Abstract
This review deals with the in vitro biosynthesis of the characteristics of polar lipids in archaea along with preceding in vivo studies. Isoprenoid chains are synthesized through the classical mevalonate pathway, as in eucarya, with minor modifications in some archaeal species. Most enzymes involved in the pathway have been identified enzymatically and/or genomically. Three of the relevant enzymes are found in enzyme families different from the known enzymes. The order of reactions in the phospholipid synthesis pathway (glycerophosphate backbone formation, linking of glycerophosphate with two radyl chains, activation by CDP, and attachment of common polar head groups) is analogous to that of bacteria. sn-Glycerol-1-phosphate dehydrogenase is responsible for the formation of the sn-glycerol-1-phosphate backbone of phospholipids in all archaea. After the formation of two ether bonds, CDP-archaeol acts as a common precursor of various archaeal phospholipid syntheses. Various phospholipid-synthesizing enzymes from archaea and bacteria belong to the same large CDP-alcohol phosphatidyltransferase family. In short, the first halves of the phospholipid synthesis pathways play a role in synthesis of the characteristic structures of archaeal and bacterial phospholipids, respectively. In the second halves of the pathways, the polar head group-attaching reactions and enzymes are homologous in both domains. These are regarded as revealing the hybrid nature of phospholipid biosynthesis. Precells proposed by Wächtershäuser are differentiated into archaea and bacteria by spontaneous segregation of enantiomeric phospholipid membranes (with sn-glycerol-1-phosphate and sn-glycerol-3-phosphate backbones) and the fusion and fission of precells. Considering the nature of the phospholipid synthesis pathways, we here propose that common phospholipid polar head groups were present in precells before the differentiation into archaea and bacteria.
INTRODUCTION
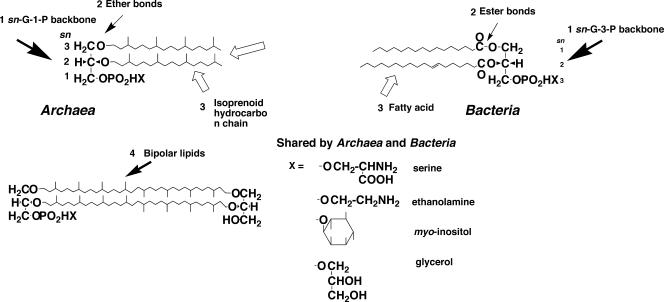
The concept of Archaea was proposed on the basis of phylogenetic analysis of small-subunit rRNA sequences (109). In addition to the rRNA sequences, a number of biochemical properties of Archaea that are distinct from those of Bacteria and Eucarya supported the concept that these three domains are the most basic taxa of all living organisms. Membrane polar lipids are some of the most remarkable features among the distinct characteristics of archaea. Archaeal lipids have been studied since the 1960s from the structural and biosynthetic points of view. Throughout this period, a great number of novel and unique structures of archaeal polar lipids were reported and reviewed (19, 47, 49, 52, 53). Regarding these structures, we recognized four structural characteristics of archaeal lipids that are distinct from their bacterial and eucaryal counterparts (Fig. 1). They are summarized as follows. (i) The stereostructure of the glycerophosphate backbone: hydrocarbon chains are bound at the sn-2 and sn-3 positions of the glycerol moiety in archaeal lipids, while bacterial and eucaryal lipids have sn-1 and 2-diradyl chains. That is, the glycerophosphate backbone of archaeal phospholipids is sn-glycerol-1-phosphate (G-1-P), which is an enantiomer of the sn-glycerol-3-phosphate (G-3-P) backbone of bacterial and eucaryal phospholipids. (ii) Ether linkages: hydrocarbon chains are bound to the glycerol moiety exclusively by ether linkages in archaeal lipids, in contrast to the situation for bacterial polar lipids, most of which have ester linkages between fatty acids and a glycerol moiety. (iii) Isoprenoid hydrocarbon chains: hydrocarbon chains of polar lipids are highly methyl-branched isoprenoids in archaea, while their bacterial counterparts are mostly straight-chain fatty acids. (iv) Bipolar tetraether lipids: bipolar lipids with a tetraether core are present in significant numbers in archaeal species. These tetraether polar lipids span the membrane to form a membrane monolayer. Among these characteristics, the stereostructure of the glycerophosphate backbone is the most specific to organisms of each domain in terms of structure. It is the most crucial feature of archaeal lipids of phylogenetic and evolutionary significance (see below). However, the enantiomeric difference appears to be insignificant for the physicochemical properties of the lipid membrane of archaea, because the enantiomers are equivalent in physicochemical properties except for chirality. The ether linkages, isoprenoid chains, and bipolar tetraether lipids are significant for the physicochemical properties of archaeal lipid membranes, for example, their phase behavior and the permeability of the membranes (52).
FIG. 1.
Characteristics of archaeal polar lipids. Archaeal phospholipids are characterized by (i) G-1-P backbone, (ii) ether bonds, (iii) isoprenoid hydrocarbon chains, and (iv) bipolar tetraether lipids. Most of the polar head groups of phospholipids are shared by archaea and bacteria.
In contrast to the four differences, most of the polar head groups of phospholipids are shared by organisms of the three domains, with minor exceptions. Ethanolamine, l-serine, glycerol, myo-inositol, and even choline are found throughout the three domains as phosphodiester-linked polar head groups in phospholipid (31, 52, 53). Some minor unique polar groups, such as di- and trimethylaminopentanetetrols (30), glucosaminyl-myo-inositol (78), and glucosyl-myo-inositol (71), are found in a limited number of species of archaea.
The biosynthetic mechanisms by which these characteristic lipid structures are formed have been a subject of interest for a long time. As early as 1970, the first metabolic study on archaeal lipids was reported (50). It comprised in vivo incorporation experiments of radioactive glycerol into lipids in Halobacterium cutirubrum (later renamed Halobacterium salinarum [104]), which allowed exploration of the mechanism of the formation of the enantiomeric glycerophosphate backbone. However, only in vivo studies were reported occasionally in the 20 years after 1970. In 1990, the first in vitro study of archaeal lipid biosynthesis was published (115). The enzymatic activity of ether bond formation was reported for Methanobacterium thermoautotrophicum strain Marburg (recently reclassified Methanothermobacter marburgensis [107]). In vitro studies of the major pathway of polar lipid biosynthesis in archaea have been published in the 15 years since the in vivo studies were carried out during the preceding two decades (1970 to 1990). To the best of our knowledge, no review specifically dealing with the in vitro biosynthesis of archaeal lipids has yet been published. Recently, phylogenetic analyses and evolutionary considerations of the enzymes for lipid biosynthesis were carried out (10), but polar lipid biosynthesis was not discussed. Although previous reviews mainly dealing with archaeal lipid structures 10 years or more ago partly described biosynthetic aspects, those were restricted to in vivo studies (48, 53). Therefore, this is the first review that focuses mainly on in vitro biosynthetic studies of archaeal polar lipids. The present review assembles collected knowledge on the recent progress in in vitro studies of the biosynthesis of the four characteristic structures of polar lipids in archaea along with the preceding in vivo studies (isoprenoid biosynthesis, sn-glycerol-1-phosphate formation, ether bond formation, phospholipid polar head group attachment, glycolipid synthesis, and tetraether lipid formation), and it also provides a comparison with bacterial polar lipid biosynthesis.
In spite of the limited number of enzymatic studies of polar lipid biosynthesis in archaea, quite a number of genes relevant to lipid biosynthesis have been found in the genome sequences of archaea. Homology searches for enzymes that are expected to be involved in archaeal polar lipid biosynthesis have revealed many homologs, which have been used for the detection of activities of unknown enzymes and for the evolutionary analysis of the polar lipid synthetic pathway. The evolutionary aspects of polar lipid biosynthesis are also discussed in this review. Because polar lipids are the principal and essential constituents of the cytoplasmic membranes of all cells, fundamental differences in polar lipid structure likely reflect the history of diversification of fundamental cellular lines. The uniformity and diversity of the membrane polar lipid structures of archaea and bacteria are discussed. The nomenclature for archaeal polar lipids proposed by Nishihara et al. (77) is used throughout this paper. Scientific names of archaea as they appear in the original references or have been changed most recently are used in this text. The changes in nomenclature of the archaea appearing in the present text are listed in Table 1.
TABLE 1.
Nomenclatural changes in archaea discussed in the present text
| Original name | Strain | Name most recently proposed | Reference |
|---|---|---|---|
| Caldariella acidophila | Sulfolobus solfataricus | 116 | |
| Halobacterium cutirubrum | Halobacterium salinarum | 104 | |
| Halobacterium halobium | Halobacterium salinarum | 104 | |
| Halobacterium mediterranei | Haloferax mediterranei | 102 | |
| Halobacterium vallismortis | Haloarcula vallismortis | 102 | |
| Methanobacterium thermoautotrophicum | ΔH | Methanothermobacter thermautotrophicus | 107 |
| Methanobacterium thermoautotrophicum | Marburg | Methanothermobacter marburgensis | 107 |
| Methanobacterium thermoformicicum | SF-4 | Methanothermobacter wolfeii | 107 |
| Methanococcus igneus | Methanotorris igneus | 108 | |
| Natronobacterium pharaonis | Natronomonas pharaonis | 45 | |
| Pseudomonas salinaria | Halobacterium salinarum | 32 |
ISOPRENOID BIOSYNTHESIS
The hydrocarbon portions of archaeal diether lipids are exclusively isoprenoids (C20 phytanyl, C25 sesterterpenyl or farnesylgeranyl groups). While isoprenoids are found only in archaea as a component of polar lipids, more than 25,000 naturally occurring isoprenoid derivatives are known to exist throughout the organisms of the three domains. As isoprenoid biosynthesis is a more ordinary subject than the other aspects of the biosynthesis of archaeal polar lipids, the subject has been more extensively studied and reviewed (9, 93). Therefore, this section is limited to Archaea-specific topics of isoprenoid biosynthesis.
MVA Pathway from Acetyl-CoA to DMAPP
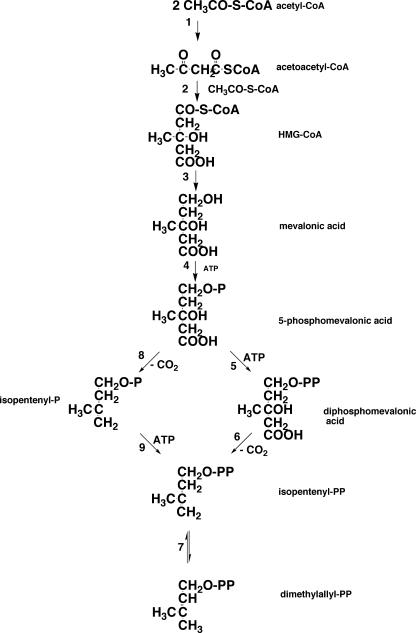
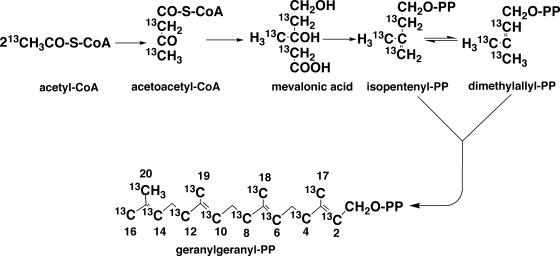
The isoprenoid biosynthetic pathway is divided into two sections. The first half is the synthesis from acetyl-coenzyme A (CoA) to isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP), and the second half is the synthesis of polyprenyl diphosphate from the two C5 units (IPP and DMAPP). For IPP synthesis, two independent pathways are known: the classical mevalonate (MVA) pathway (Fig. 2) and a more recently discovered MVA-independent (1-deoxy-d-xylulose 5-phosphate [DOXP]) pathway (92). The former is usually found in eucarya and the latter in bacteria, algae, and higher plants. Which pathway is functional in archaea for the formation of the isoprenoid chains of polar lipids is a fundamental question. This was first evidenced by in vivo incorporation experiments of exogenously supplied, isotopically labeled acetate into isoprenoid chains. When [13CH3]acetate was incorporated into isoprenoid moieties of polar lipids in Methanospirillum hungatei (28) and Sulfolobus solfataricus (18), 13C appeared at the positions of methyl carbons (C-17, C-18, C-19, and C-20) and carbons (C-2, C-4, C-6, C-8, C-10, C-12, C-14, and C-16). In contrast, 13C was detected at the methine carbons (C-3, C-7, C-11, and C-15) and at the carbon positions C-1, C-5, C-9, and C-13 when [13COOH]acetate was fed to the same archaeal cultures. These results are consistent with the positions expected from labeling via the MVA isoprenoid synthesis pathway from three molecules of acetyl-CoA known in eucarya (Fig. 3). However, Ekiel et al. (29) reported that in Halobacterium cutirubrum, neither [13COOH]acetate nor [13CH3]acetate was incorporated into branch-methyl and methine carbons in phytanyl chains; instead, they were derived from lysine. Because [14C]mevalonic acid was efficiently incorporated into lipids, and carbons at positions other than branch and methine were labeled by acetate, a new route for the formation of MVA from the two molecules of acetate (acetyl-CoA) and lysine was presumed. However, this result has not been adequately evaluated because no specific biochemical reaction was delineated.
FIG. 2.
MVA pathway for synthesis of isopentenyl diphosphate and dimethylallyl diphosphate. 1, acetyl-CoA acetyltransferase; 2, HMG-CoA synthase; 3, HMG-CoA reductase; 4, MVA kinase; 5, phosphomevalonate kinase; 6, diphosphomevalonate decarboxylase; 7, isopentenyl diphosphate isomerase; 8, hypothetical phosphomevalonate decarboxylase; 9, isopentenyl phosphate kinase. The classical MVA pathway proceeds from reaction 1 through reaction 7 via reactions 5 and 6, while a modified MVA pathway goes through reactions 8 and 9 (33). P and PP in the structural formula are phosphate and pyrophosphate, respectively.
FIG. 3.
Expectation of 13C incorporation from [13CH3]acetate into GGPP via the MVA pathway (18, 28). PP in the structural formula is pyrophosphate.
It was reported that farnesol inhibits the growth of Haloferax volcanii and lipid synthesis (98). The inhibition is attributed to an inhibition of acetate incorporation into lipids, which is recovered by the addition of MVA. Farnesol did not affect the incorporation of MVA. Accordingly, it was concluded that farnesol inhibits the synthesis of MVA from acetate. This phenomenon suggests the existence of a regulatory mechanism of isoprenoid synthesis by farnesol in this organism. It is not known whether farnesol inhibition occurs in other archaea.
An early in vitro work on C40 carotene synthesis in Halobacterium cutirubrum also showed that isoprenoids are synthesized from MVA via IPP, even though carotenes are not components of polar lipids (57). In vitro assays of enzyme activities of the pathway supported the conclusion led by the in vivo evidence. 3-Hydroxy-3-methyl-glutaryl CoA (HMG-CoA) reductase, a key enzyme of the MVA pathway, was detected, purified, and characterized from Haloferax volcanii (6) and Sulfolobus solfataricus (7). The Haloferax enzyme was found to be sensitive to lovastatin, an inhibitor of HMG-CoA reductase in mammals. The genes encoding HMG-CoA reductase in Haloferax volcanii and Sulfolobus solfataricus were cloned and expressed in Escherichia coli cells. Sulfolobus HMG-CoA reductase showed more than 40% similarity to eucaryal homologs (7). The purified enzymes from both archaeal species displayed similar kinetic properties to the mammalian enzyme. The HMG-CoA reductases from various eucarya and bacteria were divided into two classes based on amino acid sequences: class I includes the eucaryal enzymes, and class II includes mainly the enzymes from bacteria (8). Archaeal HMG-CoA reductases belong to class I, with the exception of the enzyme from Archaeoglobus fulgidus, which is a class II enzyme and is hypothesized to be laterally transferred from bacteria (9).
The seven enzymes and their genes in the MVA pathway from acetyl-CoA to DMAPP are most completely characterized for yeast. When a BLAST search was performed with these sequences as queries, three enzymes had no match in any archaeal genome (93). Orthologous genes for the four enzymes in the first half of the MVA pathway (acetoacetyl-CoA synthase, HMG-CoA synthase, HMG-CoA reductase, and MVA kinase) were detected, while the three orthologs for phosphomevalonate kinase, diphosphomevalonate decarboxylase, and IPP isomerase were not. Smit and Mushegian (93), based on an analysis of the sequence motifs of the known enzymes, found candidate enzymes for the missing steps in superfamilies of galactokinase, nucleoside monophosphate kinase, and MutT protein (8-oxo-7,8-dihydro-dGTP pyrophosphohydrolase [62]) in archaeal genomes. They inferred three genes encoding the three enzymes based on linkages, phylogenetic relationships, and sequence similarities. On the other hand, Growchouski et al. (33) have identified isopentenyl phosphate (IP) kinase as an MJ0044 gene product that is one of the isoprene biosynthesis genes of the hyperthermophilic methanoarchaeon Methanocaldococcus jannaschii. The presence of IP kinase, in conjunction with the absence of phosphomevalonate kinase and diphosphomevalonate decarboxylase, led them to infer that an alternative route for the formation of IPP may be operating in this organism and some related archaea. Their alternative route includes formation of IP from phosphomevalonate by phosphomevalonate decarboxylase and conversion of IP to IPP by IP kinase. The former enzyme is speculative and needs biochemical confirmation. Thus, modification of the MVA pathway is a possible candidate for the IPP synthetic mechanism, at least in seven species of archaea.
Although IPP isomerase is not essential for E. coli, in which IPP is synthesized via the DOXP pathway (34), IPP isomerase is essential for organisms with the MVA pathway to form DMAPP, which is the starting allylic C5 precursor for polyprenyl diphosphate synthesis. IPP isomerase activity, however, could not be detected in any archaea until 2004, when genes from two archaeal species, Methanothermobacter thermautotrophicus (2) and Sulfolobus shibatae (110), homologous to the Streptococcus sp. strain CL190 type 2 IPP isomerase gene fni (46), were cloned and expressed in E. coli cells. This new type of IPP isomerase requires NAD(P)H and Mg2+ and is strictly dependent on flavin mononucleotide without a net redox change (37). The previously known type 1 IPP isomerase does not require these coenzymes. fni homologs are found in the whole-genome sequences of many archaea. Thus, it has been verified that in archaea the MVA pathway is responsible for the formation of the two essential C5 intermediates (IPP and DMAPP) for the biosynthesis of isoprenoids, and no evidence for the DOXP pathway has been obtained.
Polyprenyl Diphosphate Synthesis
The involvement of polyprenyl diphosphate in polar lipid synthesis was supported by the inhibition of growth and polar lipid synthesis in Halobacterium cutirubrum by bacitracin. The inhibitory effect is attributed to its complexing with polyprenyl diphosphates (67).
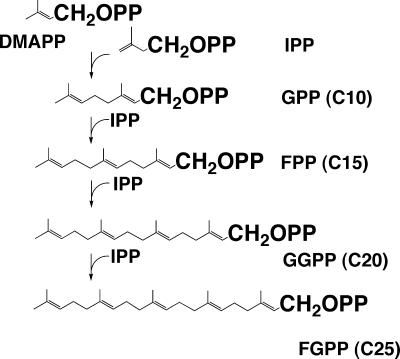
DMAPP is consecutively condensed with several IPP molecules by prenyltransferase (polyprenyl synthase). The products are geranyl (C10), farnesyl (C15), geranylgeranyl (C20), and farnesylgeranyl (C25) diphosphate (Fig. 4). The products are all in the trans form. On the other hand, cis-polyprenyl synthase has also been characterized (39); cis-polyprenyl diphosphate is not a precursor of membrane polar ether lipids but is possibly a glycosyl carrier precursor. Therefore, we do not discuss cis-prenyl diphosphate synthase in the present paper. The mechanism of each condensing reaction is quite similar; i.e., the allyl diphosphate (DMAPP) serves as an acceptor of IPP. The product of the first reaction is also an allyl diphosphate, which can in turn react with another molecule of IPP to form a product that is a single C5 unit longer. These enzymes with definite product specificity can synthesize products of shorter chain lengths. Polyprenyl synthases are common throughout the three domains of living organisms, i.e., yeast, Archaea, and Bacteria. In Archaea, geranylgeranyl diphosphate (GGPP) synthase was found in Methanobacterium thermoformicicum SF-4 (100) (reclassified Methanothermobacter wolfeii [107]), Methanothermobacter marburgensis (12), Sulfolobus acidocaldarius (85), and Pyrococcus horikoshii OT-3 (64). GGPP synthase also produces farnesyl diphosphate from DMAPP and IPP. The catalytic mechanism of a GGPP synthase from Methanothermobacter thermoautotrophicum SF-4 was shown to be an ordered-sequential Bi-Bi mechanism. Potassium ions stimulate the enzyme activity by expediting binding of the substrates (99). Farnesylgeranyl diphosphate synthase was found in Natronobacterium pharaonis (97) (later reclassified Natronomonas pharaonis [45]) and Aeropyrum pernix (101), whose polar lipids contain C25 farnesylgeranyl chains (20, 71). The gene encoding GGPP synthase (idsA) was cloned, identified, and expressed from Methanothermobacter marburgensis (11), Sulfolobus acidocaldarius (85, 87), and Pyrococcus horikoshii (64). These enzymes are characterized by aspartate-rich motifs in their sequences, which are commonly found in polyprenyl transferases.
FIG. 4.
Synthetic pathway of polyprenyl diphosphate. PP in the structural formula is pyrophosphate.
As the molecular mechanism for regulation of the chain length of the polyprenyl products of GGPP synthase of bacteria and archaea has already been reviewed by Liang et al. (61), and that article discussed the mechanism of chain length determination in Sulfolobus acidocaldarius by Ohnuma et al. (82-84), along with bacteria and eucarya, this subject is no longer dealt with in this review.
A salvage pathway for polyprenols was detected in Sulfolobus acidocaldarius, in which geranylgeraniol and geranylgeranyl monophosphate were phosphorylated with ATP by separate enzymes (86).
In summary, archaeal isoprenoid synthesis proceeds via the classical MVA pathway that is shared with Eucarya, or its modified form; however, the archaeal MVA pathway is a mosaic composed of the enzymes common to Archaea and Eucarya, along with enzymes unique to Archaea and Bacteria. In particular, type 2 IPP isomerase and IP kinase, which are the latter enzymes, are remarkably novel. This illustrates that elucidation not only of a metabolic pathway but also of the specifically relevant enzymes is important for obtaining a deeper insight into the biochemistry of lipid metabolism.
FORMATION OF THE G-1-P BACKBONE
In Vivo Evidence
The difference in the stereoconfiguration of the glycerophosphate backbone is one of the most remarkable characteristics that distinguishes archaea from bacteria and eucarya. G-1-P is the enantiomer of bacterial G-3-P. Formation of the G-1-P backbone of polar lipids was investigated in vivo in 1970. Kates et al. (50) first reported radiolabeled glycerol incorporation into an extremely halophilic archaeon, Halobacterium cutirubrum. When [1(3)-14C]glycerol and [2-3H]glycerol were incorporated into a glycerol moiety of polar lipids, the 3H/14C ratio was greatly reduced compared with the ratio of the precursors, while [1(3)-3H]glycerol was incorporated into lipids without a loss of 3H. Kates et al. also detected glycerophosphate dehydrogenase and glycerol kinase activities in this organism, and these were both G-3-P specific (106). They postulated that retention of 3H at the 1 position of glycerol excluded the involvement of dihydroxyacetonephosphate (DHAP), which undergoes keto-aldehyde isomerization between DHAP and d-glyceraldehyde-3-phosphate (GAP) by the high level of activity of triose phosphate isomerase. These observations led to the hypothesis that exogenously supplied glycerol was oxidized at the 2 position, forming dihydroxyacetone (DHA), on which one ether linkage is then formed at the 1 position. The alkylation of DHA is followed by rereduction at the 2 position, a second ether bond formation, and phosphorylation. At the rereduction stage, the stereoconfiguration of the new hydroxyl group is formed so that the first ether bond should be located at the sn-3 position. G-1-P-forming enzymes (glycerophosphate dehydrogenase and glycerol kinase) would not be involved in this pathway. Instead, glycerol dehydrogenase activity was detected in the cell extract of the same organism (4) (the organism called Pseudomonas salinaria at that time [1954] was later renamed Halobacterium cutirubrum and then Halobacterium salinarum [32]). However, the occurrence of glycerol dehydrogenase was species dependent in the genus Halobacterium. While Halobacterium salinarum, Halobacterium cutirubrum, and Halobacterium halobium (these were later reclassified as the same species [104]) have high activities of glycerol dehydrogenase, Halobacterium mediterranei (Haloferax mediterranei [102]) and Halobacterium vallismortis (Haloarcula vallismortis [102]) do not (91). If the speculated pathway is actually operating in halobacterial cells, all of these species must have this enzyme.
Kakinuma et al. (44) conducted similar experiments using [2H]glycerol and nuclear magnetic resonance (NMR) detection. They synthesized position-specific labeled glycerol. 2H of [sn-1-2H]glycerol supplied to a culture of Halobacterium appeared at the sn-3 position of lipid glycerol, while the 2H of the [sn-3-2H]glycerol precursor was incorporated into the sn-1 position of the lipid. This implies that a prochiral glycerol molecule is inverted during lipid biosynthesis. Based on this result, they proposed that an asymmetrical intermediate should be involved in lipid biosynthesis, and the involvement of DHAP instead of DHA was suggested to be the candidate for the asymmetrical intermediate. Kakinuma's pathway of glycerol incorporation into lipid is as follows: glycerol → G-3-P → DHAP → alkyl DHAP → alkyl G-1-P → dialkyl G-1-P (archaetidic acid). This model is similar to the Kates model but is different at the phosphorylation step in the pathway. Enzymes that alkylate DHAP are present in mammalian cells (103) but have not been found in any archaea (see below). They included G-3-P but not G-1-P in their proposal, since Kates et al. (106) reported that the glycerophosphate-forming enzyme of the organism was specific to G-3-P. Although the pathway was thought to be unnatural because of the presence of unnecessary or fruitless oxidation and rereduction of the glycerol moiety at the sn-2 position in the pathway, no evidence against the pathway was presented until Poulter et al. (115) reported a G-1-P-specific ether bond-forming enzyme.
Direct Participation of G-1-P
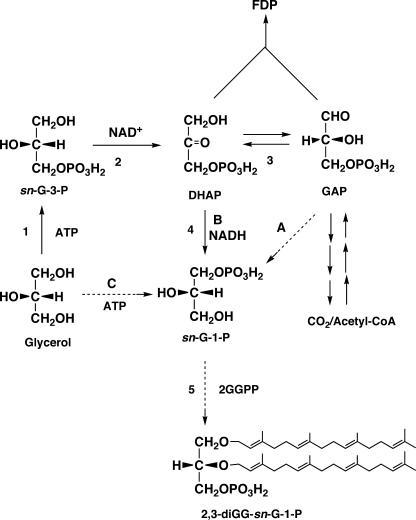
Poulter et al. (115) investigated in vitro ether bond formation in Methanothermobacter marburgensis and found a G-1-P-specific ether bond-forming enzyme (see the next section for details). This suggested that G-1-P was directly involved in the formation of ether lipids. Therefore, direct formation of G-1-P came to be the focus as the next problem. Three reactions were considered to be candidate mechanisms for G-1-P formation (Fig. 5). One was the reduction of GAP at the 1 position (Fig. 5A). Because d-GAP has the same configuration as that of G-1-P at the 2 position, simple reduction of its aldehyde group can form G-1-P. The second possibility for the G-1-P-forming reaction was reduction of DHAP at the 2 position (Fig. 5B). This reaction is similar to the G-3-P dehydrogenase reaction except for the stereospecificity. The last possibility was direct phosphorylation of glycerol at the sn-1 position by ATP (Fig. 5C).
FIG. 5.
Three possible reactions for the direct formation of G-1-P: A, reduction of d-GAP; B, reduction of DHAP; C, phosphorylation of glycerol (G-1-P forming). Enzyme 1, glycerol kinase (G-3-P forming); enzyme 2, G-3-P dehydrogenase; enzyme 3, triose phosphate isomerase; enzyme 4, G-1-P dehydrogenase. Reaction 5, ether lipid synthesis. FDP, fructose diphosphate.
Discovery of G-1-P Dehydrogenase (EC 1.1.1.261)
Nishihara et al. (75) detected glycerophosphate-forming activity from DHAP in a cell-free homogenate of Methanothermobacter thermautotrophicus. The homogenate also contained extremely high triose phosphate isomerase activity, which catalyzes interconversion between GAP and DHAP. Therefore, glycerophosphate was apparently formed also from GAP when GAP and NADH were incubated with the unfractionated homogenate. At this stage, the first and second possibilities could not be discriminated. Glycerophosphate formation from GAP was proven to be comprised of combined reactions of triose phosphate isomerase and glycerophosphate dehydrogenase by fractionation of the two enzymes with a DEAE-cellulose column. Because the fractions of cell-free homogenates including no triose phosphate isomerase activity did not exhibit glycerophosphate formation activity from GAP, the possibility of an enzyme catalyzing direct formation of glycerophosphate from GAP was excluded. The activity that catalyzes glycerophosphate formation from DHAP was purified to homogeneity (74). The reaction product from DHAP was confirmed to be G-1-P, and G-1-P but not G-3-P was oxidized in the presence of NAD [DHAP + NAD(P)H → G-1-P + NAD(P)]. NADH acted as a coenzyme, while NADPH was also shown to be active but at a significantly lower level than NADH. Therefore, this enzyme was established as a new enzyme of G-1-P dehydrogenase and has been designated EC 1.1.1.261.
Interpretation of the In Vivo Phenomena Observed in Halobacterium
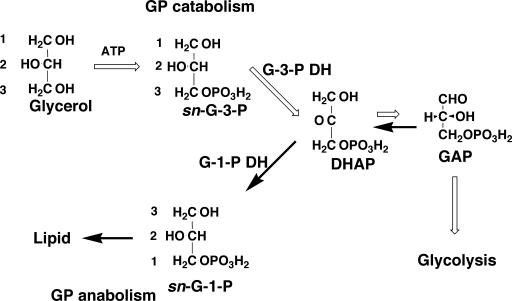
G-1-P-forming activity has been detected in cell extracts of all of the archaeal species so far examined, such as Methanothermobacter thermautotrophicus, Methanosarcina barkeri, Halobacterium salinarum, Pyrococcus furiosus, Pyrococcus sp. strain KS8-1, and Thermoplasma acidophilum strain HO-62 (79, 111). G-3-P was also formed from DHAP by incubation with NADH or NADPH and cell extracts from some of the species (Methanothermobacter thermautotrophicus, Halobacterium salinarum, Pyrococcus sp. strainKS8-1, and Thermoplasma acidophilum strain HO-62). G-1-P dehydrogenase activity was also confirmed in recombinant proteins from Aeropyrum pernix (36) and Sulfolobus tokodaii (54). G-1-P has never formed from glycerol and ATP, while G-3-P is formed from glycerol and ATP by cell extracts from Halobacterium, Pyrococcus, and Thermoplasma. These are all heterotrophs. Accordingly, the third possibility for G-1-P formation has been excluded. Nishihara et al. (79) discussed the fact that only heterotrophic archaea contain a G-3-P-specific enzyme set (both G-3-P dehydrogenase and G-3-P-forming glycerol kinase), as follows (Fig. 6). When these heterotrophic archaea utilize glycerol as a carbon or energy source, they convert glycerol to G-3-P but not to G-1-P. The produced G-3-P is further metabolized to DHAP by G-3-P dehydrogenase, and DHAP enters the central metabolic pathway after conversion to GAP. In this scenario, G-3-P is used for glycerol catabolism. G-1-P is used for lipid biosynthesis in archaea. DHAP is the crossing point of both pathways. These two pathways hence might confuse the results of the in vivo incorporation experiments performed by Kates et al. and Kakinuma et al. Apparent inversion of the prochiral stereostructure of glycerol should be seen only in the case of the incorporation of exogenously supplied glycerol into lipids in heterotrophic archaea that is not essential for lipid synthesis itself. Although Kates et al. regarded retention of H at the 1 position of glycerol as evidence against the involvement of DHAP because DHAP is in equilibrium with GAP, the absence of any loss of the 3H of [1-3H]glycerol during incorporation into lipids might be interpreted by the large equilibrium constant between DHAP and GAP (K = [DHAP]/[GAP] = 22 [5]). Because the equilibrium greatly favors DHAP, it is conceivable that the DHAP produced from glycerol via G-3-P is quickly reduced to G-1-P without substantial conversion to GAP. Alternatively, there might be separate pools of DHAP for lipid synthesis and catabolism.
FIG. 6.
Glycerol metabolism and lipid biosynthesis in archaea. The reactions indicated with open arrows are the catabolic pathway of glycerol in heterotrophic archaea. Reactions shown with closed arrows are the synthetic pathway of phospholipid in archaea (79).
Kakinuma et al. (42) also reported that when d-[6,6-2H]glucose was fed to a Halobacterium culture, deuterium appeared at the sn-1 position of lipid glycerol. Because glucose is metabolized via a modified Entner-Doudoroff pathway, carbons at the 4, 5, and 6 positions of glucose are converted to the carbons 1, 2, and 3 of the d-GAP that is isomerized to DHAP. Therefore, this result of Kakinuma is consistent with the involvement of G-1-P dehydrogenase proposed by Nishihara et al.
Phylogenetic Relationships and Molecular Mechanisms of G-1-P Dehydrogenases
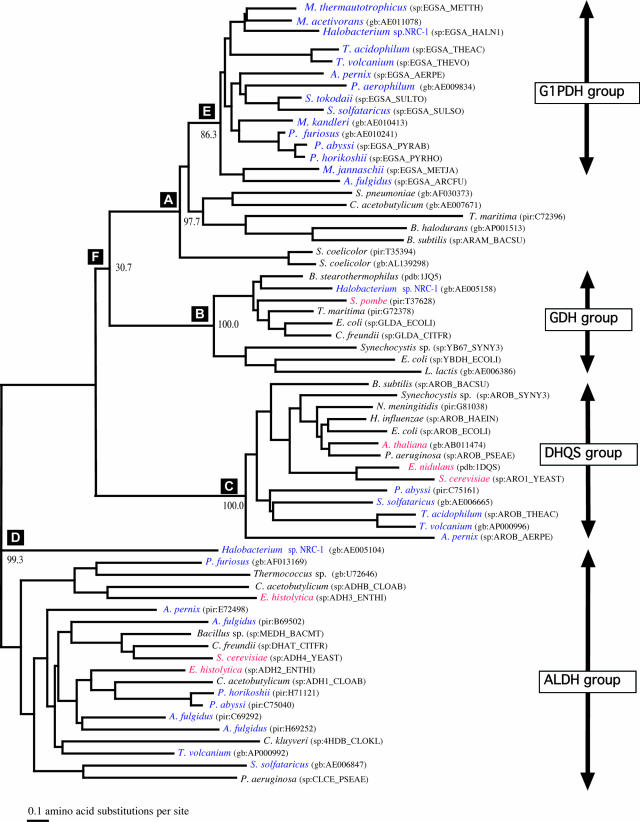
The gene encoding G-1-P dehydrogenase of Methanothermobacter thermautotrophicus was cloned, sequenced, named egsA (51), and heterologously expressed in E. coli (81). The deduced amino acid sequence of G-1-P dehydrogenase from Methanothermobacter thermautotrophicus is composed of 347 amino acid residues with a molecular weight of 36,963. Archaeal G-1-P dehydrogenase and bacterial G-3-P dehydrogenase share little sequence similarity, showing that they belong to different enzyme families. A database search detected open reading frames (ORFs) homologous to the egsA gene in all 21 species of archaea whose whole genomes had been sequenced up until that time, and it was shown that the archaeal enzyme exhibited sequence similarity to glycerol dehydrogenase, dehydroquinate synthase, and alcohol dehydrogenase IV (16). A phylogenetic tree of G-1-P dehydrogenases and their homologs was constructed (Fig. 7). Glycerol dehydrogenase, with coordinates available at that time, was shown to be closely related to G-1-P dehydrogenase. Using the structure of glycerol dehydrogenase as the template, Daiyasu et al. (16) built a model structure of G-1-P dehydrogenase that predicts the following structure and function characteristics of the enzyme: (i) the chirality of the product, (ii) the requirement of the Zn2+ ion for the enzyme reaction, (iii) the transfer of pro-R hydrogen of NADH during the enzyme reaction, (iv) the putative active site and the reaction mechanism, and (v) that G-1-P dehydrogenase does not share an evolutionary origin with G-3-P dehydrogenase from bacteria. The pro-R hydrogen transfer and Zn2+ requirement were experimentally verified (35, 55). It is known that G-3-P dehydrogenase transfers the pro-S hydrogen of NADH (23). Crystallographic data showed that pro-R stereospecific enzymes and pro-S stereospecific enzymes bind the nicotinamide ring of the coenzyme at the opposite orientation, and this is apparently the basis for the enzyme stereospecificity (112). Therefore, it is assumed that G-1-P dehydrogenase (pro-R type) and G-3-P dehydrogenase (pro-S type) have symmetrical ternary structures, at least in the coenzyme-binding sites. G-1-P dehydrogenase in Aeropyrum pernix has been kinetically studied (36). The enzyme uses NADH or NADPH as a coenzyme in DHAP reduction with preference for NADPH but does not use NADP in G-1-P oxidation. This fact, along with the far lower Km value for DHAP than for G-1-P (74), suggests that G-1-P dehydrogenase is really functioning in the direction of G-1-P formation from DHAP. A kinetic analysis revealed that the catalytic reaction by the enzyme follows an ordered Bi-Bi mechanism (36).
FIG. 7.
Phylogenetic tree of G-1-P dehydrogenase and its homologs. The sequence is indicated by the source name of the sequence database (sp, SwissProt; pir, PIR; gb, GenBank; pdb, PDB) and the identification code. G1PDH, sn-glycerol-1-phosphate dehydrogenase; GDH, glycerol dehydrogenase; DHQS, dehydroquinate synthase; ALDH, alcohol dehydrogenase type IV. (Reprinted from reference 16 by permission of Oxford University Press.)
Thus, G-1-P dehydrogenase has been established at the enzyme and genetic levels as a key enzyme responsible for the formation of the enantiomeric glycerophosphate backbone structure of archaeal phospholipids.
G-1-P Formation in Sulfolobus
An example that cannot be explained by the G-1-P pathway is the case of Sulfolobus. [U-14C,1(3)-3H]glycerol and [U-14C,2-3H]glycerol were incorporated into lipid glycerol without any loss of 3H in in vivo labeling experiments in Caldariella acidophila (Sulfolobus solfataricus [116]) (21). Kakinuma et al. confirmed this result by using stereospecifically labeled glycerol with 2H and reported no inversion of the glycerol moiety of lipids during biosynthesis in Sulfolobus acidocaldarius (41). One possible explanation for these results may be direct phosphorylation of glycerol by an unknown G-1-P-forming glycerol kinase. Only one study, presented orally at a meeting, has thus far reported the product of glycerol kinase in S. acidocaldarius to be G-3-P (81a). Although the phenomena described by De Rosa et al. and Kakinuma et al. remain to be explained, the universality of the presence of G-1-P dehydrogenase was verified by the detection of G-1-P dehydrogenase in a species of Sulfolobus (S. tokodaii) (54).
ETHER BOND FORMATION
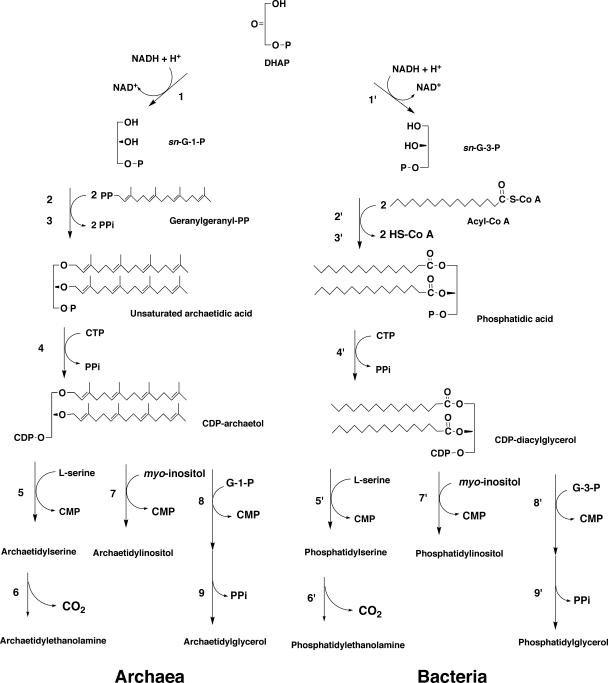
The pathways for biosynthesis of the ether-type and ester-type phospholipids from archaea and bacteria, respectively, are depicted in Fig. 8.
FIG. 8.
Possible biosynthetic pathway for phospholipids in archaea compared with their bacterial counterpart. Enzymes confirmed by in vitro experiments are as follows: 1, G-1-P dehydrogenase; 2, GGGP synthase; 3, DGGGP synthase; 4, CDP-archaeol synthase; 5, archaetidylserine synthase. Reactions and enzymes 6 to 9 are indicated by in vivo experiments or database searches of the relevant genes. 6, archaetidylserine decarboxylase; 7, archaetidylinositol synthase; 8, archaetidylglycerophosphate synthase; 9, archaetidylglycerophosphate phosphatase. The established enzymes for phospholipid biosynthesis in bacteria are as follows; 1′, G-3-P dehydrogenase; 2′, lysophosphatidic acid synthase; 3′, phosphatidic acid synthase; 4′, CDP-diacylglycerol synthase; 5′, phosphatidylserine synthase; 6′, phosphatidylserine decarboxylase; 7′, phosphatidylinositol synthase; 8′, phosphatidylglycerophosphate synthase; 9′, phosphatidylglycerophosphate phosphatase. P and PP in the structural formula are phosphate and pyrophosphate, respectively.
First Ether Bond Formation (G-1-P: GGPP Geranylgeranyltransferase; GGGP Synthase; EC 2.5.1.41)
Ether lipid biosynthesis was studied by in vivo incorporation of several lipid components into Methanospirillum hungatei cells (90). Archaeol (2,3-di-O-phytanyl-sn-glycerol) and caldarchaeol (2,3,2′,3′-di-O-bisphytandiyl-di-sn-glycerol) were incorporated into mainly the nonpolar lipid fraction, and no interconversion between them was found. Among the C20 polyprenols, fully unsaturated geranylgeraniol was most efficiently incorporated into polar lipids in intact cells of the methanogenic archaeon. Monounsaturated phytol was poorly incorporated, and fully saturated phytanol was not incorporated. The results suggested that an ether bond was formed from unsaturated prenyl precursors (90). This was confirmed by an in vitro enzymatic experiment with sonicates of Methanothermobacter marburgensis cells (115). Ether bond formation is catalyzed by two prenyl transferases: one is responsible for formation of the first ether bond between the sn-3 hydroxyl group of G-1-P and GGPP (GGGP synthase), and the other catalyzes the formation of the second ether bond at the sn-2 position to form di-O-geranylgeranyl-G-1-P (DGGGP, or unsaturated archaetidic acid) (DGGGP synthase) (114). The first enzyme was found in sonic extracts of Methanothermobacter marburgensis cells and Halobacterium halobium cells (113). The enzyme showed 30 to 40 times higher activity to G-1-P than to G-3-P and did not react with DHAP. This evidence directly contradicts Kakinuma's hypothesis. GGGP synthase exhibited the same order of specificity of geranylgeranyl-, phytyl-, and phytanyl diphosphate as the incorporation efficiencies of these prenyl alcohols exhibited in the in vivo incorporation experiments (115). Farnesyl diphosphate could not serve as a substrate for GGGP synthase (114).
GGGP synthase is a soluble enzyme, while the second ether bond-forming enzyme is associated with the membrane fraction (114). GGGP synthase was first purified to homogeneity from the Methanothermobacter marburgensis cytosolic fraction, and its properties were described (13). The genes encoding GGGP synthase from Methanothermobacter marburgensis (94) and Thermoplasma acidophilum (72) were cloned and expressed in E. coli cells. Each recombinant enzyme was purified and characterized. Homologs of the Methanothermobacter marburgensis gene were found in Methanothermobacter thermautotrophicus ΔH, Methanocaldococcus jannaschii, Pyrococcus horikoshii, Pyrococcus abyssi, Thermoplasma acidophilum, Aeropyrum pernix, and Archaeoglobus fulgidus (94). The Methanothermobacter enzyme is a pentamer composed of a monomer of 245 amino acid residues (94), while the Thermoplasma enzyme is a dimer (72).
The mechanism of ether bond formation was suggested by incorporation of [18O]glycerol into the lipid of intact cells of Halobacterium halobium (43). Because 18O was retained in the lipid, the oxygen atom of glycerophosphate is suggested to react as an acceptor of an electrophilic reagent of the slightly positively charged carbon atom at the 1′ position of the geranylgeranyl chain. The pyrophosphate group attracts an electron pair of the carbon atom at the 1′ position of the geranylgeranyl chain to give a partially positive charge, which attacks the glycerol oxygen. The crystal structure of GGGP synthase from Archaeoglobus fulgidus has been reported (88). It presents the first triose phosphate isomerase barrel structure with a prenyltransferase function. The ternary structural basis for G-1-P selectivity and GGPP binding, as well as the catalytic reaction mechanism of GGGP synthase, were also presented. It is worth noting that this enzyme commits the step at which three major unique characteristics of archaeal polar lipid structure (G-1-P backbone, ether bond, and isoprenoid group) are assembled into one molecule (G-1-P + GGPP → GGGP [lysoarchaetidic acid] + PPi). It imparts the specific G-1-P stereochemistry of the lipid backbone. GGPP synthase is involved in the formation of an ether bond by transferring an isoprenoid group to a nonlipid acceptor. The authors (88) emphasized that the evolutionary history of GGGP synthase reflects the emergence of archaea.
Second Ether Bond Formation (GGGP: GGPP Geranylgeranyltransferase; DGGGP Synthase; EC 2.5.1.42)
The second ether bond-forming enzyme was studied for two species of archaea. Digeranylgeranyl G-1-P (unsaturated archaetidic acid, or DGGGP) synthase activity was found in the membrane fraction of Methanothermobacter marburgensis (114). It was also specific to the G-1-P-derived substrate, monogeranylgeranyl G-1-P, but did not react with 1-monogeranylgeranyl-G-3-P (GGGP + GGPP → DGGGP [archaetidic acid] + PPi). Hemmi et al. (38) found that Sulfolobus solfataricus DGGGP synthase was one of the three enzymes that are members of the UbiA prenyl transferase family, which, excluding DGGGP synthase, transfers prenyl groups to hydrophobic ring structures such as quinones, hemes, chlorophylls, vitamin E, or shikonin. One of the encoding genes, SSO0583, was cloned and expressed in E. coli, and the recombinant protein was confirmed to have DGGGP synthase activity. They discussed the phylogenetic relationship of DGGGP synthase with the other UbiA homologs, such as the side chain production enzymes of respiratory quinones and hemes. These ether bond-forming enzymes, in addition to G-1-P dehydrogenase, help establish and maintain the G-1-P structure in polar lipids in archaea by their substrate specificity.
ATTACHMENT OF POLAR HEAD GROUPS
In Vivo Pulse-Labeling Experiments
Kates and coworkers explored the biosynthetic pathways of polar lipids (phospholipid, glycolipid) in Halobacterium cutirubrum in vivo (66). Three kinds of radioactive precursors ([14C]MVA, [14C]glycerol, and 32Pi) were separately supplied to the culture of the organism for a short time (1 to 60 min). The labeled lipids were separated by thin-layer chromatography (TLC) and identified by various chemical degradation procedures {acid methanolysis of phosphodiester linkages and glycosyl linkages, alkaline hydrolysis of phosphodiester linkages, and Vitride hydrogenolysis [reductive degradation of allyl ether compounds with sodium dihydrobis(2-methoxyethoxy)aluminate]} and mass spectrometry. The biosynthetic relationship of the intermediates was inferred from the time course of labeling of each lipid. The intermediates of the short-term pulse labeling were classified into two types: “early” and “later.” The early intermediates represent short-term precursors that appear within the first few minutes of biosynthesis and are converted to the later intermediates. The latter are composed of the same polar head groups as the final polar lipid products but with acid-labile allyl ether hydrocarbon chains. These later intermediates were termed “pre-PGP,” “pre-PG,” “pre-PGS,” and “pre-S-TGD.” PGP, PG, PGS, are saturated archaetidylglycerophosphate, archaetidylglycerol, and archaetidylglycerosulfate, respectively. S-TGD is sulfated triglycosylarchaeol. The early intermediates probably include unsaturated prenyl phosphate, unsaturated archaetidic acid, unsaturated CDP-archaeol, and unidentified intermediates. On the basis of these results, the authors presented the following tentative pathway for the biosynthesis of the major polar lipids in Halobacterium cutirubrum. Glycerol or dihydroxyacetone is alkylated with phytyl diphosphate or GGPP to give di-isoprenylglycerol or the substituted glycerol derivative, which is then phosphorylated to “pre-PA.” This early intermediate appears to be converted to “pre-PGP” via putative unsaturated CDP-archaeol. Dephosphorylation of “pre-PGP” and then the sulfating of it give “pre-PG” and “pre-PGS.” Hydrogenation of the “pre-” intermediates gives the saturated final products. Dephosphorylation of “pre-PA” enters the biosynthetic pathway for glycolipids. It is significant that Moldoveanu et al. (66) pointed out the involvement of unsaturated intermediates in polar lipid synthesis in archaea before Poulter et al. (115) showed the results of in vitro experiments that the first ether bond-containing intermediate was unsaturated GGGP.
CDP-Archaeol Synthase (CTP:2,3-di-O-Geranylgranyl-sn-Glycero-1-Phosphate Cytidyltransferase)
The above results by Kates et al. (66) outlined the biosynthetic route of polar lipids in archaea, although some parts of the proposed pathway were not confirmed by subsequent in vitro enzymatic investigations. In vitro experiments to clarify ether polar lipid biosynthesis reactions were performed mainly with cell-free homogenates of Methanothermobacter marburgensis (ether bond formation) or Methanothermobacter thermautotrophicus ΔH (polar lipid synthesis). As described above, two ether bonds form on the G-1-P molecule but not on DHA or glycerol. The prenyl donor was GGPP rather than phytyl diphosphate. The first diether-type phospholipid intermediate was found to be digeranylgeranyl G-1-P (unsaturated archaetidic acid). This was designated for spot 11, which is one of the “early” intermediates cited by Moldoveanu and Kates (66). CDP-archaeol was found to be involved in the polar lipid biosynthetic pathway of archaea by Morii and Koga (70). This was nominated as the entity of another early intermediate, spot 4. In contrast to these intermediates and reactions, the identity of the nonphosphorylated early intermediate spot 12 has yet to be delineated by in vitro experiments. Although these differences in polar lipid biosynthetic mechanisms can be attributed to the difference of species (an extreme halophile and a methanogen), it is reasonable to consider that a general biosynthetic mechanism is shared by both archaeal species, since the enzyme activities and the genes of G-1-P dehydrogenase, ether bond-forming enzymes, and acid-labile unsaturated intermediates are all held in common.
Morii and Koga (70) detected, in the Methanothermobacter thermautotrophicus cell membrane fraction, an activity that catalyzes the synthesis of CDP-archaeol from unsaturated archaetidic acid and CTP (CTP:2,3-di-O-geranylgeranyl-sn-glycerol-1-phosphate cytidyltransferase) (DGGGP [archaetidic acid] + CTP → CDP-archaeol + PPi). The product of the reaction was identified as CDP-digeranylgeranylglycerol by chromatographic mobility, chemical analysis, acid lability, and mass spectrometry. A tiny amount of radioactive CDP-archaeol was detected in growing cells, which was briefly labeled with 32Pi, suggesting that this intermediate is really involved in phospholipid biosynthesis in vivo. Similar to the bacterial CDP-diacylglycerol synthase that plays a central role in the biosynthesis of phospholipids in bacteria (22), CDP-archaeol synthase must be an essential intermediate of archaeal ether phospholipid synthesis. In order to determine whether this enzyme recognizes the stereostructure of the G-1-P backbone of polar lipids, the substrate specificity of CDP-archaeol synthase was investigated by using chemically synthesized substrate analogs with different hydrocarbon chains, with ester or ether bonds between glycerophosphate and hydrocarbon chains, and with G-1-P or G-3-P backbone enantiomers (Table 2). The enzyme recognized and was active specifically to archaetidic acid analogs with geranylgeranyl chains regardless of ether/ester bonds or the stereoconfiguration of the glycerophosphate backbone. Analogs with saturated or monounsaturated hydrocarbon chains exhibited low or no activity as substrates of the enzyme. This suggests that the stereochemical structure of a glycerophosphate backbone is established and maintained by G-1-P dehydrogenase and two ether-bond forming enzymes.
TABLE 2.
Substrate specificity of CDP-archaeol synthase from Methanothermobacter thermautotrophicusa
| Substrate | Hydrocarbon | No. of double bonds/side chain | Linkage | GP backbone | CDP-archaeol synthase activity (%) |
|---|---|---|---|---|---|
| 2,3-GG-GP ether | Geranylgeranyl | 4 | Ether | G-1-P | 100 |
| 1,2-GG-GP ether | Geranylgeranyl | 4 | Ether | G-3-P | 94 |
| 2,3-Phyta-GP ether | Phytanyl | 0 | Ether | G-1-P | 3 |
| 2,3-Phyto-GP ether | Phytyl | 1 | Ether | G-1-P | 0 |
| 2,3-Ole-GP ether | Oleyl | 1 | Ether | G-1-P | 0 |
| 2,3-GG-GP ester | Geranylgeranoyl | 4 | Ester | G-1-P | 122 |
| 1,2-GG-GP ester | Geranylgeranoyl | 4 | Ester | G-3-P | 50 |
| rac-GG-GP ester | Geranylgeranoyl | 4 | Ester | rac-GP | 81 |
| 2,3-Ole-GP ester | Oleoyl | 1 | Ester | G-1-P | 13 |
| rac-Ole-GP ester | Oleoyl | 1 | Ester | rac-GP | 3 |
See reference 70. Each substrate (2,3-di-O-geranylgeranyl-sn-glycerol-1-phosphate) analog synthesized was incubated with [5-3H]CTP and the membrane fraction of Methanothermobacter thermautotrophicus at 55°C for 2 h. After the reaction, chloroform-extractable 3H was counted.
Archaetidylserine Synthase (CDP-2,3-Di-O-Geranylgeranyl-sn-Glycerol:l-Serine O-Archaetidyltransferase)
Methanothermobacter thermautotrophicus homogenate exhibited the activity of archaetidylserine synthase (CDP-2, 3-di-O-geranylgeranyl-sn-glycerol:l-serine O-archaetidyltransferase), which catalyzes the replacement of CMP in CDP-archaeol by l-serine to give archaetidylserine (CDP-archaeol + l-serine → archaetidylserine + CMP) (69). This reaction is analogous to phosphatidylserine synthase in bacteria. The reaction product of archaetidylserine synthase was identified as 2,3-di-O-geranylgeranyl-sn-glycerol-1-phospho-l-serine from the data of chromatographic mobility, mass spectrometry, and chemical degradations. The activities toward various substrate analogs with different hydrocarbon chains, different stereochemical configurations of the glycerophosphate backbone, and ether/ester bonds between the glycerophosphate backbone and hydrocarbon chains were measured. Archaetidylserine synthase was active to all these substrate analogs, especially with the highest activity of the ester-type CDP-diacylglycerols with G-1-P and G-3-P backbones (Table 3). The substrate specificity of this enzyme was similar to that of Bacillus subtilis phosphatidylserine synthase (type 2) but quite different from E. coli phosphatidylserine synthase (type 1). Although the gene encoding archaetidylserine synthase has not been cloned, a gene homologous to the gene of Bacillus subtilis phosphatidylserine synthase was found in the genomes of Methanothermobacter thermautotrophicus and Methanocaldococcus jannaschii. The methanogen genes displayed no similarity to the E. coli phosphatidylserine synthase gene. The detected methanogen's homologs are likely structural genes for archaetidylserine synthase, and this gene belongs to the type 2 phosphatidylserine synthases (65).
TABLE 3.
Substrate specificity of archaetidylserine synthase from Methanothermobacter thermautotrophicusa
| Substrateb | Hydrocarbon | No. of double bonds/side chain | Linkage | GP backbone | Relative CDP-archaeol synthase activity (%) |
|---|---|---|---|---|---|
| CDP-2,3-GG-Gro ether | Geranylgeranyl | 4 | Ether | G-1-P | 100 |
| CDP-1,2-GG-Gro ether | Geranylgeranyl | 4 | Ether | G-3-P | 149 |
| CDP-2,3-Phyta-Gro ether | Phytanyl | 0 | Ether | G-1-P | 86 |
| CDP-1,2-Ole-Gro ether | Oleyl | 1 | Ether | G-3-P | 64 |
| CDP-2,3-Ole-Gro ester | Oleoyl | 1 | Ester | G-1-P | 280 |
| CDP-1,2-acyl-Gro ester | Fatty acyl | Ester | G-3-P | 199 |
See reference 69. Each substrate (CDP-2,3-di-O-geranylgeranyl-sn-glycerol or CDP-archaeol) analog synthesized was incubated with [3-3H]l-serine and cell-free homogenate of Methanothermobacter thermautotrophicus at 60°C for 10 min. After the reaction, chloroform-extractable 3H was counted.
Abbreviations: GG, geranylgeranyl; Gro, glycerol; Phyta, phytanyl; Ole, ole(o)yl; acyl, mixed fatty acyl.
The biosynthetic pathway of polar lipids in archaea has now been confirmed in vitro from DHAP to archaetidylserine. The sequence of reactions in the pathway resembles the bacterial phospholipid synthesis pathway except for the stereostructure of glycerophosphate, ether bonds, and isoprenoid chains. Both pathways consist of the reduction of DHAP, attachment of two radyl chains to glycerophosphate, activation by CTP, and replacement of CMP by various polar groups, in that order.
Homology Search for the CDP-Alcohol Phosphatidyltransferase Family
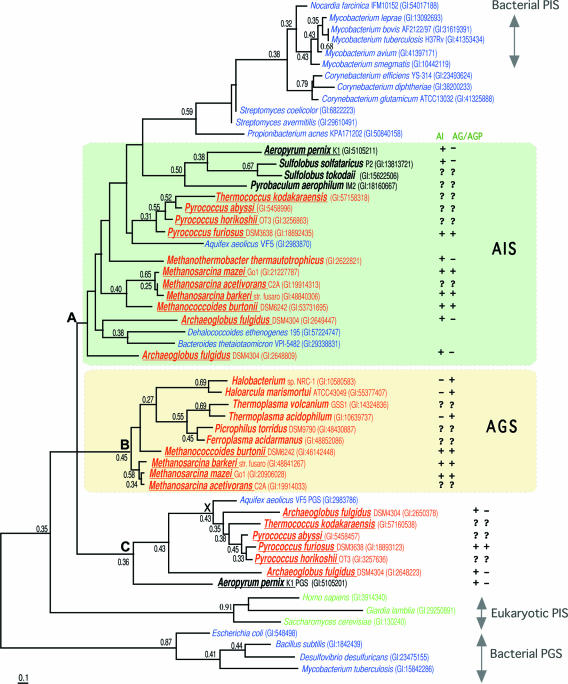
Although only the archaetidylserine synthase from Methanothermobacter thermautotrophicus has been biochemically characterized among the archaeal polar lipid-synthesizing enzymes and other enzymes have not been examined, Daiyasu et al. (17) tried a bioinformatics approaches to obtaining more information on polar lipid biosynthesis in archaea. A first search for CDP-diacylglycerol synthase homologs in archaea did not yield significant hits (Y. Koga, unpublished results). From a database search of 22 archaeal species whose entire genomes had been sequenced, many archaeal hypothetical proteins were found to display sequence similarity to members of the CDP-alcohol phosphatidyltransferase family, including phosphatidylserine synthase, phsophatidylglycerol synthase, and phosphatidylinositol synthase derived from bacteria and eucarya (Fig. 9 and 10) (17). These enzymes may catalyze the following reactions: CDP-archaeol + l-serine → archaetidylserine + CMP; CDP-archaeol + G-1-P → archaetidylglycerophosphate + CMP; and CDP-archaeol + myo-inositol → archaetidyl-myo-inositol + CMP. These archaeal proteins were classified into two groups based on sequence similarity. The first group included archaetidylserine synthase from Methanothermobacter thermautotrophicus and members that were closely related to phosphatidylserine synthase (Fig. 9). Most of the archaeal species that had these proteins contained serine phospholipid (52). These proteins were thus suggested to be archaetidylserine synthase. The second group of archaeal hypothetical proteins was related to phosphatidylglycerophosphate synthase and phosphatidylinositol synthase (Fig. 10). By constructing a phylogenetic tree of these proteins and considering the distribution of glycerol phospholipid and inositol phospholipid (52), these were divided into two clusters, one of which corresponds to archaetidylglycerophosphate synthase and the other to archaetidylinositol synthase (Fig. 10). This widespread distribution of genes for possible archaetidylglycerophosphate synthase and archaetidylinositol synthase suggests that archaeal glycerol phospholipid and inositol phospholipid would probably be synthesized by the reactions depicted in the above equations and in Fig. 8 and catalyzed by these enzymes, although in vitro enzymatic evidence has not yet been obtained.
FIG. 9.
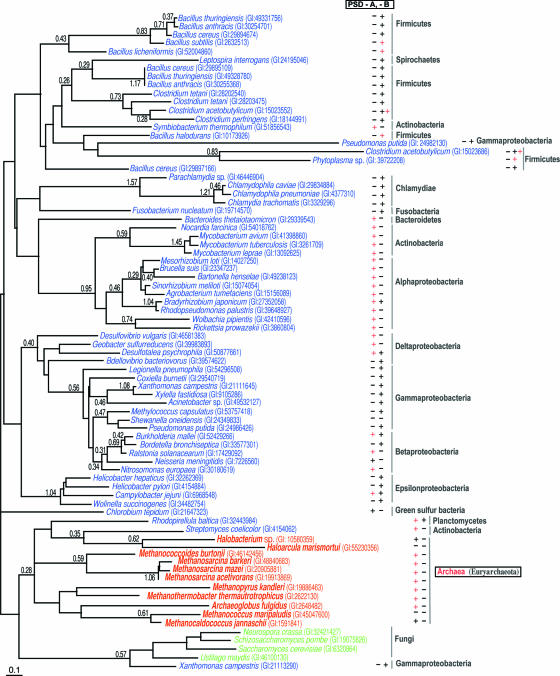
Phylogenetic tree of archaetidylserine synthase and phosphatidylserine synthase constructed by the maximum-likelihood method. The sequence is indicated by the source name and GI number from the National Center for Biotechnology Information. PSD-A and PSD-B are two groups (A and B) of phosphatidylserine decarboxylase that are divided by phylogenetic analysis (17). (Reprinted from reference 17 by permission of the publisher.)
FIG. 10.
Phylogenetic tree of archaetidylinositol synthase and archaetidylglycerol synthase constructed by the maximum-likelihood method. The sequence is indicated by the source name and GI number from the National Center for Biotechnology Information. The presence or absence of archaetidylinositol (AI) or archaetidylglycerol (AG)/archaetidylglycerophosphate (AGP) in each species is indicated by a symbol, “+” or “−,” after the source name and GI number. The symbol “?” indicates that the lipid composition has not been reported for the species. For the characters “A,” “B,” “C,” and “X,” see reference 17. PIS, phosphatidylinositol synthase; PGS, phosphatidyglycerol synthase; AIS, archaetidylinositol synthase; AGS, archaetidylglycerol synthase. (Reprinted from reference 17 by permission of the publisher.)
Archaetidylethanolamine Synthesis
Ethanolamine phospholipid biosynthesis was studied kinetically by in vivo pulse-chase experiments with 32Pi, which was reviewed previously (53). The results suggested that archaetidylethanolamine was synthesized from archaetidylserine by decarboxylation. It was suggested that an ORF encodes archaetidylserine decarboxylase from the data showing sequence similarity with phosphatidylserine decarboxylase and the conserved adjacent location of and the order of the ORF and archaetidylserine synthase in the genome (17). Probable archaetidylserine decarboxylase is expected to transform archaetidylserine to archaetidylethanolamine: archaetidylserine → archaetidylethanolamine + CO2.
If archaetidylserine decarboxylase is included in the cell-free homogenate and archaetidylserine has been formed from CDP-archaeol and l-serine during archaetidylserine synthase reaction, it would be expected that a portion of the archaetidylserine formed might be converted to archaetidylethanolamine in the reaction mixture. When the archaetidylserine synthase reaction was carried out using cell-free homogenates of Methanothermobacter thermautotrophicus with proper substrates under optimal conditions, archaetidylserine was readily synthesized but no trace of archaetidylethanolamine was detected in the reaction mixture after the archaetidylserine synthase reaction was completed (H. Morii, unpublished results). The fact that no archaetidylethanolamine was formed suggests that the reaction conditions for the enzymes might be different. The conditions for the archaetidylethanolamine synthase reaction are not known at present.
Serine, ethanolamine, glycerol, and myo-inositol as the polar head groups of phospholipids are shared by Archaea, Bacteria, and Eucarya, in contrast to other structural aspects of phospholipids, such as ether bonds, isoprenoid side chains, and the G-1-P backbone structure. In accordance with the distinctive structural traits of lipids, G-1-P-forming enzymes and ether bond-forming enzymes are specific to Archaea, whereas polar head group-attaching enzymes are common to organisms throughout the three domains. This is a hybrid nature in the lipid synthetic pathway; we discuss below what this implies.
Although choline phospholipid has been reported to be present in a species of methanogenic archaeon (Methanopyrus kandleri), no homolog for a choline phospholipid-synthesizing enzyme was detected in a database search (17). Therefore, at present no means to discuss choline phospholipid biosynthesis in archaea is available. The unrelatedness of the choline phospholipid-synthesizing enzymes of archaea and bacteria may reflect the deepest branching of M. kandleri in phylogeny.
1l-myo-Inositol-1-Phosphate Synthase (EC 5.5.1.4) and 1l-myo-Inositol-1-Phosphate Phosphatase (EC 3.1.3.25)
Although no biochemical information on archaetidylinositol synthase and archaetidylglycerol synthase has been made known to date, archaetidylinositol might most likely be synthesized from CDP-archaeol and myo-inositol, because putative archaetidylinositol synthase belongs to the same enzyme family (the CDP-alcohol phosphatidyltransferase family, as described above). One of the substrates for this enzyme reaction in eucarya is myo-inositol, which is generated from 1l-myo-inositol-1-phophate by dephosphorylation. 1l-myo-Inositol-1-phophate is a precursor of di-myo-inositol-monophosphate, which is a compatible solute of certain archaeal species, including Archaeoglobus fulgidus, Pyrococcus woesei, Pyrococcus furiosus, and Methanococcus igneus (later reclassified Methanotorris igneus [108]). Chen et al. (15) characterized 1l-myo-inositol-1-phophate synthase as the product of the ips gene of Archaeoglobus fulgidus expressed in E. coli. Even though it is an enzyme reported to be involved in the compatible solute biosynthesis, it is most likely that the enzyme reaction product can also be the substrate of archaetidylinositol synthase. The enzyme catalyzes conversion of glucose-6-phophate to myo-inositol-1-phosphate in the presence of NAD (d-glucose-6-P + NAD → l-myo-inositol-1-P + NAD). The stereochemistry of the product was identified as 1l-myo-inositol-1-phophate by 1H-NMR. A phylogenetic tree of 1l-myo-inositol-1-phophate synthase was constructed, and the evolutionary relationships were discussed (1). The three-dimensional structure of the Archaeoglobus enzyme was analyzed crystallographically, on the basis of which the reaction mechanism was studied in detail (95).
A conversion activity of glucose-6-phosphate to inositol phosphate (l-myo-inositol-1-P → myo-inositol + Pi) was found by the NaIO4 oxidation-Pi release method (3) in crude cell homogenates of Methanothermobacter thermautotrophicus (H. Morii and Y. Koga, unpublished results). The stereostructure of the product was identified as 1l-myo-inositol-1-phosphate by use of chiral column-gas-liquid chromatography-mass spectrometry. Because the organism does not contain di-myo-inositol-monophosphate as a compatible solute, the 1l-myo-inositol-1-phosphate produced would probably be the precursor of archaetidylinositol synthesis in this methanoarchaeon.
If archaetidylinositol is synthesized from CDP-archaeol and myo-inositol, 1l-myo-inositol-1-phophate should be dephosphorylated. The 1l-myo-inositol-1-phophate phosphatase gene has been cloned as the suhB gene from the Archaeoglobus fulgidus genome and expressed heterologously (14). Based on the above results, archaetidylinositol would be synthesized from CDP-archaeol and myo-inositol, which is a reaction analogous to phosphatidylinositol synthesis.
Hydrogenation of Unsaturated Intermediates
The intermediates thus far identified for phospholipid biosynthesis in archaea are all unsaturated or geranylgeranyl chain-containing phospholipids. On the other hand, the final products or membrane phospholipids in most archaea contain fully saturated chains. Therefore, hydrogenation or saturation reactions must be operating somewhere along the steps leading these intermediates to final products.
Nishimura and Eguchi (80) have purified and characterized an enzyme in Thermoplasma acidophilum that catalyzes reduction (hydrogenation) of the geranylgeranyl chains of unsaturated archaetidic acid. Because NADH was required for the reduction and flavin adenine dinucleotide (FAD) increased the activity, NADH would act as a hydrogen donor via FAD. The N-terminal amino acid sequence was determined and the encoding gene was cloned. Although the activity was detected in only Thermoplasma acidophilum cells, homologous genes were found in several archaeal cells. The enzyme also catalyzed the reduction of unsaturated archaetidylglycerophosphate, unsaturated archaetidylglycerol, and unsaturated archaetidylethanolamine. If unsaturated archaetidic acid were saturated, it could not react with CDP-archaeol synthase, because CDP-archaeol synthase reacts preferentially with unsaturated archaetidic acid, as described above, supposing that Thermoplasma acidophilum and Methanothermobacter thermautotrophicus synthesize their phospholipids by the same biosynthetic pathway. Saturation of the geranylgeranyl chains would therefore take place after the formation of CDP-archaeol. The real substrate of the new reductase might possibly be phospholipids with polar head groups. A gene (af0464) of Archaeoglobus fulgidus encoding the homologous enzyme has been expressed in E. coli cells, and the enzyme has been purified (71a).
It was not possible to determine the exact step at which saturation occurs, as unsaturated and saturated CDP-archaeols are of almost comparable activity to archaetidylserine synthase. However, unsaturated CDP-archaeol and unsaturated archaetidylserine were detected in intact cells of Methanothermobacter thermautotrophicus, suggesting that the saturation reaction may take place somewhere after attachment of the polar head groups.
Glycolipid Synthesis
Morii and Koga (unpublished data) have also studied the in vitro biosynthesis of gentiobiosyl (β-d-glucosyl-(1→6)-β-d-glucosyl) archaeol. The gentiobiosyl moiety is the only glycosyl group of glycolipids and phosphoglycolipids of Methanothermobacter thermautotrophicus (76). Cell-free homogenates contained activities that catalyze the transfer of glucose from UDP-glucose to saturated archaeol and the transfer of another glucose unit to monoglucosylarchaeol from UDP-glucose to generate monoglucosylarchaeol (monoglucosylarchaeol synthase) (archaeol + UDP-glucose → monoglucosylarchaeol + UDP) and diglucosylarchaeol (diglucosylarchaeol synthase) (monoglucosylarchaeol + UDP-glucose → diglucosylarchaeol + UDP). Both activities are loosely associated with membranes, monoglucosylarchaeol synthase being more loosely associated. The two activities are, although not yet separated, distinguished by the requirement of the Mg2+ ion for activity and cellular localization. Both activities strictly require archaetidylinositol, similar to Streptococcus pneumoniae diglucosyl diacylglycerol synthase, which requires phosphatidylglycerol or cardiolipin (24). Monoglucosylarchaeol synthase is also active to caldarchaeol and caldarchaetidylinositol (both are tetraether-type lipids), as well as to 2,3-diphytanyl-sn-glycerol (saturated archaeol). 2,3-Digeranylgeranyl-sn-glycerol (unsaturated archaeol) was 30% active compared to saturated archaeol. It is not clear whether the natural substrate of monoglucosylarchaeol synthase is saturated or unsaturated archaeol, since the natural source of saturated or unsaturated archaeol is unknown. The mechanism of biosynthesis of glycolipid with two same hexose units are classified into two cases: in the first, one enzyme catalyzes the transfer of both the first and second (or even third or more) sugar units, and in the second, individual specific enzymes are responsible for the formation of monoglycosyl lipid and diglycosyl lipid. In the case of Methanothermobacter thermautotrophicus, the latter type is likely as seen in Acholeplasma (24). The former example is known in Bacillus subtilis gentiobiosyl diacylglycerol synthase (40).
Compared with the glycolipids of Methanothermobacter thermautotrophicus, which are composed of a rather simple glycosyl moiety (with only glucose as a hexose unit), the glycolipids of thermoacidophilic and halophilic archaea are generally multifarious. The biosynthesis of each glycolipid from those archaea is not understood at all.
BIOSYNTHESIS OF TETRAETHER POLAR LIPIDS
The formation of tetraether lipids is one of the most challenging and interesting problems in all of biochemistry. The ultimate reaction of tetraether lipid synthesis is C-C bond formation between the two methyl termini of phytanyl chains or their precursor chains. Since this is an extremely unusual reaction, never seen previously in biochemistry, no one presently has a clue regarding how to make it clear in an in vitro reaction. Only the results from in vivo incorporation experiments are currently available. Four kinds of in vivo experiments for the study of tetraether lipid synthesis have been reported.
Simple Kinetic Studies between Diether and Tetraether (Polar) Lipids
When Thermoplasma cells were labeled with [14C]MVA for a short period, radioactivity was first incorporated into archaeol core lipid, and incorporation of radioactivity into caldarchaeol core lipid increased after the time point at which the labeling of archaeol reached its maximum (59). A pulse-chase experiment revealed a precursor-product relationship between the archaeol and caldarchaeol cores. Langworthy proposed from these results that C20 phytanyl diethers might serve as the immediate precursor of C40 tetraether biosynthesis via direct condensation of two C20 diethers in the membrane (59). Since the author prepared archaeol from diether-type polar lipids, the discussion implies that diether-type polar lipids are the precursors of tetraether-type polar lipid formation but not that the precursor is the bare archaeol core lipid itself. As described above, when archaeol and caldarchaeol were incorporated into the mainly nonpolar lipid fraction of Methanospirillum hungatei cells, no interconversion between them took place (90). This suggests that tetraether lipid is not synthesized by condensation of two molecules of diether core lipid (archaeol) without the polar head groups.
A similar type of kinetic study of Pi incorporation instead of MVA in a methanogen species was conducted. As this has been discussed in a previous review (53), we summarize it here briefly. Incorporation of Pi into phospholipids in growing Methanothermobacter thermautotrophicus cells suggested a precursor-product relationship between diether polar lipids and tetraether polar lipids which had already been substituted by phosphoric esters and glycosyl moieties (76). The previous review (53) discussed other lines of evidence for the synthesis of tetraether polar lipids from diether polar lipids, such as the structural regularity of polar lipids in Methanothermobacter thermautotrophicus (76) and Methanospirillum hungatei (58), as well as the asymmetric and similar distribution of diether and tetraether polar lipids in the cytoplasmic membranes of Methanothermobacter thermautotrophicus (68).
Inhibition of Tetraether Lipid Synthesis
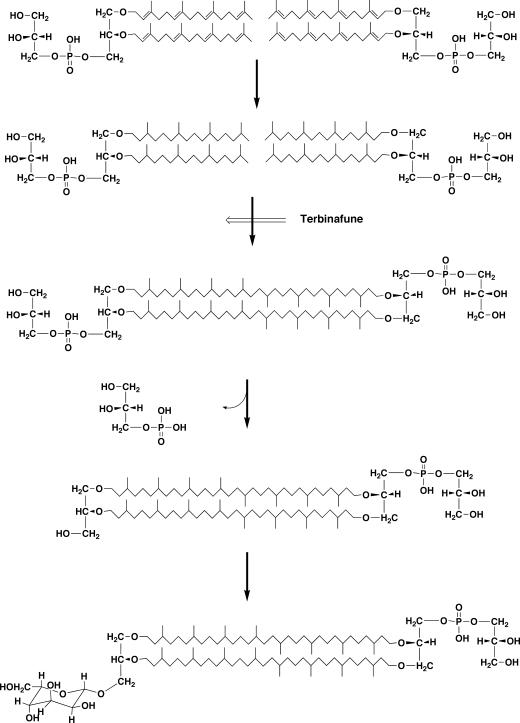
A recent significant advance in tetraether lipid biosynthesis has been achieved in pulse-chase experiments using an inhibitor (terbinafine) of tetraether lipid formation (56, 73) (Fig. 11). Terbinafine is originally a squalene epoxidase inhibitor. Although it is not known why a squalene epoxidase inhibitor inhibits tetraether lipid synthesis, it in fact does inhibit the formation of tetraether lipids, and a diether polar lipid precursor accumulates as a result. After removal of the inhibitor, the precursor was converted to tetraether polar lipids. A time course of appearing and disappearing radioactivity after chasing in each intermediate lipid spot on a TLC plate established the biosynthetic relationships of the intermediates. The structures of the accumulated intermediates were analyzed. As a result, the following results were obtained. The diether phospholipid which accumulated in the presence of terbinafine is saturated archaetidylglycerol, which is converted to bisarchaetidylglycerol (caldarchaeol with two phosphoglycerols on both of the hydroxyl groups) after removal of the inhibitor. One of the two phosphoglycerol groups on the caldarchaeol core of bisarchaetidylglycerol is removed to give caldarchaetidylglycerol, which is then gulosylated to form gulosyl caldarchaetidylglycerol (the so-called “main polar lipid” of Thermoplasma acidophilum [96]). It is most notable that the diether precursor of tetraether lipid formation has saturated isoprenoid chains. However, even though the reaction sequence for tetraether lipid biosynthesis has been outlined by this work, the mechanism of C-C bond formation remains unknown. It is not easy to conceive what sort of reaction would lead to the establishment of a C-C bond between two highly unreactive saturated methyl groups. However, an unpublished result by H. Morii and Y. Koga (as described above) that tetraether-type lipids (caldarchaeol and caldarchaetidyl-myo-inositol) were glucosylated with UDP-glucose in the presence of Methanothermobacter thermautotrophicus cell extracts is consistent with the results of the terbinafine inhibition experiments with Thermoplasma acidophilum.
FIG. 11.
Proposed biosynthetic route of tetraether lipids in Thermoplasma acidophilum inferred from inhibition experiments with terbinafine (73).
Possible Involvement of Radical Intermediates?
The mechanism of head-to-head C-C bond formation during tetraether lipid synthesis was explored by incorporation of deuterium-labeled precursors and NMR analysis by Eguchi et al. (25-27). 1H-NMR analysis of the deuterium-labeled lipid synthesized from mevalonolactone-d9 in growing cells of Haloarcula japonica showed again that the isoprenoid was synthesized via the MVA pathway and that hydrogenation of the double bonds in the geranylgeranyl chains took place through the addition of hydrogens in a syn manner (two hydrogen molecules attacked from the same side of a double bond) (25). Similar experiments with Methanocaldococcus jannaschii gave similar results except that significant proton (1H) incorporation into the C-4, C-8, C-12, and C-16 positions was observed. The protons at these positions were designated pro-S protons. It was suggested that the proton incorporation at those positions occurred regio- and stereospecifically in each phytanyl chain. This protonation pattern may well suggest the involvement of migration of the double bonds (isomerization), and this double bond migration would have to occur after the formation of the digeranylgeranylglyceryl group (27). Because the authors took note of the fact that this migration occurs only in archaea that have macrocyclic ether lipids with C40 biphytandiyl chains (macrocyclic archaeol or caldarchaeol core), they considered the isomerized intermediate having a terminal methylene functionality to be potentially important for the C-C bond formation between two C20 isoprenoid chains. However, in contrast, deuterium-labeled archaetidic acid analogs with terminal double bonds were not incorporated into diether and tetraether lipids in Methanothermobacter thermautotrophicus cells (26). The most efficient precursor was an archaetidic acid analog with a terminal isopropylidene group [(CH3)2CH=] for the synthesis of tetraether lipid, and this contradicts Nemoto's result, which showed that the precursor was fully saturated archaetidylglycerol. The authors suggested the involvement of a radical trigger reaction in the C-C bond formation in connection with the biosynthesis of a similar compound, diabolic acid (15,16-dimethyltraicontanedioic acid), in a bacterium, Butyrivibrio fibrisolvens. Diabolic acid was shown to be synthesized from fully saturated [16-2H]palmitic acid or [14-2H]palmitic acid without the loss of 2H, suggesting unrelatedness with a double bond. Both experiments were done in vivo. Since in vivo experiments include inevitable ambiguity, the contradiction must be settled by appropriate in vitro experiments. However, the substrates, a detection method for the products, and the assay conditions required for in vitro experiments are all still unknown.
EVOLUTION OF MEMBRANE LIPID AND DIFFERENTIATION OF ARCHAEA AND BACTERIA
Hybrid Nature of the Phospholipid Synthesis Pathway
The enantiomeric glycerophosphate backbone, ether linkages, and isoprenoid chains are distinguishing characteristics of archaeal lipids. These structural features are synthesized in the first half of the biosynthetic pathway of the archaeal phospholipids. In bacteria, the characteristic structures of polar lipids (G-3-P backbone, ester linkages, and fatty acid chains) are also synthesized in the first half of the biosynthetic pathway. Furthermore, the orders of the reactions are arranged in a similar manner, that is, glycerophosphate backbone formation first, followed by two steps of hydrophobic chain binding.
In contrast with the core portion, several kinds of polar groups (phosphodiester-linked ethanolamine, l-serine, glycerol, myo-inositol, and choline) are membrane phospholipid constituents in all kinds of organisms. The polar groups of phospholipids are shared by organisms of all three domains. Incorporation of common polar groups into phospholipids in archaea and bacteria proceeds by the same reaction mode and is catalyzed by the homologous enzymes, that is, activation of the first lipidous intermediate by CTP and replacement of CMP by various kinds of polar compounds.
Throughout the biosynthetic pathways of phospholipids in organisms of both domains, the overall reaction sequences are analogous. Although the biosynthesis of core lipids is carried out by analogous reaction sequences, specific enzymes are at work to give the specific products of the reactions for the respective domains. On the other hand, polar head group-attaching enzymes belong to the same enzyme family and are homologous. The enzymes (at least the serine phospholipid synthases) are interchangeable, at least in terms of substrate specificities and reaction conditions. In short, the phospholipid synthesis pathway of Archaea consists of Archaea-specific reactions and Archaea-Bacteria-common reactions. This can be regarded as the hybrid nature of the phospholipid synthesis pathway.
Differentiation of Archaea and Bacteria Caused by Segregation of Enantiomeric Membrane Phospholipid, and the Evolution of Phospholipids
Koga et al. (51) considered the difference in the phospholipid backbones (G-1-P and G-3-P) to be important for the emergence of archaea and bacteria. Wächtershäuser had adopted the significance of the difference in the backbone stereostructure in the evolution of archaea and bacteria and proposed a hypothesis. According to Wächtershäuser's hypothesis (105), precells (common ancestral cells assumed to be present before the speciation of archaea and bacteria) contain glycerophospholipids as major cell membrane constituents with racemic glycerophosphate backbone. Spontaneous physicochemical phase separation resulted in more homochiral membrane segments, and frequent and active fusion and fission of precells resulted in advanced precells with increased homochiral lipid membranes. These precells with greater homochirality and more-stable lipid membranes (with G-1-P and G-3-P backbones) would be the ancestors of archaea and bacteria, respectively. The fact that the structures of the polar head groups and the attaching enzymes are shared by archaea and bacteria suggests that the precells, before the diversification of the two domains, possessed common polar groups in their phospholipids. That is, the membrane phospholipids at that stage were glycerophospholipids with a racemic glycerophosphate backbone and several common polar head groups, as found in contemporary phospholipids. H. Morii and Y. Koga (69) speculated that the gene encoding the ancestral phosphatidylserine synthase was transferred from a gram-positive bacterium to a group of archaea based on the similarities in amino acid sequences and enzymatic properties. Now, we consider the polar head group structures and polar head group-attaching enzymes common to archaea and bacteria to be derived from a common ancestor; that is, the common phospholipids and the common enzymes are assumed to be present in precells before the differentiation of archaea and bacteria. Differentiation of the domains would be driven by spontaneous phase separation of hetrochiral phospholipids, and the polar head groups would be carried on to the descendant archaea and bacteria.
Another theory that connects the differentiation of archaea and bacteria with lipid backbone chirality has been developed by Martin and Russell (63) and by Pereto et al. (89). This theory more or less emphasizes the evolutionary significance of the enantiomeric glycerophosphate backbone of membrane phospholipid and proposes the origin of the two stereospecific glycerophosphate dehydrogenases by recruitment of the dehydrogenases already present in the universal cenancestor, which constitute the concurrent superfamilies of G-1-P dehydrogenase and G-3-P dehydrogenase.
The structures of phospholipids that constitute biological membranes have certain common features, which include having one polar head and two hydrophobic tails connected by a three-carbon backbone. This is the minimum requirement for membrane phospholipid structure. All membrane lipids possess this structure. This is presumed to be the overarching uniformity in the polar lipid structure (Fig. 12). Actual phospholipids are diverse in structure within the limited range of this structural uniformity. Hydrocarbon chains and linkages between a glycerophosphate backbone and hydrocarbon chains may be diverse; ether, ester, and alk-1′-enyl ether linkages (plasmalogen) are all possible. In a rare case, the C-C bond as a connection linkage of the two parts is possible in the long-chain 1,2-diol lipid in Thermomicrobium roseum (60). In this case, glycerol lipids are completely absent and the first three carbons with two hydroxyl groups of the long-chain diol play a role in the backbone of the lipid. Hydrocarbon chains may be straight fatty acid or highly methyl-branched isoprenoids. Either stereoconfiguration of a glycerophosphate backbone is possible for formation of a membrane. The stereostructure of the glycerophosphate backbone, one example of the diversity of lipid structures, became a trigger of speciation in Archaea and Bacteria. At the time of the segregation of the chiral core lipid, both descendants had common polar groups. The fact that present-day membrane phospholipids have common polar groups is thus a matter of course. Discussions put forward by Wächtershäuser (105) and Koga et al. (51) considered only the differences in the stereostructure of glycerophosphate backbones. However, the membrane lipids in ancestral precells must have carried polar head groups as well as core lipids. They did not address the issue of the polar groups. Now, this issue of polar head groups in the membranes of precells has been addressed by studies of the structure and biosynthesis of phospholipids of archaea compared with their bacterial counterparts.
FIG. 12.
Uniformity and diversity of membrane lipids.
FUTURE SUBJECTS OF LIPID BIOSYNTHESIS IN ARCHAEA AND CONCLUDING REMARKS
The present paper has reviewed in vivo and in vitro studies conducted on polar lipid biosynthesis in archaea, along with a phylogenetic analysis of biosynthetic enzymes. Although in vivo experiments are an efficient means of garnering insight into completely unknown mechanisms, sometimes in vivo results are complicated by unexpected living phenomena (such as metabolisms). One such example is the subject of the mechanism of G-1-P structure formation. Whereas in vitro experiments can to a degree compensate for the vulnerable points of in vivo experiments, it is important to exercise caution that a detected reaction is confirmed to be physiologically functional in living organisms. We have learned such lessons on the strong and weak points of the two methodologies from the reconstruction of the history of biosynthetic research of archaeal polar lipids traced in this review.
Studies of the structures and biosynthesis of archaeal polar lipids and comparisons with bacteria have afforded insight into early cellular evolution, especially the early differentiation of Archaea and Bacteria. These studies may also shed light on the biochemistry of bacterial lipids from a perspective that has not received attention.
Many problems concerning lipid biosynthesis in archaea remain to be investigated. Although the general main pathway of phospholipid biosynthesis has been outlined in vitro, the in vitro biosynthesis of ethanolamine-, glycerol-, and myo-inositol-containing phospholipids has not been determined. Because the final products of most archaeal polar lipids are fully saturated, there should be saturation (hydrogenation) steps somewhere after the formation of unsaturated archaeal phospholipids. Studies of the mechanism for the hydrogenation reaction are under way, as the result of the discovery of a hydrogenating enzyme. This is also a unique feature of archaeal polar lipid biosynthesis that is not seen in bacteria. The mechanism of tetraether lipid biosynthesis, i.e., head-to-head condensation of the isoprenoid chain at the methyl ends, must be a novel reaction. It is, as mentioned already, one of the most challenging subjects in general biochemistry. In addition, most enzymes in the pathway have neither been purified nor characterized. Finally, while many relevant genes have been detected from database searches, these genes have not been cloned.
Hydroxyarchaeol, cyclic archaeol, cyclopentane ring-containing caldarchaeol, and H-shaped caldarchaeol may also be synthesized by novel mechanisms and hence be worthy of study. Additionally, how the polar groups of the lipids unique to archaea (e.g., aminopentanetetrols, glucosaminylinositol, and glucosylinositol) are synthesized is a remaining challenge. However, these diverse problems may be too-particular subjects.
The purification and characterization of relevant enzymes, cloning and sequencing of the genes encoding the enzymes, phylogenetic studies of the enzymes, and comparative studies of the structure and synthesis of the lipids of archaea and bacteria all show great promise for yielding novel and valuable insights into biological lipids and membranes.
Acknowledgments
We are particularly indebted to the late Masateru Nishihara for his ardent and long-standing contribution to and support of our research on archaeal lipid structures and biosynthesis. Pacific Edit reviewed the manuscript prior to submission.
This work was partly supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science.
REFERENCES
- 1.Bachhawat, N., and S. C. Mande. 2000. Complex evolution of the inositol-1-phosphate synthase gene among archaea and eubacteria. Trends Genet. 16:111-113. [DOI] [PubMed] [Google Scholar]
- 2.Barkley, S. J., R. M. Cornish, and C. D. Poulter. 2004. Identification of an archaeal type II isopentenyl diphosphate isomerase in Methanothermobacter thermautotrophicus. J. Bacteriol. 186:1811-1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnett, J. E. G., R. E. Brice, and D. L. Corina. 1970. A colorimetric determination of inositol monophosphates as an assay for d-glucose 6-phosphate-1-l-myoinositol 1-phosphate cyclase. Biochem. J. 119:183-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baxter, R. M., and N. E. Gibbons. 1954. The glycerol dehydrogenases of Pseudomonas salinaria, Vibrio costicolus, and Escherichia coli in relation to bacterial halophilism. Can. J. Biochem. Physiol. 32:206-217. [PubMed] [Google Scholar]
- 5.Beisenherz, G. 1955. Triose phosphate isomerase from calf muscle. Methods Enzymol. 1:387-391. [Google Scholar]
- 6.Bischoff, K. M., and V. W. Rodwell. 1996. 3-Hydroxy-3-methylglutaryl-coenzyme A reductase from Haloferax volcanii: purification, characterization, and expression in Escherichia coli. J. Bacteriol. 178:19-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bochar, D. A., J. R. Brown, W. F. Doolittle, H. P. Klenk, W. Lam, M. E. Schenk, C. V. Stauffacher, and V. W. Rodwell. 1997. 3-Hydroxy-3-methylglutaryl coenzyme A reductase of Sulfolobus solfataricus: DNA sequence, phylogeny, expression in Escherichia coli of the hmgA gene, and purification and kinetic characterization of the gene product. J. Bacteriol. 179:3632-3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bochar, D. A., C. V. Stauffacher, and V. W. Rodwell. 1999. Sequence comparisons reveal two classes of 3-hydroxy-3-methylglutaryl coenzyme A reductase. Mol. Genet. Metabol. 66:122-127. [DOI] [PubMed] [Google Scholar]
- 9.Boucher, Y., and W. F. Doolittle. 2000. The role of lateral gene transfer in the evolution of isoprenoid biosynthesis pathway. Mol. Microbiol. 37:703-716. [DOI] [PubMed] [Google Scholar]
- 10.Boucher, Y., M. Kamekura, and W. F. Doolittle. 2004. Origins and evolution of isoprenoid lipid biosynthesis in archaea. Mol. Microbiol. 52:515-527. [DOI] [PubMed] [Google Scholar]
- 11.Chen, A. J., and C. D. Poulter. 1994. Isolation and characterization of idsA: the gene for the short chain isoprenyl diphosphate synthase from Methanobacterium thermoautotrophicum. Arch. Biochem. Biophys. 314:399-404. [DOI] [PubMed] [Google Scholar]
- 12.Chen, A. J., and C. D. Poulter. 1993. Purification and characterization of farnesyl diphosphate/geranylgeranyl diphosphate synthase. J. Biol. Chem. 268:11002-11007. [PubMed] [Google Scholar]
- 13.Chen, A. J., D. L. Zhang, and C. D. Poulter. 1993. (S)-Geranylgeranylglyceryl phosphate synthase—purification and characterization of the first pathway-specific enzyme in archaebacterial membrane lipid biosynthesis. J. Biol. Chem. 268:21701-21705. [PubMed] [Google Scholar]
- 14.Chen, L. J., and M. F. Roberts. 2000. Overexpression, purification, and analysis of complementation behavior of E-coli SuhB protein: comparison with bacterial and archaeal inositol monophosphatases. Biochemistry 39:4145-4153. [DOI] [PubMed] [Google Scholar]
- 15.Chen, L. J., C. Zhou, H. Y. Yang, and M. F. Roberts. 2000. Inositol-1-phosphate synthase from Archaeoglobus fulgidus is a class II aldolase. Biochemistry 39:12415-12423. [DOI] [PubMed] [Google Scholar]
- 16.Daiyasu, H., T. Hiroike, Y. Koga, and H. Toh. 2002. The analysis of membrane sterochemistry with the homology modeling of sn-glycerol-1-phosphate dehydrogenase. Protein Eng. 15:987-995. [DOI] [PubMed] [Google Scholar]
- 17.Daiyasu, H., K. Kuma, T. Yokoi, H. Morii, Y. Koga, and H. Toh. 2005. A study on archaeal enzymes involved in polar lipids synthesis by an approach linking the information about amino acid sequences, genomic contexts and lipid composition. Archaea 1:399-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Rosa, M., and A. Gambacorta. 1986. Lipid biogenesis in archaebacteria. Syst. Appl. Microbiol. 7:278-285. [Google Scholar]
- 19.De Rosa, M., A. Gambacorta, and A. Gliozzi. 1986. Structure, biosynthesis, and physicochemical properties of archaebacterial lipids. Microbiol. Rev. 50:70-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Rosa, M., A. Gambacorta, B. Nicolaus, H. N. M. Ross, W. D. Grant, and J. D. Bu'Lock. 1982. An asymmetric archaebacterial diether lipid from alkaliphilic bacterial halophiles. J. Gen. Microbiol. 128:343-348. [Google Scholar]
- 21.De Rosa, M., A. Gambacorta, B. Nicolaus, and S. Sodano. 1982. Incorporaton of labelled glycerols into ether lipids in Caldariella acidophila. Phytochemistry 21:595-599. [Google Scholar]
- 22.Dowhan, W. 1997. CDP-diacylglycerol synthase of microorganisms. Biochim. Biophys. Acta 1348:157-165. [DOI] [PubMed] [Google Scholar]
- 23.Edgar, J. R., and R. M. Bell. 1980. Biosynthesis in Escherichia coli of sn-glycerol 3-phosphate, a precursor of phospholipid. Further kinetic characterization of wild type and feedback-resistant forms of the biosynthetic sn-glycerol-3-phosphate dehydrogenase. J. Biol. Chem. 255:3492-3497. [PubMed] [Google Scholar]
- 24.Edman, M., S. Berg, P. Storm, M. Wikström, S. Vikström, A. Öhman, and A. Wieslander. 2003. Structural features of glycosyltransferases synthesizing major bilayer and nonbilayer-prone membrane lipids in Acholeplasma laidlawii and Streptococcus pneumoniae. J. Biol. Chem. 278:8420-8428. [DOI] [PubMed] [Google Scholar]
- 25.Eguchi, T., M. Morita, and K. Kakinuma. 1998. Multigram synthesis of mevalonolactone-d(9) and its application to stereochemical analysis by H-1 NMR of the saturation reaction in the biosynthesis of the 2,3-di-O-phytanyl-sn-glycerol core of the archaeal membrane lipid. J. Am. Chem. Soc. 120:5427-5433. [Google Scholar]
- 26.Eguchi, T., Y. Nishimura, and K. Kakinuma. 2003. Importance of the isopropylidene terminal of geranylgeranyl group for the formation of tetraether lipid in methanogenic archaea. Tetrahedron Lett. 44:3275-3279. [Google Scholar]
- 27.Eguchi, T., H. Takyo, M. Morita, K. Kakinuma, and Y. Koga. 2000. Unusual double-bond migration as plausible key reaction in the biosynthesis of the isoprenoid membrane lipids of methanogenic archaea. J. Chem. Soc. Chem. Commun. 2000:1545-1546. [Google Scholar]
- 28.Ekiel, I., I. C. P. Smith, and G. D. Sprott. 1983. Biosynthetic pathway in Methanospirillum hungatei as determined by 13C nuclear magnetic resonance. J. Bacteriol. 156:316-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ekiel, I., G. D. Sprott, and I. C. P. Smith. 1986. Mevalonic acid is partially synthesized from amino acids in Halobacterium cutirubrum: a 13C nuclear magnetic resonance study. J. Bacteriol. 166:559-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferrante, G., I. Ekiel, and G. D. Sprott. 1987. Structures of diether lipids of Methanospirillum hungatei containing novel head groups N,N-dimethylamino- and N,N,N-trimethylaminopentanetetrol. Biochim. Biophys. Acta 921:281-291. [DOI] [PubMed] [Google Scholar]
- 31.Goldfine, H. 1982. Lipids of procaryote—structure and distribution, p. 1-43. In F. Bronner and A. Kleinzeller (ed.), Current topics in membranes and transport, vol. 17. Academic Press, New York, NY. [Google Scholar]
- 32.Grant, W. D. 2001. Genus I. Halobacterium Elazari-Volcani 1957, 207, AL emend. Larsen and Grant 1989, 2222, p. 301-305. In D. R. Boone and R. W. Castenholz (ed.), Bergey's manual of systematic bacteriology—the Archaea and the deeply branching and phototrophic bacteria, vol. 1. Springer-Verlag, New York, NY. [Google Scholar]
- 33.Grochowski, L. L., H. M. Xu, and R. H. White. 2006. Methanocaldococcus jannaschii uses a modified mevalonate pathway for biosynthesis of isopentenyl diphosphate. J. Bacteriol. 188:3192-3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hahn, F. M., A. P. Hurlburt, and C. D. Poulter. 1999. Eschrichia coli open reading frame 696 is idi, a nonessential gene encoding isopentenyl diphosphate isomerase. J. Bacteriol. 181:4499-4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han, J.-S., and K. Ishikawa. 2005. Active site of Zn2+-dependent sn-glycerol-1-phosphate dehydrogenae from Aeropyrum pernix K1. Archaea 1:311-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han, J. S., Y. Kosugi, H. Ishida, and K. Ishikawa. 2002. Kinetic study of sn-glycerol-1-phosphate dehydrogenase from the aerobic hyperthermophilic archaeon, Aeropyrum pernix K1. Eur. J. Biochem. 269:969-976. [DOI] [PubMed] [Google Scholar]
- 37.Hemmi, H., Y. Ikeda, S. Yamashita, T. Nakayama, and T. Nishino. 2004. Catalytic mechanism of type 2 isopentenyl diphosphate:dimethylalyl diphosphate isomerase: verification of a redox role of the flavin cofactor in a reaction with no net redox change. Biochem. Biophys. Res. Commun. 322:905-910. [DOI] [PubMed] [Google Scholar]
- 38.Hemmi, H., K. Shibuya, Y. Takahashi, T. Nakayama, and T. Nishino. 2004. (S)-2,3-Di-O-geranylgeranylglyceryl phosphate synthase from the thermoacidophilic Archaeon Sulfolobus solfataricus—molecular cloning and characterization of a membrane-intrinsic prenyltransferase involved in the biosynthesis of archaeal ether-linked membrane lipids. J. Biol. Chem. 279:50197-50203. [DOI] [PubMed] [Google Scholar]
- 39.Hemmi, H., S. Yamashita, T. Shimoyama, T. Nakayama, and T. Nishino. 2001. Cloning, expression, and characterization of cis-polyprenyl diphosphate synthase from the thermoacidophilic archaeon Sulfolobus acidocaldarius. J. Bacteriol. 183:401-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jorasch, P., F. P. Wolter, U. Zahringer, and E. Heinz. 1998. A UDP glucosyltransferase from Bacillus subtilis successively transfers up to four glucose residues to 1,2-diacylglycerol: expression of ypfP in Escherichia coli and structural analysis of its reaction products. Mol. Microbiol. 29:419-430. [DOI] [PubMed] [Google Scholar]
- 41.Kakinuma, K., Y. Obata, T. Matsuzawa, T. Uzawa, and T. Oshima. 1990. The stereochemical fate of glycerol during the biosynthesis of membrane lipids in thermoacidophilic archaebacteria Sulfolobus acidocaldarius. J. Chem. Soc. Chem. Commun. 1990:925-927. [Google Scholar]
- 42.Kakinuma, K., I. Takagi, and T. Eguchi. 1992. The fate of the hydrogens of d-glucose during the lipid biosynthsesis and stereochemistry of the triose-phosphate isomerase reaction in archaebacteria Halobacterium halobium. Tetrahedron Lett. 33:6479-6482. [Google Scholar]
- 43.Kakinuma, K., M. Yamagishi, Y. Fujimoto, N. Ikekawa, and T. Oshima. 1990. Biosynthetic mechanism of sn-2,3-di-O-phytanylglycerol, core membrane lipid of the archaebacterium Halobacterium halobium. J. Am. Chem. Soc. 112:2740-2745. [Google Scholar]
- 44.Kakinuma, K., M. Yamagishi, Y. Fujimoto, N. Ikekawa, and T. Oshima. 1988. Stereochemistry of the biosynthesis of sn-2,3-O-diphytanyl glycerol, membrane lipid of archaebacteria Halobacterium halobium. J. Am. Chem. Soc. 110:4861-4863. [Google Scholar]
- 45.Kamekura, M., M. L. DyallSmith, V. Upasani, A. Ventosa, and M. Kates. 1997. Diversity of alkaliphilic halobacteria: proposals for transfer of Natronobacterium vacuolatum, Natronobacterium magadii, and Natronobacterium pharaonis to Halorubrum, Natrialba, and Natronomonas gen. nov., respectively, as Halorubrum vacuolatum comb. nov., Natrialba magadii comb. nov., and Natronomonas pharaonis comb. nov., respectively. Int. J. Syst. Bacteriol. 47:853-857. [DOI] [PubMed] [Google Scholar]
- 46.Kaneda, K., T. Kuzuyama, M. Takagi, Y. Hayakawa, and H. Seto. 2001. An unusual isopentenyl diphosphate isomerase found in the mevalonate pathway gene cluster from Streptomyces sp. strain CL190. Proc. Natl. Acad. Sci. USA 98:932-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kates, M. 1993. Membrane lipids of archaea, p. 261-295. In M. Kates, D. J. Kushner, and A. T. Matheson (ed.), The biochemistry of Archaea (Archaebacteria). Elsevier Science Publishers B.V., Amsterdam, The Netherlands.
- 48.Kates, M. 1993. Membrane lipids of extreme halophiles: biosynthesis, function and evolutionary significance. Experientia 49:1027-1036. [DOI] [PubMed] [Google Scholar]
- 49.Kates, M. 1978. The phytanyl ether-linked polar lipids and isoprenoid neutral lipids of extremely halophilic bacteria. Prog. Chem. Fats Other Lipids 15:301-342. [DOI] [PubMed] [Google Scholar]
- 50.Kates, M., M. K. Wassef, and E. L. Pugh. 1970. Origin of the glycerol moieties in the glycerol diether lipids of Halobacterium cutirubrum. Biochim. Biophys. Acta 202:206-208. [DOI] [PubMed] [Google Scholar]
- 51.Koga, Y., T. Kyuragi, M. Nishihara, and N. Sone. 1998. Did archaeal and bacterial cells arise independently from noncellular precursors? A hypothesis stating that the advent of membrane phospholipid with enantiomeric glycerophosphate backbones caused the separation of the two lines of descent. J. Mol. Evol. 46:54-63. [DOI] [PubMed] [Google Scholar]
- 52.Koga, Y., and H. Morii. 2005. Recent advances in structural research on ether lipids from archaea including comparative and physiological aspects. Biosci. Biotechnol. Biochem. 69:2019-2034. [DOI] [PubMed] [Google Scholar]
- 53.Koga, Y., M. Nishihara, H. Morii, and M. Akagawa-Matsushita. 1993. Ether polar lipids of methanogenic bacteria: structures, comparative aspects, and biosynthesis. Microbiol. Rev. 57:164-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koga, Y., M. Ohga, M. Tsujimura, H. Morii, and Y. Kawarabayasi. 2006. Identification of sn-glycerol-1-phosphate dehydrogenase activity from genomic information of a hyperthermophilic archaeon Sulfolobus tokodaii strain 7. Biosci. Biotechnol. Biochem. 70:282-285. [DOI] [PubMed] [Google Scholar]
- 55.Koga, Y., N. Sone, S. Noguchi, and H. Morii. 2003. Transfer of pro-R hydrogen from NADH to dihydroxyacetonephosphate by sn-glycerol-1-phosphate dehydrogenase from the archaeon Methanothermobacter thermautotrophicus. Biosci. Biotechnol. Biochem. 67:1605-1608. [DOI] [PubMed] [Google Scholar]
- 56.Kon, T., N. Nemoto, T. Oshima, and A. Yamagishi. 2002. Effects of a squalene epoxidase inhibitor, terbinafine, on ether lipid biosynthesis in a thermoacidophilic archaeon, Thermoplasma acidophilum. J. Bacteriol. 184:1395-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kushwaha, S. C., M. Kates, and J. W. Porter. 1976. Enzymatic synthesis of C40 carotenes by cell-free preparation from Halobacterium cutirubrum. Can. J. Biochem. 54:816-823. [DOI] [PubMed] [Google Scholar]
- 58.Kushwaha, S. C., M. Kates, G. D. Sprott, and I. C. P. Smith. 1981. Novel polar lipids from the methanogen Methanospirillum hungatei GP1. Biochim. Biophys. Acta 664:156-173. [DOI] [PubMed] [Google Scholar]
- 59.Langworthy, T. A. 1982. Turnover of di-O-phytanylglycerol in Thermoplasma. Rev. Infect. Dis. 4:S266. [Google Scholar]
- 60.Langworthy, T. A., and J. L. Pond. 1986. Membranes and lipids of thermophiles, p. 107-135. In T. D. Brock (ed.), Thermophiles: general, molecular, and applied microbiology. John Wiley & Sons, New York, NY.
- 61.Liang, P.-H., T.-P. Ko, and A. H.-J. Wang. 2002. Structure, mechanism and function of prenyltransferase. Eur. J. Biochem. 269:3339-3354. [DOI] [PubMed] [Google Scholar]
- 62.Maki, H., and M. Sekiguchi. 1992. MutT protein specifically hydrolyses a potent mutagenic substrate for DNA synthesis. Nature 355:273-275. [DOI] [PubMed] [Google Scholar]
- 63.Martin, W., and M. J. Russell. 2003. On the origin of cells: a hypothesis for the evolutionary transitions from abiotic geochemistry to chemoautotrophic prokaryotes, and from prokaryotes to nucleated cells. Philos. Trans. R. Soc. Lond. Biol. Sci. 358:59-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Masuchi, Y., A. Sugai, N. Minaka, Y. H. Itoh, T. Itoh, and I. Karube. 1998. Isoprenoid synthesis gene for geranylgeranyl diphosphate synthase from a hyperthermophile, Pyrococcus sp. strain OT3. J. Mar. Biotechnol. 6:201-205. [PubMed] [Google Scholar]
- 65.Matsumoto, K. 1997. Phosphatidylserine synthase from bacteria. Biochim. Biophys. Acta 1348:214-227. [DOI] [PubMed] [Google Scholar]
- 66.Moldoveanu, N., and M. Kates. 1988. Biosynthetic studies of the polar lipids of Halobacterium cutirubrum. Formation of isoprenyl ether intermediate. Biochim. Biophys. Acta 960:164-182. [Google Scholar]
- 67.Moldoveanu, N., and M. Kates. 1989. Effect of bacitracin on growth and phospholipid, glycolipid and bacterioruberin biosynthesis in Halobacterium cutirubrum. J. Gen. Microbiol. 135:2503-2508. [Google Scholar]
- 68.Morii, H., and Y. Koga. 1994. Asymmetric topology of diether and tetraether type polar lipids in the membrane of Methanobacterium thermoautotrophicum cells. J. Biol. Chem. 269:10492-10497. [PubMed] [Google Scholar]
- 69.Morii, H., and Y. Koga. 2003. CDP-2,3-di-O-geranylgeranyl-sn-glycerol:l-serine O-archaetidyltransferase (archaetidylserine synthase) in the methanogenic archaeon Methanothermobacter thermautotrophicus. J. Bacteriol. 185:1181-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Morii, H., M. Nishihara, and Y. Koga. 2000. CTP:2,3-di-O-geranylgeranyl-sn-glycero-1-phosphate cytidyltransferase in the methanogenic archaeon Methanothermobacter thermoautotrophicus. J. Biol. Chem. 275:36568-36574. [DOI] [PubMed] [Google Scholar]
- 71.Morii, H., H. Yagi, H. Akutsu, N. Nomura, Y. Sako, and Y. Koga. 1999. A novel phosphoglycolipid archaetidyl(glucosyl)inositol with two sesterterpenyl chains from the aerobic hyperthermophilic archaeon Aeropyrum pernix K1. Biochim. Biophys. Acta 1436:426-436. [DOI] [PubMed] [Google Scholar]
- 71a.Murakami, M., H. Hemmi, K. Shibuya, T. Nakayama, T. Nishino, and T. Yoshimura. 2006. Abstr. 19th Meet. Jpn. Soc. Archaea, abstr. 13-14.
- 72.Nemoto, N., T. Oshima, and A. Yamagishi. 2003. Purification and characterization of geranylgeranylglyceryl phosphate synthase from a thermoacidophilic archaeon, Thermoplasma acidophilum. J. Biochem. 133:651-657. [DOI] [PubMed] [Google Scholar]
- 73.Nemoto, N., Y. Shida, H. Shimada, T. Oshima, and A. Yamagishi. 2003. Characterization of the precursor of tetraether lipid biosynthesis in the thermoacidophilic archaeon Thermoplasma acidophilum. Extremophiles 7:235-243. [DOI] [PubMed] [Google Scholar]
- 74.Nishihara, M., and Y. Koga. 1997. Purification and properties of sn-glycerol-1-phosphate dehydrogenase from Methanobacterium thermoautotrophicum: characterization of the biosynthetic enzyme for the enantiomeric glycerophosphate backbone of ether polar lipids of archaea. J. Biochem. 122:572-576. [DOI] [PubMed] [Google Scholar]
- 75.Nishihara, M., and Y. Koga. 1995. sn-Glycerol-1-phosphate dehydrogenase in Methanobacterium thermoautotrophicum: key enzyme in biosynthesis of the enantiomeric glycerophosphate backbone of ether phospholipids of archaebacteria. J. Biochem. 117:933-935. [DOI] [PubMed] [Google Scholar]
- 76.Nishihara, M., H. Morii, and Y. Koga. 1989. Heptads of polar ether lipids of an archaebacterium, Methanobacterium thermoautotrophicum: structure and biosynthetic relationship. Biochemistry 28:95-102. [Google Scholar]
- 77.Nishihara, M., H. Morii, and Y. Koga. 1987. Structure determination of a quartet of novel tetraether lipids from Methanobacterium thermoautotrophicum. J. Biochem. 101:1007-1015. [DOI] [PubMed] [Google Scholar]
- 78.Nishihara, M., M. Utagawa, H. Akutsu, and Y. Koga. 1992. Archaea contain a novel diether phosphoglycolipid with a polar head group identical to the conserved core of eucaryal glycosyl phosphatidylinositol. J. Biol. Chem. 267:12432-12435. [PubMed] [Google Scholar]
- 79.Nishihara, M., T. Yamazaki, T. Oshima, and Y. Koga. 1999. sn-Glycerol-1-phosphate-forming activities in archaea: separation of archaeal phospholipid biosynthesis and glycerol catabolism by glycerophosphate enantiomers. J. Bacteriol. 181:1330-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nishimura, Y., and T. Eguchi. 2006. Biosynthesis of archaeal membrane lipids: digeranylgeranylglyceryphospholipid reductase of the thermophilic archaeon Thermoplasma acidophilum. J. Biochem. 139:1073-1081. [DOI] [PubMed] [Google Scholar]
- 81.Noguchi, S., M. Maeda, M. Nishihara, Y. Koga, and N. Sone. 1998. Expression and use of Methanobacterium thermoautotrophicum sn-glycerol 1-phosphate dehydrogenase for the assay of sn-glycerol 1-phosphate in archaea. J. Ferment. Bioeng. 86:266-270. [Google Scholar]
- 81a.Ohnuma, S., T. Kato, and T. Nishino. 1995. Abstr. 68th Ann. Meet. Jpn. Biochem. Soc., p. 895. (In Japanese.)
- 82.Ohnuma, S., K. Hirooka, H. Hemmi, C. Ishida, C. Ohto, and T. Nishino. 1996. Conversion of product specificity of archaebacterial geranylgeranyl-diphosphate synthase—identification of essential amino acid residues for chain length determination of prenyltransferase reaction. J. Biol. Chem. 271:18831-18837. [DOI] [PubMed] [Google Scholar]
- 83.Ohnuma, S., K. Hirooka, C. Ohto, and T. Nishino. 1997. Conversion from archaeal geranylgeranyl diphosphate synthase to farnesyl diphosphate synthase—two amino acids before the first aspartate-rich motif solely determine eukaryotic farnesyl diphosphate synthase activity. J. Biol. Chem. 272:5192-5198. [DOI] [PubMed] [Google Scholar]
- 84.Ohnuma, S., K. Hirooka, N. Tsuruoka, M. Yano, C. Ohto, H. Nakane, and T. Nishino. 1998. A pathway where polyprenyl diphosphate elongates in prenyltransferase. J. Biol. Chem. 273:26705-26713. [DOI] [PubMed] [Google Scholar]
- 85.Ohnuma, S., M. Suzuki, and T. Nishino. 1994. Archaebacterial ether-linked lipid biosynthetic gene—expression, cloning, sequencing, and characterization of geranylgeranyl diphosphate synthase. J. Biol. Chem. 269:14792-14797. [PubMed] [Google Scholar]
- 86.Ohnuma, S., M. Watanabe, and T. Nishino. 1996. Identification and characterization of geranylgeraniol kinase and geranylgeranyl phosphate kinase from the archaebacterium Sulfolobus acidocaldarius. J. Biochem. 119:541-547. [DOI] [PubMed] [Google Scholar]
- 87.Ohto, C., H. Nakane, H. Hemmi, S. Ohnuma, S. Obata, and T. Nishino. 1998. Overexpression of an archaeal geranylgeranyl diphosphate synthase in Escherichia coli cells. Biosci. Biotechnol. Biochem. 62:1243-1246. [DOI] [PubMed] [Google Scholar]
- 88.Payandeh, J., M. Fujihashi, W. Gillon, and E. F. Pai. 2006. The crystal structure of (S)-3-O-geranylgeranylglyceryl phosphate synthase reveals an ancient fold for an ancient enzyme. J. Biol. Chem. 281:6070-6078. [DOI] [PubMed] [Google Scholar]
- 89.Pereto, J., P. Lopez-Garcia, and D. Moreira. 2004. Ancestral lipid biosynthesis and early membrane evolution. Trends Biochem. Sci. 29:469-477. [DOI] [PubMed] [Google Scholar]
- 90.Poulter, C. D., T. Aoki, and L. Daniels. 1988. Biosynthesis of isoprenoid membranes in the methanogenic archaebacterium Methanospirillum hungatei. J. Am. Chem. Soc. 110:2620-2624. [Google Scholar]
- 91.Rawal, N., S. M. Kelkar, and W. Altekar. 1988. Alternative routes of carbohydrate metabolism in halophilic archaebacteria. Ind. J. Biochem. Biophys. 25:674-686. [PubMed] [Google Scholar]
- 92.Rohmer, M. 1999. The discovery of a mevalonate-independent pathway for isoprenoid biosynthesis in bacteria, algae and higher plants. Nat. Prod. Rep. 16:565-574. [DOI] [PubMed] [Google Scholar]
- 93.Smit, A., and A. Mushegian. 2000. Biosynthesis of isoprenoids via mevalonate in archaea: the lost pathway. Genome Res. 10:1465-1484. [DOI] [PubMed] [Google Scholar]
- 94.Soderberg, T., A. J. Chen, and C. D. Poulter. 2001. Geranylgeranylglyceryl phosphate synthase. Characterization of the recombinant enzyme from Methanobacterium thermoautotrophicum. Biochemistry 40:14847-14854. [DOI] [PubMed] [Google Scholar]
- 95.Stieglitz, K. A., H. Y. Yang, M. F. Roberts, and B. Stec. 2005. Reaching for mechanistic consensus across life kingdoms: structure and insights into catalysis of the myo-inositol-1-phosphate synthase (mIPS) from Archaeoglobus fulgidus. Biochemistry 44:213-224. [DOI] [PubMed] [Google Scholar]
- 96.Swain, M., J. R. Brisson, G. D. Sprott, F. P. Cooper, and G. B. Patel. 1997. Identification of β-l-glucose as the sugar moiety of the main polar lipid Thermoplasma acidophilum. Biochim. Biophys. Acta. 1345:56-64. [DOI] [PubMed] [Google Scholar]
- 97.Tachibana, A. 1994. A novel prenyltransferase, farnesylgeranyl diphosphate synthase, from the haloalkaliphilic archaeon, Natronobacterium pharaonis. FEBS Lett. 341:291-294. [DOI] [PubMed] [Google Scholar]
- 98.Tachibana, A., T. Tanaka, M. Taniguchi, and S. Oi. 1996. Evidence for farnesol-mediated isoprenoid synthesis regulation in a halophilic archaeon, Halferax volcanii. FEBS Lett. 379:43-46. [DOI] [PubMed] [Google Scholar]
- 99.Tachibana, A., T. Tanaka, M. Taniguchi, and S. Oi. 1993. Potassium-stimulating mechanism of geranylgeranyl diphosphate synthase of Methanobacterium thermoformicicum. J. Biochem. 114:389-392. [DOI] [PubMed] [Google Scholar]
- 100.Tachibana, A., T. Tanaka, M. Taniguchi, and S. Oi. 1993. Purification and characterization of geranylgeranyl diphosphate synthase from Methanobacterium thermoautotrophicum SF-4. Biosci. Biotechnol. Biochem. 57:1129-1133. [DOI] [PubMed] [Google Scholar]
- 101.Tachibana, A., Y. Yano, S. Otani, N. Nomura, Y. Sako, and M. Taniguchi. 2000. Novel prenyltransferase gene encoding farnesylgeranyl diphosphate synthase from a hyperthermophilic archaeon, Aeropyrum pernix—molecular evolution with alteration in product specificity. Eur. J. Biochem. 267:321-328. [DOI] [PubMed] [Google Scholar]
- 102.Torreblanca, M., F. Rodoriguez-Valera, G. Juez, A. Ventosa, M. Kamekura, and M. Kates. 1986. Classification of non-alkaliphilic halobacteria based on numerical taxonomy and polar lipid composition, and description of Haloarcula gen. nov. and Haloferax gen. nov. Syst. Appl. Microbiol. 8:89-99. [Google Scholar]
- 103.van den Bosch, H., and E. C. J. M. de Vet. 1997. Alkyl-dihydroxyacetonephosphate synthase. Biochim. Biophys. Acta 1348:35-44. [DOI] [PubMed] [Google Scholar]
- 104.Ventosa, A., and A. Oren. 1996. Halobacterium salinarum nom. corrig., a name to replace Halobacterium salinarium (Elazari-Volcani) and to include Halobacterium halobium and Halobacterium cutirubrum. Int. J. Syst. Bacteriol. 46:347. [Google Scholar]
- 105.Wächtershäuser, G. 2003. From pre-cells to Eukarya—a tale of two lipids. Mol. Microbiol. 47:13-22. [DOI] [PubMed] [Google Scholar]
- 106.Wassef, M. K., J. Sarner, and M. Kates. 1970. Stereospecificity of the glycerol kinase and the glycerophosphate dehydrogenase in Halobacterium cutirubrum. Can. J. Biochem. 48:69-73. [DOI] [PubMed] [Google Scholar]
- 107.Wasserfallen, A., J. Nolling, P. Pfister, J. Reeve, and E. C. de Macario. 2000. Phylogenetic analysis of 18 thermophilic Methanobacterium isolates supports the proposals to create a new genus, Methanothermobacter gen. nov., and to reclassify several isolates in three species, Methanothermobacter thermautotrophicus comb. nov., Methanothermobacter wolfeii comb. nov., and Methanothermobacter marburgensis sp. nov. Int. J. Syst. Evol. Microbiol. 50:43-53. [DOI] [PubMed] [Google Scholar]
- 108.Whitman, W. B. 2001. Genus II. Methanotorris gen. nov., p. 245-246. In D. R. Boone and R. W. Castenholz (ed.), Bergey's manual of systematic bacteriology—the archaea and the deeply branching and phototrophic bacteria, vol. 1. Springer-Verlag, New York, NY. [Google Scholar]
- 109.Woese, C. R., O. Kandler, and M. L. Wheelis. 1990. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc. Natl. Acad. Sci. USA 87:4576-4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yamashita, S., H. Hemmi, Y. Ikeda, T. Nakayama, and T. Nishino. 2004. Type 2 isopentenyl diphosphate isomerase from a thermoacidophilic archaeon, Sulfolobus shibatae. Eur. J. Biochem. 271:1087-1093. [DOI] [PubMed] [Google Scholar]
- 111.Yasuda, M., H. Oyaizu, A. Yamagishi, and T. Oshima. 1995. Morphological variation of new Thermoplasma acidophilum isolates from Japanese hot springs. Appl. Environ. Microbiol. 61:3482-3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.You, K. 1985. Stereospecificity for nicotinamide nucleotide in enzymatic and chemical hydride transfer reactions. CRC Crit. Rev. Biochem. 17:313-451. [DOI] [PubMed] [Google Scholar]
- 113.Zhang, D., and C. D. Poulter. 1993. Biosynthesis of archaebacterial ether lipid in Halobacterium halobium and Methanobacterium thermoautotrophicum. J. Org. Chem. 58:3919-3922. [Google Scholar]
- 114.Zhang, D., and C. D. Poulter. 1993. Biosynthesis of archaebacterial ether lipids—formation of ether linkages by prenyltransferases. J. Am. Chem. Soc. 115:1270-1277. [Google Scholar]
- 115.Zhang, D.-L., L. Daniels, and C. D. Poulter. 1990. Biosynthesis of archaebacterial membranes. Formation of isoprene ethers by a prenyl transfer reaction. J. Am. Chem. Soc. 112:1264-1265. [Google Scholar]
- 116.Zillig, W., K. O. Stetter, S. Wunderl, W. Schulz, H. Priess, and I. Scholz. 1980. The Sulfolobus-“Caldariella” group: taxonomy on the basis of the structure of DNA-dependent RNA polymerases. Arch. Microbiol. 125:259-269. [Google Scholar]