Abstract
CRH, the main regulator of the systemic response to stress, is also expressed in the skin where it is incorporated into a local homolog of the hypothalamic-pituitary-adrenal axis. To investigate the mechanisms of the induction of the CRH-proopiomelanocortin (POMC) response in human melanocytes, we used UVB as an epidermal-specific stressor. Human normal melanocytes cultured in vitro were irradiated with graded doses of UVB, and the CRH-POMC responses were measured in cell extracts and/or supernatants. UVB stimulated the CRH promoter, the CRH mRNA expression, and peptide release. The UVB-induced stimulation of the CRH promoter was suppressed by pharmacological inhibitors of protein kinase A or by plasmid overexpressing a dominant mutant cAMP response element (CRE)-binding protein (CREB). UVB also stimulated phosphorylation of CREB, binding of phosphorylated CREB to CRE sites in the CRH promoter, and activity of the reporter gene construct driven by consensus CRE sites. Mutation in the CRE site in the CRH promoter rendered the corresponding reporter gene construct less responsive to UVB in both normal and malignant melanocytes. In addition to CRH effects, UVB activated the POMC promoter, POMC mRNA expression, and ACTH release, whereas an antagonist of the CRH receptor 1 abrogated the UVB-stimulated induction of POMC. In conclusion, UVB induces CRH production in human melanocytes through stimulation of the protein kinase A pathway, with sequential involvement of CRH-CRH receptor 1 in the stimulation of POMC expression.
STRESS, ADAPTATION, AND homeostasis are concepts fundamental for survival. Recognition of their coexistence in a potentially noxious environment led to the discovery of an organized response system (1), which in humans involves three separate organs: the hypothalamus, pituitary, and adrenal glands (the HPA axis) (2). Furthermore, because this system is activated by nonphysiological conditions, the homeostasis-modifying responses might overshoot, contributing to the development of subsequent pathology.
We recently uncovered a functional homolog of the HPA axis that was fully expressed in single skin cells (3–5). Because the skin is the organ most exposed to environmental stressors, it is possible that this HPA axis homolog would participate in the local reaction to stress. If that were the case, its functional dysregulation could also contribute to the development of cutaneous disorders. To evaluate this possibility we investigated the molecular events involved in the activation of the cutaneous HPA axis homolog using as a stimulating agent its main natural stressor, i.e. UVB radiation.
The epidermis functions as a barrier for protection against environmental noxious stimuli, the most important of which is the sunlight with its UVB spectrum. UVB causes epidermal cell DNA damage, cell cycle arrest, apoptosis, and release of inflammation mediators (6). In response to UVB, epidermal melanocytes produce the protective skin pigmentation (melanin) (7, 8), and also neurohormonal signaling molecules that act on neighboring keratinocytes (4, 5, 7, 9). Melanocyte recognition of incoming damaging stimuli and presumed regulatory responses are probably related to their neuroectodermal origin (7, 9). Indeed, melanocytes respond to UVB with increased expression of tyrosinase, tyrosinase-related proteins 1, 2 (10), endothelin, stem cell factor (11); with proopiomelanocortin (POMC) gene expression, production and release of ACTH and α-MSH; and with increased expression and activity of MSH receptors (7, 8, 12–16). Pawelek et al. (8, 14) proposed that the skin pigmentary responses to UVB are mediated through MSH receptors via protein kinase A (PKA)-dependent pathway(s).
In addition, melanocytes also respond to UVB with production and release of CRH (17). CRH, a 41-amino acid peptide, is the main regulator of the systemic response to stress (2, 18), and its hypothalamic production is mediated by the cAMP-PKA-cAMP response element (CRE)-binding protein (CREB) pathway (19). However, the mechanism of production of CRH has not been characterized in melanocytes. Therefore, we investigated the following: 1) steps leading to CRH production in response to UVB, 2) local role of the cAMP-PKA-CREB pathway, which mediates the activation of CRH promoter in the central nervous system, and 3) involvement of the CRH-CRH receptor 1 (CRH-R1) system in the UVB activation of POMC activity.
RESULTS AND DISCUSSION
Dynamics of CRH and POMC Responses to UVB
Both CRH and ACTH peptides were released incrementally into the supernatant of human neonatal me-lanocytes cultured in vitro, in a UVB dose-dependent manner (Fig. 1). UVB at 100 mJ/cm2 increased basal production of CRH from 300 ± 57.7 pg/ml (per 7 × 105 cells) to 1050 ± 350 pg/ml (3.5-fold). Basal production of ACTH that was 1.2 ± 0.2 pg/ml (per 3 × 105 cells) also increased (1.7-fold), to 2.1 ± 0.1 pg/ml at the same UVB dose.
Fig. 1. UVB Stimulates Release of CRH and ACTH.
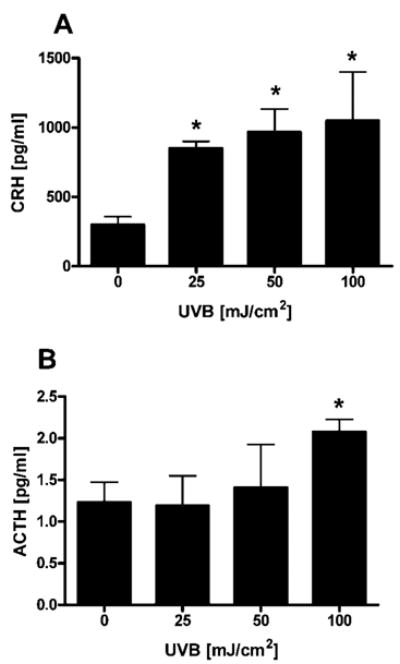
Normal human melanocytes were irradiated with UVB (0–100 mJ/cm2). After 24 h, the supernatants were collected, concentrated, and CRH (A) and ACTH (B) were determined by ELISA. Data are presented as mean ± SEM (n = 3).
CRH and POMC mRNA expression also increased in human neonatal melanocytes in a time- and UVB dose-dependent manner (Fig. 2). The CRH mRNA rise was already significant at 1 h after UVB (2.3- ± 0.3-fold) and reached a peak of 75- ± 0.1-fold increase at 24 h after the UVB dose of 50 mJ/cm2 (Fig. 2, A and B). POMC mRNA levels reached a maximum increase of 2-± 0.1-fold at 24 h in response to 100 mJ/cm2 UVB (Fig. 2, C and D).
Fig. 2. UVB Stimulates CRH and POMC mRNA Expression in Normal Human Melanocytes.
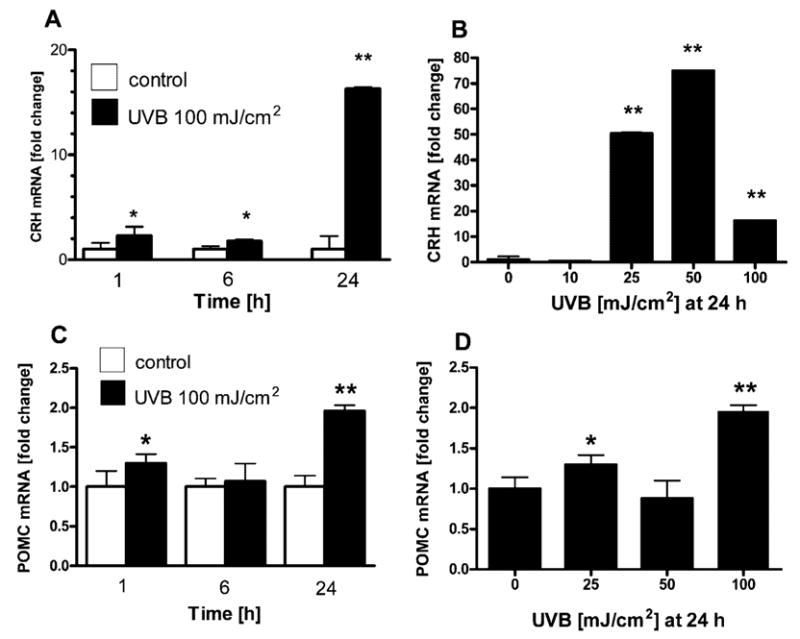
Normal human melanocytes were irradiated with UVB (0–100 mJ/cm2). Cells were lysed, and RNA was extracted at the time points indicated on the figure, and real-time PCRs were performed for CRH (A and B) and POMC (C and D). Data are presented as mean ± SEM (n = 3).
To further characterize the intra- and intercellular events that lead to activation of CRH and POMC genes, we prepared luciferase reporter constructs containing the promoter sequences of CRH (−969 to +7) and POMC (−771 to −8). CRH promoter activity increased significantly (2.4- ± 0.3-fold) by 6 h after the dose of 100 mJ/cm2 UVB. When measured at 24 h, it increased already after the dose of 10 mJ/cm2 UVB (2.7- ± 0.2-fold), with maximal stimulation occurring after the dose of 100 mJ/cm2 UVB (55- ± 25.1-fold) (Fig. 3A). As regards the POMC promoter, it followed stimulation dynamics similar to POMC mRNA, with maximal stimulation (3.6- ± 0.5-fold) in response to 100 mJ/cm2 UVB at 24 h (Fig. 3B).
Fig. 3. UVB Stimulates Transcriptional Activity of CRH (A) and POMC (B) Promoters.
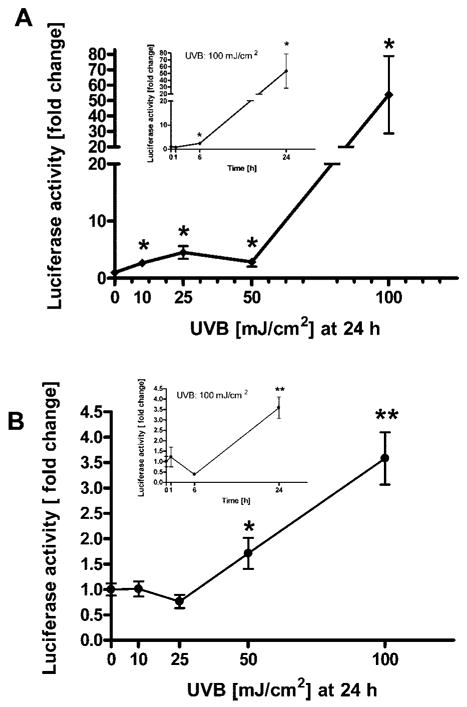
Normal human melanocytes were transfected with a reporter gene construct driven by the sequence of human CRH promoter (−969 to +7; p-CRH-Luc) or human POMC promoter (−771 to −8; p-POMC-Luc) and with PhRL-TK (transfection efficiency control). Cells were irradiated with UVB (0–100 mJ/cm2) and lysed at the time points indicated on the figure, when activity of the promoter was measured. Data are presented as mean ± SEM (n = 3).
It would be expected that the responses at the promoter, transcriptional, and peptide levels would follow the same dose-response patterns, as was indeed the case for POMC. As regards CRH, we noted increases that were significant statistically (P < 0.005) and biologically (75- and 16-fold) at the mRNA levels, with UVB doses of 50 and 100 mJ/cm2, respectively. The observed higher levels of CRH mRNA with 50 mJ/cm2 than at 100 mJ/cm2, as opposed to the peptide levels and promoter activity, which were higher at 100 mJ/cm2, may be explained by secondary processes involving cellular RNA. Thus, at high UVB dose (100 mJ/cm2), the cell viability becomes compromised and total RNA is subject to degradation; nevertheless, both promoter activity and peptide release were still higher at 100 mJ/cm2 than at 50 mJ/cm2. As expected, the increased promoter activity occurred gradually. As regards the increased peptide release, this would depend on increased translation (pro-CRH production), on intracellular convertase activity, and exocytosis itself. Therefore, although the mRNA levels were lower at 100 mJ/cm2 than 50 mJ/cm2, the expected consequences may be hidden by additional UVB effects on translation, convertase activity, and exocytosis that may be up-regulated, overriding direct effects on mRNA.
We observed significant differences in the responses to UVB between CRH and POMC genes. Thus, the CRH gene response was stronger (up to ~75-fold increase of promoter activity, up to ~16-fold increase of mRNA levels and up to ~3.5-fold of released peptide), than that of the POMC gene (up to ~3.6-fold increase of promoter activity, up to ~2-fold increase of mRNA levels, and up to ~1.7-fold of released ACTH peptide). These activity differences plus the significant release of CRH into the medium raised the possibility of their dependence on extracellular CRH signaling, acting through CRH-R1 for the activation of POMC promoter, after the irradiation of melanocytes with UVB. We therefore examined whether UVB could stimulate POMC production in the presence of a CRH-R1 antagonist in the medium.
CRH Receptor Blockade Attenuates the POMC Promoter Response to UVB
Melanocytes transfected with POMC-Luc responded to UVB with a 6.2- ± 2.5-fold increase in POMC promoter-driven reporter gene activity (in this separate experiment, POMC-Luc activity was higher than in the experiment presented in Fig. 3, but still significantly lower than that of CRH promoter under similar conditions). Conversely, cells transfected with p-Luc (empty vector) did not show changes in reporter gene activity. In the presence of antalarmin [a specific nonpeptide CRH-R1 antagonist (20–22)], the effect of UVB on the activity of POMC promoter decreased by 87 ± 8.2% (Fig. 4). Antalarmin also suppressed the UVB effect on POMC mRNA levels by 95 ± 0.2% (data not shown).
Fig. 4. Effect of UVB on POMC Expression Is Abrogated by a CRH Receptor Antagonist.
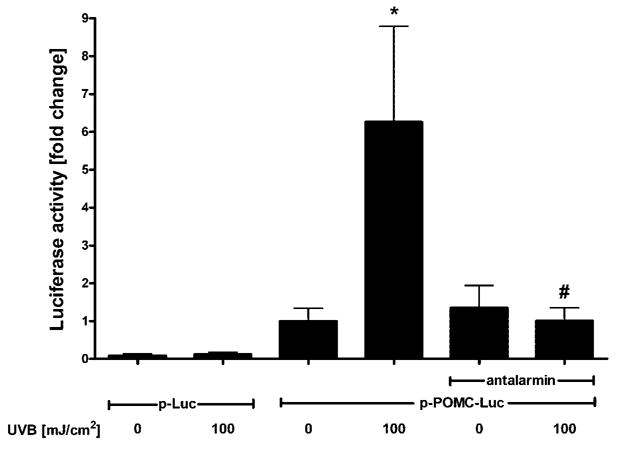
Normal human melanocytes were transfected with a reporter gene construct driven by the sequence of the human POMC promoter (−771 to −8; p-POMC-Luc) and PhRL-TK (transfection efficiency control) and irradiated with UVB (100 mJ/cm2). Some cells were incubated with CRH receptor antagonist (10−5 antalarmin) after irradiation. Cells were lysed 24 h after UVB and activity of the promoter measured. Data are presented as mean ± SEM (n = 3).
Therefore, CRH-R1 is involved in the stimulation of POMC in human neonatal melanocytes. It must be noted that CRH-R2 expression has not been documented in the human epidermis (23), and that CRH-R1 expresses several isoforms whose significance in in-traepidermal interactions is not yet fully determined (24, 25). For example, the CRH-R1 isoforms c, d, e, and g are expressed in SKMEL-188 melanoma cells and HaCaT keratinocytes, but only after stimulation with UVB (24). In pituitary cells, the signaling pathway leading from CRH receptor to POMC promoter includes transcription factors Nurr1 and Nur77 (26), although operation of the same pathway in human melanocytes remains to be determined. Production of ACTH in response to CRH was originally described in corticotrophs (2), but also takes place in the skin (27, 28), at least, in melanocytes (22) and in fibroblasts (29). The biological significance of CRH in central nervous system is triggering the activation of the HPA axis, which ultimately suppresses immune responses among other systemic responses (30). As regards the cutaneous HPA axis homolog, this may fulfill similar functions at a different scale (3, 5). For example, cutaneous ACTH could potentially counteract or prevent autoimmune responses against self antigens exposed by UVB (31).
Activation of the CRH Promoter in Human Melanocytes Is Mediated by the PKA Pathway
Because UVB has a more rapid effect on CRH than on the POMC promoter, we evaluated sequentially the steps preceding activation of the CRH promoter. Thus, the CRH promoter possesses binding sites for several transcription factors that could be modified by yet other regulatory molecules (19). Moreover, UVB activates all the major intracellular pathways including those mediated by PKA, PKC, and MAPKs (32). We focused on the cAMP-PKA-CREB pathway, because it is engaged in the classic activation of the CRH promoter by systemic stressors in the hypothalamus (19).
In the PKA pathway, cAMP after binding to the regulatory subunits of PKA causes dissociation of the catalytic subunits and their translocation to the nucleus, where the activated catalytic subunits phosphorylate CREB at serine 133 (33). Phosphorylation of CREB leads to its increased binding to asymmetric and to symmetric CREB-binding sites such as the site at the CRH promoter (34). As regards UVB, this has been shown to increase the amount of phospho-CREB in keratinocytes (35) and melanocytes (36). Our experiments with Western blotting did confirm that UVB increased the levels of phospho-CREB levels in melanocytes (Fig. 5A). Thus, the amount of phospho-CREB rose abruptly at 30 min, remained elevated at 1–3 h, and decreased back to basal levels at 6 h. Interestingly, flow cytometry analysis showed that malignant melanocytes (line WM 164) also responded to UVB (100 mJ/cm2) with increased levels of phospho-CREB (at 1 h; Fig. 5B). We then examined the effects of UVB on the binding of proteins to the CRE site in the CRH promoter of normal melanocytes. As shown on Fig. 5C, UVB increased in a time-dependent manner the binding of proteins to CRE with maximal binding occurring at 1–3 h. The bound protein was identified as phospho-CREB with supershift assay (Fig. 5D).
Fig. 5. UVB Activates CREB in the Melanocytes.
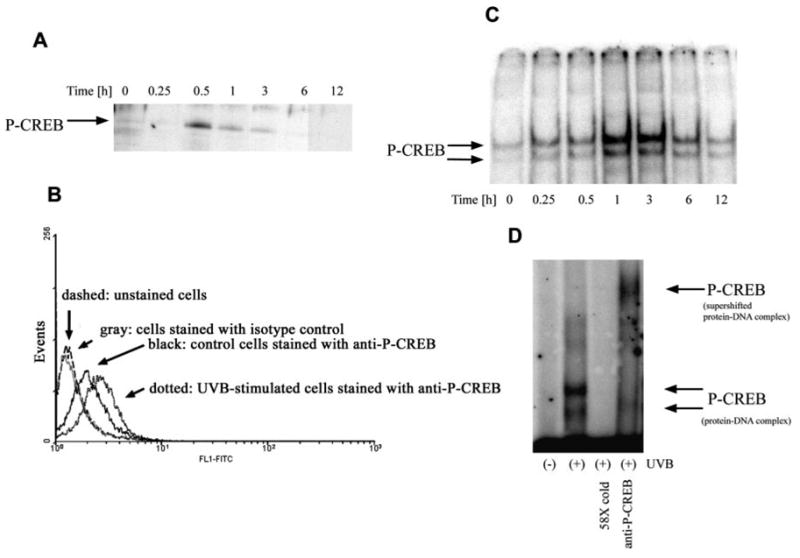
A, Normal human melanocytes were irradiated with UVB (100 mJ/cm2). Cells were lysed at consecutive time points, and extracts were subjected to SDS-PAGE followed by Western blot with the use of anti-phospho-CREB antibodies. B, WM 164 malignant melanocytes were exposed to UVB at 100 mJ/cm2 and, after 1 h, fixed, stained with anti-phospho-CREB antibody, and read with a flow cytometer. Dashed, Unstained cells; gray, cells stained with isotype control antibody; black, unstimulated cells stained with anti-phospho-CREB; dotted, stimulated cells stained with anti-phospho-CREB. C, Content of nuclei of normal melanocytes was extracted after irradiation with UVB (100 mJ/cm2) at the time points indicated on the figure. Nuclear extracts were subjected to EMSA with the 32P-labeled probe containing CRE and flanking nucleotides from CRH promoter. D, Nuclear extract from cells irradiated for 1 h was subjected to supershift assay with the use of antibodies directed against phospho-CREB. 58X cold indicates extract preincubated with excess cold probe.
Normal melanocytes transfected with CRH-Luc and preincubated with inhibitors of both the catalytic and regulatory domain of PKA showed reduced CRH promoter response to stimulation with UVB at 100 mJ/ cm2 (Fig. 6A). Also, the transfection of normal melanocytes with KCREB (dominant mutant CREB) resulted in increased expression of CREB immunoreactivity on Western blot (Fig. 6A, inset). Overexpression of KCREB in normal melanocytes reduced the UVB-stimulated CRH promoter activity (Fig. 6A). Of note, neither pharmacological inhibitors of PKA nor KCREB altered the basal activity of the CRH construct in nonstimulated cells (Fig. 6A).
Fig. 6. UVB Activates PKA Pathway in Melanocytes.
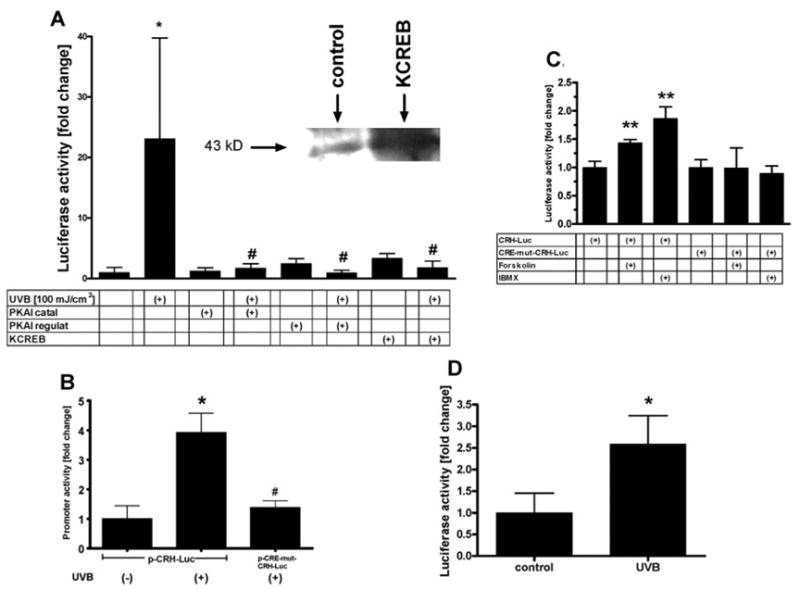
A, Normal melanocytes were transfected with a reporter gene construct driven by wild-type human CRH promoter and cotransfected with PhRL-TK. Cells were preincubated with pharmacological inhibitors of catalytic or regulatory domain of PKA or cotransfected with KCREB. The cells were then irradiated with UVB (100 mJ/cm2) and lysed at 24 h before measuring the activity of the promoter. Inset, Melanocytes not transfected or transfected with KCREB. After 24 h, cellular lysates were prepared, and equal amounts of cellular proteins were then separated on a gel. Proteins transferred onto the membrane were probed with anti-CREB antibody. B and C, WM 164 malignant melanocytes were transfected with a reporter gene construct driven either by either sequence of wild-type human CRH promoter (−969 to +7; p-CRH-Luc), or by the sequence of the human CRH promoter mutated at the CRE site (−969 to +7; C-224-G, A-220-T; CRE-mut p-CRH-Luc) and cotransfected with PhRL-TK. Cells were then irradiated with UVB (100 mJ/cm2) or stimulated with forskolin (100 μM) or IBMX (100 μM) and lysed at 24 h before measuring the activity of the promoter. D, Normal melanocytes were transfected with CRE-Luc and cotransfected with PhRL-TK. Cells were then irradiated with UVB (100 mJ/cm2) and lysed at 24 h before measuring the activity of the promoter. Data are presented as mean ± SEM (n = 4–6).
In malignant melanocytes, UVB stimulated the CRH promoter-driven reporter gene activity by 3.9- ± 0.7-fold at 24 h (Fig. 6B). The response to UVB of a reporter gene construct driven by a CRH promoter with a mutated CRE site was 62 ± 5.2% lower than in the nonmutated control (Fig. 6B). Concordantly, normal melanocytes transfected with a construct containing the CRH promoter sequence mutated at the CRE site did not respond to UVB treatment, as opposed to cells transfected with the nonmutated CRH-Luc (data not shown). Of note, when transfected into malignant melanocytes, the CRH-Luc construct, but not the CRH-Luc construct mutated at CRE site, did respond to the classical PKA stimulators forskolin and 3-isobutyl-1-methylxanthine (IBMX) (Fig. 6C). UVB (24 h; 100 mJ/cm2) also increased by 2.6- ± 0.6-fold the transcriptional activity of a construct driven by four consensus CRE sites (p-CRE-Luc) transfected into normal melanocytes (Fig. 6D).
Activation of the CRE site by phosphorylated CREB is therefore essential for activation of the CRH promoter in both normal and malignant melanocytes. This functional restriction in the cis site for activation of the CRH promoter had been initially found in neurons, and can now be extended to other cells of neuroectodermal origin that include melanocytes, even their malignant variants.
Involvement of PKA pathway in the response of melanocytes to UVB was proposed by Pawelek et al. (8) based on the studies in S91 melanoma cells and in rodent skin. Because UVB induces production of reactive oxygen species and causes DNA damage (32), it may activate myriad signaling pathways. According to our results, there is the involvement of cAMP in the response to UVB because the PKA-CRE cascade is activated, although the mechanism(s) for UVB induction of cAMP remains to be elucidated.
To conclude, the natural stressor UVB does induce human melanocytes to produce and release CRH through the PKA pathway. Extracellularly released CRH acts then in paracrine fashion to stimulate POMC production and, in turn, ACTH release. This endocrine cutaneous homolog of a systemic organization is therefore emerging as a fundamental component of a skin stress response system, whose activities are probably indispensable for the organ most exposed to the environment.
MATERIALS AND METHODS
Cell Culture
Normal human epidermal melanocytes from moderately pigmented skin (Cascade Biologics, Portland, OR) were cultured as described (23). Cells were seeded into multiwell plates in growth medium (EpiLife with Human Melanocyte Growth Supplement) that was changed after 24 h to Epilife medium with EpiLife Defined Growth Supplement free of serum and pituitary extract (EDGS; Cascade Biologics). After incubation for further 24 h, the cells were washed with PBS, exposed to UVB as described (37), and then incubated in EpiLife medium with EDGS. UVB at 100 mJ/cm2 caused 48% decrease of viability of the cells as measured with trypan blue exclusion assay 24 h after treatment. Where indicated, a CRH-R1 antagonist antalarmin (10 μM; Sigma, St. Louis, MO) was added to the medium after the cells had been irradiated with UVB. Inhibitors of the catalytic domain of PKA (cell-permeable, myristoylated PKA inhibitor amide 14–22; 40 nM; Calbiochem, San Diego, CA) or of the regulatory domain of PKA (cell-permeable, resistant to mammalian cyclin nucleotide phosphodiesterases, adenosine 3′,5′-cyclin monophosphorothioate, 8-bromo-Rp-isomer, sodium salt; 10 nM; Alexis Biochemicals, Lausen, Switzerland) were added 1 h before UVB treatment.
WM 164 malignant melanocytes were cultured in DMEM supplemented with insulin 5 μg/ml and antibiotic/antimycotic solution. Where indicated, forskolin (100 μM) and IBMX (100 μM; Sigma) were added for 24 h.
CRH and ACTH Production
Supernatants from the above UVB-treated cells were concentrated in C18 SEP-COLUMNS as described by the manufacturer (Peninsula Laboratories, San Carlos, CA). Peptide concentration in the supernatants was measured with ELISA kits for CRH (Phoenix Pharmaceuticals, Belmont, CA) and ACTH (Alpco Diagnostics, Windham, NH) and normalized for total protein content in cell lysates (quantified with BCA reagent; Pierce Biotechnology, Rockford, IL).
Real-Time RT-PCR
RNA was extracted using Absolutely RNA RT-PCR Miniprep kit (Stratagene, La Jolla, CA). Reverse transcription was performed using Taqman Reverse Transcription Reagents. CRH (GenBank accession no. NM_000756) primers and probe were designed using Assays-by-Design Service (custom product symbol CRH2E_SL) with sequences as follows: forward, 5′-GCG GCG CTA GGA ATG C-3′; reverse, 5′-GAA GGT GAG ATC CAG GGA GAT G-3′, FAM dye-labeled probe 5′-ACC GCC TTT CTC TCT CC-3′. 18SrRNA (GenBank accession no. X03205) was quantitated using the ABI catalog product Hs99999901_s1. The reaction was performed with Taqman Universal PCR Master Mix; data were collected on ABI Prism 7700 and analyzed on Sequence Detector 1.9.1. All real-time PCR products were purchased from Applied Biosystems (Foster City, CA). CRH amounts were related to 18SrRNA by comparative CT method. Quantification of POMC mRNA by real-time PCR was performed as reported (22).
Reporter Gene Constructs
Reporter gene plasmids were constructed by inserting promoter sequences into firefly luciferase pLuc vector (construction of pLuc was described in Ref. 25). P-CRH-Luc contained the sequence −969 to +7 of the human CRH promoter [this sequence contains all the response elements reported to be essential for activation of the CRH gene in the hypothalamus (nGRE, CRE, CDXRE, TATA box; reviewed in Ref. 19)]; p-POMC-Luc contained the sequence −771 to −8 of the human POMC promoter [this sequence contains all response elements reported to be essential for activation of POMC gene in the pituitary gland (NeuroD1, TPIT, PTX-1, Nurr77, TATA box, PO-B; reviewed in Ref. 38)]. p-CRE-Luc was described in Ref. 25. CRE-mut p-CRH-Luc contains the CRH promoter (from −969 to +7) mutated at CRE site (C-224-G, A-220-T). Site-directed mutagenesis was performed using QuikChange II Site-Directed Mutagenesis kit (Stratagene) and the following primers: forward, 5′-CCA TTT TAG GGC TCG TTG AG GTC TCC AAG AGG CG-3′; reverse, 5′-CGC CTC TTG GAG ACC TCA ACG AGC CCT AAA ATG G-3′; and conditions: 95 C, 30 sec, 16× (95 C, 30 sec; 6.5 min, 72 C). Plasmid correctness was checked with restriction enzyme analysis and sequencing. The plasmid expressing the mutant CREB [KCREB; blocks the ability of wild-type CREB to mediate induction by PKA (39)] was cotransfected with p-CRH-Luc where indicated (0.05 μg/cm2).
Reporter Gene Constructs Activity
Melanocytes were transfected using transfection reagents (sc-29528 and sc-36868; Santa Cruz Biotechnology, Santa Cruz, CA) in EpiLife medium with EDGS with firefly luciferase reporter gene plasmid and with phRL-TK (expresses Renilla luciferase and serves as normalization control; Promega, Madison, WI). After transfection, cells were incubated for 24 h in EpiLife medium with EDGS, and then treated with UVB as above. The firefly luciferase and Renilla luciferase signals were recorded with a TD-20/20 luminometer (Turner Designs, Sunnyvale, CA); background luminescence was subtracted and the resulting promoter-specific firefly signal was divided by the Renilla signal (proportional to the number of transfected cells). The values obtained were divided by the mean of control (untreated) cells.
Western Blot
Cell lysates were separated on 12.5% SDS-PAGE gel and transferred to the polyvinylidene difluoride membrane. After blocking for 1 h with Tris-buffered saline, 0.1% Triton X-100, and 5% milk, the membranes were incubated overnight at 4 C with rabbit anti-phospho-CREB (Ser133) (87G3) (no. 9198; Cell Signaling Technology, Beverly, MA) diluted 1:1000 or with mouse anti-CREB (X-12) (sc-240; Santa Cruz Biotech-nology) diluted 1:250. The incubation buffer was Tris-buffered saline, 0.1% Triton X-100, and 5% BSA. After incubation for 3 h with horseradish peroxidase-conjugated goat antirab-bit or antimouse IgG diluted 1:2000 in incubation buffer, phospho-CREB or CREB were visualized with Lumiglo reagent and peroxide (Cell Signaling Technology).
Flow Cytometry
Equal number of cells (5 × 105) were fixed with 2% paraformaldehyde (10 min at 37 C), and then ice-cold methanol was added to a final concentration of 90%. After washing with PBS, cells were incubated for 1 h at room temperature with rabbit anti-phospho-CREB (Ser133) (87G3) (no. 9198; Cell Signaling Technology) diluted 1:250 in incubation buffer (0.5% BSA in PBS). This was followed by incubation for 1 h with goat antirabbit IgG-FITC (sc-2012; Santa Cruz Biotechnology, Santa Cruz, CA) diluted 1:200 in incubation buffer. Control cells were stained with rabbit IgG isotype control antibody (no. 0111-01; Southern Biotech, Birmingham, AL). The FL-1 signal (collected from 10,000 events in side scatter/ forward scatter window after debris exclusion) was recorded by FACSCalibur flow cytometer (BD Biosciences, San Diego, CA). Signal intensities were analyzed with CellQuest (BD Biosciences) and graphic representations of FL-1 signal were prepared with WinMdi 2.8 (shareware from Joseph Trotter, The Scripps Research Institute, La Jolla, CA).
EMSA
Nuclei extracts were prepared as described (40) and used for EMSA. Sequence of the sense CRE-containing probe from CRH promoter was as follows: 5′-CCA TTT TAG GGC TCG TTG ACG TCA CCA AGA GGC G-3′. The sense oligonucleotide was mixed 1:1 with complementary single-strand oligonucleotide, boiled for 20 min, and cooled down at room temperature overnight. The double-stranded probe was end-labeled with [γ-32P]ATP (Amersham Biosciences, Piscat-away, NJ) using DNA 5′-end Labeling System (Promega). The binding reaction was performed as described (21), and the protein-DNA complexes were separated on 5% polyacryl-amide gel. For supershift assays, nuclear extracts were incubated with anti-phospho-CREB antibody (no. 9198; Cell Signaling Technology). Binding specificity was determined using a 48× excess of unlabeled double-stranded probe. Radioactivity was measured with a Packard Cyclone phosphorimager, and data analyzed with Optiquant (PerkinElmer Life Sciences, Boston, MA) and Adobe Photoshop (San Jose, CA) software.
Statistical Analysis
Data were analyzed with Student’s t test using Prism 4.00 (GraphPad Software, San Diego, CA) and presented as mean ± SEM (n = 3–6). Statistical differences are denoted with asterisks (*, P < 0.05; **, P < 0.005).
Acknowledgments
The technical assistance of Dr. M. Zmijewski is greatly appreciated. We also thank Dr. Richard H. Goodman (Oregon Health and Sciences University, Portland, OR) for the generous gift of plasmid expressing mutant CREB, and Dr. Meenhard Herlyn (Wistar Institute, Philadelphia, PA) for providing the WM 164 melanoma cell line. Real-time RT-PCR was performed on ABI Prism 7770 in the Molecular Resource Center at the University of Tennessee (Memphis, TN).
Abbreviations
- CRE
cAMP response element
- CREB
CRE-binding protein
- CRH-R1
CRH receptor 1
- HPA
hypothalamus-pituitary-adrenal
- IBMX
3-isobutyl-1-methylxan-thine
- PKA
protein kinase A
- POMC
proopiomelanocortin
Footnotes
The authors have nothing to disclose.
This work was supported by National Institutes of Health Grants AR047079 and AR052190 (to A.S.); Johnson and Johnson Skin Research Training Grant (to B.Z.; A.S. as mentor) and William J. Cunliffe Scientific Prize (to A.S.).
References
- 1.Selye HA. Syndrome produced by various noxious agents. Nature. 1936;138:32–33. [Google Scholar]
- 2.Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992;267:1244–1252. [PubMed] [Google Scholar]
- 3.Slominski A, Mihm MC. Potential mechanism of skin response to stress. Int J Dermatol. 1996;35:849–851. doi: 10.1111/j.1365-4362.1996.tb05049.x. [DOI] [PubMed] [Google Scholar]
- 4.Slominski A, Wortsman J. Neuroendocrinology of the skin. Endocr Rev. 2000;21:457–487. doi: 10.1210/edrv.21.5.0410. [DOI] [PubMed] [Google Scholar]
- 5.Slominski A, Wortsman J, Luger T, Paus R, Solomon S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev. 2000;80:979–1020. doi: 10.1152/physrev.2000.80.3.979. [DOI] [PubMed] [Google Scholar]
- 6.Hruza LL, Pentland AP. Mechanisms of UV-induced inflammation. J Invest Dermatol. 1993;100:35S–41S. doi: 10.1111/1523-1747.ep12355240. [DOI] [PubMed] [Google Scholar]
- 7.Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84:1155–1228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- 8.Pawelek J, Chakraborty A, Osber MP, Orlow SJ, Min KK, Rosenzweig KE, Bolognia JL. Molecular cascades in UV-induced melanogenesis: acentral role for melanotropins? Pigment Cell Res. 1992;5:34–356. doi: 10.1111/j.1600-0749.1992.tb00561.x. [DOI] [PubMed] [Google Scholar]
- 9.Slominski A, Paus R, Schanderdorf D. Melanocytes as sensory and regulatory cells in the epidermis. J Theor Biol. 1993;164:103–120. doi: 10.1006/jtbi.1993.1142. [DOI] [PubMed] [Google Scholar]
- 10.Nishioka E, Funasaka Y, Kondoh H, Chakraborty AK, Mishima Y, Ichihashi M. Expression of tyrosinase, TRP-1 and TRP-2 in ultraviolet-irradiated human melanomas and melanocytes: TRP-2 protects melanoma cells from ultraviolet B induced apoptosis. Melanoma Res. 1999;9:433–443. doi: 10.1097/00008390-199910000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Hachiya A, Kobayashi A, Yoshida Y, Kitahara T, Takema Y, Imokawa G. Biphasic expression of two paracrine melanogenic cytokines, stem cell factor and endothelin-1, in ultraviolet B-induced human melanogenesis. Am J Pathol. 2004;165:2099–2109. doi: 10.1016/S0002-9440(10)63260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chakraborty AK, Funasaka Y, Slominski A, Ermak G, Hwang J, Pawelek JM, Ichihashi M. Production and release of proopiomelanocortin (POMC) derived peptides by human melanocytes and keratinocytes in culture: regulation by ultraviolet B. Biochim Biophys Acta. 1996;1313:130–138. doi: 10.1016/0167-4889(96)00063-8. [DOI] [PubMed] [Google Scholar]
- 13.Slominski A, Ermak G, Hwang J, Chakraborty A, Mazurkiewicz JE, Mihm M. Proopiomelanocortin, corticotropin releasing hormone and corticotropin releasing hormone receptor genes are expressed in human skin. FEBS Lett. 1995;374:113–116. doi: 10.1016/0014-5793(95)01090-2. [DOI] [PubMed] [Google Scholar]
- 14.Chakraborty AK, Funasaka Y, Slominski A, Bolognia J, Sodi S, Ichihashi M, Pawelek JM. UV light and MSH receptors. Ann NY Acad Sci. 1999;885:100–116. doi: 10.1111/j.1749-6632.1999.tb08668.x. [DOI] [PubMed] [Google Scholar]
- 15.Wintzen M, Gilchrest BA. Proopiomelanocortin, its derived peptides, and the skin. J Invest Dermatol. 1996;106:3–10. doi: 10.1111/1523-1747.ep12326950. [DOI] [PubMed] [Google Scholar]
- 16.Luger T, Paus R, Lipton JM, Slominski A. Cutaneous neuromodulation “The Proopiomelanocortin System. Ann NY Acad Sci. 1999;885:1–479. doi: 10.1111/j.1749-6632.1999.tb08661.x. [DOI] [PubMed] [Google Scholar]
- 17.Slominski A, Baker J, Ermak G, Chakraborty A, Pawelek J. Ultraviolet B stimulates production of corticotropin releasing factor (CRF) by human melanocytes. FEBS Lett. 1996;399:175–176. doi: 10.1016/s0014-5793(96)01315-4. [DOI] [PubMed] [Google Scholar]
- 18.Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and β-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 19.Nicholson RC, King BR, Smith R. Complex regulatory interactions control CRH gene expression. Front Biosci. 2004;9:32–39. doi: 10.2741/1204. [DOI] [PubMed] [Google Scholar]
- 20.Webster EL, Lewis DB, Torpy DJ, Zachman EK, Rice KC, Chrousos GP. In vivo and in vitro characterization of antalarmin, a nonpeptide corticotropin-releasing hormone (CRH) receptor antagonist: suppression of pituitary ACTH release and peripheral inflammation. Endocrinology. 1996;137:5747–5750. doi: 10.1210/endo.137.12.8940412. [DOI] [PubMed] [Google Scholar]
- 21.Zbytek B, Pfeffer LM, Slominski AT. Corticotropin-releasing hormone stimulates NF-κB in human epidermal keratinocytes. J Endocrinol. 2004;181:R1–R7. doi: 10.1677/joe.0.181r001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slominski A, Zbytek B, Szczesniewski A, Semak I, Kaminski J, Sweatman T, Wortsman J. CRH stimulation of corticosteroids production in melanocytes is mediated by ACTH. Am J Physiol. 2005;288:E701–E706. doi: 10.1152/ajpendo.00519.2004. [DOI] [PubMed] [Google Scholar]
- 23.Slominski A, Pisarchik A, Tobin DJ, Mazurkiewicz JE, Wortsman J. Differential expression of a cutaneous corticotropin-releasing hormone system. Endocrinology. 2004;145:941–950. doi: 10.1210/en.2003-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pisarchik A, Slominski AT. Alternative splicing of CRH-R1 receptors in human and mouse skin: identification of new variants and their differential expression. FASEB J. 2001;15:2754–2756. doi: 10.1096/fj.01-0487fje. [DOI] [PubMed] [Google Scholar]
- 25.Pisarchik A, Slominski A. Molecular and functional characterization of novel CRFR1 isoforms from the skin. Eur J Biochem. 2004;271:2821–2830. doi: 10.1111/j.1432-1033.2004.04216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mynard V, Latchoumanin O, Guignat L, Devin-Leclerc J, Bertagna X, Barre B, Fagart J, Coqueret O, Catelli MG. Synergistic signaling by CRH and leukemia inhibitory factor bridged by phosphorylated CREB at the NurRE-STAT element of the proopiomelanocortin promoter. Mol Endocrinol. 2004;18:2997–3010. doi: 10.1210/me.2003-0417. [DOI] [PubMed] [Google Scholar]
- 27.Ito N, Ito T, Kromminga A, Bettermann A, Takigawa M, Kees F, Straub RH, Paus R. Human hair follicles display a functional equivalent of the hypothalamic-pituitary-adrenal axis and synthesize cortisol. FASEB J. 2005;19:1332–1334. doi: 10.1096/fj.04-1968fje. [DOI] [PubMed] [Google Scholar]
- 28.Slominski A, Zbytek B, Zmijewski M, Slominski RM, Kauser S, Wortsman J, Tobin DJ. Corticotropin releasing hormone and the skin. Front Biosci. 2006;11:2230–2248. doi: 10.2741/1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slominski A, Zbytek B, Semak I, Sweatman T, Wortsman J. CRH stimulates POMC activity and corticosterone production in dermal fibroblasts. J Neuroimmunol. 2005;162:97–102. doi: 10.1016/j.jneuroim.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 30.Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995;332:1351–1363. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- 31.Caricchio R, McPhie L, Cohen PL. Ultraviolet B radiation-induced cell death: critical role of ultraviolet dose in inflammation and lupus autoantigen redistribution. J Immunol. 2003;171:5778–5786. doi: 10.4049/jimmunol.171.11.5778. [DOI] [PubMed] [Google Scholar]
- 32.Assefa Z, Van Laethem A, Garmyn M, Agostinis P. Ultraviolet radiation-induced apoptosis in keratinocytes: on the role of cytosolic factors. Biochim Biophys Acta. 2005;1755:90–106. doi: 10.1016/j.bbcan.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 33.Cooper DM. Regulation and organization of adenylyl cyclases and cAMP. Biochem J. 2003;375:517–529. doi: 10.1042/BJ20031061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nichols M, Weih F, Schmid W, DeVack C, Kowenz-Leutz E, Luckow B, Boshart M, Schutz G. Phosphorylation of CREB affects its binding to high and low affinity sites: implications for cAMP induced gene transcription. EMBO J. 1992;11:3337–3346. doi: 10.1002/j.1460-2075.1992.tb05412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gonzales M, Bowden GT. Ultraviolet B (UVB) induction of the c-fos promoter is mediated by phospho-cAMP response element binding protein (CREB) binding to CRE and c-fos activator protein 1 site (FAP1) cis elements. Gene. 2002;293:169–179. doi: 10.1016/s0378-1119(02)00723-0. [DOI] [PubMed] [Google Scholar]
- 36.Tada A, Pereira E, Beitner-Johnson D, Kavanagh R, Abdel-Malek ZA. Mitogen- and ultraviolet-B-induced signaling pathways in normal human melanocytes. J Invest Dermatol. 2002;118:316–322. doi: 10.1046/j.0022-202x.2001.01694.x. [DOI] [PubMed] [Google Scholar]
- 37.Fischer T, Zbytek B, Sayre RM, Apostolov EO, Basnakian AG, Sweatman TW, Wortsman J, Elsner JP, Slominski A. Melatonin increases survival of HaCaT keratinocytes by suppressing UV induced apoptosis. J Pineal Res. 2006;40:18–26. doi: 10.1111/j.1600-079X.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 38.Newell-Price J. Proopiomelanocortin gene expression and DNA methylation: implications for Cushing’s syndrome and beyond. J Endocrinol. 2003;177:365–372. doi: 10.1677/joe.0.1770365. [DOI] [PubMed] [Google Scholar]
- 39.Walton KM, Rehfuss RP, Chrivia JC, Lochner JE, Good-man RH. A dominant repressor of cyclic adenosine 3′,5′-monophosphate (cAMP)-regulated enhancer-binding protein activity inhibits the cAMP-mediated induction of the somatostatin promoter in vivo. Mol Endocrinol. 1992;6:647–655. doi: 10.1210/mend.6.4.1350057. [DOI] [PubMed] [Google Scholar]
- 40.Yang CH, Murti A, Pfeffer SR, Basu L, Kim JG, Pfeffer LM. IFNα/β promotes cell survival by activating NF-κB. Proc Natl Acad Sci USA. 2000;97:13631–13636. doi: 10.1073/pnas.250477397. [DOI] [PMC free article] [PubMed] [Google Scholar]


