Abstract
Interaction of anxiety and memory represents an essential feature of CNS functioning. This paper reviews experimental data coming from neurogenetics, neurochemistry, and behavioral pharmacology (as well as parallel clinical findings) reflecting different mechanisms of memory-anxiety interplay, including brain neurochemistry, circuitry, pharmacology, neuroplasticity, genes, and gene-environment interactions. It emphasizes the complexity and nonlinearity of such interplay, illustrated by a survey of anxiety and learning/memory phenotypes in various genetically modified mouse models that exhibit either synergistic or reciprocal effects of the mutation on anxiety levels and memory performance. The paper also assesses the putative role of different neurotransmitter systems and neuropeptides in the regulation of memory processes and anxiety, and discusses the role of neural plasticity in these mechanisms.
1. INTRODUCTION
Pathologic anxiety is a complex stress-related disorder which includes generalized anxiety, panic, social anxiety, agoraphobia, posttraumatic stress, and obsessive-compulsive disorders [1–5]. There are many animal (experimental) paradigms that model different subtypes of human anxiety [6–10]. In addition to anxiety, stress has long been known to affect animal and human cognitions [11–14], raising the possibility that memory and anxiety interact.
Numerous studies have outlined behavioral, physiological, pharmacological, and genetic aspects of memory-anxiety interaction [13, 15–20]. Since memory consolidation and anxiety both require brain arousal, it has been considered as promnestic and anxiogenic, whereas brain inhibition is amnestic and anxiolytic; review [12, 21, 22]. However, classic works of Yerkes and Dodson [14], as well as many subsequent studies [23–30], have shown that memory and stress interplay in a more complex, type-specific, and nonlinear manner. Here we will analyze the available clinical and experimental data in order to examine (with a particular focus on neurogenetics) the links between anxiety and memory functions.
Transgenic and mutant animals, including tissue-specific and inducible knockout mice, represent a valuable tool for biomedical brain research [31–34] powered by extensive on-line databases [8, 9]. Table 1 summarizes anxiety and memory/learning phenotypes in various genetically modified mouse models, including mutant mice lacking or over-expressing receptors of various neuromediators, neuropeptides, and some brain proteins mediating neuroplasticity. Several important conclusions can be made based on these findings. A common situation when the same mutation leads to altered anxiety and memory phenotypes (Table 1) confirms overlapping of the two domains at genetic (in addition to behavioral and pharmacological [12, 13]) levels. While many mutants show synergetic alterations of memory and anxiety, there are also data on reciprocal effects of some mutations (Table 1), confirming a complex nonlinear nature of memory-anxiety interplay. Moreover, as can be seen in this table, different subtypes of memory seem to be differentially influenced by altered anxiety, further contributing to the complexity of the problem discussed here. While this paper will not offer a simple solution for complex animal or human phenotypes, its aim is to discuss how different brain systems may interact in determining anxiety and memory phenotypes.
Table 1.
Mouse mutagenesis data on memory and anxiety phenotypes [8]; see text for details. KO: knockout (−/−), HZ: heterozygous (+/−) mice. (↑: increased, ↓: reduced, 0: no effects, ↔: mixed or unclear results. CRF: corticotropin-releasing factor, MAO: monoamine oxidase A/B, FXR1: fragile X-related protein 1, PACAP: pituitary adenylate cyclase activating polypeptide, Rab3a: ras-associated binding 3a protein.)
| Mouse models | Effects on | References | ||
| Anxiety | Memory/learning | |||
|
| ||||
| Neurotransmitters Acetylcholine | N-receptor α4 subunit KO mice | ↑ | ↓ within-trial habituation | [35] |
| N-receptor α7 subunit KO mice | 0 (↓) | 0 fear conditioning, spatial learning | [36] | |
| N-receptor β2 subunit KO mice | — | ↓ avoidance learning, 0 spatial learning | [37] | |
|
|
||||
| Serotonin | 5HT-1B receptor KO mice | ↓ | ↑ long-term and short-term memory, 0 habituation | [38–42] |
| 5HT-1A receptor KO mice | ↑ | ↓ hippocampal-dependent learning, 0 habituation | [40, 43–45] | |
| 5HT-5A receptor KO mice | ↓ | 0 inter- and within-trial habituations | [46] | |
| Serotonin transporter KO mice | ↑ | ↔ within-trial habituation | [47] | |
|
|
||||
| GABA (also see Table 2) | GABA-A α5 subunit KO mice | 0 | ↑ hippocampal-dependent trace conditioning, 0 delayed or contextual conditioning | [48] |
| GABA-A γ2 subunit HZ mice | ↑ | ↑ cued fear conditioning, 0 spatial memory | [49] | |
|
|
||||
| Histamine | Histamine H3 receptor KO mice | ↓ | 0 habituation, ↑ spatial memory and learning, higher resistance to amnestic effects of scopolamine | [50, 51] |
|
|
||||
| Glycine | Glycine transporter 1 brain-selective disruption | 0 | ↑ aversive Pavlovian conditioning | [52] |
|
|
||||
| Glutamate | B subunit ionotropic receptor KO mice | ↓ olfactory memory (rescued by selective expression in hippocampus) | [53] | |
| Metabotropic subtype 7 receptor KO mice | ↓ | ↓ cued fear response and conditioned taste aversion | [54] | |
| A type receptor KO mice | ↑ | ↓ spatial working memory (alternation) | [55] | |
|
|
||||
| Related models | MAO B targeted inactivation | ↑ | 0 working memory, ↓ long-term memory | [56] |
| MAO A/B KO mice | ↑ | 0 within-trial habituation | [57] | |
|
|
||||
| Neuropeptides and other brain proteins | CRF receptor 1 KO mice | ↓ | ↓ spatial recognition memory | [58] |
| Thyroid hormone α1 receptor mutations | ↑ | ↓ olfactory recognition memory, contextual fear conditioning | [59, 60] | |
| Neuropeptide Y KO mice | ↓ | ↓ attention training test performance | [61] | |
| Brain-derived neurotrophic factor (mice) | ↔ | ↔ | Table 3 | |
| Glial protein S100B KO mice | ↑ fear conditioning, spatial memory | [62] | ||
| Protein kinase Cγ KO mice | ↓ | ↓ spatial and contextual learning | [63, 64] | |
| FXR1 KO mice | ↓ | ↓ fear conditioning, spatial memory, 0 habituation | [65] | |
| Modified β-amyloid precursor KO mice | ↑ | ↓ spatial learning, habituation | [66] | |
| PACAP-type 1 receptor KO mice | ↓ | ↓ associative learning | [67, 68] | |
| Rab3a KO mice | 0 ↓ | ↓ cued fear conditioning 0 acquisition, mild ↓ spatial reversal learning and spatial working memory | [69] [70] | |
| Rab3a loss-of-function mutant mice | ↓ | ↓ cued fear conditioning | [69] | |
2. NEUROCHEMISTRY AND NEUROGENETICS OF MEMORY AND ANXIETY
Cholinergic synaptic transmission has long been implicated in learning, memory, and anxiety [36, 92]. Neuronal nicotinic (N) acetylcholine (ACh) receptors are hetero-oligomers (formed by five of 11 known α and β subunits) mediating anxiolytic-like effect of nicotine [35]. Their loss has also been noted for Altzheimer's and Parkinson's patients with impaired cognitive functions [35], collectively implicating these receptors in both memory and anxiety. In line with this, increased anxiety and impaired memory were reported in mice lacking α4 subunit of N-type Ach receptor (Table 1). Mice lacking the receptor's β2 subunits (predominant in hippocampus) showed impaired avoidance learning, but normal spatial learning in Morris water maze [37]. Surprisingly, ablation of α7 subunits (also rich in hippocampus) leads to no or very mild alterations in anxiety (open field test) and memory (unaltered acoustic startle habituation and Pavlovian conditioning, but faster finding a platform in the Morris water maze) [36]. Taken together, this suggests that various subtypes of ACh receptors may play different roles in memory-anxiety interplay. Notably, RS-1259, a newly synthesized inhibitor of acetylcholinesterase [93], elevated ACh levels in hippocampus and improved memory in mice, suggesting that targeting brain ACh may lead to effective therapy of neurodegenerative disorders. The same drug also inhibited serotonin transport [93], implying that altered serotonergic system may also contribute to these effects (see further).
Gamma-amino butyric acid (GABA) is the primary mediator of inhibitory neurotransmission, acting through ionotropic A and metabotropic B type receptors. GABA-A receptors are Cl-channels composed of five subunits (from eight families: α1-α6, β1-β3, γ1-γ3, δ, ɛ, π, θ, and ρ1-ρ3) with multiple binding sites for positive (GABA agonists, barbiturates, benzodiazepines, steroids, and ethanol) and negative (GABA-A antagonists, neurosteroid antagonists, benzodiazepine inverse agonists, and chloride channel blockers) modulators [4, 12, 94–97]. GABA has long been implicated in anxiety [80, 97–101]. In both humans and animals, positive modulators of GABA receptors generally possess anxiolytic activity while negative modulators produce anxiogenic-like effects. Moreover, various GABA analogs and agents affecting transmitter metabolism to enhance GABAergic tone have been reported to exert anxiolytic effects [98, 102–107]. The role of GABA in learning and memory has also been widely recognized [28–30, 90, 100, 108–112]. Three comprehensive reviews particularly [12, 17, 113] emphasize the role of central GABA in memory-anxiety interplay, noting amnestic/anxiolytic effects of positive, and opposite profiles of negative, GABA modulators (also see [27–30, 111, 114, 115] for details).
Mounting neurogenetic data further implicates GABA in memory and anxiety. GABAergic genes are associated with anxiety (α2, α3, α4, α6, β1, γ1, and γ2) [95, 96, 116, 117] and memory (α5) [48, 49, 118]; see Table 1. Downregulation of α1, α4, α5, α6, γ1, δ genes was reported in anxious versus nonanxious rat strains [119]. Other studies show reduced expression of rat α2, γ1, or δ subunits after fear conditioning [79] and chronic unpredictable stress [120]. In humans, treatment-resistant depression with anxiety was linked to a mutant β1 subunit gene [121], whereas positive genetic associations were found between GABA-A subunits genes and neuroticism (α6 [122]), posttraumatic stress disorder with anxiety and depression (β3 [123]), and hormonal/autonomic stress responses (α6 [124]).
Recent clinical and experimental data outline the role of GABA and GABA-ergic genes in amygdala and hippocampus (Table 2); the brain areas involved in the regulation of both memory and anxiety [125, 126]. In addition to receptors, these domains are also influenced by GABA metabolism. While specific amygdalar reduction in expression of GABA-synthesizing enzyme was observed in animals during learning [126], spatial learning was impaired in rats following anxiolytic GABA transporter inhibitor tiagabine [127]. Collectively, these findings confirm that central GABA is a key mediator regulating anxiety and memory, and that GABAergic genes, metabolism, and/or subunit-specific GABAergic drugs [100, 128–132] may modulate such interplay.
Table 2.
Clinical and preclinical data linking common GABAergic brain areas to pathogenesis of anxiety and depression.
| Clinical data | Animal data |
|
| |
| Amygdala (anxiety, memory) | |
| Activation in patients with posttraumatic stress disorder [71], during anticipatory anxiety [72], in adults and adolescents viewing fearful faces; also positive correlation of amygdalar activation and social anxiety scores [73–75]. | Reduced anxiety and memory in rats following muscimol injection [76–78]. Reduced expression of GABA-A receptor associated protein (a) after fear conditioning in rats [79]. Increased c-fos expression (b) in rats following anxiogenic drugs [10]. Correlation between anxiety phenotype and reduced GABA-A receptor densities, benzodiazepine binding, and γ2 subunit mRNA levels in mice and rats [80–82]. Altered amygdalar electric activity during fear conditioning in mice [83]. Reduced extracellular GABA in mice exposed to conditioned fear stimulus [84]. |
|
| |
| Hippocampus (memory, anxiety) | |
| Reduced blood flow in anxious volunteers during phobogenic (versus neutral) visual stimulation [85]. Decreased blood flow in right hippocampus in women with posttraumatic stress disorder [86] | Reduced expression of α2 GABA-A receptor subunit 6 hours after fear conditioning in rats [79]. Correlation between anxiety and altered benzodiazepine binding in rats [27, 82]. Reduced expression of α1 and α2 subunits mRNA in punished rats [87]. Altered volume in anxious HAB (versus low-anxiety LAB) rats [88]. Increased c-fos expression in rats following administration of anxiogenic drugs [10]. Reduced hippocampal allopregnanolone levels in anxious high-vocalizing rats [89]. Correlation between mouse spatial learning abilities and GABA-A receptor densities [90]. Disrupted context-specific fear memory in rats following muscimol injection [91]. |
(a) Modulates channel kinetics and neurotransmission by promoting GABA-A receptor clustering.
(b)Genetic marker of neuronal activation.
Glutamate receptors mediate most excitatory CNS neurotransmission. There are several known subtypes of metabotropic glutamate receptors which are coupled to G-proteins and exert their effects via second messenger signaling pathways. Genetic ablation of glutamate subtype 7 receptors in mice impairs their performance in two distinct amygdala-dependent paradigms [54] and inhibits hippocampal neurotransmission [133], suggesting that both structures are involved in glutamate-mediated mechanisms of memory and anxiety. Consistent with this, glutamate receptor densities positively correlate with spatial learning abilities in mice [90].
Several recent clinical and experimental data also show that central dopaminergic system plays a role in the regulation of memory and anxiety, including fear conditioning [134, 135]. In line with this, a recent quantitative trait loci study showed that cognitive functions (intertrial habituation) of 25 inbred mouse strains were linked to a region on chromosome 15 mapping dopamine D1 and D2 receptors [136].
Serotonin and its receptors have long been implicated in memory and anxiety in both humans [38, 122, 134, 137, 138] and animals [1, 139–144]. In addition to receptors (Table 1), other factors include serotonin homeostasis and metabolism. Serotonin is removed from the synaptic cleft by a specific membrane transporter protein (SERT [31, 145]), representing an important target for various manipulations. For example, pharmacological inhibition of SERT leads to elevated hippocampal serotonin levels and improved memory [93]. While genetic ablation of SERT in mice is widely used as a model of anxiety [47, 145–148], these mice display increased poststress responsivity [149], indirectly implying a better memory for aversive stimuli. Clearly, further studies are needed to assess the link between SERT and cognitive abilities in animals, and its relevance to human brain dysfunctions. Overall, human anxiety-related traits seem to generally facilitate cognitive functions (e.g., acquisition of conditioned fear), and such interplay is partially serotonergically mediated [134].
Strengthening this notion, genetic variations in SERT have been linked to strain differences in emotional learning in rats [150]. In humans, SERT has also been implicated in anxiety and cognitions. For example, SERT polymorphisms have been associated with anxiety-related personality traits [122, 151], amygdalar reactivity [152–154], cognitive abilities [36, 155], and altered hippocampal neurochemistry [137]. In line with this, Caspi et al. [156] recently established that human SERT polymorphisms modulate the effect of life stress on stress-related CNS pathogenesis, while Fox et al. [157] found association of SERT polymorphisms with children behavioral inhibition—a temperamental construct predicting anxiety.
Importantly, brain catecholamines do not act individually in the brain, interact at different levels with each other, and with other brain molecules [147, 148]. Antipanic drug phenelzine (a nonselective inhibitor of monoamine oxidase MAO A/B which elevates brain norephinephrine, dopamine, and serotonin levels) also exerts mnemotropic effects [19]. MAO A/B knockout mice (demonstrating phenotype similar to the effect of phenelzine) display robust anxiety phenotype but unaltered working memory (Table 1), as assessed by their open field habituation [57]. In contrast, MAO B inactivation in mice leads to increased anxiety, unaltered spatial working memory in Y-maze, but reduced habituation to the forced swim test 4 weeks after the initial trial [56]. Collectively, these data confirm the notion that anxiety and memory phenotypes are heterogeneous and may be determined by interactions of various mediator systems. For example, Birzniece et al. [114] recently analyzed the interplay between GABA-active steroids and serotonin in modulating cognitive functions, and Sibille et al. [45] found reduced GABAergic tone in anxious serotonin 5HT-1A receptor knockout mice, also displaying memory deficits [44].
3. NEUROPEPTIDES AND NEURAL PLASTICITY ISSUES
In addition to mediators, brain neuropeptides play a key role in modulation of memory and anxiety. For example, mutants lacking receptors of “anxiogenic” cotricotropin releasing factor (CRF) display a predictable reduction of anxiety accompanied by reduced cognitive performance during the retrieval trial in the Y-maze (Table 1). Overall, these findings are in line with numerous data implicating CRF in both anxiety and memory, and suggest that novel antistress mnemotropic drugs may be created based on targeting central CRH system [58, 167]. In contrast, mutant mice with reduced sensitivity of thyroid receptors [60] display increased anxiety but reduced memory (Table 1), demonstrating that not always various manipulations exert synergetic effects on these two processes. Interestingly, while CRF has been traditionally linked to memory and anxiety, nonanxiogenic doses of CRF type 1 and 2 receptor agonist urocortin produced anxiety (accompanied by amygdalar hyperexcitability) after 5 daily intra-amygdalar infusions in rats [168]. These results indicate that CRF-induced synaptic plasticity, in addition to anxiety and memory processes, may be involved in pathogenesis of emotional disorders (also see [169] for review).
Pituitary adenylate cyclase-activating polypeptide (PACAP) is another important regulator of synaptic plasticity, neurotrophins, neuromediators, and neuronal differentiation [67, 68]. It binds to a highly selective type 1 receptor (PAC1), widely distributed in the limbic system, including amygdala and hippocampus. Since mice lacking PAC1 demonstrate reduced anxiety and impaired memory (Table 1), PACAP/PAC1 system may be directly involved in the regulation of memory-anxiety interplay. Clearly, further studies are needed to explore this interesting aspect in detail, including its relation to PACAP/PAC1-mediated neuroimmuno-modulation and neuroprotection [170] and impairment in mossy fiber long-term potentiation [68].
Glial Ca-binding protein S100B also plays an important modulatory role in memory. S100B knockout mice display strengthened synaptic plasticity, enhanced long-term potentiation, and spatial memory in Morris water maze, while mice over-expressing this protein exhibit the opposite phenotype [62]. Importantly, these findings show that both neurons and glial cells modulate brain synaptic plasticity, and that glial-neuronal interactions must also be considered in examining memory-anxiety interplay in the CNS.
Protein kinase C (PKC) γ is an enzyme highly expressed in the limbic system—the brain structure that regulates both memory and anxiety [63, 64]. Since PKCγ plays an important role in neural plasticity, modulation of neurotransmitter release, and neuronal excitability, its genetic ablation in mice predictably affects their anxiety and learning (Table 1). Mechanisms underlying these effects are still unknown but most likely include postsynaptic modulation of central GABA-A and serotonergic 5HT2 receptors [64].
From various brain proteins essential for synaptic vesicle trafficking, ras-associated binding proteins, such as Rab3a [70, 171], deserve special attention in relation to memory and anxiety. Using Rab3a knockout (−/−) and Ebd (loss-of-function) Rab3a mutant mice, a recent study has shown that Rab3a −/− mice display reduced cued fear conditioning, while Ebd mutants show both reduced anxiety and cued fear conditioning (Table 1), accompanied by altered hippocampal and cortical expression of Rab3a [69]. D'Adamo et al. [70] reported that Rab3a −/− mice display deficits in short- and long-term synaptic plasticity in the mossy fiber pathway, normal acquisition but several mild impairments in other memory tasks (Table 1), accompanied by increased locomotion and reduced anxiety. Collectively, these data implicate protein modulators of synaptic transmission (such as Rab3a) in the regulation of memory and anxiety, also enabling further dissection of molecular domains involved in their regulation.
Another recent study demonstrated that Rab3a is required for brain-derived neurotrophic factor (BDNF)-induced synaptic plasticity [172], implying functional interplay between the two molecules involved in brain plasticity. Indeed, BDNF is a key neurotrophic factor, acting through trkB receptor to regulate brain growth, differentiation, and functioning [32, 160, 173]. While an early study showed no anxiety or memory effects of BDNF genetic ablation in mice, numerous other data did reveal such actions (see Table 3 for details), also implying BDNF role in aversive memories [158, 162]. Consistent with this, spatial learning induces BDNF and trkB expression in activated brain areas, while BDNF inactivation markedly impairs spatial learning [32, 165]. In addition, mutant mice with reduced BDNF levels display impaired learning and memory in some tasks [159], whereas increased mouse BDNF signaling by trkB overexpression improves memory [165].
Table 3.
Summary of data showing the role of BDNF in memory and anxiety. KO: knockout (−/−), HZ: heterozygous (+/−) mice. (?: unclear effects. ∗: although authors claimed that anxiety was unaltered in this study, it contradicts the original anxiogenic interpretation of the social defeat model (also see [158]).)
| Model | Effects on | References | |
| Anxiety | Memory/learning | ||
|
| |||
| BDNF HZ mice | 0 | ↓ learning (but 0 spatial learning and memory, fear conditioning) | [159], but see [160, 161] |
|
| |||
| Repeated aggression accompanied by increased BDNF expression in mice | ↑* | ↑ long-term social aversion | [162] |
|
| |||
| Mesolimbic-specific BDNF knockdown | ↑* | ↓ long-term social aversion | [162] |
| BDNF intrahippocampal injection in rats | ↓↑ | ↑ short-term spatial memory | [163] |
| BDNF injection to the cortex in rats | ↑ long-term memory | [164] | |
| BDNF receptor overexpression in mice | ↓ | ↑ spatial memory and learning | [165] |
|
| |||
| Forebrain-specific BDNF KO mice | 0 ↑? | ↓ spatial and nonspatial discrimination learning, 0 contextual fear | [166] |
|
| |||
| Brain conditional BDNF KO mice | ↑ | — | [33] |
BDNF is rich in hippocampus and amygdala, and its administration improves rat short-term spatial memory and reduces anxiety [163]. In contrast, the same study revealed increased anxiety on trial 2 in BDNF-treated rats, suggesting that different types of anxiety may differently interplay with BDNF-modulated memories. In line with this, increased BDNF signaling in mice over-expressing trkB produced anxiolysis [165], while stress and anxiety correlate with memory deficits and reduction in brain BDNF [174, 175]. Moreover, Rattiner et al. [176, 177] have recently outlined the crucial role of BDNF and its receptors in hippocampal and amygdala-dependent learning (including fear conditioning—another potential mechanism underlying BDNF modulation of memory and anxiety).
Overall, human data strikingly parallel animal data on BDNF role in memory and anxiety (Table 3). For example, functional BDNF polymorphisms have been associated with anxiety-related personality traits [178], hippocampal volume in healthy volunteers [179], and episodic memory [180]. Taken together, these data confirm the important role of BDNF in memory, anxiety, and their interplay. Given the important role of BDNF in brain plasticity [173], behavior-modulating properties of this molecule seem to be particularly intriguing.
Importantly, brain mediators seem to cooperate with BDNF in modulating brain functions. For example, BDNF interacts with cholinergic, dopaminergic, serotonergic systems, and SERT [181–184] whose involvement in memory and anxiety has already been discussed. Analyses of human quantitative trait loci associated with cognitive functions also pointed to genes encoding BDNF, ACh, and glutamate receptors [185]. From this point of view, it is interesting that heterozygous BDNF knockout mice display unaltered or little anxiety and rather mild alterations in memory (Table 3), accompanied by altered hippocampal ACh but unaltered catecholamine levels [160]. In contrast, simultaneous ablation of BDNF and SERT alleles exacerbates anxiety in double knockout mice and reduces hippocampal serotonin levels [147, 186], confirming an important functional interplay between BDNF and serotonin in the brain [181]. Extending original findings of Caspi et al. [156], a recent study has examined BDNF/SERT genes' interactions in depressed children, reporting that a combination of met-BDNF allele with two short SERT alleles was associated with higher depression in maltreated children [187]. Notably, this situation strikingly resembles experiments of Ren-Patterson et al. [186] in mice, indirectly supporting the notion that depression as well as specific anxiety-related traits (i.e., social anxiety or posttraumatic stress) may also be involved in BDNF-SERT interplay; also see [158, 162] for discussion.
4. CONCLUSIONS
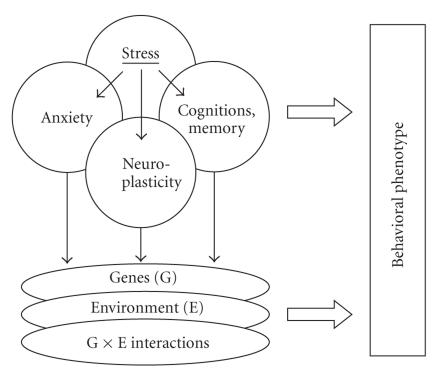
As already mentioned, memory and anxiety do not always follow synergetic “high anxiety-better memory” rule, indicating that more complex nonlinear relations exist between these behavioral domains. Moreover, not always altered anxiety is seen together with altered memory, as vise versa (Table 1), suggesting that under certain circumstances both domains may be affected independently. Likewise, memory (as well as anxiety) must not be considered as a single entity, and clearly represents a complex multidimensional domain. However, it is important to understand that memory and anxiety represent two overlapping CNS processes that closely interact at different levels, including brain neurochemistry, circuitry, pharmacology, and various genes, as discussed here in detail. For such interactions, clinical findings strikingly parallel animal experimentation data, showing how these factors (in addition to environmental influences) may affect memory and anxiety. Both neuronal and glial cells, as well as brain mediators, neuropeptides, and other key proteins, cooperate in the regulation of memory and anxiety (Figure 1). Finally, brain plasticity factors (Figure 1) appear to play an important role in fine-tuning of memory-anxiety interplay, collectively contributing to the complexity of behavioral phenotypes.
Figure 1.
Stress, memory, and anxiety interplay.
ACKNOWLEDGMENT
This study is supported by the NIMH/NIH Intramural Research Program.
References
- 1.Clement Y, Chapouthier G. Biological bases of anxiety. Neuroscience and Biobehavioral Reviews. 1998;22(5):623–633. doi: 10.1016/s0149-7634(97)00058-4. [DOI] [PubMed] [Google Scholar]
- 2.Nutt DJ. Neurobiological mechanisms in generalized anxiety disorder. Journal of Clinical Psychiatry. 2001;62(supplement 11):22–27. [PubMed] [Google Scholar]
- 3.Nutt DJ. Overview of diagnosis and drug treatments of anxiety disorders. CNS Spectrums. 2005;10(1):49–56. doi: 10.1017/s1092852900009901. [DOI] [PubMed] [Google Scholar]
- 4.Nutt DJ, Malizia AL. New insights into the role of the GABAA-benzodiazepine receptor in psychiatric disorder. British Journal of Psychiatry. 2001;179:390–396. doi: 10.1192/bjp.179.5.390. [DOI] [PubMed] [Google Scholar]
- 5.Nutt DJ, Ballenger JC, Sheehan D, Wittchen H-U. Generalized anxiety disorder: comorbidity, comparative biology and treatment. International Journal of Neuropsychopharmacology. 2002;5(4):315–325. doi: 10.1017/S1461145702003048. [DOI] [PubMed] [Google Scholar]
- 6.Bailey KR, Rustay NR, Crawley JN. Behavioral phenotyping of transgenic and knockout mice: practical concerns and potential pitfalls. ILAR Journal. 2006;47(2):124–131. doi: 10.1093/ilar.47.2.124. [DOI] [PubMed] [Google Scholar]
- 7.Crawley JN. Behavioral phenotyping of transgenic and knockout mice: experimental design and evaluation of general health, sensory functions, motor abilities, and specific behavioral tests. Brain Research. 1999;835(1):18–26. doi: 10.1016/s0006-8993(98)01258-x. [DOI] [PubMed] [Google Scholar]
- 8.MGI Mouse Genome Informatics. 2006. http://www.informatics.jax.org/.
- 9.MPD Mouse Phenome Database. 2006. http://phenome.jax.org/pub-cgi/phenome/mpdcgi.
- 10.Singewald N, Salchner P, Sharp T. Induction of c-Fos expression in specific areas of the fear circuitry in rat forebrain by anxiogenic drugs. Biological Psychiatry. 2003;53(4):275–283. doi: 10.1016/s0006-3223(02)01574-3. [DOI] [PubMed] [Google Scholar]
- 11.Dagnino-Subiabre A, Orellana JA, Carmona-Fontaine C, et al. Chronic stress decreases the expression of sympathetic markers in the pineal gland and increases plasma melatonin concentration in rats. Journal of Neurochemistry. 2006;97(5):1279–1287. doi: 10.1111/j.1471-4159.2006.03787.x. [DOI] [PubMed] [Google Scholar]
- 12.Kalueff AV, Nutt DJ. Role of GABA in memory and anxiety. Depression and Anxiety. 1996;4(3):100–110. doi: 10.1002/(SICI)1520-6394(1996)4:3<100::AID-DA2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 13.Wall PM, Messier C. Concurrent modulation of anxiety and memory. Behavioural Brain Research. 2000;109(2):229–241. doi: 10.1016/s0166-4328(99)00177-1. [DOI] [PubMed] [Google Scholar]
- 14.Yerkes RM, Dodson JD. The relation of strength of stimulus to rapidity of habit-formation. Journal of Comparative Neurology and Psychology. 1908;18(5):459–482. [Google Scholar]
- 15.Barad M. Fear extinction in rodents: basic insight to clinical promise. Current Opinion in Neurobiology. 2005;15(6):710–715. doi: 10.1016/j.conb.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Beuzen A, Belzung C. Link between emotional memory and anxiety states: a study by principal component analysis. Physiology and Behavior. 1995;58(1):111–118. doi: 10.1016/0031-9384(95)00013-9. [DOI] [PubMed] [Google Scholar]
- 17.Chapouthier G, Venault P. GABAA receptor complex and memory processes. Medicinal Chemistry Reviews. 2004;1(1):91–99. doi: 10.2174/1568026023393552. [DOI] [PubMed] [Google Scholar]
- 18.Gerlai R. Memory enhancement: the progress and our fears. Genes, Brain and Behavior. 2003;2(4):187–190. doi: 10.1034/j.1601-183x.2003.00019.x. [DOI] [PubMed] [Google Scholar]
- 19.Parent MB, Habib MK, Baker GB. Time-dependent changes in brain monoamine oxidase activity and in brain levels of monoamines and amino acids following acute administration of the antidepressant/antipanic drug phenelzine. Biochemical Pharmacology. 2000;59(10):1253–1263. doi: 10.1016/s0006-2952(00)00244-6. [DOI] [PubMed] [Google Scholar]
- 20.Quirk GJ, Gehlert DR. Inhibition of the amygdala: key to pathological states? Annals of the New York Academy of Sciences. 2003;985:263–272. doi: 10.1111/j.1749-6632.2003.tb07087.x. [DOI] [PubMed] [Google Scholar]
- 21.Izquierdo I, Medina JH. GABAA receptor modulation of memory: the role of endogenous benzodiazepines. Trends in Pharmacological Sciences. 1991;12(7):260–265. doi: 10.1016/0165-6147(91)90567-c. [DOI] [PubMed] [Google Scholar]
- 22.Izquierdo I, Medina JH. Correlation between the pharmacology of long-term potentiation and the pharmacology of memory. Neurobiology of Learning and Memory. 1995;63(1):19–32. doi: 10.1006/nlme.1995.1002. [DOI] [PubMed] [Google Scholar]
- 23.Bannerman DM, Rawlins JNP, McHugh SB, et al. Regional dissociations within the hippocampus—memory and anxiety. Neuroscience and Biobehavioral Reviews. 2004;28(3):273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Bierman EJM, Comijs HC, Jonker C, Beekman ATF. Effects of anxiety versus depression on cognition in later life. American Journal of Geriatric Psychiatry. 2005;13(8):686–693. doi: 10.1176/appi.ajgp.13.8.686. [DOI] [PubMed] [Google Scholar]
- 25.El Hage W, Peronny S, Griebel G, Belzung C. Impaired memory following predatory stress in mice is improved by fluoxetine. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2004;28(1):123–128. doi: 10.1016/j.pnpbp.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 26.El Hage W, Griebel G, Belzung C. Long-term impaired memory following predatory stress in mice. Physiology and Behavior. 2006;87(1):45–50. doi: 10.1016/j.physbeh.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 27.Ribeiro RL, Andreatini R, Wolfman C, Viola H, Medina JH, Da Cunha C. The ‘anxiety state’ and its relation with rat models of memory and habituation. Neurobiology of Learning and Memory. 1999;72(2):78–94. doi: 10.1006/nlme.1998.3891. [DOI] [PubMed] [Google Scholar]
- 28.Savić MM, Obradović DI, Ugrešić ND, Bokonjić DR. Memory effects of benzodiazepines: memory stages and types versus binding-site subtypes. Neural Plasticity. 2005;12(4):289–298. doi: 10.1155/NP.2005.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Savić MM, Obradović DI, Ugrešić ND, Cook JM, Sarma PVVS, Bokonjić DR. Bidirectional effects of benzodiazepine binding site ligands on active avoidance acquisition and retention: differential antagonism by flumazenil and β-CCt. Psychopharmacology. 2005;180(3):455–465. doi: 10.1007/s00213-005-2170-1. [DOI] [PubMed] [Google Scholar]
- 30.Savić MM, Obradović DI, Ugrešić ND, Cook JM, Yin W, Bokonjić DR. Bidirectional effects of benzodiazepine binding site ligands in the passive avoidance task: differential antagonism by flumazenil and β-CCt. Behavioural Brain Research. 2005;158(2):293–300. doi: 10.1016/j.bbr.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 31.Gingrich JA, Hen R. Dissecting the role of the serotonin system in neuropsychiatric disorders using knockout mice. Psychopharmacology. 2001;155(1):1–10. doi: 10.1007/s002130000573. [DOI] [PubMed] [Google Scholar]
- 32.Monteggia LM, Barrot M, Powell CM, et al. Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(29):10827–10832. doi: 10.1073/pnas.0402141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rios M, Fan G, Fekete C, et al. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Molecular Endocrinology. 2001;15(10):1748–1757. doi: 10.1210/mend.15.10.0706. [DOI] [PubMed] [Google Scholar]
- 34.Wehner JM, Balogh SA. Genetic studies of learning and memory in mouse models. In: Plomin R, DeFries J, Craig I, McGuffin P, editors. Behavioral Genetics in the Postgenomic Era. Washington, DC, USA: APA; 2002. pp. 103–121. [Google Scholar]
- 35.Ross SA, Wong JYF, Clifford JJ, et al. Phenotypic characterization of an α4 neuronal nicotinic acetylcholine receptor subunit knock-out mouse. Journal of Neuroscience. 2000;20(17):6431–6441. doi: 10.1523/JNEUROSCI.20-17-06431.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paylor R, Nguyen M, Crawley JN, Patrick J, Beaudet A, Orr-Urtreger A. α7 nicotinic receptor subunits are not necessary for hippocampal- dependent learning or sensorimotor gating: a behavioral characterization of Acra7-deficient mice. Learning and Memory. 1998;5(4-5):302–316. [PMC free article] [PubMed] [Google Scholar]
- 37.Picciotto MR, Zoli M, Léna C, et al. Abnormal avoidance learning in mice lacking functional high-affinity nicotine receptor in the brain. Nature. 1995;374(6517):65–67. doi: 10.1038/374065a0. [DOI] [PubMed] [Google Scholar]
- 38.Buhot M-C, Malleret G, Segu L. Serotonin receptors and cognitive behaviour—an update. IDrugs. 1999;2(5):426–437. [PubMed] [Google Scholar]
- 39.Buhot M-C, Wolff M, Savova M, Malleret G, Hen R, Segu L. Protective effect of 5-HT1B receptor gene deletion on the age-related decline in spatial learning abilities in mice. Behavioural Brain Research. 2003;142(1-2):135–142. doi: 10.1016/s0166-4328(02)00400-x. [DOI] [PubMed] [Google Scholar]
- 40.Dirks A, Pattij T, Bouwknecht JA, et al. 5-HT1B receptor knockout, but not 5-HT1A receptor knockout mice, show reduced startle reactivity and footshock-induced sensitization, as measured with the acoustic startle response. Behavioural Brain Research. 2001;118(2):169–178. doi: 10.1016/s0166-4328(00)00326-0. [DOI] [PubMed] [Google Scholar]
- 41.López-Rubalcava C, Hen R, Cruz SL. Anxiolytic-like actions of toluene in the burying behavior and plus-maze tests: differences in sensitivity between 5-HT(1B) knockout and wild-type mice. Behavioural Brain Research. 2000;115(1):85–94. doi: 10.1016/s0166-4328(00)00241-2. [DOI] [PubMed] [Google Scholar]
- 42.Wolff M, Savova M, Malleret G, Hen R, Segu L, Buhot M-C. Serotonin 1B knockout mice exhibit a task-dependent selective learning facilitation. Neuroscience Letters. 2003;338(1):1–4. doi: 10.1016/s0304-3940(02)01339-3. [DOI] [PubMed] [Google Scholar]
- 43.Parks CL, Robinson PS, Sibille E, Shenk T, Toth M. Increased anxiety of mice lacking the serotonin1A receptor. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(18):10734–10739. doi: 10.1073/pnas.95.18.10734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sarnyai Z, Sibille EL, Pavlides C, Fenster RJ, McEwen BS, Tóth M. Impaired hippocampal-dependent learning and functional abnormalities in the hippocampus in mice lacking serotonin1A receptors. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(26):14731–14736. doi: 10.1073/pnas.97.26.14731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sibille E, Pavlides C, Benke D, Toth M. Genetic inactivation of the serotonin(1A) receptor in mice results in downregulation of major GABAA receptor α subunits, reduction of GABAA receptor binding, and benzodiazepine-resistant anxiety. Journal of Neuroscience. 2000;20(8):2758–2765. doi: 10.1523/JNEUROSCI.20-08-02758.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grailhe R, Waeber C, Dulawa SC, et al. Increased exploratory activity and altered response to LSD in mice lacking the 5-HT (5A) receptor. Neuron. 1999;22(3):581–591. doi: 10.1016/s0896-6273(00)80712-6. [DOI] [PubMed] [Google Scholar]
- 47.Holmes A, Murphy DL, Crawley JN. Abnormal behavioral phenotypes of serotonin transporter knockout mice: parallels with human anxiety and depression. Biological Psychiatry. 2003;54(10):953–959. doi: 10.1016/j.biopsych.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 48.Crestani F, Keist R, Fritschy J-M, et al. Trace fear conditioning involves hippocampal α5 GABAA receptors. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(13):8980–8985. doi: 10.1073/pnas.142288699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crestani F, Lorez M, Baer K, et al. Decreased GABAA-receptor clustering results in enhanced anxiety and a bias for threat cues. Nature Neuroscience. 1999;2(9):833–839. doi: 10.1038/12207. [DOI] [PubMed] [Google Scholar]
- 50.Rizk A, Curley J, Robertson J, Raber J. Anxiety and cognition in histamine H3receptor−/− mice. European Journal of Neuroscience. 2004;19(7):1992–1996. doi: 10.1111/j.1460-9568.2004.03251.x. [DOI] [PubMed] [Google Scholar]
- 51.Toyota H, Dugovic C, Koehl M, et al. Behavioral characterization of mice lacking histamine H3 receptors. Molecular Pharmacology. 2002;62(2):389–397. doi: 10.1124/mol.62.2.389. [DOI] [PubMed] [Google Scholar]
- 52.Yee BK, Balic E, Singer P, et al. Disruption of glycine transporter 1 restricted to forebrain neurons is associated with a procognitive and antipsychotic phenotypic profile. Journal of Neuroscience. 2006;26(12):3169–3181. doi: 10.1523/JNEUROSCI.5120-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shimshek DR, Bus T, Kim J, et al. Enhanced odor discrimination and impaired olfactory memory by spatially controlled switch of AMPA receptors. PLoS Biology. 2005;3(11):e354. doi: 10.1371/journal.pbio.0030354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Masugi M, Yokoi M, Shigemoto R, et al. Metabotropic glutamate receptor subtype 7 ablation causes deficit in fear response and conditioned taste aversion. Journal of Neuroscience. 1999;19(3):955–963. doi: 10.1523/JNEUROSCI.19-03-00955.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bannerman DM, Deacon RMJ, Brady S, et al. A comparison of GluR-A-deficient and wild-type mice on a test battery assessing sensorimotor, affective, and cognitive behaviors. Behavioral Neuroscience. 2004;118(3):643–647. doi: 10.1037/0735-7044.118.3.643. [DOI] [PubMed] [Google Scholar]
- 56.Grimsby J, Toth M, Chen K, et al. Increased stress response and beta-phenylethylamine in MAOB-deficient mice. Nature Genetics. 1997;17(2):206–210. doi: 10.1038/ng1097-206. [DOI] [PubMed] [Google Scholar]
- 57.Chen K, Holschneider DP, Wu W, Rebrini I, Shih JC. A spontaneous point mutation produces monoamine oxidase A/B knock-out mice with greatly elevated monoamines and anxiety-like behavior. Journal of Biological Chemistry. 2004;279(38):39645–39652. doi: 10.1074/jbc.M405550200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Contarino A, Dellu F, Koob GF, et al. Reduced anxiety-like and cognitive performance in mice lacking the corticotropin-releasing factor receptor 1. Brain Research. 1999;835(1):1–9. doi: 10.1016/s0006-8993(98)01158-5. [DOI] [PubMed] [Google Scholar]
- 59.Guadaño-Ferraz A, Benavides-Piccione R, Venero C, et al. Lack of thyroid hormone receptor α1 is associated with selective alterations in behavior and hippocampal circuits. Molecular Psychiatry. 2003;8(1):30–38. doi: 10.1038/sj.mp.4001196. [DOI] [PubMed] [Google Scholar]
- 60.Venero C, Guadaño-Ferraz A, Herrero AI, et al. Anxiety, memory impairment, and locomotor dysfunction caused by a mutant thyroid hormone receptor α1 can be ameliorated by T3 treatment. Genes and Development. 2005;19(18):2152–2163. doi: 10.1101/gad.346105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Greco B, Carli M. Reduced attention and increased impulsivity in mice lacking NPY Y2 receptors: relation to anxiolytic-like phenotype. Behavioural Brain Research. 2006;169(2):325–334. doi: 10.1016/j.bbr.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 62.Nishiyama H, Knöpfel T, Endo S, Itohara S. Glial protein S100B modulates long-term neuronal synaptic plasticity. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(6):4037–4042. doi: 10.1073/pnas.052020999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abeliovich A, Paylor R, Chen C, Kim JJ, Wehner JM, Tonegawa S. PKCγ mutant mice exhibit mild deficits in spatial and contextual learning. Cell. 1993;75(7):1263–1271. doi: 10.1016/0092-8674(93)90614-v. [DOI] [PubMed] [Google Scholar]
- 64.Bowers BJ, Collins AC, Tritto T, Wehner JM. Mice lacking PKCγ exhibit decreased anxiety. Behavior Genetics. 2000;30(2):111–121. doi: 10.1023/a:1001951104208. [DOI] [PubMed] [Google Scholar]
- 65.Bontekoe CJM, McIlwain KL, Nieuwenhuizen IM, et al. Knockout mouse model for Fxr2: a model for mental retardation. Human Molecular Genetics. 2002;11(5):487–498. doi: 10.1093/hmg/11.5.487. [DOI] [PubMed] [Google Scholar]
- 66.Müller U, Cristina N, Li Z-W, et al. Behavioral and anatomical deficits in mice homozygous for a modified β-amyloid precursor protein gene. Cell. 1994;79(5):755–765. doi: 10.1016/0092-8674(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 67.Otto C, Martin M, Wolfer DP, Lipp H-P, Maldonado R, Schütz G. Altered emotional behavior in PACAP-type-I-receptor-deficient mice. Molecular Brain Research. 2001;92(1-2):78–84. doi: 10.1016/s0169-328x(01)00153-x. [DOI] [PubMed] [Google Scholar]
- 68.Otto C, Kovalchuk Y, Wolfer DP, et al. Impairment of mossy fiber long-term potentiation and associative learning in pituitary adenylate cyclase activating polypeptide type I receptor-deficient mice. Journal of Neuroscience. 2001;21(15):5520–5527. doi: 10.1523/JNEUROSCI.21-15-05520.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang S, Farias M, Kapfhamer D, et al. Biochemical, molecular and behavioral phenotypes of Rab3A mutations in the mouse. doi: 10.1111/j.1601-183X.2006.00235.x. to appear in Genes, Brain and Behavior. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.D'Adamo P, Wolfer DR, Kopp C, Tobler I, Toniolo D, Lipp H-P. Mice deficient for the synaptic vesicle protein Rab3a show impaired spatial reversal learning and increased explorative activity but none of the behavioral changes shown by mice deficient for the Rab3a regulator Gdi1. European Journal of Neuroscience. 2004;19(7):1895–1905. doi: 10.1111/j.1460-9568.2004.03270.x. [DOI] [PubMed] [Google Scholar]
- 71.Rauch SL, Van Der Kolk BA, Fisler RE, et al. A symptom provocation study of posttraumatic stress disorder using positron emission tomography and script-driven imagery. Archives of General Psychiatry. 1996;53(5):380–387. doi: 10.1001/archpsyc.1996.01830050014003. [DOI] [PubMed] [Google Scholar]
- 72.Phelps EA, O'Connor KJ, Gatenby JC, Gore JC, Grillon C, Davis M. Activation of the left amygdala to a cognitive representation of fear. Nature Neuroscience. 2001;4(4):437–441. doi: 10.1038/86110. [DOI] [PubMed] [Google Scholar]
- 73.Morris JS, Friston KJ, Büchel C, et al. A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain. 1998;121(1):47–57. doi: 10.1093/brain/121.1.47. [DOI] [PubMed] [Google Scholar]
- 74.Killgore WDS, Yurgelun-Todd DA. Activation of the amygdala and anterior cingulate during nonconscious processing of sad versus happy faces. NeuroImage. 2004;21(4):1215–1223. doi: 10.1016/j.neuroimage.2003.12.033. [DOI] [PubMed] [Google Scholar]
- 75.Killgore WDS, Yurgelun-Todd DA. Social anxiety predicts amygdala activation in adolescents viewing fearful faces. NeuroReport. 2005;16(15):1671–1675. doi: 10.1097/01.wnr.0000180143.99267.bd. [DOI] [PubMed] [Google Scholar]
- 76.Bueno CH, Zangrossi H, Jr, Nogueira RL, Soares VP, Viana MB. Panicolytic-like effect induced by the stimulation of GABAA and GABAB receptors in the dorsal periaqueductal grey of rats. European Journal of Pharmacology. 2005;516(3):239–246. doi: 10.1016/j.ejphar.2005.04.045. [DOI] [PubMed] [Google Scholar]
- 77.Bueno CH, Zangrossi H, Jr, Viana MB. The inactivation of the basolateral nucleus of the rat amygdala has an anxiolytic effect in the elevated T-maze and light/dark transition tests. Brazilian Journal of Medical and Biological Research. 2005;38(11):1697–1701. doi: 10.1590/s0100-879x2005001100019. [DOI] [PubMed] [Google Scholar]
- 78.Spanis CW, Bianchin MM, Izquierdo I, McGaugh JL. Excitotoxic basolateral amygdala lesions potentiate the memory impairment effect of muscimol injected into the medial septal area. Brain Research. 1999;816(2):329–336. doi: 10.1016/s0006-8993(98)01104-4. [DOI] [PubMed] [Google Scholar]
- 79.Mei B, Li C, Dong S, Jiang CH, Wang H, Hu Y. Distinct gene expression profiles in hippocampus and amygdala after fear conditioning. Brain Research Bulletin. 2005;67(1-2):1–12. doi: 10.1016/j.brainresbull.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 80.Yilmazer-Hanke DM, Roskoden T, Zilles K, Schwegler H. Anxiety-related behavior and densities of glutamate, GABAA, acetylcholine and serotonin receptors in the amygdala of seven inbred mouse strains. Behavioural Brain Research. 2003;145(1-2):145–159. doi: 10.1016/s0166-4328(03)00107-4. [DOI] [PubMed] [Google Scholar]
- 81.Caldji C, Diorio J, Anismam H, Meaney MJ. Maternal behavior regulates benzodiazepine/GABAA receptor subunit expression in brain regions associated with fear in BALB/c and C57BL/6 mice. Neuropsychopharmacology. 2004;29(7):1344–1352. doi: 10.1038/sj.npp.1300436. [DOI] [PubMed] [Google Scholar]
- 82.Da Cunha C, De Stein ML, Wolfman C, Koya R, Izquierdo I, Medina JH. Effect of various training procedures on performance in an elevated plus-maze: possible relation with brain regional levels of benzodiazepine-like molecules. Pharmacology Biochemistry and Behavior. 1992;43(3):677–681. doi: 10.1016/0091-3057(92)90395-v. [DOI] [PubMed] [Google Scholar]
- 83.Rogan MT, Leon KS, Perez DL, Kandel ER. Distinct neural signatures for safety and danger in the amygdala and striatum of the mouse. Neuron. 2005;46(2):309–320. doi: 10.1016/j.neuron.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 84.Stork O, Ji F-Y, Obata K. Reduction of extracellular GABA in the mouse amygdala during and following confrontation with a conditioned fear stimulus. Neuroscience Letters. 2002;327(2):138–142. doi: 10.1016/s0304-3940(02)00387-7. [DOI] [PubMed] [Google Scholar]
- 85.Wik G, Fredrikson M, Ericson K, Eriksson L, Stone-Elander S, Greitz T. A functional cerebral response to frightening visual stimulation. Psychiatry Research - Neuroimaging. 1993;50(1):15–24. doi: 10.1016/0925-4927(93)90020-i. [DOI] [PubMed] [Google Scholar]
- 86.Bremner JD, Narayan M, Staib LH, Southwick SM, McGlashan T, Charney DS. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. American Journal of Psychiatry. 1999;156(11):1787–1795. doi: 10.1176/ajp.156.11.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang L, Rubinow DR, Ma W, et al. GABA receptor subunit mRNA expression in brain of conflict, yoked control and control rats. Molecular Brain Research. 1998;58(1-2):16–26. doi: 10.1016/s0169-328x(98)00061-8. [DOI] [PubMed] [Google Scholar]
- 88.Kalisch R, Schubert M, Jacob W, et al. Anxiety and hippocampus volume in the rat. Neuropsychopharmacology. 2006;31(5):925–932. doi: 10.1038/sj.npp.1300910. [DOI] [PubMed] [Google Scholar]
- 89.Zimmerberg B, Brunelli SA, Fluty AJ, Frye CA. Differences in affective behaviors and hippocampal allopregnanolone levels in adult rats of lines selectively bred for infantile vocalizations. Behavioural Brain Research. 2005;159(2):301–311. doi: 10.1016/j.bbr.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 90.Zilles K, Wu J, Crusio WE, Schwegler H. Water maze and radial maze learning and the density of binding sites of glutamate, GABA, and serotonin receptors in the hippocampus of inbred mouse strains. Hippocampus. 2000;10(3):213–225. doi: 10.1002/1098-1063(2000)10:3<213::AID-HIPO2>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 91.Hobin JA, Ji J, Maren S. Ventral hippocampal muscimol disrupts context-specific fear memory retrieval after extinction in rats. Hippocampus. 2006;16(2):174–182. doi: 10.1002/hipo.20144. [DOI] [PubMed] [Google Scholar]
- 92.Jerusalinsky D, Kornisiuk E, Izquierdo I. Cholinergic neurotransmission and synaptic plasticity concerning memory processing. Neurochemical Research. 1997;22(4):507–515. doi: 10.1023/a:1027376230898. [DOI] [PubMed] [Google Scholar]
- 93.Abe Y, Aoyagi A, Hara T, et al. Pharmacological characterization of RS-1259, an orally active dual inhibitor of acetylcholinesterase and serotonin transporter, in rodents: possible treatment of Alzheimer's disease. Journal of Pharmacological Sciences. 2003;93(1):95–105. doi: 10.1254/jphs.93.95. [DOI] [PubMed] [Google Scholar]
- 94.Korpi ER, Gründer G, Lüddens H. Drug interactions at GABAA receptors. Progress in Neurobiology. 2002;67(2):113–159. doi: 10.1016/s0301-0082(02)00013-8. [DOI] [PubMed] [Google Scholar]
- 95.Korpi ER, Sinkkonen ST. GABAA receptor subtypes as targets for neuropsychiatric drug development. Pharmacology and Therapeutics. 2006;109(1-2):12–32. doi: 10.1016/j.pharmthera.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 96.Rosahl TW. Validation of GABAA receptor subtypes as potential drug targets by using genetically modified mice. Current Drug Targets. CNS and Neurological Disorders. 2003;2(4):207–212. doi: 10.2174/1568007033482823. [DOI] [PubMed] [Google Scholar]
- 97.Vicini S, Ortinski P. Genetic manipulations of GABAA receptor in mice make inhibition exciting. Pharmacology and Therapeutics. 2004;103(2):109–120. doi: 10.1016/j.pharmthera.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 98.Argyropoulos SV, Nutt DJ. The use of benzodiazepines in anxiety and other disorders. European Neuropsychopharmacology. 1999;9(supplement 6):S407–S412. doi: 10.1016/s0924-977x(99)00052-8. [DOI] [PubMed] [Google Scholar]
- 99.Lydiard RB. The role of GABA in anxiety disorders. Journal of Clinical Psychiatry. 2003;64(supplement 3):21–27. [PubMed] [Google Scholar]
- 100.Rudolph U, Möhler H. GABA-based therapeutic approaches: GABAA receptor subtype functions. Current Opinion in Pharmacology. 2006;6(1):18–23. doi: 10.1016/j.coph.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 101.Sandford JJ, Argyropoulos SV, Nutt DJ. The psychobiology of anxiolytic drugs - Part 1: basic neurobiology. Pharmacology and Therapeutics. 2000;88(3):197–212. doi: 10.1016/s0163-7258(00)00082-6. [DOI] [PubMed] [Google Scholar]
- 102.Argyropoulos SV, Sandford JJ, Nutt DJ. The psychobiology of anxiolytic drugs. Part 2: pharmacological treatments of anxiety. Pharmacology and Therapeutics. 2000;88(3):213–227. doi: 10.1016/s0163-7258(00)00083-8. [DOI] [PubMed] [Google Scholar]
- 103.Beleboni RO, Carolino ROG, Pizzo AB, et al. Pharmacological and biochemical aspects of GABAergic neurotransmission: pathological and neuropsychobiological relationships. Cellular and Molecular Neurobiology. 2004;24(6):707–728. doi: 10.1007/s10571-004-6913-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.De-Paris F, Busnello JV, Vianna MRM, et al. The anticonvulsant compound gabapentin possesses anxiolytic but not amnesic effects in rats. Behavioural Pharmacology. 2000;11(2):169–173. doi: 10.1097/00008877-200004000-00009. [DOI] [PubMed] [Google Scholar]
- 105.Lang AP, De Angelis L. Experimental anxiety and antiepileptics: the effects of valproate and vigabatrin in the mirrored chamber test. Methods and Findings in Experimental and Clinical Pharmacology. 2003;25(4):265–271. doi: 10.1358/mf.2003.25.4.769674. [DOI] [PubMed] [Google Scholar]
- 106.Nemeroff CB. The role of GABA in the pathophysiology and treatment of anxiety disorders. Psychopharmacology Bulletin. 2003;37(4):133–146. [PubMed] [Google Scholar]
- 107.Stahl SM. Anticonvulsants as anxiolytics, part 1: tiagabine and other anticonvulsants with actions on GABA. The Journal of Clinical Psychiatry. 2004;65(3):291–292. doi: 10.4088/jcp.v65n0301. [DOI] [PubMed] [Google Scholar]
- 108.Akirav I, Raizel H, Maroun M. Enhancement of conditioned fear extinction by infusion of the GABAA agonist muscimol into the rat prefrontal cortex and amygdala. European Journal of Neuroscience. 2006;23(3):758–764. doi: 10.1111/j.1460-9568.2006.04603.x. [DOI] [PubMed] [Google Scholar]
- 109.Chhatwal JP, Myers KM, Ressler KJ, Davis M. Regulation of gephyrin and GABAA receptor binding within the amygdala after fear acquisition and extinction. Journal of Neuroscience. 2005;25(2):502–506. doi: 10.1523/JNEUROSCI.3301-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McCabe C, Shaw D, Atack JR, et al. Subtype-selective GABAergic drugs facilitate extinction of mouse operant behaviour. Neuropharmacology. 2004;46(2):171–178. doi: 10.1016/j.neuropharm.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 111.Zarrindast MR, Bakhsha A, Rostami P, Shafaghi B. Effects of intrahippocampal injection of GABAergic drugs on memory retention of passive avoidance learning in rats. Journal of Psychopharmacology. 2002;16(4):313–319. doi: 10.1177/026988110201600405. [DOI] [PubMed] [Google Scholar]
- 112.Zarrindast M-R, Noorbakhshnia M, Motamedi F, Haeri-Rohani A, Rezayof A. Effect of the GABAergic system on memory formation and state-dependent learning induced by morphine in rats. Pharmacology. 2006;76(2):93–100. doi: 10.1159/000089934. [DOI] [PubMed] [Google Scholar]
- 113.Chapouthier G, Venault P. GABAA receptor complex and memory processes. Current Topics in Medicinal Chemistry. 2002;2(8):841–851. doi: 10.2174/1568026023393552. [DOI] [PubMed] [Google Scholar]
- 114.Birzniece V, Bäckström T, Johansson I-M, et al. Neuroactive steroid effects on cognitive functions with a focus on the serotonin and GABA systems. Brain Research Reviews. 2006;51(2):212–239. doi: 10.1016/j.brainresrev.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 115.Blum S, Hebert AE, Dash PK. A role for the prefrontal cortex in recall of recent and remote memories. NeuroReport. 2006;17(3):341–344. doi: 10.1097/01.wnr.0000201509.53750.bc. [DOI] [PubMed] [Google Scholar]
- 116.Chandra D, Korpi ER, Miralles CP, de Blas AL, Homanics GE. GABAA receptor γ2 subunit knockdown mice have enhanced anxiety-like behavior but unaltered hypnotic response to benzodiazepines. BMC Neuroscience. 2005;6 doi: 10.1186/1471-2202-6-30. Article ID 30, 13 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Marowsky A, Fritschy J-M, Vogt KE. Functional mapping of GABAA receptor subtypes in the amygdala. European Journal of Neuroscience. 2004;20(5):1281–1289. doi: 10.1111/j.1460-9568.2004.03574.x. [DOI] [PubMed] [Google Scholar]
- 118.Yee BK, Hauser J, Dolgov VV, et al. GABAA receptors containing the α5 subunit mediate the trace effect in aversive and appetitive conditioning and extinction of conditioned fear. European Journal of Neuroscience. 2004;20(7):1928–1936. doi: 10.1111/j.1460-9568.2004.03642.x. [DOI] [PubMed] [Google Scholar]
- 119.Wang H, Zhu YZ, Wong PT-H, et al. cDNA microarray analysis of gene expression in anxious PVG and SD rats after cat-freezing test. Experimental Brain Research. 2003;149(4):413–421. doi: 10.1007/s00221-002-1369-1. [DOI] [PubMed] [Google Scholar]
- 120.Verkuyl JM, Hemby SE, Jöels M. Chronic stress attenuates GABAergic inhibition and alters gene expression of parvocellular neurons in rat hypothalamus. European Journal of Neuroscience. 2004;20(6):1665–1673. doi: 10.1111/j.1460-9568.2004.03568.x. [DOI] [PubMed] [Google Scholar]
- 121.Kosel M, Rudolph U, Wielepp P, et al. Diminished GABAA receptor-binding capacity and a DNA base substitution in a patient with treatment-resistant depression and anxiety. Neuropsychopharmacology. 2004;29(2):347–350. doi: 10.1038/sj.npp.1300353. [DOI] [PubMed] [Google Scholar]
- 122.Sen S, Villafuerte S, Nesse R, et al. Serotonin transporter and GABAA alpha 6 receptor variants are associated with neuroticism. Biological Psychiatry. 2004;55(3):244–249. doi: 10.1016/j.biopsych.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 123.Feusner J, Ritchie T, Lawford B, Young RM, Kann B, Noble EP. GABAA receptor β3 subunit gene and psychiatric morbidity in a post-traumatic stress disorder population. Psychiatry Research. 2001;104(2):109–117. doi: 10.1016/s0165-1781(01)00296-7. [DOI] [PubMed] [Google Scholar]
- 124.Uhart M, McCaul ME, Oswald LM, Choi L, Wand GS. GABRA6 gene polymorphism and an attenuated stress response. Molecular Psychiatry. 2004;9(11):998–1006. doi: 10.1038/sj.mp.4001535. [DOI] [PubMed] [Google Scholar]
- 125.Maren S, Quirk GJ. Neuronal signalling of fear memory. Nature Reviews Neuroscience. 2004;5(11):844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- 126.Pape H-C, Stork O. Genes and mechanisms in the amygdala involved in the formation of fear memory. Annals of the New York Academy of Sciences. 2003;985:92–105. doi: 10.1111/j.1749-6632.2003.tb07074.x. [DOI] [PubMed] [Google Scholar]
- 127.Schmitt U, Hiemke C. Tiagabine, a γ-amino-butyric acid transporter inhibitor impairs spatial learning of rats in the Morris water-maze. Behavioural Brain Research. 2002;133(2):391–394. doi: 10.1016/s0166-4328(02)00008-6. [DOI] [PubMed] [Google Scholar]
- 128.Atack JR. Anxioselective compounds acting at the GABAA receptor benzodiazepine binding site. Current Drug Targets. CNS and Neurological Disorders. 2003;2(4):213–232. doi: 10.2174/1568007033482841. [DOI] [PubMed] [Google Scholar]
- 129.Atack JR. The benzodiazepine binding site of GABAA receptors as a target for the development of novel anxiolytics. Expert Opinion on Investigational Drugs. 2005;14(5):601–618. doi: 10.1517/13543784.14.5.601. [DOI] [PubMed] [Google Scholar]
- 130.Atack JR, Hutson PH, Collinson N, et al. Anxiogenic properties of an inverse agonist selective for α3 subunit-containing GABAA receptors. British Journal of Pharmacology. 2005;144(3):357–366. doi: 10.1038/sj.bjp.0706056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hu J-H, Ma Y-H, Jiang J, et al. Cognitive impairment in mice over-expressing γ-aminobutyric acid transporter 1 (GAT1) NeuroReport. 2004;15(1):9–12. doi: 10.1097/00001756-200401190-00003. [DOI] [PubMed] [Google Scholar]
- 132.Möhler H, Fritschy J-M, Crestani F, Hensch T, Rudolph U. Specific GABAA circuits in brain development and therapy. Biochemical Pharmacology. 2004;68(8):1685–1690. doi: 10.1016/j.bcp.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 133.Bushell TJ, Sansig G, Shigemoto R, et al. An impairment of hippocampal synaptic plasticity in mice lacking mGlu7 receptors. Neuropharmacology. 1996;35(6):A6. [Google Scholar]
- 134.Garpenstrand H, Annas P, Ekblom J, Oreland L, Fredrikson M. Human fear conditioning is related to dopaminergic and serotonergic biological markers. Behavioral Neuroscience. 2001;115(2):358–364. [PubMed] [Google Scholar]
- 135.Pezze MA, Feldon J. Mesolimbic dopaminergic pathways in fear conditioning. Progress in Neurobiology. 2004;74(5):301–320. doi: 10.1016/j.pneurobio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 136.Bolivar V, Flaherty L. A region on chromosome 15 controls intersession habituation in mice. Journal of Neuroscience. 2003;23(28):9435–9438. doi: 10.1523/JNEUROSCI.23-28-09435.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gallinat J, Ströhle A, Lang UE, et al. Association of human hippocampal neurochemistry, serotonin transporter genetic variation, and anxiety. NeuroImage. 2005;26(1):123–131. doi: 10.1016/j.neuroimage.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 138.Maron E, Nikopensius T, Kõks S, et al. Association study of 90 candidate gene polymorphisms in panic disorder. Psychiatric Genetics. 2005;15(1):17–24. doi: 10.1097/00041444-200503000-00004. [DOI] [PubMed] [Google Scholar]
- 139.Graeff FG, Silveira MC, Nogueira RL, Audi EA, Oliveira RMW. Role of the amygdala and periaqueductal gray in anxiety and panic. Behavioural Brain Research. 1993;58(1-2):123–131. doi: 10.1016/0166-4328(93)90097-a. [DOI] [PubMed] [Google Scholar]
- 140.Groenink L, Van Bogaert MJV, Van Der Gugten J, Oosting RS, Olivier B. 5-HT1A receptor and 5-HT1b receptor knockout mice in stress and anxiety paradigms. Behavioural Pharmacology. 2003;14(5-6):369–383. doi: 10.1097/01.fbp.0000087737.21047.75. [DOI] [PubMed] [Google Scholar]
- 141.Hensler JG. Serotonergic modulation of the limbic system. Neuroscience and Biobehavioral Reviews. 2006;30(2):203–214. doi: 10.1016/j.neubiorev.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 142.Kusserow H, Davies B, Hörtnagl H, et al. Reduced anxiety-related behaviour in transgenic mice overexpressing serotonin1A receptors. Molecular Brain Research. 2004;129(1-2):104–116. doi: 10.1016/j.molbrainres.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 143.Lesch KP. Neurotism and serotonin: a developmental genetic perspective. In: Plomin R, DeFries J, Craig I, McGuffin P, editors. Behavioral Genetics in the Postgenomic Era. Washington, DC, USA: American Psychological Association; 2002. pp. 389–423. [Google Scholar]
- 144.Lesch KP, Zeng Y, Reif A, Gutknecht L. Anxiety-related traits in mice with modified genes of the serotonergic pathway. European Journal of Pharmacology. 2003;480(1–3):185–204. doi: 10.1016/j.ejphar.2003.08.106. [DOI] [PubMed] [Google Scholar]
- 145.Murphy DL, Lerner A, Rudnick G, Lesch K-P. Serotonin transporter: gene, genetic disorders, and pharmacogenetics. Molecular Interventions. 2004;4(2):109–123. doi: 10.1124/mi.4.2.8. [DOI] [PubMed] [Google Scholar]
- 146.Holmes A, Yang RJ, Lesch K-P, Crawley JN, Murphy DL. Mice lacking the serotonin transporter exhibit 5-HT1A receptor-mediated abnormalities in tests for anxiety-like behavior. Neuropsychopharmacology. 2003;28(12):2077–2088. doi: 10.1038/sj.npp.1300266. [DOI] [PubMed] [Google Scholar]
- 147.Murphy DL, Uhl GR, Holmes A, et al. Experimental gene interaction studies with SERT mutant mice as models for human polygenic and epistatic traits and disorders. Genes, Brain and Behavior. 2003;2(6):350–364. doi: 10.1046/j.1601-1848.2003.00049.x. [DOI] [PubMed] [Google Scholar]
- 148.Zhao S, Edwards J, Carroll J, et al. Insertion mutation at the C-terminus of the serotonin transporter disrupts brain serotonin function and emotion-related behaviors in mice. Neuroscience. 2006;140(1):321–334. doi: 10.1016/j.neuroscience.2006.01.049. [DOI] [PubMed] [Google Scholar]
- 149.Adamec R, Burton P, Blundell J, Murphy DL, Holmes A. Vulnerability to mild predator stress in serotonin transporter knockout mice. Behavioural Brain Research. 2006;170(1):126–140. doi: 10.1016/j.bbr.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 150.Zhang S, Amstein T, Shen J, Brush FR, Gershenfeld HK. Molecular correlates of emotional learning using genetically selected rat lines. Genes, Brain and Behavior. 2005;4(2):99–109. doi: 10.1111/j.1601-183X.2004.00099.x. [DOI] [PubMed] [Google Scholar]
- 151.Mizuno T, Aoki M, Shimada Y, et al. Gender difference in association between polymorphism of serotonin transporter gene regulatory region and anxiety. Journal of Psychosomatic Research. 2006;60(1):91–97. doi: 10.1016/j.jpsychores.2005.06.068. [DOI] [PubMed] [Google Scholar]
- 152.Bertolino A, Arciero G, Rubino V, et al. Variation of human amygdala response during threatening stimuli as a function of 5′HTTLPR genotype and personality style. Biological Psychiatry. 2005;57(12):1517–1525. doi: 10.1016/j.biopsych.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 153.Hariri AR, Drabant EM, Weinberger DR. Imaging genetics: perspectives from studies of genetically driven variation in serotonin function and corticolimbic affective processing. Biological Psychiatry. 2006;59(10):888–897. doi: 10.1016/j.biopsych.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 154.Stein DJ, Matsunaga H. Specific phobia: a disorder of fear conditioning and extinction. CNS Spectrums. 2006;11(4):248–251. doi: 10.1017/s1092852900020721. [DOI] [PubMed] [Google Scholar]
- 155.Payton A, Gibbons L, Davidson Y, et al. Influence of serotonin transporter gene polymorphisms on cognitive decline and cognitive abilities in a nondemented elderly population. Molecular Psychiatry. 2005;10(12):1133–1139. doi: 10.1038/sj.mp.4001733. [DOI] [PubMed] [Google Scholar]
- 156.Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 157.Fox NA, Nichols KE, Henderson HA, et al. Evidence for a gene-environment interaction in predicting behavioral inhibition in middle childhood. Psychological Science. 2005;16(12):921–926. doi: 10.1111/j.1467-9280.2005.01637.x. [DOI] [PubMed] [Google Scholar]
- 158.Kalueff AV, Avgustinovich DF, Kudryavtseva NN, Murphy DL. BDNF in anxiety and depression. Science. 2006;312(5780):1598–1599. doi: 10.1126/science.312.5780.1598. [DOI] [PubMed] [Google Scholar]
- 159.Linnarsson S, Björklund A, Ernfors P. Learning deficit in BDNF mutant mice. European Journal of Neuroscience. 1997;9(12):2581–2587. doi: 10.1111/j.1460-9568.1997.tb01687.x. [DOI] [PubMed] [Google Scholar]
- 160.Chourbaji S, Hellweg R, Brandis D, et al. Mice with reduced brain-derived neurotrophic factor expression show decreased choline acetyltransferase activity, but regular brain monoamine levels and unaltered emotional behavior. Molecular Brain Research. 2004;121(1-2):28–36. doi: 10.1016/j.molbrainres.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 161.Montkowski A, Holsboer F. Intact spatial learning and memory in transgenic mice with reduced BDNF. NeuroReport. 1997;8(3):779–782. doi: 10.1097/00001756-199702100-00040. [DOI] [PubMed] [Google Scholar]
- 162.Berton O, McClung CA, DiLeone RJ, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311(5762):864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- 163.Cirulli F, Berry A, Chiarotti F, Alleva E. Intrahippocampal administration of BDNF in adult rats affects short-term behavioral plasticity in the Morris water maze and performance in the elevated plus-maze. Hippocampus. 2004;14(7):802–807. doi: 10.1002/hipo.10220. [DOI] [PubMed] [Google Scholar]
- 164.Alonso M, Bekinschtein P, Cammarota M, Vianna MRM, Izquierdo I, Medina JH. Endogenous BDNF is required for long-term memory formation in the rat parietal cortex. Learning and Memory. 2005;12(5):504–510. doi: 10.1101/lm.27305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Koponen E, Võikar V, Riekki R, et al. Transgenic mice overexpressing the full-length neurotrophin receptor trkB exhibit increased activation of the trkB-PLCγ pathway, reduced anxiety, and facilitated learning. Molecular and Cellular Neuroscience. 2004;26(1):166–181. doi: 10.1016/j.mcn.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 166.Gorski JA, Balogh SA, Wehner JM, Jones KR. Learning deficits in forebrain-restricted brain-derived neurotrophic factor mutant mice. Neuroscience. 2003;121(2):341–354. doi: 10.1016/s0306-4522(03)00426-3. [DOI] [PubMed] [Google Scholar]
- 167.Schulkin J, Morgan MA, Rosen JB. A neuroendocrine mechanism for sustaining fear. Trends in Neurosciences. 2005;28(12):629–635. doi: 10.1016/j.tins.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 168.Rainnie DG, Bergeron R, Sajdyk TJ, Patil M, Gehlert DR, Shekhar A. Corticotrophin releasing factor-induced synaptic plasticity in the amygdala translates stress into emotional disorders. Journal of Neuroscience. 2004;24(14):3471–3479. doi: 10.1523/JNEUROSCI.5740-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Shekhar A, Truitt W, Rainnie D, Sajdyk T. Role of stress, corticotrophin releasing factor (CRF) and amygdala plasticity in chronic anxiety. Stress. 2005;8(4):209–219. doi: 10.1080/10253890500504557. [DOI] [PubMed] [Google Scholar]
- 170.Kojro E, Postina R, Buro C, Meiringer C, Gehrig-Burger K, Fahrenholz F. The neuropeptide PACAP promotes the alpha-secretase pathway for processing the Alzheimer amyloid precursor protein. FASEB Journal. 2006;20(3):512–514. doi: 10.1096/fj.05-4812fje. [DOI] [PubMed] [Google Scholar]
- 171.Kapfhamer D, Valladares O, Sun Y, et al. Mutations in Rab3a alter circadian period and homeostatic response to sleep loss in the mouse. Nature Genetics. 2002;32(2):290–295. doi: 10.1038/ng991. [DOI] [PubMed] [Google Scholar]
- 172.Thakker-Varia S, Alder J, Crozier RA, Plummer MR, Black IB. Rab3A is required for brain-derived neurotrophic factor-induced synaptic plasticity: transcriptional analysis at the population and single-cell levels. Journal of Neuroscience. 2001;21(17):6782–6790. doi: 10.1523/JNEUROSCI.21-17-06782.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Binder DK, Scharfman HE. Brain-derived neurotrophic factor. Growth Factors. 2004;22(3):123–131. doi: 10.1080/08977190410001723308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Francia N, Cirulli F, Chiarotti F, Antonelli A, Aloe L, Alleva E. Spatial memory deficits in middle-aged mice correlate with lower exploratory activity and a subordinate status: role of hippocampal neurotrophins. European Journal of Neuroscience. 2006;23(3):711–728. doi: 10.1111/j.1460-9568.2006.04585.x. [DOI] [PubMed] [Google Scholar]
- 175.Song L, Che W, Min-Wei W, Murakami Y, Matsumoto K. Impairment of the spatial learning and memory induced by learned helplessness and chronic mild stress. Pharmacology Biochemistry and Behavior. 2006;83(2):186–193. doi: 10.1016/j.pbb.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 176.Rattiner LM, Davis M, Ressler KJ. Differential regulation of brain-derived neurotrophic factor transcripts during the consolidation of fear learning. Learning and Memory. 2004;11(6):727–731. doi: 10.1101/lm.83304. [DOI] [PubMed] [Google Scholar]
- 177.Rattiner LM, Davis M, Ressler KJ. Brain-derived neurotrophic factor in amygdala-dependent learning. Neuroscientist. 2005;11(4):323–333. doi: 10.1177/1073858404272255. [DOI] [PubMed] [Google Scholar]
- 178.Lang UE, Hellweg R, Kalus P, et al. Association of a functional BDNF polymorphism and anxiety-related personality traits. Psychopharmacology. 2005;180(1):95–99. doi: 10.1007/s00213-004-2137-7. [DOI] [PubMed] [Google Scholar]
- 179.Bueller JA, Aftab M, Sen S, Gomez-Hassan D, Burmeister M, Zubieta J-K. BDNF Val66Met allele is associated with reduced hippocampal volume in healthy subjects. Biological Psychiatry. 2006;59(9):812–815. doi: 10.1016/j.biopsych.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 180.Dempster E, Toulopoulou T, McDonald C, et al. Association between BDNF val66 met genotype and episodic memory. American Journal of Medical Genetics. 2005;134 B(1):73–75. doi: 10.1002/ajmg.b.30150. [DOI] [PubMed] [Google Scholar]
- 181.Mattson MP, Maudsley S, Martin B. BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends in Neurosciences. 2004;27(10):589–594. doi: 10.1016/j.tins.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 182.Rios M, Lambe EK, Liu R, et al. Severe deficits in 5-HT2A-mediated neurotransmission in BDNF conditional mutant mice. Journal of Neurobiology. 2006;66(4):408–420. doi: 10.1002/neu.20233. [DOI] [PubMed] [Google Scholar]
- 183.Siuciak JA, Boylan C, Fritsche M, Altar CA, Lindsay RM. BDNF increases monoaminergic activity in rat brain following intracerebroventricular or intraparenchymal administration. Brain Research. 1996;710(1-2):11–20. doi: 10.1016/0006-8993(95)01289-3. [DOI] [PubMed] [Google Scholar]
- 184.Szapacs ME, Mathews TA, Tessarollo L, Ernest Lyons W, Mamounas LA, Andrews AM. Exploring the relationship between serotonin and brain-derived neurotrophic factor: analysis of BDNF protein and extraneuronal 5-HT in mice with reduced serotonin transporter or BDNF expression. Journal of Neuroscience Methods. 2004;140(1-2):81–92. doi: 10.1016/j.jneumeth.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 185.Payton A. Investigating cognitive genetics and its implications for the treatment of cognitive deficit. Genes, Brain and Behavior. 2006;5(supplement 1):44–53. doi: 10.1111/j.1601-183X.2006.00194.x. [DOI] [PubMed] [Google Scholar]
- 186.Ren-Patterson RF, Cochran LW, Holmes A, et al. Loss of brain-derived neurotrophic factor gene allele exacerbates brain monoamine deficiencies and increases stress abnormalities of serotonin transporter knockout mice. Journal of Neuroscience Research. 2005;79(6):756–771. doi: 10.1002/jnr.20410. [DOI] [PubMed] [Google Scholar]
- 187.Kaufman J, Yang B-Z, Douglas-Palumberi H, et al. Social supports and serotonin transporter gene moderate depression in maltreated children. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(49):17316–17321. doi: 10.1073/pnas.0404376101. [DOI] [PMC free article] [PubMed] [Google Scholar]