Abstract
Hypothesis
A minimal threshold of S. pneumoniae is required to induce meningitis in healthy animals for intraperitoneal (hematogenous), middle ear and inner ear inoculations and this threshold may be altered by recent inner ear surgery.
Background
There has been an increase in the number of reported cases of cochlear implant-related pneumococcal meningitis since 2002. The pathogenesis of pneumococcal meningitis is complex and not completely understood. The bacteria can reach the central nervous system (CNS) from the upper respiratory tract mucosa via either hematogenous route or via the inner ear. The establishment of a threshold model for all potential routes of infection to the CNS in animals without cochlear implantation is an important first step to help us understand the pathogenesis of the disease in animals with cochlear implantation.
Methods
54 otologically normal, adult Hooded Wistar rats (27 receiving cochleostomy and 27 controls) were inoculated with different amounts of bacterial counts via three different routes (intraperitoneal, middle ear and inner ear). Rats were monitored over 5 days for signs of meningitis. Blood, CSF and middle ear swabs were taken for bacterial culture and brains and cochleae were examined for signs of infection.
Results
The threshold of bacterial counts required to induce meningitis is lowest in rats receiving direct inner ear inoculation compared to both intraperitoneal and middle ear inoculation. There is no change in threshold between the group of rats with cochleostomy and the control (Fisher exact test; p < 0.05).
Conclusion
A minimal threshold of bacteria is required to induce meningitis in healthy animals and is different for three different routes of infection (intraperitoneal, middle ear and inner ear). Cochleostomy performed 4 weeks prior to the inoculation did not reduce the threshold of bacteria required for meningitis in all three infectious routes. This threshold model will also serve as a valuable tool, assisting clinicians to quantitatively determine if the presence of a cochlear implant or other central nervous system (CNS) prostheses alter the risk of meningitis.
Keywords: pneumococcal meningitis, routes of infection, Streptococcus pneumoniae, threshold model
Introduction
Meningitis was considered to be a rare complication post cochlear implantation. However, since 2002, an increase in the number of cases of cochlear implant-related meningitis has been reported to the US Food and Drug Administration1. The most common organism identified was Streptococcus pneumoniae2. The incidence of pneumococcal meningitis was found to be greater than that of an age matched cohort in the general population3. Risk factors identified from the clinical records of patients with cochlear implants included an implant with a positioner, inner-ear malformations with and without cerebral spinal fluid (CSF) leak, the presence of a CSF leak after cochlear implantation, a history of ventriculoperitoneal-shunt placement, and a history of otitis media3–5. In some implanted patients, acute otitis media (AOM) was diagnosed concurrently with meningitis. It should be noted, however, that profoundly deaf patients without cochlear implantation also can have a higher risk of acquiring meningitis compared with normal population because of their pre-existing risk factors1.
Pathogenesis of pneumococcal meningitis is very complex and is not completely understood6. An individual’s susceptibility to pneumococcal meningitis is determined by the interaction between the host’s immune response and the virulence factors of S. pneumoniae. It is well documented that adults with chronic illness or conditions, individuals with a history of congenital CSF fistula or head trauma with basilar skull fracture with or without CSF leak, and adults over 65 years of age and children under the age of 2 who are otherwise healthy, are associated with increased frequency and/or severity of serious pneumococcal infections7–13. However, an immunocompetent individual with no pre-existing risk factors can acquire pneumococcal meningitis and this may be due to increase virulence of the bacteria in a particular host. Although the polysaccharide capsular antigen is the key virulence factor of the bacteria14, other components including the enzymes (e.g. hyaluronidase, pneumolysin, neuraminidases) and cellular wall components (e.g. PspA, PspC, PsaA) have been implicated as important virulence factors for the pathogenesis of pneumococcal disease15–23.
A review of the literature for patients who acquired pneumococcal meningitis and have not received a cochlear implant suggests that S. pneumoniae can spread to the meninges either directly from the middle ear or from the hematogenous seeding of bacteria. The tympanogenic or otogenic spread of infection can be further sub-classified into either a direct invasion of the meninges by the bacteria (via direct communication between the middle ear/mastoid cavity and the CNS as a result of congenital temporal bone malformation, trauma or neoplasm or chronic infection of temporal bone) or indirect invasion via the inner ear24,25. When meningitis occurs in the presence of acute otitis media (AOM), there is usually insufficient evidence to suggest a direct spread of S. pneumoniae from the middle ear to the inner ear then to the meninges as the only means of infection. The role of the hematogenous spread of the bacteria from the middle ear to the meninges must also be considered.
Previous work investigating Haemophilus influenzae meningitis in rats demonstrates that a minimal threshold of bacteria is required to induce meningitis via intranasal inoculation of bacteria26,27. The development of meningitis as a result of H. influenzae infection is determined by the intensity of bacteremia which correlates with the concentration of the intranasal inoculum27. The threshold theory for CNS infection for different routes of infection has not been studied in S. pneumoniae. This concept may be important in the development of pneumococcal meningitis in humans. We propose that the number of bacteria exposed to a human subject is one of the important factors for determining whether a person acquires pneumococcal meningitis. A minimum or threshold level of the bacteria is required to induce meningitis in a healthy individual. This threshold may be reduced in patients with existing risk factors for acquiring pneumococcal meningitis. We have established an animal model to examine whether a threshold relationship exists between the rate of pneumococcal CNS infection and the concentration of the infective inoculum.
As there are different routes by which S. pneumoniae reach the meninges, our previous work showed that animals with cochlear implantation acquired pneumococcal meningitis following three separate routes of infection (hematogenous, middle and inner ear)28. This study builds on the previous work by examining the threshold of bacteria required to induce meningitis for each route of infection in healthy non-implanted animals. The understanding of these relationships is an important first step to determine whether cochlear implantation increases the risk of pneumococcal meningitis. The threshold model established in this paper will help us to determine whether the cochlear implant or other surgical interventions shift the threshold for all possible routes of CNS infection, and may provide further understanding of pathogenesis of pneumococcal meningitis in patients with other CNS prostheses.
Material and methods
Source of the animals
All the experimental animals were bred and housed in the animal house within the Department of Otolaryngology, University of Melbourne. All procedures and animal handling were conducted in accordance with guidelines set by the Animal Research Ethics Committee of the Royal Victorian Eye and Ear Hospital and “The Australian code of practice for the care and use of animals for scientific purposes” from the National Health and Medical Research Council 2004.
In total, 54 otologically normal adult Hooded Wisters rats (10 to 16 weeks old) weighing between 100g to 400g, were used in the study. One cohort of 27 rats received a cochleostomy (a small opening of the cochlea that is created surgically to allow insertion of a cochlear implant electrode array) to the left ear 4 weeks prior to inoculation with bacteria. A second control cohort of 27 rats had no surgical procedures performed prior to inoculation. The cochleostomy was selected as the surgical intervention because the breach of the bony and mucosa barrier between the middle and inner ear during cochlear implantation has been considered a potential risk factor for meningitis post cochlear implantation29. The understanding of the effect of cochleostomy on the threshold of infection will provide important background information for subsequent study of the effects of cochlear implantation on the risk of pneumococcal meningitis.
Eighteen rats (9 operated and 9 non-operated control animals) were randomly allocated to each of the three different routes of bacterial inoculation (middle ear, inner ear and intra-peritoneal (i/p); Table 1). Within each infection route group, 6 rats (3 non-operated, 3 operated) were allocated to one of three concentrations of the bacteria used for each route (Table 1).
Table 1.
Summary of meningitis threshold study
| Routes of inoculation | Concentrations of the bacteria : Colony-forming Units (CFU) | Number of rats | |
|---|---|---|---|
| Cochleostomy | Control | ||
| Intra-peritoneal (i/p) | 4 x 1010 | 3 | 3 |
| 4 x 108 | 3 | 3 | |
| 4 x 106 | 3 | 3 | |
| Inner ear | 1 x 107 | 3 | 3 |
| 1 x 105 | 3 | 3 | |
| 1 x 103 | 3 | 3 | |
| Middle ear | 3 x 108 | 3 | 3 |
| 3 x 106 | 3 | 3 | |
| 3 x 104 | 3 | 3 | |
Cochleostomy surgery
Twenty-seven adult rats underwent a cochleostomy of the left inner ear. Under anesthesia (see below), a postauricular skin incision was made and subcutaneous tissues and muscles of the head and neck were dissected and retracted to expose the auditory bulla. Care was taken not to damage the facial nerve, which lies antero-superior to the bulla. A 2.0mm cutting burr was used to open the bulla and expose the round window membrane (RWM). The bulla cavity was inspected for any abnormality of the middle ear mucosa; only animals with a macroscopically normal mucosa were used in this study. Both the stapedial artery and the round window niche were identified. The stapedial artery, which is located just below the round window niche, was cauterized with a Zencor MF1 bipolar coagulator (Zencor, Australia) and a cochleostomy was performed. The scala tympani was entered by using a straight Kirschner wire (diameter 0.8mm) (KA0R5171, Kaisers, WA, Australia) and temporalis fascia used to seal the cochleostomy site. The animal had two doses of prophylactic antibiotics Enrofloxacin (Baytril 50®, Bayer Australia Ltd., NSW, Australia) 10mg/kg subcutaneously (s/c) diluted 1:1 with saline, one dose immediately after operation and the second dose 12 hours later.
Surgical anesthesia
Rats were anesthetized with an i/p injection of a mixture of 8mg/kg xylazine (Ilium Xylazil-20®, Troy Laboratories Pty. Ltd. NSW, Australia) and 75mg/kg ketamine hydrochloride (Ketamine®, Parnell Laboratories, NSW, Australia). A local anesthetic agent (0.1 ml of lignocaine hydrochloride with 0.0182 mg/ml of adrenalin tartrate, Troy Laboratories, NSW, Australia) was injected subcutaneously around the surgical incision. The animals were then placed on a heated pad maintained at 37° C throughout the surgery. The animals were given 0.03–0.05mg/kg s/c Buprenorphine (Temgesic®, Reckitt Benckiser, NSW, Australia) for analgesia immediately after surgery. They were assessed continuously for signs of post-operative pain and discomfort, and Buprenorphine was given on an 8 to 12 hourly basis if there were signs of post-operative pain or discomfort. The animals were given 10ml/kg of normal saline s/c during recovery from the surgery for fluid replacement.
Inoculation of Streptococcus pneumoniae
The preparation of inoculum has been described in detail in the previous study28. In brief, Streptococcus pneumoniae 447A, which carries type 2 capsular antigen, and was originally isolated from the CSF of a child with meningitis, was selected for the study28. The bacteria were stored at −70°C in aliquots of nutrient broth containing 30% (V/V) glycerol. The inoculum for each experiment was prepared from bacteria grown on horse blood agar (HBA) at 37°C, overnight in air containing 5% CO2. The isolated colonies from these plates were thoroughly emulsified in pre-warmed phosphate buffered saline (PBS) until they matched a turbidity standard equivalent (McFarland Equivalence Turbidity Standards) of the desired concentrations. Three graded concentrations of inoculum for each of the infectious routes were chosen (Table 1). The amounts of bacteria for each route of inoculation were derived from the maximum infective dose of our previous established rat meningitis model and titrated down at a decrement of 2 log scale28.
Retrospective viable counts of the inoculum were performed by making serial ten fold dilutions in PBS using the Miles and Misra method30 for quantifying bacteria just prior to inoculation. The bacteria were spotted onto HBA plates (10 μl / dilution), allowed to dry and incubated at 37°C overnight. Colonies were counted following incubation and the actual inoculum calculated (Colony-forming Unit (CFU)/ml). Retrospective viable counts of the inoculum confirmed that each rat consistently received the desired amount of bacteria as indicated in Table 1.
Methods of bacterial inoculation
Bacteremia as a result of i/p inoculation was introduced to study hematogenous spread of infection without the possible confounding effect of direct invasion of the meninges from middle ear infection. Direct inoculation of the bacteria into the inner ear was introduced to study the direct route of infection from the middle ear to the meninges via inner ear without the bacteremia of the middle ear infection. The attack rate of meningitis from middle ear inoculation is compared with the inner ear and hematogenous route of infection.
Intraperitoneal inoculation (i/p) (Hematogenous spread of infection to the meninges)
Eighteen rats were anesthetized as described above and three groups of 6 rats (3 operated and 3 non-operated controls) received 3 different concentrations of the bacteria in 1 ml inoculum via direct injection into the intra-peritoneal cavity using a sterile 20 Gauge (G) needle and 1ml syringe (Table 1).
Middle ear inoculation
Under general anesthesia, the left bullae of 18 rats were surgically exposed for direct inoculation of 3 different concentrations of the bacteria in a10 μl inoculum (Table 1). To retain the micro-organisms in the bulla, the cavity was first filled with Gelfoam®(Pharmacia & Upjohn, Michigan, USA). After the inoculation of the bacteria, the opening of the bulla was covered with temporalis fascia and the wound sutured in 2 layers.
Inner ear inoculation
Under general anesthesia, the left bulla was surgically exposed and a cochleostomy to access the scala tympani was performed with a straight Kirschner wire. Two microliters of perilymph were removed and 1 μl of bacterial inoculum was inoculated into the scala tympani over 1 minute using an infusion catheter, 5 μl micro-syringe (ILS, Stützerbach, Germany), and a micro-syringe pump controller (World Precision Instruments Inc, Sarasota, FL, USA; Table 1). The cochleostomy was then covered with temporalis fascia. The opening of the bulla was covered with temporalis fascia and the wounds sutured in 2 layers.
Post infection monitoring
Following the inoculation each animal was examined, at a minimum, twice daily for clinical signs of meningitis over 5 days. The clinical assessment was recorded in a 12 point scored monitoring sheet as described previously28.
Microbiological specimen collection and tissue preparation
Isoflurane was used to deeply anesthetize rats, once they developed early signs of meningitis, to allow collection of CSF, middle ear fluid and blood for microscopy and culture (described in detail in the previous study)28. Briefly, a 30G needle with a 1ml syringe was used to perform a cisterna puncture to aspirate CSF using sterile techniques. Approximately 10 to 50 μl of CSF was collected for bacterial culture. Biochemistry, microscopy and cell counts could not be performed due to the small sample of CSF collected; not performing these tests did not alter the interpretation and outcome of the results28. One millilitre of blood was aspirated by intracardiac puncture using a 23G needle and sent for blood culture. While animals were still fully anesthetized, bacterial swabs were taken from the left bulla under a strict aseptic technique and were sent for bacteriological analysis.
The animals were then given a lethal dose of pentobarbitone sodium, 120mg/kg of body weight, intramuscularly (Lethabarb®, Virbac Pty. Ltd. NSW, Australia) and were transcardially perfused with 0.9% saline then 10% neutral buffered formalin (NBF) pH 7.4 at 4° C. The brain, meninges and the cochleae were harvested and placed in 10% NBF for further processing.
Fifty-four brains including meninges were harvested and stored in 10% NBF for 48 hours then embedded in paraffin. The specimens were sectioned 10 μm thick, stained with both Haematoxylin and Eosin (H &E) and Gram stain and examined under light microscopy for presence of inflammation and gram-positive cocci.
Twelve pairs of randomly selected cochleae were harvested from the temporal bones and fixed in 10% NBF. They were decalcified in a solution of 10% ethylene diamine tetra-acetic acid in 0.1M phosphate buffer (pH 7.4) on an agitation platform. Excessive bone was trimmed. They were then processed and embedded in Spurr’s resin. The embedded cochleae were orientated and twenty-one 2 μm sections were collected at 126 μm intervals throughout the cochlea. Three sections were stained with H &E and representative sections with Gram stain.
The outcome of the study was to detect the presence of meningitis. CSF, blood and middle ear cultures were collected to detect the presence of the bacteria. The meninges were harvested for histological analysis and were used to confirm the culture results. Serotypes of S. pneumoniae isolated from the cultures were reexamined to ensure that the strain causing the disease was the same as the initial inoculum.
Histology analysis
The sections of histological specimens were examined under a light microscope. The brain and the meninges were examined for presence of an inflammatory cell response within the subarachnoid space and brain tissue, thickening and hyperplasia of the meningeal cells, gram-positive cocci within the subarachnoid space and brain tissue. The cochleae were examined for the presence of bacteria and inflammatory cells.
Statistical analysis
The effects of cochleostomy on the threshold of infection for the three different routes of inoculation have been evaluated statistically using Fisher’s exact test which calculates the exact probability of observing a particular 2 x 2 table, and those in which more extreme values would be obtained. The null hypothesis is that there is no difference in the incidence of meningitis when comparing rats with a cochleostomy to that of a control group of rats for each of the three different inoculating routes. If the sum of probabilities calculated using Fisher’s test is less than the significance level required (i.e., p<0.05), the null hypothesis is rejected and a significant difference between the groups has been demonstrated not to have occurred by chance.
Results
In control rats with normal cochleae the thresholds of S. pneumoniae required to induce meningitis differed for each of the three different routes of inoculation. The following symptoms were observed when rats acquired meningitis: tiredness, lethargy, unresponsiveness to sound and light stimulations, a hunched body posture, poor grooming, weight loss and rectal temperature rising above 38°C. When these signs developed, the histology of the brain consistently showed evidence of meningitis with the infiltration of inflammatory cells and gram-positive diplococci within the subarachnoid space. Rats that did not exhibit the above clinical signs showed no histological evidence of meningitis.
The attack rate of meningitis was reduced and the time required to induce the disease increased as the number of bacteria in the inoculum was lowered for all routes of infection (Table 2). With the highest numbers of bacteria (4 x 1010 CFU) via the i/p route, all rats developed meningitis within 15 hours. Inoculating with lower numbers (4 x 106 CFU) of S. pneumoniae via the same route, only 2 out of 6 rats developed meningitis and this occurred within 51 to 82 hours post inoculation. Similar trends were seen in rats receiving bacteria via the inner and middle ears. Inoculating with 1 x 107 CFU S. pneumoniae via the inner ear, all 6 rats developed meningitis within 26 hours. When the number of bacteria for the inner ear inoculation was reduced to 1 x 103 CFU, only 1 rat developed meningitis at 72 hours post inoculation. In rats receiving a middle ear inoculation at a level of 3 x 108 CFU, only 4 rats developed meningitis 72 to120 hours post inoculation. When the amount of bacteria was reduced to 3 x 104 CFU, no rats developed meningitis 5 days post inoculation. The attack rates of meningitis for rats with and without cochleostomy are also shown in Table 2. A cochleostomy performed 4 weeks prior to inoculation did not appear to increase the attack rate of meningitis compared with the non-operated group.
Table 2.
Summary of the results
| Routes of inoculation | Amount of S. pneumoniae: Colony- forming Units (CFU) | Number of rats exhibited meningitis after 5 days | Ratio of meningitic rats with cochleostomy vs. control | Time from inoculation to meningitis (hours) | Number of rats with positive blood culture (S. pneumoniae) | Number of rats with positive CSF culture (S. pneumoniae) | Number of rats with positive left middle ear culture (S. pneumoniae) | Number of rats with positive histological evidence of meningitis |
|---|---|---|---|---|---|---|---|---|
| Intra-peritoneal (i/p) | 4 x 1010 | 6 | 3:3 | 14 – 15 | 6 | 6 | 0 | 6 |
| 4 x 108 | 3 | 2:1 | 28 – 30 | 4 | 4 | 4 | 3 | |
| 4 x 106 | 2 | 0:2 | 51 – 82 | 5 | 3 | 1 | 2 | |
| Inner ear | 1 x 107 | 6 | 3:3 | 22 – 26 | 6 | 6 | 6 | 6 |
| 1 x 105 | 5 | 2:3 | 50 – 54 | 5 | 5 | 6 | 5 | |
| 1 x 103 | 1 | 0:1 | 72 | 1 | 1 | 1 | 1 | |
| Middle ear | 3 x 108 | 4 | 1:3 | 72 – 120 | 6 | 5 | 6 | 4 |
| 3 x 106 | 2 | 1:1 | 96 | 5 | 2 | 6 | 2 | |
| 3 x 104 | 0 | N/A | N/A | 2 | 2 | 6 | 0 |
N/A: no rats from both cohort of control and cochleostomy exhibited meningitis.
Blood culture, CSF culture and left middle ear swab culture results are summarized in Table 2. Serotyping of the bacteria from positive blood, CSF and middle ear fluid cultures showed the bacteria to be serotype 2 (the same strain of S. pneumoniae contained within the inoculum).
For i/p inoculation, all rats developed bacteremia and meningitis with the infectious dosage of 4 x 1010 CFU. When the amount of bacteria was lowered to the level of 4 x 108 CFU, 4 rats were found to have bacteremia. All these 4 rats had a positive middle ear swab culture for S. pneumoniae but only 3 of these 4 rats had both clinical and histological evidence of meningitis as indicated by the presence of gram-positive cocci within the subarachnoid space. Five rats that received 4 x 106 CFU of S. pneumoniae i/p, showed a positive blood culture while 3 animals had a positive CSF culture and one exhibited a positive middle ear swab culture. Only 2 of the 3 rats with a positive blood culture had both clinical and histological evidence of meningitis.
S. pneumoniae were isolated from blood, CSF and the middle ear of all 6 rats inoculated with 1 x 107 CFU of the bacteria directly into the inner ear. All these animals demonstrated both clinical and histological evidence of meningitis. When the amount of the bacteria was lowered to 1 x 105 CFU, 5 out of 6 rats exhibited S. pneumoniae in blood and CSF cultures. All these 5 rats showed clinical and histological evidence of meningitis. The bacteria were isolated in the middle ear swab of all 6 rats receiving 1 x 105 CFU of the bacteria via the inner ear. At a much lower amount of bacteria, 1 x 103 CFU, via inner ear inoculation, only 1 rat showed a positive culture for S. pneumoniae in all specimens collected. This was the only rat in this group to develop clinical and histological evidence of meningitis.
Following middle ear inoculation, S. pneumoniae were isolated from the middle ear cavity of all rats with and without clinical signs of meningitis. The 6 rats receiving 3 x 108 CFU of the bacteria via the middle ear developed bacteremia. Five of these 6 rats were found to have S. pneumoniae in the CSF. However, only 4 rats had both clinical and histological evidence of meningitis. When the number of bacteria was lowered to 3 x 106 CFU, 5 rats developed bacteremia. Only 2 of these animals exhibited positive CSF culture for S. pneumoniae and both also had clinical and histological evidence of meningitis. At a much lower bacterial count, 3 x 104 CFU, via middle ear inoculation, only 2 rats showed positive culture for S. pneumoniae in both the blood and CSF. However, none of the 6 rats in this group developed clinical or histological evidence of meningitis.
Histology
In rats with clinical and histological evidence of meningitis, gram-positive bacteria and inflammatory cells were found within both cochleae but the histopathological pattern of infection was depend upon the route of inoculation (described in detail in the previous study)28. The amount and distribution of the inflammatory cells within both cochleae were similar in rats with i/p inoculation (Fig. 1). Bacteria were found symmetrically within the internal acoustic meatus (IAM) and modiolus but were not present within scalae. However, the inflammatory changes within the cochleae were asymmetrical in meningitic animals following middle (Fig. 2) and inner ear inoculation (Fig. 3). In these cases a more severe labyrinthitis was observed in the cochlea ipsilateral to the inoculation and minimal inflammatory changes were evident in the contralateral cochlea. When inflammatory cells were present in the contralateral ear, they were found more in the scala tympani than the scala vestibuli. Few bacteria were seen in the stria vascularis of rats with i/p and middle ear inoculation. In rats without meningitis, the histological appearance of the cochleae was normal for i/p (hematogenous) inoculation. Few inflammatory cells and scant serofibrinous exudate was seen in the basal turn of the cochleae in rats that did not develop meningitis following direct middle or inner ear inoculation.
Figure 1.
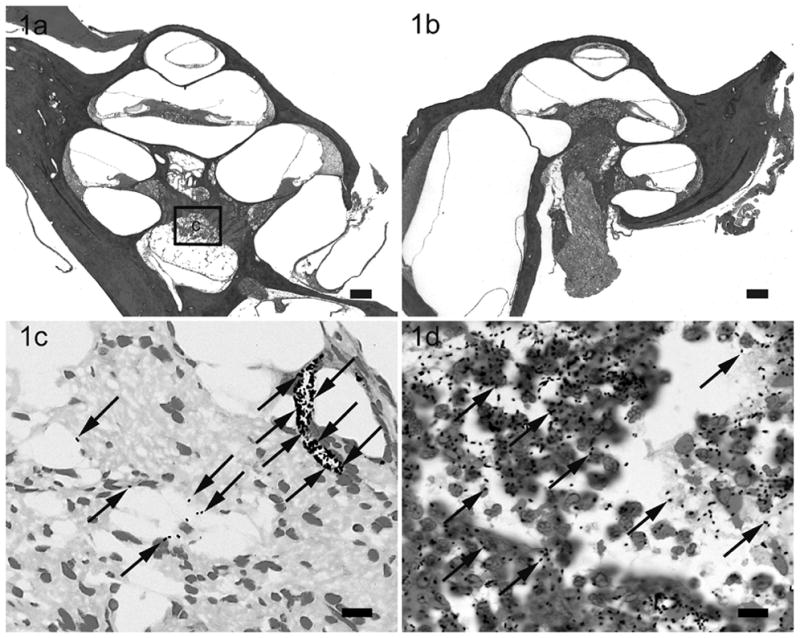
Lower power H & E photomicrographs illustrating the left (a) cochlea with prior cochleostomy and the right (b) cochleae of a rat 28 hours following i/p inoculation of 4 x 108 CFU S. pneumoniae. This animal exhibited clinical and histological (CNS) evidence of meningitis. However the scalae of both cochleae were devoid of gross infection. Higher power photomicrograph of Gram stain from the modiolus (c) illustrates the presence of bacteria (arrows). Higher power photomicrograph of Gram stain of the subarachnoid space around the brain (d) illustrates the presence of bacteria (arrows) and inflammatory cells with phagocytosed bacteria. The approximate location of the higher power micrograph (c) is illustrated in (a). Scale bar: (a) & (b) 200 μm; (c & d) 10 μm.
Figure 2.
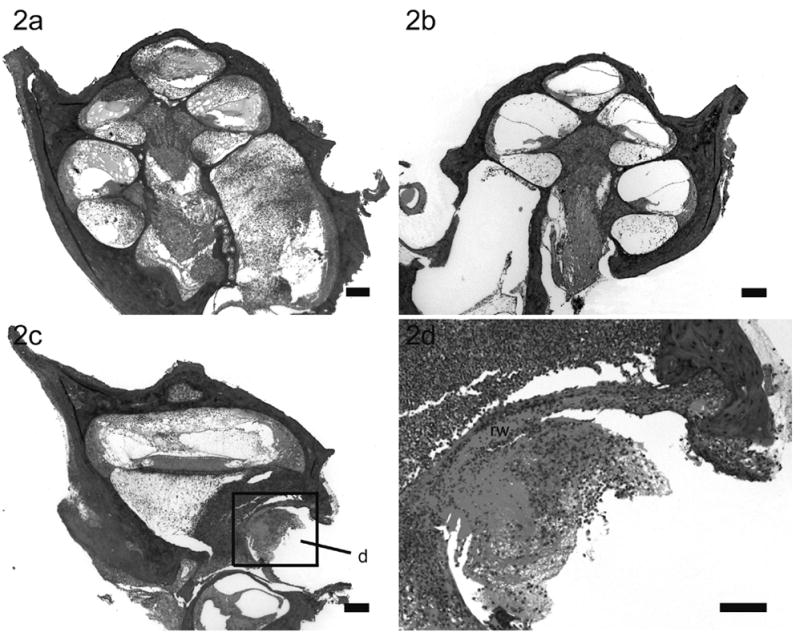
Lower power H & E photomicrographs illustrating the left (a) cochlea with prior cochleostomy and the right (b) non-operated cochlea of a rat 72 hours following left middle ear inoculation of 3 x 108 CFU S. pneumoniae. This animal exhibited clinical and histological (CNS) evidence of meningitis. In this example there is severe labyrinthitis of the left inoculated ear (a) while the contralateral cochlea exhibits evidence of infection predominantly localized to the scala tympani (b). Lower power H & E photomicrograph of a horizontal section taken at the level of the round window niche of the left (c) cochlea of the same animal illustrating the inflammatory cells mass infiltrating the round window membrane (rw). Higher power H &E micrograph of the round window niche (d) was taken from region (d) in lower power micrograph (c). Scale bar: (a – c) 200 μm; (d) 10 μm.
Figure 3.
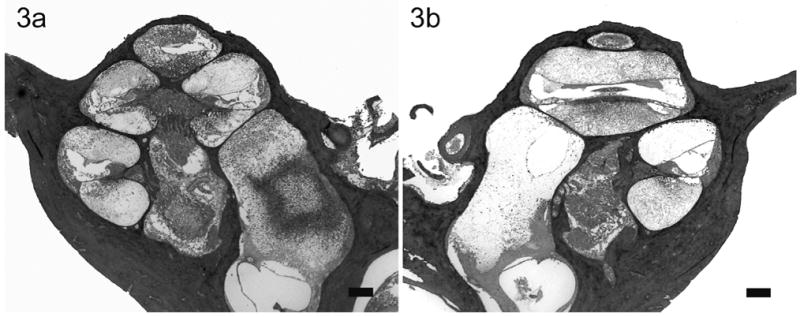
Lower power H & E photomicrographs illustrating the left (a) cochlea with previous cochleostomy and right (b) non-operated cochleae of a rat 22 hours following direct left inner ear inoculation of 1 x 107 CFU of S. pneumoniae. This animal exhibited clinical and histological (CNS) evidence of meningitis. Extensive labyrinthitis of the inoculated left inner ear involved all three scalae. In contrast, the contralateral cochlea exhibited a less severe labyrinthitis. Scale bar: 200 μm.
Macroscopic examination of the ipsilateral middle ear mucosa in the region of the round window niche revealed evidence of inflammation in rats following direct middle ear inoculation. When present, the inflammation was mild in the ipsilateral middle ear mucosa of rats following direct inner ear inoculation. The contralateral control bullae showed no evidence of middle ear inflammation for both middle and inner ear inoculations. There were no inflammatory changes within the middle ear mucosa in rats following an i/p inoculation.
Effects of cochleostomy on the threshold of infection
Statistical analysis of the results was performed using the Fisher’s exact test of independence. To determine the effect of cochleostomy on the risk of acquiring pneumococcal meningitis, a comparison of attack rate of meningitis between a group of rats receiving cochleostomy to that of a control group for each of the inoculating routes was performed (Table 3). No significant difference was observed at the 95% confidence level for all three different routes of inoculation (p>0.05; two tailed test).
Table 3.
| Routes of inoculation | Attack rate of meningitis in rats with cochleostomy (per 9 rats) | Attack rate of meningitis in rats without cochleostomy (per 9 rats) (Control Group) | Fisher exact test: calculated p value at the 95% confidence interval; two-tailed test |
|---|---|---|---|
| Intraperitoneal (i/p) | 5/9 | 6/9 | P = 1.000 * |
| Inner ear | 5/9 | 7/9 | P=0.620 * |
| Middle ear | 2/9 | 4/9 | P=0.620* |
: P > 0.05, there is no statistical difference between the two groups. Fail to reject null hypothesis
Discussion
A quantitative threshold model of pneumococcal meningitis has been established in healthy rats with no pre-existing risk factors for meningitis. The results showed that the number of bacteria that healthy animals were exposed to is an important factor influencing the outcome of the infection. A minimal threshold of bacteria is required to achieve meningitis in healthy animals. Moreover, this threshold value varies depending on the route of inoculation.
Previous animal models have shown that meningitis rarely occurred after direct inoculation of 1 x 103 CFU of virulent pneumococci into the subarachnoid space31. This observation suggests that a minimal number of bacteria must be present in the CSF to achieve meningitis. Our work supports and expands this finding by demonstrating that minimal numbers of bacteria are required to induce meningitis for hematogenous, inner and middle ear based infection.
When the i/p inoculum of S. pneumoniae was reduced from 4 x 1010 to 4 x 106 CFU, the attack rate of meningitis in rats dropped from 6/6 to 2/6. The number of rats with bacteremia was similar across the three groups of rats receiving i/p inoculums, suggesting that the intensity of the bacteremia must be reduced in rats inoculated with fewer bacteria, resulting in a reduced attack rate of meningitis. We hypothesize that a greater concentration of i/p inoculum leads to a greater magnitude of bacteremia and a greater number of the organisms reaching the subarachnoid space. Even though the magnitude of bacteremia for each i/p inoculum was not measured, our data using S. pneumoniae is consistent with previous work with Haemophilus influenzae. The magnitude of the bacteremia with H. influenzae has been shown to be directly related to the size of the intranasal inoculum and the incidence of meningitis26,32.
There is a sharp drop in the attack rate of meningitis when the inner ear inoculum was titrated down from 1 x 105 to 1 x 103 CFU. It is likely that an inoculum of S. pneumoniae below 1 x 103 CFU will not induce meningitis via the inner ear inoculation in this species. Similar results were observed in rats with middle ear inoculation as the attack rate dropped when the bacterial count was titrated from 3 x 106 to 3 x 104 CFU.
The attack rate of meningitis increased when animals were exposed to a higher concentration of the bacteria. Moreover, the time required to develop meningitis was reduced as the infective dose of the bacteria was increased. This is clearly demonstrated by rats receiving the highest bacterial counts via i/p or the inner ear compared to rats receiving smaller quantities of the bacteria. Previous work with H. influenzae, in rhesus monkeys with intranasal inoculation of the bacteria also demonstrated that time to onset of meningitis after inoculation is a function of the density of the bacteremia33.
Based on our data, the threshold bacteria required to induce meningitis is lowest with direct inner ear inoculation and is higher in rats with middle ear and hematogenous infection (Table. 2). As the inner ear has a close anatomical association with the CNS via the cochlear aqueduct and the modiolus through the canaliculi perforantes and the internal acoustic meatus, the threshold required to induce meningitis is expected to be lowest for this route. The route by which S. pneumoniae spread from the intraperitoneal cavity to CNS infection after i/p inoculation is via the blood circulation. Unlike the inner ear, the intraperitoneal cavity has no direct anatomical association with the CNS. Bacteremia was observed in 15 of the 18 rats receiving i/p inoculation. Eleven i/p inoculated rats developed meningitis in the presence of a positive blood culture. This suggested a direct bacterial invasion of the blood-brain barrier as a consequence of bacteremia. Likewise, no rats developed meningitis in the absence of a positive blood culture. Finally, there are two possible routes for the bacteria to reach the CNS via the middle ear cavity; either from the local or systemic blood circulation or via the inner ear through the round or oval windows. It is also possible that the bacteria may enter the CNS via a combination of the described routes. Despite having two potential routes of infection, the attack rate of meningitis in rats with middle ear inoculation was similar to the group of rats with i/p inoculation. However, a longer time was required to induce meningitis following middle ear inoculation compared to the i/p inoculation of similar bacterial counts. There are two possible explanations. First, bacteremia as a result of AOM is not as intense compared to i/p inoculation. Second, the bony and soft tissue barriers between the inner and middle ear are effective in either preventing or reducing the amount of bacteria reaching the inner ear.
Any breach of the bony and mucosal barrier is considered to increase the risk of infection spreading from the middle ear to the inner ear29 and this may reduce the threshold of bacteria required in the middle ear to induce meningitis as a result of AOM. However, 4 weeks following cochleostomy surgery, rats with cochleostomy did not show a greater incidence of meningitis when compared to control rats without a cochleostomy. This can be explained by the fact that after 4 weeks, the cochleostomy had healed either by fibrosis of the fascial seal, and in some instances, a complete regeneration of the bony capsule. Any type of repair at the cochleostomy may act as a physical barrier to prevent infection from easy access to the inner ear and then to the CNS. Not surprisingly, a cochleostomy did not reduce the threshold of bacteria required for meningitis for the i/p or inner ear routes of inoculation.
Blood, CSF and middle ear fluid culture specimens were collected and the results were used as an adjunct to the histology of the brain and meninges. The histology of the brain demonstrating meningitis is the gold standard for the diagnosis of the disease in our study. Clinical signs of meningitis in rats correlate closely with the histological appearance of the brain/meninges. Five rats were found to have positive CSF and blood culture but no histological evidence of inflammatory cells or bacteria within the subarachnoid space. It is possible that the CSF from these animals was contaminated with blood during collection, due to the small size of the animal and the small volume of CSF collected (less than 50 μl). Similarly, 5 rats with i/p inoculation were found to have positive left middle ear swabs. Microscopic examination of the middle ear mucosa showed no evidence of otitis media. In order to obtain a middle ear swab, skin/soft tissue dissections and drilling of the bulla are required to access the middle ear mucosa. The presence of positive blood culture in these rats suggests that the swab may have been contaminated by blood within the surgical field during specimen collection. Left middle ear swabs were also positive in 12 out the 18 rats receiving the bacteria via the inner ear with no histological evidence for AOM. One possible explanation is that a small number of bacteria (insufficient to cause AOM) leaked from the cochleostomy at the time of inoculation. Twelve rats with direct inner ear inoculation of the bacteria developed meningitis and bacteremia. Positive blood culture in the majority of rats with inner ear inoculation is more likely to be a consequence of the subsequent meningitis than a result of direct inner ear inoculation as the threshold of bacteria required to induce meningitis from inner ear inoculation is significantly less than that of the i/p hematogenous route. This observation supports direct spread of bacteria from the inner ear to the meninges as a predominate route of infection rather than from the inner ear to blood circulation then to the meninges.
The histological appearance of the cochleae in rats with meningitis was dependent upon the route of infection28. A symmetrical distribution of gram-positive bacteria and inflammatory cells within the internal acoustic meatus and modiolus were found in both cochleae in rats with i/p inoculation. Although a few isolated bacteria were seen within the stria vascularis, there were no bacteria or inflammatory cells seen within the scala tympani and vestibuli and no evidence to suggest that bacteria traversed blood vessels to enter the scala media. An asymmetrical distribution of the bacteria and inflammatory cells was seen in meningitic rats with both middle and inner ear inoculations. In a number of rats with middle ear infection and meningitis, bacteria were found to infiltrate the round window membrane to reach the scala tympani. This provides support for the notion that meningitis might have been caused by direct spread of infection through round window membrane into scala tympani and then to the CNS. While we cannot completely exclude the hematogenous spread to the meninges following middle ear infection because bacteremia was identified in these rats, one would have expected a more symmetrical distribution of the bacteria and inflammatory cells within their cochleae if this was the dominant route.
Whether a threshold level of S. pneumoniae is required to cause meningitis in human subjects following bacteremia or AOM has not been established, and if so the threshold is likely to be dependent on the interaction between the host’s immunity and the virulence of the bacteria. Patients with pneumococcal bacteremia do not always develop meningitis, as these bacteria are commonly grown from the blood of febrile infants and children in whom no obvious focus of infection is determined34. Similarly pneumococcal meningitis as a result of AOM is a very rare complication in patients with anatomically normal middle and inner ears, despite the fact that pneumococcal AOM is commonplace. Even without antibiotic treatment the spontaneous resolution of AOM has been reported to be as high as 75% within 14 days of infection35. Our result demonstrates that under experimental conditions a threshold number of bacteria is required to cause meningitis. This finding suggests that the threshold may be one of the factors determining whether a human subject develops meningitis following pneumococcal bacteremia or AOM.
The use of rat model to study human disease is reasonable as it has been instrumental in the study of bacterial meningitis. The rat model of meningitis has enabled investigators to identify the pathogenic and pathophysiologic mechanisms responsible for the neurologic damage observed in patients with bacterial meningitis36. Rats have been shown to exhibit meningitis with features that closely resemble human disease37,38. Furthermore, the surgical approach to cochlear implantation in rats has been established28,39 and this provides an excellent opportunity to study implant related CNS infection.
The principle of a threshold model, as described in the present study, could be utilized to assess whether the risk of pneumococcal meningitis is influenced by the presence of a cochlear implant or by the presence of other neurosurgical prostheses. Furthermore, the model could also be used to test whether the design of prostheses and/or modifications in surgical technique effectively reduces the risk of implant-related CNS infection.
Conclusion
There has been an increase in the number of reported cases of pneumococcal meningitis post cochlear implantation. The pathogenesis of pneumococcal meningitis is complex even in the absence of cochlear implantation and the relative importance of different infection routes remains unknown. Improved knowledge of these areas is an important first step in determining the effect of cochlear implantation on the risk of acquiring pneumococcal meningitis. A quantitative threshold model for pneumococcal meningitis has been established and demonstrates that a minimal number of bacteria in a healthy animal is required to achieve meningitis. The thresholds of bacteria required are significantly different for each route of infection. Inner ear surgery (cochleostomy without cochlear implantation) performed four weeks prior to inoculation does not alter the threshold for infection. This model can be used to assess the risk of acquiring pneumococcal infection in animals with cochlear implantation or other neurosurgical implantable devices.
Acknowledgments
We would like to thank staff from the Departments of Otolaryngology, and Microbiology and Immunology, University of Melbourne and Bionic Ear Institute for their support and help in the research project. We are grateful to Dimitra Stathopoulos and Rachael Richardson for editorial comments; Prue Nielsen and Maria Clarke for histology; Dr. Sue Pierce for veterinary support and Elisa Borg for animal maintenance (Department of Otolaryngology); and Susie Germano and Kristy Azzopardi for preparation of the bacteria (Department of Microbiology).
Footnotes
Source of financial support: The Garnett Passe and Rodney Williams Memorial Foundation Scholarship in Otolaryngology Head and Neck Surgery; The Wagstaff Fellowship, Royal Victorian Eye & Ear Hospital; NIH-NIDCD-N01-DC-3-1005; the Bionic Ear Institute and the Department of Otolaryngology, University of Melbourne.
References
- 1.FDA. [Accessed October 21, 2005];Public health web notification: risk of bacterial meningitis in children with cochlear implants. Available at www.fda.gov/cdrh/safety/cochlear.html.
- 2.Callanan V, Poje C. Cochlear implantation and meningitis. Int J Pediatr Otorhinolaryngol. 2004;68:545–550. doi: 10.1016/j.ijporl.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Reefhuis J, Honein MA, Whitney CG, et al. Risk of bacterial meningitis in children with cochlear implants. N Engl J Med. 2003;349:435–445. doi: 10.1056/NEJMoa031101. [DOI] [PubMed] [Google Scholar]
- 4.Cohen N, Roland JT, Jr, Marrinan M. Meningitis in cochlear implant recipients : the north american experience. Otol Neurotol. 2004;25:275–281. doi: 10.1097/00129492-200405000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Angeli S, Balkany T. Post-cochlear implant meningitis. Oper Tech Otolaryngol Head Neck surg. 2003;14:293–296. [Google Scholar]
- 6.Meli DN, Christen S, Leib SL, Tauber MG. Current concepts in the pathogenesis of meningitis caused by Streptococcus pneumoniae. Curr Opin Infect Dis. 2002;15:253–257. doi: 10.1097/00001432-200206000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Janoff EN, Rubins JB. Invasive pneumococcal disease in the immunocompromised host. In: Tomasz A, editor. Streptococcus pneumoniae: molecular biology & mechanisms of disease. New York: Mary Ann Liebert; 2000. pp. 321–341. [Google Scholar]
- 8.Lau YL, Kenna AP. Post-traumatic meningitis in children. Injury. 1986;17:407–409. doi: 10.1016/0020-1383(86)90082-3. [DOI] [PubMed] [Google Scholar]
- 9.Kline MW. Review of recurrent bacterial meningitis. Pediatr Infect Dis J. 1989;8:630–634. doi: 10.1097/00006454-198909000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Sudhoff H, Linthicum FH., Jr Temporal bone fracture and latent meningitis: temporal bone histopathology study of the month. Otol Neurotol. 2003;24:521–522. doi: 10.1097/00129492-200305000-00029. [DOI] [PubMed] [Google Scholar]
- 11.Baltas I, Tsoulfa S, Sakellariou P, Vogas V, Fylaktakis M, Kondodimou A. Posttraumatic meningitis: bacteriology, hydrocephalus, and outcome. Neurosurgery. 1994;35:422–426. doi: 10.1227/00006123-199409000-00009. discussion 426–427. [DOI] [PubMed] [Google Scholar]
- 12.Brahams D. Hairline fracture and undiagnosed meningitis. Lancet. 1991;337:605–606. doi: 10.1016/0140-6736(91)91659-i. [DOI] [PubMed] [Google Scholar]
- 13.Brodie HA, Thompson TC. Management of complications from 820 temporal bone fractures. Am J Otol. 1997;18:188–197. [PubMed] [Google Scholar]
- 14.Kamerling JP. Pneumococcal polysaccharides: a chemical view. In: Tomasz A, editor. Streptococcus pneumoniae: molecular biology & mechanisms of disease. New York: Mary Ann Liebert; 2000. pp. 81–114. [Google Scholar]
- 15.Kostyukova NN, Volkova MO, Ivanova VV, Kvetnaya AS. A study of pathogenic factors of Streptococcus pneumoniae strains causing meningitis. FEMS Immunol Med Microbiol. 1995;10:133–137. doi: 10.1111/j.1574-695X.1995.tb00022.x. [DOI] [PubMed] [Google Scholar]
- 16.Balachandran P, Hollingshead SK, Paton JC, Briles DE. The Autolytic Enzyme LytA of Streptococcus pneumoniae Is Not Responsible for Releasing Pneumolysin. J Bacteriol. 2001;183:3108–3116. doi: 10.1128/JB.183.10.3108-3116.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cockeran R, Theron AJ, Steel HC, et al. Proinflammatory interactions of pneumolysin with human neutrophils. J Infect Dis. 2001;183:604–611. doi: 10.1086/318536. [DOI] [PubMed] [Google Scholar]
- 18.Paton JC. The contribution of pneumolysin to the pathogenicity of Streptococcus pneumoniae. Trends Microbiol. 1996;4:103–106. doi: 10.1016/0966-842X(96)81526-5. [DOI] [PubMed] [Google Scholar]
- 19.Zysk G, Schneider-Wald BK, Hwang JH, et al. Pneumolysin is the main inducer of cytotoxicity to brain microvascular endothelial cells caused by Streptococcus pneumoniae. Infect Immun. 2001;69:845–852. doi: 10.1128/IAI.69.2.845-852.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Briles D, Hollingshead SK, Swiatlo E, et al. Pneumococcal proteins PspA and PspC : their potentials for use as vaccines. In: Tomasz A, editor. Streptococcus pneumoniae: molecular biology & mechanisms of disease. New York: Mary Ann Liebert; 2000. pp. 253–260. [Google Scholar]
- 21.Paton JC, Berry AM, Lock RA. Molecular analysis of putative pneumococcal virulence proteins. In: Tomasz A, editor. Streptococcus pneumoniae: molecular biology & mechanisms of disease. New York: Mary Anne Liebert; 2000. pp. 261–270. [Google Scholar]
- 22.Andrew PW, Mitchell TJ, Morgan P, Gilbert RJC. Relationship of structure to function in pneumolysin. In: Tomasz A, editor. Streptococcus pneumoniae: molecular biology & mechanisms of disease. New york: Mary Ann Liebert; 2000. pp. 271–277. [Google Scholar]
- 23.Mitchell TJ, Andrew PW. Biological properties of pneumolysin. In: Tomasz A, editor. Streptococcus pneumoniae: molecular biology & mechanisms of disease. New York: Mary Ann Liebert; 2000. pp. 279–286. [Google Scholar]
- 24.Schuknecht HF, Montandon PB. Pathology of the ear in pneumoccocal meningitis. Arch Klin Exp Ohr Nas Kehlkheilk. 1970;195:207–225. doi: 10.1007/BF00302950. [DOI] [PubMed] [Google Scholar]
- 25.Barry B, Delattre J, Vie F, Bedos JP, Gehanno P. Otogenic intracranial infections in adults. Laryngoscope. 1999;109:483–487. doi: 10.1097/00005537-199903000-00026. [DOI] [PubMed] [Google Scholar]
- 26.Moxon ER, Smith AL, Averill DR, Smith DH. Haemophilus influenzae meningitis in infant rats after intranasal inoculation. J Infect Dis. 1974;129:154–162. doi: 10.1093/infdis/129.2.154. [DOI] [PubMed] [Google Scholar]
- 27.Moxon ER, Ostrow PT. Haemophilus influenzae meningitis in infant rats: role of bacteremia in pathogenesis of age-dependent inflammatory responses in cerebrospinal fluid. J Infect Dis. 1977;135:303–307. doi: 10.1093/infdis/135.2.303. [DOI] [PubMed] [Google Scholar]
- 28.Wei BPC, Shepherd RK, Robins-Browne R, Clark G, O'Leary SJ. Pneumococcal meningits post cochlear implantation: development of an animal model. Otol Neurotol. 2005 under review. [Google Scholar]
- 29.Clark G. Cochlear implants in children: safety as well as speech and language. Int J Pediatr Otorhinolaryngol. 2003;67:S7–S20. doi: 10.1016/j.ijporl.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 30.Miles A, Misra SS. Miles and Misera technique. J Hyg. 1938;38:372. [Google Scholar]
- 31.Moxon ER. Experimental infections of animals in the study of Streptococcus pneumoniae. Rev Infec Dis. 1981;3:354–357. doi: 10.1093/clinids/3.2.354. [DOI] [PubMed] [Google Scholar]
- 32.Moxon ER, Glode MP, Sutton A, Robbins JB. The infant rat as a model of bacterial meningitis. J Infect Dis. 1977;136:S186–S190. doi: 10.1093/infdis/136.supplement.s186. [DOI] [PubMed] [Google Scholar]
- 33.Scheifele DW, Daum RS, Syriopoulou VP, Averill DR, Smith AL. Haemophilus influenzae bacteremia and meningitis in infant primates. J Lab Clin Med. 1980;95:450–462. [PubMed] [Google Scholar]
- 34.Finland M. Conference on the pneumococcus: summary and comments. Rev Infect Dis. 1981;3:358–371. doi: 10.1093/clinids/3.2.358. [DOI] [PubMed] [Google Scholar]
- 35.Rosenfeld RM. Natural history of untreated otitis media. In: Rosenfeld RM, Bluestone CD, editors. Evidenced-based otitis media. Hamilton: B.C. Decker Inc.; 1999. pp. 157–177. [Google Scholar]
- 36.Townsend GC, Scheld WM. Adult rat model of meningitis. In: Zak O, Sande MA, editors. Handbook of animal models of infection : experimental models in antimicrobial chemotherapy. London: Academic Press; 1999. pp. 627–629. [Google Scholar]
- 37.Rodriguez AF, Kaplan SL, Hawkins EP, Mason EO., Jr Hematogenous pneumococcal meningitis in the infant rat: description of a model. J Infect Dis. 1991;164:1207–1209. doi: 10.1093/infdis/164.6.1207. [DOI] [PubMed] [Google Scholar]
- 38.Vogel U, Frosch M. Infant rat model of acute meningitis. In: Zak O, Sande MA, editors. Handbook of animal models of infection : experimental models in antimicrobial chemotherapy. London: Academic Press; 1999. pp. 619–626. [Google Scholar]
- 39.Lu W, Xu J, Shepherd RK. Cochlear implantation in rats: a new surgical approach. Hear Res. 2005;205:115–122. doi: 10.1016/j.heares.2005.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]


