Abstract
Antioxidant enzymes perform a variety of vital functions including the reduction of life-shortening oxidative damage. We used the honey bee genome sequence to identify the major components of the honey bee antioxidant system. A comparative analysis of honey bee with Drosophila melanogaster and Anopheles gambiae shows that although the basic components of the antioxidant system are conserved, there are important species differences in the number of paralogs. These include the duplication of thioredoxin reductase and the expansion of the thioredoxin family in fly; lack of expansion of the Theta, Delta and Omega GST classes in bee and no expansion of the Sigma class in dipteran species. The differential expansion of antioxidant gene families among honey bees and dipteran species might reflect the marked differences in life history and ecological niches between social and solitary species.
Keywords: Antioxidant genes, honey bee genome
Introduction
Reactive oxygen species (ROS) are constantly generated as by-products of aerobic metabolism. Accumulated evidence suggests that oxidative damage to cellular components induced by ROS is a major contributive cause of degenerative diseases and ageing. ROS generation occurs mainly in mitochondria in which more than 90% of the oxygen used by the cell is consumed (Perez-Campo et al., 1998). Aerobic organisms have evolved a complex network of enzymatic and non-enzymatic antioxidant systems to avoid oxidative damage. Key components of the antioxidant defence system are conserved throughout evolution, but there are unique adaptations among different groups. The major changes in insects in comparison with vertebrates and other phylogenetic groups include the loss of genes encoding functional glutathione reductase (GR) and glutathione peroxidase (GPX). Homologous genes for thioredoxin reductase (TrxR) (Kanzok et al., 2001) and thioredoxin peroxidase (TPX) (Radyuk et al., 2001) activities, respectively, act in their place.
There are both primary and secondary antioxidant enzymes, which act directly or indirectly on ROS molecules. The first line of defence against ROS attack is provided by three different kinds of primary antioxidant enzymes that act directly on ROS: (1) superoxide dismutases (SODs), which rearrange superoxide to oxygen and hydrogen peroxide; (2) catalase, which prevents free hydroxyl radical formation by breaking down hydrogen peroxide into oxygen and water; and (3) peroxidases, which catalyse an analogous reaction in which hydrogen peroxide is reduced to water by a reductant that acts as an electron donor, normally reduced thioredoxin (TRX) or glutathione (GSH). In addition, insects have three families of genes that encode antioxidant enzymes that act as peroxidases: TPXs, also known as peroxiredoxins (Radyuk et al., 2001), phospholipid-hydroperoxide GPX homologs with thioredoxin peroxidase activity (GTPX) (Missirlis et al., 2003), and glutathione S-transferases (GSTs) (Tang & Tu, 1994; Toba & Aigaki, 2000). Secondary antioxidant enzymes that act indirectly on ROS include TrxR, which recycles both TRX and GSH (Kanzok et al., 2001), and methionine sulphoxide reductases (MsrA and MsrB), which are involved in protein reparation by catalysing the TRX-dependent reduction of methionine sulphoxide to methionine (Moskovitz et al., 1996; Kumar et al., 2002).
Honey bee antioxidant enzymes are of particular interest because of their potential involvement in some of the exceptional biological characteristics of the queen honey bee, especially its longevity relative to worker bees (10 × longer; e.g. Page & Peng, 2001). Elevated expression of several traditional antioxidant-encoding genes occurs in young queens and old workers (Corona et al., 2005), suggesting that queen longevity is not related to higher expression of these particular genes, a result consistent with findings for Sod1 in Lasius niger ant queens (Parker et al., 2004). However, traditional antioxidants likely play roles in other processes. For example, Weirich et al. (2002) and Collins et al. (2004) reported that catalase, GST and SOD might contribute to the ability of queens to store sperm in their spermatheca for several years without loss of viability.
The recent release of the honey bee genome sequence provides the first opportunity to compare the whole set of antioxidant genes between insect orders. In this report we present the results of the manual annotation of the main antioxidant genes of Apis mellifera, a hymenopteran social insect, and a comparative analysis with the dipteran Anopheles gambiae and Drosophila melanogaster.
Results and discussion
We identified 38 antioxidant genes in the honey bee genome, which include all major components of the enzymatic antioxidant system. This report does not include the annotation of genes encoding proteins thought to have indirect antioxidant effects mediated by metal binding capacities, such as vitellogenin (Seehuus et al., 2006), transferrin (Kucharski & Maleszka, 2003; do Nascimento et al., 2004), ferritin (Dunkov & Georgieva, 1999; Geiser et al., 2003) and metallothioneins (Egli et al., 2006).
In general, antioxidant genes encode small proteins less than 250 amino acids, with the exception of TrxR, catalase and proteins of unknown function such as Rsod and Trx/Grx-like proteins, which probably diverged by duplication of ancestral Cu/ZnSOD and Trx/glutaredoxin (Grx) genes. Most of the honey bee's antioxidant genes have protein-encoding regions with high A/T content (64% average, Table 1), a characteristic that is not specific to antioxidant genes, but rather is a general attribute of the honey bee genome. The honey bee genome is reported to contain 67% A/T, compared with 58% in D. melanogaster and 56% in Anopheles gambiae (Honey Bee Genome Consortium, 2006). It has been postulated that genes from organisms with high rates of metabolism use more A-ending codons than those from organisms with lower rates (Xia, 1996). This hypothesis has not yet been studied in insect species, which in general have very high metabolic rates (Suarez et al., 2000).
Table 1.
Summary of honey bee antioxidant gene annotation. Gene localization based on the scaffolds_assembly_2 database. Apis mellifera GstO2 and Gstu1 are partial sequences
| Gene | aa | Location | introns | ORF AT% |
|---|---|---|---|---|
| Sod2 | 218 | Group11.11 | 2 | 65.3 |
| Sod1 | 152 | Group8.3 | 3 | 61.0 |
| Sod3 | 178 | GroupUn | 2 | 64.2 |
| CCS | 266 | GroupUn.5386 | 6 | 70.8 |
| Rsod | 1100 | GroupUn.153 | 18 | 69.7 |
| Cat | 513 | Group6.23 | 7 | 61.6 |
| Gtpx1 | 168 | GroupUn.5 | 3 | 68.4 |
| Gtpx2 | 201 | Group5.15 | 1 | 70.3 |
| Tpx1 | 194 | GroupUn.29 | 2 | 63.4 |
| Tpx3 | 242 | Group15.12 | 2 | 66.9 |
| Tpx4 | 220 | GroupUn.1374 | 4 | 61.8 |
| Tpx5 | 220 | Group12.14 | 3 | 67.0 |
| Tpx6 | 219 | Group9.2 | 1 | 57.2 |
| GstT1 | 230 | GroupUn.336 | 3 | 72.0 |
| GstD1 | 217 | Group15.2 | 4 | 56.1 |
| GstS1 | 204 | GroupUn.1306 | 3 | 66.0 |
| GstS2 | 202 | GroupUn.1306 | 2 | 63.4 |
| GstS3 | 207 | GroupUn.898 | 3 | 60.3 |
| GstS4 | 206 | Group4.16 | 3 | 58.6 |
| GstZ1 | 217 | Group5.15 | 4 | 63.3 |
| GstO1 | 241 | Group1.28 | 4 | 66.9 |
| GstO2 | partial | GroupUn.264 | 4 | ND |
| Gstu1 | partial | GroupUn.176 | ND | ND |
| Gstmic1 | 149 | Group2.5 | 1 | 70.4 |
| Gstmic2 | 156 | Group1.56 | 1 | 53.6 |
| Trxr-1 | 494 | GroupUn.68 | 7 | 64.6 |
| Trx-1 | 105 | GroupUn.35 | 3 | 68.3 |
| Trx-2 | 136 | Group6.16 | 2 | 64.9 |
| Trx-3 | 103 | GroupUn.125 | 0 | 73.5 |
| Trx-like1 | 287 | Group14.6 | 3 | 66.1 |
| Trx-like2 | 488 | Group3.21 | 5 | 48.0 |
| Trx-like3 | 411 | Group13.2 | 5 | 65.6 |
| Grx1 | 98 | GroupUn.505 | 1 | 67.0 |
| Grx2 | 133 | Group11.6 | 2 | 65.2 |
| Grx-like1 | 711 | Group6.26 | 0 | 43.2 |
| Trx/Gtx | 222 | Group15.14 | 1 | 67.9 |
| MsrA | 217 | GroupUn.104 | 3 | 65.7 |
| MsrB | 137 | GroupUn.304 | 2 | 59.6 |
ND, not determined.
Comparative analysis of A. mellifera, D. melanogaster and A. gambiae antioxidant genes
Superoxide dismutases
SOD converts radical superoxide to oxygen and hydrogen peroxide, providing the first line of defence against ROS produced in the mitochondria. SODs normally exist in two forms in eukaryotic cells; the two forms differ in cellular localization and in the structure of their active sites. MnSOD (SOD2) is present in the inner mitochondrial space and Cu/ZnSOD (SOD1) in the cytoplasm. Like most eukaryotes, honey bees have a single mitochondrial MnSOD gene located on chromosome 11. Vertebrate orthologs, including those in Tetraodon and human, have higher overall identity with the honey bee ortholog (66.21 and 62.33% ID) than dipteran species (Drosophila, 59.09, Anopheles 59.17). Possible explanations for this phylogenetic discordance include rapid divergence of the dipteran orthologs (Honey Bee Genome Sequencing Consortium, 2006).
The Cu/ZnSOD family includes five members in Drosophila and Anopheles and four members in Apis (Table 2). In Drosophila this group includes the canonical cytoplasmatic Cu/ZnSOD (CG11793), extracellular SOD (Sod3, CG9027), copper chaperone (CCS, CG17753), related to Sod (Rsod, CG31028), and Sodesque (Sodq, CG5948). Extracellular CuZnSODs are present in several animal groups, from nematode to mammals. In insects, they have been identified in D. melanogaster, Anopheles gambiae (Landis and Tower, 2005) and Lasius niger (Parker et al., 2004). The honey bee has an extracellular Cu/ZnSOD (SOD3) of 178 amino acids.
Table 2.
Major components of the enzymatic antioxidant system of Apis mellifera, Drosophila melanogaster and Anopheles gambiae. Gene identification numbers: for bee, the BeeBase ID; for mosquito, the Genbank accession number; for fly, the Flybase gene ID. NP indicates genes with no automatic prediction in bees. For the GST Delta and Epsilon classes of Drosophila and Anopheles, only four representative members are shown
| Gene | Apis | Anopheles | Drosophila |
|---|---|---|---|
| Sod2 | GB14346 | AAS17758 | CG8905 |
| Sod1 | GB10133 | AAR90328 | CG11793 |
| Sod3 | NP | AAS17758 | CG9027 |
| CCS | GB14210 | XP_308747 | CG17753 |
| Rsod | GB14567 | EAA00894 | CG31028 |
| Sodq | Not identified | EAA04552 | CG5948 |
| Cat | GB11518 | XP_314995 | CG6871 |
| Gtpx1 | GB14138 | XP_313166 | CG12013 |
| Gtpx2 | GB18955 | XP_562772 | Not identified |
| Gpx-like | Not identified | Not identified | CG15116 |
| Tpx1 | GB19380 | XP_308081 | CG1633 |
| Tpx2 | Not identified | XP_308336 | CG1274 |
| Tpx3 | GB10972 | XP_565975 | CG5826 |
| Tpx4 | GB10498 | XP_320690 | CG12405 |
| Tpx5 | GB10803 | XP_308753 | CG3083 |
| Tpx6 | NP | Not identified | CG6888 |
| GstT1 | GB12047 | AAM61893, AAM61892 | CG1702, CG30005 |
| CG30000, CG1681 | |||
| GstD1 | GB18045 | AAC79995 | CG10045 |
| GstD2-12 | Not identified | CAA96104, AAM53610 | CG4181, CG4381 |
| AAM53607, AAM53607 | CG11512, CG12242 | ||
| GstS1 | GB16959 | AAA29358 | CG8938 |
| GstS2 | NP | Not identified | Not identified |
| GstS3 | GB19254 | Not identified | Not identified |
| GstS4 | GB14372 | Not identified | Not identified |
| GstZ1 | GB17672 | AF515522 | CG9363 |
| GstZ2 | Not identified | Not identified | CG9362 |
| GstO1 | GB11466 | AAP13482 | CG6781 |
| GstO2 | GB19678 | CG6662 | |
| GstO3-4 | Not identified | Not identified | CG6776 CG6673 |
| GSTu1 | GB15512 | AAM61888 | CG33546 |
| GstE1-13 | Not identified | AAG45163, AAG45164 | CG5164 CG17524 |
| AAL59653, AAL59654 | CG17523 CG17525 | ||
| GSTmic1 | GB12371 | AAP37003 | CG1742 |
| GSTmic2 | GB10566 | AAP37005 | CG33178 |
| Trxr-1 | GB14972 | CAD30858 | CG2151 |
| Trxr-2 | Not identified | Not identified | CG11401 |
| Trx-1 | GB17503 | EAA04498 | CG8993, CG8517 |
| Trx-2 | GB15855 | EAA14495 | CG31884, CG3315 |
| CG4193, CG13473 | |||
| Trx-3 | GB19972 | EAA09650 | CG3719 |
| Trx1-like1 | GB15457 | EAA11972 | CG5495 |
| Trx1-like2 | GB15572 | XP_320264 | CG14221 |
| Trx1-like3 | GB19276 | XP_316887 | CG9911 |
| Grx1 | GB10598 | XP_309539 | CG6852, CG7975 |
| Grx2 | GB18700 | XP_312440 | CG14407 |
| Grx-like1 | GB11664 | EAA06446 | CG31559, CG12206 |
| Trx/Gtx | GB12870 | EAA07378 | CG6523 |
| MsrA | GB10196 | XP_320164 | CG7266 |
| MsrB | GB15486 | XP_311902 | CG6584 |
Phylogenetic analysis (Fig. 1) shows that the extracellular SODs of insects and vertebrates form different monophyletic clades. This suggests the possibility that they evolved independently in each group, for example, by the addition of a signal peptide to cytoplasmatic SOD (Landis and Tower, 2005). Copper chaperone (CCS) has, in addition to the SOD domain, a N-terminal heavy-metal-associated domain (HMA) involved in the transport of copper to Cu/ZnSOD. As insect and vertebrate homologs form a single monophyletic clade, CCS proteins seem to have diverged from cytoplasmatic SOD before the separation of these two lineages.
Figure 1.
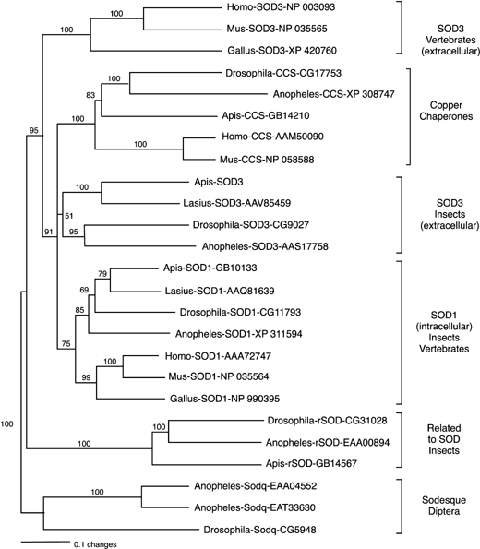
Neighbour joining tree showing the relationships of the CuZn SOD family. The GenBank accession number (Anopheles gambiae), Flybase ID (Drosophila melanogaster) and BeeBase ID (Apis mellifera) are shown for each sequence. Values above the branches represent bootstrap support.
A putative ortholog for the Drosophila Sodesque (Sodq) gene is present in Anopheles gambiae; however, it encodes a rapid evolving protein, with only 42% identity between these dipteran species. As a Sodq-related protein is also present in Aedes aegypti (EAT33630), but orthologs for this gene are absent in honey bee, other insects, and vertebrates, it is possible that this gene has diverged from cytoplasmatic SOD only in dipteran species. Sodq function in Drosophila is uncertain, because the fly ortholog lacks several conserved residues essential for catalytic function while possessing a signal peptide for extracellular targeting (Landis and Tower, 2005).
The Drosophila related to Sod gene (Rsod) is an atypical member in the Cu/ZnSOD family. It has a duplicated SOD domain and an unusually high number (18) of introns (Table 1). Homologous genes (with two or three SOD domains) are present in Anopheles, Apis, protozoa (Dictyostelium discoideum XP_639320 and XP_639300), fish (Tetraodon nigroviridis, CAF89944), but not in mammals. Rsod function is unknown in insects. However, a homologous protein (pernin, AAK20952) in Perna canaliculus (Mollusca) does not show SOD activity but might be involved in the transport of divalent metal cations (Scotti et al., 2001).
Catalase
Catalase prevents free hydroxyl radical formation by breaking down hydrogen peroxide into oxygen and water. A single catalase gene is normally present in eukaryotes, with the exception of C. elegans, in which this gene is duplicated. Honey bee catalase encodes a protein of 513 amino acids and is localized on chromosome 6. Catalase in Apis, as in other eukaryotes, is located in the cytosol and lacks a signal peptide necessary for secretion. Interestingly, catalase activity has been reported to be present in honey (White, 1975), which perhaps acts to keep H2O2 levels in honey (produced by bees as a preservative) below toxic levels. Since in the honey bee genome the only catalase is not extracellular, the source of the catalase in honey remains to be determined. It has been assumed that it comes from plants (White, 1975), but extracellular catalases are apparently only found in some bacteria and fungi. An intriguing possibility is that catalase in honey originates from endosymbiotic bacteria.
Thioredoxin peroxidases
TPXs, also known as peroxiredoxins, are a type of peroxidase that reduces H2O2 using electrons provided by TRX (Chae et al., 1994). Based on the number of conserved cysteins, TPXs are classified into two subfamilies: 1-Cys and 2-Cys. In contrast to the 1-Cys, the 2-Cys subfamily has a second conserved Cys in the C-terminus (Trivelli et al., 2003) (Fig. 3A). The TPX family has five members in humans, which include cytosolic, mitochondrial and extracellular forms (Chang et al., 2004). The Drosophila genome also contains five TPX homologs (Radyuk et al., 2001) that comprise three cytosolic variants (Tpx1, CG1633, Tpx4, CG12405, Tpx5, CG3083), one mitochondrial (Tpx3, CG5826) and one secretable (Tpx2, CG1274).
Figure 3.

Neighbour joining tree showing the phylogenetic relationships of Apis mellifera (Am), Anopheles gambiae (Ag), Drosophila melanogaster (Dm) and Homo sapiens (Hs) peroxidases homologs. (A) Thioredoxin family. (B) Glutathione peroxidase homologs. Values above the branches represent bootstrap support.
We identified a new putative TPX homolog in Drosophila (DmTpx6, CG6888), five Tpx members in Anopheles and five homologs in Apis (Table 2). Compared with dipteran species, honey bee seems to have lost the secretable variant (Tpx-2). AmTpx6 and DmTpx6 are the more diverged members of the Cys-1 subfamily; there is no mosquito homolog (Fig. 2A and 3A). Phylogenetic analysis (Fig. 3A), showed that the different insect and human homologs are grouped in separate phylogenetic groups. Three of them are included in the 2-Cys subfamily and two in the 1-Cyst subfamily. This distribution suggests that the major members of the TPX family could have diverged before the separation of the insect and vertebrate metazoan ancestor. Consistent with this analysis is the finding that each of the phylogenetic groups contain members that seem to have conserved their particular subcellular localization. Clades A, D and E contain cytoplasmic, clade B contains mitochondrial, and clade C contains extracellular variants (as inferred in Apis mellifera and Anopheles gambiae by the presence of predicted mitochondrial targeting and signal peptides).
Figure 2.
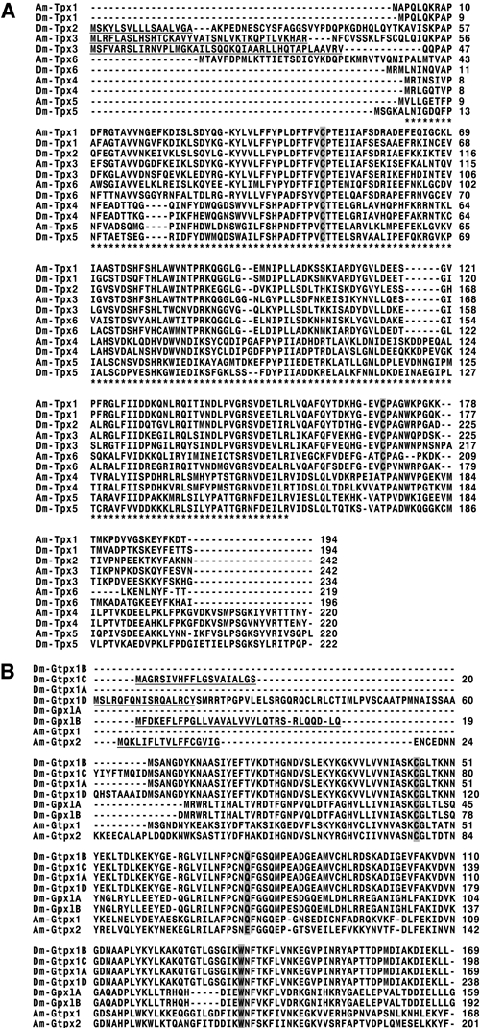
Apis mellifera and Drosophila melanogaster thioredoxin-dependent peroxidase homologs. (A) Thioredoxin peroxidase family (peroxiredoxins). Predicted signal peptide for Dm Tpx-2 (Dpx4156) and mitochondrial targeting peptide of AmTpx3 and DmTpx-3 (Dpx5037) are underlined. Asterisks mark the peroxiredoxin domain. Conserved cysteins are highlighted. (B) Glutathione peroxidase homologs with thioredoxin peroxidase activity. Predicted signal peptides (AmGtpx2, DmGtpx1C) and mitochondrial targeting peptides (DmGtpx1D) are shown underlined. Amino acids of the catalytic site (Ursini et al., 1995) are highlighted. Amino acid colour follows the ClustalW code.
Glutathione peroxidase homologs
GPX catalyses the reduction of hydrogen peroxide and organic hydroperoxides. In mammals, GPX catalyses the reduction of hydroxyperoxides utilizing GSH as an electron donor (Ursini et al., 1995). Early work (Smith & Shrift, 1979) found that insects lack GPX activity. However, the Drosophila genome contains two GPX homologs. One of these genes encodes for an enzyme that uses TRX, rather than GSH, as an electron donor and was therefore referred to as a GPX homolog with TPX activity, Gtpx-1 (CG12013) (Missirlis et al., 2003). This gene also is known as DmPHGpx and has been shown to be highly expressed in testis (Li et al., 2003). The second Drosophila GPX homolog remains to be biochemically characterized and is referred to as GPX-like gene (Gpxl, CG15116).
We found that both Apis mellifera and Anopheles gambiae also have a pair of GPX homologs (Table 2), although one of the honey bee homologs (AmGtpx-2, GB18955) lacks one of the three conserved residues of the catalytic site (Fig. 2B) (Ursini et al., 1995). Homologs in each species share more identity with each other than with homologs in other species, suggesting that they are paralogs that diverged after speciation. As might be expected, the dipteran homologs are more closely related to each other (Fig. 3B, clade A) compared with those of the honey bee, which form a monophyletic group (clade B) with human Gpx4. Our phylogenetic analysis also shows that each pair of homologous genes in mosquito and bee are more closely related to each other compared with the pair of GPX homologs in Drosophila. This could be due to several causes, including the possibilities that the duplication event occurred early in Drosophila or there was rapid sequence divergence of the Gpxl gene.
Humans have six GPX homologs, some with cytosolic, mitochondrial or extracellular localization. In Drosophila there are four Gpx-1 isoforms, two of them with putative cytosolic (CG12013-PA, CG12013-PB), one with mitochondrial (CG12013-PD) and one with extracellular localization (CG12013-PC), as inferred by computational identification of putative mitochondrial targeting sequences and signal peptides. This suggests that diversity in subcellular localization in Drosophila is achieved via alternative splicing rather than gene duplication, and honey bee may share a similar gene expression strategy (Table 3).
Table 3.
Predicted subcellular localization and available expression data for honey bee antioxidant genes. Putative mitochondrial and extracellular variants were inferred by computational identification of predicted mitochondrial targeting and secretory signal peptides
| Gene | Localization | W | Q |
|---|---|---|---|
| Sod2 | M | BTA | BTA |
| Sod1 | C | BTA | BTA |
| Sod3 | E | B | |
| CCS | C | ||
| Rsod | E | ||
| Cat | C | BTA | BTA |
| Gtpx1 | C | BTA | BTA |
| Gtpx2 | E | ||
| Tpx1 | C | B | |
| Tpx3 | M | BTA | BTA |
| Tpx4 | C | B | |
| Tpx5 | C | ||
| Tpx6 | C | ||
| GstT1 | C | ||
| GstD1 | C | BTA | BTA |
| GstD2-12 | C | ||
| GstS1 | C | B | |
| GstS2 | C | B | |
| GstS3 | C | B | |
| GstS4 | C | ||
| GstZ1 | C | ||
| GstZ2 | C | ||
| GstO1 | M | B | |
| GstO2 | Unk | ||
| GstO3-4 | C | ||
| Gstu1 | C | ||
| GstE1-13 | C | ||
| Gstmic1 | Mic | ||
| Gstmic2 | Mic | ||
| Trxr-1 | C | BTA | BTA |
| Trx-1 | M | ||
| Trx-2 | C | ||
| Trx-3 | C | ||
| Trx1-like1 | C | ||
| Trx1-like2 | C | ||
| Trx1-like3 | C | B | |
| Grx1 | C | ||
| Grx2 | M | ||
| Grx-like1 | N | B | |
| Trx/Gtx | C | B | |
| MsrA | C | BTA | BTA |
| MsrB | C |
Cellular localization: C, cytosolic; M, mitochondria; E, extracellular. N, nuclear; Mic, microsomal; Unk, unknown (5′ truncated genes). Honey bee castes: W, workers; Q, queens. Tissues: B, brain; T, thorax; A, abdomen.
Like Gpx-1, the second Drosophila GPX homolog (Gpxl) also has a splicing variant with a putative signal peptide sequence (CG15116-PB), and a splice variant with a putative signal peptide sequence occurs for at least one of the Apis (AmGtpx1, GB18955) and Anopheles (Ag Gtpx-1, XP_313166) Gpx-like genes (Table 3). Thus, it is likely that at least one of the two paralogs in each species have an extracellular function, as it is the case for four of six human Gpx genes (Lee et al., 2005). At present the function of the putative extracellular GPX-like proteins in insects is unknown. Interestingly, an extracellular GPX homolog with no enzymatic activity was found in the parasitic wasp Venturia canescens that is included in a virus-like particle injected with the eggs into the host, and is probably involved in protection of the egg (Li et al., 2003).
Thioredoxin reductase
TrxR is an essential enzyme that in insects transfers reducing equivalents from NADPH to thioredoxin (TrxS2) and GSH disulphide (GSSG). The resulting products, Trx (SH)2 and GSH, respectively, act as thiol-based reductants and powerful intracellular antioxidants (Holmgren, 1989). Mammal TrxR carries a distinctive COOH-Terminal extension that includes a tetrapeptide motif (Gly-Cys-Sec-Gly-OH) containing a selenium in the form of selenocysteine (s residue) involved in TRX reduction. This motif distinguishes TrxR proteins from other structural and functionally closely related flavoprotein disulphide oxidoreductases such as lipoaminede hydrogenases and ferredoxin reductases (Nordberg & Arner, 2001). The Drosophila ortholog (Trxr-1) has a cysteine instead of selenocysteine, with equivalent function (Kanzok et al., 2001). As Anopheles orthologs also have a cysteine residue in this site (Bauer et al., 2003) the absence of selenium-containing TrxR might be general characteristic of dipteran species.
In contrast with human, which has three Trxr genes, and Drosophila, which has two, Apis and Anopheles have only a single Trxr gene (Table 2, Fig. 4A). In Drosophila, Trxr-1 encodes three splice variants that include one mitochondrial and two cytoplasmic forms (Missirlis et al., 2002). The functional significance of the second Drosophila Trxr gene (Trxr2) is unknown, but it encodes a protein with a potential mitochondrial targeting peptide. Anopheles has a single Trxr gene, and, as in the Drosophila ortholog has three splice variants encoding for one mitochondrial and two cytoplasmic forms (Bauer et al., 2003). Apis also has a single Trxr gene (Table 2); we identified two putative splice variants, but none of them appear to encode a mitochondrial variant. We were unable to localize an alternative 5′ exon encoding a mitochondrial targeting peptide. However, as a mitochondrial TrxR is necessary to provide reduced TRX for mitochondrial peroxidases (including at least Tpx3) and given that catalase is not expressed in mitochondria to reduce H2O2, a mitochondrial variant should be present.
Figure 4.

Alignments for thioredoxin reductases and thioredoxins from Apis mellifera (Am), Drosophila melanogaster (Dm) and Anopheles gambiae (Ag). (A) Thioredoxin reductase family. The sequences of redox-active centres are highlighted. (B) Fragment of an alignment of thioredoxin family proteins. The conserved active site (CXXC) (Holmgren, 1989) is highlighted.
Thioredoxins
TRXs are small, highly conserved oxidoreductase proteins required to maintain the redox homeostasis of the cell. TRX is reduced by TrxR through NADPH (Holmgren et al., 2005). In mammals seven TRX/TRX-like proteins have been identified, including tissue-specific and ubiquitously expressed forms with cytoplasmic, mitochondrial and Golgi apparatus-associated variants (Spyrou et al., 1997; Miranda-Vizuete et al., 2001; Jimenez et al., 2004, 2006). In Drosophila three Trx genes have been characterized: Trx-1 (deadhead gene, CG4193) (Pellicena-Palle et al., 1997; Kanzok et al., 2001), Trx-2 (CG31884) (Bauer et al., 2002) and TrxT (CG3315) (Svensson et al., 2003). Whereas Trx-1 and TrxT are localized in the nucleus and are ovary- and testis-specific, respectively, Trx-2 is localized in the cytoplasm of somatic tissues. This distribution suggests that Trx-2 plays a major part in whole-body redox homeostasis. Accordingly, Trx-2 but not Trx-1, functions as a substrate for TrxR (Bauer et al., 2002).
The Drosophila genome contains four additional genes (CG8993, CG8517, CG3719, CG13473) that contain both an overall TRX-like fold domain (Martin, 1995) and the conserved motif Cys-X1X2-Cys of the active site (Holmgren et al., 2005). Two of these genes (CG8993 and CG8517) encode for proteins with probable mitochondrial targeting peptides. The Anopheles genome contains at least three putative Trx genes, one with cytoplasmic localization (Txr-1, EAA14495) (Bauer et al., 2002) and two with probable mitochondrial localization (Trx-2, EAA04498 and Trx-3, XP_314234).
As in Anopheles, the Apis genome contains three genes encoding putative TRX homologs: Am Trx-1 (GB17503) with predicted mitochondrial localization and an apparent ortholog of Drosophila CG8993 and Anopheles Trx-2 (clade C, Fig. 5); AmTrx-2 (GB15855), a putative ortholog of Drosophila Trx-2 (60.38% ID) and Anopheles Trx-1 (56.6% ID) (clade E) and the intronless gene Am Trx3 (GB19972), putative ortholog of Drosophila CG3719 and Anopheles Trx3, suggesting that it is not of bacterial origin (clade D). Thus, each TRX homolog in honey bee and mosquito has a corresponding putative ortholog in fly. But Drosophila melanogaster has four additional genes with no apparent ortholog in honey bee and mosquito. These genes include CG8517, which seems to have duplicated from CG8993, Trx-1, TrxT and CG13473, which possibly diverged from Drosophila Trx-2 after fly and mosquito diverged from the common dipteran ancestor. Thus, compared with Apis and Anopheles, the TRX subfamily in Drosophila was clearly expanded.
Figure 5.
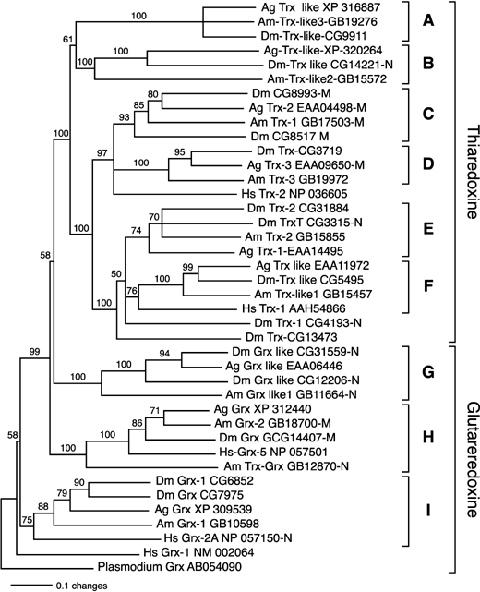
Phylogenetic tree of the thioredoxin/glutharedoxin protein family. ‘M’ and ‘N’ after the accession number indicate mitochondrial or nuclear predicted subcellular localization. Values above the branches represent bootstrap support.
As in other organisms, insect genomes also contain a large group of genes encoding TRX-related proteins containing one or multiple TRX domains, which include protein disulphure isomerases (Arner & Holmgren, 2000) and other proteins of unidentified function. One group of these proteins, which have higher identity to bona fide TRX, contain a single N-terminal TRX domain, but have an additional C-terminal extension of unknown function. One homolog of this protein in humans, TRX-like-1 (TXL-1), is a substrate for the cytosolic selenoprotein TrxR-1 (Jimenez et al., 2006). We identified three genes encoding this kind of TRX-like protein with homologs in Apis, Anopheles and Drosophila genomes (Table 2, Fig. 5 clades A, B and F). Only two of them (Trx-like-1 and Trx-like 2) have a TRX domain with a conserved CXXC active site (Fig. 4B).
Glutaredoxin
GRXs are both structurally and functionally related to TRXs. Insect genomes contain genes encoding GRX homologs, although at present their products have not been characterized. In most organisms oxidized GRX proteins are regenerated by reduced GSH, and the resulting oxidized GSH (GSSG) is reduced by GSH reductase (Holmgren et al., 2005). However, in insects the reduction of GSSG is performed by TrxR (Kanzok et al., 2001). In vertebrates, the products of three Grx genes have been characterized: GRX1, GRX2 (Johansson et al., 2004) and the more distantly related, GRX5 (Wingert et al., 2005). In humans, GRX1 is localized primarily in the cytoplasm, whereas Grx2 encodes for both nuclear and mitochondrial variants (Johansson et al., 2004; Holmgren et al., 2005). In zebrafish GRX5 is primarily localized in mitochondria (Wingert et al., 2005), although in human the reported uncharacterized homolog (NP_057501) lacks a potential mitochondrial targeting peptide.
In Apis, we identified two GRX homologs that we named Grx1 (GB10598) and Grx2 (GB18700), with predicted cytoplasmic and mitochondrial localizations, respectively. Grx1 forms a monophyletic group (Clade I, Fig. 5) with one human (Grx2, NP_057150), one Anopheles (XP_309539) and two Drosophila (CG6852, CG7975) homologs. This suggests that Grx1 was duplicated only in flies. Grx2 has putative orthologs in human (Grx5, NM_016417), Drosophila (GCG14407) and Anopheles (XP_312440). Although this group of proteins shares a clear common evolutionary origin with other GRX proteins, members of this group contain a single cysteine residue at the putative active site (Rodriguez-Manzaneque et al., 1999).
Insect genomes contain two additional groups of genes encoding GRX-related proteins of unknown function (Grx-like genes). The first group contains a GRX domain in the C-terminal of the predicted protein and has a predicted nuclear localization. In honey bees this group is represented by Grx-like-1, which forms a monophyletic group with two Drosophila and one Anopheles homologs (Clade G, Fig. 5). The other group of Grx-like genes, with orthologs in honey bee (GB12870), fly (CG6523) and mosquito (EAA07378), is interesting because it encodes proteins that contain a TRX domain in the N-terminal region and a GRX domain in C-terminal region.
Glutathione S-transferases
GSTs are multifunctional proteins essential for xenobiotic metabolism and protection against peroxidative damage. The GST superfamily can be divided into several structurally and functionally classes that show unique variations among different phylogenetic groups. Plants have exclusive Tau and Phi classes, whereas mammalian have the mitochondrial Kappa class. In insects eight different classes have been identified: Epsilon (GSTe), Delta (GSTd), Theta (GSTt), Zeta (GSTz), Omega (Gsto) and Sigma (GSTs), the structurally unrelated microsomal class (GSTmic) and the denominated unclassified class (u), so designated for the lack or precise immunological or biochemical data (Ding et al. 2003). Most studies of GSTs in insects have been focused on their role in conferring insecticide resistance. (Claudinos et al. in press) have recently analysed the GST family in honey bees from this perspective. GST can be considered a primary antioxidant enzyme, given the fact that at least the Delta (Tang & Tu, 1994), microsomal (Toba & Aigaki, 2000), and Sigma classes (Singh et al., 2001) exhibit GPX activity with cumene hydroperoxide.
The GST superfamily includes 43 members in Drosophila and 37 in Anopheles. (Ding et al., 2003). In contrast, we only identified 12 genes in the Apis genome (two of them with partial sequences, Table 1) Compared with dipteran species, which experienced considerable expansion of the Delta and Epsilon GTS subfamilies, the bee genome contains a single ortholog of the Delta class and no members of the Epsilon class. Another difference includes double and single duplications in the Omega and Zeta classes that occurred only in fly. In addition, the Theta class ortholog that experienced two duplications in fly and one in mosquito was apparently not duplicated in bee (Table 1 and Fig. 6).
Figure 6.
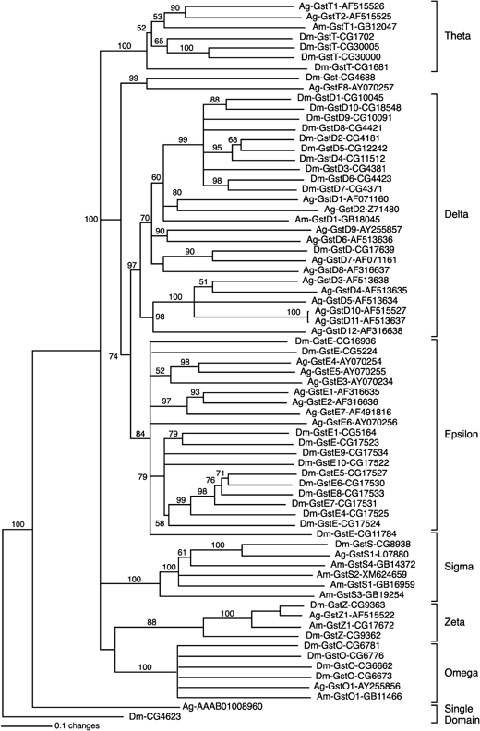
Phylogenetic relationships of GST family. GSTs belonging to the unclassified (Ding et al., 2003) class were not included. Values above the branches represent bootstrap support. Each entry has a species name (Am, for A. mellifera; Ag, for A. gambiae; Dm, for D. melanogaster), GST class, number if assigned, and accession number.
The Sigma class is the only GST lineage larger in honey bees in comparison with dipteran species. There are four members of this group in bee and a single ortholog in fly and mosquito. This is also the group with the higher conservation in intron position (Fig. 7). In addition, two members of this group (GstS1–2) are the only antioxidant genes so far found to be physically located close to each other (Table 1). Both findings suggest that in bees the GST Sigma class could have been expanded by a recent duplication event, as seems to be the case for the Delta and Epsilon classes in Drosophila (Sawicki et al., 2003) and Anopheles (Ding et al., 2003). Lack of knowledge of endogenous insect GST substrates makes it difficult to interpret the functional consequences deriving from the differential expansion of GST subfamilies between dipteran species and honey bees. Perhaps they reflect both differences in metabolic activity and variation in the quantity of pro-oxidant molecules ingested with the food. For example, the Epsilon class (expanded in dipteran but lost in bees) is involved with DDT resistance (Ranson et al., 2000; Lumjuan et al., 2005) and is expected to be related to the detoxification of xenobiotics in general. It is reasonable to expect a higher quantity of xenobiotics in the food of a solitary species, with no parental care or sociality, compared with the food received by honey bees, especially during the larval stages and the first 2 weeks of adulthood, when their food is restricted to honey, pollen and glandular secretions provided by other members of the colony (Winston, 1987). In addition, honey bees feed on angiosperms in a highly mutualistic relationship; angiosperms have evolved many traits to attract bees for pollination purposes. Bees are much less likely to be exposed to naturally occurring feeding deterrents or toxins.
Figure 7.
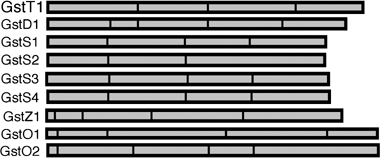
Intron position in Apis mellifera GST family members. With the exception of the third intron of GstS2, intron positions are conserved between the members of the Gst Sigma class. GstO2 genomic sequences is truncated toward the deduced C terminal region.
The expansion of the Sigma class, which occurred only in bees, seems to be involved with protection against oxidants produced by aerobic metabolism, rather than xenobiotics. In flies, these proteins are primarily located in the indirect flight muscles (Franciosa & Berge, 1995) and have been reported to play an important part in the detoxification of lipid peroxidation products (Singh et al., 2001). Honey bees take foraging trips that may last up to 1 h and they carry heavy loads of nectar and pollen during this time (Winston, 1987), so they likely produce a high level of free radicals (Young & Robinson, 1983). Perhaps this aspect of their life-style exerted selection on these detoxification genes.
Methionine-R-sulphoxide reductases
Methionine-R-sulphoxide reductases (Msr) are secondary antioxidant enzymes involved in protein repair, catalysing the TRX-dependent reduction of methionine sulphoxide to methionine (Moskovitz et al., 1996). Methionine sulphoxides can be reduced to methionines by methionine-S-sulphoxide reductase (MsrA) and methionine-R-sulphoxide reductase (MsrB), two structurally unrelated proteins (Kumar et al., 2002). A single gene for each of these enzymes is present in the analysed insect species (Table 2).
Validation by gene expression
The expression of 16 of the 38 antioxidant genes annotated in this paper (Sod2, Sod3, Cat, Gtpx1, Tpx1, Tpx3, Tpx4, GstD1, GstS1, GstS2, GstS3, GstO1, Trxr-1, Trx-like 3, Trx/Gtx and MsrA) was validated by their identification in a brain expressed sequence tag library (Whitfield et al., 2002). In addition, age and tissue specific expression profiles for eight of these genes (Sod1, Sod2, Cat, Tpx3, Trx-1, GstD1, Gtpx-1 and MsrA) encoding representative members of the main antioxidant families were reported for both workers and queens (Corona et al., 2005) (Table 3).
Bacterial genes
During the annotation of honey bee antioxidant genes, we also found several genes encoding putative bacterial-like antioxidant enzymes, including catalase, Mn SOD, TPX, GST and TRX (Supplementary material, Table 1). In the case of the catalase gene, a fragment was amplified by PCR only in samples from the thorax and abdomen of worker pupae and adult (but not larvae), and was not detectable in worker heads or any body part of adult queens (data not shown). These results suggest that this gene is not integrated into the bee's genomic DNA and might therefore come from endosymbiotic bacteria infecting the digestive tract of the larva. This gene is distinct from the bona fide Apis catalase gene discussed above.
We also identified a bacterial-like gene encoding a putative TRX (XP_561198) in the Anopheles genomic sequence, which is also presumably the product of bacterial DNA contamination. These examples show that contamination from endosymbiotic bacterial genomes are a common phenomenon present in insect genomic sequence projects, as has been shown for Wolbachia in Drosophila species (Salzberg et al., 2005).
Conclusions
We presented the results of manual annotation of the main component of the enzymatic antioxidant system of Apis mellifera and a comparative analysis with Anopheles gambiae and Drosophila melanogaster. This report represents the first systematic comparison of antioxidant genes between insect orders and between social vs. solitary insects. We found that although the basic components of the antioxidant system are conserved, there are important differences in the number of paralogs between species. The main differences include the absence of one of the five members of CuZn SOD family (Sodesque) in bee; duplication of TrxR in fly; expansion of the TRX family in fly; expansion of the Theta, Delta and Omega GST classes in fly and mosquito, and expansion of the Sigma GST class in bee. We have also speculated on how the differential expansion of antioxidant gene families among these species could reflect both differences in their life-style and the quantity of pro-oxidant molecules ingested with the food.
Experimental procedures
Annotation of Apis mellifera antioxidant genes
Identification of putative orthologs
We initially identified genes encoding known components of the enzymatic antioxidant system in organisms with well-characterized genomes, primarily human and Drosophila melanogaster. Searches were performed using both key-word searches or protein queries vs. translated DNA databases (tblastn) at NCBI (http://www.ncbi.nlm.nih.gov/), ENSEMBL (http://www.ensembl.org/index.html), and Flybase (http://www.flybase.indiana.edu). Then, we searched the Apis mellifera genome for candidate antioxidant genes using the tblastn program with the scaffolds_assembly_2 database at BEEBASE (http://racerx00.tamu.edu/bee_resources.html). This database included a number of gene prediction sets as well as a combined prediction data set (Glean3). Identification of putative antioxidant gene orthologs was completed by multiple protein sequence alignments followed by phylogenetic analysis (see details in next section). As in some cases overall protein homology does not always determine similar function and therefore the identity of an ortholog, additional bioinformatics support for the identification of putative orthologs were performed using the Conserved Domain Architecture Retrieval Tool (CDART) (http://www.ncbi.nlm.nih.gov/Structure/lexington/lexington.cgi?cmd=rps) and by identifying reported conserved residues of the catalytic site for each predicted enzyme.
Verification and correction of gene predictions
Verification of automatic gene predictions derived from the honey bee genome project (Honey Bee Genome Sequencing Consortium, 2006) were performed using protein alignments with existing gene prediction sets, selected orthologs (including known isoforms) and if available, EST sequences (http://titan.biotec.uiuc.edu/cgi-bin/ESTWebsite/estima_blastui?seqSet=bee). When conflicts in gene structure were detected between existing gene predictions or with respect to homologs across species, they were resolved using a combination of protein alignments, splice prediction algorithms (http://www.fruitfly.org/seq_tools/splice.html) and manual verification of splicing consensus sequences. A similar approach was followed to build the structure of genes with no automatic predictions (Sod3, Tpx6).
Classification and nomenclature of Apis mellifera antioxidant genes
After the identification of a putative Apis ortholog, the gene was named following the closest Drosophila ortholog. In the case of genes with no assigned names in this Drosophila (as in the case of several members of the GST family) we followed the Anopheles classification (Ding et al., 2003). In the case of bee genes with no identified orthologs in other species, we assigned a name using the family and subfamily abbreviation plus a number (for example, GstS2–4). When members of a gene family have both conserved structural domains and conserved residues of the catalytic site, but are atypical family members (for example, by containing other structural domains) we used in addition the term ‘like’ as in Trx-like1 and Trx-like2.
Phylogenetic analysis
Initial protein alignments were performed using CLUSTALW and then edited using the jalview program (http://www.ebi.ac.uk/clustalw/). We removed the predicted N-term and C-term regions when they were extended relative to other homologs in the alignment. Edited sequences were re-aligned using the ClustalX 1.81 program (Thompson et al., 1997) with the following parameters. Pair-wise: gap opening = 35.0, gap extension = 0.75; Alignment: gap opening = 15, gap extension = 0.3, protein weight matrix, Gonnet series. Phylogenetic trees were made with the Neighbour Joining method (Saitou & Nei, 1987) using the paup 4.0 b10 program (Swofford, 2002). Trees were rooted using as outgroup the most divergent sequence in each group. The statistical significance of branch order was estimated by the generation of 1000 replications of bootstrap re-sampling of the original aligned amino acid sequences.
Prediction of subcellular localization
Prediction of subcellular protein localization was performed for all identified antioxidant genes using four programs: PSORT II (http://psort.ims.u-tokyo.ac.jp/form2.html), iPSORT (http://hc.ims.u-tokyo.ac.jp/iPSORT/) (Bannai et al., 2002); TargetP (http://www.cbs.dtu.dk/services/TargetP/) (Emanuelsson et al., 2000) and SignalP (http://www.cbs.dtu.dk/services/SignalP/) (Bendtsen et al., 2004).
Acknowledgments
We thank Hugh Robertson for assistance with phylogenetic analysis, Hilary Ranson for collaboration on the annotation of the GST family, and Axel Brockmann, David Nanney, Rodrigo Velarde, James Whitfield, and anonymous reviewers for reviewing the manuscript. Supported by R01 AG 022824–04 (GER).
Supplementary material
The following material is available for this article online:
Deduced protein sequences of bacterial-like antioxidant genes found in the honey bee genomic sequence databases.
This material is available as part of the online article from http://www.blackwell-synergy.com
References
- Arner ES, Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. Eur J Biochem. 2000;267:6102–6109. doi: 10.1046/j.1432-1327.2000.01701.x. [DOI] [PubMed] [Google Scholar]
- Bannai H, Tamada Y, Maruyama O, Nakai K, Miyano S. Extensive feature detection of N-terminal protein sorting signals. Bioinformatics. 2002;18:298–305. doi: 10.1093/bioinformatics/18.2.298. [DOI] [PubMed] [Google Scholar]
- Bauer H, Kanzok SM, Schirmer RH. Thioredoxin-2 but not thioredoxin-1 is a substrate of thioredoxin peroxidase-1 from Drosophila melanogaster: isolation and characterization of a second thioredoxin in Drosophila melanogaster and evidence for distinct biological functions of Trx-1 and Trx-2. J Biol Chem. 2002;277:17457–17463. doi: 10.1074/jbc.M200636200. [DOI] [PubMed] [Google Scholar]
- Bauer H, Gromer S, Urbani A, Schnolzer M, Schirmer RH, Muller HM. Thioredoxin reductase from the malaria mosquito Anopheles gambiae. Eur J Biochem. 2003;270:4272–4281. doi: 10.1046/j.1432-1033.2003.03812.x. [DOI] [PubMed] [Google Scholar]
- Bendtsen JD, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides: SignalP 3.0. J Mol Biol. 2004;340:783–795. doi: 10.1016/j.jmb.2004.05.028. [DOI] [PubMed] [Google Scholar]
- Chae HZ, Chung SJ, Rhee SG. Thioredoxin-dependent peroxide reductase from yeast. J Biol Chem. 1994;269:27670–27678. [PubMed] [Google Scholar]
- Chang TS, Cho CS, Park S, Yu S, Kang SW, Rhee SG. Peroxiredoxin III, a mitochondrion-specific peroxidase, regulates apoptotic signaling by mitochondria. J Biol Chem. 2004;279:41975–41984. doi: 10.1074/jbc.M407707200. [DOI] [PubMed] [Google Scholar]
- Claudianos C, Ranson H, Feyereisen R, Berenbaum M, Johnson R, Oakeshott J. A defecit of metabolic enzymes: Pesticide sensitivity and environmental response in the honey bee. Genome Res. doi: 10.1111/j.1365-2583.2006.00672.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AM, Williams V, Evans JD. Sperm storage and antioxidative enzyme expression in the honey bee, Apis mellifera. Insect Mol Biol. 2004;13:141–146. doi: 10.1111/j.0962-1075.2004.00469.x. [DOI] [PubMed] [Google Scholar]
- Corona M, Estrada E, Zurita M. Differential expression of mitochondrial genes between queens and workers during caste determination in the honeybee Apis mellifera. J Exp Biol. 1999;202:929–938. doi: 10.1242/jeb.202.8.929. [DOI] [PubMed] [Google Scholar]
- Corona M, Hughes KA, Weaver DB, Robinson GE. Gene expression patterns associated with queen honey bee longevity. Mech Ageing Dev. 2005;126:1230–1238. doi: 10.1016/j.mad.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Ding Y, Ortelli F, Rossiter LC, Hemingway J, Ranson H. The Anopheles gambiae glutathione transferase supergene family: annotation, phylogeny and expression profiles. BMC Genomics. 2003;4:4–35. doi: 10.1186/1471-2164-4-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkov BC, Georgieva T. Organization of the ferritin genes in Drosophila melanogaster. DNA Cell Biol. 1999;18:937–944. doi: 10.1089/104454999314791. [DOI] [PubMed] [Google Scholar]
- Egli D, Yepiskoposyan H, Selvaraj A, et al. A family knockout of all four Drosophila metallothioneins reveals a central role in copper homeostasis and detoxification. Mol Cell Biol. 2006;26:2286–2296. doi: 10.1128/MCB.26.6.2286-2296.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuelsson O, Nielsen H, Brunak S, von Heijne G. Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J Mol Biol. 2000;300:1005–1016. doi: 10.1006/jmbi.2000.3903. [DOI] [PubMed] [Google Scholar]
- Franciosa H, Berge JB. Glutathione S-transferases in housefly (Musca domestica): location of GST-1 and GST-2 families. Insect Biochem Mol Biol. 1995;25:311–317. doi: 10.1016/0965-1748(94)00053-k. [DOI] [PubMed] [Google Scholar]
- Geiser DL, Chavez CA, Flores-Munguia R, Winzerling JJ, Pham DQ. Aedes aegypti ferritin. Eur J Biochem. 2003;270:3667–3674. doi: 10.1046/j.1432-1033.2003.03709.x. [DOI] [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin and glutaredoxin systems. J Biol Chem. 1989;264:13963–13966. [PubMed] [Google Scholar]
- Holmgren A, Johansson C, Berndt C, Lonn ME, Hudemann C, Lillig CH. Thiol redox control via thioredoxin and glutaredoxin systems. Biochem Soc Trans. 2005;33:1375–1377. doi: 10.1042/BST0331375. [DOI] [PubMed] [Google Scholar]
- Honeybee Genome Sequencing Consortium. Insights into social insects from the genome of the honeybee Apis mellifera. Nature. in press. [DOI] [PMC free article] [PubMed]
- Jimenez A, Zu W, Rawe VY, et al. Spermatocyte/spermatid-specific thioredoxin-3, a novel Golgi apparatus-associated thioredoxin, is a specific marker of aberrant spermatogenesis. J Biol Chem. 2004;279:34971–34982. doi: 10.1074/jbc.M404192200. [DOI] [PubMed] [Google Scholar]
- Jimenez A, Pelto-Huikko M, Gustafsson JA, Miranda-Vizuete A. Characterization of human thioredoxin-like-1: potential involvement in the cellular response against glucose deprivation. FEBS Lett. 2006;580:960–967. doi: 10.1016/j.febslet.2006.01.025. [DOI] [PubMed] [Google Scholar]
- Johansson C, Lillig CH, Holmgren A. Human mitochondrial glutaredoxin reduces S-glutathionylated proteins with high affinity accepting electrons from either glutathione or thioredoxin reductase. J Biol Chem. 2004;279:7537–7543. doi: 10.1074/jbc.M312719200. [DOI] [PubMed] [Google Scholar]
- Kanzok SM, Fechner A, Bauer H, et al. Substitution of the thioredoxin system for glutathione reductase in Drosophila melanogaster. Science. 2001;291:643–646. doi: 10.1126/science.291.5504.643. [DOI] [PubMed] [Google Scholar]
- Kucharski R, Maleszka R. Transcriptional profiling reveals multifunctional roles for transferrin in the honeybee, Apis mellifera. J Insect Sci. 2003;3:27–27. doi: 10.1093/jis/3.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar RA, Koc A, Cerny RL, Gladyshev VN. Reaction mechanism, evolutionary analysis, and role of zinc in Drosophila methionine-R-sulfoxide reductase. J Biol Chem. 2002;277:37527–37535. doi: 10.1074/jbc.M203496200. [DOI] [PubMed] [Google Scholar]
- Landis GN, Tower J. Superoxide dismutase evolution and life span regulation. Mech Ageing Dev. 2005;126:365–379. doi: 10.1016/j.mad.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Lee OJ, Schneider-Stock R, McChesney PA, et al. Hypermethylation and loss of expression of glutathione peroxidase-3 in Barrett's tumorigenesis. Neoplasia. 2005;7:854–861. doi: 10.1593/neo.05328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Blasevich F, Theopold U, Schmidt O. Possible function of two insect phospholipid-hydroperoxide glutathione peroxidases. J Insect Physiol. 2003;49:1–9. doi: 10.1016/s0022-1910(02)00189-0. [DOI] [PubMed] [Google Scholar]
- Lumjuan N, McCarroll L, Prapanthadara LA, Hemingway J, Ranson H. Elevated activity of an Epsilon class glutathione transferase confers DDT resistance in the dengue vector, Aedes aegypti. Insect Biochem Mol Biol. 2005;35:861–871. doi: 10.1016/j.ibmb.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Martin JL. Thioredoxin – a fold for all reasons. Structure. 1995;3:245–250. doi: 10.1016/s0969-2126(01)00154-x. [DOI] [PubMed] [Google Scholar]
- Miranda-Vizuete A, Ljung J, Damdimopoulos AE, Gustafsson JA, Oko R, Pelto-Huikko M, Spyrou G. Characterization of Sptrx, a novel member of the thioredoxin family specifically expressed in human spermatozoa. J Biol Chem. 2001;276:31567–31574. doi: 10.1074/jbc.M101760200. [DOI] [PubMed] [Google Scholar]
- Missirlis F, Ulschmid JK, Hirosawa-Takamori M, Gronke S, Schafer U, Becker K, Phillips JP, Jackle H. Mitochondrial and cytoplasmic thioredoxin reductase variants encoded by a single Drosophila gene are both essential for viability. J Biol Chem. 2002;277(13):11521–11526. doi: 10.1074/jbc.M111692200. [DOI] [PubMed] [Google Scholar]
- Missirlis F, Rahlfs S, Dimopoulos N, et al. A putative glutathione peroxidase of Drosophila encodes a thioredoxin peroxidase that provides resistance against oxidative stress but fails to complement a lack of catalase activity. Biol Chem. 2003;384:463–472. doi: 10.1515/BC.2003.052. [DOI] [PubMed] [Google Scholar]
- Moskovitz J, Weissbach H, Brot N. Cloning the expression of a mammalian gene involved in the reduction of methionine sulfoxide residues in proteins. Proc Natl Acad Sci USA. 1996;93:2095–2099. doi: 10.1073/pnas.93.5.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- do Nascimento AM, Cuvillier-Hot V, Barchuk AR, Simoes ZL, Hartfelder K. Honey bee (Apis mellifera) transferrin-gene structure and the role of ecdysteroids in the developmental regulation of its expression. Insect Biochem Mol Biol. 2004;34:415–424. doi: 10.1016/j.ibmb.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Nordberg J, Arner ES. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic Biol Med. 2001;31:1287–1312. doi: 10.1016/s0891-5849(01)00724-9. [DOI] [PubMed] [Google Scholar]
- Page RE, Jr, Peng CY. Aging and development in social insects with emphasis on the honey bee, Apis mellifera L. Exp Gerontol. 2001;36:695–711. doi: 10.1016/s0531-5565(00)00236-9. [DOI] [PubMed] [Google Scholar]
- Parker JD, Parker KM, Sohal BH, Sohal RS, Keller L. Decreased expression of Cu-Zn Superoxide Dismutase 1 in ants with extreme life-span. Proc Nat Acad Sci USA. 2004;101:3486–3489. doi: 10.1073/pnas.0400222101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicena-Palle A, Stitzinger SM, Salz HK. The function of the Drosophila thioredoxin homolog encoded by the deadhead gene is redox-dependent and blocks the initiation of development but not DNA synthesis. Mech Dev. 1997;62:61–65. doi: 10.1016/s0925-4773(96)00650-8. [DOI] [PubMed] [Google Scholar]
- Perez-Campo R, Lopez-Torres M, Cadenas S, Rojas C, Barja G. The rate of free radical production as a determinant of the rate of aging: evidence from the comparative approach. J Comp Physiol [B] 1998;168:149–158. doi: 10.1007/s003600050131. [DOI] [PubMed] [Google Scholar]
- Radyuk SN, Klichko VI, Spinola B, Sohal RS, Orr WC. The peroxiredoxin gene family in Drosophila melanogaster. Free Radic Biol Med. 2001;31:1090–1000. doi: 10.1016/s0891-5849(01)00692-x. [DOI] [PubMed] [Google Scholar]
- Ranson H, Jensen B, Wang X, Prapanthadara L, Hemingway J, Collins FH. Genetic mapping of two loci affecting DDT resistance in the malaria vector, Anopheles gambiae. Insect Mol Biol. 2000;9:499–507. doi: 10.1046/j.1365-2583.2000.00214.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Manzaneque MT, Ros J, Cabiscol E, Sorribas A, Herrero E. Grx5 glutaredoxin plays a central role in protection against protein oxidative damage in Saccharomyces cerevisiae. Mol Cell Biol. 1999;19:8180–8190. doi: 10.1128/mcb.19.12.8180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Salzberg SL, Hotopp JC, Delcher AL, Pop M, Smith DR, Eisen MB, Nelson WC. Serendipitous discovery of Wolbachia genomes in multiple Drosophila species. Genome Biol. 2005;6:R23–R23. doi: 10.1186/gb-2005-6-3-r23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicki R, Singh SP, Mondal AK, Benes H, Zimniak P. Cloning, expression and biochemical characterization of one Epsilon-class (GST-3) and ten Delta-class (GST-1) glutathione S-transferases from Drosophila melanogaster, and identification of additional nine members of the Epsilon class. Biochem J. 2003;370:661–669. doi: 10.1042/BJ20021287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotti PD, Dearing SC, Greenwood DR, Newcomb RD. Pernin: a novel, self-aggregating haemolymph protein from the New Zealand green-lipped mussel, Perna canaliculus (Bivalvia: Mytilidae) Comp Biochem Physiol B Biochem Mol Biol. 2001;128:767–779. doi: 10.1016/s1096-4959(01)00301-3. [DOI] [PubMed] [Google Scholar]
- Seehuus SC, Norberg K, Gimsa U, Krekling T, Amdam GV. Reproductive protein protects functionally sterile honey bee workers from oxidative stress. Proc Natl Acad Sci USA. 2006;103:962–967. doi: 10.1073/pnas.0502681103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SP, Coronella JA, Benes H, Cochrane BJ, Zimniak P. Catalytic function of Drosophila melanogaster glutathione S-transferase DmGSTS1–1 (GST-2) in conjugation of lipid peroxidation end products. Eur J Biochem. 2001;268:2912–2923. doi: 10.1046/j.1432-1327.2001.02179.x. [DOI] [PubMed] [Google Scholar]
- Smith J, Shrift A. Phylogenetic distribution of glutathione peroxidase. Comp Biochem Physiol B. 1979;63:39–44. doi: 10.1016/0305-0491(79)90231-1. [DOI] [PubMed] [Google Scholar]
- Spyrou G, Enmark E, Miranda-Vizuete A, Gustafsson J. Cloning and expression of a novel mammalian thioredoxin. J Biol Chem. 1997;272:2936–2941. doi: 10.1074/jbc.272.5.2936. [DOI] [PubMed] [Google Scholar]
- Suarez RK, Staples JF, Lighton JR, Mathieu-Costello O. Mitochondrial function in flying honeybees (Apis mellifera): respiratory chain enzymes and electron flow from complex III to oxygen. J Exp Biol. 2000;203:905–911. doi: 10.1242/jeb.203.5.905. [DOI] [PubMed] [Google Scholar]
- Svensson MJ, Chen JD, Pirrotta V, Larsson J. The ThioredoxinT and deadhead gene pair encode testis- and ovary-specific thioredoxins in Drosophila melanogaster. Chromosoma. 2003;112:133–143. doi: 10.1007/s00412-003-0253-5. [DOI] [PubMed] [Google Scholar]
- Swofford DL. Sunderland, MA: Sinauer Associates; 2002. Phylogenetic Analysis Using Parsimony (*and Other Methods) Version 4. [Google Scholar]
- Tang AH, Tu CP. Biochemical characterization of Drosophila glutathione S-transferases D1 and D21. J Biol Chem. 1994;269:27876–27884. [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toba G, Aigaki T. Disruption of the microsomal glutathione S-transferase-like gene reduces lifespan of Drosophila melanogaster. Gene. 2000;253:179–187. doi: 10.1016/s0378-1119(00)00246-8. [DOI] [PubMed] [Google Scholar]
- Trivelli X, Krimm I, Ebel C, Verdoucq L, Prouzet-Mauleon V, Chartier Y, Tsan P, Lanquin G, Meyer Y, Lancelin JM. Characterization of the yeast peroxiredoxin Ahp1 in its reduced active and overoxidized inactive forms using NMR. Biochemistry. 2003;42:14139–14149. doi: 10.1021/bi035551r. [DOI] [PubMed] [Google Scholar]
- Ursini F, Maiorno M, Brigelius-Flohe R, Aumann KD, Roveri A, Schomburg D, Flohe L. Diversity of glutathione peroxidases. Methods Enzymol. 1995;252:38–53. doi: 10.1016/0076-6879(95)52007-4. [DOI] [PubMed] [Google Scholar]
- Weirich GF, Collins AM, Williams VP. Antioxidant enzymes in the honey bee, Apis mellifera. Apidologie. 2002;33:3–14. [Google Scholar]
- White JW. Composition of honey. In: Crane E, editor. Honey: A Comprehensive Survey. Chalfont St Peter, London: Bee Research Association; 1975. pp. 157–206. [Google Scholar]
- Whitfield CW, Band MR, Bonaldo MF, et al. Gene expression profiles in the brain predict behavior in individual honey bees. Genome Res. 2002;12:555–566. [Google Scholar]
- Wingert RA, Galloway JL, Barut B, et al. Deficiency of glutaredoxin 5 reveals Fe-S clusters are required for vertebrate haem synthesis. Nature. 2005;436:1035–1039. doi: 10.1038/nature03887. [DOI] [PubMed] [Google Scholar]
- Winston ML. Biology of the Honey Bee. Cambridge, MA: Harvard University Press; 1987. [Google Scholar]
- Xia X. Maximizing transcription efficiency causes codon usage bias. Genetics. 1996;144:1309–1320. doi: 10.1093/genetics/144.3.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RG, Robinson GE. Age and oxygen toxicity related fluorescence in the honey bee thorax. Exp Gerontol. 1983;18:471–447. doi: 10.1016/0531-5565(83)90026-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Deduced protein sequences of bacterial-like antioxidant genes found in the honey bee genomic sequence databases.


