Abstract
Histamine H1 receptor antagonists have some behavioral effects that predict abuse liability. In the present study, diphenhydramine and cocaine each maintained i.v. self-administration under a progressive-ratio schedule in rhesus monkeys. When cocaine and DPH were combined in a 1:1 ratio of the ED50s, the combination was super-additive in all monkeys. The data predict that the combination of cocaine and histamine H1 receptor antagonists would have enhanced potential for abuse relative to either drug alone.
Keywords: cocaine, diphenhydramine, drugs interaction
In preclinical models, histamine H1 receptor antagonists can have effects that are associated with abuse liability. Monkeys will self-administer some H1 receptor antagonists (Beardsley and Balster, 1992), and H1 receptor antagonists can have amphetamine-like discriminative stimulus effects (Evans and Johanson, 1989). In humans, H1 receptor antagonists tend to be sedative (Weiler et al., 2000). Abuse has not been particularly problematic, and the compounds are available over the counter. However, the antihistamine tripelennamine has been abused in a mixture with the opioid partial agonist pentazocine ("Ts and Blues"; Shannon and Su, 1982). The present study was designed to examine the possibility of an interaction between cocaine and the H1 receptor antagonist diphenhydramine (DPH) as positive reinforcers.
The experiments were approved by the University of Mississippi Medical Center’s Animal Care and Use Committee and in accordance with National Institutes of Health Guidelines. Subjects were four male rhesus monkeys (Macaca mulatta, 10.4–11.3 kg). Each was implanted with an i.v. silastic catheter under isoflurane anesthesia. In baseline sessions, cocaine (0.1 or 0.2 mg/kg/injection) or saline was available as a consequence of lever pressing under a progressive-ratio (PR) schedule of reinforcement (see Wilcox et al., 2000). When responding was stable, test sessions were added to the daily sequence between two saline and two cocaine sessions. After every second test session, a randomly determined cocaine or saline was conducted. In test sessions, a dose of cocaine (0.03–0.3mg/kg/injection), DPH (0.1–3.0 mg/kg/injection) or the cocaine-DPH combination in a 1:1 ratio of the individual ED50s (total dose 0.04–0.8 mg/kg/injection) was available under conditions identical to baseline sessions. Doses were tested at least twice, once each with a saline or cocaine session the day before.
The mean number of injections per session was calculated individually from the test sessions. ED50 values were calculated individually using the ascending limb of log dose-response functions and non-linear regression analysis (GraphPad Prism 4.0). Predicted additive dose-response functions of the cocaine-DPH combination were calculated and statistically compared to the experimentally-determined cocaine-DPH effects using ANOVA test (PharmToolsPro 1.1.27; The McCary Group, Inc.). The interaction index, defined as the ratio of experimentally-determined dose combinations to the predicted additive combinations (Zmix/Zadd; Tallarida, 2000), were calculated at levels of 6, 8, 10 and 12 injections/session. An interaction index significantly less than 1.0 indicates super-additivity.
Cocaine and DPH functioned as reinforcers in a dose-dependent manner in all monkeys. For cocaine, the mean maximum injections/session (± S.E.M.) was 19.1 (± 0.4) and the ED50 was 0.062 (± 0.005 mg/kg/inj). For DPH, the mean maximum injections/session was 11.9 (± 1.6) and the ED50 was 0.51 (± 0.12 mg/kg/inj). The dose-response function for combinations of cocaine:DPH was to the left of the function predicted by additivity in all monkeys (Figure 1). The interaction indexes at 6, 8, 10, and 12 injections/session level were 0.42 (± 0.08), 0.38 (± 0.07), 0.35 (± 0.08) and 0.33 (± 0.09) respectively (P < 0.05 at all levels).
Fig. 1.
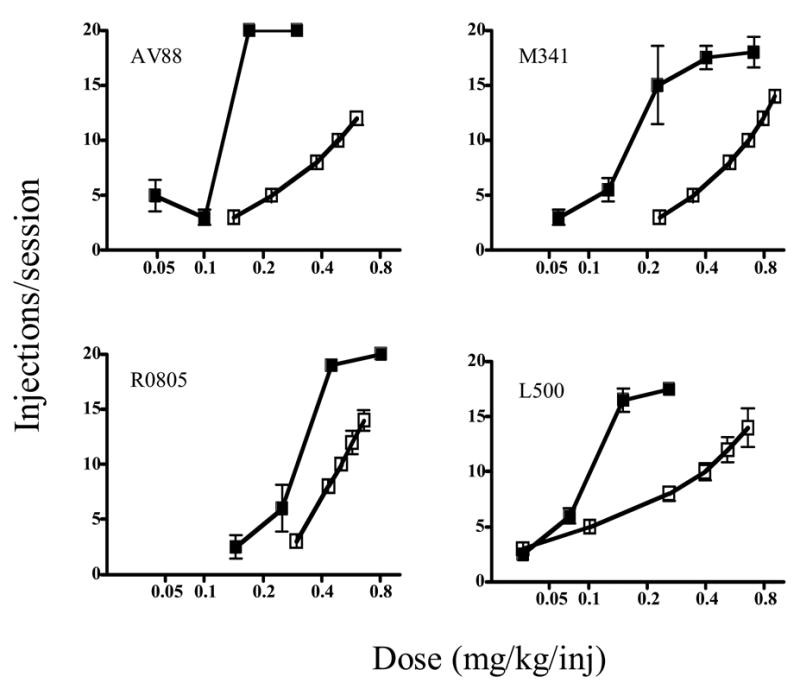
Dose-response curves of self-administration of cocaine/DPH combinations for individual monkeys under a progressive-ratio schedule of reinforcement. Solid squares represent experimentally-determined effects of cocaine/DPH combinations; open squares are effects of dose combinations predicted by additivity. Vertical lines are S.E.M. Doses are the total dose of cocaine+DPH. Numbers in panels are monkey identification numbers.
Consistent with previous research, both cocaine and DPH served as i.v. positive reinforcers in monkeys. That cocaine maintained more responding than DPH under a PR schedule extends previous findings to suggest that DPH is a weaker reinforcer than cocaine, consistent with lower liability for abuse. When cocaine and DPH were combined they were super-additive with regard to potency. Maximum responding maintained by the combination, a measure of reinforcing strength, was also higher than predicted by addivity and comparable to that of cocaine alone. Since both cocaine and the combination approximated the 20-injection maximum for the assay, additional research will be required to establish whether the two can differ in strength. In any case, the present results suggest that cocaine and H1-antihistamines can be synergistic in terms of reinforcing effects and that the combination may have significant potential for abuse.
The reinforcing effects of cocaine seem to involve increased dopamine neurotransmission in the brain. Histamine H1 receptor antagonists increase dopamine levels in the neostriatum and nucleus accumbens (Dringenberg et al., 1998) and block histamine–induced excitation GABAergic cells, indirectly inhibiting dopamine neurons (Korotkova et al., 2002). It is possible, that dopaminergic actions are involved in the reinforcing effect of the combination. However, additional research will also be required to establish the mechanism of the cocaine-antihistamine interaction.
Acknowledgments
Supported by NIDA grant DA-10352. W.L.W. is the recipient of NIDA Senior Scientist Award K05DA-15343. We gratefully acknowledge the technical assistance of Karah Godfrey.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beardsley PM, Balster RL. The intravenous self-administration of antihistamines by rhesus monkeys. Drug Alcohol Depend. 1992;30:117–126. doi: 10.1016/0376-8716(92)90016-6. [DOI] [PubMed] [Google Scholar]
- Dringenberg HC, de Souza-Silva MA, Schwarting RK, Huston JP. Increased levels of extracellular dopamine in neostriatum and nucleus accumbens after histamine H1 receptor blockade. Naunyn Schmiedebergs Arch Pharmacol. 1998;358:423–429. doi: 10.1007/pl00005274. [DOI] [PubMed] [Google Scholar]
- Evans SM, Johanson CE. Discriminative stimulus properties of histamine H1- antagonists in animals trained to discriminate d-amphetamine or pentobarbital. J Pharmacol Exp Ther. 1989;250:779–787. [PubMed] [Google Scholar]
- Korotkova TM, Haas HL, Brown RE. Histamine excites GABAergic cells in the rat substantia nigra and ventral tegmental area in vitro. Neurosci Lett. 2002;320:133–136. doi: 10.1016/s0304-3940(02)00050-2. [DOI] [PubMed] [Google Scholar]
- Shannon HE, Su TP. Effects of the combination of tripelennamine and pentazocine at the behavioral and molecular levels. Pharmacol Biochem Behav. 1982;17:789–795. doi: 10.1016/0091-3057(82)90362-8. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ. Drug Synergism and Dose-Effect Data Analysis. Chapman & Hall/CRC; Washington, D.C: 2000. pp. 169–170. [Google Scholar]
- Weiler JM, Bloomfield JR, Woodworth GG, Grant AR, Layton TA, Brown TL, McKenzie DR, Baker TW, Watson GS. Effects of fexofenadine, diphenhydramine, and alcohol on driving performance. A randomized, placebo-controlled trial in the Iowa driving simulator. Ann Intern Med. 2000;32:354–63. doi: 10.7326/0003-4819-132-5-200003070-00004. [DOI] [PubMed] [Google Scholar]
- Wilcox KM, Rowlett JK, Paul IA, Ordway GA, Woolverton WL. On the relationship between the dopamine transporter and the reinforcing effects of local anesthetics in rhesus monkeys: practical and theoretical concerns. Psychopharmacology. 2000;53:139–147. doi: 10.1007/s002130000457. [DOI] [PubMed] [Google Scholar]


