Abstract
It has been shown previously using in vivo and ex vivo animal models, that cyclical mechanical stimulation is capable of maintaining osteocyte viability through the control of apoptotic cell death. Here we have studied the effect of mechanical stimulation on osteocyte viability in human trabecular bone maintained in a 3-D bioreactor system. Bone samples, maintained in the bioreactor system for periods of 3, 7 and 27 days, were subjected to either cyclical mechanical stimulation which engendered a maximum of 3,000 μstrain in a waveform corresponding to physiological jumping exercise for 5 minutes daily or control unloading. Unloading resulted in a decrease in osteocyte viability within 3 days that was accompanied by increased levels of cellular apoptosis. Mechanical stimulation significantly reduced apoptosis (p≤0.032) and improved the maintenance of osteocyte viability in bone from all patient samples. The percentage Alkaline Phosphatase (ALP) labelled bone surface was significantly increased (p≤0.05) in response to mechanical stimulation in all samples as was the Bone Formation Rate (BFR/BS) (p=0.005) as determined by calcein label incorporation in the 27-day experiment. These data indicate that in this model system, mechanical stimulation is capable of maintaining osteocyte viability in human bone.
Keywords: Human Bone, Mechanical Stimulation, Osteocyte, Viability, Apoptosis
Introduction
The role of the osteocyte in skeletal health and disease has become the topic of a large amount of research effort. Until relatively recently, the osteocyte was considered to be comparatively inactive. Despite being the most common cell type in bone, occupying an intriguing anatomical location within mineralised matrix with the clear formation of a syncitial network, the osteocyte was without a defined physiological purpose. However it is now appreciated that not only are osteocytes highly responsive to circulating hormone levels1-3 and cytokines4, there is now growing evidence to support the hypothesis that the osteocyte is the mechanosensor in bone being able to sense and respond to load-induced strains5 and to translate this information to cells at the bone surface6. The loss of these cells from our bones is associated with the human ageing process7,8 and the consequence of having too few live osteocytes appears to include an inability to efficiently remove microdamage9, reduced levels of remodelling activity10-13 and may also relate to a reduced response to exercise in the elderly. In a range of studies it has been shown that apoptotic osteocytes co-localise with regions of bone resorption14-16. These findings have led workers to hypothesise that the apoptotic osteocyte might in some way be signalling to osteoclasts or their progenitors in order to target remodelling activity to specific areas in our skeleton15,17.
Osteocyte death by apoptosis has been associated with a range of conditions including glucocorticoid treatment18, estrogen withdrawal3,19, elevated PTH1, Klotho deficiency20, androgen action21 and both underloaded15, 17,22 and overloaded bone15, 16. Osteogenic levels of cyclical loading have been shown to decrease the proportion of apoptotic osteocytes and osteoclastic activity at the midshaft of the rat ulna while high, microdamage inducing levels of mechanical stimulation increased apoptosis and subsequent targeted osteoclastic resorption15 .Unloading mouse17 and rat23 bones was also associated with increased levels of osteocyte apoptosis that were reduced upon reloading. In addition, 3-D cultures of bovine bone have been used to demonstrate that dynamic hydrostatic pressure was capable of increasing the levels of osteocyte viability24 as was mechanical stimulation of rat bone in ex vivo culture25. While the potential importance of osteocyte apoptosis in the regulation of bone remodelling is clear and the role of mechanical stimulation has been demonstrated in animal models, it is important that we acknowledge potential species-related differences in the response and its physiological significance before we infer a role in human bone function. For example, our previous work has demonstrated that while relatively high levels of osteocyte apoptosis can be tolerated in rat bone without a resultant loss of osteocytes due to the rapid turnover rate in this species26, in human bone, similar levels of apoptosis resulting from pharmacologically-induced oestrogen withdrawal lead to a significant decrease in osteoctyte viability in the longer term3. Presumably this is simply a result of the differences in turnover rate and resultant cell replacement in these species but it serves to illustrate the danger of over-extrapolation. It will be particularly difficult to obtain bone pre and post-exercise in human subjects in order to study in more detail the relevance of mechanical stimulation in keeping our bones viable and responsive in old age. Therefore in order to study within human bone the response of the osteocyte population to mechanical stimulation, we have used a 3-D bioreactor system (Zetos™) which allows the controlled application of force and/or compression to 3-D explants of cancellous bone maintained within specific chambers ex vivo for prolonged periods of time27,28.
Materials and methods
All chemicals were purchased from Sigma-Aldrich (Dorset, UK) unless otherwise stated.
Patient samples
Excised femoral head tissue was obtained, with written informed consent and with the approval of the local ethical committee, from patients undergoing elective arthroplasty. A total of 8 patient samples were collected (Table 1) with n=4 male and n=4 female patients with an age range between 34 and 90 (median age 60).
Table 1.
Patient samples.
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Gender | Female | Female | Male | Female | Male | Male | Female | Male |
| Age | 34 | 65 | 46 | 90 | 55 | 75 | 47 | 65 |
Sample preparation
The individual patient samples were processed within 2 hours post-surgery and all subsequent manipulations were carried out under sterile conditions. Eight mm slices were prepared from femoral head tissue using an Exackt 310 cutting system which incorporates a diamond coated band saw, constantly irrigated with PBS at 4°C in order to minimise tissue damage during cutting. Bone cores 10 mm in diameter were drilled from each bone slice using a diamond coated hollow drill bit and cut flat plan parallel to a height of 5 mm (±2 μm). The bone cores were then washed 3 x 10 minutes at 37°C with shaking in HANKS Balanced Salt Solution (HBSS) followed by a final 20 minute wash in HBSS containing Gentamycin (10 mg/ml), Amphothericin B (4 μg/ml) and Penicillin/Strptomycin (50 IU/ml). The bone cores were then randomly assigned into three groups: time zero control (T0) (cores which were flash frozen in super cooled hexane immediately following sample preparation from each individual patient in order to give an indication of baseline parameters), unloading or mechanical stimulation.
Ex vivo culture
Individual bone cores were maintained within bioreactor chambers, which were themselves connected to individual media reservoirs via a peristaltic pump system that allowed the continual perfusion (7 ml/hour) of the bone tissue with culture media. A detailed description of the Zetos™ 3-D bioreactor system has been described previously by Davies et al.27. The bioreactor culture media consisted of Dulbecco’s Modified Eagle medium (DMEM) (Gibco, UK) supplemented with 10% FCS (Autogen Bioclear) L-Glutamine (2 mM), penicillin (50IU/ml) streptomycin (50 μgml), HEPES (10 mM) L-ascorbic acid-2-phosphate (10 μg/ml), β-glycerophosphate disodium hydrate (5 mM), NaHCO3 (0.14 mM). Samples were maintained within the bioreactor system for varying experimental periods of either 3 days (n= 2 patients), 7 days (n= 5 patients) or 27 days (n= 1 patient) (Table 2) within a 37°C environmental chamber with media being replaced every third day.
Table 2.
Osteocyte viability was quantified from 7 μm cryo-sections taken at ≥1.0 mm distance, the region of interest, through each bone core at Time zero (T0) or maintained within the 3-D bioreactor system with or without mechanical stimulation for 7 days (n=5 Patients), 3 days (n=2 Patients) and 27 days (n=1 Patient). The results are expressed as the average number of LDH positive osteocytes per mm2 from n=4 bone cores per treatment group±SEM.
| Patient | Treatment | |||||||
|---|---|---|---|---|---|---|---|---|
| Sex | Age | Length of Experiment (days) | TO | Unloading | TO vs. Unloading p value | Mechanical Stimulation | Unloading vs. Mechanical Stimulation p value | |
| 1 | Female | 34 | 7 | 124.8±8.9 | 25.1±6.0 | p=4.4 x 10-6 | 48.9±8.1 | p=0.021 |
| 2 | Female | 65 | 7 | 119±2.9 | 38.7±2.3 | p=8.4 x 10-5 | 74.5±5.2 | p=3.6 x 10-5 |
| 3 | Male | 46 | 7 | 160±11 | 41±12.4 | p=0.0004 | 84.4±6.5 | p=0.0076 |
| 4 | Female | 90 | 7 | 113.5±9.2 | 20.3±3.9 | p=3.3 x 10-6 | 64.4±11 | p=0.00032 |
| 5 | Male | 55 | 7 | 138.3±14.1 | 29.1±5.1 | p=2.2 x 10-5 | 46.2±13.9 | p=0.03 |
| 6 | Male | 75 | 3 | 95.5±7.5 | 33.7±2.9 | p=0.00025 | 70.9±3.1 | p=0.018 |
| 7 | Female | 47 | 3 | 162±1.6 | 65.8±4.6 | p=4.5 x 10-6 | 106±4.91 | p=0.019 |
| 8 | Male | 65 | 27 | 118.5±27 | 28.3±1.4 | p=0.0007 | 48±9 | p=0.009 |
Mechanical stimulation
For each experiment the human bone cores were divided into two groups (n= 4 cores per group per patient sample) and maintained in the bioreactor system either without mechanical stimulation (unloading control group) or with mechanical stimulation which was carried out for 5 minutes on a daily basis for the length of the experiment, using forces that engendered peak strains of 3,000 με at a frequency of 1Hz in wave patterns corresponding to physiological jumping exercise (high impact) (Figure 1) based on preliminary studies in bovine bone29.
Figure 1.
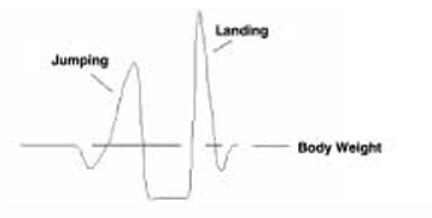
Physiological mechanical stimulation. The Zetos™ bioreactor system is capable of producing dynamic mechanical stimulation in specific waveforms mimicking physiological relevant patterns of exercise. We have used a high impact stimulus using the repetition of the waveform shown above for a jumping signal as obtained from force platform measurements.
Cell viability
Bone samples were flash frozen in super cooled hexane (-80°C) either at the time of collection of the femoral head (Time Zero; T0 n=4) in order to get an indication of baseline parameters for each individual or at the end of each experimental period (n=4 per treatment group unloading or mechanical stimulation). In order to broadly identify appropriate regions for subsequent analysis of osteocyte viability and osteogenic activity, consecutive 7 μm undecalcified cryosections of bone were collected at depths of 0.1mm, 0.5 mm, 1.0 mm, and 2.5 mm from the upper surface of each of 2 individual patients bone cores (n= 4 cores per treatment= 12 cores per patient) maintained in the bioreactor for 3 days and 7 days, respectively (Figure 2A). Cell viability was determined using in situ histochemical analysis of Lactate Dehydrogenase (LDH) activity30. The cryo-sections were incubated for 3 hours at 37°C in 40 % Polypep solution (pH 8.0) containing 1.75 mg/ml Nicotinamide Adenine Dinucleotide (NAD) Disodium salt (Roche Diagnostics), 60 mM Lactic acid, 3 mg/ml Nitroblue Tetrazolium (NBT). Following incubation the sections were rinsed in warm water to remove the reaction mixture prior to fixation in 4% paraformaldehyde. For the analysis of LDH activity (osteocyte viability) transmitted light images were captured with a DXM1200 camera mounted on Eclipse E800 microscope (Nikon) using 20X/0.50 ∝/0.17WD2.1 magnification lens. Six image fields were analysed per section from two consecutive sections taken at each specified distance through each of the bone cores (n=4 cores per treatment) and the results are expressed as the number of LDH positive osteocytes corrected for area (mm2) which was calculated using Bioquant Osteo Histomorphometry software (Bioquant Image Analysis Corporation, USA).
Figure 2.
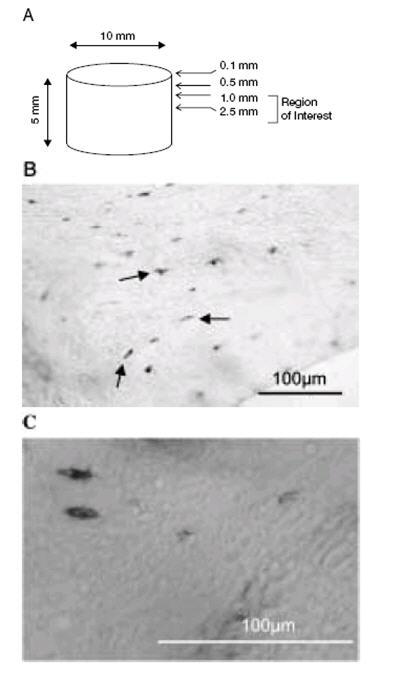
Cell viability as demonstrated by Lactate Dehydrogenase (LDH) Activity. (A) 7 μm cryo-sections were cut at distances of 0.1 mm, 0.5 mm, 1.0 mm and 2.5 mm through each 10 mm x 5 mm bone core (image not to scale). (B) A representative image showing LDH positive osteocytes stained dark blue in a cryo-section from a bone core maintained in the 3-D bioreactor system for 7 days and subjected to mechanical stimulation. (C) High power image of LDH positive osteocytes. Scale bar 100 μm.
Cellular apoptosis
Consecutive cryo-sections were analysed for osteocyte apoptosis using a Nick Translation assay31. The sections were fixed in 4% paraformaldehyde and demineralised in 50mM Tris/HCl solution containing 0.25 mM Ethylenediamine tetra-acetate (EDTA) pH 7.4. The sections were then washed in PBS and incubated for 45 minutes at 37°C with the nick translation mixture (50 mmol/L Tris HCl solution pH 7.5 containing 3 mM digoxigenin (DIG)-labelled dUTP; 3 mM each of dGTP, dATP, and dCTP; 5 mM MgCl2, 0.1 mM dithiothreitol and 0.5 μl/ml DNA polymerase I). Following PBS washing the sections were incubated with fluorescein isothiocyanate (FITC)-labelled anti-DIG antibody for 1 hour at room temperture, washed in PBS and incubated with 4,6-Diamidino-2-phenylindole (DAPI) (1 μg/ml) nuclear counter stain. Fluorescent images were captured using the camera system described above. Six image fields were analysed per section from two consecutive sections taken at the central region of interest through the bone cores (n=4 cores per treatment) and apoptotic osteocytes, detected by FITC positive signal (490 nm excitation, emission 520 nm) were expressed as a percentage of the total osteocyte numbers as detected by DAPI nuclear counter-stain (358 nm excitation, 461 nm emission).
Histochemical analysis
Alkaline Phosphatase positive bone surfaces were detected histochemically in 7 μm cryo-sections taken at the central region of interest. Sections were fixed in 4% paraformaldehyde before incubation at room temperature for 15 minutes in a solution of 1 mg/ml Fast Blue containing 40 μl (v/v) Naphthol-ASMX-mix. Transmitted light images were captured and five image fields were analysed from two consecutive sections taken at the central region of interest through each of the individual bone cores (n=4 cores per treatment i.e., 40 fields). The percentage of Alkaline Phosphatase (ALP) positive surface to total bone surface per field of view was measured using Bioquant Osteo Histomorphometry software (Bioquant Image Analysis Corporation, USA).
Bone histomorphometric parameters
During the 27-day bioreactor experiment (n=1 patient; 4 cores unloading; 4 cores mechanical stimulation) calcein label (final concentration 2 mg/ml) was added to the culture media on day 6 and allowed to re-circulate in the system for 16 hours after which the media was removed and fresh media replaced. Hence, 21 days elapsed after the addition of this label. Any bone material existing above the label at the end of the experimental period was therefore synthesised during that 21-day period. Three consecutive 7 μm cryo-sections were analysed at the region of interest within the individual bone cores from each treatment group (n=4 cores from each group; unloading and mechanical stimulation). Fluorescent FITC (490 nm excitation, emission 520 nm) images were captured using a DXM1200 camera mounted on Eclipse E800 microscope (Nikon) through a 20X/0.50 ∝/0.17WD2.1 magnification lens. Nine image fields were analysed per section using Bioquant Osteo Histomorphometry software (Bioquant Image Analysis Corporation, USA). Measurements were made for Bone Surface mm (BS); Mineralised Surface mm for single label (MS) and the Mineral Apposition Rate μm/day (MAR) estimated by the mean distance between the calcein label to bone surface divided by the time period of 21 days. The Bone Formation Rate per Bone Surface (BFR/BS μm3/μm2/year) was derived using the formula: BFR/BS=MAR × MS/BS.
Statistical analysis
Statistical differences between treatment groups was determined using one-way ANOVA followed by Tukey-Kramer test post hoc and results were considered significant when p<0.05 denoted by * when exercise treatment was compared to disuse and p<0.05 denoted by † when experimental treatments were compared to T0 control. The results are presented as means ± SEM.
Results
Osteocyte viability and apoptosis in response to mechanical stimulation
The numbers of LDH positive osteocytes per mm2 (Figure 2B, C) were quantified at each distance from the upper surface - 0.1 mm, 0.5 mm, 1.0 mm and 2.5 mm - (Figure 2A) through individual cores taken either at T0 or maintained within the 3-D bioreactor for 3 days (Patient 6; Male 75 years) (Figure 3A) or 7 days (Patient 3; Male 46 years) (Figure 3B) under both unloaded or mechanical stimulated conditions. There was no significant difference in osteocyte viability (as detected by LDH activity) throughout the depth of the T0 samples within either patient. However, there was a significant reduction in osteocyte viability (p≤0.0004) at all depths, following unloading, in both patients. In these patient samples the upper 0.1 mm cross-section showed the greatest reduction in osteocyte viability (Figure 3A, B). In both patients, at all depths throughout the core, mechanical stimulation significantly increased osteocyte viability (p≤0.04). In the 3-day experiment cell viability at depths of 1.0 mm and 2.5 mm from the core surface in the mechanically stimulated samples approached that of fresh T0 samples (Figure 3A). Because osteocyte viability within the central regions between 1-2.5 mm from the surface of the 5 mm high cores showed the greatest osteocyte viability all further analysis of subsequent patient samples were carried out at this region of interest within each core.
Figure 3.
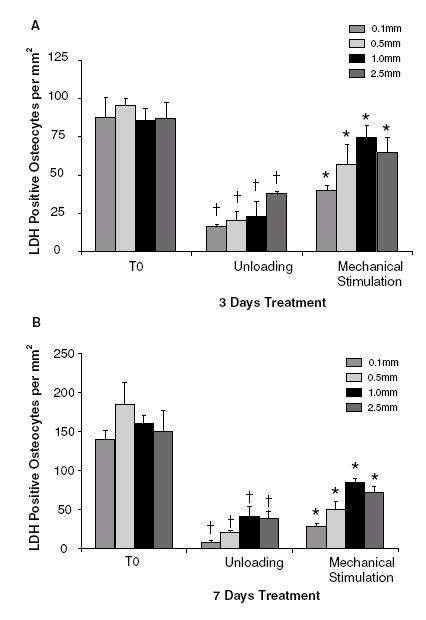
Osteocyte viability in different regions of the bone core.(A) LDH positive osteocytes per mm2 in T0, unloaded and mechanically stimulated bone cores at 0.1, 0.5, 1.0 and 2.5 mm from the core surface after 3 days in culture (n=4 cores per group, Patient 6; Male 75 years). (B) As above, in cores after 7 days in culture (n=4 cores per group, Patient 3; Male 46 years).
Within the initial T0 cores the number of viable osteocytes per mm2 was shown to vary between individual patients and ranged from 95.5±7.5 cells per mm2 up to 162±1.6 cells per mm2 (Table 2). The samples were derived from male (n=4) and female (n=4) patients with an age range between 34 years - 90 years (median age 60 years). When patients were grouped into those below or above the median age of 60 years there was a significant difference (p=0.016) in the average number of viable osteocytes per mm2 at T0 (osteocyte viability at age <60; 146.3±10.3 cells per mm2 vs. osteocyte viability at age >60; 111.6±6.4 cells per mm2).
When no mechanical stimulation was applied to the cores for periods of 3 days, 7 days and 27 days, a significant reduction (p≤0.0007) in osteocyte viability at the region of interest was observed in all patient samples (Table 2). When mechanical stimulation was applied to the patient samples maintained during the experimental period there was a significant, approximately 2-fold, increase in osteocyte viability per mm2 (p≤0.03) when compared to unloaded samples (Table 2).
After 7 days in the bioreactor the mean patient percentage reduction in osteocyte viability when compared to fresh T0 samples was 75.9±3.8% in unloaded vs. 47.2±5.7%; in mechanically stimulated samples (p=0.001; n=5) (Figure 4). The small number of patients in the 3-day (n=2 experiments) and 27-day (n=1 experiment) precluded this type of analysis being applied to these samples.
Figure 4.
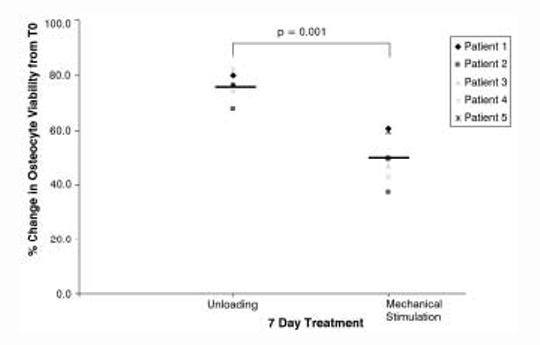
Percentage change in osteocyte viability relative to T0 samples. The percentage change in osteocyte viability in cores from 5 individual patients’ samples maintained within the bioreactor for 7 days (n=4 cores per treatment group for each patient).
When the percentage of apoptotic osteocytes was examined (Figures 5A-D) in T0 samples the group mean of 1.88±0.186% was similar to levels previously reported in human bone3 (Figure 5E). Apoptosis was increased in all patient samples in the unloaded cores giving a group mean of 10.15±2.16 % apoptotic cells (Figure 5E). Upon application of mechanical stimulation osteocyte apoptosis was significantly decreased (p=0.0021) in all patient samples giving a group mean of 3.422±0.745% apoptotic cells that was approaching a similar magnitude to that of the healthy T0 samples (Figure 5E).
Figure 5.
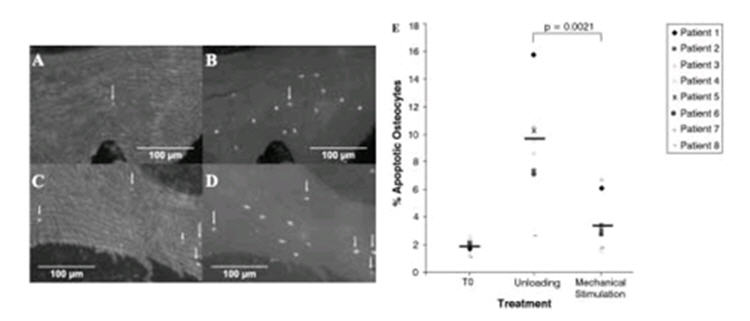
Apoptotic osteocytes in the bone cores-response to mechanical stimulation. The percentage of apoptotic osteocytes were quantified in cryo-sections after nick translation assay. (A) Apoptotic cells in a mechanically stimulated sample (B) corresponding total cell DAPI stain. (C) Apoptotic cells in unloaded sample (D) corresponding total cell DAPI stain. (E) Unloading increased the percentage of apoptotic osteocytes in all patient samples, while mechanical stimulation reduced the percentage of apoptotic osteocytes.
Markers of bone formation
Mechanical stimulation was shown to increase Mineral Apposition Rate (MAR) and Bone Formation Rate (BFR) as determined by calcein label/distance to bone surface in the 27-day experiment - MAR (μm/day) unloading 0.0025±0.0026 vs. mechanical stimulation 0.2564±0.0854; p=0.008 (Figure 6C) and BFR/BS (μmm3/μmm2/year) unloading 0.548±0.577 vs. mechanical stimulation 9.7881±2.990; p=0.005 (Figure 6D). In the 3-day and 7-day patient samples the percentage of Alkaline Phosphatase (ALP) labelled bone surface (Figure 7B, D) was used to give an indication of bone formation in response to mechanical stimulation since it would not be feasible to use calcein label in these shorter experiments. In all patient samples there was a significant increase in the percentage ALP labelled bone surface on mechanical stimulation when compared to unloading (p≤0.05). This is represented in Figure 7E where the line joining the data points is used to highlight the difference between unloading and mechanical stimulation for each individual and does not represent an actual change with time.
Figure 6.
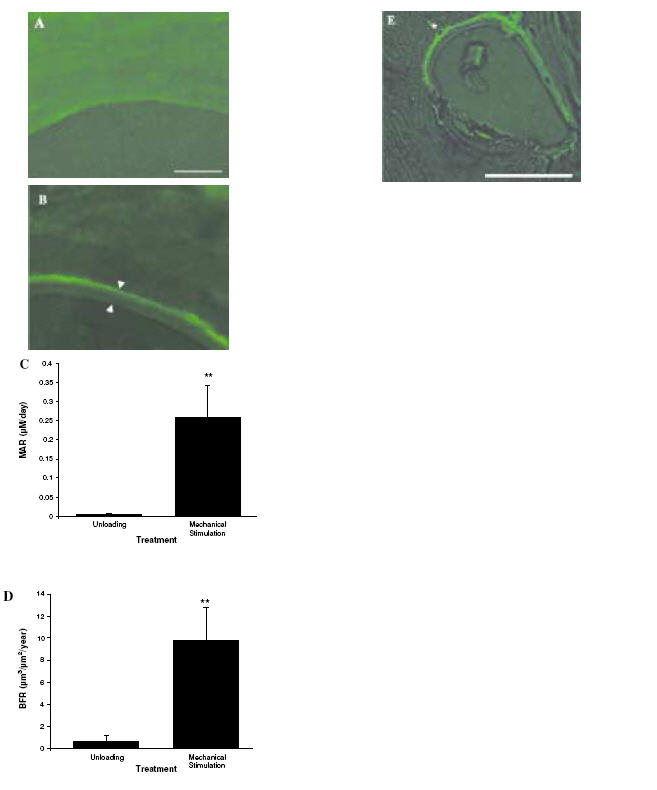
Calcein label and bone formation rate. Calcein label was administered on day 6 of the 27-day experiment and all bone material overlying the label was measured using histomorphometry software. (A) Unloaded sample showing no label. (B) Mechanically stimulated sample showing representative calcein label and “newly formed bone material” between arrowheads. (C) Mineral Apposition Rate and (D) Bone Formation Rate were shown to increase in mechanically stimulated samples relative to unloading. Scale bar=100 μm. (E)A newly formed osteocyte has become embedded in the newly formed bone (as indicated by * in the image). Scale bar=100 μm.
Figure 7.
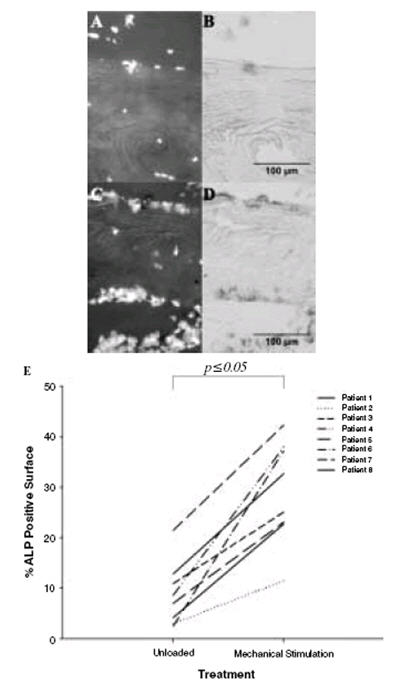
Alkaline phosphatase (ALP) positive bone surfaces. (A) (C) The total cells present are stained using DAPI. (B) ALP was stained using standard techniques and can be seen to be almost absent in a representative image from an unloaded bone sample (D) ALP clearly visible in a loaded sample. (E) Quantification of the percentage ALP positive bone surface revealed an increase (as indicated by the line joining individual patients between treatment groups) in this marker of osteogenic activity in all mechanically stimulated patient cores relative to the unloaded cores.
Discussion
Here we have used a 3-D bioreactor system (Zetos™) to apply cyclical mechanical loads to human trabecular bone from 8 patients over periods of 3, 7 and 27 days to assess the viability and active apoptotic cell death of osteocytes in unloaded (disuse) and mechanically stimulated human bone. The effect of unloading was to decrease markers of bone formation, increase the apoptosis of osteocytes and to reduce the viability of the bone. The application of mechanical stresses capable of engendering osteogenic responses in vivo increased evidence of bone formation, dramatically decreased osteocyte apoptosis and improved the viability of these human bone samples. The human bone tissue used in these studies was obtained from patients undergoing elective hip arthroplasty and responses should be interpreted accordingly. While the individuals were receiving various prophylaxes for pain management none was receiving any treatments that are known to overtly affect bone turnover such as estrogen in the form of Hormone Replacement Therapy (HRT) or bisphosphonates. The degree of between-patient variability in the initial viability of trabecular bone samples seen here is in agreement with previous findings3,7 in which a range of viabilities are reported. Interestingly, when samples were grouped into those from individuals either above or below the median patient age of 60 years, osteocyte viability at baseline (T0) was found to be lower in the older >60 individuals. Such an age-related decrease in osteocyte viability was first described during 1960 in the work of Frost8 and has been substantiated in a number of subsequent studies7,32.
Bone formation rates measured in the trabecular bone samples using pseudo double label in the 27-day experiment were of the order 0.548±0.577 μm3/μm2/year in unloading and 9.7881±2.990 μm3/μm2/year in mechanically stimulated samples. While further and fuller histomorphometric analysis is required on a greater number of patient samples to compare anabolic and catabolic activities of bone in the bioreactor with that in vivo, it is notable that our initial estimates of bone formation rates (BFR/BS) under mechanically stimulated conditions are at the lowest range of those described in the human iliac crest33,34. Unfortunately bone formation rate estimates are not available for human femoral head bone in order to act as a comparison. The reduced bone formation seen under unloaded conditions would broadly be in agreement with the response seen in animal models in vivo in which decreases in BFR of approximately 30% have been observed35. The mechanism behind the reduced BFR has not been fully investigated in the current studies but might include direct effects of reduced mechanical deformation or fluid flow and nutrient delivery or secondary effects related to loss of osteocyte viability and signalling. Alkaline phosphatase (ALP) positive bone surface was reduced along with BFR in the unloaded samples. The application of mechanical stimulus increased BFR and ALP positive bone surface in all patient samples demonstrating a significant mechano-responsiveness in the bioreactor system.
Application of mechanical stimuli to human bone cores occurred in the absence of supplementation with putative osteogenic/mechano-permissive factors such as estrogens, PTH or 1,25 Vitamin D3. For this reason we consider the conditions as sub-optimal for engendering a mechanical response, however it provides a robust baseline from which to study the effect of mechanical stimulation alone on osteocyte behaviour. Future studies will address the combinations of secondary factors that will optimise the response in terms of viability or bone formation.
Osteocyte viability was clearly responsive to the degree of mechanical stimulation in this bioreactor system. The loss of viability in the trabecular cores over the short experimental periods of 3 days was substantial but appeared not to increase further over the longer periods in the bioreactor (7 and 27 days). Loss of viability in other ex vivo 3-D culture systems has frequently been attributed to the formation of an anoxic core due to lack of diffusion of bathing medium36. For this reason, bathing medium is actively perfused in the bioreactor system and the mechanical loading cycles will further distribute fluids in the chamber. If distribution of fluids within the sample proved insufficient for the maintenance of viability one would expect higher viability at the surface of the sample with an increasing loss of viability towards the centre. Our data suggest that throughout the thickness of the trabecular bone core the viability did not drop towards the centre, in fact it increased somewhat. These findings indicate a relatively homogeneous supply of nutrients across the samples in both the unloaded and the loaded state. The lower viability at the outside edge of the samples is likely caused by damage associated with the cutting of the samples prior to culture29.
The application of cyclical mechanical loads, sufficient to engender strains in the known osteogenic range, improved viability in the bone cores up to 2-fold. Despite a strongly positive effect of mechanical stimulation on osteocyte viability in all patient samples tested none of the ‘exercised’ samples were able to attain T0 levels of viability although some did approach these. The ability of mechanical stimulation to improve cell viability in ex vivo cultures of rat bone has been demonstrated in the past where a bi-phasic influence of load depending on magnitude was seen24,25.
The loss of cell viability in disuse appeared to have been the result of increased levels of osteocyte apoptosis, while the application of mechanical loads reduced apoptotic activity. These findings in human bone are in accord with those found in rat bone in vivo in which the application of cyclical loading to rat ulnae decreased osteocyte apoptosis at the midshaft and the level of osteocyte apoptosis was closely related to the calculated strain magnitude across this anatomical region37. The molecular mechanism by which mechanical stimulation decreases osteocyte apoptosis in this system has not been determined in these experiments. It is possible that the reduction in apoptosis is simply a product of increased supply of nutrients and oxygen to the osteocytes or a related improved removal of toxic molecules. Mechanical loading is known to improve fluid flow and nutrient transport within rodent cortical bones in vivo38 and to reduce local hypoxia as indicated by HIF-1α production39.In the study by Gross et al. the production of HIF-1α by osteocytes in underloaded bone was rapidly reversed upon cyclical loading. Alternatively, apoptosis might be controlled through the production of signal molecules generated as a result of mechanotransduction. These topics will be the subject of future experiments using the bioreactor.
Footnotes
The authors have no conflict of interest.
References
- 1.Bringhurst FR. PTH receptors and apoptosis in osteocytes. J Musculoskelet Neuronal Interact. 2002;2:245–251. [PubMed] [Google Scholar]
- 2.Divieti P, Inomata N, Chapin K, Singh R, Juppner H, Bringhurst FR. Receptors for the carboxyl-terminal region of pth(1-84) are highly expressed in osteocytic cells. Endocrinology. 2001;142:916–925. doi: 10.1210/endo.142.2.7955. [DOI] [PubMed] [Google Scholar]
- 3.Tomkinson A, Reeve J, Shaw RW, Noble BS. The death of osteocytes via apoptosis accompanies estrogen withdrawal in human bone. J Clin Endocrinol Metab. 1997;82:3128–3135. doi: 10.1210/jcem.82.9.4200. [DOI] [PubMed] [Google Scholar]
- 4.Ahuja SS, Zhao S, Bellido T, Plotkin LI, Jimenez F, Bonewald LF. CD40 ligand blocks apoptosis induced by tumor necrosis factor alpha, glucocorticoids, and etoposide in osteoblasts and the osteocyte-like cell line murine long bone osteocyte-Y4. Endocrinology. 2003;144:1761–1769. doi: 10.1210/en.2002-221136. [DOI] [PubMed] [Google Scholar]
- 5.Rubin J, Rubin C, Jacobs CR. Molecular pathways mediating mechanical signaling in bone. Gene. 2006;367:1–16. doi: 10.1016/j.gene.2005.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aarden EM, Burger EH, Nijweide PJ. Function of osteocytes in bone. J Cell Biochem. 1994;55:287–299. doi: 10.1002/jcb.240550304. [DOI] [PubMed] [Google Scholar]
- 7.Dunstan CR, Somers NM, Evans RA. Osteocyte death and hip fracture. Calcif Tissue Int. 1993;53(Suppl.1):S113–S116. doi: 10.1007/BF01673417. [DOI] [PubMed] [Google Scholar]
- 8.Frost HM. In vivo osteocyte death. J Bone Joint Surg Am. 1960;42-A:138–143. [PubMed] [Google Scholar]
- 9.Mori S, Harruff R, Ambrosius W, Burr DB. Trabecular bone volume and microdamage accumulation in the femoral heads of women with and without femoral neck fractures. Bone. 1997;21:521–526. doi: 10.1016/s8756-3282(97)00200-7. [DOI] [PubMed] [Google Scholar]
- 10.Kakizaki I, Zechner G, Altmann F. The osteocytes of the human labyrinthine capsule. Arch Otolaryngol. 1971;94:139–150. doi: 10.1001/archotol.1971.00770070375010. [DOI] [PubMed] [Google Scholar]
- 11.Kamijou T, Nakajima T, Ozawa H. Effects of osteocytes on osteoinduction in the autogenous rib graft in the rat mandible. Bone. 1994;15:629–637. doi: 10.1016/8756-3282(94)90311-5. [DOI] [PubMed] [Google Scholar]
- 12.Marotti G. The structure of bone tissues and the cellular control of their deposition. Ital J Anat Embryol. 1996;101:25–79. [PubMed] [Google Scholar]
- 13.Marotti G, Farneti D, Remaggi F, Tartari F. Morphometric investigation on osteocytes in human auditory ossicles. Ann Anat. 1998;180:449–453. doi: 10.1016/S0940-9602(98)80106-4. [DOI] [PubMed] [Google Scholar]
- 14.Gu G, Mulari M, Peng Z, Hentunen TA, Vaananen HK. Death of osteocytes turns off the inhibition of osteoclasts and triggers local bone resorption. Biochem Biophys Res Commun. 2005;335:1095–1101. doi: 10.1016/j.bbrc.2005.06.211. [DOI] [PubMed] [Google Scholar]
- 15.Noble BS, Peet N, Stevens HY, Brabbs A, Mosley JR, Reilly GC, Reeve J, Skerry TM, Lanyon LE. Mechanical loading: biphasic osteocyte survival and targeting of osteoclasts for bone destruction in rat cortical bone. Am J Physiol Cell Physiol. 2003;284:C934–C943. doi: 10.1152/ajpcell.00234.2002. [DOI] [PubMed] [Google Scholar]
- 16.Verborgt O, Gibson GJ, Schaffler MB. Loss of osteocyte integrity in association with microdamage and bone remodeling after fatigue in vivo. J Bone Miner Res. 2000;15:60–67. doi: 10.1359/jbmr.2000.15.1.60. [DOI] [PubMed] [Google Scholar]
- 17.Aguirre JI, Plotkin LI, Stewart SA, Weinstein RS, Parfitt AM, Manolagas SC, Bellido T. Osteocyte apoptosis is induced by weightlessness in mice and precedes osteoclast recruitment and bone loss. J Bone Miner Res. 2006;21:605–615. doi: 10.1359/jbmr.060107. [DOI] [PubMed] [Google Scholar]
- 18.Gu G, Hentunen TA, Nars M, Harkonen PL, Vaananen HK. Estrogen protects primary osteocytes against glucocorticoid-induced apoptosis. Apoptosis. 2005;10:583–595. doi: 10.1007/s10495-005-1893-0. [DOI] [PubMed] [Google Scholar]
- 19.Weinstein RS, Nicholas RW, Manolagas SC. Apoptosis of osteocytes in glucocorticoid-induced osteonecrosis of the hip. J Clin Endocrinol Metab. 2000;85:2907–2912. doi: 10.1210/jcem.85.8.6714. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki H, Amizuka N, Oda K, Li M, Yoshie H, Ohshima H, Noda M, Maeda T. Histological evidence of the altered distribution of osteocytes and bone matrix synthesis in klotho-deficient mice. Arch Histol Cytol. 2005;68:371–381. doi: 10.1679/aohc.68.371. [DOI] [PubMed] [Google Scholar]
- 21.Wiren KM, Toombs AR, Semirale AA, Zhang X. Osteoblast and osteocyte apoptosis associated with androgen action in bone: requirement of increased Bax/Bcl-2 ratio. Bone. 2006;38:637–651. doi: 10.1016/j.bone.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 22.Burger EH, Klein-Nulend J. Microgravity and bone cell mechanosensitivity. Bone. 1998;22:127S–130S. doi: 10.1016/s8756-3282(98)00010-6. [DOI] [PubMed] [Google Scholar]
- 23.Basso N, Heersche JN. Effects of hind limb unloading and reloading on nitric oxide synthase expression and apoptosis of osteocytes and chondrocytes. Bone. 2006;39:807–814. doi: 10.1016/j.bone.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 24.Takai E, Mauck RL, Hung CT, Guo XE. Osteocyte viability and regulation of osteoblast function in a 3D trabecular bone explant under dynamic hydrostatic pressure. J Bone Miner Res. 2004;19:1403–1410. doi: 10.1359/JBMR.040516. [DOI] [PubMed] [Google Scholar]
- 25.Lozupone E, Palumbo C, Favia A, Ferretti M, Palazzini S, Cantatore FP. Intermittent compressive load stimulates osteogenesis and improves osteocyte viability in bones cultured “in vitro”. Clin Rheumatol. 1996;15:563–572. doi: 10.1007/BF02238545. [DOI] [PubMed] [Google Scholar]
- 26.Tomkinson A, Gevers EF, Wit JM, Reeve J, Noble BS. The role of estrogen in the control of rat osteocyte apoptosis. J Bone Miner Res. 1998;13:1243–1250. doi: 10.1359/jbmr.1998.13.8.1243. [DOI] [PubMed] [Google Scholar]
- 27.Davies CM, Jones DB, Stoddart MJ, Koller K, Smith E, Archer CW, Richards RG. Mechanically loaded ex vivo bone culture system ‘Zetos’: systems and culture preparation. Eur Cell Mater. 2006;11:57–75. doi: 10.22203/ecm.v011a07. [DOI] [PubMed] [Google Scholar]
- 28.Jones DB, Broeckmann E, Pohl T, Smith EL. Development of a mechanical testing and loading system for trabecular bone studies for long term culture. Eur Cell Mater. 2003;5:48–59. doi: 10.22203/ecm.v005a05. [DOI] [PubMed] [Google Scholar]
- 29.Mann V, Jones DB, Noble BS. The effect of mechanical stimulation on osteocyte viability in bone. J Musculoskelet Neuronal Interact. 2004;4(2):210–211. [PMC free article] [PubMed] [Google Scholar]
- 30.Noble BS, Stevens HY. Techniques for the study of apoptosis in bone. Methods Mol Med. 2003;80:225–236. doi: 10.1385/1-59259-366-6:225. [DOI] [PubMed] [Google Scholar]
- 31.Noble BS, Stevens H, Loveridge N, Reeve J. Identification of apoptotic changes in osteocytes in normal and pathological human bone. Bone. 1997;20:273–282. doi: 10.1016/s8756-3282(96)00365-1. [DOI] [PubMed] [Google Scholar]
- 32.Qiu S, Rao DS, Fyhrie DP, Palnitkar S, Parfitt AM. The morphological association between microcracks and osteocyte lacunae in human cortical bone. Bone. 2005;37:10–15. doi: 10.1016/j.bone.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 33.Compston JE, Vedi S, Stephen AB, Bord S, Lyons AR, Hodges SJ, Scammell BE. Reduced bone formation in UK Gulf War veterans: a bone histomorphometric study. J Clin Pathol. 2002;55:897–899. doi: 10.1136/jcp.55.12.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rauch F, Travers R, Glorieux FH. Pamidronate in children with osteogenesis imperfecta: histomorphometric effects of long-term therapy. J Clin Endocrinol Metab. 2006;91:511–516. doi: 10.1210/jc.2005-2036. [DOI] [PubMed] [Google Scholar]
- 35.Chow JW, Jagger CJ, Chambers TJ. Characterization of osteogenic response to mechanical stimulation in cancellous bone of rat caudal vertebrae. Am J Physiol. 1993;265:E340–E347. doi: 10.1152/ajpendo.1993.265.2.E340. [DOI] [PubMed] [Google Scholar]
- 36.Trowell OA. A modified technique for organ culture in vitro. Exp Cell Res. 1954;6:246–248. doi: 10.1016/0014-4827(54)90169-x. [DOI] [PubMed] [Google Scholar]
- 37.Noble BS, Peet N, Stevens HY, Brabbs A, Mosley JR, Reilly GC, Reeve J, Skerry TM, Lanyon LE. Mechanical loading: biphasic osteocyte survival and targeting of osteoclasts for bone destruction in rat cortical bone. Am J Physiol Cell Physiol. 2003;284:C934–C943. doi: 10.1152/ajpcell.00234.2002. [DOI] [PubMed] [Google Scholar]
- 38.Knothe Tate ML, Knothe U. An ex vivo model to study transport processes and fluid flow in loaded bone. J Biomech. 2000;33:247–254. doi: 10.1016/s0021-9290(99)00143-8. [DOI] [PubMed] [Google Scholar]
- 39.Gross TS, Akeno N, Clemens TL, Komarova S, Srinivasan S, Weimer DA, Mayorov S. Selected Contribution: Osteocytes upregulate HIF-1alpha in response to acute disuse and oxygen deprivation. J Appl Physiol. 2001;90:2514–2519. doi: 10.1152/jappl.2001.90.6.2514. [DOI] [PubMed] [Google Scholar]


