Abstract
The intrinsic (mitochondria-dependent) pathway of apoptosis is one of the major pathways leading to cell death. We evaluated cytochrome c/apoptotic protease activation factor-1 (Apaf-1)-dependent activation of caspase-3 in brain and liver of different strains of rodents at different stages of development. In cell-free extracts from brain and liver of Sprague-Dawley rats, caspase was activated by cytochrome c/2′-deoxyadenosine 5′-tri-phosphate at both neonatal and adult stages. In adult brain extracts from Wistar rats, no activation of caspase was observed while extracts from neonatal brain and liver and from adult liver were activated. In CD-1 mouse, only neonatal extracts were activated. Alteration in levels of endogenous inhibitors of apoptosis were not responsible for the lack of activation observed. Instead, decrease in the content of Apaf-1 and caspase-3 and some degradation of caspase-9 during brain ageing were observed. These results suggest that a decrease in apoptosis activation during ageing is not tissue-specific, but rather displays a complex dependence on species and strains of animals.
Keywords: Age-dependent caspase activation, Apoptosis-resistant tissues, Cytochrome c/2′-deoxyadenosine 5′-triphosphate activation, Intrinsic pathway, Mitochondrial pathway, Strain-dependent caspase activation
1. Introduction
Cell death is critical for the development and orderly maintenance of cellular homeostasis in metazoans. Apoptosis is an evolutionarily conserved cell death program executed by cysteine proteases (caspases) and regulated by the anti-apoptotic members of the Bcl-2 protein family, among other regulators [1,2].
The intrinsic pathway of apoptosis (IPA) is one of the main apoptotic pathways, and is initiated by triggering events within the cell, such as stress signals like DNA damage and hypoxia, or the loss of survival signals [3,4]. A distinctive feature of this pathway is the involvement of mitochondria, where the core of the pathway is the apoptosome [5] consisting of cytochrome c (cyt.c), 2′-deoxyadenosine 5′-triphosphate (dATP) and the apoptotic protease activation factor-1 (Apaf-1), which promotes the assembly of a caspase-activating complex [6]. The role of cyt.c in apoptosis was recently established by the generation of “knock-in” mice expressing a cyt.c mutant (KA allele), which retains normal electron transfer function but fails to activate Apaf-1 [7]. It was widely accepted that Bcl-2 protein also prevented cyt.c release by safeguarding the mitochondrial membrane integrity; however, it seems that Bcl-2 can also regulate a caspase activation programme independently of the cyt.c/Apaf-1/caspase-9 ‘apoptosome’, which appears to amplify rather than initiate the caspase cascade [8].
The implication of mitochondria in apoptosis has important consequences for the understanding of the normal physiology of cell death, its deregulation in cancer and degenerative diseases, and the development of novel cytotoxic and cytoprotective drugs [9–11].
However, there is increasing evidence for the lack of activation of the intrinsic pathway under physiological [12,13] and pathological [12,14–16] conditions.
In order to understand the regulation of the IPA during ageing, we assessed several factors that could influence its activation in rodents: developmental stage, tissue specificity, genetic background, potential upregulation of endogenous inhibitors of apoptosis and regulation of the components of the apoptosome complex.
2. Materials and methods
All chemicals were of analytical grade and were purchased from Sigma–Aldrich unless otherwise stated.
2.1. Preparation of cell-free extracts from tissues
Healthy rodents, namely CD-1 mouse, Sprague-Dawley and Wistar rat strains were purchased from Charles River Laboratories and paired at the vivarium of the Buck Institute. The experimental animals used in these studies were handled according the NIH Guide for the care and use of Laboratory Animals and the Institutional Animal Care and Use Committee (IACUC) policies.
The animals were selected at various stages of maturation. The fetus were 14–16 days old, neonatal ranged from birth to 24 h after birth, young from 15 to 30 days old, adult from 3 to 4 months and old from 8 to 12 months old. The animals were euthanized on a carbon dioxide chamber prior to use. All steps were carried at 4 °C unless otherwise stated. The selected organ was immediately removed, washed with ice-cold PBS, chopped and homogenized at the appropriate ratio in hypotonic buffer (20 mM PIPES buffer, pH 7.4, containing 10 mM KCl, 5 mM sodium EGTA, 2 mM MgCl2 and 1 mM dithiothreitol). Fetal and neonatal brain tissue were homogenized in a glass homogenizer (KONTES) and a teflon pestle homogenizer (KONTES) was used for the other tissues. Debris and nuclei were removed by centrifugation at 1000 × g for 10 min. The supernatant was further centrifuged at 16000 × g for 30 min and the pellet was discarded. The S16 extract was aliquoted and stored at −80 °C.
For Western-blotting, a cocktail of inhibitors (Roche) was added in order to prevent unwanted proteolysis.
2.2. Determination of protein concentration
Protein concentration was determined using a Coomassie plus protein reagent (Pierce) based on a Bradford assay. The measurements were done on a Shimadzu UV-2401PC spectrophotometer calibrated using a bovine serum albumin standard curve.
2.3. Caspase activation
The kinetics of activation was performed as described [17]. The effect of cyt.c/dATP non-activatable extract on IPA activation was studied as following: based on the response of S16 cell-free extracts to cyt.c/dATP activation, two types of extracts were found. Activatable extract (A) responded positively to cyt.c/dATP activation and was found in neonate extracts whereas the non-activatable ones (NA) were resistant to cyt.c/dATP activation and were found mainly in adult tissue extracts.
Five microliters of neonate CD-1 mouse brain (or liver) cell-free extracts were incubated with increasing volumes (5–40 μl) of adult mouse brain (or liver) cell-free extracts during 60 min at 37 °C. The samples were then activated with 10 μM cyt.c and 1 mM dATP for 30 min at 37 °C in a reaction volume of 90 μl.
Granzyme B activation was studied by addition of the enzyme (Sigma, #G9278) (0.1 μM final concentration) to 50 μg of S16 extract of mouse brain tissue. A control experiment in the absence of granzyme B was run under the same conditions. In both cases, the reaction was started by addition of 10 μl of Ac-DEVD-AMC (acetyl-Asp-Glu-Val-Asp-7-amino-4-methylcoumarin) substrate (Anaspec, #25262) (50 μM final concentration) and the residual activity was monitored continuously for 30 min at 37 °C in a thermostated Molecular Devices SpectraMax Gemini spectrofluorimeter, at excitation and emission wavelengths of 370 and 460 nm. The steady-state rates of substrate hydrolysis were obtained from the linear parts of the curves. Activation was measured in terms of DEVDase activity and expressed in relative units of fluorescence (RU), except in the granzyme activation experiment where the results were expressed in μM/min and standardized using a calibration curve based on known concentrations of AMC (7-amino-4-methylcoumarin) fluorogen (Anaspec, #23482). Means ± S.D. were calculated from independent measurements of three different lysates.
2.4. Electrophoresis and Western blotting
Electrophoresis and Western blotting were carried out on the Nu-PAGE Bis-Tris system following the instructions provided by the supplier (Invitrogen). Positive controls for caspases -9 and -3 consisted of cell free extracts of HEK 293T overexpressing cells. The following antibodies were used: Apaf-1 antibody (QED Bioscience, #2015), anti-caspase-9 (Santa Cruz Biotechnology, #H-83), anti-caspase-3 (Santa Cruz Biotechnology, #H-277), and horseradish peroxidase (HRP)-conjugated goat anti-rabbit antibody (Santa Cruz Biotechnology). The protein/antibody complexes were detected using enhanced chemiluminescence (ECL) Plus Western Blotting Detection Reagents (Amersham Biosciences, #RPN2132). Densitometric analysis was carried out using the NIH Image J software at http://rsb.info.nih.gov/nih-image/. The y-axis represents protein relative area quantified at each maturation stage. Means ± S.D. were calculated from three repeated measurements of density from the same experiment.
3. Results and discussion
We recently reported that the cyt.c/dATP activation of cell-free extracts of primary cortical neurons is age-dependent, whereas extracts of astrocytes were activated to a similar extent at all stages evaluated [17]. In the current work, we evaluated the effects of several factors that might influence the activation of the caspase cascade, such as the rodent’s genetic background, tissue specificity, developmental stage and the presence and activity of endogenous inhibitors of apoptosis.
Out-bred rodents, Sprague-Dawley and Wistar rats and CD-1 mice, were used in these studies. Liver and brain tissues were chosen as mitotic and post-mitotic models, respectively. The rodents’ ages were postnatal day 1 (neonate) and 4-month-old (adult), respectively. Sprague-Dawley brain and liver extracts were activated by cyt.c/dATP to different extents at both developmental stages (Fig. 1A). In contrast, no activation was observed in Wistar brain extracts (Fig. 1B) or in both adult CD-1 extracts (Fig. 1C). The pattern of activation of the ‘IPA’ was different in the three rodents studied (Fig. 1). These differences were therefore quantified for each organism. Degrees of activation in brain and liver were compared at both developmental stages (neonate and adult) and for each developmental stage (neonate or adult) both tissues (brain and liver) were compared. In Sprague-Dawley rats, the neonate: adult ratio for the IPA activation was 10-fold in brain and 1.5-fold in liver cell-free extracts, respectively (Fig. 1A). The corresponding ratios were 48-fold and 2.8-fold in Wistar rats (Fig. 1B) and 54-fold and 17-fold in CD-1 mice (Fig. 1C). In neonatal extracts the IPA activation was greater in brain than in liver by factors of 2.2-fold in Sprague-Dawley (Fig. 1A), 1.5-fold in Wistar (Fig. 1B) and 3.2-fold in CD-1 strains (Fig. 1C). In contrast, for adult animals, the liver:brain ratio for IPA activation was 3.2-fold for Sprague-Dawley (Fig. 1A), 11-fold for Wistar (Fig. 1B) strains; no activation was observed in CD-1 mice in either tissue (Fig. 1C).
Fig. 1.
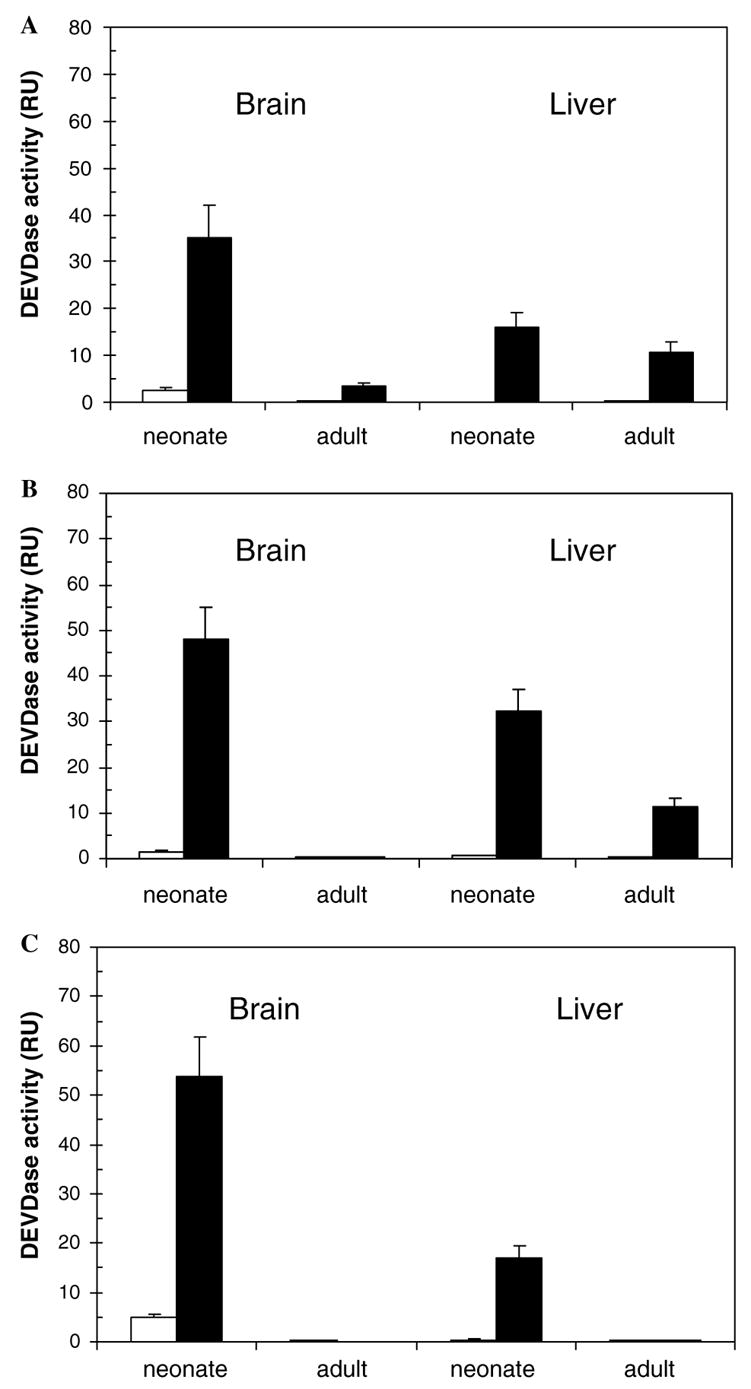
Cyt.c/dATP activation of brain and liver extracts of Sprague-Dawley rat (A), Wistar rat (B) and CD-1 mouse (C). Brain and liver cell-free extracts of neonatal and adult rodents (20 μg/μl protein concentration) were activated with cyt.c/dATP. A control experiment was carried out in the absence of cyt.c/dATP. The control and cyt.c/dATP treated samples are indicated by open and filled bars, respectively.
The fact that cyt.c/dATP activation showed a distinct pattern in all three rodents evaluated (Fig. 1) suggests that the lack of activation of the IPA is not clearly tissue-specific or age-specific, but that the strain background appears to be a major determinant. Our findings indicate that the IPA does not demonstrate a completely consistent pattern, being neither tissue-specific, as previously suggested [13], nor uniformly age dependent; rather, it is strain-dependent and species-dependent (Fig. 1). These data are compatible with those of a recent report that showed that, in certain strains of caspase-9, Apaf-1 or cyt.c-ablated mice, alternative cell death routes are available, despite the growth and developmental abnormalities observed in these mice [7].
Recently, strain-dependent differential susceptibilities to cell death stimuli that target the intrinsic [7, this work] and/or the extrinsic [18] apoptotic pathway have been reported. For example, cortical cultures from the inbred mouse strain C57BL/6 exhibited a 28% greater neuronal death following doxorubicin treatment than C57BL/10, and the greater resistance to death receptor-mediated apoptotic cell death of the latter was related to Fas, FasL, and MMP expression [18]. In addition, the importance of the strain selected was found to be crucial to the phenotype observed [19,20]. A detailed study aimed to uncover the importance of various genes to apoptosis in the ovary was carried out in one outbred (CD-1) and several inbred (129/Sv, DBA/2, C57BL/6, FVB, AKR/J) strains of mice at day 4 (neonatal) and day 42 (young adult) postpartum [21]. In the absence of genetic manipulation the size of the primordial oocyte reserve was affected by mouse strain whereas genetic modifiers play a major role in follicle endowment, development and atresia in the mouse ovary [21]. Therefore, strain-dependent differences in response to apoptotic stimuli should be taken into consideration when developing transgenic models to understand the molecular basis of disease.
Since the response to cyt.c/dATP activation of the different extracts studied here differed significantly, we hypothesized that resistance of an extract to cyt.c/dATP activation might be caused by the presence of an endogenous inhibitor. Numerous such inhibitors exist, such as the IAP (inhibitor of apoptosis) proteins (e.g., IAP-1, IAP-2, NAIP, XIAP, and survivin) [22], some Bcl-2 family members, some chaperones, and others. Previous results have indicated that alterations in such inhibitors underlie apoptotic shifts in some pathological conditions: for example, degradation of cellular IAP-1 and XIAP was found in post-mortem Huntington’s disease brain tissue, in association with an excess of apoptosis [23]. On the other hand, IAP-2 conferred resistance to apoptosis in hypoxic cells [24]. In addition, in a study carried out on 6- and 26-month-old Fischer 344 rats it was found that caloric restriction may provide neuroprotection to the ageing brain by partially suppressing the activation of caspase-3 and PARP cleavage, and by further upregulating XIAP inhibitor, the latter probably involved in the increased resistance to apoptosis observed in caloric restricted animals [25].
The anti-apoptotic Bcl-2 protein was reported to be responsible for the cardiac fibroblast resistance to apoptosis [26]. On the other side, despite low Bcl-2 levels, senescent cells develop resistance to apoptosis, even though no significant changes were observed between the ratio of anti- and pro-apoptotic proteins [27]. Since senescent cells are halted permanently in the G0/G1-phase of the cell cycle, their resistance to cell death was argued to be related to their non-cycling nature [27]. Thus, numerous strategies have been shown to be utilized by different cell types, under different conditions, to achieve modulation of the activation of apoptosis, and in some cases these involve an upregulation of apoptosis inhibitors such as the IAP proteins.
Therefore, to evaluate the hypothesis that the observed decline in IPA activation may be due to the age-related upregulation of apoptotic inhibitors, we performed mixing studies to reveal the possible presence of inhibitors of caspase activation. Two extracts originating from the same strain and tissue, differing only in the stage of development, were incubated for 60 min at 37 °C. The extract from the neonate (extract A) was effectively activated by cyt.c/dATP, whereas that from the adult (extract NA) was cyt.c/dATP resistant (Fig. 2A). The two extracts were mixed in different proportions, the effect of cyt.c/dATP observed for 30 min, and residual activities monitored on a spectrofluorometer after addition of the substrate. At a 1:1 ratio (v/v) no inhibitory effect was observed in mouse brain or mouse liver, since the activities of the activatable and non-activatable mixture (A + NA) were of the same magnitude as that observed for the A fraction (Fig. 2A). In order to exclude the possibility of the lack of inhibition being due to a potential component being at a low concentration in the NA extract, increasing volumes of the NA extract (5–40 μl) were added, and found to cause no inhibitory effect on the A fraction (Fig. 2B). An analogous pattern was obtained for brain extracts of Sprague-Dawley and Wistar strains (results not shown), thus further confirming that endogenous inhibitors were not responsible for the observed decrease in IPA activation.
Fig. 2.
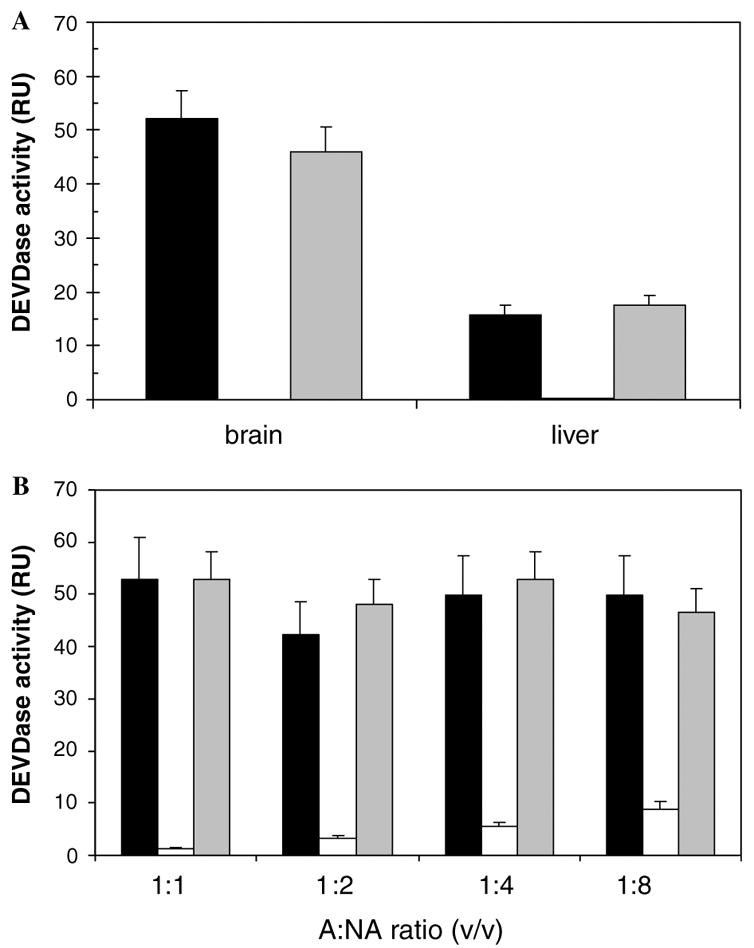
Effect of an extract non-activatable by cyt.c/dATP on caspase activation. The experiment was carried out on CD-1 mouse extracts as described in Section 2.3. The activatable extract (A) was from neonatal and the non-activatable (NA) from adult tissues. (A) Effect of a non-activatable extract on an activatable one for brain and liver at a ratio 1:1 (v/v). (B) Effect of concentration of NA extract on the IPA activation. Brain extracts were used at ratios of 1:1, 1:2, 1:4 and 1:8 (v/v). The activatable extracts (A) are indicated by a filled bar, the non-activatable (NA) by an open bar, and the mixtures of activatable and non-activatable extracts (A + NA) by a grey bar.
The Apaf-1 · cyt.c complex is the functional apoptosome that activates procaspase-9 [6]. Therefore, Apaf-1 expression was examined as a function of brain ageing by Western blotting. After the neonatal stage, a 130 kDa band corresponding to the Apaf-1 precursor, as detected with Apaf-1 antibody raised against residues 1158–1177 of Apaf-1 molecule, was less intense, indicating either downregulation or proteolytic cleavage (Fig. 3A, lanes 4–6). Apaf-1 expression observed at the neonatal stage was 2-fold greater than at the following stages (Fig. 3B) thus being at least one of the causes of the lack of activation observed (Fig. 1). The deficiency or absence of Apaf-1 was found to confer resistance to cyt.c-dependent apoptosis [12,14,16,28]. Apaf-1 inactivation has been reported as being due to methylation [15] and caspase-3 cleavage [29,30]. The relevance of Apaf-1 for normal development has been confirmed in gene-targeted Apaf-1−/− mice which exhibited brain overgrowth and severe craniofacial malformations [31,32]. The latter were linked to the forebrain overgrowth mutation (fog) involved in the pathogenesis of spina bifida, a common congenital malformation [33]. Other alterations included male infertility [34], persistence of interdigital webs and dramatic alterations of the lens and retina [32].
Fig. 3.
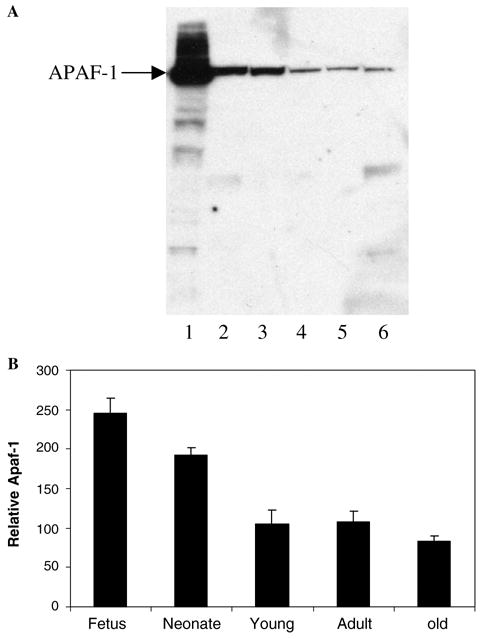
Expression profile of Apaf-1 during brain ageing. (A) Fifty micrograms of mouse brain cell-free extracts at several stages of maturation: fetus (lane 2), neonate (lane 3), young (lane 4), adult (lane 5) and old (lane 6) were subjected to electrophoresis and Western blotting. The positive control consisted of a cell-free extract of HEK 293T cells overexpressing Apaf-1 (lane 1). (B) The Apaf-1 band intensities in (A) were determined as stated in Section 2.4.
In the same extracts we also measured the protein expression of the two main regulators of the pathway, namely caspases-9 and -3 (Figs. 4A and 5A). Caspase-9 antibody recognized the ~46 kDa precursor form (pro-C9), a major band of ~35 kDa and a minor band of ~11 kDa (Fig. 4A, lane 1). C9 p35 (the ~35 kDa band) includes the enzyme linker region (Asn92-Leu151, 6446.37 Da) bound to its catalytic domain (Ala152-Ser416, 29199.22 Da). The ~11 kDa band corresponds to the small subunit (C9 p11) (Ala316-Ser416, 11548.11 Da). The ~4 kDa difference between human [35] (Fig. 4A, lane 1) and mouse [36] (Fig. 4, lanes 2–6) caspase-9 zymogens is due to the 38 amino acids shorter linker region in the former. Sequence alignment of the two primary structures indicated 72% amino acid identity (results not shown). The antibody raised against human caspase-9 C-terminus epitope cross-reacted with the mouse counterpart and showed the ~50 kDa precursor form (pro-C9), a minor band of ~29 kDa corresponding to the catalytic domain (C9 p29) (Tyr191-Ser454, 29233 Da) and a major band of ~11 kDa corresponding to the small subunit (C9 p11) (Ala354-Ser454, 11373.98 Da) (Fig. 4A, lanes 2–6). During the course of brain ageing the expression of caspase-9 zymogen decreased ~1.5-fold after birth (Fig. 4A, lanes 4–6; Fig. 4B). The zymogen was activated, as seen by the ~2-fold increase in the expression levels of the enzyme small subunit (Fig. 4A, lanes 2–6; Fig. 4D) and the catalytic domain (Fig. 4A, lanes 4–6; Fig. 4C). The fact that the antibody used was raised against the small subunit could explain the lower recognition seen by the catalytic domain (Fig. 4A, lanes 4–6). Steric hindrance of the catalytic domain by the enzyme’s large subunit was excluded because, on electrophoresis, the sample was run under denaturing conditions. In a previous study on rat cortex using a monoclonal 5B4 anti-caspase-9 antibody (Medical and Biological Laboratories) that recognizes both rat procaspase-9 and its large subunit, nearly complete cleavage of procaspase-9 was shown in E17 (embryonic day 17), P2 (postnatal day 2) and P7 extracts, whereas the degree of caspase-9 cleavage was markedly decreased in P14 extracts and was not detected in P60 extracts [12]. Our results are in agreement with the zymogen pattern, however, the differences in the large subunit pattern could be due to the facts that we monitored animals up to 8 months old and we used a different antibody. In addition, the studies reported here were carried out on a whole brain of a CD-1 mouse strain. In the previous report [12] the authors used rat cortex and stated that similar age-dependent changes in cyt.c-dependent apoptotic susceptibility were found in developing mouse brain [12].
Fig. 4.
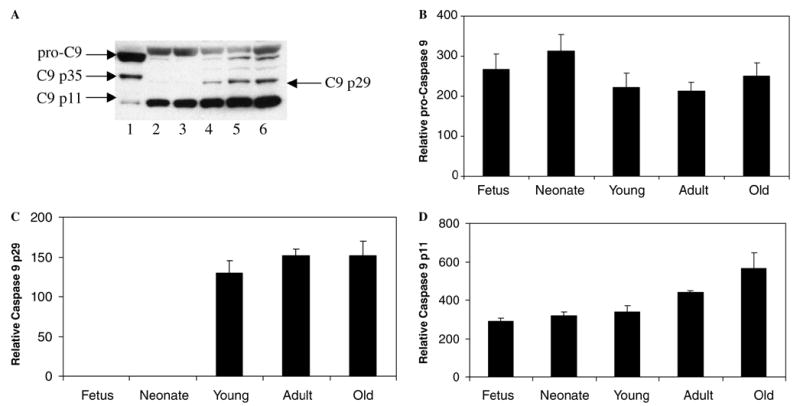
Expression profile of caspase-9 during brain ageing. (A) Representative Western blot of 50 μg of cell-free extracts of mouse brain tissue at various stages of maturation: fetus (lane 2), neonate (lane 3), young (lane 4), adult (lane 5) and old (lane 6). A positive control consisted of 50 μg of HEK 293T cells overexpressing caspase-9 (lane 1). The experiment was carried out as described in Section 2.4. Densitometric values for relative procaspase-9 protein (B), catalytic domain (caspase 9 p29) (C) and small subunit (caspase 9 p11) (D) were obtained as stated in the same section.
Fig. 5.

Expression profile of caspase-3 during brain ageing. (A) Representative Western blot of 50 μg of cell-free extracts of mouse brain tissue at various stages of maturation: fetus (lane 2), neonate (lane 3), young (lane 4), adult (lane 5) and old (lane 6). A positive control consisted of 50 μg of HEK 293T cells overexpressing caspase-3 (lane 1). The experiment was carried out as described in Section 2.4. (B) Densitometric values for relative procaspase-3 protein from (A) were obtained as stated in the same section.
Caspase-3 was evaluated by two independent methods, Western blotting (Fig. 5A) and kinetics, using the serine protease granzyme B as direct activator (Fig. 6). During brain ageing, procaspase-3 was shown to be structurally intact, as seen by the ~32 kDa precursor form (pro-C3) (Fig. 5A, lanes 2–6). However, the zymogen expression was downregulated 3-fold (Fig. 5B). In order to confirm the enzyme functionality, the extracts were induced with the canonical serine protease granzyme B [37]. Procaspase-3 was activated in the range of 52–213-fold at all stages of brain maturation (Fig. 6), in agreement with the enzyme expression levels (Fig. 5A, lanes 2–6; Fig. 5B). The positive control consisted of an extract of HEK 293T cells overexpressing caspase-3. The antibody recognized two additional bands of ~20 kDa and ~11 kDa corresponding to the enzyme large (C3 p20) and small (C3 p11) subunits (Fig. 5A, lane 1) which resulted from a cleavage at Asp175/Ser176 [38]. Human and mouse caspase-3 zymogens are 85% amino acid identical (results not shown). The lack of activation of this pathway may be due to enzyme downregulation (Fig. 5A, lanes 2–6) or to the enzyme functionality being compromised. The lack of function due to alternative inactivating cleavages was excluded because, by Western blotting, we confirmed the enzyme’s intactness (Fig. 5A, lanes 2–6). Caspase-3 downregulation represents a major regulatory step during brain ageing because it turns-off not only the ‘intrinsic’ but also the ‘extrinsic’ pathway. Downregulation of caspase-3 has been observed under physiological [13,17,39] and pathological conditions [12,40]. In addition, this experiment excludes the possibility of random lack of activation of some adult extracts, since it involves the serine peptidase granzyme B which directly activates executioner caspases [37]. It appears therefore that the resistance to cyt.c/dATP activation is cell-dependent and that there is no unique pathway.
Fig. 6.
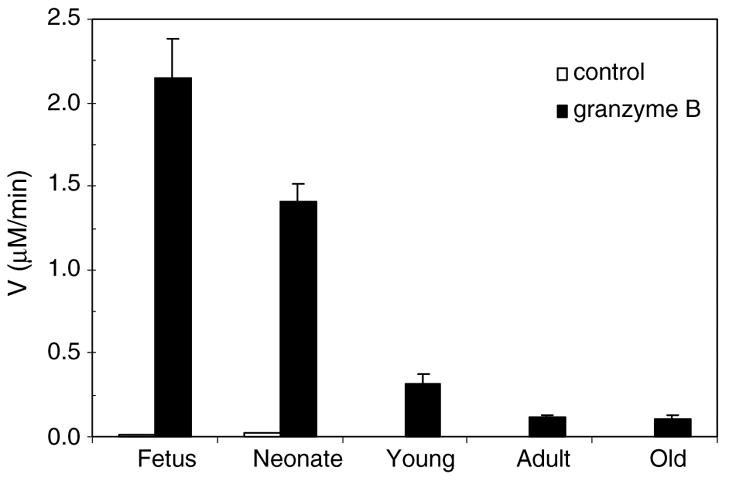
Direct activation of the apoptotic pathway during brain ageing. Fifty micrograms of cell-free extracts of mouse brain tissue at several stages of maturation, fetus, neonate, young, adult and old were activated by addition of granzyme B. A control experiment in the absence of activator was run under the same conditions. Caspase activation was measured fluorometrically as described in Section 2.3.
In conclusion, our results indicate that the rodent’s genetic background and developmental maturity play crucial roles in IPA activation/inactivation, not simply the tissue specificity, as previously suggested [13]. Furthermore, the age-related decline in IPA activation was found not to be the result of the upregulation of dominant inhibitors such as IAPs. Instead, the results are most compatible with the decline in apoptotic-activating proteins such as Apaf-1, caspase-9 and caspase-3, respectively.
Acknowledgments
We appreciate the skilled technical assistance of Sylvia F. Chen during animal tissues preparation. We acknowledge Professor Guy S. Salvesen for helpful discussion and critical comments on the manuscript and Professor Roger H. Pain for critically reading the manuscript. This work was supported by NIH Grants AG12282 and NS33376 to D.E.B. and Grant OB14PO4SK from the Ministry of Higher Education, Science and Technology of the Republic of Slovenia to V.T.
Abbreviations
- Apaf-1
apoptotic protease activation factor-1
- cyt.c
cytochrome c
- dATP
2′-deoxyadenosine 5′-triphosphate
- DEVD-AMC
acetyl-Asp-Glu-Val-Asp-7-amino-4-methylcoumarin
- IPA
intrinsic pathway of apoptosis
Footnotes
Edited by Vladimir Skulachev
References
- 1.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 2.Lindsten T, Zong WX, Thompson CB. Defining the role of the Bcl-2 family of proteins in the nervous system. Neuroscientist. 2005;11:10–15. doi: 10.1177/1073858404269267. [DOI] [PubMed] [Google Scholar]
- 3.Budihardjo I, Oliver H, Lutter M, Luo X, Wang X. Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol. 1999;15:269–290. doi: 10.1146/annurev.cellbio.15.1.269. [DOI] [PubMed] [Google Scholar]
- 4.Jin Z, El-Deiry WS. Overview of cell death signaling pathways. Cancer Biol Ther. 2005;4:139–163. doi: 10.4161/cbt.4.2.1508. [DOI] [PubMed] [Google Scholar]
- 5.Adrain C, Martin SJ. The mitochondrial apoptosome: a killer unleashed by the cytochrome seas. Trends Biochem Sci. 2001;26:390–397. doi: 10.1016/s0968-0004(01)01844-8. [DOI] [PubMed] [Google Scholar]
- 6.Zou H, Li Y, Liu X, Wang X. An APAF-1 · cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J Biol Chem. 1999;274:11549–11556. doi: 10.1074/jbc.274.17.11549. [DOI] [PubMed] [Google Scholar]
- 7.Hao Z, Duncan GS, Chang CC, Elia A, Fang M, Wakeham A, Okada H, Calzascia T, Jang Y, You-Ten A, Yeh WC, Ohashi P, Wang X, Mak TW. Specific ablation of the apoptotic functions of cytochrome c reveals a differential requirement for cytochrome c and Apaf-1 in apoptosis. Cell. 2005;121:579–591. doi: 10.1016/j.cell.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 8.Marsden VS, O’Connor L, O’Reilly LA, Silke J, Metcalf D, Ekert PG, Huang DCS, Cecconi F, Kuida K, Tomaselli KJ, Roy S, Nicholson DW, Vaux DL, Bouillet P, Adams JM, Strasser A. Apoptosis initiated by Bcl-2-regulated caspase activation independently of the cytochrome c/Apaf-1/caspase-9 apoptosome. Nature. 2002;419:634–637. doi: 10.1038/nature01101. [DOI] [PubMed] [Google Scholar]
- 9.Olson M, Kornbluth S. Mitochondria in apoptosis and human disease. Curr Mol Med. 2001;1:91–122. doi: 10.2174/1566524013364239. [DOI] [PubMed] [Google Scholar]
- 10.Mattson MP, Kroemer G. Mitochondria in cell death: novel targets for neuroprotection and cardioprotection. Trends Mol Med. 2003;9:196–205. doi: 10.1016/s1471-4914(03)00046-7. [DOI] [PubMed] [Google Scholar]
- 11.Dias N, Bailly C. Drugs targeting mitochondrial functions to control tumor cell growth. Biochem Pharmacol. 2005;70:1–12. doi: 10.1016/j.bcp.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 12.Yakovlev AG, Ota K, Wang G, Movsesyan V, Bao WL, Yoshihara K, Faden AI. Differential expression of apoptotic protease-activating factor-1 and caspase-3 genes and susceptibility to apoptosis during brain development and after traumatic brain injury. J Neurosci. 2001;21:7439–7446. doi: 10.1523/JNEUROSCI.21-19-07439.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ota K, Yakovlev AG, Itaya A, Kameoka M, Tanaka Y, Yoshihara K. Alteration of apoptotic protease-activating factor-1 (APAF-1)-dependent apoptotic pathway during development of rat brain and liver. J Biochem (Tokyo) 2002;131:131–135. doi: 10.1093/oxfordjournals.jbchem.a003067. [DOI] [PubMed] [Google Scholar]
- 14.Jia L, Srinivasula SM, Liu FT, Newland AC, Fernandes-Alnemri T, Alnemri ES, Kelsey SM. Apaf-1 protein deficiency confers resistance to cytochrome c-dependent apoptosis in human leukemic cells. Blood. 2001;98:414–421. doi: 10.1182/blood.v98.2.414. [DOI] [PubMed] [Google Scholar]
- 15.Soengas MS, Capodieci P, Polsky D, Mora J, Esteller M, Opitz-Araya X, McCombie R, Herman JG, Gerald WL, Lazebnik YA, Cordon-Cardo C, Lowe SW. Inactivation of the apoptosis effector Apaf-1 in malignant melanoma. Nature. 2001;409:207–211. doi: 10.1038/35051606. [DOI] [PubMed] [Google Scholar]
- 16.Wolf BB, Schuler M, Li W, Eggers-Sedlet B, Lee W, Tailor P, Fitzgerald P, Mills GB, Green DR. Defective cytochrome c-dependent caspase activation in ovarian cancer cell lines due to diminished or absent apoptotic protease activating factor-1 activity. J Biol Chem. 2001;276:34244–34251. doi: 10.1074/jbc.M011778200. [DOI] [PubMed] [Google Scholar]
- 17.Stoka V, Chen SF, Turk V, Bredesen DE. Developmental shift in the apostat: comparison of neurons and astrocytes. FEBS Lett. 2005;579:6147–6150. doi: 10.1016/j.febslet.2005.09.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wetzel M, Tibbitts J, Rosenberg GA, Cunningham LA. Vulnerability of mouse cortical neurons to doxorubicin-induced apoptosis is strain-dependent and is correlated with mRNAs encoding Fas, Fas-ligand, and metalloproteinases. Apoptosis. 2004;9:649–656. doi: 10.1023/B:APPT.0000038038.42809.e2. [DOI] [PubMed] [Google Scholar]
- 19.Sibilia M, Wagner EF. Strain-dependent epithelial defects in mice lacking the EGF receptor. Science. 1995;269:234–238. doi: 10.1126/science.7618085. [DOI] [PubMed] [Google Scholar]
- 20.Momoi T, Fujita E, Urase K. Strain-specific caspase-3-dependent programmed cell death in the early developing mouse forebrain. Neuroreport. 2003;14:111–115. doi: 10.1097/00001756-200301200-00021. [DOI] [PubMed] [Google Scholar]
- 21.Canning J, Takai Y, Tilly JL. Evidence for genetic modifiers of ovarian follicular endowment and development from studies of five inbred mouse strains. Endocrinology. 2003;144:9–12. doi: 10.1210/en.2002-220988. [DOI] [PubMed] [Google Scholar]
- 22.Roy N, Deveraux QL, Takahashi R, Salvesen GS, Reed JC. The c-IAP-1 and c-IAP-2 proteins are direct inhibitors of specific caspases. EMBO J. 1997;16:6914–6925. doi: 10.1093/emboj/16.23.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goffredo D, Rigamonti D, Zuccato C, Tartari M, Valenza M, Cattaneo E. Prevention of cytosolic IAPs degradation: a potential pharmacological target in Huntington’s disease. Pharmacol Res. 2005;52:140–150. doi: 10.1016/j.phrs.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 24.Dong Z, Wang JZ, Yu F, Venkatachalam MA. Apoptosis-resistance of hypoxic cells: multiple factors involved and a role for IAP-2. Am J Pathol. 2003;163:663–671. doi: 10.1016/S0002-9440(10)63693-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hiona A, Leeuwenburgh C. Effects of age and caloric restriction on brain neuronal cell death/survival. Ann NY Acad Sci. 2004;1019:96–105. doi: 10.1196/annals.1297.018. [DOI] [PubMed] [Google Scholar]
- 26.Mayorga M, Bahi N, Ballester M, Comella JX, Sanchis D. Bcl-2 is a key factor for cardiac fibroblast resistance to programmed cell death. J Biol Chem. 2004;279:34882–34889. doi: 10.1074/jbc.M404616200. [DOI] [PubMed] [Google Scholar]
- 27.Sasaki M, Kumazaki T, Takano H, Nishiyama M, Mitsui Y. Senescent cells are resistant to death despite low Bcl-2 level. Mech Ageing Dev. 2001;122:1695–1706. doi: 10.1016/s0047-6374(01)00281-0. [DOI] [PubMed] [Google Scholar]
- 28.Burgess DH, Svensson M, Dandrea T, Gronlund K, Hammarquist F, Orrenius S, Cotgreave IA. Human skeletal muscle cytosols are refractory to cytochrome c-dependent activation of type-II caspases and lack APAF-1. Cell Death Differ. 1999;6:256–261. doi: 10.1038/sj.cdd.4400489. [DOI] [PubMed] [Google Scholar]
- 29.Bratton SB, Walker G, Roberts DL, Cain K, Cohen GM. Caspase-3 cleaves Apaf-1 into an approximately 30 kDa fragment that associates with an inappropriately oligomerized and biologically inactive approximately 1.4 MDa apoptosome complex. Cell Death Differ. 2001;8:425–433. doi: 10.1038/sj.cdd.4400834. [DOI] [PubMed] [Google Scholar]
- 30.Lauber K, Appel HA, Schlosser SF, Gregor M, Schulze-Osthoff K, Wesselborg S. The adapter protein apoptotic protease-activating factor-1 (Apaf-1) is proteolytically processed during apoptosis. J Biol Chem. 2001;276:29772–29781. doi: 10.1074/jbc.M101524200. [DOI] [PubMed] [Google Scholar]
- 31.Yoshida H, Kong YY, Yoshida R, Elia AJ, Hakem A, Hakem R, Penninger JM, Mak TW. Apaf1 is required for mitochondrial pathways of apoptosis and brain development. Cell. 1998;94:739–750. doi: 10.1016/s0092-8674(00)81733-x. [DOI] [PubMed] [Google Scholar]
- 32.Cecconi F, Alvarez-Bolado G, Meyer BI, Roth KA, Gruss P. Apaf1 (CED-4 homolog) regulates programmed cell death in mammalian development. Cell. 1998;94:727–737. doi: 10.1016/s0092-8674(00)81732-8. [DOI] [PubMed] [Google Scholar]
- 33.Honarpour N, Gilbert SL, Lahn BT, Wang X, Herz J. Apaf-1 deficiency and neural tube closure defects are found in fog mice. Proc Natl Acad Sci USA. 2001;98:9683–9687. doi: 10.1073/pnas.171283198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Honarpour N, Du C, Richardson JA, Hammer RE, Wang X, Herz J. Adult Apaf-1-deficient mice exhibit male infertility. Dev Biol. 2000;218:248–258. doi: 10.1006/dbio.1999.9585. [DOI] [PubMed] [Google Scholar]
- 35.Duan H, Orth K, Chinnaiyan AM, Poirier GG, Froelich CJ, He WW, Dixit VM. ICE-LAP6, a novel member of the ICE/Ced-3 gene family, is activated by the cytotoxic T cell protease granzyme B. J Biol Chem. 1996;271:16720–16724. doi: 10.1074/jbc.271.28.16720. [DOI] [PubMed] [Google Scholar]
- 36.Fujita E, Jinbo A, Matuzaki H, Konishi H, Kikkawa U, Momoi T. Akt phosphorylation site found in human caspase-9 is absent in mouse caspase-9. Biochem Biophys Res Commun. 1999;264:550–555. doi: 10.1006/bbrc.1999.1387. [DOI] [PubMed] [Google Scholar]
- 37.Darmon AJ, Nicholson DW, Bleackley RC. Activation of the apoptotic protease CPP32 by cytotoxic T-cell-derived granzyme B. Nature. 1995;377:446–448. doi: 10.1038/377446a0. [DOI] [PubMed] [Google Scholar]
- 38.Nicholson DW, Ali A, Thornberry NA, Vaillancourt JP, Ding CK, Gallant M, Gareau Y, Griffin PR, Labelle M, Lazebnik YA, et al. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- 39.Shimohama S, Tanino H, Fujimoto S. Differential expression of rat brain caspase family proteins during development and aging. Biochem Biophys Res Commun. 2001;289:1063–1066. doi: 10.1006/bbrc.2001.6108. [DOI] [PubMed] [Google Scholar]
- 40.Hu BR, Liu CL, Ouyang Y, Blomgren K, Siesjo BK. Involvement of caspase-3 in cell death after hypoxia-ischemia declines during brain maturation. J Cereb Blood Flow Metab. 2000;20:1294–1300. doi: 10.1097/00004647-200009000-00003. [DOI] [PubMed] [Google Scholar]


