Abstract
Carnobacterium piscicola LV17 isolated from vacuum-packed meat produces bacteriocin(s) that is active against closely related lactic acid bacteria, Enterococcus spp., and a strain of Listeria monocytogenes but not against gram-negative bacteria. The bacteriocin has a bactericidal mode of action, is heat resistant, and is stable over a wide range of pH but is inactivated by proteolytic enzymes. Sensitive and resistant cells were shown to adsorb the bacteriocin, but cell death depended on contact of the bacteriocin with the cell membrane. Bacteriocin production is detected early in the growth cycle of the organism in APT broth, but it is not produced in APT broth adjusted to pH 5.5. Bacteriocin production and resistance to the bacteriocin produced are associated with two plasmids of 40 and 49 megadaltons. The possibility that two bacteriocins are produced is indicated because the inhibitory substances of the mutant strains containing either the 40- or 49-megadalton plasmids have different antimicrobial spectra.
Full text
PDF



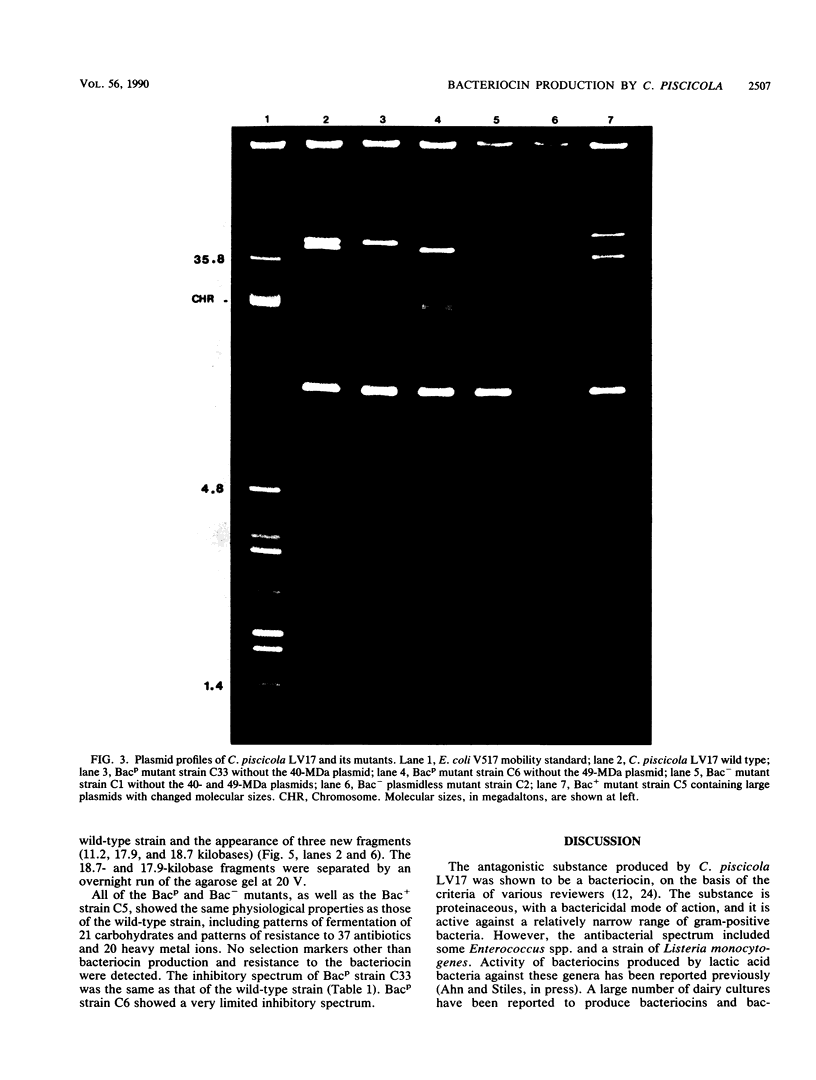
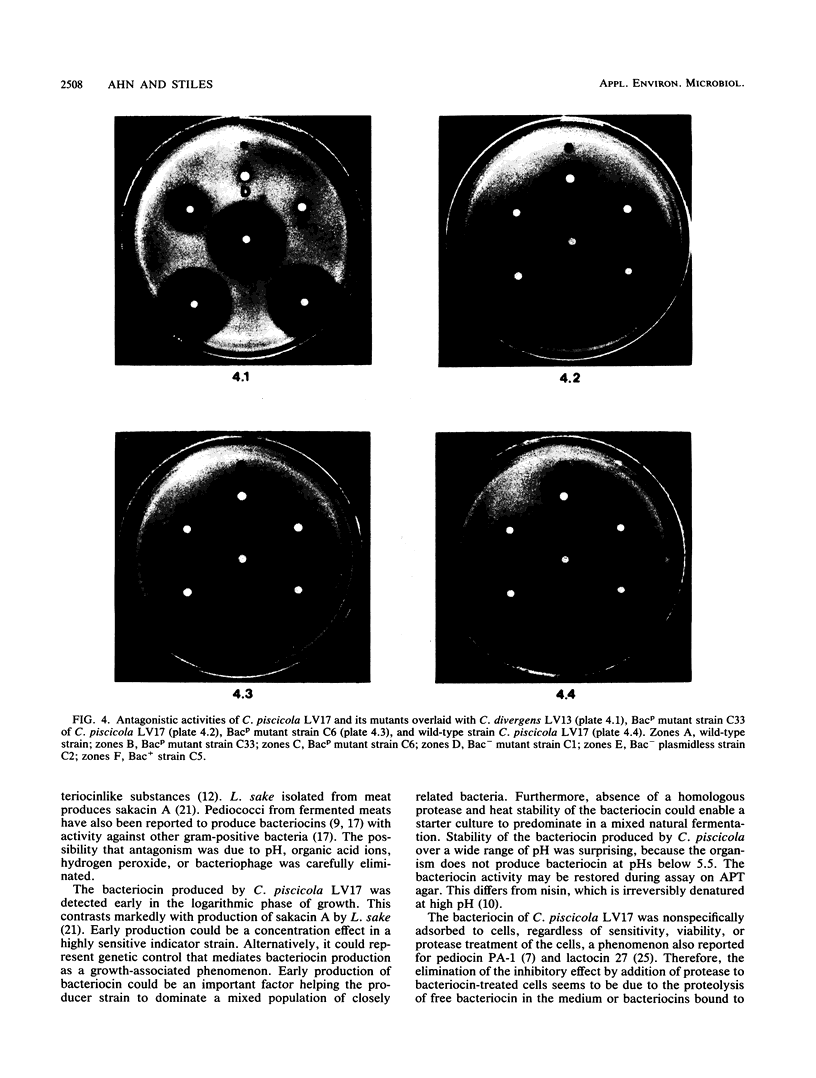
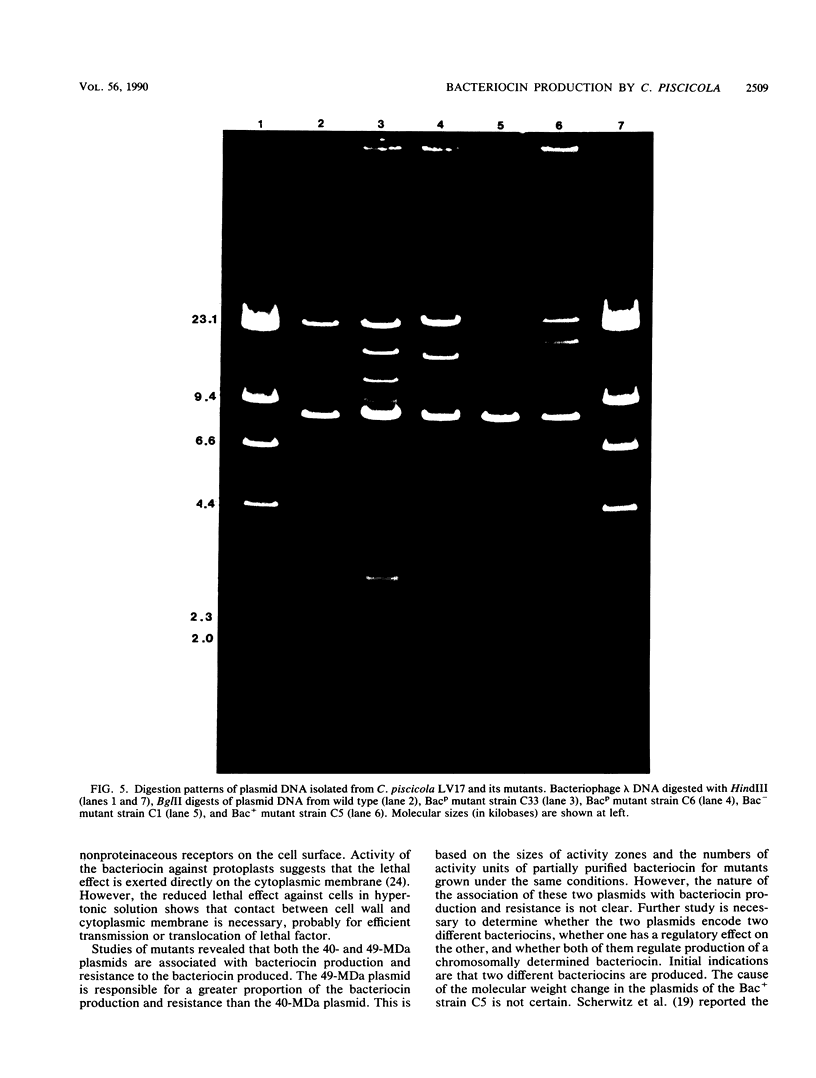

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson R. E., Daeschel M. A., Hassan H. M. Antibacterial activity of plantaricin SIK-83, a bacteriocin produced by Lactobacillus plantarum. Biochimie. 1988 Mar;70(3):381–390. doi: 10.1016/0300-9084(88)90211-8. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C. A rapid alkaline extraction method for the isolation of plasmid DNA. Methods Enzymol. 1983;100:243–255. doi: 10.1016/0076-6879(83)00059-2. [DOI] [PubMed] [Google Scholar]
- Efstathiou J. D., McKay L. L. Inorganic salts resistance associated with a lactose-fermenting plasmid in Streptococcus lactis. J Bacteriol. 1977 Apr;130(1):257–265. doi: 10.1128/jb.130.1.257-265.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasson M. J. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J Bacteriol. 1983 Apr;154(1):1–9. doi: 10.1128/jb.154.1.1-9.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez C. F., Kunka B. S. Plasmid-Associated Bacteriocin Production and Sucrose Fermentation in Pediococcus acidilactici. Appl Environ Microbiol. 1987 Oct;53(10):2534–2538. doi: 10.1128/aem.53.10.2534-2538.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst A. Nisin: its preservative effect and function in the growth cycle of the producer organism. Soc Appl Bacteriol Symp Ser. 1978;7:297–314. [PubMed] [Google Scholar]
- Klaenhammer T. R. Bacteriocins of lactic acid bacteria. Biochimie. 1988 Mar;70(3):337–349. doi: 10.1016/0300-9084(88)90206-4. [DOI] [PubMed] [Google Scholar]
- Macrina F. L., Kopecko D. J., Jones K. R., Ayers D. J., McCowen S. M. A multiple plasmid-containing Escherichia coli strain: convenient source of size reference plasmid molecules. Plasmid. 1978 Jun;1(3):417–420. doi: 10.1016/0147-619x(78)90056-2. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Roth C. Plasmid-linked resistance to inorganic salts in Staphylococcus aureus. J Bacteriol. 1968 Apr;95(4):1335–1342. doi: 10.1128/jb.95.4.1335-1342.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochelle P. A., Fry J. C., Day M. J., Bale M. J. An accurate method for estimating sizes of small and large plasmids and DNA fragments by gel electrophoresis. J Gen Microbiol. 1986 Jan;132(1):53–59. doi: 10.1099/00221287-132-1-53. [DOI] [PubMed] [Google Scholar]
- Scherwitz K. M., Baldwin K. A., McKay L. L. Plasmid linkage of a bacteriocin-like substance in Streptococcus lactis subsp. diacetylactis strain WM4: transferability to Streptococcus lactis. Appl Environ Microbiol. 1983 May;45(5):1506–1512. doi: 10.1128/aem.45.5.1506-1512.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schillinger U., Lücke F. K. Antibacterial activity of Lactobacillus sake isolated from meat. Appl Environ Microbiol. 1989 Aug;55(8):1901–1906. doi: 10.1128/aem.55.8.1901-1906.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw B. G., Harding C. D. A numerical taxonomic study of lactic acid bacteria from vacuum-packed beef, pork, lamb and bacon. J Appl Bacteriol. 1984 Feb;56(1):25–40. doi: 10.1111/j.1365-2672.1984.tb04693.x. [DOI] [PubMed] [Google Scholar]
- Tagg J. R., Dajani A. S., Wannamaker L. W. Bacteriocins of gram-positive bacteria. Bacteriol Rev. 1976 Sep;40(3):722–756. doi: 10.1128/br.40.3.722-756.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upreti G. C., Hinsdill R. D. Production and mode of action of lactocin 27: bacteriocin from a homofermentative Lactobacillus. Antimicrob Agents Chemother. 1975 Feb;7(2):139–145. doi: 10.1128/aac.7.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson B. J., Jones D. A numerical taxonomic survey of Listeria and related bacteria. J Gen Microbiol. 1977 Feb;98(2):399–421. doi: 10.1099/00221287-98-2-399. [DOI] [PubMed] [Google Scholar]