Abstract
In many developmental systems, growth factor signalling must be temporally and spatially regulated, and this is commonly achieved by growth factor antagonists. Here we describe the expression patterns of newly identified growth factor inhibitors, Sprouty and Sef, in the developing ocular lens. Sprouty and Sef are both expressed in the lens throughout embryogenesis, and become restricted to the lens epithelium, indicating that lens cell proliferation and fibre differentiation may be tightly regulated by such antagonists. Future studies will be aimed at understanding how these negative regulatory molecules modulate growth factor-induced signalling pathways and cellular processes in the lens.
Keywords: Sef, Sprouty, FGF antagonists, lens cell proliferation, differentiation
Introduction
The lens of the eye is a relatively simple tissue, comprised of a monolayer of cuboidal epithelial cells that overlies the anterior surface of elongated and precisely aligned fibre cells. The ordered spatial arrangement of these two lens cell types is established during embryogenesis and is maintained as the lens continues to grow throughout life. Lens growth is dependent on proliferation of the epithelial cells and their subsequent differentiation into fibre cells at the lens equator. Above the equator, in the germinative zone, lens epithelial cells divide. Progeny of these divisions are displaced posteriorly, into the transitional zone, where they begin to differentiate into fibre cells. Fibre differentiation is characterised by distinct molecular and morphologic changes such as exit from the cell cycle, cell elongation, loss of cytoplasmic organelles and nuclei, as well as the accumulation of fibre-specific crystallin proteins [1,2,3]. The normal architecture, and hence transparency of the lens, depends on the maintenance of this distinctive pattern of growth.
It is well established that the distinct patterns of cell behaviour in the lens are primarily regulated by the ocular environment, in particular the ocular fluids that bathe the lens; the aqueous and vitreous humour [3]. Much of the fibre-differentiating activity of the ocular fluid, namely the vitreous, has been shown to be attributed to the presence of FGFs [4]. In vitro studies have led to the identification of members of the FGF family as potent lens fibre-inducing factors [1]. It has also been shown that these FGFs stimulate lens epithelial cells to proliferate and migrate. Significantly, FGF can induce lens epithelial cell proliferation, migration and fibre differentiation in a dose-dependent manner [3,5]; a low concentration of FGF stimulates only cell division, whereas higher concentrations can induce cell migration and fibre differentiation, respectively. Biologically active FGFs are also present in the ocular media and are expressed in the lens, specifically in regions of high cell proliferative activity and early fibre differentiation [4,6,7,8]. These findings have led to the hypothesis that in situ, lens growth patterns are regulated by an antero-posterior ‘FGF gradient’, whereby FGF bioavailability is differentially distributed in the eye. In this model, anterior proliferating lens epithelial cells are exposed to a lower level of FGF activity from the aqueous, whereas differentiating fibre cells are exposed to a higher level of FGF activity in the vitreous. This is supported by the distinct expression of FGF receptors in the lens [9] which indicate that FGF influences the spatial patterns of cell proliferation and fibre differentiation. Support for this comes from in vivo studies that have directed the overexpression of different FGFs specifically to the lenses of transgenic mice [10,11]. In these mice, the anterior lens epithelial cells, now exposed to increased levels of FGF, are induced to differentiate into fibre cells, disrupting the normal polarity of the lens. Moreover, recent studies using conditional gene targeting strategies have generated FGF receptor ‘knockouts’ in the lens. The observation that these mice undergo no fibre differentiation and have impaired lens cell proliferation confirms the requirement for FGF receptor signalling in cell proliferation and fibre differentiation [12]. Taken together, these studies support the model that FGFs are important for determining the spatial patterns of cell differentiation and proliferation in the lens.
Most growth factors mediate cellular responses by binding and activating high affinity cell surface receptor tyrosine kinases (RTKs). This event leads to the activation of several intracellular signalling cascades, including the phosphatidylinositol-3 kinase (PI3-K), phospholipase C gamma (PLC-γ) and Ras to extracellular signal-regulated protein kinase (ERK) pathways; ERKs are a subclass of the MAPKs (mitogen-activated protein kinases). The ERK/MAPKs are the most abundant MAPKs in the lens [13] and their activation plays an important role in regulation of embryonic development, as well as in modulating many cellular events, including cell cycle progression and cell differentiation. Studies by our laboratory and others have recently established an important role for ERK1/2 signalling in growth factor-induced lens cell proliferation and fibre differentiation [14,15,16]. Using a lens epithelial explant system, ERK1/2 was differentially activated in response to different concentrations of FGF. Furthermore, inhibition of ERK1/2 activation blocked FGF-induced lens cell proliferation, as well as cell elongation accompanying fibre differentiation [14]. Clearly, FGF signalling in the lens is quite complex, with its activity potentially regulated at multiple levels. Not only do FGFs signal through multiple pathways, there may be cross-talk between these pathways, as well as with different signalling pathways initiated by other growth factors found in the ocular media. As mentioned earlier, FGF signalling in the lens is further complicated by the fact that activation of a specific RTK can result in different cellular responses. In many developmental systems, to ensure a physiologically appropriate cellular response, growth factor signalling must be temporally and spatially regulated, and this can be achieved by a parallel set of inhibitory signals that lead to an accurate and reproducible biological outcome. Antagonists of growth factor signalling pathways can provide such negative signals, and are reported to play important roles in developmental patterning by restricting the range of their cognate inducer [17].
One of the first identified bona fide feedback regulators of the FGF pathway was Sprouty (Spry), which was initially discovered through a genetic screen in Drosophila and later shown to be important for FGF-induced tracheal branching in this species [18]. Spry proteins are widely conserved, with up to four members identified in mammals (Spry1–4) [19], and are expressed in highly restricted patterns that correlate with known sites of FGF signalling [20,21]. Spry is recognized in many physiological and developmental processes as an antagonist of RTK signalling pathways, with its overexpression mimicking the functional loss of RTKs, including those activated by FGF [22]. In the chick, overexpression of Spry in the developing limb bud results in inhibition of cell differentiation, displaying a comparable phenotype to that reported in FGF null mutants [22]. Consistent with this, cells overexpressing Spry have also been reported to have a reduced responsiveness to exogenously applied growth factors. Moreover, in Drosophila, Spry null mutants have an ‘overactive’ FGF signalling pathway, inducing increased numbers of cells situated outside the normal range of FGF [18,23].
Further screening for genes with restricted expression patterns during early development [24] revealed other modulators of the FGF pathway, including Sef (similar expression to fgfs). Sef is a transmembrane protein that unlike Spry, is restricted to vertebrates; however, like Spry, it has been shown to function as an antagonist of FGF signalling during development [25,26]. Also similar to Spry, overexpression of Sef can inhibit FGF signalling, and impaired Sef expression leads to overstimulation of FGF signalling [17]. The co-expression of Spry and Sef with known sites of RTK signalling during embryogenesis [21] lends credence to the important role they have in cell fate specification; a close spatial and temporal interdependence between RTK signalling (e.g. FGF signalling) and Spry and Sef gene expression has been seen in many mammalian tissues, including brain, muscle, gut, heart and lung [20,21,27,28]. As a means of providing tight autoregulation, the expression of these inhibitors are induced by the pathway they antagonize; Spry and Sef have been shown to be positively regulated by FGF, more specifically FGF-induced ERK1/2 signalling [18,22,26,29]. Overall, given the strong link between FGF signalling with the expression of Spry and Sef in different developmental systems and the important role that FGF plays in lens development, there is a strong rationale for investigating the expression patterns and role of these antagonists in the developing lens.
Methods
All animal procedures were performed in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research and the animal care guidelines published by the Institute for Laboratory Animal Research (Guide for the Care and Use of Laboratory Animals). The use of human tissues adhered to the tenets of the Declaration of Helsinki. All studies were approved by the Institutional Ethics Committee of the University of Sydney.
RT-PCR
Lenses were collected from postnatal day 21 (P21) Wistar rats and dissected to separate the lens capsule (with adherent epithelial cells) from the fibre cell mass. Lung tissue, which is known to express Sef and Sprouty genes [21,28] was also collected for use as a positive control. Total RNA was extracted from each tissue type using TRI Reagent® (Molecular Research Center, OH, USA) as per the manufacturer’s instructions. DNaseI (Amersham Biosciences, NJ, USA) was used to degrade any contaminating DNA and then removed with DNase Inactivation Reagent (Ambion, TX, USA). First strand cDNA was synthesised from 5 μg of total RNA using Promega’s reverse transcription system as instructed (Promega, Sydney, Australia). AMV reverse transcriptase was omitted from control reactions. For PCR, specific primers were designed from the coding regions of Spry1, Spry2, Spry4 and Sef genes as follows: Spry1 (5′cagccaccggctgtggaagg3′, 5′atctgacctatgacctggg3′), Spry2 (5′tgtgaggactgcggcaagtgc3′, 5′tttaggcaacccttggctgg3′), Spry4 (5′acccattgaccagatgaagac3′, 5′tccagcggcttacagtgaac3′), Sef (5′ggcatgaagtactttgtagataag3′, 5′tgctgtggcctgggtgctgaccc3′). Each PCR reaction contained 4 μl of cDNA, 1.3 μM of the respective primer pair, 1.7 mM MgCl2, 1 mM dNTPs, 1 U BioTaq Red™ and 1x NH4 reaction buffer (Bioline, Alexandria, Australia). Samples were heated at 94°C for 3 minutes, then amplified for 32 cycles (94°C for 30 seconds, 58°C for 30 seconds and 72°C for 1 minute), followed by a final extension at 72°C for 2 minutes. In some instances, samples with genomic DNA as a template were included as an additional positive control.
cDNAs and Riboprobe Synthesis
PCR products for Spry1, Spry2 and Sef were agarose gel purified and cloned into either pGEM-T (Promega) or pBluescript II KS+ (Stratagene, CA, USA). The identity of each cDNA clone was confirmed by restriction endonuclease digestion and sequencing. Complementary (sense and antisense) riboprobes were generated from linearised cDNA constructs using digoxigenin labelled nucleotides (Roche, Manheim, Germany) with T7/SP6 (for pGEM-T) or T3/T7 (for pBluescript) in vitro transcription systems (Promega). Following transcription, DNA templates were degraded by DNaseI (Amersham Biosciences) and riboprobes recovered via ethanol precipitation with 0.1 M LiCl.
Histology
Embryonic tissues were obtained from mice (FvB/N) between embryonic day 9 (E9) to E18. Whole eyes were collected from postnatal stages (P0–P21) of FvB/N mice, newborn (P3) and weanling (P21) Wistar rats, as well as chick hatchlings. Intact lenses were also obtained from foetal human and adult bovine eyes. All tissues were fixed in 10% neutral buffered formalin and processed for paraffin embedding. Once embedded, 6 μm thick sections were cut for subsequent in situ hybridisation or immunofluorescence studies.
In Situ Hybridisation
The expression patterns of Spry1, Spry2 and Sef mRNA transcripts were examined by in situ hybridisation using sense and antisense riboprobes labelled with digoxigenin as previously described [30]. Following hybridization, slides were washed at 65°C in decreasing concentrations of SSC (twice in 4xSSC for 20 minutes, once in 2xSSC, 1xSSC and 0.5xSSC for 10 minutes each, and twice in 0.1xSSC for 20 minutes), with a final 0.1xSSC wash (5 minutes) at room temperature. The distribution of the hybridised riboprobes was determined using either immunofluorescence or an anti-digoxigenin alkaline phosphatase conjugated antibody (Roche) as instructed by the manufacturer. For immunofluorescence, hybridised sections were rinsed in PBS and incubated with a mouse anti-digoxin monoclonal antibody (Sigma, Sydney, Australia) diluted 1:30 000. Reactivity was detected using an anti-mouse Alexa Fluor 594 secondary antibody (2 μg ml−1; Molecular Probes, OR, USA). Sections were counterstained with 1.8 μg mL−1 bisbenzimide (Hoechst dye, Sigma) to label cell nuclei, rinsed with PBS, mounted, and examined using epi-fluorescence microscopy.
Immunofluorescence
Immunofluorescence was carried out as described previously [31]. In brief, paraffin sections were hydrated and incubated for 30 minutes in 3% normal goat serum to reduce non-specific staining. Sections were then incubated overnight at 4°C with primary antibodies specific for either Spry2 (10 μg ml−1, rabbit polyclonal IgG raised against human amino acids 26–39, GEPDPRDALTQQVH; Upstate, NY, USA) or Sef (2.5 μg ml−1, rabbit polyclonal IgG raised against human amino acids 320–357, MCRKKQ…VAVSAI; Phoenix Pharmaceuticals, Inc. CA, USA). After a brief rinse in PBS, bound primary antibodies were visualized using a Cy3-conjugated secondary anti-rabbit antibody (5 μg ml−1; Sigma). All sections were counterstained with Hoechst dye and examined using epi-fluorescence microscopy.
Results
To examine the expression of Sef and Spry in the developing lens, we first applied RT-PCR using specific primers for Spry1, Spry2, Spry4 and Sef, on RNA obtained from postnatal rat lens tissues that were separated into epithelial (lens capsule attached) and fibre cell fractions. Specific amplicons for Spry1, Spry2 and Sef, but not Spry4, were detected in reverse transcribed RNA from both the lens epithelial and fibre cell preparations (Figure 1). Note that in some cases there was a very weak amplicon for Spry4 in the lens but this was not consistently reproducible. RNA derived from rat lung tissue was used as a control, as was murine genomic DNA, and the absence of reverse transcriptase (see Figure 1). The strongest PCR-amplified fragments for the three lens-derived genes (Spry1, Spry2 and Sef) were sub-cloned into a transcription vector and used to prepare templates for DIG-labelled riboprobes for our in situ hybridization analysis of their expression in rodent lenses. In light of the fact that Spry3 has previously been reported to be absent in the developing murine embryo using northern blotting and whole-mount in situ hybridization [22], we did not examine for its expression in the developing lens.
Figure 1.
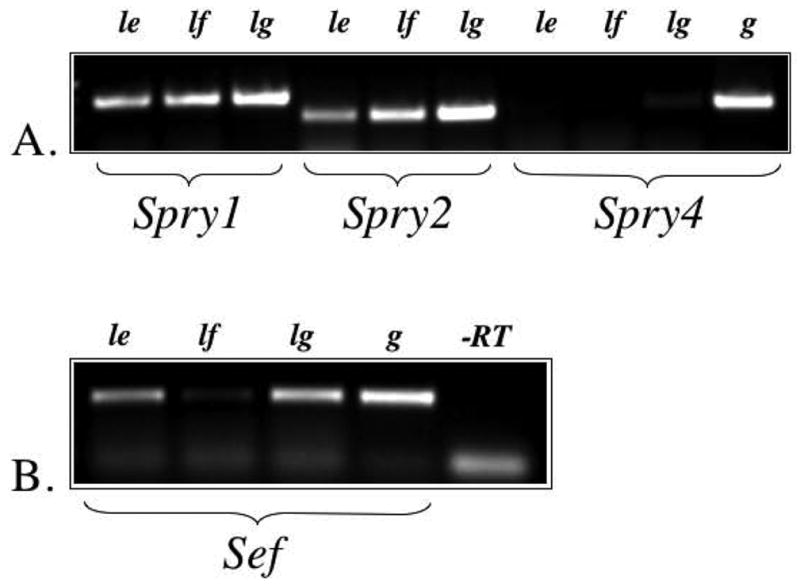
Expression of Spry1, Spry2, Spry4 and Sef using RT-PCR. A. Spry1 and Spry2, but not Spry4, were detected in the rat lens epithelium (le) and lens fibre cells (lf). Lung tissue (lg), and genomic DNA (g) were used as positive controls. B. Sef was detected in the lens epithelium, lens fibre cells (weakly) and lung. Genomic DNA was used as a positive control, and reverse transcriptase (-RT) was omitted to control for DNA contamination of RNA samples.
Spry1 mRNA expression
Alkaline phosphatase activity was used to temporally and spatially determine the pattern of Spry1 expression during lens morphogenesis and growth. From as early as embryonic day 9.5 (E9.5), it was clear that Spry1 expression was very strong throughout the embryonic head, including the embryonic surface ectoderm and underlying neuroectoderm (optic vesicle), structures that ultimately give rise to the eye (Figure 2A). A day later in development, at E10.5, the head ectoderm has invaginated, together with the optic vesicle, to give rise to the lens pit/early lens vesicle and underlying optic cup, respectively (Figure 2B). Expression for Spry1 is retained at this stage with all lens cells labelled strongly for Spry1 transcripts. At E11.5, although all the lens vesicle cells are still strongly expressing Spry1, the anterior lens vesicle cells (presumptive lens epithelium), appear to have a stronger label to that observed in the underlying elongating primary fibre cells (Figure 2C). This pattern of expression becomes more apparent later in development, at E13.5, with strongest expression for Spry1 now localized to the anterior monolayer of lens cells (Figure 2D). At E14.5 and E15.5, as the anterior epithelial cells differentiate at the lens equator to give rise to some of the first secondary fibre cells, Spry1 expression is still expressed in these early elongating cells, but it is clearly lost in the maturing primary fibre cells (Figure 2E, F). This pattern of Spry1 expression in the lens is retained throughout foetal and postnatal growth (Figure 3). At birth, the lens epithelial cells retain the strongest expression of Spry1 which is progressively lost as the cells begin to differentiate into secondary fibres (Figure 3A). Although the expression of Spry1 weakens with age, by postnatal day 14 and 21, it is still primarily strongly expressed in the anterior monolayer of lens epithelial cells (Figure 3B,C). In situ hybridisation using sense riboprobes failed to give any label at all the stages we examined (see Figure 3D).
Figure 2.
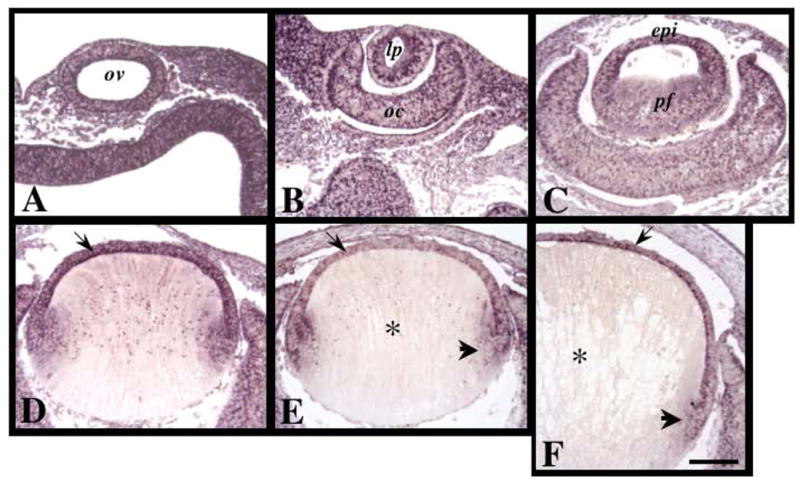
Expression of Spry1 mRNA during murine embryonic lens morphogenesis. Sections of mouse eyes at embryonic stages E9.5 (A), E10.5 (B), E12.5 (C), E13.5 (D), E14.5 (E) and E15.5 (F) were labelled for Spry1 transcripts using in situ hybridisation. Spry1 was ubiquitously expressed early in development (A–C). By E13.5, strongest expression for Spry1 was detected in the anterior monolayer of lens cells (D, arrow). At E14.5 and E15.5, Spry1 was expressed in the epithelial cells (E, F, arrow) and early secondary fibre cells (E, F, arrowhead), but not in the maturing primary fibre cells (E, F, asterisks). Abbreviations: epi, lens epithelium; lp, lens pit; oc, optic cup; ov, optic vesicle; pf, primary fibre cells. Scale bar: 100 μm.
Figure 3.
Expression of Spry1 mRNA during postnatal murine lens growth. Sections of eyes at postnatal stages P0 (A), P14 (B) and P21 (C, D) were labelled for Spry1 transcripts using in situ hybridisation. (A) At P0, Spry1 was strongly expressed in lens epithelial cells (arrow) and early secondary fibre cells (arrowhead). At P14 and P21, Spry1 was primarily expressed in the lens epithelial cells (B, C, arrows). (D) Control sections using a sense-labelled riboprobe showed no signal. Scale bar: 100 μm.
Spry2 mRNA expression
Using fluorescently-tagged secondary antibodies to detect hybridisation of the DIG- labelled riboprobes for Spry2, we observed, similar to Spry1, that Spry2 was ubiquitously expressed throughout the embryonic head at E9.5 (Figure 4A,E). Strong expression for Spry2 mRNA was found in the evaginating optic vesicle and overlying head ectoderm giving rise to the lens placode (Figure 4A,E). With subsequent invagination of the lens placode, Spry2 was strongly expressed in the early (E10.5, Figure 4B,F) and late (E12.5, Figure 4C,G) lens vesicle. It was not until E13.5 that we noted a difference in the expression of Spry2 from that previously observed for Spry1. Unlike Spry1 expression that become progressively weaker in the primary lens fibre cells, Spry2 mRNA expression persisted in the primary fibre cells, as well as in the overlying lens epithelium (Figure 4D,H). This expression of Spry2 persisted through foetal (E15.5, Figure 4I,J; E18.5, Figure 4K, L) and early stages of postnatal lens growth (P3, Figure 5A). With postnatal development, the fibre cell mass progressively became weaker for Spry2 expression while the lens epithelial cells retained their strong label (P15, Figure 5B; P21, Figure 5C). To confirm that this persistence of Spry2 expression up until neonatal lens growth, and its subsequent postnatal loss in the fibre cells, was not an artefact of our secondary fluorescent label, we repeated this in situ hybridisation analysis using alkaline phosphatase activity as described for Spry1. At all ages we obtained a comparable expression pattern throughout lens morphogenesis to that described using our fluorescent label (data not shown). As before, this ubiquitous expression persisted in all lens cells up until early postnatal development (P3, Figure 5D,G), after which expression was progressively lost in the maturing fibre cells, but retained in the lens epithelium and newly elongating secondary fibres (P15, Figure 5E,H; P21, Figure 5F,I). For all ages examined, in situ hybridisation using sense riboprobes (with either fluorescent or alkaline phosphatase detection) failed to give any label (see Figure 5J,K,L).
Figure 4.
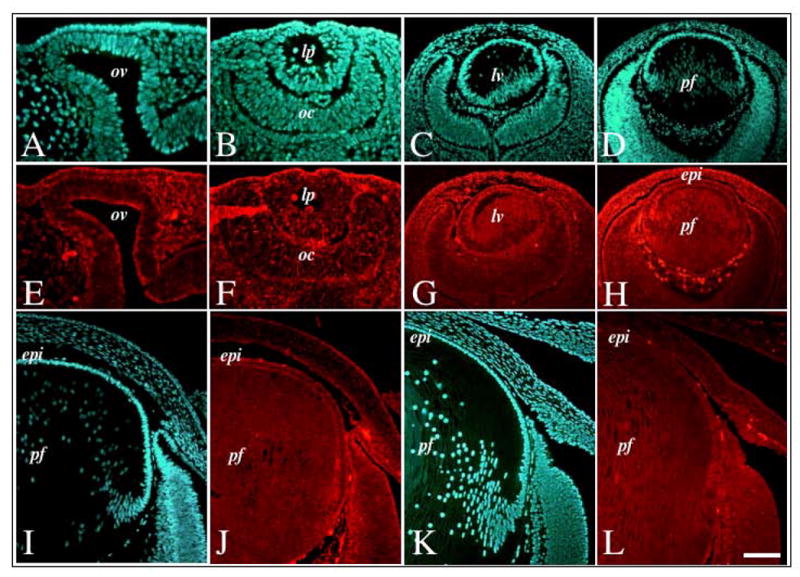
Expression of Spry2 mRNA during murine embryonic lens morphogenesis. Sections of mouse eyes at embryonic stages E9.5 (A, E), E10.5 (B, F), E12.5 (C, G), E13.5 (D, H), E15.5 (I, J) and E18.5 (K, L) were labelled for Spry2 transcripts using fluorescence in situ hybridisation (E–H, J, L) and counterstained with Hoechst dye (A–D, I, K). Spry2 was ubiquitously expressed throughout lens morphogenesis, with strong expression in both the lens epithelium and primary fibre cells. Abbreviations: epi, lens epithelium; lp, lens pit; lv, lens vesicle ; oc, optic cup; ov, optic vesicle; pf, primary fibres. Scale bar: 50 μm (A, B, E, F); 100 μm (C, D, G, H); 80 μm (I–L).
Figure 5.
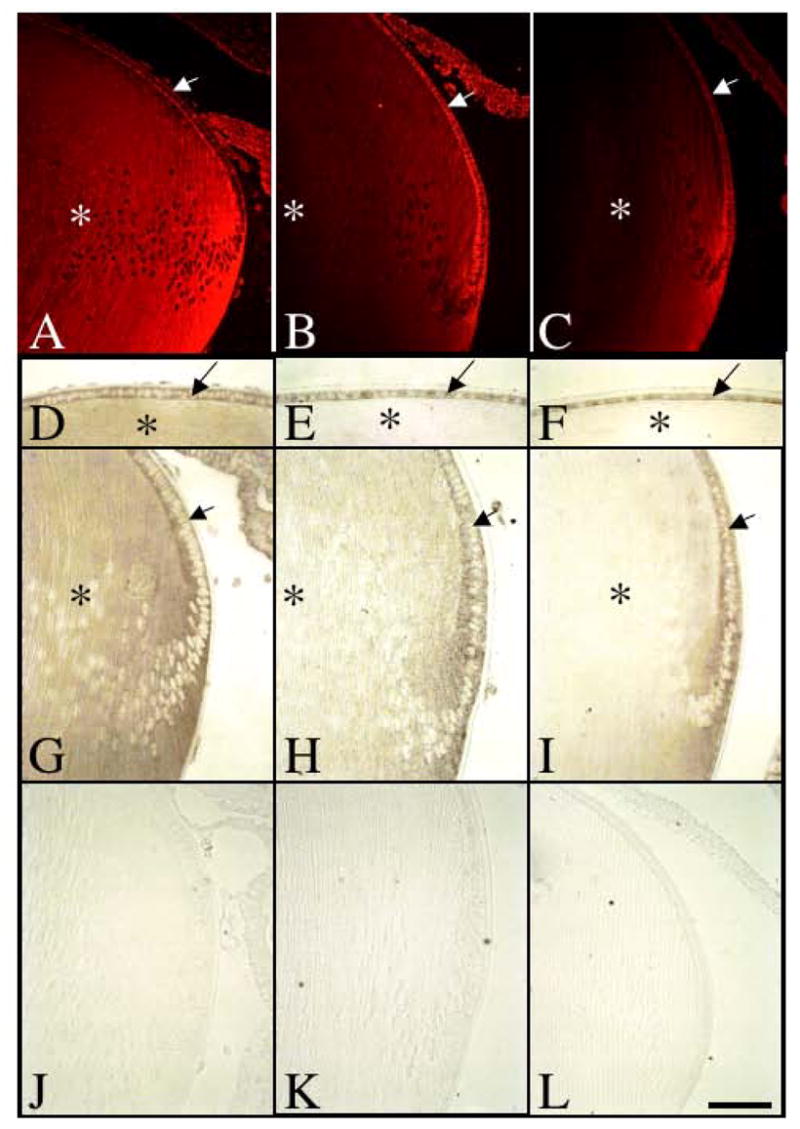
Expression of Spry2 mRNA during postnatal murine lens growth. Sections of eyes at postnatal stages P3 (A, D, G, J), P15 (B, E, H, K) and P21 (C, F, I, L) were labelled for Spry2 transcripts using either fluorescent in situ hybridisation (A–C) or alkaline-phosphatase labelled riboprobes (D–L). At P3, Spry2 was expressed throughout the lens, in both epithelial cells (A, D, G, arrows) and fibre cells (A, D, G, asterisk). At P15 and P21, Spry2 expression in the lens epithelium (B, C, E, F, H, I, arrow) was retained, but progressively lost in the maturing lens fibre cells (B, C, E, F, H, I, asterisk). Control sections using a sense-labelled riboprobe showed no signal (J–L). Scale bar: 100 μm.
Spry2 protein expression
The availability of an antibody made it possible to determine if the temporal and spatial patterns of Spry2 mRNA expression, observed during lens morphogenesis and growth, were consistent with the distribution of Spry2 protein. As for the expression of Spry2 transcripts, strong immunolabelling for Spry2 protein was detected throughout lens morphogenesis. The early lens placode, pit and vesicle all showed high levels of Spry2 protein in the majority of cells (see Figure 6A–F). At E13.5, consistent with the mRNA expression, strong levels of Spry2 protein were detected in the lens epithelium and the fibre cell mass (Figure 6G,J). By E15.5 (Figure 6H,K) and E18.5 (Figure 6I,L), the lens was still strongly reactive for Spry2. Interestingly, specific regions of the lens epithelium lost their reactivity for Spry2 (see Figure 6K,L). Single cells scattered throughout the lens epithelium at E15.5, appeared unreactive for Spry2, whereas in the more peripheral epithelium it appeared that groups of cells lost their reactivity for Spry2. By E18.5, this loss was more confined to the peripheral lens epithelial cells.
Figure 6.
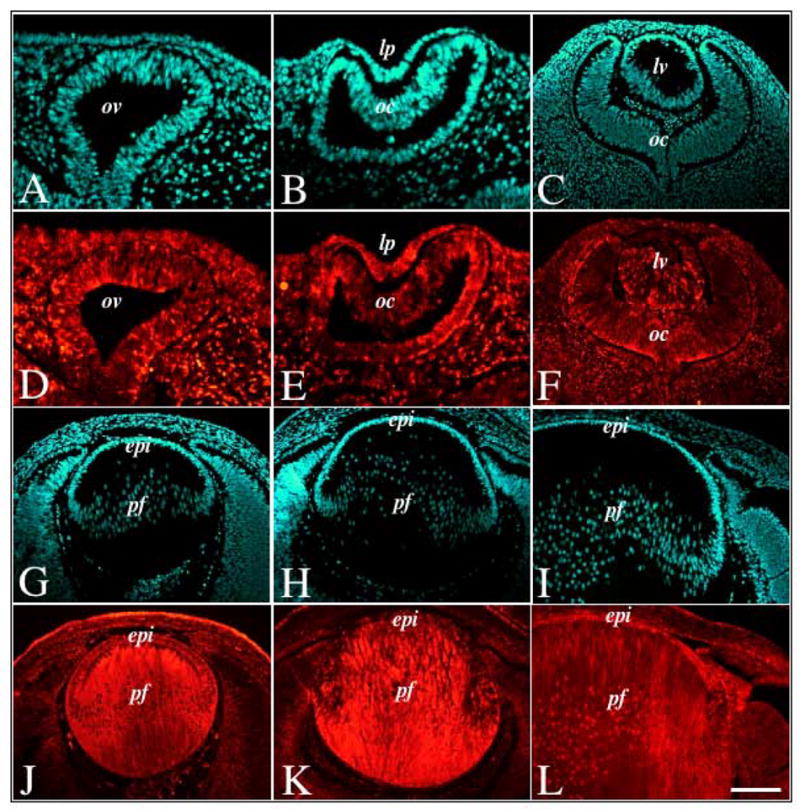
Immunofluorescent labelling of Spry2 during murine embryonic lens morphogenesis. Sections of mouse eyes at embryonic stages E11.5 (A, B, D, E), E12.5 (C, F), E13.5 (G, J), E15.5 (H, K) and E18.5 (I, L) were immunolabelled for Spry2 protein (D–F, J–L) and counterstained with Hoechst dye (A–C, G–I). Spry2 protein was ubiquitously expressed throughout lens morphogenesis, with strong expression in both the lens epithelium and primary fibre cells. Abbreviations: epi, lens epithelium; lp, lens pit; lv, lens vesicle; oc, optic cup; ov, optic vesicle; pf, primary fibres. Scale bar: 100 μm.
In neonatal mice, consistent with the strong Spry2 mRNA expression observed, immunoreactivity for Spry2 was detected throughout the lens, in both the epithelial cells and fibre cell mass (Figure 7A,B). With age, the most mature fibre cells lost their reactivity for Spry2, with its labelling primarily localised to the epithelium and lens fibre cortical region (P15, Figure 7C,D). This labelling became even more restricted in the fibre cell mass by P21 (Figure 7E,F), with strongest labelling in the epithelium and early differentiating secondary fibre cells. This same labelling pattern was observed in the lens of rats of comparable age (data not shown). Interestingly, similar to that observed in late lens embryogenesis, the labelling in the lens epithelium in the postnatal mouse lens was heterogeneously distributed, with some cells labelling strongly for Spry2 while other cells displayed little to no labelling (Figure 7D–H).
Figure 7.
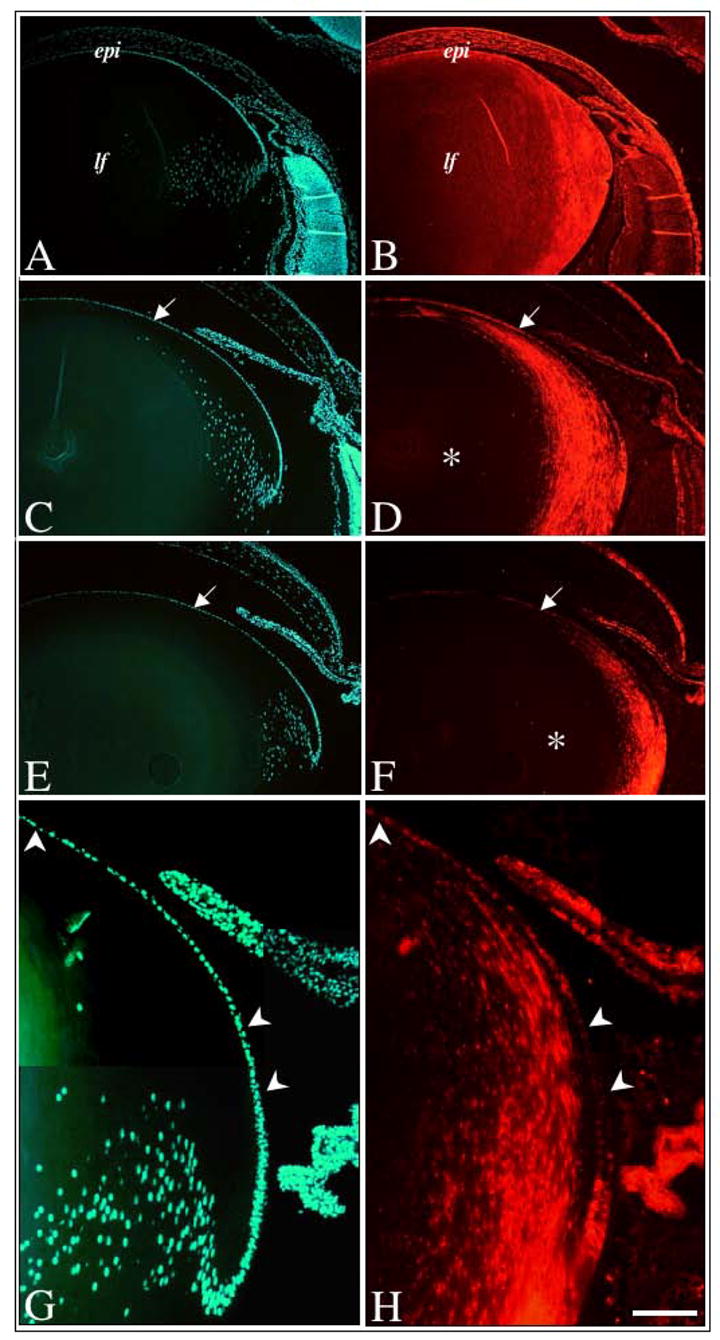
Immunofluorescent labelling of Spry2 during postnatal murine lens growth. Sections of mouse eyes at postnatal stages P3 (A, B), P10 (C, D), and P21 (E, F–H), were immunolabelled for Spry2 protein (B, D, F, H) and counterstained with Hoechst dye (A, C, E, G). In neonates, Spry2 labelling was strong throughout the lens, in both epithelial and fibre cells (B). At P10 (D) and P21 (F), Spry2 labelling was strong in the lens epithelium (arrows), but progressively lost in the 26 maturing lens fibre cells (asterisk). At higher magnification (G, H), the heterogenous labelling for Spry2 protein is more prominent with many lens epithelial cells demonstrating weak to reduced labelling (arrowheads). Abbreviations: epi, lens epithelium; lf, lens fibres. Scale bar: A–F, 200 μm, G–H, 100 μm.
Sef mRNA expression
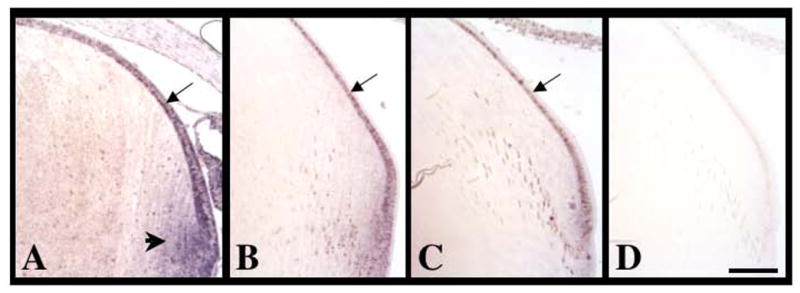
Labelling of mRNA transcripts for Sef was observed as early as those for Spry1 and Spry2 mRNA in the mouse and rat. By the late lens pit stage of the mouse, at E10.5, the invaginated head ectoderm demonstrated expression for Sef (Figure 8A). This persisted further into lens development, at E11.5 (Figure 8B) and E12.5 (Figure 8C), where we observed strong expression for Sef in the anterior lens vesicle cells and the newly elongating and differentiating primary fibre cells. By E13.5, consistent with the pattern of expression observed for Spry1, Sef was now more strongly expressed in the anterior differentiating lens epithelium, with the maturing underlying primary fibres displaying reduced expression (Figure 8D). This was more prominent slightly later in development, at E15.5, where all expression of Sef was now primarily expressed in the lens epithelium, with very little to no labelling as these cells differentiated into secondary fibre cells (Figure 8E). This same pattern of labelling in the lens persisted through to birth (Figure 8G) and beyond at P21, in lenses of both mice (data not shown) and rats (Figure 8H). For all stages examined, in situ hybridisation using sense riboprobes (with either fluorescent or alkaline phosphatase detection) did not demonstrate any label (see Figure 8F).
Figure 8.
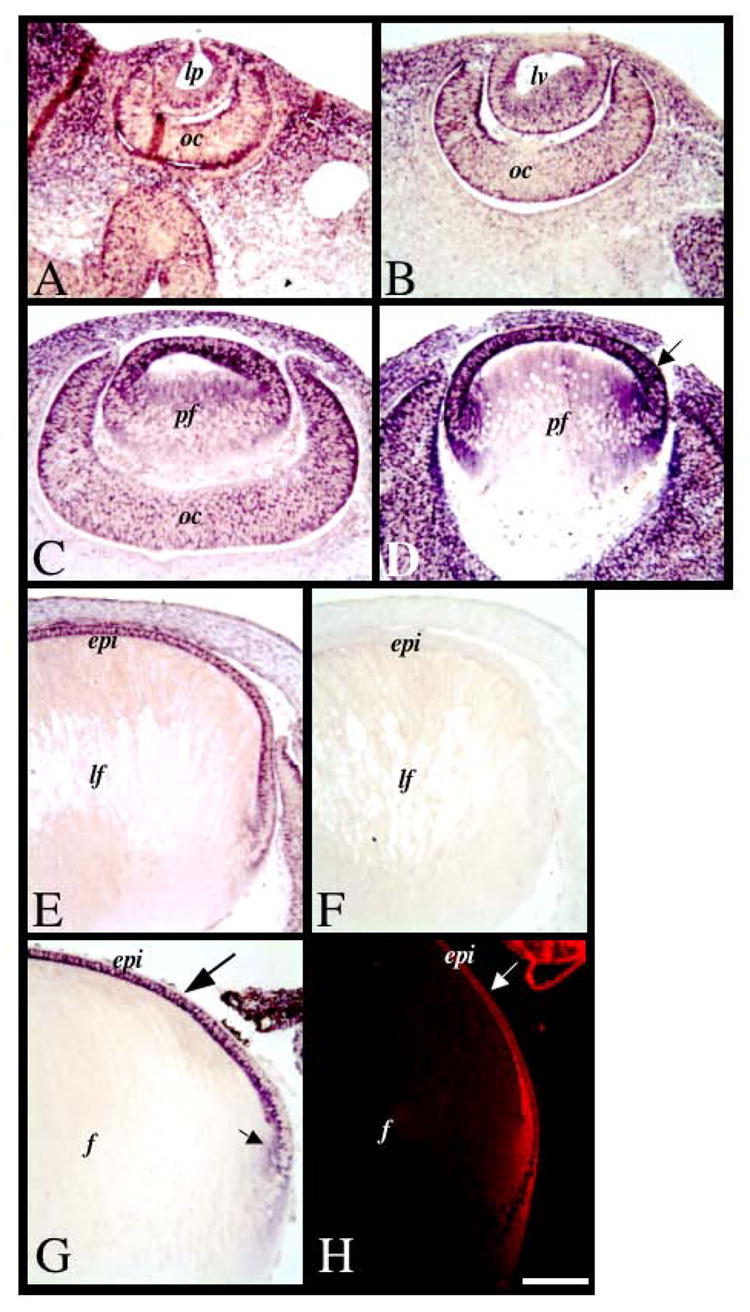
Expression of Sef mRNA during lens morphogenesis and growth. Sections of mouse eyes at embryonic stages E10.5 (A), E11.5 (B), E12.5 (C), E13.5 (D), E15.5 (E, F) and mouse postnatal stage P1 (G) and rat postnatal stage P21 (H) were labelled for Sef transcripts using alkaline phosphatase-labelled (A–G) or fluorescent (H) in situ hybridisation. Sef was ubiquitously expressed early in development (A–C). By E13.5 (D), strongest expression for Sef was detected in the differentiating anterior monolayer of lens cells (arrow). At E15.5, Sef was expressed in the epithelial cells, and not in the fibre cells. This labelling pattern persisted into postnatal development at birth (G) and weaning age (H). Control sections using a sense-labelled riboprobe showed no signal (F). Abbreviations: epi, lens epithelium; lp, lens pit; lv, lens vesicle; oc, optic cup; ov, optic vesicle; pf, primary fibre cells. Scale bar: 100 μm.
Sef protein expression
As the in situ hybridisation studies showed that Sef transcripts were primarily expressed in the lens epithelium, we wanted to determine if the protein showed a similar localisation pattern. In addition, as Sef is reported to be expressed in all vertebrates, we compared the epithelial expression in lenses of different species. In all species examined, Sef was found primarily in the lens epithelium. Consistent with mRNA labelling, Sef protein was evident in the lens epithelium of neonatal mice (Figure 9A,B), neonatal rat (Figure 9C,D) and P21 rat (Figure 9E,F). Sef was also shown to be localised to the lens epithelium and annular pad of the newborn chick (Figure 9G,H), as well as the epithelium of the adult bovine lens (Figure 9I,J) and foetal human lens (Figure 9K, L).
Figure 9.
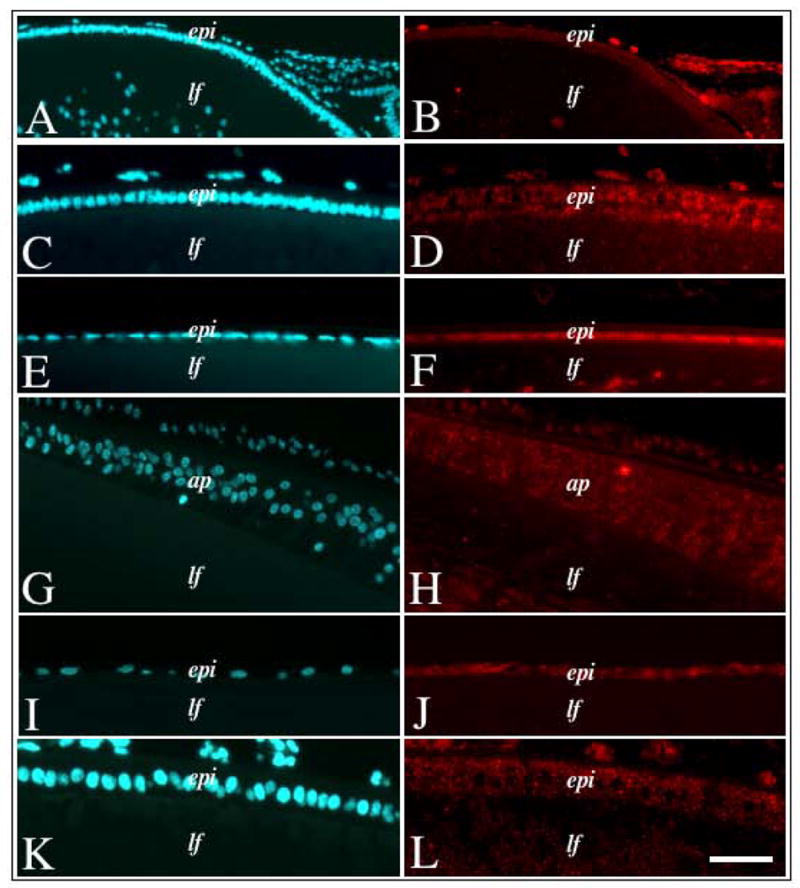
Immunofluorescent labelling of Sef in lenses of different species. Lens sections from neonatal mouse (A, B), neonatal rat (C, D), weanling rat (E, F), newborn chick (G, H), adult bovine (I, J) and foetal human (K, L) eyes, either immunolabelled for Sef protein (B, D, F, H, J, L) or counterstained with Hoechst dye (A, C, E, G, I, K). In all vertebrates examined, Sef protein labelling was strongest in the epithelial cells with little to no labelling in the fibre cells. Abbreviations: epi, lens epithelium; lf, lens fibres, ap, chick annular pad. Scale bar: 100 μm (A, B), 50 μm (C–L).
Discussion
Much of the lens research carried out to date has focused on identifying the key growth factors, and their respective signalling pathways, that influence the behavior of lens cells. Aberrant lens cell behaviour, leading to the diseased state, may result from the impaired function of these molecules, and/or the dysregulation of their signalling pathways. As a result, in normal lens development and growth, to ensure a physiologically appropriate cellular reponse, growth factor signalling events must be precisely regulated, both spatially and temporally. Due to this, a lot of interest has now focused on identifying and characterising the role of modulatory molecules that have the potential to positively and/or negatively regulate growth factor signalling in the lens. In this study, we report for the first time the detailed expression patterns of specific RTK antagonists belonging to the Sef and Spry gene families in the developing lens.
We have shown that Sef, Spry1 and Spry2 are all expressed during the earliest stages of lens development through to postnatal lens growth. During early ocular morphogenesis, we observed that Sef, Spry1 and Spry2 are ubiquitously expressed in the mouse, with strong expression in the head ectoderm and optic vesicle, prior to lens induction. As the surface head ectoderm invaginates to form the lens pit and subsequent lens vesicle, expression of Spry1, Spry2 and Sef is retained in all lens cells. This strong level of labelling is maintained through to birth, with both lens epithelial and fibre cells expressing Spry2 transcripts and protein. With age, the maturing lens fibre cells progressively lose expression of Spry2 as it becomes restricted to the lens epithelium. Similarly, in the postnatal murine lens, both Spry1 and Sef are primarily expressed in the lens epithelium, but in contrast to Spry2, this expression pattern is established much earlier in lens development. At the lens vesicle stage, once the elongating primary fibres make contact with the overlying differentiating lens epithelium, Sef and Spry1 expression is lost in the maturing fibre cells, with strong expression retained only in the anterior epithelium. This pattern of expression persists throughout embryogenesis and foetal development and continues postnatally as the lens grows. In summary, Sef, Spry1 and Spry2 are all co-expressed early in lens morphogenesis and at later postnatal stages. Spry2 expression primarily differs to that of Spry1 and Sef in that it persists in the lens fibre cells throughout embryonic and foetal development as well as early postnatal stages. Although these distinct expression patterns indicate that Spry2 may play an additional role in the regulation of primary fibre cell differentiation, without further functional studies, the significance of these differences in expression patterns is currently unclear.
Although Spry1, Spry2 and Sef transcripts are co-expressed throughout the lens epithelium, we did observe some heterogeneity in the protein labelling of Spry2. In contrast to Sef protein immunolabelling, which appeared to be uniformly expressed in all lens epithelial cells (see Figure 9), Spry2 labelling was absent in select cells of the lens epithelium. During lens embryogenesis (see Figure 6K), although all fibre cells appeared to be labelled for Spry2, many epithelial cells throughout the anterior epithelium did not label. Later in development, at E18 for example (see Figure 6L), labelling was absent in some of the more posterior cells of the epithelium. This same heterogeneity of Spry2 labelling persisted in the postnatal lens, with defined regions of epithelial cells lacking Spry2 reactivity. This is consistent with the reported accelerated degradation of Spry in response to growth factors, such as FGF, via a c-Cbl ubiquitin-dependent proteosome pathway [32]. This localized temporal degradation or downregulation of Spry2 in response to growth factor signalling may limit the repressive activity of Spry2 for a defined period, permitting the cells to appropriately respond to the ligand. It is tempting to speculate that the lens cells not reactive for Spry2 in this instance (with little to no Spry2), are in fact undergoing growth factor-induced cell division, which would implicate Spry2 as a negative regulator of lens cell proliferation. It will be interesting to determine whether the expression of Spry2 indeed alters in actively proliferating cells, with Spry2 downregulation or degradation required for lens cell proliferation to proceed. On a similar note, given that both Sef and Spry1 are downregulated with primary lens fibre differentiation and become localized to the lens epithelium throughout development, it is also just as tempting to speculate that the downregulation of these specific antagonists is required for primary fibre cell maturation and secondary fibre cell differentiation.
The expression patterns of Sef and Spry in the lens are similar to those previously reported for FGFs [8]. In other tissues, such as the lung, FGF synexpression groups have been described: these are defined as a group of genes that share the same expression pattern as FGF and are known to function in a common biological pathway [33]. In light of this we can propose that a FGF synexpression group comprising FGFs, Spry1, Spry2 and Sef is present in the lens. Given the important role that FGF plays in the regulation of lens development [3], it is important to understand if and how these growth factors are regulated in the eye. Our recent findings that antagonists such as Sef and Spry are co-expressed with FGF in the lens clearly suggest that FGF-signalling may be negatively regulated, most likely contributing to the maintenance of the distinct patterns of lens cell behaviour. As mentioned earlier, the main cellular processes in the lens, namely lens cell proliferation and fibre cell differentiation, are thought to be regulated by an ‘antero-posterior’ gradient of FGF [4], with lower levels of FGF activity in the aqueous regulating lens cell proliferation anteriorly, and higher levels of FGF activity in the vitreous inducing fibre cell differentiation, posteriorly. In addition to these antero-posterior differences in levels of FGF bioactivity, the stronger expression of Sef, Spry1 and Spry2 in the anterior lens (epithelium) is consistent with an inverse gradient (to FGF) of these antagonists in the lens. Given that Spry and Sef have been reported to be negative-feedback regulators of FGF activity in other developmental systems [17,23], the high levels of Sef and Spry we observe in the anterior epithelium may be important for the maintenance of these cells. Their downregulation (making the cells more receptive to FGF and/or other growth factors) may be required for the epithelial cells to differentiate into fibre cells, hence providing a double assurance mechanism that promotes the lens epithelial phenotype, maintaining lens cellular architecture in the process. Overall, the presence of these specific RTK antagonists in defined regions of the lens, indicates a possible mechanism for maintaining the tight regulation of growth factor signalling in lens cells.
Acknowledgments
The authors would like to acknowledge the support of the National Health and Medical Research Council (NHMRC) and the Sydney Foundation for Medical Research, AUSTRALIA as well as NIH, USA (R01 EY0-3177). Much appreciation is also extended to Dr. Michelle Madigan for providing the human foetal lens tissue.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McAvoy JW, Chamberlain C, de Iongh R, Hales A, Lovicu FJ. Lens Development. Eye. 1999;13:425–37. doi: 10.1038/eye.1999.117. [DOI] [PubMed] [Google Scholar]
- 2.Robinson ML, Lovicu FJ. The Lens: Historical and Comparative Perspectives. In: Lovicu FJ, Robinson ML, editors. Development of the Ocular Lens. New York: Cambridge University Press; 2004. pp. 3–26. [Google Scholar]
- 3.Lovicu FJ, McAvoy JW. Growth Factor Regulation Of Lens Development. Dev Biol. 2005;280:1–14. doi: 10.1016/j.ydbio.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 4.Schulz MW, Chamberlain CG, de Iongh RU, McAvoy JW. Acidic and basic FGF in ocular media and lens: implications for lens polarity and growth patterns. Development. 1993;118:117–26. doi: 10.1242/dev.118.1.117. [DOI] [PubMed] [Google Scholar]
- 5.McAvoy JW, Chamberlain CG. Fibroblast growth factor (FGF) induces different responses in lens epithelial cells depending on its concentration. Development. 1989;107:221–28. doi: 10.1242/dev.107.2.221. [DOI] [PubMed] [Google Scholar]
- 6.Lovicu FJ, McAvoy JW. Localisation of aFGF, bFGF and HSPG in rat lens: implications for lens polarity and growth patterns. Invest Ophthalmol Vis Sci. 1993;34:3355–65. [PubMed] [Google Scholar]
- 7.Lovicu FJ, Chamberlain CG, McAvoy JW. Differential effects of aqueous and vitreous on fiber differentiation and extracellular matrix accumulation in lens epithelial explants. Invest Ophthalmol Vis Sci. 1995;36:1459–69. [PubMed] [Google Scholar]
- 8.Lovicu FJ, de Iongh RU, McAvoy JW. Expression of FGF-1 and FGF-2 mRNA during lens morphogenesis, differentiation and growth. Curr Eye Res. 1997;16:222–30. doi: 10.1076/ceyr.16.3.222.15408. [DOI] [PubMed] [Google Scholar]
- 9.de Iongh RU, Lovicu FJ, Chamberlain CG, McAvoy JW. Differential expression of fibroblast growth factor receptors during rat lens morphogenesis and growth. Invest Ophthalmol Vis Sci. 1997;38:1688–99. [PubMed] [Google Scholar]
- 10.Robinson ML, Overbeek PA, Verran DJ, Grizzle WE, Stockard CR, Friesel R, et al. Extracellular FGF-1 acts as a lens differentiation factor in transgenic mice. Development. 1995;121:505–14. doi: 10.1242/dev.121.2.505. [DOI] [PubMed] [Google Scholar]
- 11.Lovicu FJ, Overbeek PA. Overlapping effects of different members of the FGF family on lens fiber differentiation in transgenic mice. Development. 1998;125:3365–77. doi: 10.1242/dev.125.17.3365. [DOI] [PubMed] [Google Scholar]
- 12.Zhao H, Rossant J, Ornitz DM, Beebe DC, Robinson ML. Different FGFR genes play an essential but redundant role in postinduction lens development. Invest Ophthalmol Vis Sci Suppl. 2003;44:954. [Google Scholar]
- 13.Li DW, Liu JP, Wang J, Mao YW, Hou LH. Expression and activity of the signalling molecules for mitogen-activated protein kinase pathways in human, bovine, and rat lenses. Invest Ophthalmol Vis Sci. 2003;44:5277–86. doi: 10.1167/iovs.03-0348. [DOI] [PubMed] [Google Scholar]
- 14.Lovicu FJ, McAvoy JW. FGF-induced lens cell proliferation and differentiation is dependent on MAPK (ERK1/2) signalling. Development. 2001;128:5075–84. doi: 10.1242/dev.128.24.5075. [DOI] [PubMed] [Google Scholar]
- 15.Le AC, Musil LS. FGF signaling in chick lens development. Dev Biol. 2001;233:394–411. doi: 10.1006/dbio.2001.0194. [DOI] [PubMed] [Google Scholar]
- 16.Iyengar L, Patkunanathan B, Lynch OT, McAvoy JW, Rasko JEJ, Lovicu FJ. Aqueous humour- and growth factor-induced lens cell proliferation is dependent on MAPK/ERK1/2 and Akt/PI3-K signalling. Exp Eye Res. 2006;83:667–78. doi: 10.1016/j.exer.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Tsang M, Dawid IB. Promotion and attenuation of FGF signaling through the Ras-MAPK pathway. Sci STKE. 2004;228:pe17. doi: 10.1126/stke.2282004pe17. [DOI] [PubMed] [Google Scholar]
- 18.Hacohen N, Kramer S, Sutherland D, Hiromi Y, Krasnow MA. Sprouty encodes a novel antagonist of FGF signalling that patterns apical branching of the Drosophila airways. Cell. 1998;92:253–63. doi: 10.1016/s0092-8674(00)80919-8. [DOI] [PubMed] [Google Scholar]
- 19.Tefft JD, Lee M, Smith S, Leinwand M, Zhao J, Bringas P, et al. Conserved function of mSpry-2, a murine homolog of Drosophila sprouty, which negatively modulates respiratory organogenesis. Curr Biol. 1999;9:219–22. doi: 10.1016/s0960-9822(99)80094-3. [DOI] [PubMed] [Google Scholar]
- 20.Chambers D, Mason I. Expression of sprouty2 during early development of the chick embryo is coincident with known sites of FGF signalling. Mech Dev. 2000;91:361–64. doi: 10.1016/s0925-4773(99)00288-9. [DOI] [PubMed] [Google Scholar]
- 21.Zhang S, Lin Y, Itaranta P, Yagi A, Vainio S. Expression of Sprouty genes 1, 2, and 4 during mouse organogenesis. Mech Dev. 2001;109:367–70. doi: 10.1016/s0925-4773(01)00526-3. [DOI] [PubMed] [Google Scholar]
- 22.Minowada G, Jarvis LA, Chi CL, Neubuser A, Sun X, Hacohen N, et al. Vertebrate sprouty genes are induced by FGF signalling and can cause chondroplaysia when overexpressed. Development. 1999;126:4465–75. doi: 10.1242/dev.126.20.4465. [DOI] [PubMed] [Google Scholar]
- 23.Kim HJ, Bar-Sagi D. Modulation of signalling by Sprouty: a developing story. Nat Rev Mol Cell Biol. 2004;5:441–50. doi: 10.1038/nrm1400. [DOI] [PubMed] [Google Scholar]
- 24.Kudoh T, Tsang M, Hukriede NA, Chen X, Dedekian M, Clarke CJ, et al. A gene expression screen in zebrafish embryogenesis. Genome Res. 2001;11:1979–87. doi: 10.1101/gr.209601. [DOI] [PubMed] [Google Scholar]
- 25.Tsang M, Friesel R, Kudoh T, Dawid IB. Identification of Sef, a novel modulator of FGF signalling. Nat Cell Biol. 2002;4:165–9. doi: 10.1038/ncb749. [DOI] [PubMed] [Google Scholar]
- 26.Furthauer M, Lin W, Ang SL, Thisse B, Thisse C. Sef is a feedback-induced antagonist of Ras/MAPK-mediated FGF signalling. Nat Cell Biol. 2002;4:170–4. doi: 10.1038/ncb750. [DOI] [PubMed] [Google Scholar]
- 27.Mailleux AA, Tefft D, Ndiaye D, Itoh N, Thiery JP, Warburton D, et al. Evidence that SPROUTY2 functions as an inhibitor of mouse embryonic lung growth and morphogenesis. Mech Dev. 2001;102:81–94. doi: 10.1016/s0925-4773(01)00286-6. [DOI] [PubMed] [Google Scholar]
- 28.Lin W, Furthauer M, Thisse B, Thisse C, Jing N, Ang SL. Cloning of the mouse Sef gene and comparative analysis of its expression with Fgf8 and Spry2 during embryogenesis. Mech Dev. 2002;113:163–8. doi: 10.1016/s0925-4773(02)00018-7. [DOI] [PubMed] [Google Scholar]
- 29.Furthauer M, Reifers F, Brand M, Thisse B, Thisse C. Sprouty4 acts in vivo as a feedback-induced antagonist of FGF signalling in zebrafish. Development. 2001;128:2175–86. doi: 10.1242/dev.128.12.2175. [DOI] [PubMed] [Google Scholar]
- 30.de Iongh RU, Gordon-Thomson C, Chamberlain CG, Hales AM, McAvoy JW. TGFbeta receptor expression in lens: implications for differentiation and cataractogenesis. Exp Eye Res. 2001;72:649–59. doi: 10.1006/exer.2001.1001. [DOI] [PubMed] [Google Scholar]
- 31.Lovicu FJ, Steven P, Saika S, McAvoy JW. Aberrant lens fiber differentiation in anterior subcapsular cataract formation: a process dependent on reduced levels of Pax6. Invest Ophthalmol Vis Sci. 2004;45:1946–53. doi: 10.1167/iovs.03-1206. [DOI] [PubMed] [Google Scholar]
- 32.Hall AB, Jura N, DaSilva J, Jang YJ, Gong D, Bar-Sagi D. hSpry2 is targeted to the ubiquitin-dependent proteasome pathway by c-Cbl. Curr Biol. 2003;13:308–14. doi: 10.1016/s0960-9822(03)00086-1. [DOI] [PubMed] [Google Scholar]
- 33.Niehrs C, Meinhardt H. Modular feedback. Nature. 2002;417:35–6. doi: 10.1038/417035a. [DOI] [PubMed] [Google Scholar]


