during early adolescence. Neuropsychopharmacology. 2002;27:212–24. doi: 10.1016/S0893-133X(02)00295-6. [DOI] [PubMed] [Google Scholar]
- 4.Adriani W, Spijker S, Deroche-Gamonet V, Laviola G, Le Moal M, Smit AB, Piazza PV. Evidence for enhanced neurobehavioral vulnerability to nicotine during periadolescence in rats. J Neurosci. 2003;23:4712–6. doi: 10.1523/JNEUROSCI.23-11-04712.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- 6.Belluzzi JD, Lee AG, Oliff HS, Leslie FM. Age-dependent effects of nicotine on locomotor activity and conditioned place preference in rats. Psychopharmacology (Berl) 2004;174:389–95. doi: 10.1007/s00213-003-1758-6. [DOI] [PubMed] [Google Scholar]
- 7.Belluzzi JD, Wang R, Leslie FM. Acetaldehyde enhances acquisition of nicotine self-administration in adolescent rats. Neuropsychopharmacology. 2005;30:705–12. doi: 10.1038/sj.npp.1300586. [DOI] [PubMed] [Google Scholar]
- 8.Benes FM, Taylor JB, Cunningham MC. Convergence and plasticity of monoaminergic systems in the medial prefrontal cortex during the postnatal period: implications for the development of psychopathology. Cereb Cortex. 2000;10:1014–27. doi: 10.1093/cercor/10.10.1014. [DOI] [PubMed] [Google Scholar]
- 9.Benowitz NLPH, Jacob P., III . Pharmacokinetics, metabolism, and pharmacodynamics of nicotine. In: Wonnacott RMS, Stolerman IP, editors. Nicotine Pharmacology: Molecular, Celllular, and Behavioral Aspects. Oxford University Press; Oxford: 1990. pp. 112–151. [Google Scholar]
- 10.Berlanga ML, Olsen CM, Chen V, Ikegami A, Herring BE, Duvauchelle CL, Alcantara AA. Cholinergic interneurons of the nucleus accumbens and dorsal striatum are activated by the self-administration of cocaine. Neuroscience. 2003;120:1149–56. doi: 10.1016/s0306-4522(03)00378-6. [DOI] [PubMed] [Google Scholar]
- 11.Breslau N, Peterson EL. Smoking cessation in young adults: age at initiation of cigarette smoking and other suspected influences. Am J Public Health. 1996;86:214–20. doi: 10.2105/ajph.86.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campbell JO, Wood RD, Spear LP. Cocaine and morphine-induced place conditioning in adolescent and adult rats. Physiol Behav. 2000;68:487–93. doi: 10.1016/s0031-9384(99)00225-5. [DOI] [PubMed] [Google Scholar]
- 13.Carr DB, Sesack SR. Terminals from the rat prefrontal cortex synapse on mesoaccumbens VTA neurons. Ann N Y Acad Sci. 1999;877:676–8. doi: 10.1111/j.1749-6632.1999.tb09299.x. [DOI] [PubMed] [Google Scholar]
- 14.Carr DB, Sesack SR. Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J Neurosci. 2000;20:3864–73. doi: 10.1523/JNEUROSCI.20-10-03864.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160:1041–52. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen J, Millar WJ. Age of smoking initiation: implications for quitting. Health Rep. 1998;9:39–46. 39–48. (Eng) (Fre) [PubMed] [Google Scholar]
- 17.Chen K, Kandel DB. The natural history of drug use from adolescence to the mid-thirties in a general population sample. Am J Public Health. 1995;85:41–7. doi: 10.2105/ajph.85.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colby SM, Tiffany ST, Shiffman S, Niaura RS. Are adolescent smokers dependent on nicotine? A review of the evidence. Drug Alcohol Depend. 2000;59(Suppl 1):S83–95. doi: 10.1016/s0376-8716(99)00166-0. [DOI] [PubMed] [Google Scholar]
- 19.Collins SL, Izenwasser S. Chronic nicotine differentially alters cocaine-induced locomotor activity in adolescent vs. adult male and female rats. Neuropharmacology. 2004;46:349–62. doi: 10.1016/j.neuropharm.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 20.Collins SL, Wade D, Ledon J, Izenwasser S. Neurochemical alterations produced by daily nicotine exposure in periadolescent vs. adult male rats. Eur J Pharmacol. 2004;502:75–85. doi: 10.1016/j.ejphar.2004.08.039. [DOI] [PubMed] [Google Scholar]
- 21.Corrigall WA, Coen KM. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology (Berl) 1989;99:473–8. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- 22.Cragg SJ. Meaningful silences: how dopamine listens to the ACh pause. Trends Neurosci. 2006;29:125–31. doi: 10.1016/j.tins.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Davidson ES, Finch JF, Schenk S. Variability in subjective responses to cocaine: initial experiences of college students. Addict Behav. 1993;18:445–53. doi: 10.1016/0306-4603(93)90062-e. [DOI] [PubMed] [Google Scholar]
- 24.Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, Acquas E, Carboni E, Valentini V, Lecca D. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology. 2004;47(Suppl 1):227–41. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 25.Fidler JA, Wardle J, Brodersen NH, Jarvis MJ, West R. Vulnerability to smoking after trying a single cigarette can lie dormant for three years or more. Tob Control. 2006;15:205–9. doi: 10.1136/tc.2005.014894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frantz KJ, O’Dell LE, Parsons LH. Behavioral and Neurochemical Responses to Cocaine in Periadolescent and Adult Rats. Neuropsychopharmacology. 2006 doi: 10.1038/sj.npp.1301130. [DOI] [PubMed] [Google Scholar]
- 27.Horger BA, Shelton K, Schenk S. Preexposure sensitizes rats to the rewarding effects of cocaine. Pharmacol Biochem Behav. 1990;37:707–11. doi: 10.1016/0091-3057(90)90552-s. [DOI] [PubMed] [Google Scholar]
- 28.Kandel DB, Logan JA. Patterns of drug use from adolescence to young adulthood: I. Periods of risk for initiation, continued use, and discontinuation. Am J Public Health. 1984;74:660–6. doi: 10.2105/ajph.74.7.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kandel DB, Yamaguchi K, Chen K. Stages of progression in drug involvement from adolescence to adulthood: further evidence for the gateway theory. J Stud Alcohol. 1992;53:447–57. doi: 10.15288/jsa.1992.53.447. [DOI] [PubMed] [Google Scholar]
- 30.Kelley AE. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004;44:161–79. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 31.Kelley BM, Middaugh LD. Periadolescent nicotine exposure reduces cocaine reward in adult mice. J Addict Dis. 1999;18:27–39. doi: 10.1300/J069v18n03_04. [DOI] [PubMed] [Google Scholar]
- 32.Lambert NM, McLeod M, Schenk S. Subjective responses to initial experience with cocaine: an exploration of the incentive-sensitization theory of drug abuse. Addiction. 2006;101:713–25. doi: 10.1111/j.1360-0443.2006.01408.x. [DOI] [PubMed] [Google Scholar]
- 33.Leslie FM, Loughlin SE, Wang R, Perez L, Lotfipour S, Belluzzia JD. Adolescent development of forebrain stimulant responsiveness: insights from animal studies. Ann N Y Acad Sci. 2004;1021:148–59. doi: 10.1196/annals.1308.018. [DOI] [PubMed] [Google Scholar]
- 34.Levin ED, Mead T, Rezvani AH, Rose JE, Gallivan C, Gross R. The nicotinic antagonist mecamylamine preferentially inhibits cocaine vs. food self-administration in rats. Physiol Behav. 2000;71:565–70. doi: 10.1016/s0031-9384(00)00382-6. [DOI] [PubMed] [Google Scholar]
- 35.Mackesy-Amiti ME, Fendrich M, Goldstein PJ. Sequence of drug use among serious drug users: typical vs atypical progression. Drug Alcohol Depend. 1997;45:185–96. doi: 10.1016/s0376-8716(97)00032-x. [DOI] [PubMed] [Google Scholar]
- 36.Mark GP, Hajnal A, Kinney AE, Keys AS. Self-administration of cocaine increases the release of acetylcholine to a greater extent than response-independent cocaine in the nucleus accumbens of rats. Psychopharmacology (Berl) 1999;143:47–53. doi: 10.1007/s002130050918. [DOI] [PubMed] [Google Scholar]
- 37.O’Brien MS, Anthony JC. Risk of becoming cocaine dependent: epidemiological estimates for the United States, 2000–2001. Neuropsychopharmacology. 2005;30:1006–18. doi: 10.1038/sj.npp.1300681. [DOI] [PubMed] [Google Scholar]
- 38.O’Dell LE, Bruijnzeel AW, Smith RT, Parsons LH, Merves ML, Goldberger BA, Richardson HN, Koob GF, Markou A. Diminished nicotine withdrawal in adolescent rats: implications for vulnerability to addiction. Psychopharmacology (Berl) 2006 doi: 10.1007/s00213-006-0383-6. [DOI] [PubMed] [Google Scholar]
- 39.Pechmann C, Loughlin LLS, Leslie F. Self-Conscious and Impulsive: Adolescents’ Vulnerability to Advertising and Promotion. J Public Policy & Marketing. 2005;24:202–221. [Google Scholar]
- 40.Schenk S, Izenwasser S. Pretreatment with methylphenidate sensitizes rats to the reinforcing effects of cocaine. Pharmacol Biochem Behav. 2002;72:651–7. doi: 10.1016/s0091-3057(02)00735-9. [DOI] [PubMed] [Google Scholar]
- 41.Schenk S, Partridge B. Sensitization and tolerance in psychostimulant self-administration. Pharmacol Biochem Behav. 1997;57:543–50. doi: 10.1016/s0091-3057(96)00447-9. [DOI] [PubMed] [Google Scholar]
- 42.Schenk S, Partridge B. Sensitization to cocaine’s reinforcing effects produced by various cocaine pretreatment regimens in rats. Pharmacol Biochem Behav. 2000;66:765–70. doi: 10.1016/s0091-3057(00)00273-2. [DOI] [PubMed] [Google Scholar]
- 43.Schoffelmeer AN, De Vries TJ, Wardeh G, van de Ven HW, Vanderschuren LJ. Psychostimulant-induced behavioral sensitization depends on nicotinic receptor activation. J Neurosci. 2002;22:3269–76. doi: 10.1523/JNEUROSCI.22-08-03269.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Services, S.A.a.M.H. and Administration. Overview of Findings from the 2002 National Survey on Drug Use and Health. Office of Applied Studies, NHSDA Series H-21; Rockville, MD: 2003. [Google Scholar]
- 45.Slotkin TA, Tate CA, Cousins MM, Seidler FJ. Functional alterations in CNS catecholamine systems in adolescence and adulthood after neonatal chlorpyrifos exposure. Brain Res Dev Brain Res. 2002;133:163–73. doi: 10.1016/s0165-3806(02)00284-5. [DOI] [PubMed] [Google Scholar]
- 46.Tseng KY, O’Donnell P. Post-pubertal emergence of prefrontal cortical up states induced by D1-NMDA co-activation. Cereb Cortex. 2005;15:49–57. doi: 10.1093/cercor/bhh107. [DOI] [PubMed] [Google Scholar]
- 47.Vastola BJ, Douglas LA, Varlinskaya EI, Spear LP. Nicotine-induced conditioned place preference in adolescent and adult rats. Physiol Behav. 2002;77:107–14. doi: 10.1016/s0031-9384(02)00818-1. [DOI] [PubMed] [Google Scholar]
- 48.Wagner FA, Anthony JC. From first drug use to drug dependence; developmental periods of risk for dependence upon marijuana, cocaine, and alcohol. Neuropsychopharmacology. 2002;26:479–88. doi: 10.1016/S0893-133X(01)00367-0. [DOI] [PubMed] [Google Scholar]
- 49.Wilmouth CE, Spear LP. Adolescent and adult rats’ aversion to flavors previously paired with nicotine. Ann N Y Acad Sci. 2004;1021:462–4. doi: 10.1196/annals.1308.065. [DOI] [PubMed] [Google Scholar]
- 50.Wonnacott S, Sidhpura N, Balfour DJ. Nicotine: from molecular mechanisms to behaviour. Curr Opin Pharmacol. 2005;5:53–9. doi: 10.1016/j.coph.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 51.Zachariou V, Caldarone BJ, Weathers-Lowin A, George TP, Elsworth JD, Roth RH, Changeux JP, Picciotto MR. Nicotine receptor inactivation decreases sensitivity to cocaine. Neuropsychopharmacology. 2001;24:576–89. doi: 10.1016/S0893-133X(00)00224-4. [DOI] [PubMed] [Google Scholar]
- 52.Zanetti L, de Kerchove D’Exaerde A, Zanardi A, Changeux JP, Picciotto MR, Zoli M. Inhibition of both alpha7* and beta2* nicotinic acetylcholine receptors is necessary to prevent development of sensitization to cocaine-elicited increases in extracellular dopamine levels in the ventral striatum. Psychopharmacology (Berl) 2006;187:181–8. doi: 10.1007/s00213-006-0419-y. [DOI] [PubMed] [Google Scholar]


