Abstract
Aims
Recent studies suggest the importance of oxidant stress in the progression of pulmonary fibrosis. The aim of this study was to investigate extracellular superoxide dismutase (ECSOD), the major antioxidant enzyme of the extracellular matrix of human lung, in biopsy-proven idiopathic pulmonary fibrosis (IPF) related to usual interstitial pneumonia (UIP).
Methods and results
Fibrotic areas and fibroblastic foci in UIP lungs were notable for absence of ECSOD by immunohistochemistry. Western blotting showed significantly lowered immunoreactivity of ECSOD in fibrotic compared with non-fibrotic areas of the diseased lung. The only cell type that showed intense ECSOD positivity in UIP was the interstitial mast cell. In order to investigate the mechanism for ECSOD depletion in fibrotic areas, alveolar epithelial cells were exposed to tumour necrosis factor-α and transforming growth factor (TGF)-β1; TGF-β suggested a trend towards decreased synthesis. Patients with UIP were also assessed to determine whether this disease is associated with a naturally occurring mutation in ECSOD (Arg213Gly) which leads to a loss of tissue binding of ECSOD. No significant differences could be found in the allele or genotype frequencies of this polymorphism between 63 UIP patients and 61 control subjects.
Conclusion
Overall, consistent with several other antioxidant enzymes, ECSOD is very low in fibrotic areas of UIP, which may further increase the oxidant burden in this disease.
Keywords: antioxidant, extracellular superoxide dismutase, fibrosis, oxidant, usual interstitial pneumonia
Introduction
It has been suggested that free radical reactions play a contributory role in the development of interstitial lung disorders such as fibrogenesis, either directly or through multiple fibrogenetic and inflammatory stimuli. 1–4 Extracellular superoxide dismutase (ECSOD) is the major enzyme capable of inactivating superoxide radicals in the extracellular matrix.5–7 The importance of ECSOD in lung protection has been confirmed both in ECSOD transgenic8,9 and knockout mice.10,11 ECSOD may be one of the key antioxidant enzymes preventing oxidant-mediated parenchymal lung injury. However, the importance of ECSOD has not previously been investigated in human interstitial lung diseases.
Idiopathic pulmonary fibrosis (IPF) is believed to result from sequential acute lung injury possibly mediated by changes in the cellular oxidant/antioxidant balance and the resultant wound-healing response culminating in the development of pulmonary fibrosis. 12 IPF represents the clinical manifestation of usual interstitial pneumonia (UIP) in most cases.13 Typical pathological features of UIP include the presence of patchy, non-uniform interstitial changes with variable distribution, with adjacent zones of mature interstitial fibrosis, immature/ongoing fibrosis and normal lung. The areas of immature fibrosis are characterized by small aggregates of actively proliferating myofibroblasts and fibroblasts termed fibroblastic foci.13 The prognosis for IPF patients is poor and is highly associated with the appearance of these fibrotic lesions.14
The main goals of this study were to assess the cell and matrix specific localization and regulation of ECSOD in humans with biopsy-proven IPF (UIP) in vivo. Additional experiments were conducted to investigate whether the functional Arg213Gly polymorphism of ECSOD, which leads to decreased tissue binding of ECSOD, is associated with UIP. Cytokine regulation of ECSOD was also examined as a possible explanation for decreased ECSOD in IPF/UIP. Since intense expression of ECSOD in some inflammatory cells in the parenchyma of UIP could be detected, these unidentified cells were further investigated by double-staining immunohistochemistry and identified to be comprised predominantly of mast cells.
Materials and methods
TISSUE SPECIMENS
Histopathologically typical cases of UIP were retrieved from the files of the Department of Pathology, Oulu University Central Hospital. Diagnoses of all patients were based on light microscopic evaluation using the histological criteria outlined by Katzenstein and Myers.13 Diagnostic lung biopsy had been conducted because of parenchymal involvement. Uninvolved peripheral lung tissue, used as a control, was obtained from five non-smokers operated on for a malignant lung tumour or carcinoid tumour. None of the control subjects received corticosteroid treatment before the biopsy and their lung function parameters were normal. The clinical information of the patients was obtained from the patient records at the University Hospital and nearby Päivärinne Hospital. The biopsy material was fixed in 10% formalin under vacuum in order to expand the tissue and to remove air bubbles or perfused by injecting the fixative, using a small syringe, into bronchioles as described by Dail and Hammar.15 The specimens were then dehydrated and embedded in paraffin.
IMMUNOHISTOCHEMISTRY
Two anti-rabbit primary antibodies against ECSOD were used: the first has been described previously16 and the second was a gift from Dr J. D. Crapo, National Jewish Medical and Research Center, Denver, CO, USA. The colour was developed with aminoethyl carbazole substrate solution (Zymed Laboratories, S. San Francisco, CA, USA) as described.17 The sections were counterstained with a light haematoxylin stain. The negative controls consisted of substituting phosphatebuffered saline (PBS) at pH 7.2 or non-immune rabbit antisera (Zymed Laboratories). Double staining for macrophages and mast cells was performed as described by the manufacturer (Zymed Laboratories). A polyclonal antibody to c-kit (CD117) was used to identify mast cells (Dako, Glostrup, Denmark). A monoclonal antibody to CD68 (Dako) identified macrophages. An antibody to CD3 (Dako) identified T lymphocytes and a monoclonal antibody to CD20 (Dako) identified B lymphocytes.
WESTERN BLOTTING OF NORMAL AND FIBROTIC LUNG
Frozen unfixed lung samples were provided by the University of Pittsburgh Tissue Bank. Four pulmonary fibrosis lung samples were obtained from patients with pathologically identified UIP. Lung samples were embedded in OCT medium and 5-μm sections cut from the blocks. These slides were stained with haematoxylin and eosin as previously described.18 Regions of histologically normal alveolar parenchyma and areas of fibrotic lung were identified histologically and used to guide the excision of these regions of tissue from the frozen blocks. Care was taken to avoid bronchi and bronchioles as well as large blood vessels since these structures contain large amounts of ECSOD. Protein concentrations were measured with the Coommassie Plus Protein Assay Reagent from Pierce (Rockford, IL, USA). Equal protein samples were subjected to reducing sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) and subsequently blotted onto Immobilon-PSQ polyvinylidenefluoride transfer membranes (Millipore, Bedford, MA, USA).
After blocking, blots were exposed to the primary antibody against ECSOD (1 : 10 000 dilution) followed by a secondary antibody and detection with ECL Reagent (Amersham, Little Chalfont, UK). Given the evident problems with β-actin and glyceraldehyde-3-phosphate dehydrogenase in human lung diseases,19 they were not used as housekeeping genes/loading controls in the tissue specimens. Instead, the loading homogeneity was confirmed by careful protein measurement of the samples as well as confirmation by replicate analysis. Densitometry was performed with Kodak 1D Software (Eastman Kodak Companies, Rochester, NY, USA).
ASSOCIATION STUDY
Patients
Ascertainment criteria for patient recruitment were based on the American Thoracic Society (ATS)/European Respiratory Society International consensus statement (ATS 2000) and described in detail previously. 20 The patients consisted of 40 females and 23 males. Eighteen of the patients originated from multiplex families. The average age at onset of UIP was 62 years (range 26–81). At the time of diagnosis, their mean vital capacity was 71% (range 35–96%) of that predicted and gas transfer for carbon monoxide was 57% (range 29–91%) of that predicted. High-resolution computed tomography (HRCT) was available for 52 (83%) patients. When any doubt existed (early onset of the disease, atypical findings on HRCT or lung function test results), the diagnosis was confirmed by surgical biopsy (11/63 patients, 17%).
Control subjects
The control group consisted of 61 unrelated individuals: 32 males and 29 females (average age 60 years). Twenty-eight of the control subjects were the spouses of the probands and 33 were the spouses of asthma patients representing population-based controls.21 The presence of any known pulmonary disorder was excluded. The study was approved by the Ethics Committees of the University Hospitals in Finland and by the Ministry of Social Affairs and Health of Finland.
Genotyping of the Arg213Gly variant in ECSOD
DNAwas extracted from peripheral blood leucocytes by a standard non-enzymatic method. The following primer pair was used for amplification: 5′-CGCCAGGCG CGGGAACACTCAG-3′ and 5′-GGCGGACTTGCACTC GCTCTCG-3′. One mismatch was induced in both primers: one to delete the second digestion site for MwoI and the one to reduce the formation of secondary structures of the primer pair. Polymerase chain reaction (PCR) amplifications were done in 10-μl reaction volumes containing 100 ng of genomic DNA with AmpliTaqGold Polymerase (Applied Biosystems, Foster City, CA, USA). After amplification, the PCR fragments were digested with MwoI (BioLabs, Beverly, MA, USA). The lengths of the PCR products were 63 bp for the minor allele (the Gly213 allele) and 28 bp and 35 bp for the major alleles (the Arg213 allele). The reaction products were electrophoresed on a 4% agarose gel stained by Gelstar (FMC Bioproducts, Rockland, MN, USA) and photographed under UV illumination. Comparisons between the patient group and control group were done using a χ2 test.
CELL CULTURES, EXPOSURES AND WESTERN BLOTTING ANALYSIS
A549 cells (American Type Culture Collection, Rockville, MD, USA) were grown in Ham’s Nutrient mixture F-12 with L-glutamine (Gibco BLR, Life Technologies, Paisley, UK) with 15% fetal bovine serum (FBS) (Euroclone, Paignton, UK). Subconfluent cells were exposed to either tumour necrosis factor (TNF)-α or transforming growth factor (TGF)-β1 (50 ng/ml and 2 ng/ml, respectively) for 24–72 h. The exposures were conducted twice and the Western blots from each exposure were repeated twice.
Equal amounts of cell proteins were subjected to reducing SDS–PAGE. The blotted membranes were incubated with the ECSOD antibody and in a control experiment polyclonal anti-rabbit antibody to recombinant human manganese superoxide dismutase (MnSOD) (gift from J. Crapo, Jewish Medical Center) (dilution 1 : 10 000). As a loading control the blots were probed with an antibody to β-actin (Sigma Aldrich, St Louis, MO, USA) (dilution 1 : 5000). MnSOD was used as a positive control, as its regulation and intense induction by inflammatory cytokines in human lung and lung cells are well documented.6,22–24
Results
TISSUE SPECIMENS
Normal peripheral lung showed weak to moderate ECSOD expression in alveolar macrophages, bronchial epithelium, alveolar type II pneumocytes, vascular endothelium and extracellular matrix, as previously described.25,26 The lung biopsies of 10 patients with UIP indicated ECSOD immunoreactivity at a similar intensity as in the controls, in vascular endothelium, bronchial epithelium and alveolar macrophages (Figure 1A, Table 1). UIP regenerative alveolar epithelium showed variable ECSOD positivity (Figure 1A). Notably, fibrotic lesions and fibroblastic foci were negative for ECSOD (Figure 1C). To confirm this finding, fibrotic and non-fibrotic areas of UIP were excised from OCT-embedded non-fixed frozen sections of UIP lungs and compared for ECSOD immunoreactivity by Western blotting analysis. In agreement with the immunohistochemical results, ECSOD immunoreactivity was significantly lower in the fibrotic than in non-fibrotic areas of UIP lungs (Figure 2). The only cells that showed intense ECSOD staining in fibrotic areas of UIP lungs were granular cells of the interstitium (Figure 1B, Table 1). These cells were identified as mast cells by CD117 double staining (Figure 1D). Double staining with antibody against CD68 for macrophages and with ECSOD antibody showed positivity in alveolar macrophages as expected, but the ECSOD-stained cells of the interstitium were CD68– (Figure 1C). Double staining with CD3 and CD20 for T and B lymphocytes showed no ECSOD expression in lymphocytes.
Figure 1.
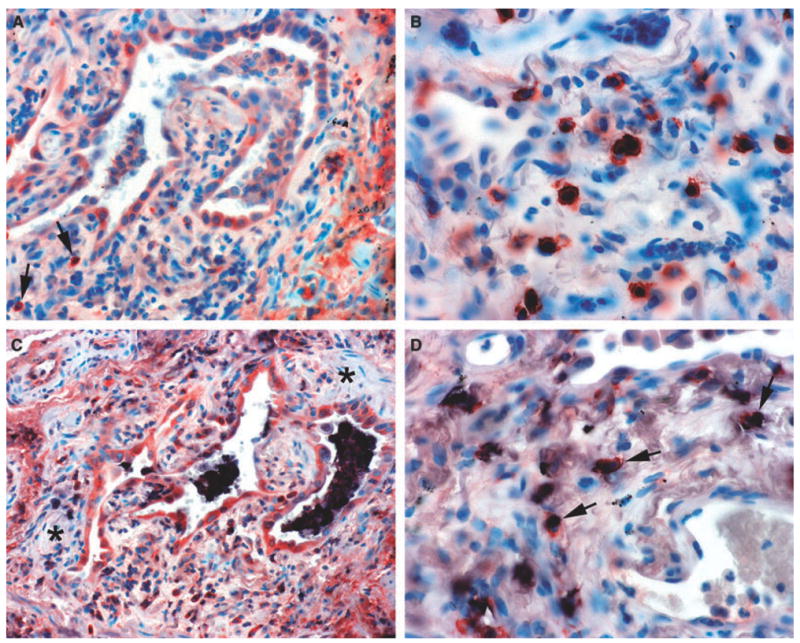
Extracellular superoxide dismutase (ECSOD) immunoreactivity in usual interstitial pneumonia and double labelling for mast cells and alveolar macrophages. A, Variable expression of ECSOD in type 2 pneumocytes, alveolar macrophages and a few interstitial cells (arrows). B, Strong expression of ECSOD in granular interstitial cells evidently representing mast cells. C, Alveolar macrophages showing staining for both CD68 (indicated by black) and ECSOD (same cells also showing red). Notice that type 2 pneumocytes and a few interstitial cells at the bottom are positive for ECSOD (red). Notice also that the fibroblast foci (asterisks) are negative for ECSOD. D, Mast cells showing staining for both ECSOD (red ) and CD117 (black) (arrows).
Table 1.
Scoring the intensity of extracellular superoxide dismutase (ECSOD) immunoreactivity in patients with usual interstitial pneumonia (UIP)
| Mast cells | Alveolar macrophages | Bronchial cells | Epithelial endothelium | Type II pneumocytes | |
|---|---|---|---|---|---|
| 1 | +++ | ++ | NA | ++ | ++ |
| 2 | +++ | ++ | + | ++ | + |
| 3 | ++ | + | ++ | + | ++ |
| 4 | ++ | ++ | + | + | + |
| 5 | ++ | + | + | + | + |
| 6 | +++ | + | ++ | ++ | ++ |
| 7 | +++ | ++ | ++ | ++ | ++ |
| 8 | +++ | ++ | + | ++ | ++ |
| 9 | +++ | ++ | + | ++ | ++ |
| 10 | +++ | ++ | + | ++ | ++ |
NA, Not present; −, negative; +, weak immunoreactivity; ++, moderate immunoreactivity; +++, intense immunoreactivity.
Note: fibroblast foci and old fibrotic lesions were mainly negative for ECSOD immunoreactivity.
Figure 2.
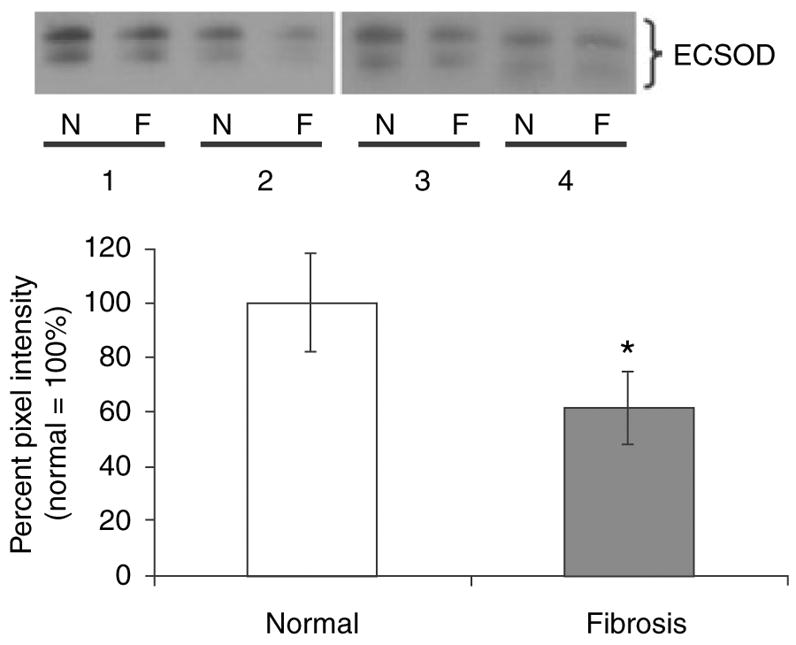
Western Blotting of normal alveolar parenchyma and fibrotic lung parenchyma from usual interstitial pneumonia (UIP) specimens. Homogenates of normal (N) and fibrotic (F) regions from four UIP specimens (nos 1–4) were subjected to reducing SDS–PAGE and Western blotting with an antibody specific for extracellular superoxide dismutase (ECSOD). Both the cut and uncut forms of ECSOD are shown. Densitometry was performed and results expressed as a percentage of normal (normal = 100%). *P = 0.021, Student’s paired t-test, two-tailed.
ASSOCIATION STUDY
A single amino acid substitution Arg213Gly in ECSOD is known to lead to decreased anchoring of the enzyme protein to the tissue matrix and, consequently, decreased ECSOD in the extracellular matrix. To determine if there is an association between IPF phenotype and the allele distribution of Arg213Gly polymorphism, we genotyped 63 UIP patients and 61 control subjects. Similar to other White populations studied36, the Gly213 allele was shown to be a rare variant in the population (2.5%). No association was detected between the study groups. One of the patients was a heterozygous carrier of the Gly213 allele and three control subjects were heterozygous carriers of the Gly213 allele (P = 0.59) (Figure 3).
Figure 3.
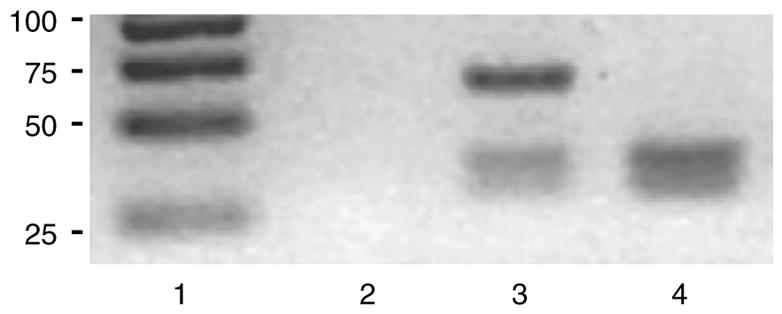
Polymerase chain reaction for the studies of extracellular superoxide dismutase polymorphism in the blood samples of controls and usual interstitial pneumonia patients. Lane 1 contains a size marker with bands of 25, 50, 75 and 100 bp. Lane 2 is negative control. Lane 3 represents a heterozygous for the minor Gly213 allele with Mwo1-digested products of 28, 35 and 63 bp. Lane 4 contains a homozygous sample for the major Arg213 allele with digested products of 28 and 35 bp.
REGULATION OF ECSOD IN CULTURED LUNG CELLS
Given that ECSOD is synthesized and expressed in type II alveolar epithelial cells and secreted to the matrix, ECSOD regulation was further investigated using the A549 alveolar epithelial cell line which retains features of type II cells in culture. In these experiments the conditions were first confirmed using MnSOD as a positive control. MnSOD was increased within the first 24 h by TNF-α (+ 130%) and decreased by TGF-β (− 19% at 24 h) (not shown). The regulation of ECSOD by TNF-α (+ 42%, 24 h, Figure 4) was modest. Exposure of the cells to TGF-β caused a slight tendency for decreased ECSOD reactivity (− 13% when calculated from the densitometry, 24 h).
Figure 4.
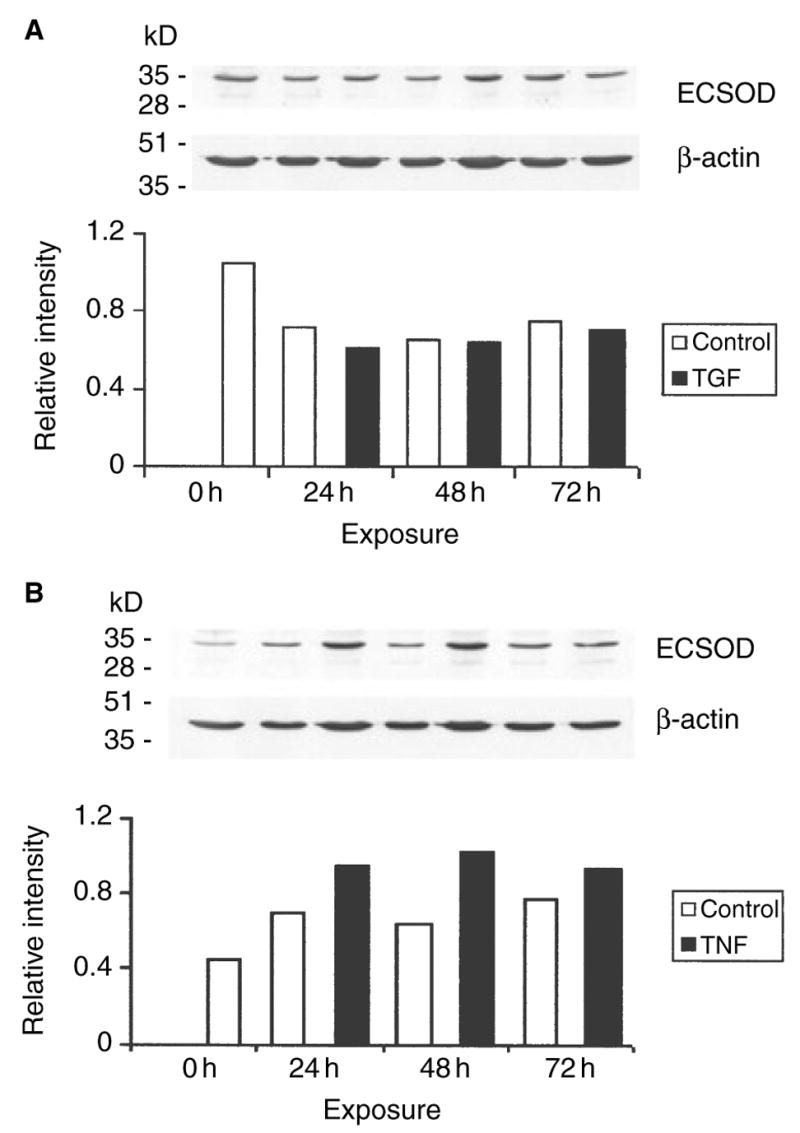
Extracellular superoxide dismutase (ECSOD) immunoreactivity in alveolar epithelial cells exposed to transforming growth factor (TGF)- β (A) and tumour necrosis factor (TNF)- α (B) for 24–76 h; the experiments were carried out in duplicate. The expression was standardized against β-actin as described. A, □, Control; ▪, TGF. B, □, Control; ▪, TNF.
Discussion
The major finding of the present study was the significant loss of ECSOD from areas of progressive fibrosis in UIP. However, there was still cell-specific expression of this enzyme in the fibrotic lung. ECSOD was positive in regenerative areas of alveolar epithelium in UIP and intense ECSOD immunoreactivity in interstitial mast cells in UIP was also observed. Low/absent ECSOD in fibrotic areas of human lungs with UIP suggests that these regions may be susceptible to increased oxidant-mediated injury during disease progression. The expression of ECSOD in regenerative alveolar areas may represent an attempt to compensate for increased oxidant stress. Present and previous results also indicate that TGF-β is incapable of inducing a major protective antioxidant response in the lung.
ECSOD is the major protective enzyme of collagen, cartilage and other extracellular matrix proteins from superoxide- or peroxynitrite-mediated degradation where the heparin-binding domain of ECSOD confers affinity for these extracellular matrix components. 16,18,25 Until now, there have been no studies concerning ECSOD in human interstitial lung diseases.
Previous experimental studies in vivo have suggested both ECSOD induction and decline and inactivation in oxidant-mediated lung disorders. Acute exposure of rats to lipopolysaccharide has suggested ECSOD induction in alveolar macrophages and neutrophils in vivo.27 On the other hand, both bleomycin and asbestos exposure of experimental animals leads to a significant loss of ECSOD from the lung matrix thought to be primarily a result of increased proteolysis of ECSOD’s heparin-binding domain.18,28 Exposure of mice to hyperoxia (100%) for 72 h has also resulted in a significant decrease in ECSOD both in lung parenchyma and bronchoalveolar lavage fluid.29 Fibrotic lung disorders such as UIP present as a patchy disease and results obtained from animal models do not translate well to the results obtained in human diseases. However, our results are in agreement with the above studies concerning bleomycin, asbestos and hyperoxia, as there is absent/weak expression of ECSOD in fibrotic areas of UIP. Notably, in animal models ECSOD has been shown to protect against bleomycin-induced pulmonary fibrosis,11 suggesting that loss of ECSOD in areas of injury in UIP lungs may contribute to the progression of this disease.
Our immunohistochemical study of UIP lungs revealed ECSOD in the regenerative areas of alveolar epithelium but not in areas of mature and immature fibrosis (fibroblast foci). Due to the heterogeneity of UIP, total tissue homogenates cannot be used reliably for the assessment of enzyme in individual cells and tissue matrices. Instead, Western blot analysis of homogenates of fibrotic lesions was performed and compared with homogenates of normal alveolar parenchyma from the same UIP patient. This confirmed a low level of ECSOD in the fibrotic areas. Observed low ECSOD immunoreactivity in fibrotic lung may be related to a number of mechanisms, including down-regulation of this enzyme by growth factors and cytokines during fibrogenesis. ECSOD polymorphism and/or proteolysis of the heparin-binding domain of ECSOD18 may also contribute to this finding. Our results have led us to examine the growth factor/cytokine regulation of ECSOD as well as functional Arg213Gly ECSOD polymorphism.
The major cytokines associated with inflammatory/fibrotic lung disorders are TNF-α and TGF-β, but the regulation of ECSOD by these cytokines is poorly understood. Our study of human alveolar epithelial cells suggests that ECSOD may be induced by TNF-α but that the induction is relatively weak, especially when compared with the induction of the mitochondrial enzyme MnSOD. On the other hand, TGF-β caused no induction of either enzyme; if anything, there was a small trend towards down-regulation of both enzymes following TGF-β exposure. In fact, antioxidant enzymes such as MnSOD and the rate-limiting enzyme in glutathione synthesis, glutamate cysteine ligase (GCL, γ-glutamylcysteine synthetase), have been shown to be down-regulated in response to TGF-β.30,31 It is therefore likely that ECSOD may also be down-regulated by this growth factor in human lung.
These results are very similar to those found with several antioxidant enzymes and related proteins such as MnSOD, thioredoxin, haeme oxygenase-1 and GCL in human pulmonary fibrosis, i.e. variable expression of these enzymes in the regenerative epithelium and low/absent expression in the fibrotic lesions of UIP.2,32–34 Thus, simultaneous absence of many intracellular antioxidant enzymes together with ECSOD, which is mainly extracellular, may be a potential contributor to the increased local oxidant stress of fibrotic lung. Overall, these results raise interesting possibilities for antioxidant therapies in pulmonary fibrosis.1,35
Individual variability and polymorphisms of various enzymes with antioxidant capacity have been found to contribute to the development of malignant lung diseases and they may also be associated with oxidant-mediated non-malignant lung disorders. The ECSOD gene contains a single nucleotide polymorphism (G→C) resulting in a functionally significant amino acid change from arginine (Arg, the 213rd amino acid of ECSOD) to glycine (Gly) (Arg213Gly). This amino-acid substitution is rare (3–6% in various populations).36 It leads to decreased anchoring of ECSOD to heparin/matrix proteins in the interstitium. This polymorphism has been hypothesized to lead to decreased protection of tissues against free radicals.37 There was no significant association of this polymorphism with this disease, suggesting that low ECSOD expression in the fibrotic lung is related to other reasons than this ECSOD polymorphism. However, larger studies are necessary to exclude completely any role for this polymorphism in UIP/IPF.
In addition to the low level of antioxidant enzymes, there are numerous studies showing that inflammatory cells of patients with interstitial lung diseases generate more radicals than the cells of healthy controls38 (reviewed by Kinnula et al.).1,4 Inflammatory cells in these disorders show elevated inducibla nitric oxide synthase (iNOS) expression, which may then cause toxicity to multiple cell types in the lung.2,3 A previous study showing distinct immunoreactivity of iNOS and endothelial nitric oxide synthase in the fibroblastic foci of UIP2 differs from the low/absent expression of ECSOD observed here. Thus, iNOS expression without compensatory ECSOD reactivity in fibrotic lesions further suggests locally increased oxidant stress in UIP as the potent peroxynitrite oxidant would be produced in the presence of nitric oxide and superoxide. Oxidants and the cellular redox state are, in turn, also known to activate fibrosis-related growth factors, one of them being TGF-β.39
The only cell type in UIP that showed intense ECSOD positivity in areas of fibrosis was the interstitial mast cell. In general, lung tissue of patients with UIP/IPF shows low-grade inflammation and low levels of macrophages, especially when compared with some other interstitial lung diseases, e.g. desquamative interstitial pneumonia.12 Little attention has, however, been paid to the observations that the lungs of IPF patients contain increased numbers of mast cells and that there is also a significant correlation between the number of mast cells and lung fibrogenesis.40 Similarly, fibrous lung tissue after chronic lymphatic obstruction and in chronic pulmonary oedema appears to be associated with proliferation of pulmonary mast cells.41 Mast cells have a variety of biological functions, including a regulatory role in tissue homeostasis, host defence, immunity, connective tissue growth and angiogenesis. Our results have shown intense intracellular ECSOD immunoreactivity in mast cells; its high expression was not due to binding to cell surface receptors as might be expected due to its high heparinbinding capacity. The finding was also confirmed by immunoelectron microscopy. The high expression of ECSOD in mast cell granules could theoretically be related to phagocytosis. However, interstitial macrophages that have greater phagocytic ability showed only weak ECSOD expression compared with mast cells (results not shown). Phagocytosis has also been shown to diminish with mast cell maturation. SOD activity has been previously described in rat mast cells, but this is the first observation of ECSOD in human mast cells. The significance of this finding remains unclear but requires more study in future investigations.
In conclusion, we have found a highly cell-specific expression of ECSOD not only in normal lung but also in UIP. ECSOD was found in the interstitial mast cells and regenerative areas of alveolar epithelium but it was very low in fibrotic lesions. The results of our current study, along with studies of ECSOD in animal models of pulmonary fibrosis, together with the increase of iNOS and the decline of several intracellular antioxidant enzymes in these same lesions in human UIP, point strongly to increased oxidant stress in the progression of IPF/UIP.
Acknowledgments
The antibodies for the MnSOD and ECSOD for the immunohistochemical studies were provided by Professor James D. Crapo (Jewish Medical and Research Center, Denver, CO, USA). The skilful technical assistance of Mr Manu Tuovinen and Ms Raija Sirviö is gratefully acknowledged. We appreciate assistance from Dr Martti Torkko, Dr Markku Pekonen, and Professor Pentti Tukiainen when the IPF patients were recruited for the genetic study. Grant support: Finnish Anti-Tuberculosis Association Foundation and Juselius Foundation (V.L.K.), Academy of Finland, Paolo Foundation, TEKES and Päivikki and Sakari Sohlberg Foundation (T.H.L., U.A.H.), Helsinki University Hospital, and National Institutes of Health Grants RO1 HL63700-04 (T.D.O.).
Abbreviations
- ECSOD
extracellular superoxide dismutase
- GLC
glutamate cysteine ligase
- HRCT
high-resolution computed tomography
- IPF
idiopathic pulmonary fibrosis
- MnSOD
manganese superoxide dismutase
- TGF
transforming growth factor
- TNF
tumour necrosis factor
- UIP
usual interstitial pneumonia
Contributor Information
V L Kinnula, Department of Medicine, Pulmonary Division, University of Helsinki, Helsinki.
U A Hodgson, Department of Medicine, Pulmonary Division, University of Helsinki, Helsinki.
E K Lakari, Department of Internal Medicine, University of Oulu.
R J Tan, Department of Pathology, University of Pittsburgh, Pittsburgh, PA, USA.
R T Sormunen, Biocentre Oulu, Oulu, Finland.
Y M Soini, Department of Pathology, University of Oulu.
S J Kakko, Department of Internal Medicine, University of Oulu.
T H Laitinen, Department of Medical Genetics, University of Helsinki, Helsinki.
T D Oury, Department of Pathology, University of Pittsburgh, Pittsburgh, PA, USA.
P K Pääkkö, Department of Pathology, University of Oulu.
References
- 1.Kinnula VL, Fattman CL, Tan RJ, Oury TD. Oxidative stress in pulmonary fibrosis: a possible role for redox modulatory therapy. Am J Respir Crit Care Med. 2005;172:417–422. doi: 10.1164/rccm.200501-017PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lakari E, Soini Y, Saily M, et al. Inducible nitric oxide synthase, but not xanthine oxidase, is highly expressed in interstitial pneumonias and granulomatous diseases of human lung. Am J Clin Pathol. 2002;117:132–142. doi: 10.1309/w7t9-hw9v-v94b-r9km. [DOI] [PubMed] [Google Scholar]
- 3.Saleh D, Barnes PJ, Giaid A. Increased production of the potent oxidant peroxynitrite in the lungs of patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1997;155:1763–1769. doi: 10.1164/ajrccm.155.5.9154889. [DOI] [PubMed] [Google Scholar]
- 4.Strausz J, Muller-Quernheim J, Steppling H, Ferlinz R. Oxygen radical production by alveolar inflammatory cells in idiopathic pulmonary fibrosis. Am Rev Respir Dis. 1990;141:124–128. doi: 10.1164/ajrccm/141.1.124. [DOI] [PubMed] [Google Scholar]
- 5.Fattman CL, Schaefer LM, Oury TD. Extracellular superoxide dismutase in biology and medicine. Free Radic Biol Med. 2003;35:236–256. doi: 10.1016/s0891-5849(03)00275-2. [DOI] [PubMed] [Google Scholar]
- 6.Kinnula VL, Crapo JD. Superoxide dismutases in the lung and human lung diseases. Am J Respir Crit Care Med. 2003;167:1600–1619. doi: 10.1164/rccm.200212-1479SO. [DOI] [PubMed] [Google Scholar]
- 7.Marklund SL. Extracellular superoxide dismutase and other superoxide dismutase isoenzymes in tissues from nine mammalian species. Biochem J. 1984;222:649–655. doi: 10.1042/bj2220649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bowler RP, Nicks M, Warnick K, Crapo JD. Role of extracellular superoxide dismutase in bleomycin-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2002;282:L719–L726. doi: 10.1152/ajplung.00058.2001. [DOI] [PubMed] [Google Scholar]
- 9.Folz RJ, Abushamaa AM, Suliman HB. Extracellular superoxide dismutase in the airways of transgenic mice reduces inflammation and attenuates lung toxicity following hyperoxia. J Clin Invest. 1999;103:1055–1066. doi: 10.1172/JCI3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlsson LM, Jonsson J, Edlund T, Marklund SL. Mice lacking extracellular superoxide dismutase are more sensitive to hyperoxia. Proc Natl Acad Sci USA. 1995;92:6264–6268. doi: 10.1073/pnas.92.14.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fattman CL, Chang LY, Termin TA, et al. Enhanced bleomycin-induced pulmonary damage in mice lacking extracellular superoxide dismutase. Free Radic Biol Med. 2003;35:763–771. doi: 10.1016/s0891-5849(03)00402-7. [DOI] [PubMed] [Google Scholar]
- 12.American Thoracic Society and European Respiratory Society. American Thoracic Society/European Respiratory Society: International multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 13.Katzenstein AL, Myers JL. Idiopathic pulmonary fibrosis: clinical relevance of pathologic classification. Am J Respir Crit Care Med. 1998;157:1301–1315. doi: 10.1164/ajrccm.157.4.9707039. [DOI] [PubMed] [Google Scholar]
- 14.Flaherty KR, Colby TV, Travis WD, et al. Fibroblastic foci in usual interstitial pneumonia: idiopathic versus collagen vascular disease. Am J Respir Crit Care Med. 2003;167:1410–1415. doi: 10.1164/rccm.200204-373OC. [DOI] [PubMed] [Google Scholar]
- 15.Dail DH, Hammar SP. Pulmonary pathology. New York: Springer-Verlag; 1994. pp. 597–678. [Google Scholar]
- 16.Oury TD, Day BJ, Crapo JD. Extracellular superoxide dismutase in vessels and airways of humans and baboons. Free Radic Biol Med. 1996;20:957–965. doi: 10.1016/0891-5849(95)02222-8. [DOI] [PubMed] [Google Scholar]
- 17.Svensk A-M, Soini Y, Pääkkö P, Hirvikoski P, Kinnula V. Differential expression of superoxide dismutases in lung cancer. Am J Clin Pathol. 2004;122:395–404. doi: 10.1309/A45Q-HB0Q-RRX6-CT9A. [DOI] [PubMed] [Google Scholar]
- 18.Fattman CL, Chu CT, Kulich SM, Enghild JJ, Oury TD. Altered expression of extracellular superoxide dismutase in mouse lung after bleomycin treatment. Free Radic Biol Med. 2001;31:1198–1207. doi: 10.1016/s0891-5849(01)00699-2. [DOI] [PubMed] [Google Scholar]
- 19.Glare EM, Divjak M, Bailey MJ, Walters EH. beta-Actin and GAPDH housekeeping gene expression in asthmatic airways is variable and not suitable for normalising mRNA levels. Thorax. 2002;57:765–770. doi: 10.1136/thorax.57.9.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hodgson U, Laitinen T, Tukiainen P. Nationwide prevalence of sporadic and familial idiopathic pulmonary fibrosis: evidence of founder effect among multiplex families in Finland. Thorax. 2002;57:338–342. doi: 10.1136/thorax.57.4.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hodgson U, Tuklainen P, Laitinen T. The polymorphism C5507G of complement receptor 1 does not explain idiopathic pulmonary fibrosis among the Finns. Respir Med. 2005;99:265–267. doi: 10.1016/j.rmed.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Kinnula VL, Crapo JD, Raivio KO. Generation and disposal of reactive oxygen metabolites in the lung. Lab Invest. 1995;73:3–19. [PubMed] [Google Scholar]
- 23.Lakari E, Paakko P, Kinnula VL. Manganese superoxide dismutase, but not CuZn superoxide dismutase, is highly expressed in the granulomas of pulmonary sarcoidosis and extrinsic allergic alveolitis. Am J Respir Crit Care Med. 1998;158:589–596. doi: 10.1164/ajrccm.158.2.9711059. [DOI] [PubMed] [Google Scholar]
- 24.Pietarinen-Runtti P, Raivio KO, Saksela M, Asikainen TM, Kinnula VL. Antioxidant enzyme regulation and resistance to oxidants of human bronchial epithelial cells cultured under hyperoxic conditions. Am J Respir Cell Mol Biol. 1998;19:286–292. doi: 10.1165/ajrcmb.19.2.2836. [DOI] [PubMed] [Google Scholar]
- 25.Oury TD, Chang LY, Marklund SL, Day BJ, Crapo JD. Immunocytochemical localization of extracellular superoxide dismutase in human lung. Lab Invest. 1994;70:889–898. [PubMed] [Google Scholar]
- 26.Su WY, Folz R, Chen JS, Crapo JD, Chang LY. Extracellular superoxide dismutase mRNA expressions in the human lung by in situ hybridization. Am J Respir Cell Mol Biol. 1997;16:162–170. doi: 10.1165/ajrcmb.16.2.9032123. [DOI] [PubMed] [Google Scholar]
- 27.Loenders B, Van Mechelen E, Nicolai S, et al. Localization of extracellular superoxide dismutase in rat lung: neutrophils and macrophages as carriers of the enzyme. Free Radic Biol Med. 1998;24:1097–1106. doi: 10.1016/s0891-5849(97)00434-6. [DOI] [PubMed] [Google Scholar]
- 28.Tan RJ, Fattman CL, Watkins SC, Oury TD. Redistribution of pulmonary EC-SOD after exposure to asbestos. J Appl Physiol. 2004;97:2006–2013. doi: 10.1152/japplphysiol.00480.2004. [DOI] [PubMed] [Google Scholar]
- 29.Oury TD, Schaefer LM, Fattman CL, et al. Depletion of pulmonary EC-SOD after exposure to hyperoxia. Am J Physiol Lung Cell Mol Physiol. 2002;283:L777–L784. doi: 10.1152/ajplung.00011.2002. [DOI] [PubMed] [Google Scholar]
- 30.Arsalane K, Dubois CM, Muanza T, et al. Transforming growth factor-beta1 is a potent inhibitor of glutathione synthesis in the lung epithelial cell line A549: transcriptional effect on the GSH rate-limiting enzyme gamma-glutamylcysteine synthetase. Am J Respir Cell Mol Biol. 1997;17:599–607. doi: 10.1165/ajrcmb.17.5.2833. [DOI] [PubMed] [Google Scholar]
- 31.Briehl MM, Gotgreave IA, Powis G. Downregulation of the antioxidant defence during glucocorticoid-mediated apoptosis. Cell Death Differ. 1995;2:41–46. [PubMed] [Google Scholar]
- 32.Lakari E, Paakko P, Pietarinen-Runtti P, Kinnula VL. Manganese superoxide dismutase and catalase are coordinately expressed in the alveolar region in chronic interstitial pneumonias and granulomatous diseases of the lung. Am J Respir Crit Care Med. 2000;161:615–621. doi: 10.1164/ajrccm.161.2.9904091. [DOI] [PubMed] [Google Scholar]
- 33.Tiitto L, Kaarteenaho-Wiik R, Sormunen R, et al. Expression of the thioredoxin system in interstitial lung disease. J Pathol. 2003;201:363–370. doi: 10.1002/path.1435. [DOI] [PubMed] [Google Scholar]
- 34.Tiitto LH, Peltoniemi MJ, Kaarteenaho-Wiik RL, et al. Cells-pecific regulation of gamma-glutamylcysteine synthetase in human interstitial lung diseases. Hum Pathol. 2004;35:832– 839. doi: 10.1016/j.humpath.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 35.Demedts M, Behr J, Buhl R, et al. IFIGENIA: effects of N-acetylcysteine (NAC) on primary end points VC and DLco. Eur Respir J. 2004;24:668s. [Google Scholar]
- 36.Zelko IN, Mariani TJ, Folz RJ. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic Biol Med. 2002;33:337–349. doi: 10.1016/s0891-5849(02)00905-x. [DOI] [PubMed] [Google Scholar]
- 37.Sandstrom J, Nilsson P, Karlsson K, Marklund SL. 10-fold increase in human plasma extracellular superoxide dismutase content caused by a mutation in heparin-binding domain. J Biol Chem. 1994;269:19163–19166. [PubMed] [Google Scholar]
- 38.Kuwano K, Nakashima N, Inoshima I, et al. Oxidative stress in lung epithelial cells from patients with idiopathic interstitial pneumonias. Eur Respir J. 2003;21:232–240. doi: 10.1183/09031936.03.00063203. [DOI] [PubMed] [Google Scholar]
- 39.Barcellos-Hoff MH, Dix TA. Redox-mediated activation of latent transforming growth factor-beta 1. Mol Endocrinol. 1996;10:1077–1083. doi: 10.1210/mend.10.9.8885242. [DOI] [PubMed] [Google Scholar]
- 40.Pesci A, Bertorelli G, Gabrielli M, Olivieri D. Mast cells in fibrotic lung disorders. Chest. 1993;103:989–996. doi: 10.1378/chest.103.4.989. [DOI] [PubMed] [Google Scholar]
- 41.Heath D, Trueman T, Sukonthamarn P. Pulmonary mast cells in mitral stenosis. Cardiovasc Res. 1969;3:467–471. doi: 10.1093/cvr/3.4.467. [DOI] [PubMed] [Google Scholar]


