Abstract
Black cohosh has become one of the most important herbal products in the U.S. dietary supplements market. It is manufactured from roots and rhizomes of Cimicifuga racemosa (Ranunculaceae). Botanical identification of the raw starting material is a key step in the quality control of black cohosh preparations. The present report summarizes a fingerprinting approach based on HPLC-PDA/MS/ELSD that has been developed and validated using a total of ten Cimicifuga species. These include three North American species, C. racemosa, C. americana, C. rubifolia, and seven Asian species, C. acerina, C. biternat, C. dahurica, C. heracleifolia, C. japonica, C. foetida, and C. simplex. The chemotaxonomic distinctiveness of the HPLC fingerprints allows identification of all ten Cimicifuga species. The triterpene glycosides cimigenol-3-O-arabinoside (3), cimifugin (12), and cimifugin-3-O-glucoside (18) were determined to be suitable species-specific markers for the distinction of C. racemosa from the other Cimicifuga species. In addition to identification, the fingerprint method provided insight into chemical interconversion processes occurring between the diverse triterpene glycosides contained in black cohosh. The reported method has proven its usefulness in the botanical standardization and quality control of black cohosh products.
Keywords: Black cohosh, Cimicifuga, Cimigenol arabinoside, Cimifugin, Species identification, Botanical standardization
1. Introduction
The dried rhizomes and roots of black cohosh, Cimicifuga racemosa L. Nutt (Ranunculaceae) (syn. Actaea racemosa L.) are widely used as a herbal dietary supplement. The plant is native to the eastern United States, growing as far south as Florida. Historically, Native American women have used black cohosh for the relief of pain during menses and childbirth. It has also been used for the treatment of general malaise, kidney ailments, rheumatism and malaria. Black cohosh was introduced to Europe in the late 19th century, and became an established phytomedicine in the early 20th century. Today, black cohosh is widely used in Europe and the United States for various female complaints, including symptoms associated with premenstrual syndrome, dysmenorrhea and menopause [1].
Raw material of black cohosh is mainly collected from plants growing in the wild in the Blue Ridge mountains. Other scattered collections are made in Indiana, Michigan, Illinois and Northern Pennsylvania. Two closely related Cimicifuga species, C. americana Michx. (syn. Actaea podocarpa DC.) and C. rubifolia Kearney (syn. A. cordifolia DC.), share the same habitat with black cohosh as they all grow in wooded areas of the eastern United States [2]. Their collection is done in batches of various sizes and has not been specifically documented. The Asian Cimicifuga species, including C. dahurica (Turcz.) Maxim., C. foetida L., and C. heracleifolia Kom., are known in traditional Chinese medicine as Sheng-Ma, and are used for their antipyretic, analgesic and anti-inflammatory properties. In Japan, C. simplex Wormsk., C. japonica (Thunb.) Sprengel, and C. acerina (Sieb. et Zucc.) C. Tanaka have been widely used for the same purposes [3].
Black cohosh is one of the most extensively studied herbal products, and is used as an herbal dietary supplement for the alleviation of menopausal symptoms [4–9]. A considerable amount of clinical research has been documented for a commercial product, Remifemin®, which is manufactured by Schaper & Brümmer in Germany, and distributed in the United States by Glaxo Smith Kline. Recent pharmacological studies of C. racemosa indicate that the biological effects of black cohosh are due to substances with dopaminergic [10] and serotonergic [11–12] activity rather than an estrogen-like activity.
Triterpenoid saponins and hydroxycinnamic or piscidic acid derivatives are the dominating two groups of chemical constituents in Cimicifuga species. The triterpenoids belong to the 9, 19-cycloartanol series and are typically glycosidated at the C-3 position. So far, D-xylose and L-arabinose are the predominant sugar moieties found in the triterpenes of all of the Cimicifuga species. Glycosides containing D-glucose and D-galactose were only isolated from some species, in particular C. simplex. Hydroxycinnamic acid derivatives are the second group of common constituents of the rhizomes/roots of Cimicifuga species. These phenolic components include caffeic, ferulic, isoferulic, fukinolic, fukiic and cimicifugic acids and their esters. Cimifugin and cimifugin glucoside are found in many Asian Cimicifuga species [3], but not in black cohosh. Cimiracemates A–D, however, are minor constituents in black cohosh [13]. Recent reports on the presence of formononetin, an estrogenic isoflavone, remain controversial. While we and others could not confirm its presence in black cohosh [14–16], there is one recent positive report [17]. Actein, 23-epi-26-deoxyactein (syn. 27-deoxyactein), acetylshengmanol-3-O-xyloside, cimigenol-3-O-arabinoside, and cimigenol-3-O-xyloside represent the major triterpene glycosides found in black cohosh [18–22].
The use of herbal dietary supplement containing black cohosh as an alternative to conventional estrogen therapy is gaining popularity among perimenopausal women. In 2004, black cohosh ranked eighth among herbal supplements sold in mainstream retail outlets in the U.S. [23]. In September 2005, the American Herbal Products Association’s trade recommendations on black cohosh adulteration came into effect in the herbal industry. This addresses a concern raised with the documentation of instances of black cohosh being adulterated with other species. Typical adulterants are C. foetida, C. dahurica and C. heracleifolia.
Most clinical trials with black cohosh have been using products made originating from C. racemosa alcoholic extract that were standardized to a certain level of content of total triterpene glycosides. Numerous efforts have been made to establish effective methods for the identifications of chemical constituents in black cohosh, including HPLC-UV/MS [24–28], HPLC-UV/ELSD [15–16, 24, 29–32], HPTLC [33], GC-MS [17] and random amplified polymorphic DNA (RAPD) analyses [34]. While most of the published chemical methods already focus on the identification of triterpene glycosides, it still remains difficult to reliably distinguish Cimicifuga species due to the large number of triterpene glycosides contained in a single species. Typically, peaks for more than 30 triterpenes are apparent in HPLC chromatograms, making it impossible to completely resolve these components and identify them unambiguously. Previously, we have developed an LC-PDA/MS method that allows differentiating between C. racemosa and C. foetida and C. simplex [24]. In the present report, this method is refined by adding an evaporative light scattering (ELSD) as a detection method and by extending the coverage to a total of ten Cimicifuga species. These include the three North American species, C. racemosa, C. americana, C. rubifolia, and the seven Asian species, C. acerina, C. biternat, C. dahurica, C. heracleifolia, C. japonica, C. foetida and C. simplex. Based on comprehensive HPLC fingerprints of all ten Cimicifuga species, unambiguous species identification can be achieved.
2. Experimental
2.1. Plant materials and standards
Nine pure Cimicifuga triterpene glycosides and eight phenolic compounds were used as authentic standards. The triterpene glycosides were actein (1), 23-epi-26-deoxyactein (2), cimigenol-3-O-arabinoside (3), cimigenol-3-O-xyloside (4), 25-O-acetylcimigenol-3-O-xyloside (5), acetyshengmanol-3-O-xyloside (6), 24-O-acetylhydroshengmanol-3-O-xyloside (7), cimiracemoside A (8), and cimicifugoside H-1 (9). The phenolic constituents were cimicifugic acids A (10) and B (11), cimifugin (12), cimiracemates A (13) and B (14), caffeic acid (15), ferulic acid (16), and isoferulic acid (17). Compounds 1–7 and 10–12 were isolated from the Cimicifuga species by procedures described in the literature [3, 24], and were identified by NMR spectroscopy. Compound 8 was purchased from Chromadex (Santa Ana, CA), 9 from Herbstandard Inc. (Clarendon Hills, IL), and 15–17 from Sigma Chemical Co. (St. Louis, MO). Compounds 13 and 14 were isolates from C. racemosa and kindly provided by Dr. Shao-Nong Chen (UIC/NIH Center for Botanical Dietary Supplements Research, University of Illinois at Chicago, Chicago, IL). The chemical structures of the standards are listed in Fig. 1, and their purities were better than 90% as determined to by HPLC and NMR. The identification of further Cimicifuga constituents by LC-PDA/MS is discussed below.
Fig. 1.
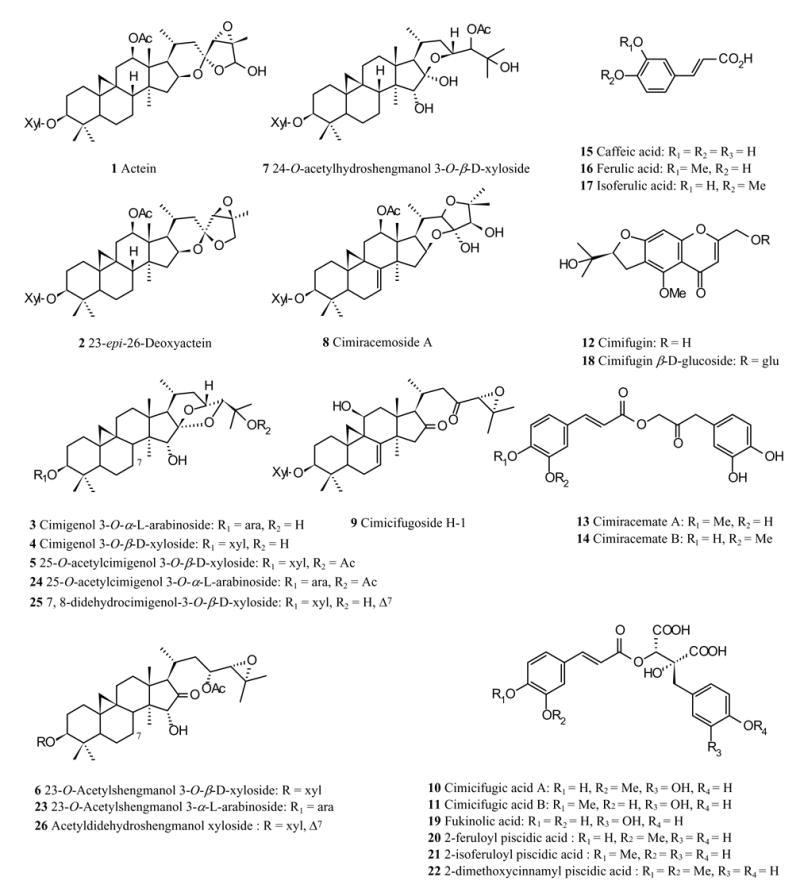
Structures of some triterpene glycosides and aromatic compounds of Cimicifuga species. 1–17 reference standards. 18–26 compounds identified by MS analysis.
The plant samples used in this study include: three C. racemosa, two C. americana and two C. rubifolia collected from North Carolina, Tennessee and Virginia in 1999, and identified by the New York Botanical Garden; two C. simplex (C. simplex-1 from Municipality of Enzan and C. simplex-2 from Mutsu, Skayu) and one C. biternat collected from Japan in 1999, and identified by T. Koyama at Nihon University, and one C. foetida collected from China in 1998 and identified by Prof. Zhaoyun Wu at Shanghai University of Traditional Chinese Medicine (SUTCM); one specimen each of C. dahurica, C. foetida and C. heracleifolia collected from China in 2001, and identified by Prof. Zheng-Tao Wang at the SUTCM and Prof. Dao-Feng Chen at the School of Pharmacy, Fudan University; one specimen each of C. simplex, C. japonica and C. acerina collected from Japan in 2002 and identified by Dr. Seiji Nagumo at Hoshi University. Voucher samples are deposited in the New York Botanical Garden, Nihon University College Museum of Bioresource Sciences, SUTCM and AndroScience’s herbariums, respectively. The commercial products analyzed include Remifemin®, purchased from a health store in New Jersey, CimiPure®, manufactured by Pure World Botanicals, Inc., and products A and B, which were obtained from the marketplace.
2.2. Apparatus
HPLC analyses were carried out on an Agilent 1100 series instrument equipped with a quaternary pump, a four-channel-online degasser, an autosampler, a column oven, a photodiode array detector (PDA), a mass detector, and an evaporative light scattering detector (ELSD). Data collection was handled by ChemStation software (Agilent). ELSD chromatograms were recorded on a Sedere Sedex-75 (Alfortville, France). Mass spectra were recorded on an LCQ (Thermo-Finnigan, San Jose, CA) instrument equipped with an atmospheric pressure electrospray (ESI) and chemical ionization (APCI) sources.
2.3. Chromatography and detection
Chromatography was performed on a Zorbax DBS (5 micron, 4 mm ID × 250) column with a flow rate of 1.0 mL/min. Two solvent systems were used. Solvent system I was used for the separation of phenolic components. It consisted of a step gradient starting with 5% (v/v) aqueous HPLC-grade acetonitrile (A) in 0.1% acetic acid, increasing to 100% acetonitrile over 55 minutes in the following step cycle: 0–18 min, 5–28% A; 18–36 min, 28–35% A; 36–45 min, 35–55% A; 45–55 min, 55–75% A; 55–56 min, 75–100% A. At the end of the run, 100% of acetonitrile was allowed to flush the column for 10 minutes, and an additional 10 minutes of post run time were set to allow for equilibration of the column with the starting eluant. Solvent system II, derived from the Institute for Nutraceutical Advancement (INA) Method 113.001 [29], was used for the triterpene analysis. This system consisted of a step gradient starting with 30% A in 1% formic acid with water, increasing to 40% A between 0–30 min, then increasing to 60% A between 30–60 min, and resetting to 30% A for column equilibrium between 60–70 min. The column temperature was constant at 25°C in the solvent system I, and 30°C in the solvent system II. The sample volume injected was 10 μL. The UV spectral data was collected at 203 nm for triterpene glycosides and 320 nm for phenolic constituents. For ELSD detection, nitrogen was used as the nebulizing gas at a pressure of 2.6 bar and a temperature of 50°C. Signal gain was set to 7 with an analog output at 20,000 units/Volt. HPLC grade acetonitrile and water were purchased from Fisher Scientific (Morris Plans, NJ). Atmospheric pressure chemical ionization (APCI) was performed by setting the discharge voltage to 5.0 kV. The vaporizer and capillary temperatures were set to 450 and 150°C, respectively. Both the sheath gas and the auxiliary gas were nitrogen and had flow rates of 90 and 10 units, respectively. A mass range of 50–1500 amu was scanned.
2.4. Sample preparation
The samples were prepared by precisely weighing 300.0–400.0 mg of powdered plant material or extract into a 10 mL volumetric flask. Approximately 7 mL of 75% methanol was added, and the solution was sonicated for 30 minutes for extracts and 6 hours for crude powders, respectively. The solution was allowed to cool to room temperature before filling up to the final volume of 10.00 ml, and was then filtered through a 0.45 μm membrane filter (Nylon Acrodisc® 13, Pall Corporation) before the HPLC injection.
3. Results and discussion
3.1. Analysis of phenolic constituents
The spectrum of phenolic acid derivatives contained in Cimicifuga species is characterized by the predominance of caffeic acids and their esters. Both the acids and esters show similar UV spectra mainly due to the conjugated caffeoyl system. The spectrum of caffeic acid derivatives usually comprises the strong caffeoyl absorption bands with maxima around 220–226, 246–252 (sh), 296–300 (sh), and 320–330 nm. The two shoulder (sh) absorptions at the inner side of the spectrum make these spectra characteristically different from other non-caffeic components (Fig. 2). Since there is less influence of the mobile phase on the long wavelength absorption, the use of wavelengths above 300 nm is preferred in structural analysis. Compounds 15–17 exhibited maxima around 322–324 nm, while compounds 10, 11, 13, and 14 showed maxima around 326–329 nm. The caffeic acid derivatives 10, 11, and 15–17 are contained in most of the 10 Cimicifuga species, but in different ratios. Their identification was achieved by a direct comparison with standards, and was confirmed by MS. Other compounds, including cimifugin glucoside (18), were identified by a combination of UV and MS. In the APCI mode, as expected, negative ionization resulted in higher sensitivity than positive ionization when analyzing phenolic components. Therefore, all mass spectral data discussed here was obtained in (−) APCI mode. All of the caffeic acid derivatives typically displayed molecular ions and acetic acid adducts, along with fragments of the caffeoyl units. Fukinolic acid (19) was identified by comparing the relative retention time (Rt.) with that reported in literature and by its characteristic UV spectrum maximum absorption at 330 nm, representing the longest wavelength of all caffeic acid derivatives discussed here. Its molecular ion was observed at m/z 433 [M−H]− and confirmed by m/z at 493 [M+OAc]−, 867 [2M−H]− and 1301 [3M−H]−. The fragments of m/z 271 [M-caffeoyl]−, 253 [M-caffeic acid-H]−, 179, the molecular ion of caffeic acid [M−H]− and its decarboxylation product 135 [Mcaffeic acid -H-CO2]− provided further structural information of 19. The above should be considered a plausible but tentative fragmentation pattern. The two peaks at Rt. around 21.2 and 21.6 min, with UV spectra similar to those of 10 and 11, showed a pseudomolecular ion m/z 431 [M−H]−, and fragmental ions at m/z 255 [M-feruloyl or isoferuloyl]−, 237 [M-ferulic or isoferulic acid-H]− and 193 due to the molecular ions of ferulic and/or isoferulic acid [M−H]−. Their structures were tentatively assigned as 2-feruloyl piscidic acid (20) and 2-isoferuloyl piscidic acid (21), respectively. The sequence of chromatographic elution was assigned as 20 ahead of 21. This is in line with the relative elution order of 10, and 11 and also with a chromatogram reported in the literature [3]. Compounds 20 and 21 are very abundant in C. dahurica. In C. simplex, there was one peak appearing at Rt. around 23 min, which exhibited a molecular ion at m/z 461 [M−H]− and a group of fragments at m/z 271 [M-dimethoxycinnamoyl]−, 253 [M-dimethoxycinnamic acid-H]−, 207 due to the pseudomolecular ion of dimethoxycinnamic acid [M−H]−, and 191 [Mdimethoxycinnamic acid-OH]−. Based on its mass spectral behavior, the structure of this compound is proposed to be 2-dimethoxycinnamoyl piscidic acid (22), and represents a phenolic that could only be detected in C. simplex. However, 3, 4-dimethoxycinnamic acid has previously been isolated from C. simplex [35]. Although the identification of this compound remains to be confirmed by isolation, it is likely that 22 can serve as a specific marker of C. simplex. Compounds 13 and 14 were observed in C. racemosa at around Rt. 31–32 min as very small peaks, and could not be detected in other species, leading to the conclusion that they were either absent or present in trace amounts only. Through UV and MS analysis, the individual caffeic acid derivative could be unambiguously identified (Fig. 3). Our previous study had indicated that two chromones, cimifugin (12) and cimifugin glucoside (18), were contained in C. foetida and C. simplex only, but not in C. racemosa. In the HPLC profiles of the current study, 12 and 18 eluted around Rt. 17 and 14 min, respectively. Their UV spectra exhibited absorption maxima at 298 and 300 nm, respectively, with a symmetrical shape, which easily distinguished them from the caffeic acid derivatives (Fig. 2). Compounds 12 and 18 displayed strong molecular ions in both positive and negative APCI modes. In (+)-APCI, protonated molecular ions of m/z 307 [M+H]+ for 12 and 467 [M+H]+ for 18 were observed. Compound 18 also showed a deglucosidated fragment ion at m/z 307 [M-glucosyl]+. The application of 12 and 18 for species identification will be discussed below. All of the identified phenolic constituents in the ten Cimicifuga species are summarized in Table 1.
Fig. 2.
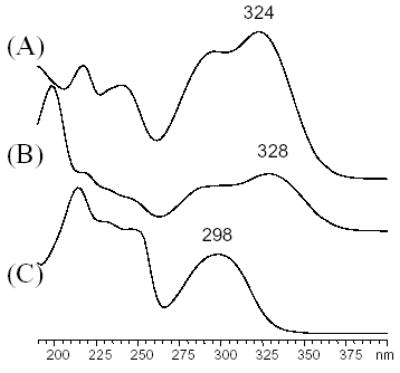
Typical UV spectra of aromatic Cimicifuga compounds, obtained on-line in mobile phase as part of the HPLC fingerprint of Cimicifuga species. (A) Isoferulic acid (17); (B) Cimicifugic acid B (11); (C) Cimifugin (12).
Fig. 3.
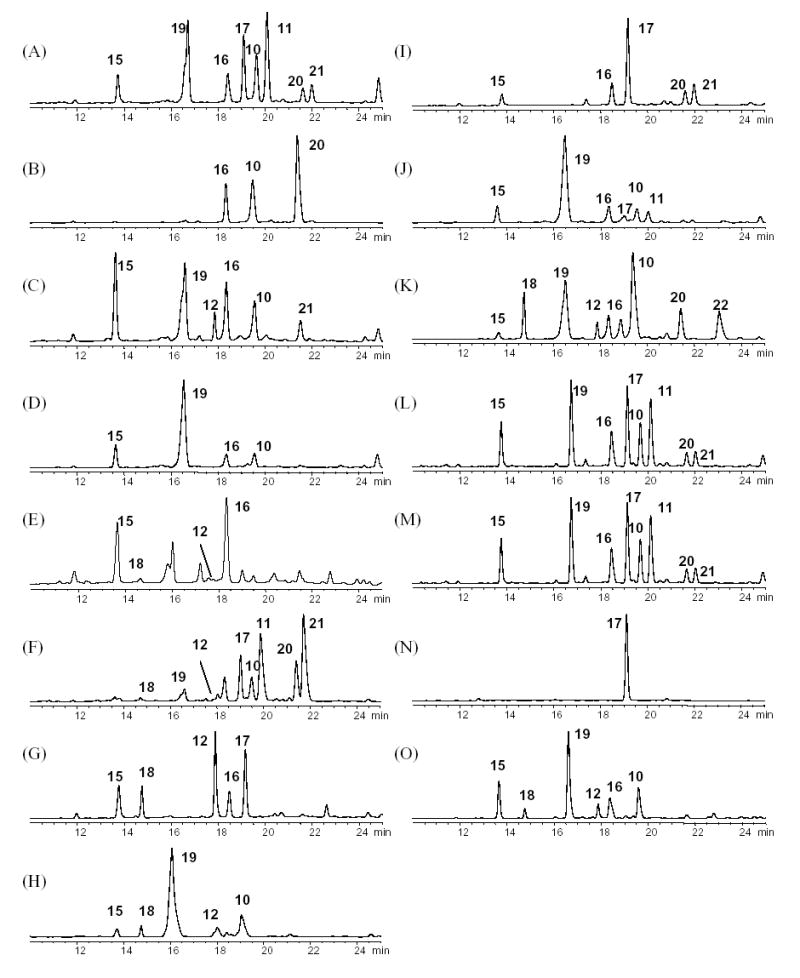
Comparison of HPLC-UV chromatograms of aromatic constituents (solvent system I). Because all major aromatic compounds elute before 25 min, only the 0–25 min windows are shown. (A) C. racemosa; (B) C. americana; (C) C. rubifolia; (D) C. acerina; (E) C. biternat; (F) C. dahurica; (G) C. foetida-1; (H) C. foetida-2; (I) C. heracleifolia; (J) C. japonica; (K) C. simplex; (L) Remifemin; (M) CimiPure; (N) Product A; (O) Product B.
Table 1.
Summary of the phenolic and triterpene glycoside constituents identified from the fingerprints of the ten different Cimicifuga species
| Compoundb | Plant speciesa | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | I | J | |
| actein (1) | x | x | ||||||||
| 23-epi-26-deoxyactein (2) | x | x | x | |||||||
| cimigenol-3-O-arabinoside (3) | x | x | ||||||||
| cimigenol-3-O-xyloside (4) | x | x | x | x | x | x | ||||
| 25-O-acetylcimigenol-3-O-xyloside (5) | x | |||||||||
| 23-O-acetyshengmanol-3-O-xyloside (6) | x | x | x | x | x | x | ||||
| 24-O-acetylhydroshengmanol-3-O-xyloside (7) | x | x | ||||||||
| cimiracemoside A (8) | x | |||||||||
| cimicifugoside H-1 (9) | x | |||||||||
| cimicifugic acid A (10) | x | x | x | x | x | x | x | |||
| cimicifugic acid B (11) | x | x | x | |||||||
| cimifugin (12) | x | x | x | x | x | |||||
| cimiracemate A (13) | x | |||||||||
| cimiracemate B (14) | ||||||||||
| caffeic acid (15) | x | x | x | x | x | x | x | x | ||
| ferulic acid (16) | x | x | x | x | x | x | x | x | x | |
| isoferulic acid (17) | x | x | x | x | x | |||||
| cimifugin glucoside (18) | x | x | x | x | ||||||
| fukinolic acid (19) | x | x | x | x | x | x | ||||
| 2-feruloyl piscidic acid (20) | x | x | x | x | x | |||||
| 2-isoferuloyl piscidic acid (21) | x | x | x | x | ||||||
| 2-dimethoxycinnamyl piscidic acid (22) | x | |||||||||
| 23-O-acetylshengmanol-3-O-arabinoside (23) | x | |||||||||
| 25-O-acetylcimigenol-3-O-arabinoside (24) | x | |||||||||
| 7, 8-didehydrocimigenol-3-O-xyloside (25) | x | x | ||||||||
| acetyldidehydroshengmanol-3-O-xyloside (26) | x | |||||||||
A = C. racemosa; B = C. americana; C = C. rubifolia; D = C. acerina; E = C. biternat; F = C. dahurica; G = C. foetida; H = C. heracleifolia; I = C. japonica; J = C. simplex.
1–17 were identified by comparison with authentic standards, and 18–26 were identified based on mass spectral analysis as well as comparison with literature reports.
“x” indicated compounds were detected in the corresponding species
3.2. Analysis of triterpene glycosides
The Cimicifuga triterpene glycosides were analyzed by LC/MS in APCI mode, with the vaporization temperature set to 450°C. At this temperature, the highly oxygenated Cimicifuga triterpene glycosides were decomposed during ionization by the loss of neutral molecules such as water, sugar, and acetic acid. The generated molecular ion, along with a group of fragmental ions, was passed through the heated capillary into the mass analyzer. As a result, both molecular ion adducts and fragmental ions were observed, simultaneously, without performing the MS/MS analysis. It was found that all of the fragmental ions generated from triterpene glycosides could be observed in a positive APCI mode, while less or no fragmentation was detected in negative mode. Nine triterpene glycoside standards were used in this study, belonging to six aglycone types: compounds 1 and 2 are based on acetyl-acteol, 3–5 on cimigenol, 6 on shengmanol, 7 on hydroshengmanol, 8 on cimiracerol, and 9 on 16,23-diketo-shengmanol. All of the nine standards contain a pentose (xyl or ara) monosaccharide attached to the C-3-OH position of the aglycone. This gave rise to losses of 132 [M-arabinosyl or xylosyl-H2O]+ or the 150 [M-arabinose or xylose]+ in (+)-APCI spectra. Six of the 9 triterpene standards contained an acetyl group, which lead to very characteristic fragments resulting from the loss of acetic acid [M-60]+. Examples are the fragment ions m/z 617 [M+H-60]+ of 1 and 8, m/z 601 [M+H -60]+ of 2 and m/z 603 [M+H -60]+ of 5, 6 and 7. The intensity of fragment signals turned out to be a helpful diagnostic tool. For example, structures with an acetyl group usually generate a stronger fragment resulting from the loss of water, such as the dehydrated ion at m/z 659 in 1 and 8, and the fragment at m/z 645 observed in the APCI MS spectra of 5, 6, and 7. While the intensity of the individual dehydration fragments varies slightly between individual acquisitions, their relative intensities within the full MS spectra is typically maintained. The mass spectral features discussed above are summarized in Fig. 4.
Fig. 4.
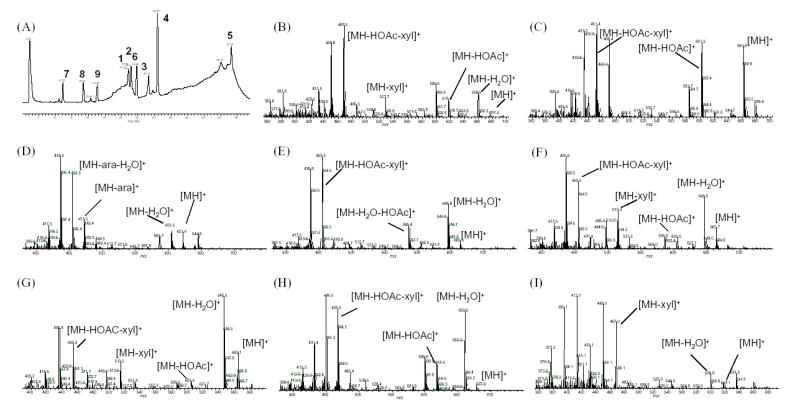
TIC and mass spectrum of reference standards obtained by hyphenated LC-MS separation. (A) TIC; (B) 1 Actein; (C) 2 23-epi-26-Deoxyactein; (D) 3 Cimigenol3-O-arabinoside; (E) 5 25-O-Acetylcimigenol xyloside; (F) 6 Acetylshengmanol3-O-xyloside; (G) 7 24-O-Acetyl hydroshengmanol3-O-xyloside; (H) 8 Cimiracemoside A; (I) 9 Cimicifugoside H-1. The mass spectrum of 4 is not displayed as it is almost identical to that of 3; xyl: xylose, ara: arabinose.
Of the more than 40 triterpene glycosides that have been reported to date from C. racemosa, acetyl-acteol, cimigenol and shengmanol represent the major aglycones. The epimeric aldopentoses arabinose and xylose are the two dominant sugar moieties in Cimicifuga triterpenes, and usually give rise to a pair of glycosides with close chromatographic retention time. While representing a chromatographical challenge, this relationship can also provide useful information for compound identification, like for the pair of 3 and 4. By directly comparing retention times and mass spectra with those of the standards, 1–8 could be identified without ambiguity. Other triterpene glycosides, including acetylshengmanol-3-O-arabinoside (23) and 25-O-acetylcimicigenol-3-O-arabinoside (24), were tentatively assigned based on the similarity of their mass spectra with those of 6 and 5, respectively, and due to their relative retention time being similar to those of the pair of 3 and 4. In two C. foetida samples, 1, 2, 4, 6 and 9 could be identified. C. simplex had been extensively studied for its chemical constituents. More than 50 triterpene glycosides have been isolated from this species [3], with the majority of triterpenoids reported belonging to the shengmanol, hydroshengmanol, and cimigenol series. However, in the two C. simplex samples collected and tested here, none of the major peaks seen in the total ion current trace (TIC) showed a mass spectrum identical to one of the 9 standards, although they all displayed triterpene glycoside fragment patterns. The major peak at Rt. of 33 min displayed a protonated molecular ion at m/z 793 [M+H]+ and a series of fragments that represent two xylosyl or arabinosyl moieties. Since no such structure has been reported from this species, the structure of the major triterpene peak seen in the TIC remains unconfirmed. The major peaks in C. dahurica, however, can be identified as 2, 6, and 4. In C. heracleifolia, 4 was shown to be one of the major components. The peak at Rt. 40 min, eluting right before 4, produced a protonated molecular ion adduct at m/z 619 [M+H]+ with a series of fragmental ions of 601 [M-H2O]+, 583 [M-2H2O]+, 469 [M-xylose]+. Due to the similarity of its fragment ion pattern to that of 4, and augmented by the fact that every fragment was 2 amu less than the corresponding ion in 4, it is reasonable to assume that this peak represents a dehyo-derivative of 4. One triterpene that fulfills the structural criteria is 24-epi-7, 8-didehydrocimigenol-3-O-xyloside (25), a compound previously isolated from this species [36]. Two peaks that appeared at Rt. around 60 and 62 min showed protonated molecules at m/z 487 [M+H]+ and 489 [M+H]+, respectively, with no sugar or acetyl group fragments, suggesting that they were triterpene aglycones.
Compounds 4, 6, and 7 were found to be the major peaks in C. japonica. In C. acerina, only 6 could be identified unambiguously. Since no chemical constituents have been previously reported from C. biternat, C. americana, and C. rubifolia, structural assignments remain tentative until the compounds are isolated. However, compounds 4 and were undoubtedly identified in C. biternat. As with C. heracleifolia, the peak eluting at Rt. 40 min was assigned to 25 based on identical mass spectra. The peak at Rt. 34 min fully resembled the mass fragment pattern of 6, except that all ions were 2 amu less compared to 6. Therefore, the structure of acetyl-didehydroshengmanol-3-O-xyloside (26) is suggested for this peak. Regarding the two North American Cimicifuga species, upon injection of the extract of C. americana all of the major peaks eluted earlier than 30 minutes, indicating that they are less likely to contain cimigenol as the aglycone. All peaks displayed the characteristic fragmentation patterns of triterpenes, but were devoid of acetyl groups and contained only one sugar moiety. The major peak found in C. rubifolia was identified as 3 on the basis of retention time (38 min) and identical mass spectra. Up to date, except for C. racemosa, this is the only other species in which 3 has been detected. The total ion current trace (TIC) obtained from 10 Cimicifuga species by (+)-APCI is shown in Fig. 5. All triterpene glycosides identified from the ten investigated Cimicifuga species are summarized in Table 1.
Fig. 5.
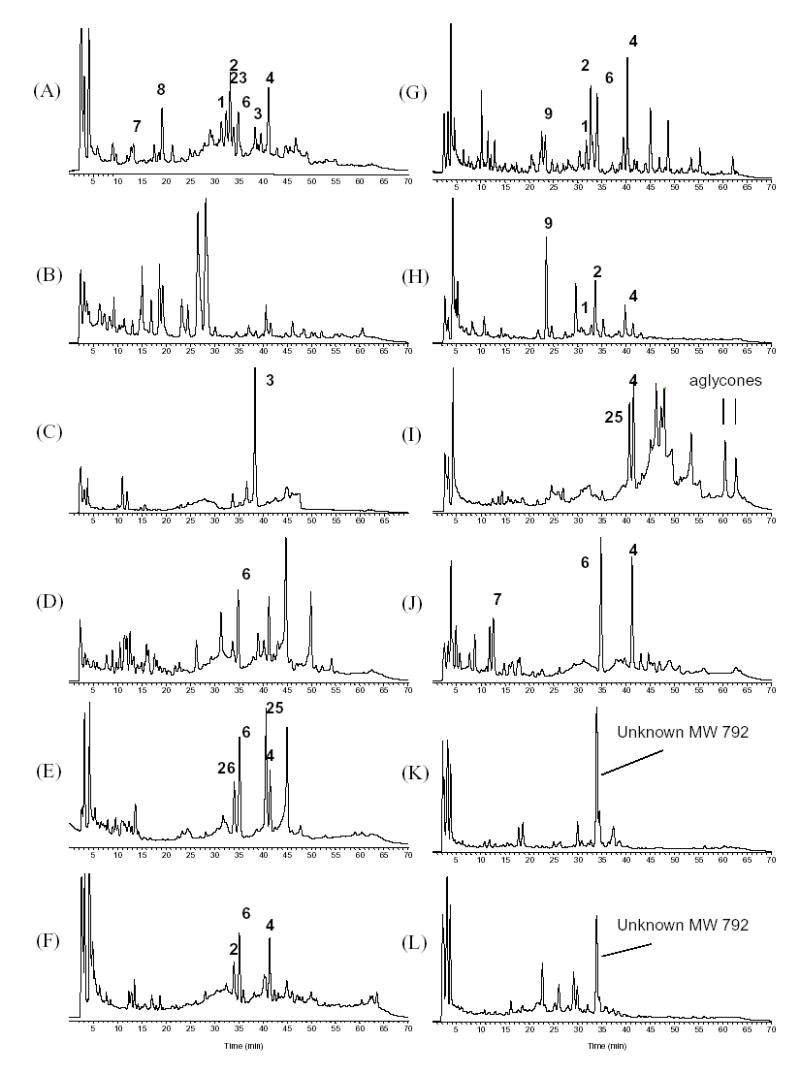
Comparison of the total ion current (TIC) traces of Cimicifuga species (solvent system II). (A) C. racemosa; (B) C. americana; (C) C. rubifolia; (D) C. acerina; (E) C. biternat; (F) C. dahurica; (G) C. foetida-1; (H) C. foetida-2; (I) C. heracleifolia; (J) C. japonica; (K) C. simplex-1; (L) C. simplex-2 (see Experimental for botanical details).
3.3. Chemical conversion of triterpene glycosides
During the production process of black cohosh botanical extracts, noteworthy changes were observed in the HPLC chromatograms, in particular with respect to the triterpene glycosides fingerprints. When analyzing the native 75% ethanol extract, a large variety of triterpene peaks was observed in the small retention time window between 37 and 43 min. However, after a concentration step, in which the extract was concentrated under the influence of heat, the peak diversity in this HPLC window was reduced, and only 3 and 4 appeared as the two dominating peaks and with increased intensity (Fig. 6). A stability test for a 75% ethanol/water extract at room temperature showed that there were no significant profile changes in the HPLC chromatograms after 10 days, while changes started to occurr after two months. Fig. 6 (A) and (B) show the comparison of the TIC obtained from LC-MS (+)-APCI with fresh extract and after 24 months of storage. The peak intensities of 3 and 4 were significantly increased in the aged compared to the freshly prepared extract. In addition, new peaks appeared at Rt. around 60 to 65 min. Two of the major peaks were identified as the 25-O-acetyl-cimigenol glycosides 5 and 24. The chemical conversion involved in the interconversion of 3–5 and 24 apparently occurs at the cimigenol aglycone level. According to literature, however, the open chain aglycones shengmanol and hydroshengmanol are biogenetically related to the spiroketal cimigenol. In studies towards the characterization of the genuine aglycone precursor of triterpene glycosides from C. simplex, 23-O-acetylshengmanol xyloside was isolated and subjected to hydrolysis with acid and base. As a result, not only hydroshengmanol, but also cimigenol were obtained (Fig. 7). Therefore, Kusano et al. hypothesized that acetyl-shengmanol glycoside is the parent component of acetyl-hydroshengmanol, 25-O-acetylcimigenol and 25-O-methylcimigenol glycosides in Cimicifuga species [3, 37]. This proposed chemical conversion pathway has led us to focus on the 23-O-acetyl-shengmanol xyloside 6 and its related compounds as stability markers to indicate changes in triterpene pattern during the production process. Freshly prepared black cohosh 75% ethanol extract was prepared with extracts heated to 90–95°C for 3 and 48 hours, respectively. After a 3 hour treatment, (Fig. 6(C)), many triterpene peaks were either reduced or had even disappeared from the chromatogram. Heating made the peaks of 3 and 4 the dominant triterpenes in the 37–43 min region. Small peaks of the rearrangement products 5 and 24 were also observed, and it is reasonable to conclude that they were produced from the same precursor(s) by chemical conversion. After 48 hours of heat treatment (Fig. 6(D)), the peak intensities of 3, 4, 5 and 24 were significantly increased, while the peaks of 6, 7 and 23 were further reduced or had even disappeared. Furthermore, two new peaks were observed at Rt. around 35.3 and 35.9 min, respectively. These compounds showed fragmentation patterns similar to those of 6, suggesting that they may represent further rearrangement products of 6 and 23. In summary, the heat treatment experiments demonstrated that chemical conversion can readily occurr and involves the triterpenes 3–6, 23, and 24.
Fig. 6.
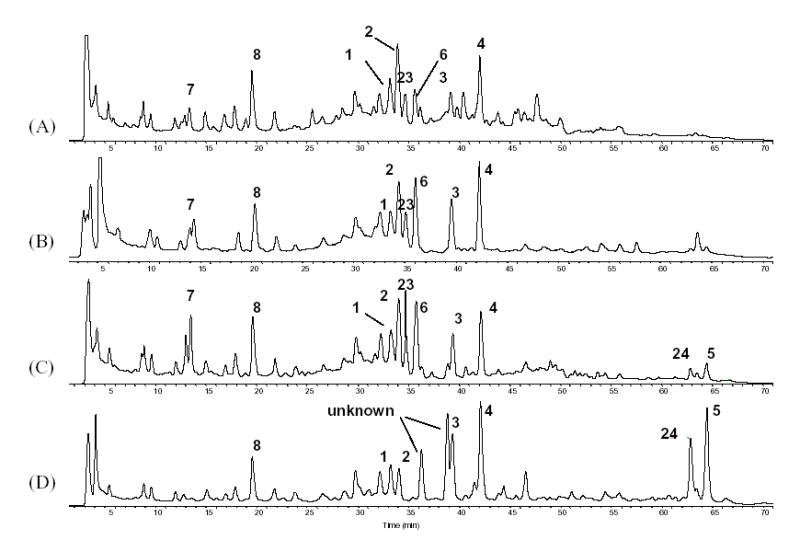
Comparison of the TIC traces of C. racemosa fingerprints demonstrating the conversion of triterpene glycosides under heat treatment. (A) Fresh 75% ethanol extract; (B) 75% ethanol extract (liquid) settled for more than 24 months; (C) Extract heated to 90–95°C for 3 hours; (D) Extract heated to 90–95°C for 48 hours.
Fig. 7.
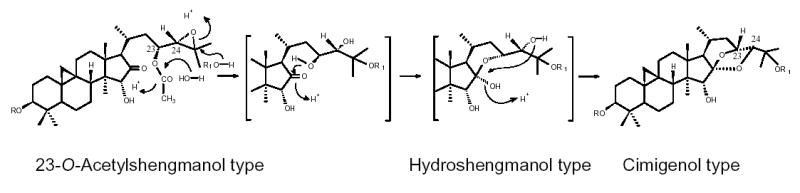
Hypothetic conversion of triterpenes: acetyl-shengmanol type triterpenes are first converted into hydroshengmanol and subsequently into cimigenol type triterpenes [3, 37].
In order to further confirm the occurrence of chemical conversion under heat treatment and possibly storage, 6 was treated in the same way as the extract at 90–95°C for 15 hours. As a result, the formation of major 4 and minor 5 was observed. The principle mechanism of the chemical conversion under heat is described in Fig. 7 [3, 37]. Other acetylated triterpenes might be the source of the acetyl reagent in the formation of the 25-O-acetyl derivatives 5 and 24. This experiment confirms that 23-O-acetyl-shengmanol and 23-O-acetyl-hydroshengmanol glycosides can convert into cimigenol glycosides in solution. While the reaction is accelerated by heat, it most likely occurs at room temperature and potentially affects the long-term stability of black cohosh extracts. Finally, it is noteworthy that we did not observe the analogous conversion to occurr with 9, which differs from 6 by bearing a carbonyl at C-23 instead of an acetyl group. This observation is on line with the lack of a C-23 hydroxyl function as the functional precursor for C-16/23 hemiacetal formation.
3.4. Cimicifuga species identification by phenolic components and triterpene glycoside profiles
In the current analysis, cimifugin (12) and its glucoside 18 were readily detected in our newly collected C. foetida and C. simplex. Fig. 8 shows the selected ion chromatograms (SIC) obtained at m/z 307 [M+H]+, representing 12 and a fragmental ion of 18. In a combination of HPLC-UV chromatograms (Fig. 3) with SIC at m/z 307, 12 and 18 can readily be detected. Their presence was also observed in other Cimicifuga species including C. biternat, C. dahurica, and C. rubifolia, but they were not detected in C. acerina, C. heracleifolia, C. japonica, and C. americana. It is noteworthy to mention (Fig. 3 (E) and (F)) that 12 and 18 give rise to very small peaks on the HPLC-UV chromatogram, which can easily be missed, while SIC clearly demonstrates the presence of the two components. C. rubifolia was determined to contain 12 as the only one of the three North American Cimicifuga species.
Fig. 8.
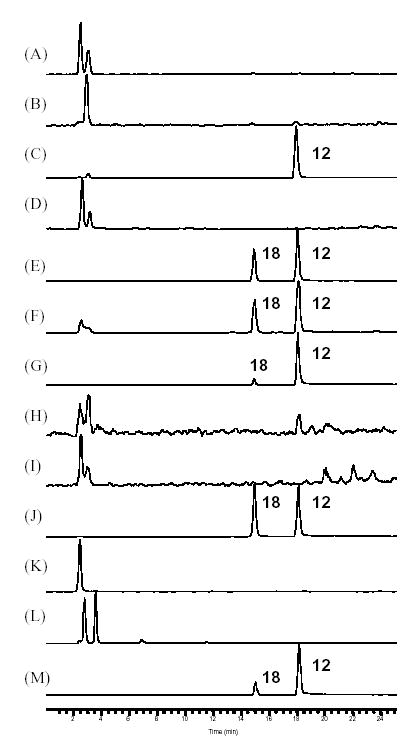
Comparison of selected ion chromatograms (SIC) at m/z 307 for cimifugin (12) and aglycone fragment of cimifugin glucoside (18). (A) C. racemosa; (B) C. americana; (C) C. rubifolia; (D) C. acerina; (E) C. biternat; (F) C. dahurica; (G) C. foetida; (H) C. heracleifolia; (I) C. japonica; (J) C. simplex; (K) Remifemin; (L) CimiPure; (M) Product B.
As discussed above, 3 could represent a conversion product from acetyl-hydroshengmanol arabinoside or acetyl-shengmanol arabinoside. Acetylhengmanol-3-O-xyloside (6) has been isolated from many Cimicifuga species, including C. simplex, C. acerina, C. japonica [3], and C. racemosa. Except for C. racemosa and C. dahurica, we are unaware of any literature reporting the isolation of acetyl-shengmanol arabinoside or cimigenol arabinoside from the abovementioned Cimicifuga species. Although 3 was reported from C. dahurica, its abundance was below the limit of detection in our current study. We did not observe an increase in the peak intensity of 3 after heating a sample of C. dahurica. Due to the presence of 12 and 18, C. dahurica can still be distinguished from C. racemosa. The SIC at m/z 621 [M+H]+ corresponding to 3 and 4 is shown on Fig. 9. In the fresh extract, both 3 and 4 were observed in C. racemosa, albeit with a low signal-to-noise ratio due to interference with other triterpene glycosides. After heating the sample to 50–60°C for 2–3 hours, the signal-to-noise ratio was improved significantly and displayed a pair of peaks similar to what is typically observed for black cohosh. Compound 3 was also found in a significant amount in C. rubifolia. Except for these two species, none of the remaining 8 species contained a detectable amount of 3 as shown in Fig. 9. To exclude the heat formation as a source of 3, as seen in C. racemosa, all of the 8 species underwent a heating process. As a result, 3 was not found to be increased to a detectable level in any of the 8 Cimicifuga species. Therefore, and in summary of the above discussion of the LC profiles, the triterpene 3, and the furanochromones 12 and 18 were determined to be three reliable markers for the identification of all 10 relevant Cimicifuga species.
Fig. 9.
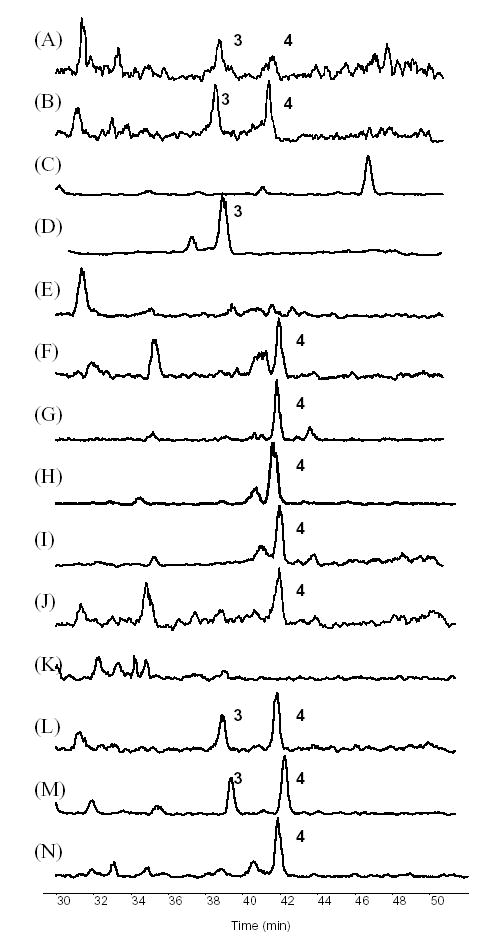
Comparison of selected ion chromatograms (SIC) at m/z 621 for cimigenol arabinoside (3). (A) fresh extract of C. racemosa; (B) heat treated C. racemosa; (C) C. americana; (D) C. rubifolia; (E) C. acerina; (F) C. biternat; (G) C. dahurica; (H) C. foetida; (I) C. heracleifolia; (J) C. japonica; (K) C. simplex; (L) Remifemin; (M) CimiPure; (N) Product B.
After the HPLC profiles of 10 different Cimicifuga species had been established, the new species identification protocol was applied to commercial black cohosh products, Remifemin®, CimiPure®, and products A and B, by comparing the phenolic and triterpene fingerprints to those of the authentic Cimicifuga species. It was clearly determined that Remifemin®, CimiPure® and product A were manufactured using C. racemosa, while product B was manufactured using C. foetida. Product A exhibited a typical C. racemosa profile (Fig. 10 (C)) with all the key constituents, including 1–8. Furthermore, the abnormal triterpene abundance with unusually large amounts of 5 and 24 suggested that the product was inadequately manufactured, probably involving overheating of the product, which caused triterpene conversion as discussed above. The use of harsh extraction conditions was also indicated by the fact that isoferulic acid (17) was the only phenolic compound detected in the HPLC-UV chromatogram, considering that 17 is likely to be the main degradation product of the condensed Cimicifuga isoferulic acid derivatives (Fig. 3 (N)).
Fig. 10.
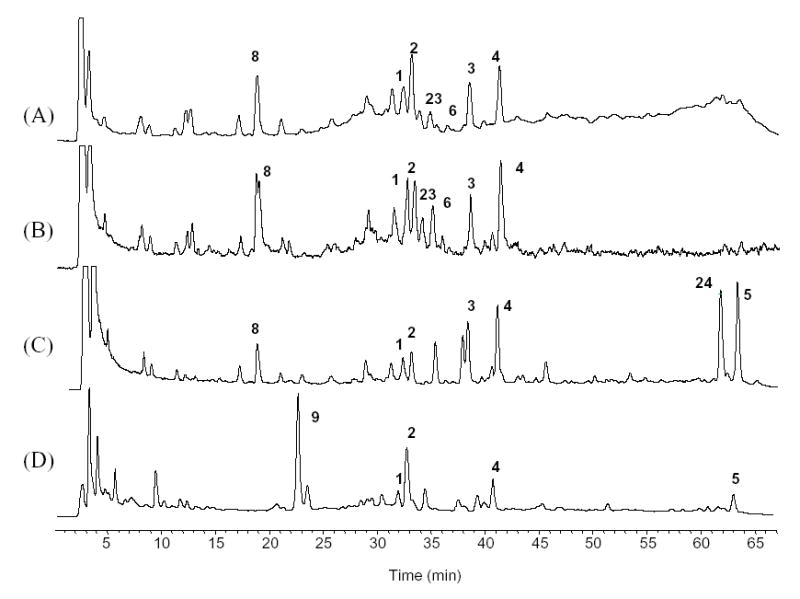
Comparison TIC fingerprints of commercial black cohosh product. (A) Remifemin; (B) CimiPure; (C) Product A; (D) Product B.
3.5. Fingerprints of HPLC-ELSD of Cimicifuga species
It is a widely accepted method for black cohosh products to be chemically standardized to their total triterpene glycoside content. Because the Cimicifuga triterpenoids essentially lack chromophores, UV detection provides very poor sensitivity and will not result in acceptable chromatograms, especially when working without previous cleanup or enrichment procedures. This problem can be overcome by using an LC-MS method, which is a powerful tool to distinguish C. racemosa from a possible adulterant using other Cimicifuga species. However, application of LC-MS as routine analytical method is limited by the fact that mass detection is still not a routine setup in the botanical and the dietary supplemental industry. However, in order to overcome the low UV sensitivity of the triterpenoids, an HPLC with an in-line evaporative light scattering detection (ELSD) has been developed in our laboratory. In addition to the LC-MS studies, HPLC-ELSD fingerprints were acquired using the established Institute for Nutraceutical Advancement (INA) method for the ten investigated Cimicifuga species. The reason for the selection of the INA method is that several laboratories, including our own, have validated it, and that it has found wide acceptance for the determination of triterpene glycoside in the herbal supplement industry. In order to match the results of the ELSD with the LC-MS analyses, and to provide most significant reference for future studies, all of the LC-MS analyses of triterpene glycosides in this work were performed using the INA method (solvent system II) as described in the experimental section. For reference purposes, the HPLC-ELSD fingerprints of the ten different Cimicifuga species are shown in Fig. 11. Interestingly, the ELSD fingerprints are quite similar to the TIC/MS traces. A typical HPLC-ELSD chromatogram of C. racemosa should include (in the order of elution) 8, 1, 2, 23, 6, 3, and 4 as the major components. Compounds 3 and 8 are very important markers, and must be considered positive markers for C. racemosa identification.
Fig. 11.
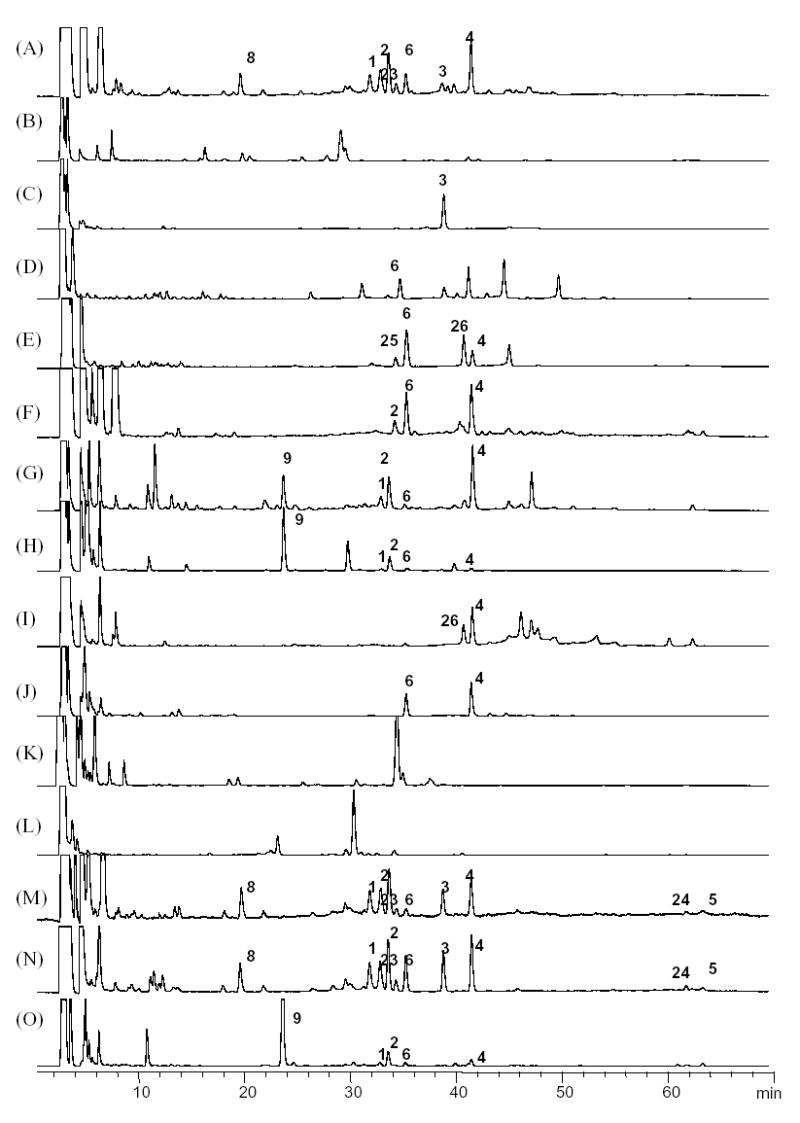
HPLC-ELSD fingerprint chromatograms of the Cimicifuga triterpene glycosides (INA method, solvent system II). (A) C. racemosa; (B) C. americana; (C) C. rubifolia; (D) C. acerina; (E) C. biternat; (F) C. dahurica; (G) C. foetida-1; (H) C. foetida-2; (I) C. heracleifolia; (J) C. japonica; (K) C. simplex-1; (L) C. simplex-2; (M) Remifemin; (N) CimiPure; (O) Product B.
Today, the most likely form of adulteration of black cohosh products found in the U.S. marketplace is C. foetida, in which 9 is the major component. ELSD detection showed high sensitivity in the analysis of these triterpene glycosides, and generally provided clean chromatograms with low baseline noise that were perfectly suitable to monitor the quality of black cohosh products. The main limitation of ELSD is its lack of structural information, which in turn limits its use in species differentiation, especially when analyzing products mixtures.
4. Conclusions
Despite of the fact that black cohosh represents one of the clinically most extensively studied botanicals, it remains a complex scientific entity with regard to its phytoconstituents and botanical species assignment. Historically, many different Cimicifuga species, containing the very similar or even identical constituents, have been used medicinally for various applications and indications. However, correct species identification and exclusion of adulteration is a key step in any clinical trial, including those that investigate how black cohosh extracts can alleviate perimenopausal and postmenopausal symptoms. This includes the establishment of reliable sources of raw material, as the use of proper and authentic plant materials is crucial to reproducible clinical results. One practical limitation that has to be taken into account is that the botanical industry buys a high volume of raw material directly from farmers and collectors, and that only the pre-processed roots and rhizomes are shipped to the manufacturer. This makes routine botanical species identification an unfeasible approach, and chemical analysis a necessity in the quality monitoring of black cohosh raw materials. Due to the complexity and variation of the chemical constituents in authentic black cohosh to start with, it has to be realized that fingerprinting methods have intrinsic limitations, especially when it comes to the identification of small amounts of adulterated product mixed into authentic material. In this study, however, hyphenated MS introduced molecular weight to the fingerprints, thus adding another layer of information to the chromatographical window for compound identification. Using LC/MS in (+)-APCI mode is preferred because APCI generates less molecular ion adducts and more fragment ions, thus providing more valuable structural information. Our results confirm that cimigenol-3-O-arabinoside (3), cimifugin (12) and cimifugin glucoside (18) can serve as the three species-specific marker compounds to distinguish authentic C. racemosa from nine other Cimicifuga species. The finding of chemical conversion among the triterpene glycosides in black cohosh provides a draft guideline for the maintenance of original “native” phytochemical profiles throughout the manufacturing process. Overall, the presented HPLC-PDA/MS/ELSD methods should be useful for Cimicifuga species identification and for quality control of black cohosh botanical products.
Acknowledgments
The authors are very thankful to Charlotte Lindqvist, Victor Albert, and Dr. Scott Mori of New York Botanical Garden, T. Koyama, Nihon University, Prof. Zhaoyun Wu, Prof. Zheng-Tao Wang, Shanghai University of Traditional Chinese Medicine, Prof. Dao-Feng Chen, School of Pharmacy, Fudan University, and Dr. Seiji Nagumo, Hoshi University, for Cimicifuga species plant collection and identification. The authors wish to thank Dr. Shao-Nong Chen, UIC/NIH Center for Dietary Supplements Research, Chicago (IL), for generously providing authentic standards. Our final thanks extend to Mrs. Nancy Madis for her proof reading of this manuscript. GFP gratefully acknowledges funding by NIH through grant #P50 AT00155 (UIC/NIH Botanical Center) from NCCAM, ODS, NIGMS, and ORWH.
References
- 1.Foster S. Herbal Gram. 1999;45:36. [Google Scholar]
- 2.Applequist WL. Flora. 2003;198:358. [Google Scholar]
- 3.Kusano G. Yakugaku Zasshi. 2001;121:497. doi: 10.1248/yakushi.121.497. [DOI] [PubMed] [Google Scholar]
- 4.Mahady GB, Fong HS, Farnsworth NR. In: Botanical Dietary Supplements: Quality, Safety and Efficacy. Swets, Zeitlinger BV, editors. Lisse; The Netherlands: 2001. p. 27. [Google Scholar]
- 5.Barrett M. The Handbook of Clinically Tested Herbal Remedies. The Haworth Press; New York: 2004. p. 185. [Google Scholar]
- 6.Borrelli F, Ernst E. Eur J Clin Pharmacol. 2002;58:235. doi: 10.1007/s00228-002-0457-2. [DOI] [PubMed] [Google Scholar]
- 7.Kronenberg F, Fugh-Berman A. Ann Intern Med. 2002;137:805. doi: 10.7326/0003-4819-137-10-200211190-00009. [DOI] [PubMed] [Google Scholar]
- 8.Dog TL, Powell KL, Weisman SM. Menopause. 2003;10:299. doi: 10.1097/01.GME.0000056039.51813.21. [DOI] [PubMed] [Google Scholar]
- 9.Wuttke W, Seidlova-Wuttke D, Gorkow C. Maturitas. 2003;44:S67. doi: 10.1016/s0378-5122(02)00350-x. Suppl 1. [DOI] [PubMed] [Google Scholar]
- 10.Borrellia F, Izzoa AA, Ernst E. Life Sciences. 2003;73:1215. doi: 10.1016/s0024-3205(03)00378-3. [DOI] [PubMed] [Google Scholar]
- 11.Burdette JE, Liu J, Chen SN, Fabricant DS, Piersen CE, Barker EL, Pezzuto JM, Mesecar A, van Breemen RB, Farnsworth NR, Bolton JL. J Agric Food Chem. 2003;51:5661. doi: 10.1021/jf034264r. [DOI] [PubMed] [Google Scholar]
- 12.Fabricant D, Nikolic D, Lankin D, Chen SN, Jaki B, Krunic A, van Breemen RB, Fong HHS, Farnsworth NR, Pauli GF. J Nat Prod. 2005;68:1266. doi: 10.1021/np050066d. [DOI] [PubMed] [Google Scholar]
- 13.Chen SN, Fabricant DS, Lu ZZ, Zhang H, Fong HS, Farnsworth N. Phytochemistry. 2002;61:409. doi: 10.1016/s0031-9422(02)00209-1. [DOI] [PubMed] [Google Scholar]
- 14.Struck D, Tegtmeier M, Harnischfeger G. Planta Med. 1997;63:289. doi: 10.1055/s-2006-957682. [DOI] [PubMed] [Google Scholar]
- 15.Li W, Chen S, Fabricant D, Angerhofer CK, Fong HHS, Farnsworth NR, Fitzloff JF. Analytica Chimica Acta. 2002;471:61. [Google Scholar]
- 16.Jiang B, Kronenberg F, Balick MJ, Kennelly EJ. Phytomedicine. 2005 doi: 10.1016/j.phymed.2005.06.007. (In press) [DOI] [PubMed] [Google Scholar]
- 17.Panossian A, Danielyan A, Mamikonyan G, Wikman G. Phytochem Anal. 2004;15:100. doi: 10.1002/pca.742. [DOI] [PubMed] [Google Scholar]
- 18.Bedir E, Khan IA. Chem Pharm Bull. 2000;48:425. doi: 10.1248/cpb.48.425. [DOI] [PubMed] [Google Scholar]
- 19.Shao Y, Harris A, Wang M, Zhang H, Cordell GA, Bowman M, Lemmo E. J Nat Prod. 2000;63:905. doi: 10.1021/np000047y. [DOI] [PubMed] [Google Scholar]
- 20.Chen SN, Li W, Fabricant DS, Santarsiero BD, Mesecar A, Fitzloff JF, Fong HHS, Farnsworth NR. J Nat Prod. 2002;65:601. doi: 10.1021/np010494t. [DOI] [PubMed] [Google Scholar]
- 21.Chen SN, Fabricant DS, Lu ZZ, Fong HS, Farnsworth NR. J Nat Prod. 2002;65:1391. doi: 10.1021/np0200818. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe K, Mimaki Y, Sakagami H, Sashida Y. Chem Pharm Bull. 2002;50:121. doi: 10.1248/cpb.50.121. [DOI] [PubMed] [Google Scholar]
- 23.Blumenthal M. Herbal Gram. 2005;66:66. [Google Scholar]
- 24.He K, Zheng B, Kim CH, Rogers L, Zheng Q. Planta Med. 2000;66:635. doi: 10.1055/s-2000-8619. [DOI] [PubMed] [Google Scholar]
- 25.Ganzera M, Bedir E, Khan IA. Chromatographia. 2000;52:301. [Google Scholar]
- 26.Onorato J, Henion JD. Anal Chem. 2001;73:4704. doi: 10.1021/ac010409m. [DOI] [PubMed] [Google Scholar]
- 27.Li W, Sun Y, Liang W, Fitzloff JF, van Breemen RB. Rapid Commun Mass Spectrom. 2003;17:978. doi: 10.1002/rcm.1008. [DOI] [PubMed] [Google Scholar]
- 28.Wang HK, Sakurai N, Shih CY, Lee KH. J Agric Food Chem. 2005;53:1379. doi: 10.1021/jf048300d. [DOI] [PubMed] [Google Scholar]
- 29. www.nsf.org/business/ina/blackcohosh.asp?program=INA.
- 30.Kong L, Li X, Zou H, Wang H, Mao X, Zhang Q, Ni J. J Chromatogr A. 2001;936:111. doi: 10.1016/s0021-9673(01)01081-0. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt AH. Journal of Liquid Chromatography & Related Technologies. 2005;28:871. [Google Scholar]
- 32.Pharmacopoeial Forum, The United States Pharmacopeial Convention, Inc. 2002;28:1455. [Google Scholar]
- 33.Upton E, editor. Black Cohosh Rhizome, monograph. American Herbal Pharmacopoeia; Scotts Valley, CA: 2002. [Google Scholar]
- 34.Xu H, Fabricant DS, Piersen CE, Bolton JL, Pezzuto JM, Fong H, Totura S, Farnsworth NR, Constantinou AI. Phytomedicine. 2002;9:757. doi: 10.1078/094471102321621403. [DOI] [PubMed] [Google Scholar]
- 35.Takahira M, Kusano A, Shibano M, Nagai M, Miyase T. Chem Pharm Bull. 1998;46:362. [Google Scholar]
- 36.Li JX, Kadota S, Hattori M, Yoshimachi S, Shiro M, Oogami N, Mizuno H, Namba T. Chem Pharm Bull. 1993;41:832. [Google Scholar]
- 37.Sakurai N, Nagai M. Yakugaku Zasshi. 1996;116:850. doi: 10.1248/yakushi1947.116.11_850. [DOI] [PubMed] [Google Scholar]


