Abstract
The Early Cambrian (≈540 million years old) Meishucun fossil assemblage of Ningqiang County (Shaanxi Province), China, contains the oldest complex skeletonized organisms known in the geological record. We here report the finding in this assemblage of an exquisitely preserved late-stage embryo of a ctenophore (“comb jelly”), its fine structure documented by confocal laser scanning microscopy and shown by Raman spectroscopy to be composed of carbonaceous kerogen permineralized in apatite. In its spheroidal morphology, the presence of eight comb rows and the absence of tentacles, this embryo resembles an adult ctenophore (Maotianoascus octonarius) known from the immediately younger Chengjiang fauna of Yunnan, China. The oldest ctenophore and the only embryonic comb jelly known from the fossil record, this exceptionally well preserved specimen provides important clues about the early evolution of the phylum Ctenophora and of metazoans in general.
Keywords: comb jelly, confocal laser scanning microscopy, Meishucun fossil assemblage, Ningqiang fossil fauna
The small shelly fossils of the Meishucun assemblage of southwestern Shaanxi, China, record the earliest radiation of the Cambrian explosion of animals (1, 2), a rapid rise in the diversity of skeletonized metazoans (all multicellular animals more advanced than sponges and characterized by having a true gut, nervous system, and musculature). Knowledge of this radiation has depended heavily on studies of disconnected microscopic skeletal components (“small shelly fossils”) preserved by phosphatic replacement during early diagenesis. Because such fossils typically lack identifiable soft tissues, they have yielded only a coarse understanding of the earliest Cambrian, ≈542 to ≈530 million years ago Meishucun pretrilobite stage of metazoan evolution.
In south China, Meishucunian phosphates contain not only abundant small shelly fossils but also metazoan eggs (2). Of several such localities, that at Ningqiang in southern Shaanxi is particularly well known, rising to prominence in 1997 when Bengtson and Zhao first interpreted globular structures preserved there to be eggs of early metazoans (3). At this locality, abundant and diverse metazoan eggs, components of the Ningqiang fossil fauna, occur in black bituminous phosphatic limestone beds of the Lower Cambrian Kuanchuanpu formation (4). Studies of such eggs have extended the documented record of metazoans to the beginning of Cambrian time and have contributed to understanding of the long-mysterious Meishucun stage of the Cambrian evolutionary explosion (2, 4–8).
Although it is well established that phosphatization played a major role in the three-dimensional preservation of such eggs (9), new techniques provide the means to document the diagenetic sequence that resulted in their preservation, the molecular-structural composition of such fossils, and fine-scale details of their morphology. Thus, as is shown here, two-dimensional Raman imagery (10) demonstrates that embryos preserved in some Ningqiang eggs have been permineralized in a mix of phosphate and kerogen (the geochemically altered carbonaceous remnants of their original organic components), augmented by secondarily emplaced calcite; and confocal scanning laser microscopy (11), applied here to such apatite-permineralized kerogenous fossils, provides high-resolution images that reveal the presence of three-dimensionally preserved anatomical structures not readily discernable by other means. The presence of kerogen in such phosphatic fossils, and the exquisitely fine structural preservation that can thus result, has not previously been documented.
The phylum Ctenophora (also called comb jellies), having <100 living species, is composed mostly of gelatinous, planktonic, spheroidal-to-oblate voracious predators (12, 13). Discovery of especially ancient fossils of this early evolved phylum, and perhaps, most notably, of the fossil reported here that reveals details about the embryonic, early ontogenetic development of such metazoans, provides important insight into the beginnings of the evolutionary history of animals. Despite the seemingly fragile nature of ctenophorans, fossils of the phylum have been reported from younger strata of the ≈530-million-year-old Lower Cambrian Maotianshan shale of Yunnan, China (14), the Middle Cambrian Burgess shale of British Columbia, Canada (15), and the Lower Devonian Hunsrück slate of Germany (16, 17). Suggested possible ctenophoran affinities of other ancient metazoans, such as Rangea (18) from the latest Precambrian (Ediacaran), and Stromatoveris (19) from the Lower Cambrian, are as yet unsubstantiated.
Results
We here describe a ctenophore embryo from the Lower Cambrian Ningqiang fossil fauna, preserved in a 10-cm-thick bed of black bituminous phosphatic limestone near the base of the Kuanchuanpu formation, a unit that directly overlies limestones containing the terminal Precambrian diagnostic fossil Cloudina (20). This specimen represents the earliest firm evidence of the Ctenophora now known.
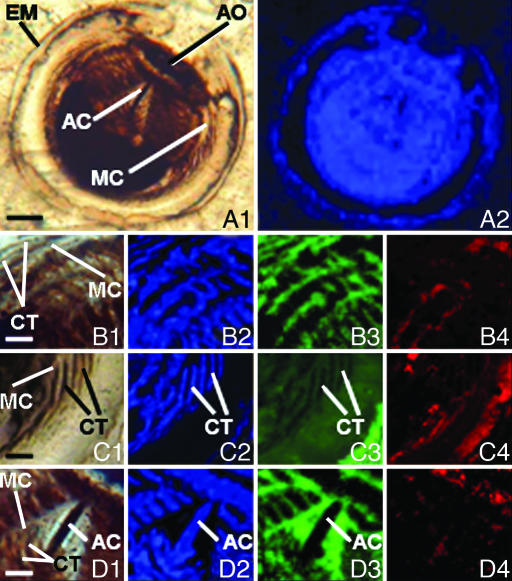
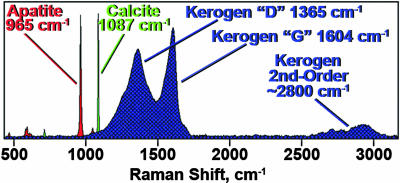
The egg containing this embryo is spheroidal, ≈190 μm in diameter, defined by an egg membrane composed of an intact outer layer (Figs. 1A1 and 3A) and a partially preserved inner layer (Fig. 3 A and C). The enclosed ball-shaped embryo is ≈150 μm in diameter and is situated centrally within the egg, prepared in a 50-μm-thick petrographic thin section that cuts obliquely through its aboral–oral axis (the plane of the section in the area opposite to the apical organ being tangent to and at or near the surface of the embryo and the remainder of the section passing through the interior of the specimen). The space between the embryo and the surrounding egg membrane is filled by early diagenetic calcite (Fig. 1C3). Studies by light microscopy (Fig. 1A1), Raman spectroscopy (Figs. 1 A2–D4 and 2), and confocal laser scanning microscopy (CLSM) (Fig. 3) show that permineralization by apatite of the kerogen that comprises this unique specimen has resulted in its exceptional preservation. Raman spectra of the specimen (Fig. 2) confirm that it is composed of kerogen, apatite, and subsidiary calcite and demonstrate that the kerogen is composed of geochemically moderately altered amorphous carbonaceous matter (interlinked polycyclic aromatic hydrocarbons) like that of other fossils of early Paleozoic and Precambrian age (10).
Fig. 1.
Optical and Raman images of a thin section-embedded ctenophore embryo from the Lower Cambrian Kuanchuanpu Formation of Ningqiang, Shaanxi Province, China. [Scale bars, 25 μm (A); 10 μm (B–D)]. The Raman images, obtained by recently documented techniques (11), are maps acquired in spectral windows centered on the major Raman bands of the materials analyzed in which varying intensities correspond to the relative concentrations of the molecular structures detected. The blue images (A2, B2, C2, and D2), acquired in a spectral window centered at ≈1,604 cm−1, show the spatial distribution of carbonaceous kerogen; green images (B3, C3, and D3), centered at ≈1,087 cm−1, show the distribution of calcite; and red images (B4, C4, and D4), centered at ≈965 cm−1, show the distribution of apatite. Optical image (A1) and Raman image (A2) of the complete embryo are shown in its kerogenous composition. AC, aboral canal; AO, apical (aboral) organ; EM, egg membrane; MC, meridional canal. Optical image (B1) and Raman images (B2–B4) of the upper left of the specimen (A1) show that it is composed of kerogen (B2) pervaded by fine-grained apatite (B4), the interstices between the structures thus preserved having been infilled by calcite (B3). Optical image (C1) and Raman images (C2–C4) of the middle-right of the specimen (A1) show that the comb plates (ctenes, denoted by CT) are composed of kerogen (C2) augmented by apatite (C4), spaces between them having been infilled by calcite (C3). Optical image (D1) and Raman images (D2–D4) are of the aboral pole of the embryo. AC, aboral canal.
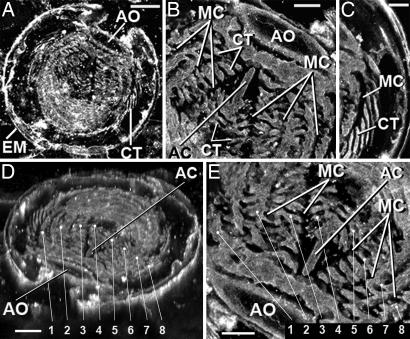
Fig. 3.
CLSM images of the thin section-embedded embryo, obtained by recently documented techniques (12). [Scale bars, 25 μm (A and D); 10 μm (B, C, and E)]. Because such CLSM images record the laser-excited fluorescence emitted by the kerogen that comprises such fossils, they provide a proxy for direct chemical analyses that show the carbonaceous composition of a specimen analyzed. (A) CLSM image of the complete embryo (see Fig. 1A). The aboral region (B) and middle-right region (C) (see Fig. 1C) of the specimen show the fine-scale morphological information provided by CLSM images. (D) Rotated CLSM image showing comb rows, numbered 1–8, that overlie meridional canals. (E) Higher-magnification image of the aboral region showing the numbered comb rows. AO, apical (aboral) organ; EM, egg membrane; CT, ctenes; AC, aboral canal; MC, meridional canal.
Fig. 2.
Overlapping Raman spectra showing the major bands of the apatite, calcite, and kerogen that comprise the embryo described here (baseline subtracted).
Two-dimensional Raman imagery shows that the fine structure of the embryo is composed of kerogen (Fig. 1 A2, B2, C2, and D2) permineralized by fine-grained apatite (Fig. 1 B4, C4, and D4), and that calcite (Fig. 1 B3, C3, and D3) has secondarily infilled interstices within this kerogen–apatite mix. Such permineralized kerogen comprises the egg membrane and such ctenophore-defining structures as comb rows, comb plates (ctenes), meridional canals, and their branches (diverticula), structures evident in optical images (Fig. 1 A1, B1, C1, and D1) and, even more so, in confocal laser scanning micrographs (Fig. 3).
This permineralized egg encloses an embryo in late development, before hatching. Enveloped by a nearly complete egg membrane, the spheroidal embryo lacks tentacles, and, although it is unhatched and much smaller, it resembles in numerous respects the ctenophore Maotianoascus octonarius from the Lower Cambrian Maotianshan shale of the immediately younger Chengjiang fauna of Yunnan, China (2, 14). On the bases of the distribution of comb rows and the free end of the comb plates in this embryo, its aboral pole, inferred to be an as yet incompletely formed apical (aboral) organ, is interpreted to be represented by its prominent circular ringed dome (Figs. 1A1 and 3 A, B, D, and E).
The comb plates diagnostic of ctenophores are represented in this embryo by meridional rows, each composed of a narrow strip-like structure that corresponds to the combs of adult ctenophores. Like the comb plates of the larvae and juveniles of modern ctenophores (21, 22), these meridional rows originate from the apical organ and are attached to the epidermis of the specimen at its aboral end. The fluid-filled, branching, tubular meridional canals that, in living ctenophores, underlie such comb plates are represented in the fossil by branching tubes filled with secondarily emplaced calcite (Fig. 1 B3, C3, and D3). Because the ≈50-μm-thick petrographic section studied (somewhat thicker than standard petrographic thin sections and about one-third of the total thickness of the original embryo) transects the medial aboral–oral axis of the specimen, it contains the portion of the embryo that exhibits a complete array of eight comb rows (Fig. 3 D and E). The total number of such comb rows thus appears to be the same as that of the Chengjiang taxon (3, 14) and most modern ctenophores (12, 13). In the medial part of the specimen (except in the area adjacent to the apical organ, as noted above), the two middle comb rows are represented by narrow transversely elongate structures (Fig. 3B). The individual comb plates (ctenes) are 15 to 20 μm long and ≈1 μm broad (Fig. 1); associated structures that were originally hollow and fluid-filled (e.g., meridional canals) and the interstices between such structures are infilled by calcite (Fig. 1 B3, C3, and D3).
Digestion of food in modern ctenophores takes place extracellularly, in the pharynx, where ingested material passes into a complex system of radiating vascular structures that include eight meridional canals, one beneath each comb row (12, 13). The meridional canals in some ctenophores are branched. The composite imagery of CLSM documents the presence of eight such branched tube-like structures in the embryo studied here (Fig. 3). The central part of the aboral hemisphere in modern ctenophores usually possesses a tube-like aboral canal (roughly comparable with the distal part of an intestine), a structure represented in this fossil by an unbranched elongate rod ≈6.5 μm in diameter that extends orally from the apical organ to a distance of 40 μm, where it is buried beneath comb rows branched meridional canals (Figs. 1A1 and 3 A, B, D, and E).
Discussion
The ctenophoran affinities of this embryo are firmly supported by the presence of eight sets of comb rows and comb plates, and of meridional and aboral canals, all having the same relative positions as those in modern ctenophores. The presence of an adult body plan and of adult-like structures, even in its prehatching embryonic condition, suggests that this ancient ctenophore embryo would have likely directly matured into a planktonic juvenile like that of most modern ctenophores.
It has been widely assumed that the ancestor of modern comb jellies closely resembled living cydippid ctenophores by being spheroidal and having eight comb rows and one pair of tentacles (12, 13, 21, 22). However, ctenophores of the Order Beroidea lack tentacles, and it has also been proposed that the ancestor of the Ctenophora was atentaculate (23). The embryo studied here closely resembles a miniaturized version of the adult atentaculate fossil ctenophore Maotianoascus octonarius from the Chengjiang fauna south China. The features shared by this Meishucun embryo and the Chengjiang taxon suggest that the common ancestor of the Ctenophora was spheroidal, had eight comb rows, and lacked tentacles. Instead of being narrow and unadorned, as in cydippids, the mouth of the Chengjiang ctenophore (2, 14) [and see supporting information (SI) Fig. 4] is surrounded by a broad skirt-like structure that, perhaps, represents an oral lobe like that of some modern ctenophores (12). Thus, members of the ancestral lineage of the Ctenophora may have had mouths surrounded by a prominent oral lobe.
The ancient ctenophore embryo studied here superficially resembles those of extant cydippidians (especially, those of Pleurobrachia) by having a globular body, but it differs by being atentaculate and having branched meridional canals. Its lack of tentacles and the presence of branched meridional canals, however, are characters shared by the embryos of extant beroidian ctenophores, with which this fossil therefore seems more closely allied. Further, its apical organ is well developed like that of the embryos of most beroidians, although its domed form resembles that of the apical organ of some cydippids (13).
On the basis of the characters exhibited by this earliest known ctenophoran embryo, we propose that the common ancestor of the phylum was an atentaculate spheroidal organism, most closely allied with extant beroidians, that had a contractible oral lobe and that used comb rows for locomotion. The pelagic habit of beroidians and most other modern ctenophores suggests that the common ancestor was also pelagic.
In contrast with this pelagic interpretation, Shu et al. (19) recently hypothesized an evolutionary link between the benthic, frondose Chengjiang fossil Stromaveris psygmoglena and modern ctenophores as well as some Ediacaran vendobionts. To link these groups, they proposed that the early evolution of ctenophores was marked by a shift from a benthic, sessile existence to a pelagic habit coupled with a change in the function of their cilia from feeding to locomotion. Such a shift would involve major changes in basic morphology and ecology and would require many (undocumented) intermediate stages. Furthermore, their interpretation of S. psygmoglena as a stem-group ctenophore is based heavily on the presence of closely spaced branches that because they are “probably ciliated” were inferred to represent precursors of the diagnostic comb rows of ctenophores (19). Given that cilia are of widespread occurrence, not only in metazoans but in protists as well, and that they have diverse functions, not only for locomotion or feeding, use of the presence of probable cilia as a prime character by which to infer a ctenophore affinity for S. psygmoglena is problematic. Similarly, the suggestion by Shu et al. (19) that the Ctenophora occupies an intermediate evolutionary position between sponges and cnidarians is inconsistent with numerous lines of evidence, both anatomical and molecular (13, 22, 24, 25).
The work presented here on the earliest ctenophoran embryo known not only documents an important find in studies of the earliest history of animal phyla, but shows also that techniques recently introduced to paleobiology, in particular, Raman imagery and CLSM, can yield important data to the understanding of life's early history.
Materials and Methods
The permineralized ctenophore embryo described here was studied in a petrographic thin section of carbonaceous phosphatic limestone collected at Ningqiang in southern Shaanxi Province, China, from a 10-cm-thick near-basal unit of the paleontologically and geologically well documented Lower Cambrian (Meishucunian) Kuanchuanpu formation (3, 4). The anatomy and morphology of this specimen were investigated by optical microscopy and CLSM (11), whereas the mineralogy of the specimen and the spatial relations between enclosing minerals and the preserved fine-structural features of the embryo were documented by Raman point spectra and two-dimensional imagery (10).
Optical Microscopy.
Studied in a ≈50-μm-thick unpolished petrographic thin section (finished by use of a slurry of 600 mesh carborundum and covered by a ≈1-μm-thick veneer of fluorescence-free microscopy immersion oil), transmitted light optical images of the fossilized embryo (Fig. 1 A1, B1, C1, and D1) were obtained by use of an Orthoplan 2 microscope (Leitz, Wetzlar, Germany) equipped with a DP12 microscope digital camera (Olympus, Melville, NY).
Raman Spectrometry.
Raman spectra of the embryo were obtained by use of a T64000 (JY Horiba, Edison, NJ) triple-stage laser-Raman system having macroRaman and confocal microRaman capability. This system permitted acquisition both of individual point spectra and of Raman images that display the two-dimensional spatial distribution of molecular-structural components of the specimen. Because of the confocal capability of the system, use of a ×50 objective (having an extended working distance of 10.6 mm and a numerical aperture of 0.5; Fig. 1A2) provided a horizontal resolution of ≈1.5 μm and a vertical resolution of ≈2–3 μm, whereas use of a ×100 objective (having an extended working distance of 3.4 mm and a numerical aperture of 0.8; Fig. 1 A2, B2–B4, C2–C4, and D2–D4) provided a horizontal resolution of ≈0.7 μm and a vertical resolution of ≈1.0–1.5 μm. As is shown in Fig. 2, use in this system of a Coherent Innova argon ion laser to provide excitation at 457.9 nm permitted data to be obtained over a range from <500 to >3,100 cm−1 by use of a single spectral window centered at 1,800 cm−1 and, thus, simultaneous acquisition of spectra of both the major bands (at ≈1,365 and ≈1,604 cm−1) and the second-order band (at ≈2,800 cm−1) of the kerogen comprising the fossil as well as the major band of the permineralizing apatite (at ≈965 cm−1) and that of secondarily emplaced calcite (at 1,087 cm−1).
For two-dimensional imaging of the specimen, the region of the thin section containing the fossilized embryo was covered by a thin veneer of fluorescence-free microscopy immersion oil (the presence of which has been shown to have no discernable effect on the Raman spectra acquired; 10), and the area of the fossil to be analyzed was centered in the path of the laser beam projected through an Olympus BX41 microscope. The laser power used was ≈1–8 mW over a ≈1-μm spot, an instrumental configuration well below the threshold resulting in radiation damage to a specimen such as that here studied (10).
CLSM.
The three-dimensional confocal fluorescence images shown in Fig. 3 were obtained by use of an Olympus Fluoview 300 confocal laser scanning biological microscope system composed of a BX51 microscope, a 488 nm 20mW-output argon ion laser (Melles Griot, Carlsbad, CA), a FV-SU303 scanning unit, and associated Fluoview software. Previously identified by optical microscopy, the fossil embryo was relocated for CLSM analysis by use of an Olympus FV5-TD transmitted light detector attached to the Fluoview System. Images were acquired by use of a ×60 PLAPO (numerical aperture = 1.4) oil-immersion objective (with the fluorescence-free microscopy immersion oil noted above) and an Olympus BA510IF filter in the light path of the system that removed wavelengths <510 nm from the kerogen-derived fluorescence emitted from the specimen. The images shown in Fig. 3 A–C and E were rendered by using the Fluoview software of the Olympus Fluoview 300 system, whereas the other CLSM-based image shown (Fig. 3D) is a derivative, processed by use of the VolView v2.0 3D-rendering computer program that permitted its manipulation in three dimensions.
Supplementary Material
Acknowledgments
We thank C. Marshall and K. Peterson for reviews of an earlier version of this article, and S. Q. Dornbos, J. Mallatt, and Y. Shen for helpful comments on the submitted manuscript. This research was supported by Chinese Academy of Science Grant KZCX3-SW-141; National Science Foundation of China Grant 40432006; National Basic Research Program of China (2006CB 806400); 111 Project and 985–2 project of Nanjing University. Raman and CLSM studies at University of California, Los Angeles (UCLA), were supported by National Aeronautics and Space Administration Exobiology Grant NAG5–12357 (to J.W.S.) and by the Institute of Geophysics and Planetary Physics, Center for the Study of Evolution and the Origin of Life, UCLA.
Abbreviation
- CLSM
confocal laser scanning microscopy.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0701246104/DC1.
References
- 1.Qian Y. Early Cambrian Small Shelly Fossils of China with Special Reference to the Precambrian–Cambrian Boundary. Nanjing, China: Nanjing Univ Press; 1989. [Google Scholar]
- 2.Chen J-Y. The Dawn of Animal World. Nanjing, China: Jiangsu Sci and Technol; 2004. [Google Scholar]
- 3.Bengtson A, Zhao Y. Science. 1997;277:1645–1648. [Google Scholar]
- 4.Chen JY, Braun A, Waloszek D, Peng Q-Q, Maas A. Prog Nat Sci. 2004;14:167–172. [Google Scholar]
- 5.Donoghue PCJ, Bengston S, Dong X-P, Gostling NJ, Huldgren T, Cunningham JA, Yin C, Yue Z, Peng F, Stampanoni M. Nature. 2006;442:680–683. doi: 10.1038/nature04890. [DOI] [PubMed] [Google Scholar]
- 6.Yue Z, Bengtson S. Lethaia. 1999;32:81–196. [Google Scholar]
- 7.Steiner M, Zhu M, Li G, Quian Y, Erdtmann B-D. Geology. 2004;32:833–836. [Google Scholar]
- 8.Steiner M, Li G-X, Zhu M. Geobios. 2004;37:259–275. [Google Scholar]
- 9.Dornbos QS, Bottjer DJ, Chen J-Y, Oliveri P, Gao F, Li C-W. Lethaia. 2005;38:101–109. [Google Scholar]
- 10.Schopf JW, Kudryavtsev AB, Agresti AG, Czaja AD, Wdowiak TJ. Astrobiology. 2005;5:333–371. doi: 10.1089/ast.2005.5.333. [DOI] [PubMed] [Google Scholar]
- 11.Schopf JW, Tripathi AB, Kudryavtsev AB. Astrobiology. 2006;6:1–16. doi: 10.1089/ast.2006.6.1. [DOI] [PubMed] [Google Scholar]
- 12.Brusca RC, Brusca GJ. Invertebrates. Sunderland, MA: Sinauer; 1990. [Google Scholar]
- 13.Ruppert ER, Fox RS, Barnes RD. Invertebrate Zoology. Ed 7. Cincinnati: Thomson Brooks Cole; 2004. [Google Scholar]
- 14.Chen JY, Zhou GQ. Bull Natl Mus Nat Sci Taichung Taiwan. 1997;10:11–105. [Google Scholar]
- 15.Conway MS, Collins DH. Philos Trans R Soc B. 1996;346:305–358. [Google Scholar]
- 16.Stanley G, Jr, Stürmer W. Nature. 1983;303:518–520. [Google Scholar]
- 17.Stanley G, Jr, Stürmer W. Nature. 1987;327:61–63. [Google Scholar]
- 18.Dzik J. J Morphol. 2002;252:315–334. doi: 10.1002/jmor.1108. [DOI] [PubMed] [Google Scholar]
- 19.Shu D-G, Morris SC, Han J, Li Y, Zhang X-L, Hua H, Zhang Z-F, Liu J-N, Guo J-F, Yao Y, et al. Science. 2006;312:731–734. doi: 10.1126/science.1124565. [DOI] [PubMed] [Google Scholar]
- 20.Chen Z, Sun W-G. Acta Micropalaeontol Sinica. 2001;18:180–202. [Google Scholar]
- 21.Hyman LH. The Invertebrates. Vol 1. New York: McGraw–Hill; 1940. pp. 662–696. [Google Scholar]
- 22.Nielsen C. Animal Evolution. Ed 2. Oxford: Oxford Univ Press; 2001. [Google Scholar]
- 23.Harbison GR. In: The Origins and Relationships of Lower Invertebrates. Morris SC, George JD, Gibson R, Platt HM, editors. Oxford: Oxford Univ Press; 1985. pp. 78–100. [Google Scholar]
- 24.Ax P. Multicellular Animals. Vol 1. New York: Springer; 1996. [Google Scholar]
- 25.Finnerty JR, Master VA, Irvine S, Kourakis MJ, Warriner S, Martindale MQ. Mol Mar Biol Biotechnol. 1996;5:249–258. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.