SYNOPSIS
Objectives.
Emerging evidence suggests that children are at higher risk for West Nile virus (WNV) exposure, but may have a lower risk for infection-related morbidity and mortality. Limited data exist regarding risk determinants of childhood WNV infection. We conducted a survey to analyze the differences between pediatric and adult behavior relevant to WNV exposure.
Methods.
Residents of participating sampled households responded to a questionnaire that measured knowledge, attitudes, personal protective behaviors, and clinical history to evaluate the association between personal behavior and exposure to WNV.
Results.
Children were more likely to have high levels of outdoor exposure compared to adults (83% vs. 70%). Children were less likely to avoid going outdoors (4% vs. 13%) and to wear long sleeves or pants compared to adults (8% vs. 19%). Both groups were highly educated about WNV. Television, not health-care provider education, was the most common source of WNV information. Participants were more concerned about WNV infection than pesticide usage.
Conclusions.
Our study demonstrates that children exhibit behaviors that could put them at greater risk for WNV infection and suggests that children could benefit from greater education about practices that can decrease WNV exposure to limit their risk for infection.
Since its arrival in the eastern United States in 1999, West Nile virus (WNV) has spread across North America, affecting all of the continental U.S. and parts of Canada by 2004.1,2 In 2002, the State of Ohio reported intense epidemic activity with 341 cases of WNV encephalitis and/or meningitis with 31 fatalities.3,4 In that same year, Cuyahoga County in Northeastern Ohio reported 221 cases of WNV illness, including 11 fatalities and 155 cases of West Nile-associated neurologic disease5 (WNND), 11.1 cases/100,000 population, all ages.
At the end of 2002, Case Western Reserve University and the Cuyahoga County Board of Health conducted a systematic household-based seroprevalence survey to determine neighborhood and countywide human WNV infection rates and risk factors for infection.5 Stratified survey sampling indicated that the countywide prevalence of West Nile seropositivity was 1.9% at that time. The risk of WNV infection was age-dependent, with children 4.5 times more likely to become infected yet 110 times less likely to develop neuroinvasive disease.5 Our hypothesis was that children may exhibit behaviors that put them at risk for WNV infection. This report contrasts the age-specific behaviors relevant to WNV exposure during the 2002 transmission season.
MATERIALS AND METHODS
A household survey of Cuyahoga County residents was performed from December 5–12, 2002, approximately 14 weeks after the WNV epidemic peak in Northeastern Ohio. Design details of this stratified survey sample have been previously published.5 Briefly, in conjunction with the previously reported serological survey, residents of participating sampled households responded to a questionnaire that measured knowledge, attitudes, personal protective behaviors, and clinical history during the months of July, August, and September 2002. The study sample consisted of 1,209 participants from 806 households. The mean participant age was 43.2 years (based on an age range of 5–94 years). The survey used a stratified cluster sample design based on WNV information from human cases and infected mosquitoes; therefore, all percentages are weighted to account for this design. Of 1,209 participants, 168 were children <18 years of age and four (6.5%, weighted) of the children were seropositive for WNV.
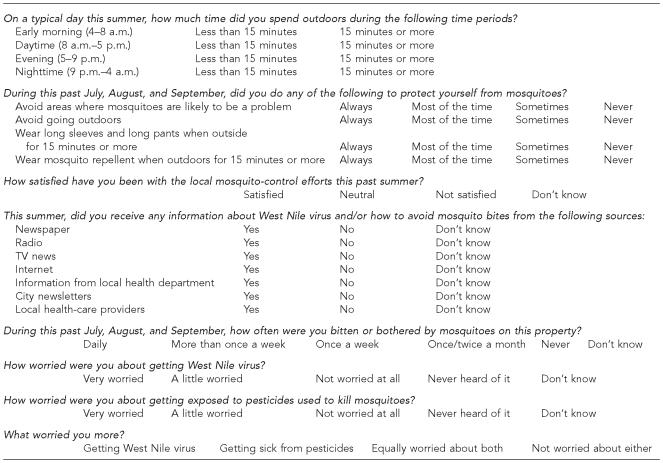
To evaluate the association between personal behavior and risk for WNV infection, all participating residents were asked detailed questions regarding the amount of time spent outdoors during different times of day and the number of mosquito bites experienced, using questionnaires previously developed by the Centers for Disease Control and Prevention (CDC)6 (Figure). Personal mosquito avoidance behavior was assessed in questions involving clothing, mosquito repellent use, and outdoor area activities. Knowledge about WNV infection was determined by asking residents what and from which source they received their information about WNV. Attitude-related questions assessed how concerned subjects were about contracting WNV infection, in contrast to their concern about local pesticide usage. Institutional Review Board (IRB) approval was obtained from University Hospitals of Cleveland.
Figure.
Knowledge, attitudes, and behaviors questionnaire
a This Centers for Disease Control and Prevention-based questionnaire was given to Cuyahoga County, Ohio, survey participants to analyze knowledge, attitudes, and practices regarding West Nile virus exposure during the 2002 epidemic transmission season.
Statistical analysis
SPSS version 11.57 was used for the preliminary analysis. Formulas that allowed weighting of the survey data were developed to account for the multistage sampling study design. Subsequently, data were analyzed using SUDAAN version 8.0.8 The standard Horvitz-Thompson estimator was used to calculate point estimates. For variance estimates, all sources of variation (i.e., households within blocks, blocks within census tracts, census tracts with municipalities, and municipalities within strata) were included using standard Taylor series approximations. To calculate the confidence interval for the true prevalence ratio (PR), we took the logarithm of the sample PR, approximated its variance using standard Taylor series methodology, and formed a confidence interval for the logarithm of PR. The end points of this interval were exponentiated to obtain the interval for PR. In this report, survey results are weighted unless otherwise indicated. The adjusted variance estimates were used to test for significant differences between child and adult behaviors, with p<0.05 taken as significant.5,9
RESULTS
Pediatric behaviors that influence WNV infection risk: age differences in knowledge, attitudes, and practices
To investigate the apparent fivefold greater risk of WNV infection among children as compared to adults, we evaluated individual behavioral factors related to mosquito exposure and use of preventive techniques. Results of our population sample survey (n=1,209) indicated the following significant factors:
Influence of outdoor activity on risk of WNV infection—daytime vs. evening.
Greater outdoor exposure was associated with higher rates of seropositivity among survey participants. Among 886 individuals who reported spending more than 15 minutes outdoors in the daytime (8 a.m. to 5 p.m.) and more than 15 minutes in the evening (5 to 9 p.m.), 26 (2.6%) were seropositive. These individuals were 20 times more likely to be seropositive than those who spent less than 15 minutes outdoors during either time period. Of 114 people who only spent time outdoors during the daytime but not during the evening, only three (0.07%) were seropositive. People who specifically avoided going outdoors also had a low seropositivity rate of 0.12%.
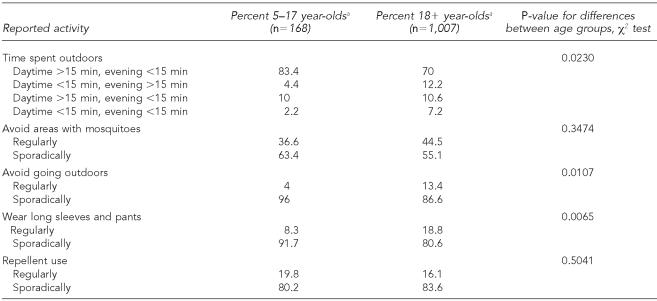
Summaries of adult and pediatric behavior characteristics are shown in Table 1. All four seropositive children captured in the county survey reported spending more than 15 minutes outside during a typical summer evening. As a group, children were more likely to have high levels of outdoor exposure than adults (83% vs. 70%). Conversely, in terms of protective behavior, children were less likely to regularly avoid going outdoors than adults (4% vs. 13%) and less likely to regularly wear long sleeves or pants when outside compared to adults (8% vs. 19%). Not shown, children reported a higher mosquito bite frequency than adults. There were no statistically significant differences between adult and child avoidance of mosquito-ridden areas or in reported use of repellents.
Table 1.
Behavioral characteristics of adults and children in Cuyahoga County, Ohio, survey: a comparison of adult and child behaviors regarding outdoor and mosquito exposure, personal protective clothing, and repellent usage in 2002
All percentages are weighted estimates derived from stratified survey samples of Cuyahoga County during the systematic household-based seroprevalence survey in 2002.
Background knowledge and attitudes regarding WNV.
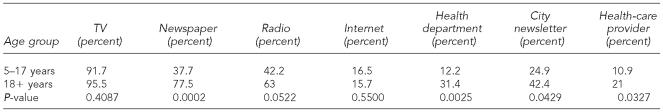
Of the 1,209 survey participants, fewer than 1% of adults and 2% of children reported never hearing about WNV infection. Both adults and children received most of their WNV information from local television news broadcasts (Table 2). Similar to the adult cohort of whom 96% received information regarding WNV via television news, 92% of children received their information from the local news. Children were less likely than adults to receive information via radio programming (42% vs. 63%) or newspaper (38% vs. 78%). Use of the Internet for information was comparable between the child and adult cohorts (17% vs. 16%). Adults were more likely than children to use their local health department, their city newsletter, and their health-care providers. Usage of health-care providers for WNV information was considerably low in both groups.
Table 2.
Respondents' reported sources of information about West Nile virus (WNV):a a comparison of adult and child information sources on WNV as reported in the Cuyahoga County, Ohio, survey
All percentages are weighted estimates derived from stratified survey samples of Cuyahoga County during the systematic household-based seroprevalence survey in 2002.
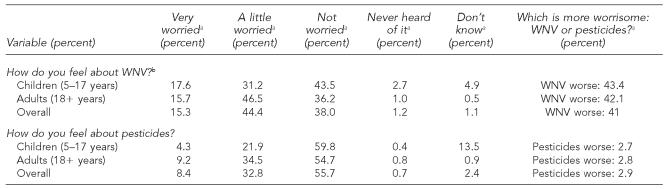
Attitudes regarding pesticide usage and concern for WNV infection are summarized in Table 3. Overall, 722 (60%) of the surveyed individuals were somewhat concerned about WNV infection, whereas 498 people (41%) worried about pesticide usage. When asked what worried them more—WNV infection or pesticide use—41% chose WNV infection, 3% chose pesticide use, 22% chose both, and 34% chose neither. Overall, 572 (47%) participants were satisfied with local mosquito control efforts. Children's attitudes about WNV and pesticides mirrored those of their parents.
Table 3.
Attitudes regarding possible West Nile virus (WNV) infection and pesticide usage: a comparison of adult and child attitudes regarding WNV and pesticides as reported in the Cuyahoga County, Ohio, survey
All percentages are weighted estimates derived from stratified survey samples of Cuyahoga County during the systematic household-based seroprevalence survey in 2002.
P=0.1397, χ2 test for difference between age groups; −p=0.1012, χ2 test for difference between age groups.
DISCUSSION
Our survey of risk-related behaviors enabled us to identify significant differences in personal protective behaviors (PPBs) between children and adults that almost certainly contributed to the children's higher WNV seroprevalence. Protective behaviors for WNV infection have been evaluated only rarely in the literature, but have been shown to decrease infection risk when utilized appropriately.10 In contrast to Aquino's British Columbia study11 and the CDC's Connecticut data,12 where approximately 60% of people practiced mosquito-avoidance behavior and used DEET-containing repellents, only 44% of our study population avoided mosquitoes and, more surprisingly, only 17% reported using repellents regularly. Although there is little published evidence that children are at higher risk for DEET toxicity than adults,13,14 concern regarding childhood toxicity from DEET may have contributed to the low repellent usage among children, despite them being outside for longer periods of time and more often than adults.15
Not surprisingly, children spent more time outside than adults and reported suffering more mosquito bites. In prior studies, one of the most prominent barriers to practicing protective behaviors included participating in outdoor leisure activities during peak mosquito hours—a behavior expected of summertime childhood play.11 Adults in our study were more likely to avoid going outdoors and to wear personal protective clothing, such as long sleeves and pants, than were children. These PPBs were likely to have been at least partially effective against WNV infection.
As in previous studies, survey respondents in our study were well informed about WNV infection.12 Both children and adults received the majority of their information about WNV from local television news broadcasting, as found by other investigators.11 This observation demonstrates the capacity of television as an effective medium to reach audiences for public health education. Whether or not WNV education improved mosquito avoidance behaviors or practices could not be elucidated from our survey. In prior studies, knowledge and concern about WNV infection has not predicted usage of PPBs.12 Unfortunately, very few survey respondents—11% of children and 21% of adults—received WNV information from their health-care providers. These figures represent a missed opportunity for physicians, especially pediatricians, to give informative and important health advice to their patients.
This study has several limitations. We did not have adequate power to detect small differences in risk of infection associated with surveyed behaviors or in clinical symptomatology between infected and uninfected subjects. Survey data is reliant on self-reporting, which may bias results by overestimation of PPBs. Also, questionnaire administration occurred 14 weeks after the mosquito season, which may introduce recall bias. The youngest survey participant may have had limited understanding of the study questions, therefore parental answers would have been used by proxy, introducing possible reporting bias in those results.
In conclusion, although differences in behaviors between adults and children cannot explain the WNV disease attack rate variation, they may determine important risk-behavior links for exposure to infection. Children in our study reported behaviors that were likely to put them at greater risk for WNV infection and may have contributed to the substantial discrepancy between child and adult WNV infection rates. As ongoing WNV transmission leads to increasing protective immunity among adults, children will become a greater proportion of the population at risk for WNV infection and disease. Finding ways to limit WNV exposure to protect this vulnerable population is essential.
WNV is still present in our community; therefore, future epidemics may be inevitable. Reducing risk of mosquito bites is currently the only way to reduce the risk of WNV infection, as no vaccine for WNV is yet available. Regular assessment of public knowledge, attitudes, and behaviors is needed to guarantee the effectiveness of public health messages. For now, the focus should remain on targeted health education outreach, especially among school-aged children, particularly via television broadcasting, in concert with ongoing, countywide mosquito-control efforts to decrease the risk of new WNV and other arboviral infections. In addition, pediatricians should take an active role in informing their patients about using personal protective measures to guard against WNV infection.
Acknowledgments
The authors would like to thank the residents of Cuyahoga County, Ohio, who participated in this study. The authors also thank the agencies that supported this effort, including the St. Luke's Foundation, the Mt. Sinai Health Care Foundation, the George Gund Foundation, the Sisters of Charity/St. Ann's Foundation, the City of Lakewood Health Department, the Ohio Department of Health, the Centers for Disease Control and Prevention, the City of Cleveland Health Department, and the Cuyahoga County Board of Health.
This work was supported in part by NIH grant K23 HD40982 and NIH grant T32 AI52067. The authors have complied with the Public Health Code of Ethics.
REFERENCES
- 1.Centers for Disease Control and Prevention (US) West Nile virus—statistics, surveillance, and control. [cited 2006 May 5]. Available from: URL: http://www.cdc.gov/ncidod/dvbid/westnile/surv&control.htm.
- 2.CDC (US) Outbreak of West Nile-like encephalitis—New York, 1999. MMWR Morb Mortal Wkly Rep. 1999;48(38):845–9. [PubMed] [Google Scholar]
- 3.O'Leary DR, Marfin AA, Montgomery SP, Kipp AM, Lehman JA, Biggerstaff BJ, et al. The epidemic of West Nile virus in the United States, 2002. Vector-Borne Zoonotic Dis. 2004;4:61–70. doi: 10.1089/153036604773083004. [DOI] [PubMed] [Google Scholar]
- 4.Mazurek JM, Winpisinger K, Mattson BJ, Duffy R, Moolenaar RL. The epidemiology and early clinical features of West Nile virus infection. Am J of Emerg Med. 2005;23:536–43. doi: 10.1016/j.ajem.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Mandalakas AM, Kippes C, Kippes C, Sedransk J, Kile JR, Garg A, McLeod J, et al. West Nile virus epidemic, northeast Ohio, 2002. Emerg Infect Dis. 2005;11:1774–7. doi: 10.3201/eid1111.040933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.CDC (US) Serosurveys for West Nile virus infection—New York and Connecticut counties, 2000. MMWR Morb Mortal Wkly Rep. 2001;50(3):37–9. [PubMed] [Google Scholar]
- 7.SPSS, Inc. Chicago: SPSS, Inc.; 2002. SPSS: Version 11.5 for Windows. [Google Scholar]
- 8.Research Triangle Park (NC): Research Triangle Institute; 2002. SUDAAN: Version 8.0.1 for Windows. [Google Scholar]
- 9.Cochran W. Sampling techniques. 3rd ed. New York: Wiley; 1977. 9A.7. [Google Scholar]
- 10.Loeb M, Elliott SJ, Gibson B, Fearon M, Nosal R, Drebot M, et al. Protective behavior and West Nile virus risk. Emerg Infect Dis. 2005;11:1433–6. doi: 10.3201/eid1109.041184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aquino M, Fyfe M, MacDougall L, Remple V. Protective behavior survey, West Nile virus, British Columbia. Emerg Infect Dis. 2004;10:1499–501. doi: 10.3201/eid1008.031053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.CDC (US) Knowledge, attitudes, and behaviors about West Nile virus—Connecticut, 2002. MMWR Morb Mortal Wkly Rep. 2003;52(37):886–8. [PubMed] [Google Scholar]
- 13.Sudakin DL, Trevathan WR. DEET: a review and update of safety and risk in the general population. J Toxicol Clin Toxicol. 2003;41:831–9. doi: 10.1081/clt-120025348. [DOI] [PubMed] [Google Scholar]
- 14.Briassoulis G, Narlioglou M, Hatzis T. Toxic encephalopathy associated with use of DEET insect repellents: a case analysis of its toxicity in children. Hum Exp Toxicol. 2001;20:8–14. doi: 10.1191/096032701676731093. [DOI] [PubMed] [Google Scholar]
- 15.Koren G, Matsui D, Bailey B. DEET-based insect repellents: safety implications for children and pregnant and lactating women. CMAJ. 2003;169:209–12. [PMC free article] [PubMed] [Google Scholar]