Abstract
The norepinephrine (NE) transporter (NET) terminates noradrenergic signaling by clearing released NE at synapses. The activity of NET can be rapidly regulated by depolarization and receptor activation via Ca2+ and kinase/phosphatase linked pathways. The SNARE protein syntaxin 1A (SYN1A) interacts with NET and influences transporter surface trafficking and catalytic activity. In this study, we establish a link between changes in intracellular Ca2+ and SYN1A/NET interactions. SYN1A influenced NE transport only in the presence of Ca2+ in brain cortical synaptosomes. Although NET/SYN1A associations were sensitive to manipulations of Ca2+ in CHO cells, in vitro binding experiments using purified NET and SYN1A fusion proteins demonstrated a lack of direct Ca2+ sensitivity. Disruption of NET/SYN1A interaction abolished inhibition of NE transport by phorbol ester (PMA) to activate protein kinase C (PKC), but had no effect on transport inhibition by the Ca2+ calmodulin kinase (CaMK) inhibitor KN93. Furthermore, PMA enhanced Ca2+ dependent modulation of NE transport in synaptosomes. Our data reveal roles for SYN1A in the Ca2+-dependent regulation of NET, likely reliant on regulation by PKC signaling, but independent of CaMK.
Introduction
Presynaptic norepinephrine (NE) transporters (NET, SLC6A2) terminate NE signals at central and peripheral synapses through Na+ and Cl− mediated neurotransmitter reuptake (Pacholczyk et al., 1991). NET is a target of tricyclic antidepressants and NET-selective reuptake inhibitors (NSRIs) as well as multiple addictive and therapeutic psychostimulants including cocaine, amphetamine and methylphenidate (Kratochvil et al., 2003; Olver et al., 2001; Pacholczyk et al., 1991). Dysfunction of NE clearance or altered NET density has been associated with mood disorders, suicide, and cardiovascular diseases (Esler et al., 1981; Hadley et al., 1995; Klimek et al., 1997; Merlet et al., 1992). A functional polymorphism causing a dominant-negative loss of NET function has been identified in subjects with Orthostatic Intolerance (Shannon et al., 2000) whereas genetic deletion of NET in mice results in basal tachycardia, hypersensitivity to anxiety provoking stimuli, and altered response to psychostimulants (Keller et al., 2006; Xu et al., 2000).
Neuronal activation and endogenous hormones rapidly regulate NET via pathways involving intracellular second messengers, kinases and phosphatases. Chronic stress increases the plasmalemmal distribution of NET in prefrontal cortex (Miner et al., 2006). Potassium-evoked depolarization also increases NET surface density in brainstem and superior cervical ganglion (SCG) primary cultures, as monitored by surface epitope antibodies (Savchenko et al., 2003). In brainstem neurons, similar rapid increases in NET surface density and transport activity are observed with acute activation of angiotensin II G-protein coupled receptors (Lu et al., 1996; Savchenko et al., 2003), coupled to Ca2+ signaling (Gelband et al., 1998). In contrast, stimulation of muscarinic acetylcholine receptors (mAChRs) reduces NE transport and surface NET density in a protein kinase C (PKC)-dependent manner (Apparsundaram et al., 1998). In placental trophoblasts, Ca2+-independent PKCs appear to participate in phorbol ester-triggered NET internalization, regulation that coincides with NET phosphorylation and endocytosis of transporters from plasma membrane lipid rafts (Jayanthi et al., 2004). Ca2+/calmodulin protein kinases (CaMK) also influence NET as well as other neurotransmitter transporters, although mechanisms are as yet ill-defined (Apparsundaram et al., 1998; Apparsundaram et al., 2001; Gadea et al., 2002; Jayanthi et al., 2000; Turetta et al., 2002; Uchida et al., 1998; Uchikawa et al., 1995; Yura et al., 1996). Catalytic function of NET also appears to be controlled by intracellular signaling pathways. For example, noradrenergic SK-N-SH cells display insulin-triggered enhancement of NE transport without altering NET surface density, supported by Ca2+ and p38 mitogen-activated protein kinase (p38 MAPK)-dependent mechanisms (Apparsundaram et al., 2001). While evidence indicates that Ca2+ is important for the activities of NET as well as other monoamine transporters, underlying mechanisms of this regulation are not well understood.
Previously (Sung et al., 2003), we reported that syntaxin 1A (SYN1A) regulates NET through two mechanisms, one involving the delivery of NET to the plasma membrane and a second involving the inactivation of NET catalytic function. How these mechanisms are linked to neuronal activation or second messenger responses remain to be elucidated. Importantly, the NET/SYN1A interaction appears not to be constitutive but can be rapidly modulated by cellular stimuli. For example, the NET/SYN1A interaction is diminished by brief agonist stimulation of mAChRs, as well as by PKC activators or protein phosphatase 2A (PP2A) inhibitors. Given the central role of Ca2+ in evoked NE release, we investigated how extracellular and intracellular Ca2+modulates NET function and whether regulation involves changes in NET/SYN1A interactions. Here we provide evidence that SYN1A is involved in Ca2+ regulation of NET through Ca2+ dependent interactions of NET and SYN1A. This regulation engages cellular pathways common to phorbol ester-triggered NET regulation as opposed to NET regulatory pathways controlled by CaMKII. Our findings support SYN1A as an important component of the regulation of NET by Ca2+ signaling events.
Results
Syntaxin 1A in Ca2+ regulation of NE transport
In order to examine the requirements for SYN1A modulation of NET, we initially examined how SYN1A inactivation, as mediated by botulinum toxin C (BoNT/C) (Schiavo et al., 2000), alters NET activity in mouse cortical synaptosomes. In vitro treatments with toxin reduced immunoreactivity of SYN1A (Fig. 1A), consistent with proteolytic loss of the SYN1A antibody epitope in the SNARE protein’s cytoplasmic domain. Consistent with our prior study (Sung et al., 2003), BoNTC treatments also produced a significant reduction in antidepressant (desipramine)-sensitive NE transport (Fig. 1B). In further studies of NET modulation by SYN1A, we observed a requirement for extracellular Ca2+ in BoNT/C actions. Basal NE transport activity is reduced in the absence of extracellular Ca2+ but cannot be further reduced by BoNT/C whereas BoNT/C effectively reduces NE transport in the presence of Ca2+ (Fig. 1C). Conversely, Ca2+ added to Ca2+-free synaptosomes triggers an enhancement of NE transport that can be partially attenuated by BoNT/C (Fig. 1C, filled bars). These findings indicate that the loss of BoNT/C action on NET in Ca2+ free medium is not a result of deleterious actions of the Ca2+-free conditions used since a complete restoration of NE transport activity is possible. Moreover, they suggest that SYN1A supports NET activity through mechanisms that require extracellular Ca2+.
Fig. 1.
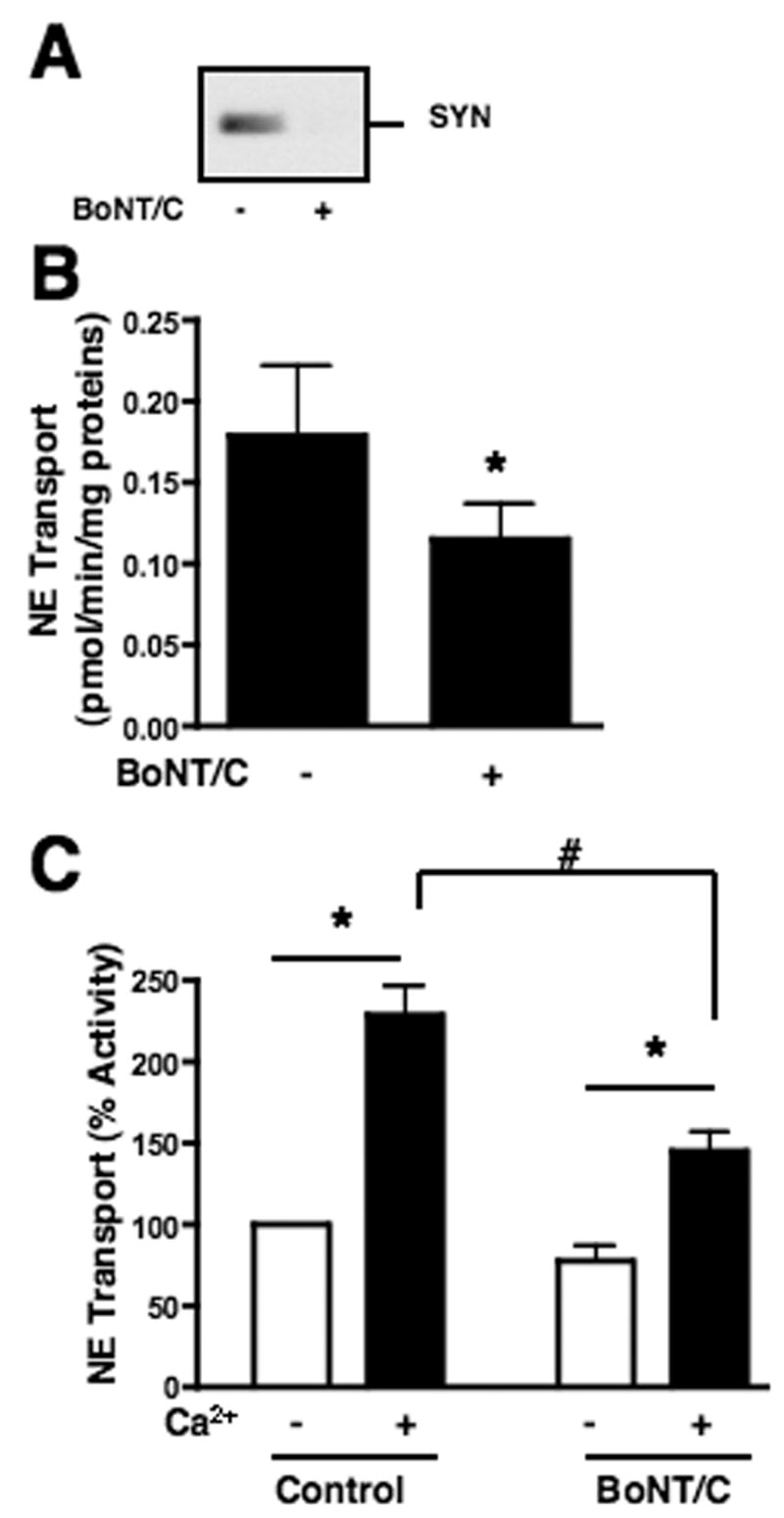
SYN1A participates in Ca2+ regulated NE transport. Synaptosomes from rat brain cortex were incubated without or with BoNT/C in HBSS as described in Materials and Methods prior to NE transport assay. Results are averages of 4 to 5 independent assays. A. Loss of SYN1A with BoNT/C treatments. Aliquots of rat cortical synaptosomes containing equal amounts of proteins were analyzed by immunoblot for SYN 1A after BoNT/C treatment. B. Treatment with BoNT/C reduced NE transport in cortical synaptosomes. Synaptosomes pre-incubated without and with BoNT/C were assessed for NE transport in KRH/Ca2+ as described in Materials and Methods. Asterisk indicates significant differences between BoNT/C-treated and untreated samples by a two-tailed Student’s t-test, P<.05. C. Effects of BoNT/C treatments on Ca2+-regulated NE transport. Synaptosomes pre-incubated without and with BoNT/C were re-suspended in KRH/EGTA and incubated at 37°C for 15 min and incubated with or without supplementation to a final concentration of 2.2 mM CaCl2 at 37°C for 10 min, followed by addition of [3H]NE for uptake assay. NE transport activities were compared to the activity of the BoNT/C-untreated synaptosomes in KRH/EGTA and analyzed by a one-way ANOVA with Newman-Keuls post hoc analysis, P<.05. Asterisk indicates significant differences between transport activities in KRH/EGTA and KRH/Ca2+, and # denotes significant differences between transport activities in KRH/Ca2+ between BoNT/C-treated and untreated samples.
Ca2+ regulates the interaction of NET and syntaxin 1A
In order to explore further how Ca2+ regulates the interaction of NET and SYN1A, we monitored functional and physical interactions of the two proteins in transiently co-transfected CHO cells. We have previously documented the utility of this expression system for demonstration of NET/SYN1A co-immunoprecipitations and regulation by mAChRs (Sung et al., 2003). In order to manipulate intracellular Ca2+ levels, we first depleted intracellular Ca2+ in KRH/EGTA (Materials and Methods) supplemented with 1 μ M thapsigargin for 10 min. The treatment of cells with thapsigargin, inhibiting SERCA (sarco/endoplasmic reticulum Ca2+ ATPase), depletes cytoplasmic Ca2+ (data not shown). Then, external Ca2+ was restored to 2.2 mM as described in the Figure Legend. This regimen has been found to achieve rapid elevations in intracellular Ca2+ levels by Ca2+ influx within physiological ranges (Fagan et al., 1996; Gailly, 1998). When Ca2+ -depleted CHO cells were supplemented with KRH/Ca2+, we monitored a rapid rise (peak in 1 min) and gradual decline of cytoplasmic Ca2+ in 10 min to the comparable level of cells in KRH/EGTA, as detected by the Ca2+ sensitive dye Fura-2 (data not shown). In parallel with this change, we observed a significant increase in antidepressant-sensitive NE transport activity upon Ca2+ supplementation (Fig. 2A), similar to our observations with cortical synaptosomes. Moreover, in co-immunoprecipitation studies, we found that intracellular Ca2+ elevations resulted in diminished recovery of NET/SYN1A complexes (Fig. 2B), observable as soon as 90 sec and up to 5 min after Ca2+ supplementation. To insure that these changes in NET/SYN1A associations were linked to cytoplasmic Ca2+ changes, we also treated cells with the Ca2+ ionophore A23187 to raise intracellular Ca2+ and with the Ca2+ chelator BAPTA/AM to reduce intracellular Ca2+ (Fig. 2C). A23187 reduced, whereas BAPTA/AM enhanced, recovery of NET/SYN1A complexes, supporting that intracellular Ca2+ negatively modulates the physical interactions between NET and SYN1A, interactions known to reduce NE transport activity (Sung et al., 2003).
Fig. 2.
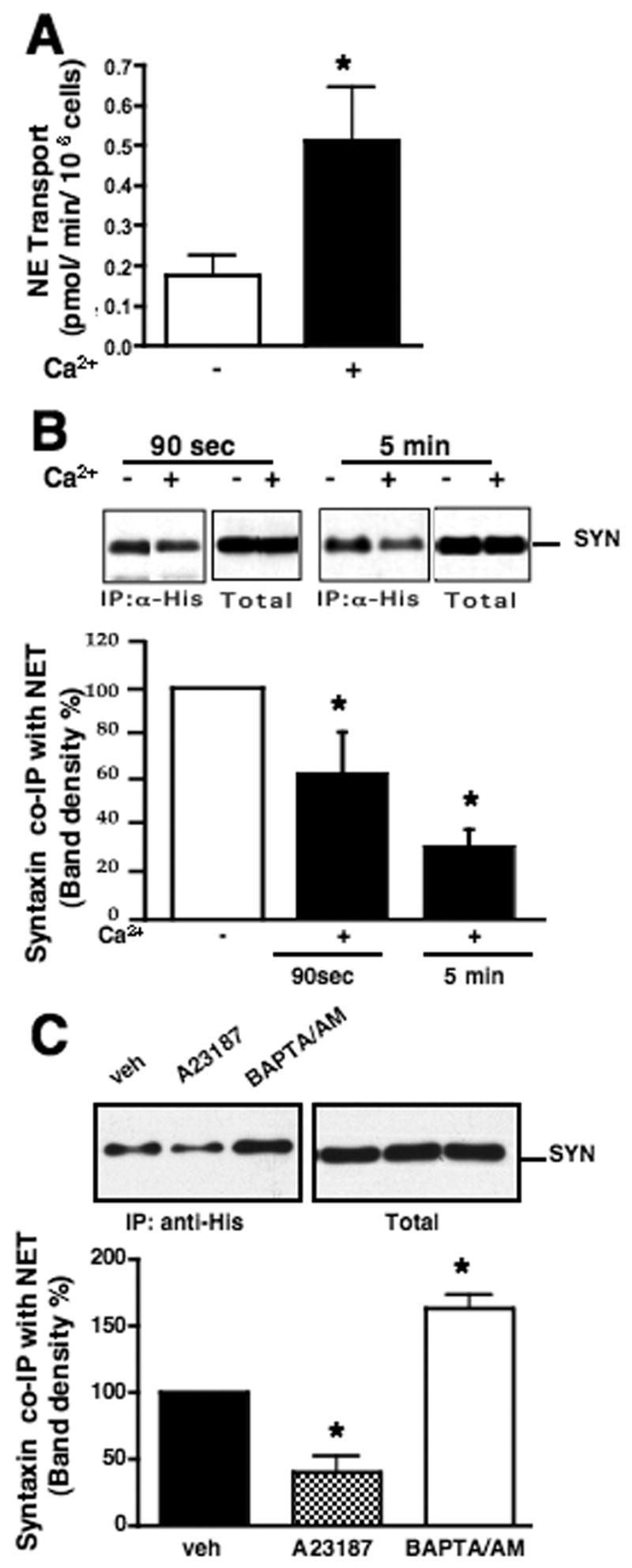
Influence of Ca2+ on NET/SYN1A complexes in transfected CHO cells. A. Ca2+ regulates NE transport in CHO cells. CHO cells, transiently transfected with NET, were pre-incubated in KRH/EGTA containing 1 μ M thapsigargin for 10 min to deplete intracellular Ca2+, replaced with KRH/EGTA or KRH/Ca2+, incubated for 5 min at 37°C, and evaluated for NE transport activity. Results are an average of 4 independent assays. Asterisk indicates significant differences between transport activities in KRH/EGTA and KRH/Ca2+ by a two-tailed Student’s t-test, P<.05. B. Increase of Ca2+ in external buffer dissociates NET/SYN1A complexes in CHO cells. CHO cells, transiently transfected with His-tagged NET and SYN1A cDNAs, were incubated in KRH/EGTA containing 1 μ M thapsigargin for 10 min at 37º C, replaced with KRH/EGTA or KRH/Ca2+, and incubated at RT for 90 sec or at 37º C 5 min prior to lysis of cells. NET was immunoprecipitated with anti-histidine, and analyzed by SDS/PAGE/immunoblotting with anti-SYN1A. Aliquots of total cell lysates show equal amounts of SYN1A expression. Lower panel: Bands of SYN1A co-immunoprecipitated with NET were quantitated. Averages of syntaxin band densities were obtained from 4 independent experiments. Data were compared to the SYN1A band density in KRH/EGTA. Asterisks indicate significant differences between NET/SYN1A complexes in KRH/EGTA and KRH/Ca2+ by a one-way ANOVA with Newman-Keuls post hoc analysis, P<.05. C. The Ca2+ ionophore A23187 and the Ca2+ chelator BAPTA/AM modulate NET/SYN1A complexes. CHO cells, transiently transfected with His-tagged NET and SYN1A cDNAs, were incubated in KRH/EGTA for 1 hour, replaced with KRH/Ca2+ with vehicle (veh), KRH/Ca2+ with 10 μ M ionophore A23187, or KRH/EGTA containing 30 μ M BAPTA/AM, and further incubated for 30 min at 37ºC. NET was immunoprecipitated with anti-histidine and analyzed by SDS/PAGE/immunoblotting with anti-SYN1A. Aliquots of total lysates show equal amounts of syntaxin 1A expression in each condition. The Ionophore A23197 dissociates and the chelator BAPTA/AM increased NET/SYN1A complexes. Averages of SYN1A band densities were obtained from 3 independent experiments. Data were compared to the SYN1A band density in KRH/Ca2+ with vehicle. Asterisks indicate significant differences between NET/SYN1A complexes in media treated with vehicle and drugs by a one-way ANOVA with Newman-Keuls post hoc analysis, P<.05.
The impact of intracellular Ca2+ regulation on NET/SYN1A complex stability could reflect a direct action of Ca2+ on interactions between the transporter and SYN1A. The NH2-terminal cytoplasmic domain of NET binds directly to the cytoplasmic domain of SYN1A in vitro whereas a 2–42 deletion of the hNET NH2 terminus eliminates SYN 1A binding in NET/SYN1A transfected cells (Sung et al., 2003). Therefore, we tested whether Ca2+ modulates directly NET/SYN1A interactions by monitoring in vitro the Ca2+ dependence of associations between a purified MBP-hNET NH2-terminal fusion (MBP-NET-N) and a purified GST-SYN1A cytoplasmic domain fusion (GST-SYNΔ TM). Across a wide range of Ca2+ concentrations that encompass the physiological range of Ca2+ experienced by neurons (Fig. 3), no impact of Ca2+ on recovery of interactions between fusion proteins of MBP-NET-N and GST-SYNΔ TM was observed. These data indicate that the Ca2+ dependence of NET/SYN1A complexes likely arises from participation of additional Ca2+ -sensitive cellular molecules.
Fig. 3.
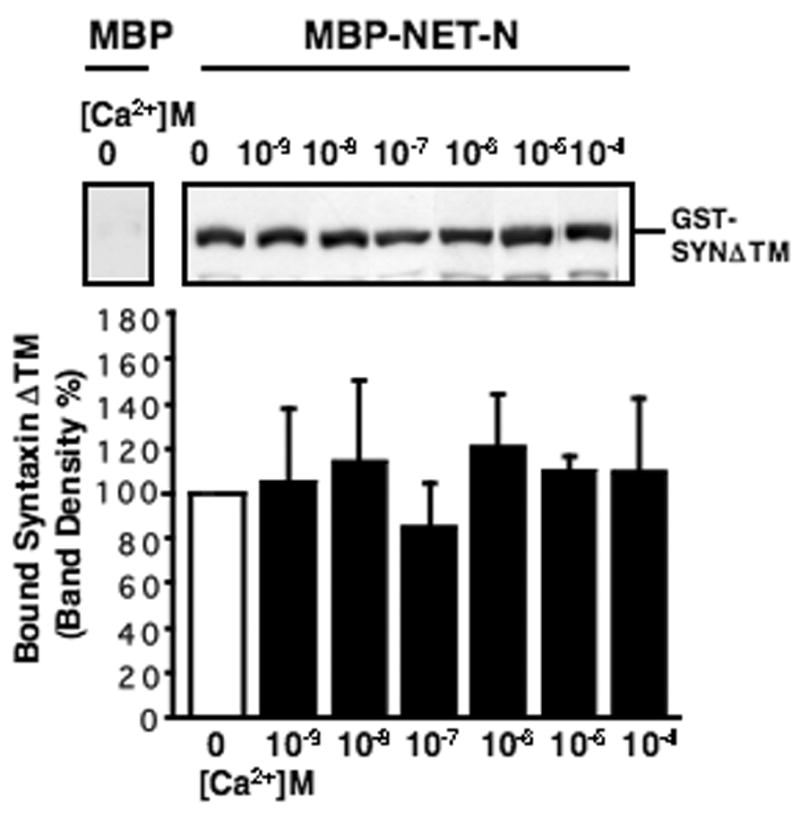
Ca2+ does not directly influence protein-protein interactions between fusion proteins of MBP-NET-N and GST-SYNΔ TM. Purified fusion protein MBP or MBP-NET-N bound to amylose resins were incubated with GST- SYNΔ TM in PBS, 0.2 mM EGTA at different concentrations of CaCl2 at 4 ° C for 1 hour as described in Materials and Methods. Bound GST-SYNΔ TM to MBP or MBP-NET-N was analyzed by immunoblotting with anti-SYN1A. Top panel: Immunoblot with anti-SYN1A shows bound GST-SYNΔ TM to MBP or MBP-NET-N. Lower panel shows averages of quantitated GST-SYNΔ TM bands recovered from 3 independent experiments.
Influences of PKC and CaMK on the interaction of NET and SYN1A
PKC and CaMKs can be activated by Ca2+ and are suggested to regulate NET in cell models (Apparsundaram et al., 1998; Sung et al., 2003; Uchida et al., 1998) and are reportedly altered in mood disorder or by psychotropic drugs influenced by NE signaling (Celano et al., 2003; Manji and Lenox, 1999). We examined an influence of these pathways on NET/SYN1A interactions by monitoring whether NET downregulation in cortical synaptosomes, as triggered by phorbol ester (β -PMA) or a CaMKII inhibitor (KN93), is influenced by BoNT/C preincubation (Fig. 4A). Consistent with prior studies (Sung et al., 2003), BoNT/C preincubation abolished the inhibition of NE transport by PMA. In contrast, inhibition of NE transport by KN93 was not influenced by toxin pre-treatment. We obtained similar results with PMA and KN93 using transiently transfected CHO cells and in this model also found that whereas PMA effects on NE transport were lost in the NETΔ 2-42 mutant, KN93 inhibited NE uptake in both wildtype and mutant NET transfected cells equivalently (Fig. 4B). Consistent with these data, KN93 failed to impact interactions of NET/SYN1A complexes in co-transfected CHO cells whereas PMA reduced NET/SYN1A complexes (Fig. 4C) without impacting total SYN1A levels. These findings reveal that suppression of NET activity, as mediated by PKC activation and CaMKII inhibition, can be differentiated by SYN1A dependence.
Fig. 4.
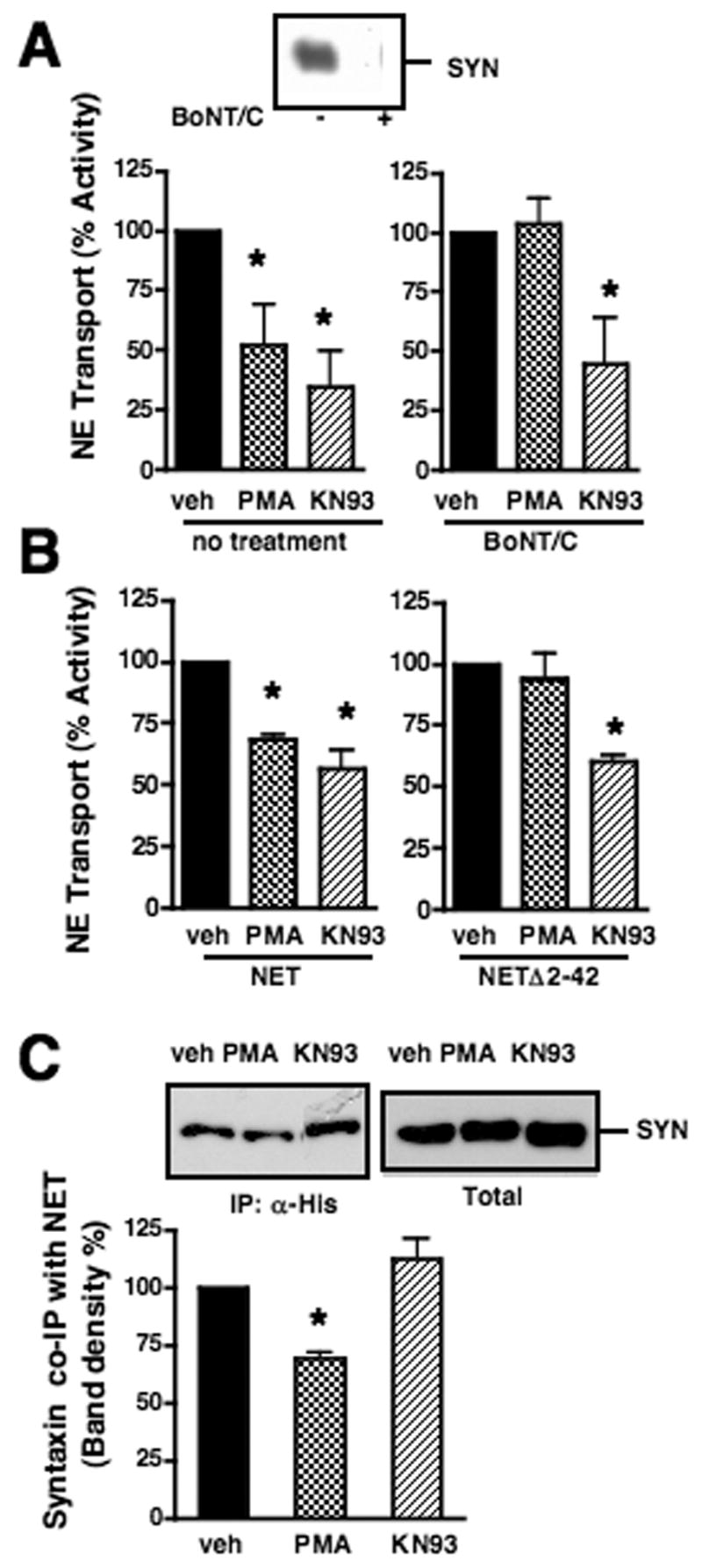
Influence of PMA and KN93 on the interaction of NET/SYN1A. A. BoNT/C pretreatment attenuates PMA-inhibition of NE transport in rat cortical synaptosomes, but did not influence KN93-inhibition of NE transport. Synaptosomes, pre-incubated without (Left panel) or with BoNT/C (Right panel), were incubated with vehicle, 1 μ M PMA or 5 μ M KN93 in KRH/Ca2+ for 30 min at 37ºC prior to the addition of [3H]NE for uptake assay. Data represent mean of 6 independent experiments. NE transport activities were compared to the activity of the synaptosomes treated with vehicle. Asterisks indicate significant differences between NE transport activities between synaptosomes treated with vehcle and drugs by a one-way ANOVA with Newman-Keuls post hoc analysis, P<.05. Top panel shows reduction of immunoreactivity of SYN1A in synaptosomes treated with BoNT/C. B. Deletion of SYN1A binding site in NET abolishes PMA-inhibition, but does not limit KN93-inhibition of NE transport in CHO cells. CHO cells were transiently transfected with SYN1A and either His-tagged NET or His-tagged NET Δ 2-42. Cells were incubated with 1 μ M PMA or 5 μ M KN93 in KRH/Ca2+ for 30 min at 37ºC prior to the addition of [3H]NE for NE transport assay. Data represent mean of 3 independent experiments. NE transport activities were compared to the activity of the cells incubated with vehicle. Asterisks indicate significant differences between NE transport activities between cells treated with vehicle and drugs by a one-way ANOVA with Newman-Keuls post hoc analysis, P<.05. C. Sensitivity of NET/SYN1A interactions to PMA and KN93. CHO cells, transfected with His-hNET and SYN1A, were incubated with vehicle, 1 μ M PMA or 5 μ M KN93 for 30 min at 37ºC prior to lysis of cells. NET was immunoprecipitated with anti-histidine and analyzed by SDS/PAGE/immunoblotting with anti-SYN1A. Aliquots of total lysates show equal expression of SYN1A in each experiment. Lower panel indicates averages of co-immunoprecipitated SYN1A bands from 4 (PMA) and 13 (KN93) independent experiments. Asterisk indicates significant differences between NET/SYN1A complexes in media treated with vehicle and drugs by a one-way ANOVA with Newman-Keuls post hoc analysis, P<.05.
PMA influences Ca2+ dependent NE transport
Having evidence that NET activity is both SYN1A and Ca2+ dependent and that PMA triggered inhibition requires SYN1A, we next monitored the impact of Ca2+on phorbol ester-inhibition of NE transport in cortical synaptosomes. As previously reported in placental cells (Jayanthi et al., 2004), we found NE transport inhibition by PMA treatment of synaptosomes to be insensitive to the status of extracellular Ca2+ (Fig. 5A). These findings suggest the involvement of Ca2+-insensitive PKCs in SYN1A-dependent NET modulation by PMA. When Ca2+ was added to synaptosomes incubated in KRH/EGTA, NE transport was recovered (Fig. 5B, left). Interestingly, the synaptosomes supplemented with Ca2+ after Ca2+-depletion did not exhibit PMA-inhibition of NE transport. Pre-incubation of the synaptosomes with PMA even slightly facilitated this recovery process (Fig. 5B, left). When we normalized the Ca2+-elevation of NE transport compared to the activity prior to Ca2+ supplementation (Fig. 5B), synaptosomes treated with vehicle recovered NE transport to 242.8 +/− 23.4 % whereas synaptosomes pre-incubated with PMA revealed a greater enhancement, reaching 374 +/−20.9 % (Fig. 5B, right).
Fig. 5.
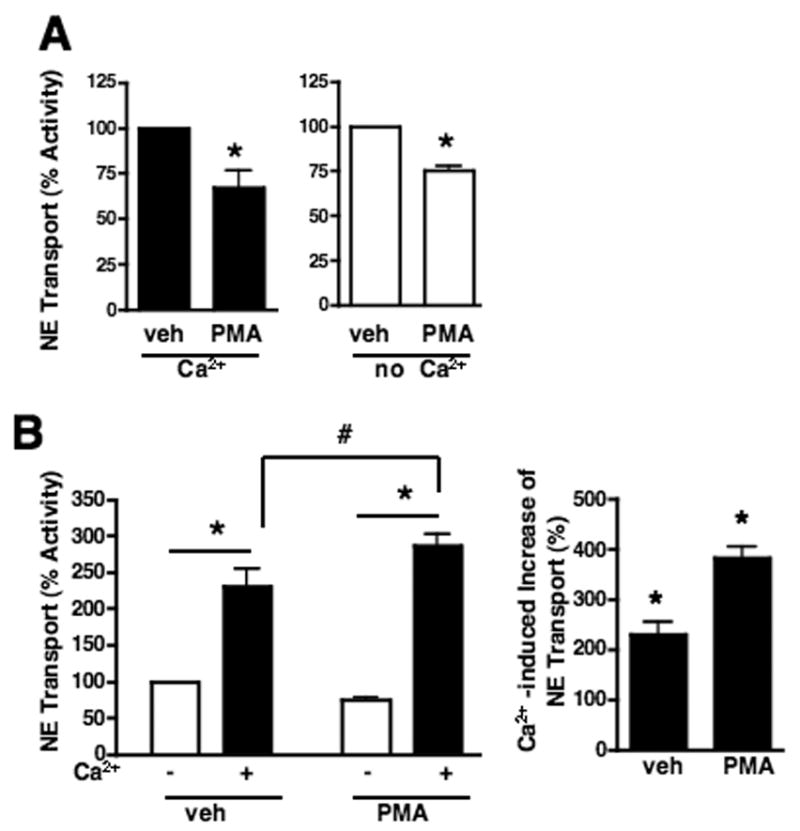
PMA influences Ca2+ recovery of NE transport in mouse cortical synaptosomes. Synaptosomes were incubated with vehicle or 1 μ M PMA in KRH/Ca2+ for 15 min at 37°C. Data are averages of 5 independent experiments. A. Synaptosomes were centrifuged and resuspended in KRH/Ca2+ (Left panel) or KRH/EGTA (right panel), and incubated at 37ºC for 5min prior to the addition of [3H]NE for NE transport assay. PMA inhibits NE transport in the presence as well as in the absence of Ca2+. Asterisks indicate significant differences between samples treated with vehicle or PMA by a two-tailed Student’s t-test, P<.05. B. PMA potentiates Ca2+-enhancement of NE transport. Synaptosomes pre-incubated with PMA in KRH/Ca2+ as described above were centrifuged and re-suspended in KRH/EGTA to wash Ca2+ out. The synaptosomes in KRH/EGTA were divided into 2 groups, one group in KRH/EGTA and the other group supplemented with Ca2+ to final concentration of 2.2 mM (ie., KRH/Ca2+). Synaptosomes in KRH/EGTA and KRH/Ca2+ were incubated at 37ºC for 5min prior to addition of [3H]NE for NE transport assay. Left Panel: NE transport activities were compared to the activity in KRH/EGTA of the vehicle-treated synaptosomes and analyzed by a one-way ANOVA with Newman-Keuls post hoc analysis. Asterisks indicate significant differences between transport activities in KRH/EGTA and KRH/Ca2+ and # denotes significant differences between transport activities in KRH/Ca2+ between samples treated with vehicle and PMA, P<.05. Right Panel: In the left panel experiments, NE transport in KRH/Ca2+ was normalized to the NE transport in KRH/EGTA under each condition. Asterisk indicates significant differences between transport activities in KRH/EGTA (100%) and KRH/Ca2+ under each condition.
PMA influences Ca2+ dependent surface trafficking of NET
SYN1A supports the surface trafficking of NET as revealed by both co-transfection and BoNT/C studies (Sung et al., 2003). Since BoNT/C treatment blunts the increase in NE transport provided by elevations in extracellular Ca2+ as well as the inhibition of transport triggered by phorbol esters, we sought to determine whether Ca2+-dependent SYN1A support for NET activity arises from SNARE-mediated NET surface trafficking. Because NET abundance in native tissues is too low for reliable quantitation using available antibodies, we monitored the influence of extracellular Ca2+ on surface trafficking of HA-tagged NET expressed in CHO cells. In this model, we found that Ca2+ manipulations can bidirectionally influence NET surface expression (Fig. 6). In CHO cells, we observed that Ca2+ depletion reduces (Fig. 6A) and Ca2+ supplementation increases (Fig. 6B) NET surface density as assessed in surface biotinylation. In parallel, we monitored multiple additional surface proteins in response and found no consistent response indicative of nonspecific alterations in surface proteins (data not shown). These findings suggest that our prior observations of Ca2+ stimulated NE uptake likely arise at least in part through elevations in NET surface density. Additionally, we uncovered sensitivities of Ca2+ induced NET trafficking to coincident PMA stimulation. Thus, pre-incubation of cells with PMA, which reduces recovery of NET/SYN1A complexes, inhibited the ability of Ca2+ depletion to reduce NET surface levels (Fig. 6A). PMA also blocked the ability of restored extracellular Ca2+ to increase NET surface density in CHO cells (Fig. 6B) whereas the same conditions display augmented NE transport activity in synaptosomes (Fig. 5B). Bisindolylmalemide I (BIM) did not influence Ca2+-induced surface trafficking of NET (Fig. 6B). These findings suggest that Ca2+ triggered changes in NET surface abundance, while in part reliant on SYN1A, arise through vesicle fusion regulatory mechanisms that are distinct from those supporting the phorbol ester regulation of NET/SYN1A complexes.
Fig. 6.
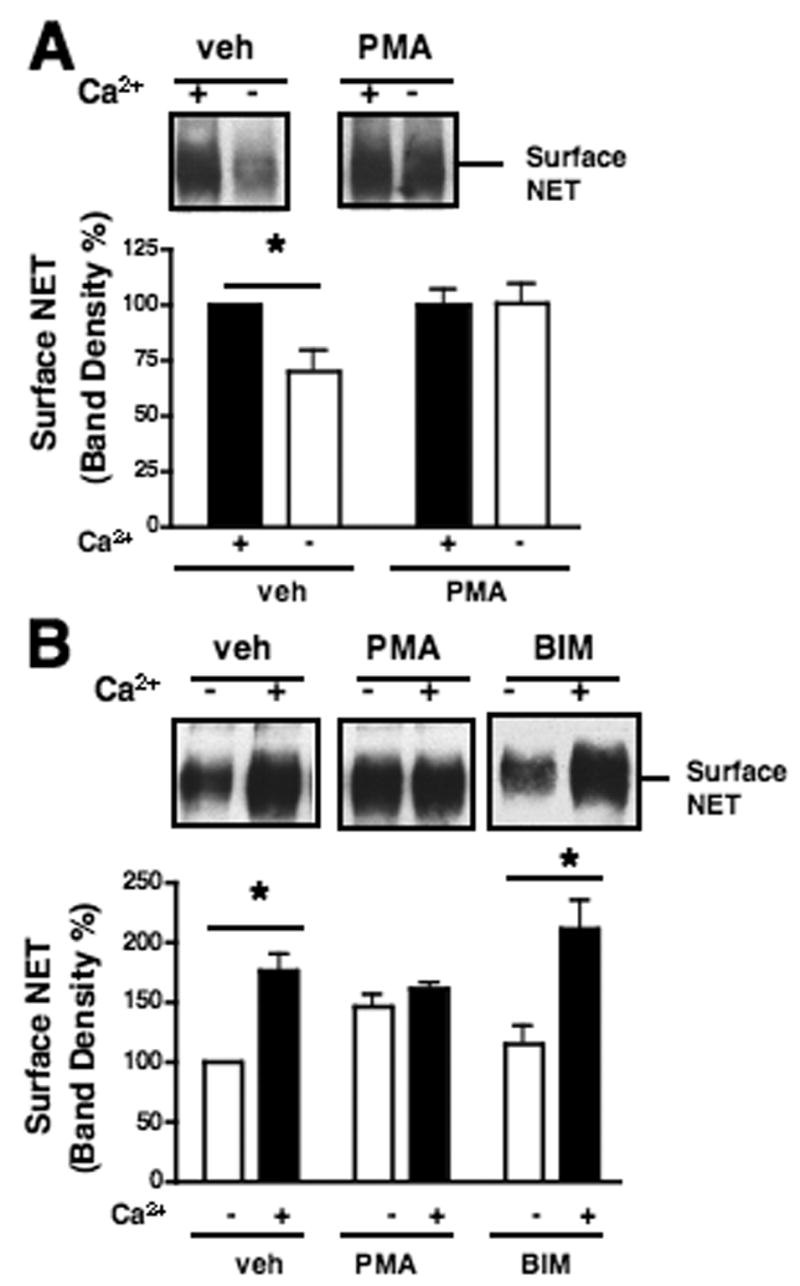
Ca2+ dependent surface trafficking of NET in CHO cells. CHO cells were transiently transfected with HA-tagged NET. Cells were incubated with vehicle, PMA at 1 μ M, or BIM at 0.1 μ M in complete media for 30 min at 37°C. A. Ca2+ depletion diminished NET at the surface in a PMS sensitive manner. Cell culture medium was replaced with complete media containing either vehicle or 10 mM EGTA/ 30 μ M BAPTA/AM and incubated for 10 min prior to surface biotinylation. A representative immunoblot of surface NET is shown. Band densities are averages from 4 independent experiments. Data were compared to the NET band density at the surface of the cells in media treated with vehicle. Asterisk indicates significant differences of surface NET between cells in media maintained in the presence of Ca2+ and cells subjected to Ca2+ depletion by a one-way ANOVA with Newman-Keuls post hoc analysis, P<.05. B. Ca2+ recovery of surface NET was sensitive to PMA, but insensitive to BIM. Cells were incubated in KRH/EGTA and 1 μ M thapsigargin at 37°C for 10 min, replaced with KRH/Ca2+ or KRH/EGTA, and incubated at RT for 1 min prior to biotinylation. PMA blocked Ca2+ triggered increase of surface NET. Pre-incubation of cells with BIM has no effect on Ca2+ triggered surface trafficking of NET. A representative immunoblot of surface NET is shown. Band densities are averages from 3 independent experiments. Data were compared to the NET band density at the surface of the cells in KRH/EGTA. Asterisks indicate significant differences between NET at the surface of the cells in KRH/EGTA and KRH/Ca2+ by a one-way ANOVA with Newman-Keuls post hoc analysis, P<.05.
Discussion
Multiple neurotransmitter transporters in the SLC6 family, including the GAT1 GABA transporter, NET, the serotonin and dopamine transporters, are known to form complexes with the SNARE protein SYN1A (Beckman et al., 1998; Haase et al., 2001; Lee et al., 2004; Quick, 2003; Sung et al., 2003). Proposed roles for these associations include coordination of transporter surface expression, modulation of transporter catalytic rates and modulation of transporter ion/substrate permeation. The control of these associations by physiologically relevant stimuli and cell signaling is likely critical for achieving transporter regulation coordinated with changes in neuronal excitability and neurotransmitter release. Previously we demonstrated that SYN1A associations influence the activity and surface trafficking of NET, interactions negatively modulated by PMA, PKC-linked mAChRs, and PP2A inhibition (Sung et al., 2003). As neuronal depolarization and receptor-triggered signal transduction cascades are linked to changes in intracellular Ca2+ homeostasis, we questioned whether Ca2+ regulation of NE transport involves SYN1A, whether interactions of NET and SYN1A are sensitive to Ca2+ dynamics, and whether Ca2+-sensitivity of NET/SYN1A interaction is supported by other signaling proteins.
As in our prior study (Sung et al., 2003), BoNT/C cleavage of endogenous SYN1A in mouse brain synaptosomes, like suppression of SYN1A expression using SYN1A antisense-oligonucleotides in neuroblastoma cells, produces a reduction of NE transport, consistent with a requirement for SYN1A to support transporter surface expression and/or intrinsic transport activity in vivo. In this study, we found that BoNT/C treatments of synaptosomes reduced the ability of external Ca2+ (added to synaptosomes in Ca2+ free buffer) to recover NE transport (Fig. 1). These findings are consistent with, at least in part, a SYN1A-mediated fusion of transporter vesicles underlying Ca2+ dependent elevation of NET surface expression. In transfected cells, where we could both validate an intracellular Ca2+ elevation and monitor transporter level at the plasmamembrane by surface biotinylation, we verified a rapid and reversible increase of NET protein at the cell surface with Ca2+ supplementation (Fig. 6). BoNT/C-treated synaptosomes could still increase NE transport following Ca2+ supplementation, though to a lesser degree. However, the fraction of SYN1A in noradrenergic terminals in our preparation may exhibit greater resistance to the toxin than the bulk of synaptic terminals, dominated by glutamatergic and GABAergic elements. We also recognize that our biochemical measurements are much slower than the time-scale associated with vesicular fusion. Depolarization-mediated vesicle fusion/neurotransmitter release occurs in hundreds of microseconds to a few milliseconds after membrane stimulation (Sabatini and Regehr, 1999). In the case of glycine transporters (GLYT), depolarization evoked surface trafficking could only be detected at low temperature (25ºC) and only within 1 sec (Geerlings et al., 2001). In order to provide for a rise in Ca2+concentrations on a time scale more compatible with our biochemical studies, we chose to manipulate external Ca2+ rather than evoke changes directly in membrane potential, a paradigm also compatible with studies in heterologous models that lack excitation-secretion coupling to support NET vesicle fusion. Under this condition, we were able to achieve reliable changes in NET activity and surface expression that could then be correlated with changes in NET/SYN1A complexes.
We showed that Ca2+, manipulated by external Ca2 supplementation as well as reagents such as the Ca2+ionophore A23187 and the chelator BAPTA/AM to disturb intracellular Ca2+, modulates NET/SYN1A associations (Fig. 2). Ca2+ is known to trigger SNARE complex formation between SYN1A, VAMP, and SNAP-25 for membrane fusion (Chen et al., 1999) and directly influences SYN1A interactions with other presynaptic proteins such as synaptotagmin, syncollin, and Ca2+ channels (Chapman et al., 1995; Edwardson et al., 1997; Sheng et al., 1996). Our in vitro studies suggest, however, that over a wide range of concentrations spanning physiological dynamics of presynaptic Ca2+, the cation does not appear to directly influence NET/SYN1A physical associations (Fig. 3). We speculate therefore that additional signaling molecules provided by an intact cellular milieu mediate Ca2+-dependent NET/SYN1A associations.
In initial attempts to identify signaling proteins responsible for Ca2+dependent NET/SYN1A interactions, we focused on PKC and CaMK (Fig. 4) as these kinases are known to influence the activity/trafficking of monoamine transporters as well as interactions of SYN1A with other presynaptic proteins including Munc18 and Ca2+ channels (Fujita et al., 1996; Yokoyama et al., 1997). SYN1A is required for PMA-mediated inhibition of GAT1 GABA transporters in primary hippocampal neurons (Beckman et al., 1998). As reported previously (Sung et al., 2003), BoNT/C abolished inhibition of NE transport by PMA. In contrast, inhibition of NE transport by KN93 was unaffected by BoNT/C (Fig. 4A). Additionally, in transfected CHO cells, PMA fails to inhibit NE transport by a NET mutant defective in SYN1A binding, whereas KN93 still inhibited transport (Fig. 4B). NET/SYN1A complexes were destabilized by PMA, but not by KN93 (Fig. 4C). Possibly, Ca2+ triggers fusion of NET-containing vesicles (or enhances vesicle recycling) and, in parallel, through PKC, NET/SYN1A complexes are destabilized to relieve SYN1A-mediated inhibition of NE transport (Fig. 7). This model is consistent with the partial attenuation of NET activity by BoNT/C cleavage of SYN1A if one assumes functional inhibition in surface NET/SYN1A complexes (Fig. 1). Although NET is known to be phosphorylated at T258 in response to PMA treatments, an event to link to internalization (Jayanthi et al., 2006), it is not clear yet whether Ca2+-dependent NET trafficking or dissociation of the NET/SYN1A complex observed in this study involves NET phosphorylation at the same site. Ca2+ dependent phosphorylation of SYN1A at S188 by the Death-associated protein kinase, a Ca2+/calmodulin-dependent serine/threonine protein kinase, is known to regulate SYN1A interactions with Munc18 (Tian et al., 2003) and R151 in SYN1A has been reported to be important for Ca2+-sensitive interactions with CaMKII (Ohyama et al., 2002). However, our mutations of SYN1A at these residues failed to block Ca2+ dependent interactions of SYN1A with NET in CHO cells (data not shown), suggesting that targets of PKC are likely either NET itself or another transporter-associated protein.
Fig. 7.
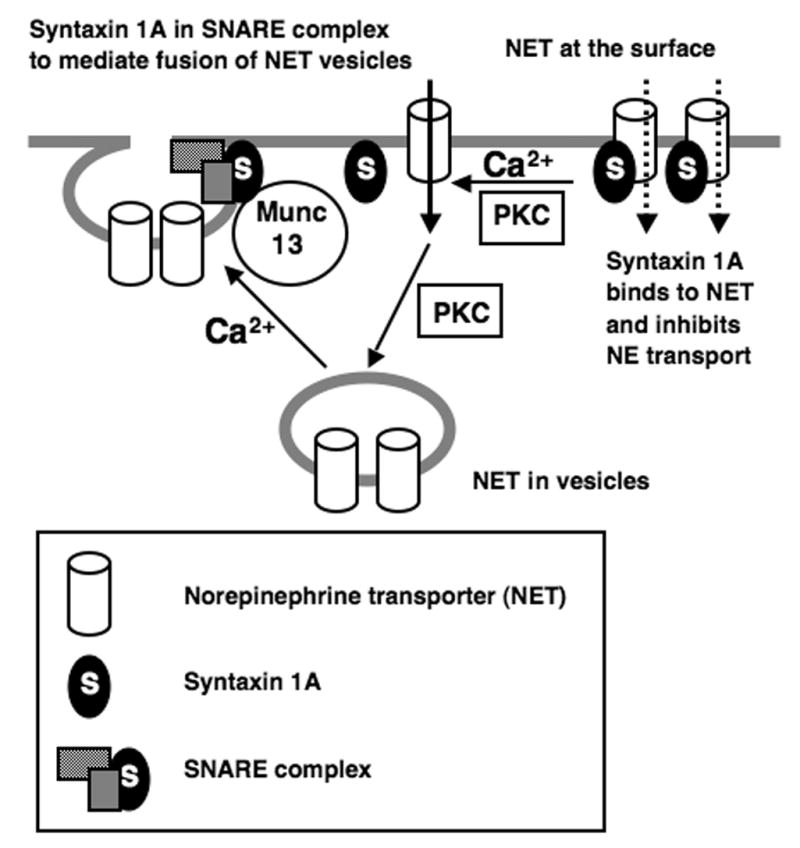
Ca2+ regulation of NET involves both SYN1A/NET interactions and NET trafficking. NET trafficking to the plasma membrane is triggered by alterations in intracellular Ca2+, mediated by SYN1A in SNARE complexes. Separately, NET and SYN1A form complexes that can be destabilized by PMA modulating NET surface activity. PMA, possibly by activating PKC, Munc-13 or other C1 domain proteins, can also modulate Ca2+ triggered NET surface trafficking.
Although PMA treatments down-regulate NET activity in synaptosomes and trigger internalization of NET proteins in cells, we found that PMA can potentiate Ca2+ triggered increases of NE transport in synaptosomes (Fig. 5), the process attenuated by BoNT/C (Fig. 1). As we cannot presently measure surface levels of NET in synaptosomes to validate that this functional change is a result of enhanced NET trafficking, we paralleled our synaptosome studies with Ca2+ manipulations of transfected cells. Interestingly, whereas Ca2+ supplementation elevates NET surface expression in transfected cells, PMA pretreatments blocked this effect (Fig. 6). Given evidence that PKC does not influence intracellular Ca2+ level in CHO cells in our experimental format (Gailly, 1998; Parekh and Putney, 2005), our observation that PKC inhibits Ca2+ dependent surface trafficking of NET supports direct actions by PKC, rather than an indirect effect by altering intracellular Ca2+ dynamics. The different observation of NET regulation by PMA in synaptosomes and in CHO cells may be explained by several mechanisms. First, the PKC and SYN1A dependence of NET function may involve two distinct mechanisms, a Ca2+-dependent trafficking of NET proteins and a Ca2+-dependent SYN1A interaction/upregulation of NET catalytic activity. Second, in CHO cells, the predominant t-SNARE is Syntaxin 4 (SYN4) which appears to be distinct from SYN1A in the interaction with other proteins such as Munc18 (Bennett et al., 1993; Hata and Sudhof, 1995; Min et al., 1999). Unlike SYN1A, SYN4 does not form physical complexes with NET (data not shown). The difference in the interactions of syntaxin isoforms with NET or other regulatory proteins may underlie differential PMA regulation in the two experimental systems. Third, differences in synaptosomes and transfected cells are likely with respect to the composition of PKC isoforms. The lack of sensitivity of NET trafficking by BIM can be interpreted to support the involvement of alternative isoforms of PKC or contributions from other non-PKC targets of PMA. In addition to PKC family members, several groups of proteins are known to contain C1 domain that binds phorbol esters (Kazanietz, 2002). In particular, Munc13 binds to SYN1A and is responsible for phorbol ester mediated enhancements in neurotransmission (Betz et al., 1998). Munc13 is expressed mainly in brain and plays a critical role in neurotransmitter release through stabilizing SYN1A open complexes that support vesicular fusion but is also known to be expressed in several non-neuronal cells such as chromaffin cells, pancreatic β cells, renal cells, and lymphocytes (Ashery et al., 2000; Brose et al., 1995; Feldmann et al., 2003; Song et al., 1998; Xia et al., 2004). The degree to which Munc13 supports the PMA-induced modulation of NET/SYN1A complexes and/or NET surface trafficking deserves further study.
In summary, we establish parallels between NET trafficking and Ca2+ enhanced transporter activity and describe a sensitivity of NET/SYN1A interactions to cellular Ca2+ levels. As illustrated in Figure 7, SYN1A likely supports Ca2+ dependent NET regulation by serving as the critical SNARE for fusion of NET vesicles. Under physiological conditions, Ca2+ elevations would likely trigger both NET insertion and a loss of inhibitory NET/SYN1A complexes. Removal of Ca2+ reduces NET surface expression. PMA can also reduce NET activity but through a Ca2+-independent trafficking mechanism. Under conditions favoring NET endocytosis, PMA-induced catalytic activation is not evident or is overwhelmed in the context of PMA-induced NET endocytosis. However, PMA-induced destabilization of NET/SYN1A complexes can synergize with elevated Ca2+-triggered transporter trafficking. This model suggests that PKC-coupled G-protein coupled receptors can either positively or negatively modulate NET activity depending on coincident Ca2+ dynamics.
Experimental Methods
Antibodies, reagents, and cDNA constructs
Monoclonal anti-histidine antibody (Clonetech, Palo Alto, CA) was used at 1 μg for immunoprecipitation of His-tagged hNET. Anti-syntaxin (SYN1A) antibody (HPC-1, Sigma, St. Louis, MO) was used at a dilution of 1:2,000 for immunoblotting. Phorbol 12-myristate 13-acetate (PMA) was obtained from Calbiochem (San Diego, CA), Alexis (Carlsbad, CA) or Sigma. Bisindolylmalemide I (BIM) was purchased from Calbiochem. Ca2+ ionophore A23187, BAPTA/AM, Thapsigargin and KN-93 were obtained from Alexis. All other chemicals were obtained from Sigma. Untagged, HA, or His-tagged hNETs, and a deletion mutant of His-tagged hNET (NETΔ 2-42) in pCDNA3, SYN1A in pCMV5, GST-SYNΔ TM (glutathione S transferase-SYN1A bearing a deletion of the transmembrane domain) in pGEX5X-1 (Pharmacia, Piscataway, NJ), and MBP-NET-N (maltose binding protein fused to the amino terminal residues 1-63 of NET) in pMAL cRI (New England Biolabs, Beverly, MA) have been previously described (Sung et al., 2003). All mutations were performed using the Quick Change site-directed mutagenesis kit (Stratagene, La Jolla, CA) and verified by dideoxynucleotide sequencing (Vanderbilt University Neurogenomics Core). Rats (Sprague Dawley) and mice (C57BL/6) were obtained from Harlan (Indianapolis, IN). All tissue harvests were performed in accordance with approved animal care protocols as monitored by the Vanderbilt Institutional Animal Care and Use Committee.
Cell culture, transfection, preparation of cortex synaptosomes
CHO cells were maintained in DMEM/10 % FBS /L-Glu/pen/strep. Cells were plated on poly-D-lysine (Chemicon, Temecula, CA) coated plates for each experiment. Transfection was performed using Fugene 6 (Roche, Indianapolis, IN) or TransIT-LT1 transfection reagent (Mirus, Madison, WI) at 3:1 reagent : DNA ratios. Cells were incubated after transfection for 24 to 48 hr before each experiment. Transfection of NET and SYN1A has been described previously (Sung et al., 2003). Synaptosomes were prepared by homogenizing dissected cortex in 10 mM HEPES, 0.32 M Sucrose, pH 7.4, using a Teflon pestle/homogenizer (Wheaton Instruments, Milville, NJ). Homogenates were centrifuged at 1,000g, 5 min at 4 ° C, with the supernatants re-centrifuged for 20 min at 16,000g at 4 ° C to obtain synaptosomal pellets. Protein assay was done using Coomassie Plus reagent (Pierce, Rockford, IL).
Transport assays, assay buffers, BoNT/C treatments, data analysis
NE transport assays were performed on cortical synaptosomes (50–100 μ g protein/assay reaction) or cells as described previously (Sung et al., 2003). All transport assays were performed using [3H]NE (1-[7,8-3H] noradrenaline, Amersham Pharmacia, Piscataway, NJ) at 50 nM final concentration, 37° C for 10 min in triplicate. Nonspecific uptake was defined using 1 μM desipramine. Uptake assays utilized KRH/Ca2+ (pH 7.4 120 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 10 mM HEPES, 1.2 mM MgSO4, 2.2 mM CaCl2, 1.8 mg/ml glucose) or KRH/EGTA (KRH without CaCl2 and with 0.2 mM ethylene glycol-bis (β -aminoethylether)-N,N,N',N'-tetraacetic acid tetra-sodium salt (EGTA)) supplemented with 0.1 mM pargyline, 1 mM tropolone, and 0.1 mM ascorbic acid. For the experiments with botulinum toxin C (BoNT/C, Calbiochem or Sigma), cortical synaptosomes from rats were incubated with the toxin at 100 nM in HBSS (Hanks’ balanced salt solution, Gibco BRL, Rockville, MD) for 30 min to 1 hr at 37°C, followed by centrifugation for 5 min at 20,000g at 4°C prior to uptake assay to remove the toxin. For experiments with drugs, synaptosomes or cells were incubated with either vehicle (DMSO: dimethylsulfoxide), β-PMA (1 μM) or KN93 (5 μM) in KRH/Ca2+ at 37°C prior to assays. All data were evaluated using a two-tailed Student’s t-test for two group comparisons or using a one-way ANOVA with Newman-Keuls post hoc analysis for multigroup comparisons, with p<0.05 taken as significant. GraphPad Prism version 4.0a (GraphPad Software, San Diego, CA) was used for data analysis.
Biochemical analysis
GST-SYNΔ TM, MBP, and MBP-NET-N fusion proteins were expressed in E.coli BL21pLysS (Gibco BRL) and induced with 1 mM IPTG for 4 hr. Fusion proteins were purified using glutathione beads (Amersham Pharmacia, Uppsala, Sweden) or amylose resin (New England Biolabs) according to manufacturer’s instructions. All protein analyses utilized 10% SDS-PAGE. In vitro pull-down experiments examining NET/SYN1A interactions were conducted using purified fusion proteins. Briefly, MBP or MBP-NET-N fusion proteins were captured on 15 μL of amylose resin. Protein-coated beads were incubated with GST-SYNΔ TM proteins at 0.5 μ M in PBS /1% TRITON X-100, containing 0.2 mM EGTA, different concentrations of CaCl2, 0.5 mM phenyl-methylsulfonylfluoride (PMSF, Sigma) at 4°C for 1 hr. Free Ca2+ concentration in each buffer was determined using the Max Chelator program (Chris Patton, Stanford University, CA., WINMAXC v2.00, http://www.stanford.edu/~cpatton/maxc.html). Beads were washed 3 times with PBS/1% TRITON X-100, 0.2 mM EGTA, each concentration of CaCl2, and 0.5 mM PMSF, subjected to SDS-PAGE, and probed with anti-SYN1A. For immunoprecipitation, CHO cells transfected with His-tagged NET and SYN1A were washed with 50mM NaH2PO4, 10mM TRIS, 100mM NaCl, 0.5mM PMSF pH 8.0, and incubated in 400 μl/well of lysis buffer (50 mM NaH2PO4, 10 mM TRIS, 100 mM NaCl, 1% TRITON X-100, 0.5 mM PMSF pH 8.0) for 1 hr at 4°C. Cell lysates were recovered by centrifugation at 20,000g for 30 min at 4°C and incubated with anti-histidine antibody for 1 hr at 4°C. Complexes were retrieved by addition of 15 μL of protein-G Sepharose (Pharmacia Amersham) and washed 3 times with lysis buffer. Bound proteins were eluted using Laemmli sample buffer (3% SDS, 2M urea, 62.5 mM TRIS, 2 mM EDTA, pH6.9) containing 3% β -mercaptoethanol (BioRad, Hercules, CA) and subjected to SDS-PAGE followed by immunoblot. For cell surface biotinylation, cells were chilled in ice cold PBS containing 1 mM MgCl2 and 0.1 mM CaCl2, pH 7.4 (PBS/Ca/Mg) or PBS with 0.1 mM MgCl2, pH 7.4 (for cells pre-incubated in Ca2+ free condition) on ice for 5 min, and then in PBS/Ca/Mg for 5 min on ice. Cells were incubated with 1 mg/ml EZ-link NHS-sulfo-S-S Biotin (Pierce) in PBS/Ca/Mg at cold for 30 min. Reactions were quenched by washing with ice cold PBS/Ca/Mg containing 0.1 M glycine, pH 7.4 and incubating in the same buffer for 10 min. Biotinylated cells were washed with PBS containing 0.5 mM PMSF, lysed in PBS, 1% TRITON X-100, and 0.5 mM PMSF pH 7.4 for 1 hr at cold. Cell lysates, recovered by centrifugation at 16,000g for 20 min, were incubated at 4°C for 1 hr with 15 μL of Streptavidin beads (Pierce). For protein quantitation in immunoblots, exposed films were scanned using an Agfa Duoscan T1200 and the captured images processed in Adobe® Photoshop® and quantitated on a Macintosh computer using the public domain NIH Image program or ImageJ version 1.32 (developed at the U.S. National Institutes of Health and available on the Internet at http://rsb.info.nih.gov/nih-image/). Multiple films were exposed for each immunoblot to insure linearity of detection.
Acknowledgments
This work was supported by NIMH Awards MH073662 (US) and MH58921 (RB). We thank Dr. A. M. Band (University of Helsinki, Finland) for providing syntaxin 4 cDNA and Dr. M. A. Knepper (NIH, MD) for providing anti-syntaxin 4. We also thank Tammy Jessen, Qiao Han and Angela Steele for general lab maintenance and Jane Wright for animal husbandry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Apparsundaram S, Galli A, DeFelice LJ, Hartzell HC, Blakely RD. Acute regulation of norepinephrine transport: I. protein kinase C-linked muscarinic receptors influence transport capacity and transporter density in SK-N-SH cells. J Pharmacol Exp Ther. 1998;287:733–743. [PubMed] [Google Scholar]
- Apparsundaram S, Sung U, Price RD, Blakely RD. Trafficking-dependent and -independent pathways of neurotransmitter transporter regulation differentially involving p38 mitogen-activated protein kinase revealed in studies of insulin modulation of norepinephrine transport in SK-N-SH cells. J Pharmacol Exp Ther. 2001;299:666–677. [PubMed] [Google Scholar]
- Ashery U, Varoqueaux F, Voets T, Betz A, Thakur P, Koch H, Neher E, Brose N, Rettig J. Munc13-1 acts as a priming factor for large dense-core vesicles in bovine chromaffin cells. EMBO J. 2000;19:3586–3596. doi: 10.1093/emboj/19.14.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman ML, Bernstein EM, Quick MW. Protein kinase C regulates the interaction between a GABA transporter and syntaxin 1A. J Neurosci. 1998;18:6103–6112. doi: 10.1523/JNEUROSCI.18-16-06103.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MK, Garcia-Arraras JE, Elferink LA, Peterson K, Fleming AM, Hazuka CD, Scheller RH. The syntaxin family of vesicular transport receptors. Cell. 1993;74:863–873. doi: 10.1016/0092-8674(93)90466-4. [DOI] [PubMed] [Google Scholar]
- Betz A, Ashery U, Rickmann M, Augustin I, Neher E, Sudhof TC, Rettig J, Brose N. Munc13-1 is a presynaptic phorbol ester receptor that enhances neurotransmitter release. Neuron. 1998;21:123–136. doi: 10.1016/s0896-6273(00)80520-6. [DOI] [PubMed] [Google Scholar]
- Brose N, Hofmann K, Hata Y, Sudhof TC. Mammalian homologues of Caenorhabditis elegans unc-13 gene define novel family of C2-domain proteins. J Biol Chem. 1995;270:25273–25280. doi: 10.1074/jbc.270.42.25273. [DOI] [PubMed] [Google Scholar]
- Celano E, Tiraboschi E, Consogno E, D'Urso G, Mbakop MP, Gennarelli M, de Bartolomeis A, Racagni G, Popoli M. Selective regulation of presynaptic calcium/calmodulin-dependent protein kinase II by psychotropic drugs. Biol Psychiatry. 2003;53:442–449. doi: 10.1016/s0006-3223(02)01491-9. [DOI] [PubMed] [Google Scholar]
- Chapman ER, Hanson PI, An S, Jahn R. Ca2+ regulates the interaction between synaptotagmin and syntaxin 1. J Biol Chem. 1995;270:23667–23671. doi: 10.1074/jbc.270.40.23667. [DOI] [PubMed] [Google Scholar]
- Chen YA, Scales SJ, Patel SM, Doung YC, Scheller RH. SNARE complex formation is triggered by Ca2+ and drives membrane fusion. Cell. 1999;97:165–174. doi: 10.1016/s0092-8674(00)80727-8. [DOI] [PubMed] [Google Scholar]
- Edwardson JM, An S, Jahn R. The secretory granule protein syncollin binds to syntaxin in a Ca2+-sensitive manner. Cell. 1997;90:325–333. doi: 10.1016/s0092-8674(00)80340-2. [DOI] [PubMed] [Google Scholar]
- Esler M, Jackman G, Bobik A, Leonard P, Kelleher D, Skews H, Jennings G, Korner P. Norepinephrine kinetics in essential hypertension. Defective neuronal uptake of norepinephrine in some patients. Hypertension. 1981;3:149–156. doi: 10.1161/01.hyp.3.2.149. [DOI] [PubMed] [Google Scholar]
- Fagan KA, Mahey R, Cooper DM. Functional co-localization of transfected Ca2+-stimulable adenylyl cyclases with capacitative Ca2+ entry sites. J Biol Chem. 1996;271:12438–12444. doi: 10.1074/jbc.271.21.12438. [DOI] [PubMed] [Google Scholar]
- Feldmann J, Callebaut I, Raposo G, Certain S, Bacq D, Dumont C, Lambert N, Ouachee-Chardin M, Chedeville G, Tamary H, et al. Munc13-4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lymphohistiocytosis (FHL3) Cell. 2003;115:461–473. doi: 10.1016/s0092-8674(03)00855-9. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Sasaki T, Fukui K, Kotani H, Kimura T, Hata Y, Sudhof TC, Scheller RH, Takai Y. Phosphorylation of Munc-18/n-Sec1/rbSec1 by protein kinase C: its implication in regulating the interaction of Munc-18/n-Sec1/rbSec1 with syntaxin. J Biol Chem. 1996;271:7265–7268. doi: 10.1074/jbc.271.13.7265. [DOI] [PubMed] [Google Scholar]
- Gadea A, Lopez E, Hernandez-Cruz A, Lopez-Colome AM. Role of Ca2+ and calmodulin-dependent enzymes in the regulation of glycine transport in Muller glia. J Neurochem. 2002;80:634–645. doi: 10.1046/j.0022-3042.2001.00735.x. [DOI] [PubMed] [Google Scholar]
- Gailly P. Ca2+ entry in CHO cells, after Ca2+ stores depletion, is mediated by arachidonic acid. Cell Calcium. 1998;24:293–304. doi: 10.1016/s0143-4160(98)90053-7. [DOI] [PubMed] [Google Scholar]
- Geerlings A, Nunez E, Lopez-Corcuera B, Aragon C. Calcium- and syntaxin 1-mediated trafficking of the neuronal glycine transporter GLYT2. J Biol Chem. 2001;276:17584–17590. doi: 10.1074/jbc.M010602200. [DOI] [PubMed] [Google Scholar]
- Gelband CH, Sumners C, Lu D, Raizada MK. Angiotensin receptors and norepinephrine neuromodulation: implications of functional coupling. Regul Pept. 1998;73:141–147. doi: 10.1016/s0167-0115(97)11050-3. [DOI] [PubMed] [Google Scholar]
- Haase J, Killian AM, Magnani F, Williams C. Regulation of the serotonin transporter by interacting proteins. Biochem Soc Trans. 2001;29:722–728. doi: 10.1042/0300-5127:0290722. [DOI] [PubMed] [Google Scholar]
- Hadley D, Hoff M, Holik J, Reimherr F, Wender P, Coon H, Byerley W. Manic-depression and the norepinephrine transporter gene. Hum Hered. 1995;45:165–168. doi: 10.1159/000154279. [DOI] [PubMed] [Google Scholar]
- Hata Y, Sudhof TC. A novel ubiquitous form of Munc-18 interacts with multiple syntaxins. Use of the yeast two-hybrid system to study interactions between proteins involved in membrane traffic. J Biol Chem. 1995;270:13022–13028. doi: 10.1074/jbc.270.22.13022. [DOI] [PubMed] [Google Scholar]
- Jayanthi LD, Annamalai B, Samuvel DJ, Gether U, Ramamoorthy S. Phosphorylation of the norepinephrine transporter at threonine 258 and serine 259 is linked to protein kinase C-mediated transporter internalization. J Biol Chem. 2006;281:23326–23340. doi: 10.1074/jbc.M601156200. [DOI] [PubMed] [Google Scholar]
- Jayanthi LD, Samuvel DJ, Ramamoorthy S. Regulated internalization and phosphorylation of the native norepinephrine transporter in response to phorbol esters. Evidence for localization in lipid rafts and lipid raft-mediated internalization. J Biol Chem. 2004;279:19315–19326. doi: 10.1074/jbc.M311172200. [DOI] [PubMed] [Google Scholar]
- Jayanthi LD, Wilson JJ, Montalvo J, DeFelice LJ. Differential regulation of mammalian brain-specific proline transporter by calcium and calcium-dependent protein kinases. Brit J Pharmacol. 2000;129:465–470. doi: 10.1038/sj.bjp.0703071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazanietz MG. Novel "nonkinase" phorbol ester receptors: the C1 domain connection. Mol Pharmacol. 2002;61:759–767. doi: 10.1124/mol.61.4.759. [DOI] [PubMed] [Google Scholar]
- Keller NR, Diedrich A, Appalsamy M, Miller LC, Caron MG, McDonald MP, Shelton RC, Blakely RD, Robertson D. Norepinephrine transporter-deficient mice respond to anxiety producing and fearful environments with bradycardia and hypotension. Neuroscience. 2006;139:931–946. doi: 10.1016/j.neuroscience.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Klimek V, Stockmeier C, Overholser J, Meltzer HY, Kalka S, Dilley G, Ordway GA. Reduced levels of norepinephrine transporters in the locus coeruleus in major depression. J Neurosci. 1997;17:8451–8458. doi: 10.1523/JNEUROSCI.17-21-08451.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratochvil CJ, Vaughan BS, Harrington MJ, Burke WJ. Atomoxetine: a selective noradrenaline reuptake inhibitor for the treatment of attention-deficit/hyperactivity disorder. Expert Opin Pharmacother. 2003;4:1165–1174. doi: 10.1517/14656566.4.7.1165. [DOI] [PubMed] [Google Scholar]
- Lee KH, Kim MY, Kim DH, Lee YS. Syntaxin 1A and receptor for activated C kinase interact with the N-terminal region of human dopamine transporter. Neurochem Res. 2004;29:1405–1409. doi: 10.1023/b:nere.0000026404.08779.43. [DOI] [PubMed] [Google Scholar]
- Lu D, Yu K, Paddy MR, Rowland NE, Raizada MK. Regulation of norepinephrine transport system by angiotensin II in neuronal cultures of normotensive and spontaneously hypertensive rat brains. Endocrinology. 1996;137:763–772. doi: 10.1210/endo.137.2.8593828. [DOI] [PubMed] [Google Scholar]
- Manji HK, Lenox RH. Ziskind-Somerfeld Research Award. Protein kinase C signaling in the brain: molecular transduction of mood stabilization in the treatment of manic-depressive illness. Biol Psychiatry. 1999;46:1328–1351. doi: 10.1016/s0006-3223(99)00235-8. [DOI] [PubMed] [Google Scholar]
- Merlet P, Dubois-Rande JL, Adnot S, Bourguignon MH, Benvenuti C, Loisance D, Valette H, Castaigne A, Syrota A. Myocardial β -adrenergic desensitization and neuronal norepinephrine uptake function in idiopathic dilated cardiomyopathy. J Cardiovasc Pharmacol. 1992;19:10–16. doi: 10.1097/00005344-199201000-00002. [DOI] [PubMed] [Google Scholar]
- Min J, Okada S, Kanzaki M, Elmendorf JS, Coker KJ, Ceresa BP, Syu LJ, Noda Y, Saltiel AR, Pessin JE. Synip: a novel insulin-regulated syntaxin 4-binding protein mediating GLUT4 translocation in adipocytes. Mol Cell. 1999;3:751–760. doi: 10.1016/s1097-2765(01)80007-1. [DOI] [PubMed] [Google Scholar]
- Miner LH, Jedema HP, Moore FW, Blakely RD, Grace AA, Sesack SR. Chronic stress increases the plasmalemmal distribution of the norepinephrine transporter and the coexpression of tyrosine hydroxylase in norepinephrine axons in the prefrontal cortex. J Neurosci. 2006;26:1571–1578. doi: 10.1523/JNEUROSCI.4450-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama A, Hosaka K, Komiya Y, Akagawa K, Yamauchi E, Taniguchi H, Sasagawa N, Kumakura K, Mochida S, Yamauchi T, Igarashi M. Regulation of exocytosis through Ca2+/ATP-dependent binding of autophosphorylated Ca2+/calmodulin-activated protein kinase II to syntaxin 1A. J Neurosci. 2002;22:3342–3351. doi: 10.1523/JNEUROSCI.22-09-03342.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olver JS, Burrows GD, Norman TR. Third-generation antidepressants: do they offer advantages over the SSRIs? CNS Drugs. 2001;15:941–954. doi: 10.2165/00023210-200115120-00004. [DOI] [PubMed] [Google Scholar]
- Pacholczyk T, Blakely RD, Amara SG. Expression cloning of a cocaine- and antidepressant-sensitive human noradrenaline transporter. Nature. 1991;350:350–354. doi: 10.1038/350350a0. [DOI] [PubMed] [Google Scholar]
- Parekh AB, Putney JW., Jr Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- Quick MW. Regulating the conducting states of a mammalian serotonin transporter. Neuron. 2003;40:537–549. doi: 10.1016/s0896-6273(03)00605-6. [DOI] [PubMed] [Google Scholar]
- Sabatini BL, Regehr WG. Timing of synaptic transmission. Annu Rev Physiol. 1999;61:521–542. doi: 10.1146/annurev.physiol.61.1.521. [DOI] [PubMed] [Google Scholar]
- Savchenko V, Sung U, Blakely RD. Cell surface trafficking of the antidepressant-sensitive norepinephrine transporter revealed with an ectodomain antibody. Mol Cell Neurosci. 2003;24:1131–1150. doi: 10.1016/s1044-7431(03)00235-5. [DOI] [PubMed] [Google Scholar]
- Schiavo G, Matteoli M, Montecucco C. Neurotoxins affecting neuroexocytosis. Physiol Rev. 2000;80:717–766. doi: 10.1152/physrev.2000.80.2.717. [DOI] [PubMed] [Google Scholar]
- Shannon JR, Flattem NL, Jordan J, Jacob G, Black BK, Biaggioni I, Blakely RD, Robertson D. Orthostatic intolerance and tachycardia associated with norepinephrine- transporter deficiency. N Engl J Med. 2000;342:541–549. doi: 10.1056/NEJM200002243420803. [DOI] [PubMed] [Google Scholar]
- Sheng ZH, Rettig J, Cook T, Catterall WA. Calcium-dependent interaction of N-type calcium channels with the synaptic core complex. Nature. 1996;379:451–454. doi: 10.1038/379451a0. [DOI] [PubMed] [Google Scholar]
- Song Y, Ailenberg M, Silverman M. Cloning of a novel gene in the human kidney homologous to rat munc13s: its potential role in diabetic nephropathy. Kidney Int. 1998;53:1689–1695. doi: 10.1046/j.1523-1755.1998.00942.x. [DOI] [PubMed] [Google Scholar]
- Sung U, Apparsundaram S, Galli A, Kahlig KM, Savchenko V, Schroeter S, Quick MW, Blakely RD. A regulated interaction of syntaxin 1A with the antidepressant-sensitive norepinephrine transporter establishes catecholamine clearance capacity. J Neurosci. 2003;23:1697–1709. doi: 10.1523/JNEUROSCI.23-05-01697.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian JH, Das S, Sheng ZH. Ca2+-dependent phosphorylation of syntaxin-1A by the death-associated protein (DAP) kinase regulates its interaction with Munc18. J Biol Chem. 2003;278:26265–26274. doi: 10.1074/jbc.M300492200. [DOI] [PubMed] [Google Scholar]
- Turetta L, Bazzan E, Bertagno K, Musacchio E, Deana R. Role of Ca2+ and protein kinase C in the serotonin (5-HT) transport in human platelets. Cell Calcium. 2002;31:235–244. doi: 10.1016/S0143-4160(02)00052-0. [DOI] [PubMed] [Google Scholar]
- Uchida J, Kiuchi Y, Ohno M, Yura A, Oguchi K. Ca2+-dependent enhancement of [3H]noradrenaline uptake in PC12 cells through calmodulin-dependent kinases. Brain Res. 1998;809:155–164. doi: 10.1016/s0006-8993(98)00850-6. [DOI] [PubMed] [Google Scholar]
- Uchikawa T, Kiuchi Y, Yura A, Nakachi N, Yamazaki Y, Yokomizo C, Oguchi K. Ca2+-dependent enhancement of [3H]dopamine uptake in rat striatum: possible involvement of calmodulin-dependent kinases. J Neurochem. 1995;65:2065–2071. doi: 10.1046/j.1471-4159.1995.65052065.x. [DOI] [PubMed] [Google Scholar]
- Xia F, Gao X, Kwan E, Lam PP, Chan L, Sy K, Sheu L, Wheeler MB, Gaisano HY, Tsushima RG. Disruption of pancreatic beta-cell lipid rafts modifies Kv2.1 channel gating and insulin exocytosis. J Biol Chem. 2004;279:24685–24691. doi: 10.1074/jbc.M314314200. [DOI] [PubMed] [Google Scholar]
- Xu F, Gainetdinov RR, Wetsel WC, Jones SR, Bohn LM, Miller GW, Wang YM, Caron MG. Mice lacking the norepinephrine transporter are supersensitive to psychostimulants. Nat Neurosci. 2000;3:465–471. doi: 10.1038/74839. [DOI] [PubMed] [Google Scholar]
- Yokoyama CT, Sheng ZH, Catterall WA. Phosphorylation of the synaptic protein interaction site on N-type calcium channels inhibits interactions with SNARE proteins. J Neurosci. 1997;17:6929–6938. doi: 10.1523/JNEUROSCI.17-18-06929.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yura A, Kiuchi Y, Uchikawa T, Uchida J, Yamazaki K, Oguchi K. Possible involvement of calmodulin-dependent kinases in Ca2+-dependent enhancement of [3H]5-hydroxytryptamine uptake in rat cortex. Brain Res. 1996;738:96–102. doi: 10.1016/0006-8993(96)00762-7. [DOI] [PubMed] [Google Scholar]


