Abstract
Published evidence suggests that adiposity in humans may be linked to impaired energy expenditure for reasons widely unresolved. We have generated mice with a systemic impairment of oxidative phosphorylation (OXPHOS) due to aP2 cre-mediated targeted disruption, and unexpectedly ubiquitous reduction of mitochondrial frataxin protein expression. Only when maintained on a high-calorie diet resembling Westernized eating habits, these animals accumulate additional body fat, leading to increased body mass, and develop diabetes mellitus, despite the fact that both calorie uptake and physical activity were identical to that in control animals. This phenotype is caused by a mild but significant reduction in total energy expenditure paralleled by increased expression of ATP citrate lyase, a rate-limiting step in de novo synthesis of fatty acids and triglycerides. Taken together, these findings indicate that a limited impairment in oxidative metabolism within the mitochondria directly predisposes mammals to excessive body weight gain.
Keywords: metabolism, mitochondria, oxidative phosphorylation
Mitochondria are subcellular organelles where conversion of nutrient intermediates into readily available energy equivalents takes place. This conversion involves a process called oxidative phosphorylation (OXPHOS) generating adenosine triphosphate (ATP). Recent scientific evidence has implicated mitochondrial dysfunction in several human diseases, including cancer (1–4), neurodegenerative disorders (5, 6), and type 2 diabetes mellitus (7–12). Of note, the latter disease is frequently associated with increased body mass (13, 14). Obesity is a major risk factor for the development of type 2 diabetes, presumably by causing insulin resistance, i.e., impaired insulin action on peripheral tissues including adipocytes and skeletal muscle (14, 15).
Excessive accumulation of body fat in humans is the most prevalent health problem in Westernized countries. It is generally accepted that obesity is caused by an imbalance of energy uptake and energy expenditure, cumulating in storage of excess calories mainly as body fat (13). Accordingly, it has been known for many years that an unexplained decrease in energy expenditure results in a predisposition to obesity in humans (16). Because most nutritive energy is ultimately converted within the mitochondria, researchers have repeatedly addressed the question whether altered mitochondrial activity might influence body mass in mammals. For example, thyroid hormones have been shown to regulate body mass by altering mitochondrial metabolism (17). Furthermore, increased expression of mitochondrial uncoupling proteins (UCPs) or a transcriptional coactivator, PGC1, has been suggested to promote systemic energy expenditure because of thermogenic effects, i.e., generation of heat (18–21) and a concurrent decrease in OXPHOS (22).
We here address the question whether a protein known to cause a neurodegenerative disorder named Friedreich ataxia (www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=229300) might be used as a regulator of OXPHOS capacity in mammals. This protein is called frataxin and has been shown to be encoded in the nucleus whereas its N-terminal localization signal directs it posttranslationally mainly into the mitochondrial matrix compartment (www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=229300). Overexpression studies have shown that frataxin increases mitochondrial membrane potential, oxygen consumption and ATP synthesis, and hence might be considered as an inducer of OXPHOS in mammalian cells in vitro (23, 24). The primary function of frataxin is to direct the mitochondrial synthesis of iron-sulfur clusters (Fe/S) (25, 26), which are essential parts of several mitochondrial enzymes, including aconitase, and complexes I, II and III of the respiratory chain (27). Accordingly, mammals with impaired frataxin expression exhibit tissue-specific impairments of the corresponding enzyme activities, whereas other mitochondrial enzymes remain unaffected (2).
By reducing expression of frataxin in mice we have now generated a model with limited impairment of OXPHOS in a non-tissue specific manner. These mice develop increased obesity and glucose intolerance only when maintained on a so-called Western diet because of impaired energy expenditure and concomitant increase of a rate-limiting step in de novo lipogenesis. These findings indicate that mildly impaired OXPHOS directly predisposes mice to obesity.
Results
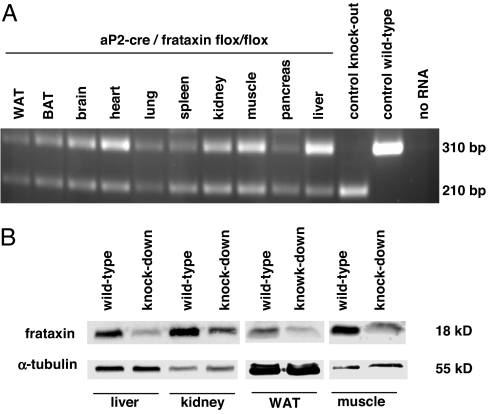
We originally aimed to disrupt expression of murine frataxin in an adipose-tissue specific manner by employing mice carrying loxP-flanked frataxin alleles (28) which were intercrossed with aP2-cre mice (29). The latter have been successfully used to disrupt expression of the glucose transporter GLUT4 (29) and the insulin receptor (30, 31) in adipose tissue only. In contrast, loxP-flanked animals intercrossed with the original aP2 cre line (29) in our hands showed an ubiquitous but incomplete disruption of the frataxin gene (Fig. 1), whereas other frataxin knockout animals generated by using an Albumin-cre line showed a complete and tissue specific disruption of the gene of interest [Fig. 1A, “control knockout” and (2)]. The reason for this inconsistency of expression patterns of this particular aP2-cre line in our hands versus previously published data (29–31) remains to be evaluated. Nevertheless, disruption of frataxin expression lead to a significantly reduced expression of frataxin protein in all tissues evaluated (Fig. 1B and additional data not shown). Of note and unlike mice with neuronal disruption of frataxin (28), our mice did not shown any signs of ataxia, indicating that these animals should not necessarily be considered a model for Friedreich ataxia.
Fig. 1.
Ubiquitous partial deletion of exon 4 of the frataxin gene after aP2 promoter controlled expression of cre recombinase. (A) Depicted is an RT-PCR using primers located in exon 3 and exon 5 of the murine frataxin gene, while loxP sites flanked exon 4 (2, 10, 28); “control knock-out” refers to an albumin-cre-controlled complete frataxin knockout (2), and “control wild-type” refers to an animal lacking any cre transgene. (B) Systemic reduction of frataxin protein expression shown by an immunoblot using a primary antibody against murine frataxin (Upper lane) and a primary antibody against alpha-tubulin (Lower lane, loading control).
Based on previously published findings that frataxin promotes synthesis of iron-sulfur clusters (Fe/S) (25, 26) and thus induces OXPHOS (23), we first questioned whether aP2-frataxin knockdown mice would show impaired ATP synthesis. Whereas complete disruption of frataxin in, e.g., hepatic tissue causes a severe reduction of ATP content by ≈80% in the fed state (2), aP2-frataxin knockdown mice showed a significant reduction of hepatic ATP content in the fasted, i.e., overnight food-deprived state only (reduction by 38%, data not depicted). Given the fact that mitochondrial activity in yeast (32) and mammals (33) is preferably induced in states of food deprivation, it is not surprising that differences in OXPHOS capacity become unmasked preferably in the fasted state.
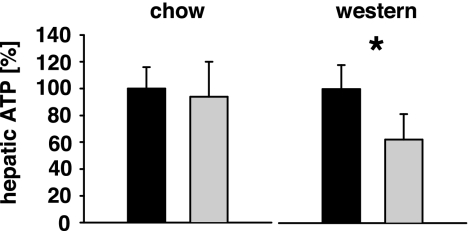
At an age of 4 weeks, aP2-frataxin knockdown mice were matched by sex and body mass, and then randomly assigned into two groups. One group was kept on fiber-rich, low-fat standard rodent chow (Table 1), whereas the other group was maintained on a so-called Western diet enriched in sugars, proteins and fat (Table 1). Based on the above-mentioned differences in e.g., hepatic ATP content in fasted state we wondered whether the dietary regimen would affect OXPHOS, i.e., ATP content. Indeed, whereas no differences in e.g., hepatic ATP within fed animals was seen between knockdown and control mice when maintained on standard chow (Fig. 2), knockdown mice showed a significant reduction in ATP content when fed a Western diet (Fig. 2). Taken together, this finding indicated that knockdown mice have a limited impairment of OXPHOS irrespective of the diet in the fasted state, and reduced OXPHOS capacity in the fed state only when maintained on a Western diet.
Table 1.
Composition of diets
| Type | Standard chow | Western diet |
|---|---|---|
| Proteins, g/kg | 190 | 265 |
| Polysaccharides, g/kg | 317 | 177 |
| Disaccharides, g/kg | 54 | 108 |
| Lipids, g/kg | 40 | 209 |
| Cholesterol, g/kg | <0.001 | 0.015 |
| Convertible energy, kJ/g | 9 | 17 |
Fig. 2.
Limited impairment of oxidative phosphorylation in aP2-frataxin knockdown mice. Relative hepatic ATP content in the fed state depicted for mice on standard rodent chow (Left) and Western diet (Right). Black bars, control animals (100%); gray bars, frataxin knockdown animals (colors apply to all subsequent panels) (n = 6 per genotype). Error bars, SEM; ∗, P < 0.05.
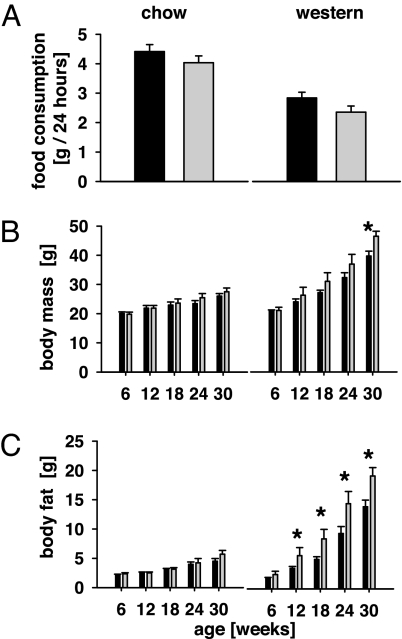
When comparing standard chow and Western diet-fed animals, daily food uptake was similar between aP2-frataxin knockdown mice and control animals in each group (Fig. 3A). Nevertheless and consistent with previously published evidence, animals regardless of the genotype consumed more food when maintained on low-calorie chow in comparison with animals kept on Western diet (Fig. 3A). Accordingly, daily energy uptake in control animals versus knockdown mice was 38.9 ± 2.2 versus 36.3 ± 2.1 kJ/day when kept on a standard chow, whereas animals maintained on a Western diet ingested 48.2 ± 3.3 versus 40.0 ± 3.6 kJ per day (controls vs. knockdown). Given the findings in regards to reduced ATP content (Fig. 2), these data (Fig. 3A) also suggest that ingested energy and OXPHOS capacity are not fully dissociated in mice with impaired mitochondrial function due to frataxin deficiency, and may reflect a functionally maintained, neuroendocrine regulation of energy uptake (34).
Fig. 3.
Eating behavior and body composition in aP2-frataxin knockdown mice. (A) Food uptake per individual mouse, depicted for mice on standard rodent chow (Left) and Western diet (Right) (n = 6 per genotype). (B) Body mass in mice with impaired mitochondrial capacity on standard rodent chow (Left) and on Western diet (Right) (n = 8 per genotype). (C) Body fat content in mice with impaired mitochondrial capacity on standard rodent chow (Left) and on Western diet (Right) (n = 8 per genotype). ∗, P < 0.05.
Next, body mass was determined noninvasively by magnetic resonance, and revealed no significant differences in animals maintained on low-calorie chow (Fig. 3B), whereas aP2-frataxin knockdown mice kept on Western diet had a significantly greater body mass starting at 30 weeks of age (Fig. 3B). Whereas lean body mass never showed any significant difference at any time point including 30 weeks (data not shown), body fat content was found to be significantly increased in aP2-frataxin knockdown mice kept on a Western diet starting a 12 weeks of age, i.e., 8 weeks after Western diet was initiated (Fig. 3C). These findings so far indicate that impaired expression of frataxin causes increased body mass because of a specific increase in body fat in animals kept on a Western diet only.
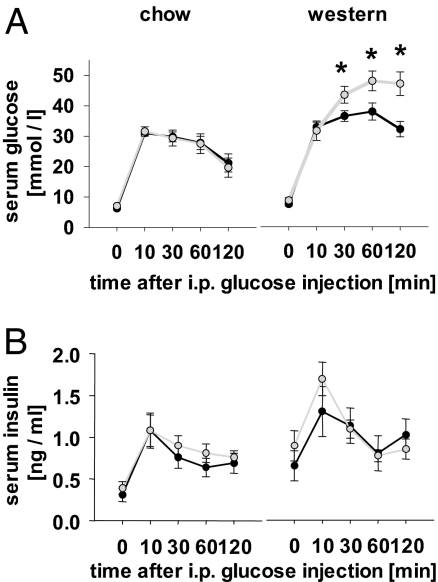
Subsequently, animals were subjected to i.p. glucose tolerance tests at an age of 24 weeks. Animals of each genotype showed no significant differences in regards to neither glucose tolerance (Fig. 4A) or insulin secretion (Fig. 4B) when maintained on low-calorie chow. In contrast, all animals on Western diet showed an impairment of glucose disposal in comparison with animals on standard chow (Fig. 4A). More importantly, aP2-frataxin knockdown mice on Western diet showed an additional impairment in glucose disposal in comparison with control littermates on Western diet (Fig. 4A). It should be noted that because body fat was already increased at 24 weeks of age in aP2-frataxin knockdown mice, the additional reduction in glucose disposal in aP2-frataxin knockdown mice might solely reflect an effect of increased body fat known to cause insulin resistance and glucose intolerance.
Fig. 4.
Glucose metabolism in aP2-frataxin knockdown mice. (A) Serum glucose excursions after i.p. injection of d-glucose into mice with impaired mitochondrial capacity on standard rodent chow (Left) and on Western diet (Right) (n = 6 per genotype). (B) Serum insulin excursions after injection of d-glucose as in A. ∗, P < 0.05.
Because complete disruption of frataxin in pancreatic beta-cells has been shown to cause progressive loss of pancreatic islets (10), whereas no alterations in individual secretory capacity of isolated islets was observed (10), we tested whether partial systemic disruption of frataxin might affect insulin secretion of aP2-frataxin knockdown mice. As shown in Fig. 4B, insulin secretion after i.p. injection of glucose was similar in aP2-frataxin knockdown mice and control littermates on a Western diet, indicating that differences in glucose tolerance (Fig. 4A) are mainly caused by insulin resistance. Nevertheless, increased circulating free fatty acids as typically observed in animals on a Western diet may have additional effects on insulin secretion and subsequently glucose metabolism.
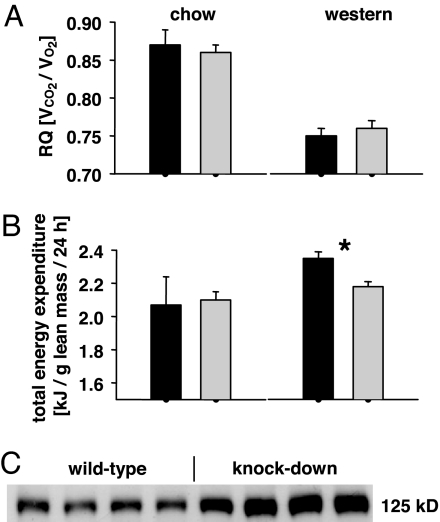
Given the fact that energy uptake was similar (Fig. 3A), we questioned whether differences in body fat content and, later on, body mass might be caused by differences in systemic energy expenditure. First, we quantified locomotor activity in all groups, and observed no differences between any genotype within the respective feeding regimen (data not shown). Hence, differences in physical activity cannot explain the observed changes in body fat content and body mass. We therefore subjected mice to indirect calorimetry. As to be expected, no differences were observed in regards to respiratory quotient within the respective feeding regimen (Fig. 5A), and increased fat content decreased the respiratory quotient in the Western diet group (Fig. 5A). Interestingly, we observed a significant decrease in energy expenditure in aP2-frataxin knockdown mice when kept on a Western diet (Fig. 5B), whereas no differences were observed in mice kept on standard chow (Fig. 5B). This finding explains the increases in body fat content and body mass despite similar food uptake rates and physical activity levels in aP2-frataxin knockdown mice, and ideally reflects the initially described decrease in mitochondrial oxidation rate (Fig. 2). Lastly, we questioned whether this frataxin-mediated impairment in mitochondrial nutrient oxidation would initiate an increase in de novo synthesis of fatty acids in animals on a Western diet. Consistent with this assumption we observed an increase expression of ATP citrate lyase (Fig. 5C), a rate-limiting step in fatty acid synthesis, in such animals only.
Fig. 5.
Metabolic and molecular consequences of aP2-frataxin knockdown. (A) Respiratory quotient of mice with impaired mitochondrial capacity on standard rodent chow (Left) and on Western diet (Right) (12 weeks of age, n = 6 per genotype). (B) Total energy expenditure of the same mice as in A. (C) Basal expression of hepatic ATP citrate lyase; protein samples from different animals (n = 4 per genotype) were used. ∗, P < 0.05.
Discussion
We here show that a limited reduction of mitochondrial energy conversion, and specifically of OXPHOS, leads to increased body fat accumulation in mice maintained on a diet resembling Westernized eating habits. This finding is explained by a concurrent decrease in systemic energy expenditure, and increased expression of ATP citrate lyase, a rate-limiting step in fatty acid synthesis.
In higher eukaryotic organisms, dissipation of nutritive energy mainly occurs via the mitochondria. Our findings suggest that increased long-term storage of nutritional energy as triglycerides may compensate for states of reduced OXPHOS capacity, leading to reduced amounts of short-term, energy-rich intermediates, namely ATP. These findings may explain why an unexplained decrease in energy expenditure results in a predisposition to obesity in humans (16). In this regard, impaired systemic substrate oxidation has been suggested to be a predisposing factor for obesity (35). Given the fact that in our model similar rates of food uptake and physical activity nevertheless cause significant differences in body mass and body triglyceride content, impaired OXPHOS capacity might possibly provide a biochemical basis for striking phenotypical differences in humans despite very similar eating habits. They might also provide a mechanistic basis for the well-known fact that human body mass typically increases with age, because OXPHOS capacity in humans has been shown to be significantly decreased in the elderly (36) suggesting that a similar tendency may apply to middle-aged individuals as well.
It has been shown that thyroid hormones as well as polyunsaturated fatty acids, both known to limit body mass gain, induce OXPHOS in e.g., isolated hepatocytes (37). Conversely, thyroid hormones induce expression of several components of the respiratory chain (38). In obese humans known to show impaired mitochondrial substrate oxidation (35), reduced thyroid function was proposed to be a potential cause for increased body weight (17) and reduced oxidative capacity (39, 40) potentially by induction of mitochondrial uncoupling (41). The latter, also named adaptive thermogenesis, was proposed to induce energy expenditure by dissipation of energy after a thermogenic proton leak reducing the mitochondrial membrane potential, ultimately leading to reduced long-term energy storage. Specifically, several UCPs, as well as subtypes of the transcriptional coactivator PGC1, have been shown to increase energy expenditure presumably by generation of heat (18–21). In regards to the present model, it should be noted that UCPs reduce OXPHOS capacity, i.e., limit ATP production (22). In contrast, there is sufficient evidence that frataxin rather induces OXPHOS by increasing mitochondrial membrane potential (23, 24). Accordingly the effects of thermogenic activators, including UCPs and PGC1 on body mass may be considered divergent from an induction of OXPHOS by other mitochondrial proteins, namely frataxin.
Taken together, our findings suggest that reduced expression of frataxin mildly decreases OXPHOS capacity to significantly promote de novo synthesis of triglycerides and hence to exacerbate obesity in mammals. These findings suggest that a limited systemic reduction in oxidative capacity due to genetic or environmental reasons may predispose mammals to body mass gain.
Materials and Methods
Targeted Disruption of Frataxin Gene.
Frataxin was disrupted by removing exon 4 of the corresponding gene in C57BL/6 mice as described (2, 10, 28) except that systemic expression of Cre recombinase was obtained by using a subline of mice carrying an aP2 promoter-driven Cre transgene (29). Expression of aP2-driven Cre recombinase was obtained with a single founder kindly provided by BIDMC (Boston, MA) reported to be >90% C57BL/6.
Hepatic ATP Content.
Methods have been described (2, 42), except that HPLC equipment was from Jasco (MD-1510; Gross-Umstadt, Germany) and a Jasco reverse phase column was used.
Housing and Physiologic Measurements of Mice.
Housing conditions and methods for determination of body mass, body composition, locomotor activity, and energy expenditure have been described (10, 43). Footprint analysis to detect possible signs of ataxia has been described (44).
Immunoblotting.
Methods have been described (2). Additional antibodies were against basal ATP citrate lyase and phosphorylated ATP citrate lyase (both from Cell Signaling Technology, Danvers, MA).
i.p. Glucose Tolerance Tests.
Methods have been described (10) and were preferred over oral glucose tolerance tests because the i.p. application of glucose requires no anesthesia known to severely affect blood glucose levels (45).
Determination of Serum Insulin Levels.
Methods have been described (10).
Statistical Analyses.
Methods have been described (10).
Acknowledgments
We thank Susann Richter and Elke Thom for excellent technical assistance; Barbara B. Kahn (Beth Israel Deaconess Medical Center) for providing the aP2-cre line; and Barbara B. Kahn, Hélène Puccio, and Randy J. Seeley for comments on the manuscript. This study was funded by grants from the Deutsche Forschungsgemeinschaft (DFG), the Wilhelm-Sander-Stiftung, and the Fritz-Thyssen-Stiftung (all to M.R.).
Abbreviations
- OXPHOS
oxidative phosphorylation
- UCP
uncoupling protein.
Footnotes
The authors declare no conflict of interest.
References
- 1.Ames BN. EMBO Rep. 2005;6:S20–S24. doi: 10.1038/sj.embor.7400426. (Spec No) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thierbach R, Schulz TJ, Isken F, Voigt A, Mietzner B, Drewes G, von Kleist-Retzow JC, Wiesner RJ, Magnuson MA, Puccio H, et al. Hum Mol Genet. 2005;14:3857–3864. doi: 10.1093/hmg/ddi410. [DOI] [PubMed] [Google Scholar]
- 3.Ristow M. Curr Opin Clin Nutr Metabol. 2006;9:339–345. doi: 10.1097/01.mco.0000232892.43921.98. [DOI] [PubMed] [Google Scholar]
- 4.Schulz TJ, Thierbach R, Voigt A, Drewes G, Mietzner BH, Steinberg P, Pfeiffer AF, Ristow M. J Biol Chem. 2006;281:977–981. doi: 10.1074/jbc.M511064200. [DOI] [PubMed] [Google Scholar]
- 5.Ames BN. Ann NY Acad Sci. 2004;1019:406–411. doi: 10.1196/annals.1297.073. [DOI] [PubMed] [Google Scholar]
- 6.Manfredi G, Beal MF. Brain Pathol. 2000;10:462–472. doi: 10.1111/j.1750-3639.2000.tb00278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van den Ouweland JM, Lemkes HH, Ruitenbeek W, Sandkuijl LA, de Vijlder MF, Struyvenberg PA, van de Kamp JJ, Maassen JA. Nat Genet. 1992;1:368–371. doi: 10.1038/ng0892-368. [DOI] [PubMed] [Google Scholar]
- 8.Ristow M, Giannakidou E, Hebinck J, Busch K, Vorgerd M, Kotzka J, Knebel B, Müller-Berghaus J, Epplen C, Pfeiffer A, et al. Diabetes. 1998;47:851–854. doi: 10.2337/diabetes.47.5.851. [DOI] [PubMed] [Google Scholar]
- 9.Hebinck J, Hardt C, Schöls L, Vorgerd M, Briedigkeit L, Kahn CR, Ristow M. Diabetes. 2000;49:1604–1607. doi: 10.2337/diabetes.49.9.1604. [DOI] [PubMed] [Google Scholar]
- 10.Ristow M, Mulder H, Pomplun D, Schulz TJ, Müller-Schmehl K, Krause A, Fex M, Puccio H, Müller J, Isken F, et al. J Clin Invest. 2003;112:527–534. doi: 10.1172/JCI18107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. N Engl J Med. 2004;350:664–671. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ristow M. J Mol Med. 2004;82:510–529. doi: 10.1007/s00109-004-0552-1. [DOI] [PubMed] [Google Scholar]
- 13.Spiegelman BM, Flier JS. Cell. 2001;104:531–543. doi: 10.1016/s0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- 14.Chiasson JL, Rabasa-Lhoret R. Diabetes. 2004;53(Suppl 3):S34–S38. doi: 10.2337/diabetes.53.suppl_3.s34. [DOI] [PubMed] [Google Scholar]
- 15.Kahn CR. Diabetes. 1994;43:1066–1084. doi: 10.2337/diab.43.8.1066. [DOI] [PubMed] [Google Scholar]
- 16.Ravussin E, Lillioja S, Knowler WC, Christin L, Freymond D, Abbott WG, Boyce V, Howard BV, Bogardus C. N Engl J Med. 1988;318:467–472. doi: 10.1056/NEJM198802253180802. [DOI] [PubMed] [Google Scholar]
- 17.Kyle LH, Ball MF, Doolan PD. N Engl J Med. 1966;275:12–17. doi: 10.1056/NEJM196607072750103. [DOI] [PubMed] [Google Scholar]
- 18.Lowell BB, S-Susulic V, Hamann A, Lawitts JA, Himms-Hagen J, Boyer BB, Kozak LP, Flier JS. Nature. 1993;366:740–742. doi: 10.1038/366740a0. [DOI] [PubMed] [Google Scholar]
- 19.Kopecky J, Clarke G, Enerback S, Spiegelman B, Kozak LP. J Clin Invest. 1995;96:2914–2923. doi: 10.1172/JCI118363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell BB, Scarpulla RC, Spiegelman BM. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 21.St Pierre J, Lin J, Krauss S, Tarr PT, Yang R, Newgard CB, Spiegelman BM. J Biol Chem. 2003;278:26597–26603. doi: 10.1074/jbc.M301850200. [DOI] [PubMed] [Google Scholar]
- 22.Cline GW, Vidal-Puig AJ, Dufour S, Cadman KS, Lowell BB, Shulman GI. J Biol Chem. 2001;276:20240–20244. doi: 10.1074/jbc.M102540200. [DOI] [PubMed] [Google Scholar]
- 23.Ristow M, Pfister MF, Yee AJ, Schubert M, Michael L, Zhang CY, Ueki K, Michael MD, II, Lowell BB, Kahn CR. Proc Natl Acad Sci USA. 2000;97:12239–12243. doi: 10.1073/pnas.220403797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonzalez-Cabo P, Vazquez-Manrique RP, Garcia-Gimeno MA, Sanz P, Palau F. Hum Mol Genet. 2005;14:2091–2098. doi: 10.1093/hmg/ddi214. [DOI] [PubMed] [Google Scholar]
- 25.Rötig A, de Lonlay P, Chretien D, Foury F, Koenig M, Sidi D, Munnich A, Rustin P. Nat Genet. 1997;17:215–217. doi: 10.1038/ng1097-215. [DOI] [PubMed] [Google Scholar]
- 26.Mühlenhoff U, Richhardt N, Ristow M, Kispal G, Lill R. Hum Mol Genet. 2002;11:2025–2036. doi: 10.1093/hmg/11.17.2025. [DOI] [PubMed] [Google Scholar]
- 27.Lill R, Mühlenhoff U. Trends Biochem Sci. 2005;30:133–141. doi: 10.1016/j.tibs.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 28.Puccio H, Simon D, Cossee M, Criqui-Filipe P, Tiziano F, Melki J, Hindelang C, Matyas R, Rustin P, Koenig M. Nat Genet. 2001;27:181–186. doi: 10.1038/84818. [DOI] [PubMed] [Google Scholar]
- 29.Abel ED, Peroni O, Kim JK, Kim YB, Boss O, Hadro E, Minnemann T, Shulman GI, Kahn BB. Nature. 2001;409:729–733. doi: 10.1038/35055575. [DOI] [PubMed] [Google Scholar]
- 30.Blüher M, Michael MD, Peroni OD, Ueki K, Carter N, Kahn BB, Kahn CR. Dev Cell. 2002;3:25–38. doi: 10.1016/s1534-5807(02)00199-5. [DOI] [PubMed] [Google Scholar]
- 31.Blüher M, Kahn BB, Kahn CR. Science. 2003;299:572–574. doi: 10.1126/science.1078223. [DOI] [PubMed] [Google Scholar]
- 32.Lin SJ, Kaeberlein M, Andalis AA, Sturtz LA, Defossez PA, Culotta VC, Fink GR, Guarente L. Nature. 2002;418:344–348. doi: 10.1038/nature00829. [DOI] [PubMed] [Google Scholar]
- 33.Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, Falcone S, Valerio A, Cantoni O, Clementi E, et al. Science. 2005;310:314–317. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- 34.Castaneda TR, Jurgens H, Wiedmer P, Pfluger P, Diano S, Horvath TL, Tang-Christensen M, Tschop MH. J Nutr. 2005;135:1314–1319. doi: 10.1093/jn/135.5.1314. [DOI] [PubMed] [Google Scholar]
- 35.Astrup A, Buemann B, Toubro S, Raben A. Proc Nutr Soc. 1996;55:817–828. doi: 10.1079/pns19960081. [DOI] [PubMed] [Google Scholar]
- 36.Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI. Science. 2003;300:1140–1142. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nogueira V, Rigoulet M, Piquet MA, Devin A, Fontaine E, Leverve XM. J Biol Chem. 2001;276:46104–46110. doi: 10.1074/jbc.M107425200. [DOI] [PubMed] [Google Scholar]
- 38.Wiesner RJ, Kurowski TT, Zak R. Mol Endocrinol. 1992;6:1458–1467. doi: 10.1210/mend.6.9.1331777. [DOI] [PubMed] [Google Scholar]
- 39.Heald FP. J Pediatr. 1962;61:327–330. doi: 10.1016/s0022-3476(62)80306-0. [DOI] [PubMed] [Google Scholar]
- 40.Astrup A, Buemann B, Toubro S, Ranneries C, Raben A. Am J Clin Nutr. 1996;63:879–883. doi: 10.1093/ajcn/63.6.879. [DOI] [PubMed] [Google Scholar]
- 41.Lebon V, Dufour S, Petersen KF, Ren J, Jucker BM, Slezak LA, Cline GW, Rothman DL, Shulman GI. J Clin Invest. 2001;108:733–737. doi: 10.1172/JCI11775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Di Pierro D, Tavazzi B, Perno CF, Bartolini M, Balestra E, Calio R, Giardina B, Lazzarino G. Anal Biochem. 1995;231:407–412. doi: 10.1006/abio.1995.0071. [DOI] [PubMed] [Google Scholar]
- 43.Jürgens H, Haass W, Castaneda TR, Schürmann A, Koebnick C, Dombrowski F, Otto B, Nawrocki AR, Scherer PE, Spranger J, et al. Obes Res. 2005;13:1146–1156. doi: 10.1038/oby.2005.136. [DOI] [PubMed] [Google Scholar]
- 44.Simon D, Seznec H, Gansmuller A, Carelle N, Weber P, Metzger D, Rustin P, Koenig M, Puccio H. J Neurosci. 2004;24:1987–1995. doi: 10.1523/JNEUROSCI.4549-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pomplun D, Mohlig M, Spranger J, Pfeiffer AF, Ristow M. Horm Metab Res. 2004;36:67–69. doi: 10.1055/s-2004-814104. [DOI] [PubMed] [Google Scholar]