Abstract
The surface of endothelial cells is decorated with a wide variety of membrane-bound macromolecules that constitute the glycocalyx. These include glycoproteins bearing acidic oligosaccharides with terminal sialic acids (SA), and proteoglycans with their associated glycosaminoglycan that include: heparan sulfate (HS), chondroitin sulfate (CS) and hyaluronic acid (HA). In this study enzymes were used to selectively degrade glycoclyx components from the surface of bovine aortic endothelial cells and the effects of these alterations on fluid shear-induced nitric oxide (NO) and prostacyclin (PGI2) production were determined. Depletion of HS, HA and SA, but not CS, blocked shear-induced NO production. Surprisingly, the same enzyme depletions that blocked NO production had no influence on shear-induced PGI2 production. The results may be interpreted in terms of a glypican-caveolae-eNOS mechanism for shear-induced NO transduction, with PGI2 being transduced in basal adhesion plaques that sense the same reaction stress whether the glycocalyx is intact or not.
Keywords: glycocalyx, shear stress, endothelial cell, sialic acid, hyaluronic acid, heparan sulfate, chondroitin sulfate, nitric oxide, prostacyclin
Introduction
The surface of endothelial cells (ECs) is decorated with a wide variety of membrane-bound macromolecules that constitute the glycocalyx (GCX) [1]. These include glycoproteins bearing acidic oligosaccharides with terminal sialic acids (SA), and proteoglycans (PG) with their associated glycosaminoglycan (GAG) side chains laden with polyanionic constituents that impart a net negative charge. This hydrophilic layer (GCX) is further extended in plasma by association with proteins and other plasma components to form an endothelial surface layer (ESL) [1,2]. Because the GCX-ESL constitutes the most apical aspect of the endothelium, it provides the interface between flowing blood and the cell membrane. Simply by virtue of its location, the ESL must be considered a possible sensor of fluid mechanical shear stress that can distribute force to other regions of the endothelial cell where transduction to biomolecular signals may occur.
To appreciate how these processes might occur, it is important to understand the basic molecular organization of the GCX-ESL. This has been described in greater detail in a recent review paper [3] and is outlined briefly here. The GAGs associated with vascular endothelium are heparan sulfate (HS), chondroitin sulfate (CS) and hyaluronic acid (HA). The transmembrane syndecans and the membrane-bound glypicans are the major protein core families on the EC plasma membrane. Syndecans (1,2 and 4) have 3 GAG attachment sites close to their N-terminus and distal to the apical surface, substituted primarily by HS. Syndecan-1 contains 2 additional sites that are close to the membrane and reserved for CS. The cytoplasmic tails of syndecans associate with the cytoskeleton through a variety of linker molecules that provide the structures required to distribute force throughout the cell. Of the glypicans, glypican-1 is the only one expressed on EC [4]. The glypican ectodomain is thought to form a compact globular tertiary structure with three to four GAG attachment sites closer to the membrane that are substituted exclusively with HS. Glypican-1 is bound directly to the plasma membrane through a C-terminal glycosylphosphatidylinositol (GPI) anchor. The GPI anchor localizes this heparan sulfate proteoglycan (HSPG) to lipid rafts including caveolae that are rich in signaling molecules including nitric oxide synthase (eNOS). Hyaluronic acid is a disaccharide polymer that is much longer than HS or CS. It is synthesized on the cell surface and is not covalently attached to a core protein. It is not sulfated, but obtains its negative charge from carboxyl groups that endow it with exceptional hydration properties. HA weaves through the glycocalyx and is anchored to the plasma membrane by interaction with the transmembrane CD44 receptor that localizes in caveolae and through binding with CS chains linked to core proteins. Completing the picture of the GCX, glycocproteins with short, branched oligosaccharides attached to their core are capped by SA, the 9-carbon monosaccharides that contribute to the net negative charge of the GCX.
Various theoretical models that treat the ESL as a porous gel layer with a characteristic hydraulic permeability (K) that can be estimated from transport experiments, predict that when the ESL is intact, the fluid shear stress that is imposed on the upper most surface is completely dissipated within the layer and the plasma membrane itself senses essentially zero fluid shear stress [5,6]. Rather, the fluid shear stress is transmitted to the solid components (GAGs and core proteins) that in turn deliver the force to the cell.
There have been a few previous experimental studies that attempted to address the role of the glycocalyx in mechanotransduction. Several studies in live animals or with intact vessels showed that depletion of SA [7,8] or HA (19)with enzymes abolished flow-dependent vasodilation or nitric oxide (NO) production. Florian et al. [9] presented the first in vitro evidence that GAGs may mediate EC mechanotransduction. They used a heparinase enzyme to selectively degrade the HS component of bovine aortic endothelial cells (BAECs), and observed that the substantial production of NO induced by steady or oscillatory shear stress could be completely inhibited by a dose of enzyme that removed only 46% of the fluorescence intensity associated with a HS antibody. Thi et al. [10] exposed monolayers of rat fat-pad ECs to steady shear stress and showed that the characteristic reorganization of peripheral actin bands in response to shear was completely blocked when protein was removed from the media or when protein was present but the monolayer was treated with heparinase as in [9].
In the present investigation, we extend the in vitro study of Florian et al. [9] by exposing BAECs to shear stress after treatment with heparinase, chondroitinase, hyaluronidase, and neuraminidase to selectively degrade key components of the glycocalyx (HS, CS, HA, SA, respectively). In addition to measuring NO production, we also measure the shear-induced production of prostacyclin (PGI2).
Materials and Methods
Chemicals
All chemicals were obtained from Sigma Chemical Co., unless indicated otherwise.
Cell Culture
Primary ECs were harvested from bovine thoracic aortas and cultured as described previously [9]. Cells between passage five and nine were plated at a density of 0.125 million cells/cm2 and grown for four days to confluent monolayers on slides pre-coated with 30 μg of fibronectin. Before cell seeding, however, the slides were glued to the bottom of 24.5 mm diameter Transwell filter holders (after removal of the filter) with a silicone elastomer (Sylgard, Dow Corning). The cylindrical filter holder could then be used to support the cells when defined shear stress was applied using the rotating disk apparatus described below. 30 μmol/L of tetrahydrobiopterin, a co-factor for eNOS, was added to the growth media 24 hours before experimentation [11]. For the shear experiments the media was changed to 2 ml of phenol red free MEM (Mediatech) supplemented with 1% bovine serum albumin.
Shear Experiments
A defined shear stress profile was applied to the endothelial monolayers using a rotating disk apparatus that has been thoroughly described previously [9,12]. All parameters were adjusted in order to achieve a maximum steady shear stress of 20 dyn/cm2. The average shear stress across the monolayer was 2/3 of the maximum. The experimental apparatus was maintained at 37 °C with a supply of 5% CO2, and 300 μl samples were collected at 0, 5, 15, 30, 60, 120 and 180 minutes after application of shear, and were replaced with an equal volume of fresh media. For the set of experiments testing the effects of agonists under static (no shear) conditions, histamine or bradykinin was kept at a concentration of 100 μmol/L (HS) or 5 μmol/L (BK) throughout the three hours using the same apparatus with the rotating disk in place but maintained stationary.
NO and PGI2 Determination
For each time point, the fluorometric assay described by Misko et al. [13] with accuracy limits down to 10 nmol/L was used to detect nitrite, the major stable metabolite of nitric oxide. Similarly, the stable metabolite of PGI2, 6-keto-prostaglandin F1α, was measured using an enzyme immunoassay kit from Amersham BioSciences following the manufacturer’s directions. Cumulative concentrations are reported that correct for sample dilutions and evaporation.
Enzyme Treatments
A two hour pretreatment with each of the following enzymes - F. heparinum heparinase III (15 mU/mL, Sigma Chemical or Associates of Cape Cod), P. vulgaris chondroitinase ABC (15 mU/mL), C. welchii neuraminidase (15 mU/mL), or S. hyalurolyticus hyaluronidase (1.5 U/mL) was used to degrade each of the four components of interest: heparan sulfate, chondroitin sulfate, sialic acid, and hyaluronic acid, respectively.
Bradykinin and Histamine Treatments
Bradykinin (BK) and histamine, NO agonists, were utilized to test the viability of the endothelial cell NO production machinery after enzymatic treatments. For experiments involving BK a concentration of 5 nmol/L was employed and the media used to replace samples also contained the same concentration of BK. Related experiments with histamine utilized a concentration of 100 μmol/L.
Verification of Enzyme Activity
Fluorescent imaging was employed in separate static experiments in order to estimate the specific activity of the heparinase, chondroitinase and neuraminidase enzymes. Heparan sulfate was stained using 1 μg/ml of a monoclonal primary mouse antibody (10E4 epitope or HepSS-1, US Biologicals), followed by a 2 μl/ml treatment with the Alexa Fluor 488 goat anti-mouse secondary antibody (Molecular Probes). The biotinylated lectins B. simplicifolia and SNA-I (Vector Labs), followed by Neutravidin tetramethylrhodamine (Molecular Probes), all at a 5 μg/ml concentration, were used for chondroitin sulfate and sialic acid visualization, respectively. All images were obtained at 40X using a Nikon TE-2000-E microscope, and the average fluorescent intensity was analyzed using MetaVue software. Comparison of the average intensity of a series of image fields, twenty to thirty for each experiment, for the untreated and treated cases provided an estimate of the degree of enzymatic removal for each glycocalyx component.
Removal of hyaluronan was verified using the ELISA kit from Echelon Inc. [14]. A series of increasing doses of hyaluronidase was used in separate treatments, and the amount of hyaluronan released in the media was quantified by the ELISA. In this case, the reference value (100% removal) was defined as the maximum possible amount of hyaluronan released without having the integrity of the monolayer affected by the strength of the hyaluronidase treatment.
Data Presentation and Statistical Analysis
Significant differences between group means were evaluated by repeated measures analysis of cumulative concentrations (NO and PGI2) using a two-way (time and concentration) ANOVA (MiniTab), followed by Tukey’s method to discern significance. Cumulative concentrations are reported as mean ± SE. A level of p<0.05 was considered significant for statistical analysis.
RESULTS
For brevity, nitric oxide and prostacyclin production data for all treatments are presented as the three hour cumulative endpoint values, rather than the complete time series (Figures 1–3). Although not displayed here, the characteristic biphasic nitric oxide production response to a step onset of shear stress was observed as demonstrated previously [9]. The time course for prostacyclin response to a step onset of shear stress was also biphasic and similar to previous reports in the literature [15]. In all cases, three hours of steady shear stress induced a significant increase in nitric oxide and prostacyclin production compared to static controls (Figures 1 and 2, p<0.01 for all experimental sets). Differences in the basal level of NO or PGI2 production can be attributed to the characteristic variability arising from use of primary culture BAECs.
Figure 1.
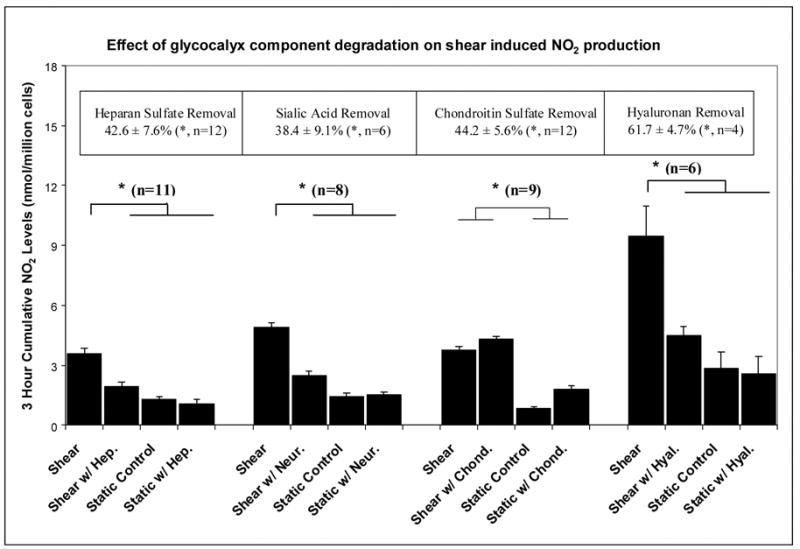
Histograms displaying the three hour cumulative shear-induced nitric oxide production after manipulation of a specific component of the endothelial glycocalyx with: heparinase (Hep.), neuraminidase (Neur.), chondroitinase (Chond.) or hyaluronidase (Hyal.). The boxed values at the top represent the level of reduction of each glycocalyx component, deduced from fluorescent imaging or ELISA assay (hyaluronan). n is the number of replications; * denotes statistical significance (p<0.05).
Figure 3.
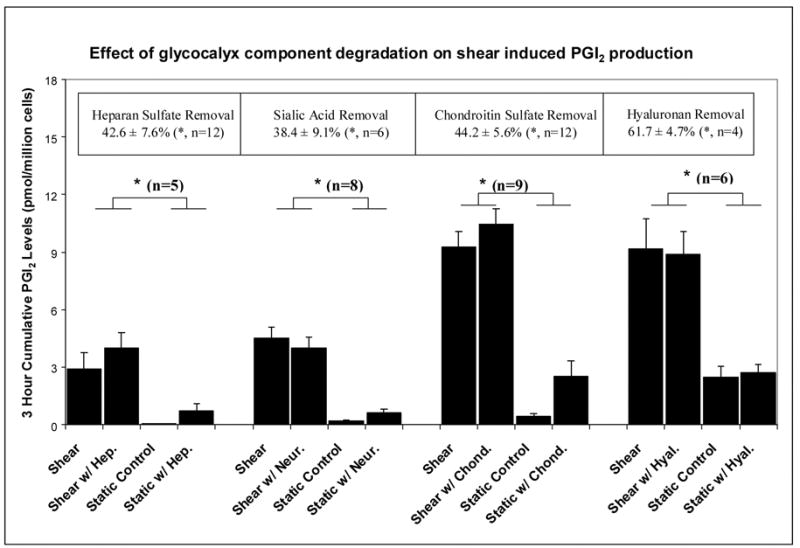
Histograms displaying the three hour cumulative shear-induced prostacyclin production after manipulation of a specific component of the endothelial glycocalyx with: heparinase (Hep.), neuraminidase (Neur.), chondroitinase (Chond.) or hyaluronidase (Hyal.). The boxed values at the top represent the level of reduction of each glycocalyx component, deduced from fluorescent imaging or ELISA assay (hyaluronan). n is the number of replications; * denotes statistical significance (p<0.05).
Figure 2.
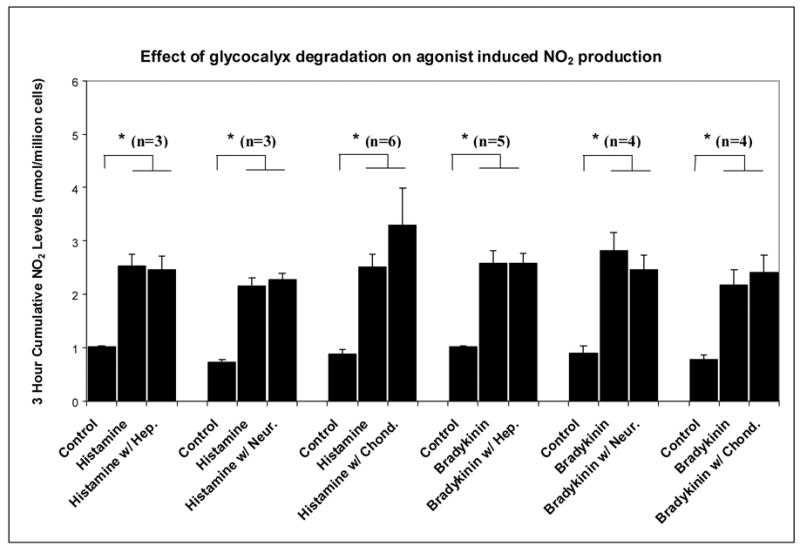
Histograms displaying the three hour cumulative histamine or bradykinin induced nitric oxide production after manipulation of a specific component of the endothelial glycocalyx with: heparinase (Hep.), neuraminidase (Neur.), chondroitinase (Chond.) or hyaluronidase (Hyal.). The boxed values at the top represent the level of reduction of each glycocalyx component, deduced from fluorescent imaging or ELISA assay (hyaluronan). n is the number of replications; * denotes statistical significance (p<0.05).
The enzymatic doses chosen significantly degraded their target glycocalyx components as demonstrated by the reduction of the fluorescent intensity associated with highly specific antibodies or lectins in the case of heparan sulfate, sialic acid and chondroitin sulfate or the release of hyaluronan into the media (Figure 1 – top boxes). It should be noted that these fluorescence intensity reductions for HS, SA and CS probably underestimate the actual mass removed. For example, Dull et al. [16] demonstrated that the same heparinase III dose employed here actually removed 67% of HS GAGs as determined by radio-labeling experiments. Although the data is not shown, none of the enzyme treatments affected the morphological appearance of endothelial monolayers as determined by visual observation (40X) nor did any enzyme remove more than 5% of the fluorescence associated with a non-target component.
The most important results displayed in Figure 1 are the observations that shear-induced NO production was significantly reduced after the removal of specific glycocalyx components: heparan sulfate (p<0.01, n=11), sialic acid (p<0.01, n=8), and hyaluronan (p<0.01, n=6), but not chondroitin sulfate. None of the enzymes had a significant influence on the static (no shear) NO production rate, and for HS, SA and HA, there was no significant difference in NO production between the static and the shear cases when the enzyme was present. On the other hand, for CS, there was no difference in NO production under shear stress whether or not the enzyme was present.
Figure 2 shows that although the NO agonists histamine and bradykinin were capable of inducing a significant increase in NO production under no-flow conditions (p<0.01 for all sets), none of the enzymatic treatments was able to significantly alter these responses. This indicates that the NO production apparatus of the cells was not impaired by the enzyme treatments.
In dramatic contrast to the results for nitric oxide, none of the enzymes inhibited shear induced PGI2 production (Figure 3). The enzyme treatments had no significant influence on the static PGI2 production levels or the shear-induced levels, although these two groups were significantly different.
DISCUSSION
Shear-induced nitric oxide production is a hallmark of endothelial mechanotransduction that is of central importance in flow-mediated vasodilation and is a significant marker of vascular well being [11]. The experimental results displayed in figure 1 indicate that partial removal of the heparan sulfate, hyaluronic acid and sialic acid components of the surface glycocalyx layer can block shear-induced NO production while comparable removal of chondroitin sulfate has no effect. The enzymes employed to degrade specific components have been shown to be quite selective. They do not remove more than 5% of the fluorescence associated with any component other than the target constituent, and they do not impair the NO production machinery of the cell as demonstrated in figure 2 where the receptor-mediated production of NO by the agonists, bradykinin and histamine, are unaffected by heparinase, neuraminidase and chondroitinase.
The results for chondroitinase, hyaluronidase and neuraminidase extend the earlier observations of Florian et al. [9] who found that heparinase blocked shear-induced NO production in BAECs. In another study, neuraminidase was used to remove sialic acid residues from saline-perfused rabbit mesenteric arteries, and it was observed that flow-dependent vasodilation was abolished by a 30 minute pretreatment [7]. Because flow-dependent vasodilation is mediated by NO release in many arteries, this study suggested that neuraminidase blocks shear-induced NO production as we report here. More directly, Hecker et al. [8] showed that when intact segments of rabbit femoral arteries were pretreated with neuraminidase, shear-induced NO production was inhibited. In a more recent study, hyaluronidase was applied to isolated canine femoral arteries and a significant reduction of shear-induced NO production was demonstrated [17]. It seems, therefore, that our in vitro experiments using cultured cells are consistent with other studies using intact vessels – heparinase, neuraminidase and hyaluronidase all inhibit shear-induced NO production. There has been no other study that considered the influence of chondroitinase on shear-induced NO production.
The results of the initial studies of glycocalyx enzymes and shear-induced NO production suggested that the glycocalyx, or at least some of its components, might be “The Mechanotransducer” for fluid shear stress. To further test this hypothesis, we considered the shear-induced PGI2 response of BAECs after the same enzyme treatments, realizing that shear-induced PGI2 is another hallmark of mechanotransduction in endothelial cells [15]. To our initial surprise, we observed that none of the enzymes that blocked shear-induced NO production had any inhibitory effect on shear-induced PGI2 production (Fig. 3). This is consistent with one other study in the literature [8] that showed that the neuraminidase treatment that inhibited shear-induced NO production had no effect on shear-induced PGI2 production.
Because the glycocalyx is a complex, multicomponent chemical structure, the results of the experiments reported in the present study are subject to several interpretations. First we will discuss possible mechanisms relating the glycocalyx to NO production. Heparan sulfate proteoglycans can be linked to both the decentralized and centralized mechanisms of mechanotransduction put forth by Davies [18]. Syndecans that contain both HS and CS have an established association with the cytoskeleton [19], and through it can decentralize the signal by distributing it to multiple sites within the cell (i.e., nucleus, organelles, focal adhesions, intercellular junctions). Significantly, the platelet-endothelial cell adhesion molecule (PECAM-1) associates with the cytoskeleton through catenins, and has been linked to shear-induced eNOS activation [20–22]. In terms of central transduction, it is noteworthy that glypicans which contain HS, but not CS, are linked to caveolae where eNOS resides along with many other signaling molecules [3]. Our observations that depletion of HS, but not CS, inhibits shear-induced NO production (Fig. 1), favor a glypican-caveolae-eNOS mechanism. It is also important to note that hyaluronic acid binds to its CD44 receptor that is localized in caveolae [23–25]. This provides a link between HA and shear-induced NO. The role of sialic acid that is removed by neuraminidase is less clear, but it is known that CD44 can have oligosaccharides (that are capped by SA) attached to it [23].
Of course all of the glycocalyx components that we have investigated provide net negative charges to the surface layer that enhance hydration and extension of the multicomponent structure in aqueous media. Loss of charge through enzyme degradation could lead to partial collapse of the integrated structure and reduction of fluid shear sensing [10]. The lack of influence of chondroitinase may be associated with its location that is closer to the plasma membrane than the other components, thus allowing the more apical drag sensing elements to remain extended in its absence.
The fact that shear-induced PGI2 production was not inhibited by any of the glycocalyx enzymes (Fig. 3) suggests that the transduction machinery for this molecule resides in a location distinct from the NO machinery. One possibility was presented in studies of shear-induced prostaglandin release from cultured osteoblasts, where it was shown that none of the 3 major cytoskeletal networks (actin microfilaments, microtubules, or intermediate filaments) is required, but rather, fibronectin-induced focal adhesions promote shear-induced prostaglandin release and upregulation of COX-2 protein [26,27]. If a similar mechanism was operative in our studies with BAECs, then the presence or absence of the glycocalyx would not be expected to affect mechanotransduction at the basal side of the cell. This follows because at mechanical equilibrium (steady state), the applied stress at the apical surface (20 dyn/cm2) must be balanced by an equal reaction stress at the basal surface. Therefore, a focal adhesion at the basal surface of the cell would feel the same mechanical stress regardless of whether the glycocalyx were present or not, and any mechanotransduction events mediated by basal adhesion plaques would not be sensitive to the status of the glycocalyx. Further studies will be required to clarify this hypothesis in BAECs and endothelial cells in general.
Acknowledgments
Work supported by NIH grant RO1 HL 57093.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pries AR, Secomb TW, Gaehtgens P. The endothelial surface layer. Pflugers Arch. 2000;440:653–66. doi: 10.1007/s004240000307. [DOI] [PubMed] [Google Scholar]
- 2.Adamson RH, Clough G. Plasma proteins modify the endothelial cell glycocalyx of frog mesenteric microvessels. J Physiol. 1992;445:473–86. doi: 10.1113/jphysiol.1992.sp018934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tarbell JM, Pahakis MH. Mechanotransduction and the glycoclyx. J Internal Med. 2006;259:339–350. doi: 10.1111/j.1365-2796.2006.01620.x. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg RD, Shworak NW, Liu J, Schwartz JJ, Zhang L. Heparan sulfate proteoglycans of the cardiovascular system. Specific structures emerge but how is synthesis regulated? J Clin Invest. 1997;99:2062–70. doi: 10.1172/JCI119377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weinbaum S, Zhang X, Han Y, Vink H, Cowin SC. Mechanotransduction and flow across the endothelial glycocalyx. Proc Natl Acad Sci USA. 2003;100:7988–95. doi: 10.1073/pnas.1332808100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Secomb TW, Hsu R, Pries AR. Effect of the endothelial surface layer on transmission of fluid shear stress to endothelial cells. Biorheology. 2001;28:143–150. [PubMed] [Google Scholar]
- 7.Pohl U, Herlan K, Huang A, Bassenge E. EDRF-mediated shear-induced dilation opposes myogenic vasoconstriction in small rabbit arteries. Am J Physiol Heart Circ Physiol. 1991;261:H2016–23. doi: 10.1152/ajpheart.1991.261.6.H2016. [DOI] [PubMed] [Google Scholar]
- 8.Hecker M, Mulsch A, Bassenge E, Busse R. Vasoconstriction and increased flow: two principal mechanisms of shear stress-dependent endothelial autacoid release. Am J Physiol Heart Circ Physiol. 1993;265:H828–33. doi: 10.1152/ajpheart.1993.265.3.H828. [DOI] [PubMed] [Google Scholar]
- 9.Florian JA, Kosky JR, Ainslie K, Pang Z, Dull RO, Tarbell JM. Heparan sulfate proteoglycan is a mechanosensor on endothelial cells. Circ Res. 2003;93:e136–42. doi: 10.1161/01.RES.0000101744.47866.D5. [DOI] [PubMed] [Google Scholar]
- 10.Thi MM, Tarbell JM, Weinbaum S, Spray DC. The role of the glycocalyx in reorganization of the actin cytoskeleton under fluid shear stress: a "bumper-car" model. Proc Natl Acad Sci USA. 2004;101:16483–8. doi: 10.1073/pnas.0407474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boo YC, Jo H. Flow-dependent regulation of endothelial nitric oxide synthase: role of protein kinases. Am J Physiol Cell Physiol. 2003;285:C499–C508. doi: 10.1152/ajpcell.00122.2003. [DOI] [PubMed] [Google Scholar]
- 12.Garanich JS, Pahakis M, Tarbell JM. Shear stress inhibits smooth muscle cell migration via nitric oxide-mediated downregulation of matrix metalloproteinase-2 activity. Am J Physiol Heart Circ Physiol. 2005;288:H2244–2252. doi: 10.1152/ajpheart.00428.2003. [DOI] [PubMed] [Google Scholar]
- 13.Misko TP, Schilling RJ, Salvemini D, Moore WM, Currie MG. A fluorometric assay for the measurement of nitrite in biological samples. Anal Biochem. 1993;214:11–16. doi: 10.1006/abio.1993.1449. [DOI] [PubMed] [Google Scholar]
- 14.Gouverneur M, Spaan JA, Pannekoek H, Fontijn RD, Vink H. Fluid shear stress stimulates incorporation of hyaluronan into the endothelial cell glycocalyx. Am J Physiol Heart Circ Physiol. 2006;290:H458–62. doi: 10.1152/ajpheart.00592.2005. [DOI] [PubMed] [Google Scholar]
- 15.Frangos JA, Eskin SG, McIntire LV, Ives CL. Flow effects on prostacyclin production by cultured human endothelial cells. Science. 1985;227:1477–9. doi: 10.1126/science.3883488. [DOI] [PubMed] [Google Scholar]
- 16.Dull RO, Dinavahi R, Schwartz L, Humphries DE, Berry D, Sasisekharan R, Garcia JGN. Lung endothelial heparan sulfates mediate cationic peptide-induced barrier dysfunction: a new role for the glycocalyx. Am J Physiol Lung Cell Mol Physiol. 2003;285:L986–L995. doi: 10.1152/ajplung.00022.2003. [DOI] [PubMed] [Google Scholar]
- 17.Mochizuki S, Vink H, Hiramatsu O, Kajita T, Shigeto F, Spaan JA, Kajiya F. Role of hyaluronic acid glycosaminoglycans in shear-induced endothelium-derived nitric oxide release. Am J Physiol Heart Circ Physiol. 2003;285:H722–6. doi: 10.1152/ajpheart.00691.2002. [DOI] [PubMed] [Google Scholar]
- 18.Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev. 1995;75:519–60. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tkachenko E, Rhodes JM, Simons M. Syndecans: new kids on the signaling block. Circ Res. 2005;96:488–500. doi: 10.1161/01.RES.0000159708.71142.c8. [DOI] [PubMed] [Google Scholar]
- 20.Dusserre N, L'Heureux N, Bell KS, et al. PECAM-1 interacts with nitric oxide synthase in human endothelial cells: implication for flow-induced nitric oxide synthase activation. Arterioscler Thromb Vasc Biol. 2004;24:1796–802. doi: 10.1161/01.ATV.0000141133.32496.41. [DOI] [PubMed] [Google Scholar]
- 21.Ilan N, Cheung L, Pinter E, Madri JA. Platelet-endothelial cell adhesion molecule-1 (CD31), a scaffolding molecule for selected catenin family members whose binding is mediated by different tyrosine and serine/threonine phosphorylation. J Biol Chem. 2000;275:21435–43. doi: 10.1074/jbc.M001857200. [DOI] [PubMed] [Google Scholar]
- 22.Govers R, Bevers L, de Bree P, Rabelink TJ. Endothelial nitric oxide synthase activity is linked to its presence at cell-cell contacts. Biochem J. 2002;361:193–201. doi: 10.1042/0264-6021:3610193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forster-Horvath C, Meszaros L, Raso E, et al. Expression of CD44v3 protein in human endothelial cells in vitro and in tumoral microvessels in vivo. Microvasc Res. 2004;68:110–8. doi: 10.1016/j.mvr.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Singleton PA, Bourguignon LY. CD44 interaction with ankyrin and IP3 receptor in lipid rafts promotes hyaluronan-mediated Ca2+ signaling leading to nitric oxide production and endothelial cell adhesion and proliferation. Exp Cell Res. 2004;295:102–118. doi: 10.1016/j.yexcr.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 25.Eggli PS, Graber W. Association of hyaluronan with rat vascular endothelial and smooth muscle cells. J Histochem Cytochem. 1995;43:689–97. doi: 10.1177/43.7.7608523. [DOI] [PubMed] [Google Scholar]
- 26.Norvell SM, Ponik SM, Bowen DK, Gerard R, Pavalko FM. Fluid shear stress induction of COX-2 protein and prostaglandin release in cultured MC3T3-E1 osteoblasts does not require intact microfilaments or microtubules. J Appl Physiol. 2004;96:957–966. doi: 10.1152/japplphysiol.00869.2003. [DOI] [PubMed] [Google Scholar]
- 27.Ponik SM, Pavalko FM. Formation of focal adhesions on fibronectin promotes fluid shear stress induction of COX-2 and PGE2 release in MC3T3-E1 osteoblasts. J Appl Physiol. 2004;97:135–42. doi: 10.1152/japplphysiol.01260.2003. [DOI] [PubMed] [Google Scholar]


