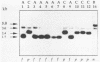
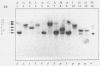
Abstract
Recombinant DNA clones carrying high-copy or low-copy sequences from Aspergillus nidulans and Neurospora crassa were used to identify restriction fragment length polymorphisms (RFLPs) diagnostic for members of the A. flavus group: A. flavus, A. parasiticus, and A. nomius. These fungi were resolved into three distinct categories when they were grouped according to RFLP patterns. Subgroups within these categories were also evident. This limited RFLP analysis of nuclear DNA of members of the A. flavus group did not identify any RFLPs that differentiate these isolates on the basis of toxin production, but limited correlation with geographic location was observed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abovich N., Rosbash M. Two genes for ribosomal protein 51 of Saccharomyces cerevisiae complement and contribute to the ribosomes. Mol Cell Biol. 1984 Sep;4(9):1871–1879. doi: 10.1128/mcb.4.9.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner J. W., Cole R. J., Diener U. L. The relationship of Aspergillus flavus and Aspergillus parasiticus with reference to production of aflatoxins and cyclopiazonic acid. Mycopathologia. 1984 Aug 30;87(1-2):13–15. doi: 10.1007/BF00436617. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Free S. J., Rice P. W., Metzenberg R. L. Arrangement of the genes coding for ribosomal ribonucleic acids in Neurospora crassa. J Bacteriol. 1979 Mar;137(3):1219–1226. doi: 10.1128/jb.137.3.1219-1226.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreader C. A., Heckman J. E. Isolation and characterization of a Neurospora crassa ribosomal protein gene homologous to CYH2 of yeast. Nucleic Acids Res. 1987 Nov 11;15(21):9027–9042. doi: 10.1093/nar/15.21.9027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtzman C. P., Horn B. W., Hesseltine C. W. Aspergillus nomius, a new aflatoxin-producing species related to Aspergillus flavus and Aspergillus tamarii. Antonie Van Leeuwenhoek. 1987;53(3):147–158. doi: 10.1007/BF00393843. [DOI] [PubMed] [Google Scholar]
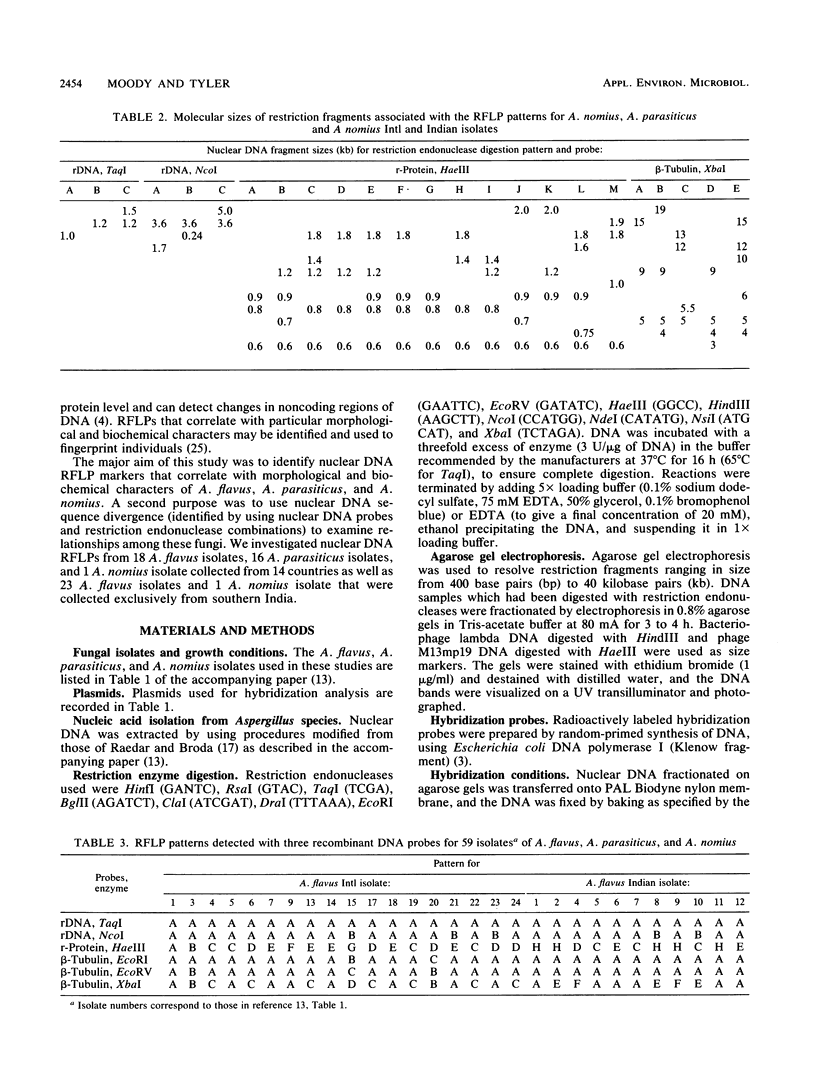
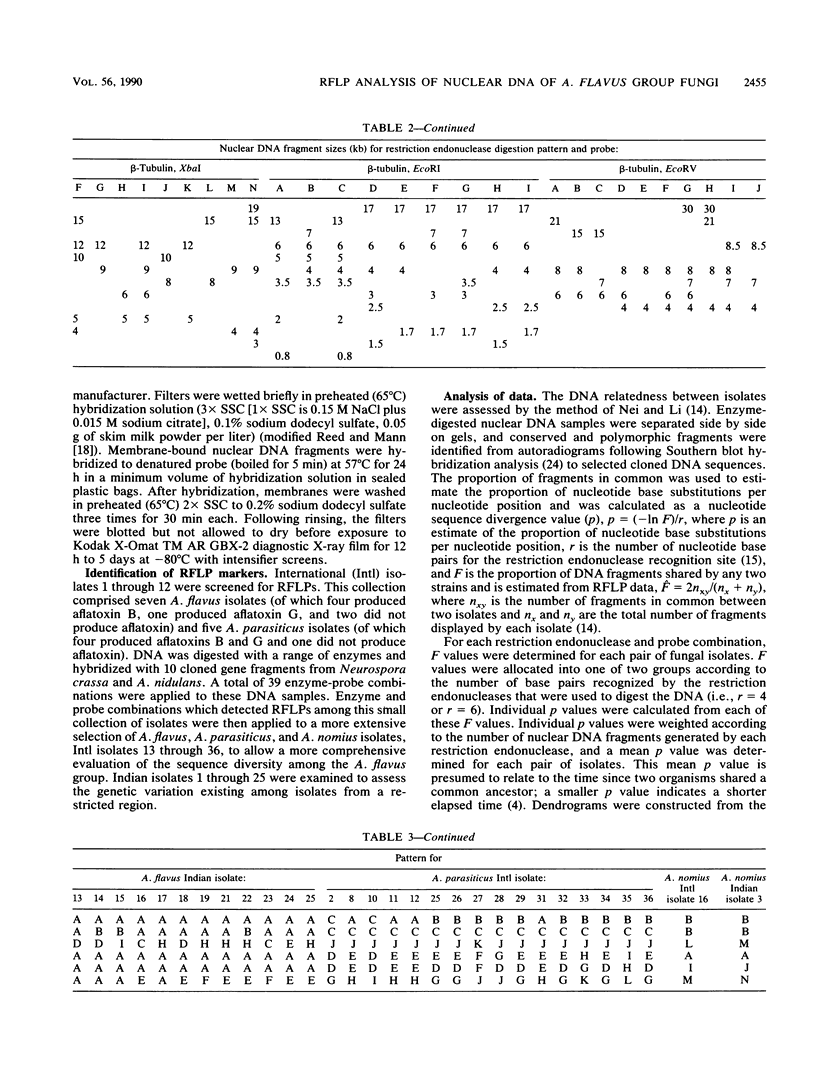
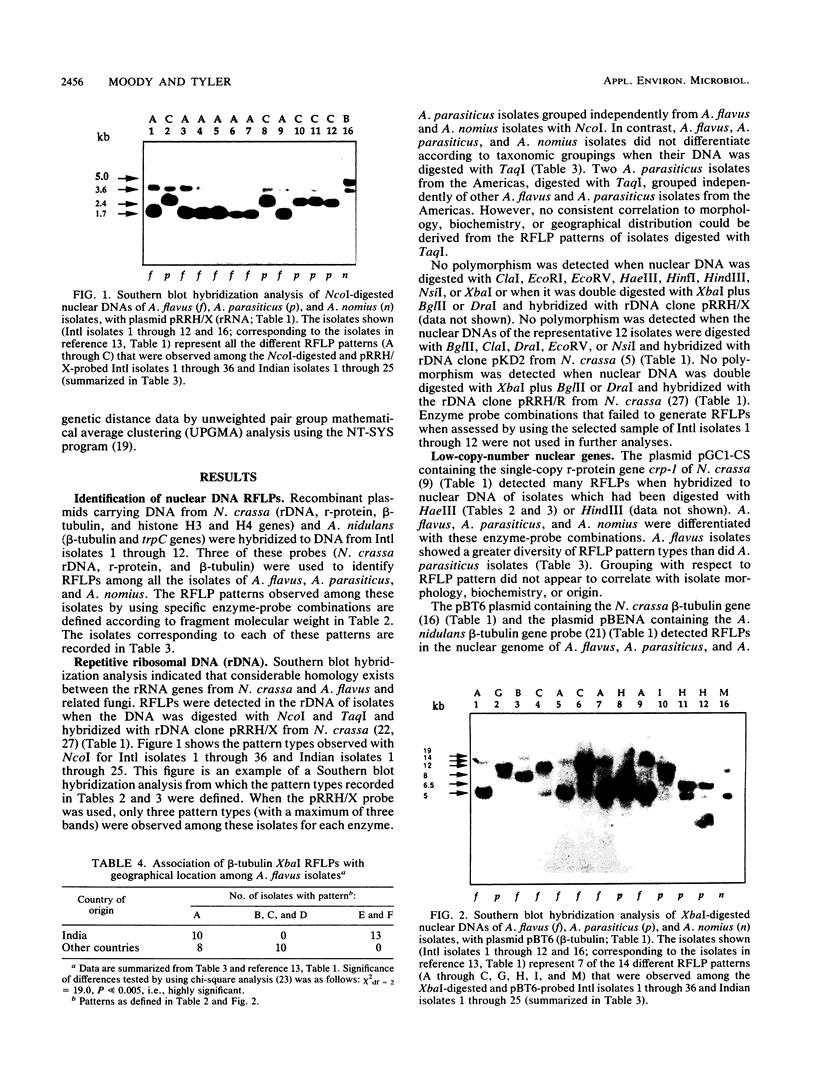
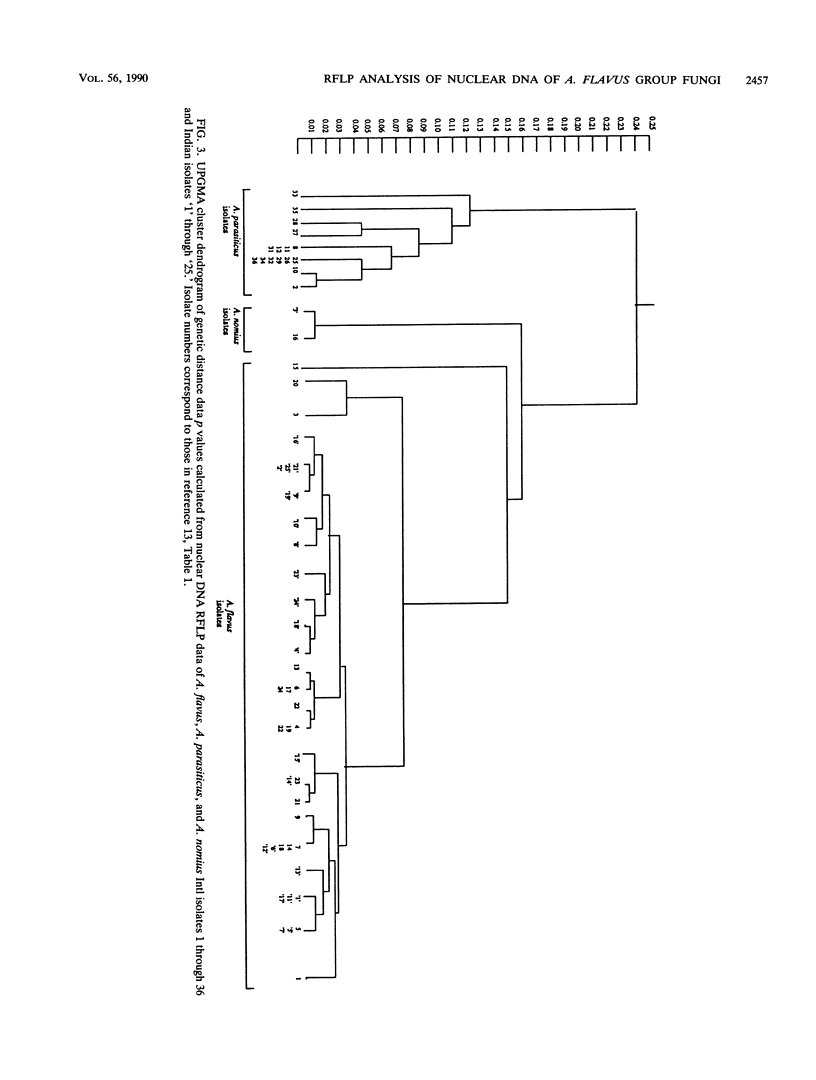
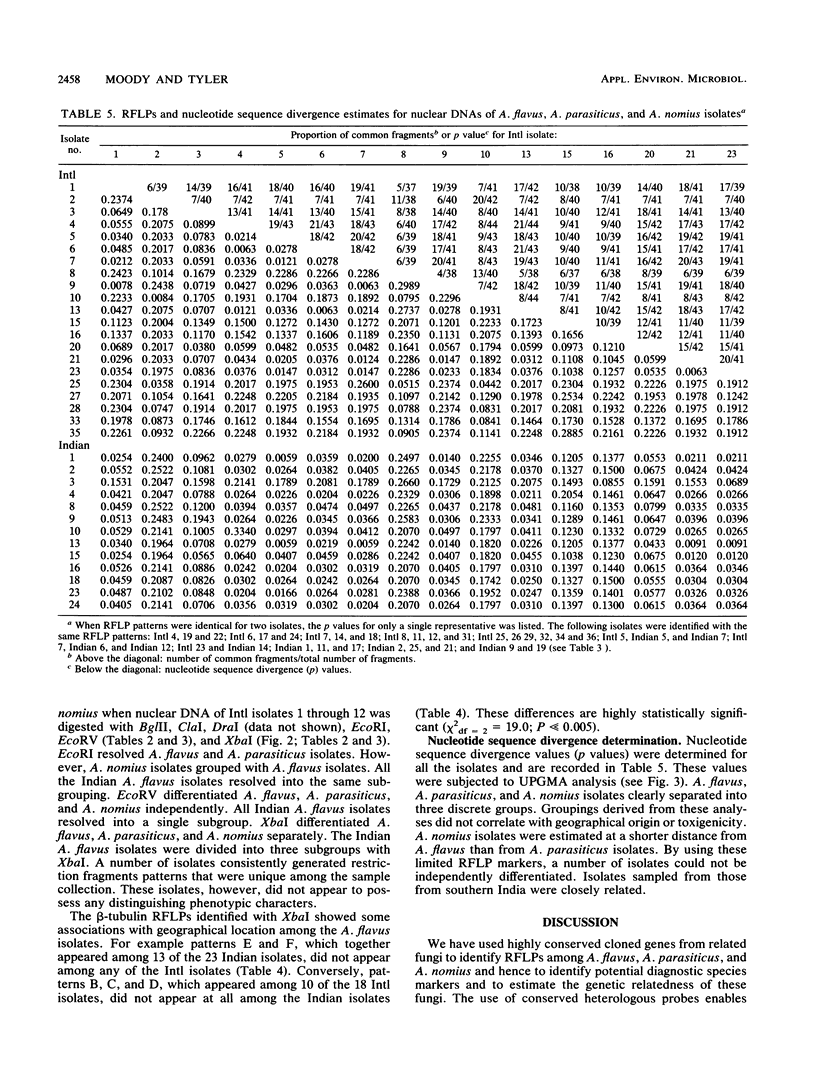
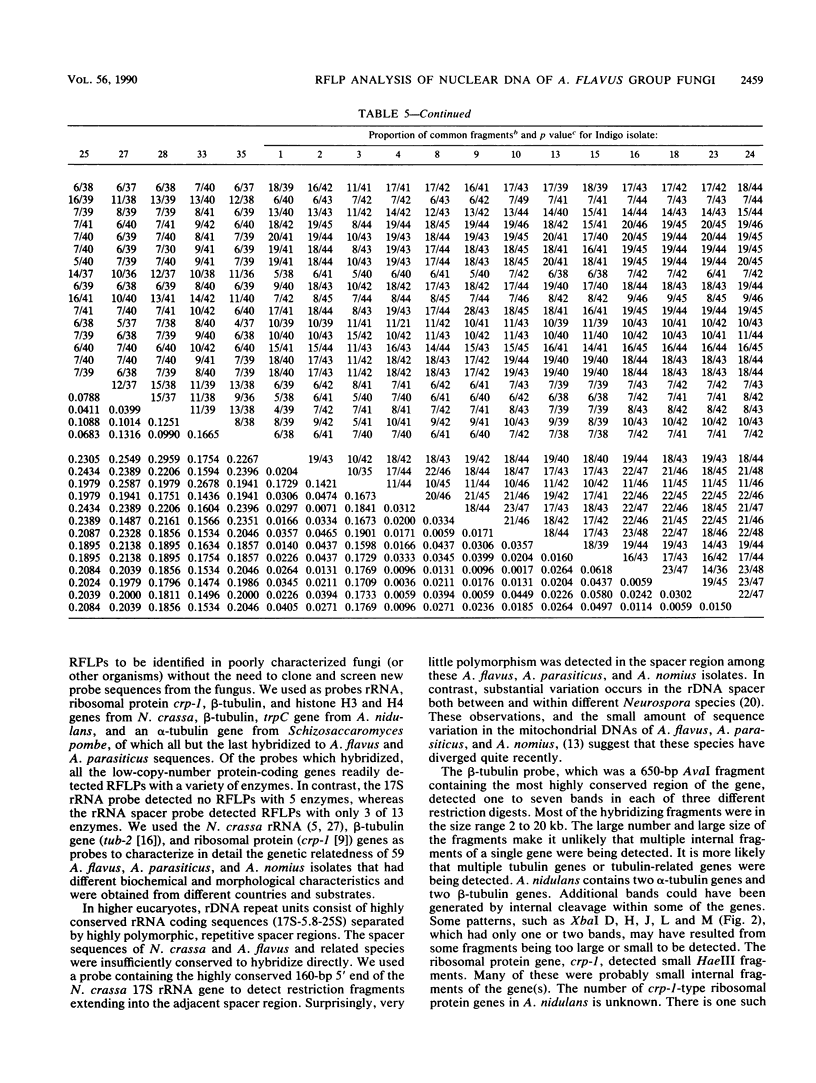
- Moody S. F., Tyler B. M. Restriction enzyme analysis of mitochondrial DNA of the Aspergillus flavus group: A. flavus, A. parasiticus, and A. nomius. Appl Environ Microbiol. 1990 Aug;56(8):2441–2452. doi: 10.1128/aem.56.8.2441-2452.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M., Li W. H. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5269–5273. doi: 10.1073/pnas.76.10.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M., Tajima F. Maximum likelihood estimation of the number of nucleotide substitutions from restriction sites data. Genetics. 1983 Sep;105(1):207–217. doi: 10.1093/genetics/105.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orbach M. J., Porro E. B., Yanofsky C. Cloning and characterization of the gene for beta-tubulin from a benomyl-resistant mutant of Neurospora crassa and its use as a dominant selectable marker. Mol Cell Biol. 1986 Jul;6(7):2452–2461. doi: 10.1128/mcb.6.7.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell P. J., Wagner S., Rodland K. D., Feinbaum R. L., Russell J. P., Bret-Harte M. S., Free S. J., Metzenberg R. L. Organization of the ribosomal ribonucleic acid genes in various wild-type strains and wild-collected strains of Neurospora. Mol Gen Genet. 1984;196(2):275–282. doi: 10.1007/BF00328060. [DOI] [PubMed] [Google Scholar]
- Sheir-Neiss G., Lai M. H., Morris N. R. Identification of a gene for beta-tubulin in Aspergillus nidulans. Cell. 1978 Oct;15(2):639–647. doi: 10.1016/0092-8674(78)90032-6. [DOI] [PubMed] [Google Scholar]
- Sogin M. L., Miotto K., Miller L. Primary structure of the Neurospora crassa small subunit ribosomal RNA coding region. Nucleic Acids Res. 1986 Dec 9;14(23):9540–9540. doi: 10.1093/nar/14.23.9540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Toda T., Adachi Y., Hiraoka Y., Yanagida M. Identification of the pleiotropic cell division cycle gene NDA2 as one of two different alpha-tubulin genes in Schizosaccharomyces pombe. Cell. 1984 May;37(1):233–242. doi: 10.1016/0092-8674(84)90319-2. [DOI] [PubMed] [Google Scholar]
- Tyler B. M., Giles N. H. Structure of a Neurospora RNA polymerase I promoter defined by transcription in vitro with homologous extracts. Nucleic Acids Res. 1985 Jun 25;13(12):4311–4332. doi: 10.1093/nar/13.12.4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woudt L. P., Pastink A., Kempers-Veenstra A. E., Jansen A. E., Mager W. H., Planta R. J. The genes coding for histone H3 and H4 in Neurospora crassa are unique and contain intervening sequences. Nucleic Acids Res. 1983 Aug 25;11(16):5347–5360. doi: 10.1093/nar/11.16.5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelton M. M., Hamer J. E., Timberlake W. E. Transformation of Aspergillus nidulans by using a trpC plasmid. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1470–1474. doi: 10.1073/pnas.81.5.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]