Abstract
Background/Aims
A recently-determined target of lipopolysaccharide (LPS) and cytokine signaling in liver is the central Type II nuclear receptor (NR) heterodimer partner, Retinoid X receptor α (RXRα). We sought to determine if rosiglitazone (Rosi) a peroxisome proliferator activated receptor γ(PPARγ) agonist with anti-inflammatory properties, can attenuate LPS and cytokine-induced molecular suppression of RXRα-regulated genes.
Methods
In vivo, mice were gavage-fed Rosi for 3 days, prior to intraperitoneal injection of LPS, followed by harvest of liver and serum. In vitro, HepG2 cells were treated with IL-1β, ± short-term Rosi pretreatment. RNA was analyzed by quantitative RT-PCR, while nuclear and cytoplasmic proteins were analyzed by immunoblotting and gel shifts.
Results
Rosi attenuated LPS-mediated suppression of RNA levels of several Type II NR-regulated genes, including bile acid transporters and the major drug metabolizing enzyme, Cyp3a11, without affecting cytokine expression, suggesting a novel, direct anti-inflammatory effect in hepatocytes. Rosi suppressed the inflammation-induced nuclear export of RXRα, in both LPS-injected mice and IL-1β-treated HepG2 cells, leading to maintenance of nuclear RXRα levels and heterodimer binding activity.
Conclusions
Rosi directly attenuates the suppressive effects of inflammation-induced cell signaling on nuclear RXRα levels in liver.
Keywords: liver, inflammation, RXR, JNK, Rosiglitazone, PPARγ, nuclear export
1. Introduction
Inflammation induces the negative hepatic APR, which is characterized by disruption of critical physiological processes in the liver [1,2]. LPS-induced APR involves Kupffer cell (KC)-mediated release of the pro-inflammatory cytokines, interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α, which results in the activation of cell-signaling pathways leading to the suppression of hepatic genes [3,4]. APR leads to the pathogenesis and progression of a variety of liver diseases, including cholestasis, which results from altered expression of the bile acid transporters, including sodium/taurocholate cotransporter (Ntcp/Slc10a1), bile acid salt exporter pump (Bsep, Abcb11), and the multi-drug resistance-related proteins (Mrp, Abcc) 2 & 3 [2,5]. The effects of LPS on hepatic genes are attenuated in rodent models upon inactivation or depletion of KCs, or by administration of anti-cytokine antibodies [6-9]. This suggests that counteracting either the production or intracellular action of inflammatory mediators secreted by KCs may attenuate the pathogenesis of inflammation in liver diseases.
The expression of many of the genes which are repressed during negative hepatic APR are regulated by Type II nuclear receptors (NRs), which require heterodimerization with retinoid X receptor (RXR) to activate gene transcription [10,11]. RXRα is the most highly expressed RXR isoform in the liver and plays a central role in regulating major physiological processes in the liver, including endobiotic/xenobiotic metabolism and homeostasis [12,13]. We have recently demonstrated that reduction of nuclear RXRα protein levels by LPS administration in vivo and IL-1β in vitro, appear to be a major contributor to the repression of hepatic genes during the negative hepatic APR [14-16]. Thus, maintaining RXRα levels in the nucleus may be a new and potent means to attenuate LPS-mediated suppression of hepatic genes.
In addition to playing major roles in lipid metabolism, members of the PPAR (Nr1c1-3) subfamily of Type II NRs (α,β/δ,γ), may possess potent anti-inflammatory properties [17-19]. PPARγ ligands can inhibit the expression of inflammatory genes such as IL-1β, TNFα, IL-6, iNOS, MMP-9 and scavenger receptor A in macrophages and monocytes [17-20]. There is increasing evidence to support a protective role of PPARγ in various pathophysiological conditions including cancer, atherosclerosis, diabetes and hepatogastroenterological diseases [19,21]. PPARγ ligands can inhibit LPS-induced NO and TNFα production in cultured KCs and the inhibition was potentiated by co-treatment with RXR agonists [22]. However, it is not known whether PPARγ agonists have any role in reducing the effects of inflammation on NR genes in hepatocytes, although recent studies in humans with non-alcoholic steatohepatitis (NASH) support PPARγ ligands as potential anti-inflammatory agents [23,24].
In this study we sought to determine whether the PPARγ ligand, Rosiglitazone (Rosi) can attenuate the deleterious effects of inflammation on the expression of genes regulating endobiotic/xenobiotic transport and metabolism in liver. Rosi attenuated the effects of LPS on the expression of critical RXRα-regulated hepatic genes (Ntcp, Bsep, Cyp3a11 and Lfabp), while inhibiting LPS-mediated RXRα nuclear export, resulting in increased nuclear binding activity of RXRα heterodimers in vivo. Surprisingly, Rosi did not affect LPS-mediated induction of cytokine expression, but appears to have a direct anti-inflammatory effect in hepatocytes. In vitro studies indicate that Rosi can act intracellularly in liver-derived HepG2 cells to prevent IL-1β-mediated nuclear export and degradation of RXRα. This suggests that PPARγ agonists can be utilized as novel therapeutic agents to modulate hepatic inflammatory responses in acute and chronic liver diseases.
2. Materials and methods
2.1. Mice
Male C57BL/6 mice from Charles River Laboratories (Wilmington, MA) were maintained in a temperature and humidity-controlled environment and provided with water and rodent chow ad lib. Mice were gavage-fed 50 mg/kg/d of Rosiglitazone (Alexis Biochemicals, San Diego, CA) or corn-oil once daily for 3 days. On day 3, the animals were intraperitoneally (IP) injected with 2 mg/kg body weight of LPS (Salmonella typhimurium; Sigma Chemical Co., St. Louis, MO) or saline and livers were harvested after 1, 4, 8 and 16 hours [16]. All animal protocols were approved by the Baylor College of Medicine Institutional Animal Care and Use Committee. Experiments were performed in triplicate and repeated three to four times.
2.2. Real time quantitative PCR analysis
Total RNA was isolated from liver tissues using the RNeasy kit from Qiagen, and cDNA was synthesized using the ProSTAR™ First-Strand RT-PCR Kit (Stratagene, La Jolla, CA). Real time quantitative PCR was performed using an ABI PRISM 7000 Sequence Detection System (Applied Biosystems, Inc., Foster City, CA). Quantitative expression values were extrapolated from standard curves and were normalized to cyclophilin. The sequences of the primers and probes are listed as supplemental data. All data were analyzed by Kruskal Wallis ANOVA followed by Mann-Whitney test. P-values less than 0.05 were used as the criteria of significance.
2.3. Plasma cytokine analysis
Plasma levels of IL-1β, IL-6 and TNFα were determined simultaneously using xMAP technology (Luminex Corporation, Austin, TX) with a commercially available kit (Linco Research, St.Charles, MO).
2.4. Cell fractionation and immunoblotting
Cell extracts were prepared as previously described [15,16,25]. The following antibodies were used in immunoblot analysis: JNK, phospho-JNK and phospho-c-Jun antibodies (Ser 63) (Cell Signaling, Beverly, MA), IκBα and anti-RXRα (D-20) (Santa Cruz Biotechnology, Santa Cruz, CA). Signals were developed using Tropix luminescence following the manufacturer’s protocol (Applied Biosystems, Foster City, CA).
2.5. Electrophoretic gel mobility shift assay (EMSA)
Nuclear extracts were prepared according to Timchenko et al. with some modifications [26]. 10 μg of nuclear extracts were incubated on ice for 30 min with 32P end-labeled oligonucleotide as described previously [15]. After binding, the samples were electrophoresed through a non-denaturing 6% polyacrylamide gel, dried and exposed to x-ray film.
2.6. Cell culture
The human hepatoma cell line, HepG2, was maintained in MEM containing Earle’s salts and supplemented with 10 % certified fetal bovine serum (FBS), penicillinstreptomycin and L-Glutamine. The cells were plated at 2.5 × 105 cells/ml and maintained in serum-containing media for 48 hours and then serum starved for 20 hours prior to treatment with 10 μM Rosi or DMSO. After 30 minutes of Rosi treatment, cells were treated with either 10ng/ml IL-1β or vehicle control (0.0001% BSA in PBS) for 30 minutes.
2.7. Immunofluorescent analysis
Mice were pre-treated with Rosi or vehicle, followed by saline or LPS injection, and livers were harvested after 1 hour. Livers were fixed in 10% buffered neutral formalin overnight at 4 °C and then stored in 70% ethanol. Fluorescent detection was performed by using anti-RXRα (D-20) antibody and fluorescein isothiocyanate (FITC)- labeled secondary antibody and nuclei was stained with 4’-6-diamidino-2-phenylindole (DAPI). Visualization was performed with a Deltavision Spectris Deconvolution Microscope System (Applied Precision, Inc.).
HepG2 cells were grown on cover slips, treated with Rosi or DMSO for 30 minutes, followed by IL-1β or vehicle treatment for another 30 minutes. Cells were washed with cold phosphate buffered saline, and immunostaining was performed as described previously [14]. The cells were stained with anti-RXRα antibody and Alexa Fluor 555 goat anti-rabbit secondary antibody (Invitrogen, Eugene, Oregon).
3. Results
3.1. Rosiglitazone pre-treatment attenuates LPS-mediated suppression of RXRα-regulated hepatic genes
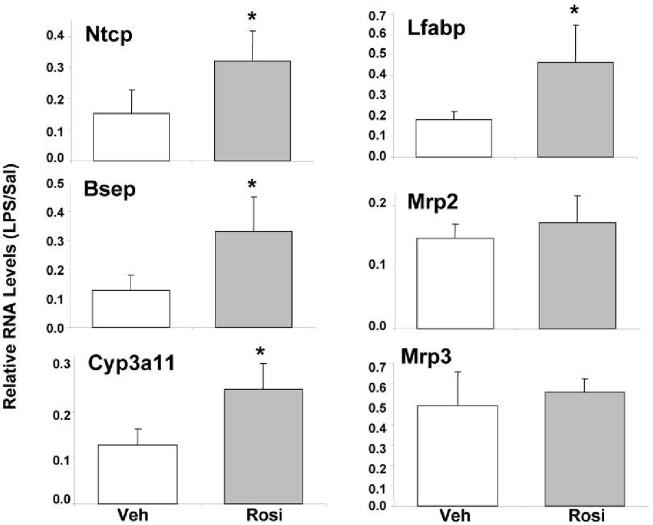
Administration of LPS leads to the down-regulation of hepatic genes involved in bile acid metabolism and transport [27,28]. To determine whether the PPARγ agonist, Rosi can attenuate the effect of LPS on hepatic gene expression, four groups of mice were tested—vehicle feeding followed by saline injection (Veh/Sal), vehicle feeding followed by LPS (Veh/LPS), Rosi feeding followed by saline injection (Rosi/Sal), and Rosi feeding followed by LPS injection (Rosi/LPS). RNA was isolated from livers harvested at 16 hours after injection and analyzed by real-time PCR (Fig. 1). The RNA levels of Veh/LPS and Rosi/LPS samples were determined relative to their controls, Veh/Sal and Rosi/Sal, respectively. RNA levels of the major bile acid transporters, Ntcp and Bsep, from Rosi/LPS treated mice increased 2-3 fold compared to Veh/LPS treated control mice (Ntcp: 15% → 30%; Bsep: 12% → 31%). RNA levels of the major bile acid and drug metabolizing enzyme, cytochrome P450 3a11 (Cyp3a11) increased ∼2-fold (12% → 25%), with Rosi pre-treatment as did RNA levels of the liver fatty acid binding protein (lfabp) (20% → 45%). Rosi did not affect the LPS-mediated suppression of 2 NR-regulated transporter genes, Mrp2 and Mrp3, suggesting that Rosi exhibited gene-specific responses.
Figure 1.
Rosiglitazone attenuates suppression of hepatic genes by LPS. C57BL/6 male mice were gavage-fed 50 mg/kg/d of Rosi or corn-oil for 3 days. On day 3, the animals were intraperitoneally (IP) injected with 2 mg/kg body weight of Salmonella LPS or saline and livers were harvested after 16 hours (n=6 per group). RNA was isolated from the livers and analyzed by TaqMan real-time PCR. All data were presented as ± SD and standardized for cyclophilin RNA levels. The expression of the genes after LPS treatment is shown here. In case of vehicle or Rosi pre-treatment, expression in saline-injected animals was set to 1, and fold change after LPS treatment was compared to vehicle or Rosi controls respectively. The asterisks indicate significant difference (p < 0.05). See supplemental information for primers and probes.
A direct hepatocyte target gene of PPARγ, fatty acid translocase (FAT)/CD36 [29] was induced by Rosi (data not shown) indicating that 50 mg/kg/d of Rosi treatment activates PPARγ in hepatocytes. Thus, Rosi is capable of activating PPARγ in liver and attenuating LPS-mediated down-regulation of key genes involved in bile acid homeostasis.
3.2. Rosi attenuates effects of LPS on RXRα subcellular localization in mouse liver
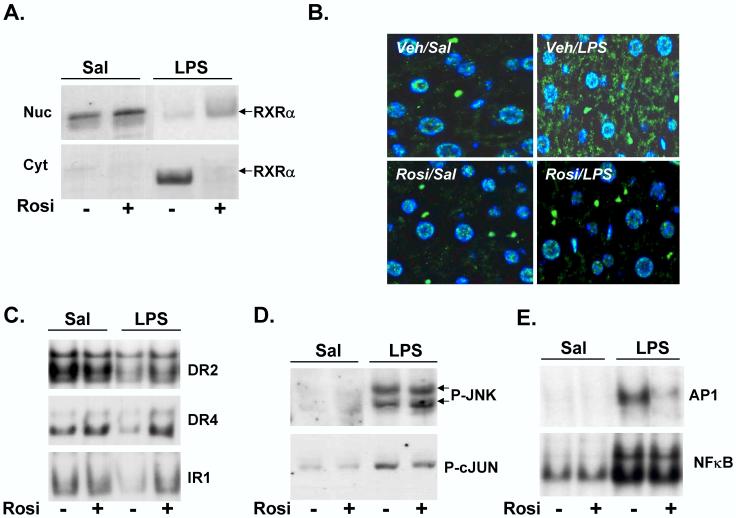
Recent results indicate that LPS reduces RXRα target gene expression by inducing its nuclear export [16]. We wanted to determine whether Rosi attenuated LPS-mediated suppression of RXRα-regulated hepatic gene expression by reducing its nuclear export and maintaining nuclear RXRα levels. As reported by us [16], in Veh/LPS-treated mice, nuclear RXRα levels were significantly reduced with a corresponding increase in cytosolic RXRα levels, compared to Veh/Sal controls (Fig. 2A). However, LPS-mediated reduction in nuclear levels of RXRα was attenuated by Rosi pre-treatment (10% in Veh/LPS→ 20% in Rosi/LPS). LPS-mediated induction in cytosolic RXRα levels was also attenuated by Rosi (80% in Veh/LPS→ 1% in Rosi/LPS), indicating that Rosi pre-treatment attenuates LPS-induced nuclear export of RXRα. Rosi treatment alone did not affect the nuclear or cytosolic levels of RXRα (Fig. 2A). Immunofluorescent analysis of formalin-fixed liver tissues show that RXRα was localized in the nucleus in Veh/Sal treated sample, and was detected in the cytosol after LPS treatment (Fig. 2B). Rosi treatment alone had no effect on nuclear RXRα, however, Rosi pre-treatment blocked LPS-induced nuclear export of RXRα, as evidenced by the lack of cytoplasmic RXRα in Rosi/LPS-treated panel (Fig. 2B).
Figure 2.
Rosiglitazone attenuates effects of LPS on RXRα localization and binding activity in vivo. C57BL/6 male mice were gavage-fed 50 mg/kg/d of Rosi or corn-oil for 3 days prior to saline or LPS (2 μg/g bw) injection on day 3. Livers were isolated at the 1, 4 and 16h and nuclear and cytosolic extracts were prepared. Extracts from 4-5 animals were analyzed individually and combined to account for inter-animal variability. (A) Nuclear (Nuc) and cytosolic (Cyt) extracts from 1h samples were analyzed by immunoblotting with antibodies to RXRα to determine the effects of Rosi on subcellular localization of RXRα in the presence of saline or LPS. (B) Immunofluorescence analysis of formalin-fixed liver tissues. RXRα was stained with FITC-labeled secondary antibody, nuclei were stained with DAPI, and the merged images are shown. (C) Electrophoretic mobility shift assay analysis of 16h samples where radiolabeled DR2, DR4 and IR1 elements were incubated with hepatic nuclear extracts. The samples were electrophoresed through a 6% non-denaturing polyacrylamide gel, dried and analyzed by autoradiography. (D) Phosphorylation of JNK (P-JNK) and c-JUN (P-cJUN) was determined by immunoblotting cell lysates from 1h samples with phospho-JNK and phospho-c-JUN antibodies respectively. (E) In order to determine the effects of Rosi on LPS-mediated AP1 or NF-κB activation, binding activity of nuclear extracts (prepared from 4h samples) to consensus AP1 or NF-κB elements was measured by EMSA.
In order to determine if attenuation of LPS-mediated nuclear export of RXRα by Rosi affects DNA binding activity of RXRα and its partners, EMSA was performed. Nuclear extracts were incubated with oligonucleotides containing canonical DNA elements scanning Type II NR binding sites--direct repeats of the hexad AGGTCA, separated by 2 and 4 nucleotides (DR2 & DR4), or an inverted repeat separated by 1 nucleotide (IR1)—sequences in promoter regions that regulate many genes involved in metabolism and transport in hepatocytes [10,16]. In response to LPS treatment, binding to these RXRα-containing conserved sequences was reduced (Fig. 2C), while, Rosi pre-treatment increased binding activity to all three target sequences back to baseline levels (DR2: 50% → 85%, DR4: 40% → 90% and IR1: 60% → 100% in Rosi/LPS compared to Veh/LPS).
3.3. Effect of Rosi on cell-signaling pathways in vivo
We next examined the role of LPS-activated cell-signaling on nuclear RXRα export in mouse liver. The MAP kinase, c-Jun N-terminal kinase, JNK was recently shown to be involved in nuclear export of RXRα during activation of inflammatory pathways [14,16]. Rosi treatment did not alter the LPS-mediated increase in phosphorylation of JNK, or its substrate, c-Jun in nuclear extracts, thus indicating that Rosi has no effect on activation of JNK by LPS (Fig. 2D).
PPARγ ligands inhibit NF-κβ and AP1 signaling pathways in LPS-treated peritoneal macrophages, although the mechanisms are unclear [18,20]. Whether or not such mechanisms are active in whole liver is unknown. We examined the effects of Rosi on LPS-induced activation of AP1 and NF-κβ by EMSA (Fig. 2E). Interestingly, Rosi inhibits AP1 activation, but had no effect on NF-κβ activation in the presence of LPS, distinguishing the effects of Rosi in isolated macrophages from whole liver.
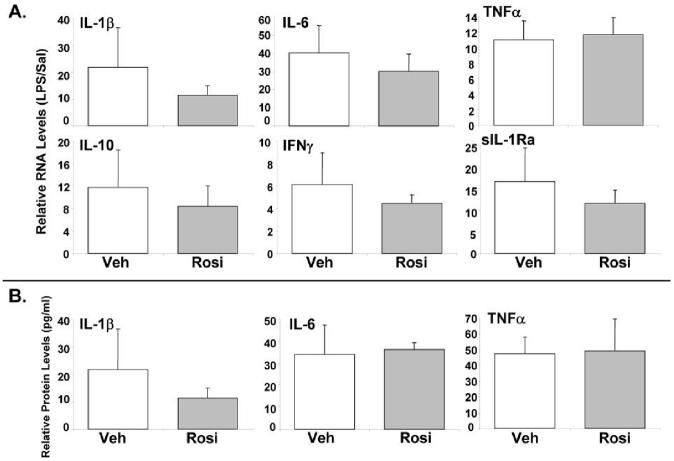
3.4. Rosi does not affect induction of hepatic cytokines by LPS
Rosi inhibits the expression of pro-inflammatory cytokines in cultured macrophages and in isolated KCs [18,20,22]. We hypothesized that the anti-inflammatory effects of Rosi in vivo were due to either impaired KC activity, or direct hepatocellular targeting. Compared to Veh/LPS, there was no reduction in IL-1β, TNFα, IL-6 and IFNγ RNA levels in Rosi/LPS mice. The induction of the anti-inflammatory cytokine, IL-10 and the secreted form of the IL-1 receptor antagonist (sIL-1Ra) by LPS was also not affected by Rosi (Fig. 3A). Plasma protein levels of the cytokines IL-1β, TNFα and IL-6 were significantly increased after LPS, but were not affected by Rosi (Fig. 3B). Thus, the effect of Rosi on LPS-mediated hepatic gene expression is not mediated by alteration of cytokine RNA and protein levels, but likely may be due to inhibition of inflammation-mediated signaling within hepatocytes.
Figure 3.
Rosiglitazone has no effect on the induction of cytokines by LPS. C57BL/6 male mice were gavage-fed 50 mg/kg/d of Rosi or corn-oil for 3 days. On day 3, the animals were IP injected with 2 mg/kg body weight of Salmonella LPS or saline and livers were harvested after 4 hours (n=5 per group). (A) RNA was isolated from the livers and analyzed by TaqMan real-time PCR. All data were presented as ± SD and standardized for cyclophilin RNA levels. The expression of the genes after LPS treatment is shown here. In case of vehicle pre-treatment, expression in saline-injected animals was set to 1, and fold change after LPS treatment was compared to vehicle controls. See supplemental information for primers and probes. (B) Plasma levels of the cytokines were determined by xMAP technology. In case of vehicle or Rosi pre-treatment, expression in saline-injected animals was set to 1, and fold change after LPS treatment was compared to vehicle or Rosi controls respectively. The asterisks indicate significant difference (p < 0.05).
3.5. Rosi attenuates cytokine-mediated nuclear export of RXR α in cell culture
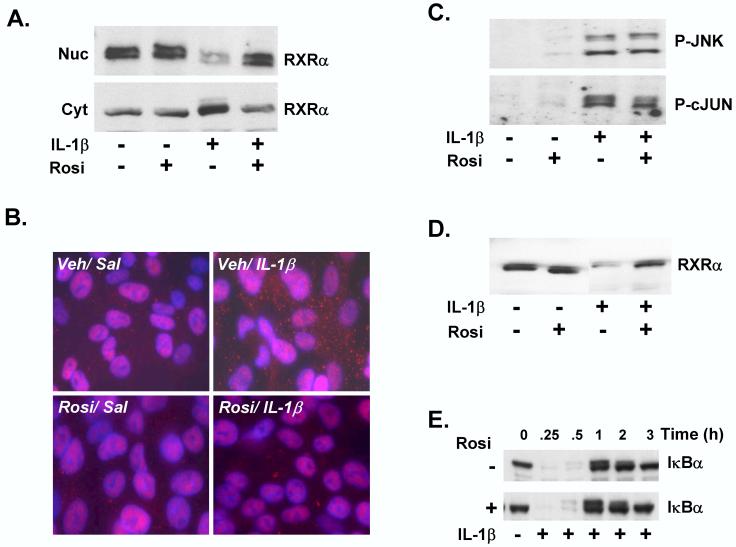
LPS administration to mice or cytokine treatment of HepG2 cells leads to rapid nuclear export of RXRα [14,16]. Since Rosi did not affect induction of cytokines by LPS (Fig. 3), we explored potential direct hepatocellular mechanisms by utilizing IL-1β-treated HepG2 cells, which models the negative effects of inflammation on RXRα-regulated gene expression [15]. IL-1β treatment of HepG2 cells resulted in rapid JNK-mediated nuclear export of RXRα, while short-term (30 min) pre-treatment with Rosi inhibited this export (Fig. 4A). Thus, Rosi inhibits IL-1β-mediated nuclear export of RXRα in HepG2 cells, in agreement with the in vivo results. This was confirmed by immunofluorescent analysis of HepG2 cells (Fig. 4B), where RXRα was detected in the cytosol after IL-1β treatment (Veh/IL-1β versus Veh/Sal). RXRα remains in the nucleus in Rosi/Sal-treated cells, indicating that Rosi by itself does not affect RXRα protein levels in the nucleus. RXRα was not detected in the cytosol in IL-1β-treated cells, which has been pre-treated with Rosi (Rosi/IL-1β), indicating that Rosi pre-treatment blocked nuclear export of RXRα by IL-1β.
Figure 4.
Rosiglitazone attenuates IL-1β -mediated RXRα nuclear export and degradation in vitro. HepG2 cells were pre-treated for 30 mins. with 10 μM Rosi or DMSO vehicle, followed by treatment with IL-1β (10 ng/ml) or vehicle control (0.0001% BSA in PBS) for 30 minutes. (A) Nuclear (Nuc) and cytosolic (Cyt) extracts were analyzed by immunoblotting with antibodies to RXRα to determine the effects of Rosi on subcellular localization of RXRα in the presence of IL-1β. (B) Immunofluorescence analysis of saline or IL-1β-treated HepG2 cells, pre-treated with vehicle or Rosi. The cells were stained with Alexa-Fluor-labeled antibody detecting RXRα, DAPI-staining of the nuclei, and the merged images are shown. (C) Nuclear protein levels of P-JNK (upper panel) or P-c-JUN were determined at 30 and 60 mins. of IL-1β treatment, preceeded by pre-incubation with Rosi. (D) Whole cell extracts (WCEs) were probed with RXRα antibodies to determine the effect of Rosi on protein levels of RXRα in total cell extracts, after IL-1β treatment. (E) Total cell lysates were prepared from HepG2 cells treated with DMSO or Rosi, prior to treatment with IL-1β from 0 - 3 hours. The samples were analyzed by immunoblotting with IκBα antibodies.
IL-1β-induced JNK activation contributed to reduced nuclear levels and activity of RXRα with the consequent down-regulation of target gene expression [14,15]. Rosi did not attenuate IL-1β-induced phosphorylation of JNK, or its substrate, c-jun, indicating that Rosi affects IL-1β mediated RXRα nuclear export without altering activation of the JNK pathway (Fig. 4C).
The fate of RXRα after nuclear export is unknown, but likely involves proteasome-mediated degradation in the cytosol [30]. In HepG2 cells, IL-1β treatment led to reduced RXRα levels in whole cell extracts, which was significantly reversed by pre-incubation with Rosi (Fig. 4D). These inhibitory effects on proteasome-dependent degradation of RXRα is unlikely due to a global, non-specific interference of proteasomal activity by Rosi, since Rosi had no effect on IL-1β-dependent degradation of prototypic proteasome target, IκBα [31] (Fig. 4E). These results demonstrate that Rosi prevents cytokine-mediated nuclear export of the central NR, RXRα, sequestering it in the nucleus, resulting in attenuation of suppression of hepatic genes during inflammation.
4. Discussion
Induction of the negative hepatic APR by inflammation is characterized by suppression of hepatic genes, resulting in broad defects in liver function. Effective treatment for inflammation-induced pathogenesis of liver diseases is lacking, and warrants continued exploration into novel mechanisms for therapeutic intervention. Agonists for PPAR family members have anti-inflammatory properties, although any role for direct effects on hepatocyte function is unknown. This study demonstrates that the PPARγ agonist, Rosiglitazone, attenuates the effects of inflammation on hepatic gene expression. The protective action of Rosi is in part mediated by blocking inflammation-mediated nuclear export of RXRα, the common and essential heterodimer partner for type II NRs [10]. Rosi pretreatment of mice led to a marked inhibition of the suppressive effects of LPS on target gene expression in liver, correlating with a mechanism involving retention of RXRα in the nucleus and maintenance of nuclear binding activities of RXRα-containing heterodimer pairs. Rosi had no effect on cytokine expression, suggesting a direct effect on cytokine-mediated cell signaling events in hepatocytes rather than the possibility of an indirect action by inhibiting cytokine-production by KCs. In IL-1β-treated HepG2 cells, short-term exposure to Rosi markedly attenuated IL-1β-mediated nuclear export of RXRα. Taken together, Rosi potentially and directly interferes with inflammation-based cell signaling pathways in liver cells.
One component of our initial working hypothesis was that Rosi blocked KC activation by LPS. This appears to be a minor player, since liver cytokine RNA and serum cytokine levels were unchanged by Rosi pre-treatment, raising the possibility that Rosi can act directly on hepatocytes. Rosi blocked inflammation-mediated RXRα nuclear export both in vivo and in vitro (Figs 2 & 4) without affecting JNK activation, as illustrated by comparable levels of P-JNK and P-cjun regardless of the presence of Rosi. This suggests that there is an effect of Rosi on phospho-JNK targeting of RXRα, or on the RXRα protein itself, rendering it less accessible to phosphorylation by activated JNK. There are several possibilities, involving either PPARγ-dependent or PPARγ-independent mechanisms. The anti-inflammatory effects of Rosi are unlikely to require Rosi-activated PPARγ-dependent gene expression, since short-term 30 minute pre-treatment in HepG2 cells blocked IL-1β’s effects on RXRα. Rosi’s effects may still involve PPARγ, perhaps via Rosi-activated PPARγ sequestering of RXRα in PPARγ:RXRα heterodimers, altering RXRα’s conformation, or the amount of non-dimerized RXRα in the nucleus accessible to activated JNK. Another possible role for Rosi and PPARγ involves direct association with other nuclear regulators, as has been reported whereby PPARγ associates with JunD in hepatic stellate cells to decrease JunD binding to the AP1 site [32]. Rosi-liganded PPARγ may associate with c-fos, c-Jun, JunD or other AP1-binding proteins, thus preventing one or more of these factors to bind to the AP1 promoter element. Since P-cJun levels were comparable in Rosi/LPS and Veh/LPS nuclear extracts, PPARγ binding to c-Jun is unlikely, however, association of PPARγ to other AP1-binding factors remain to be explored.
There are several possible PPARγ-independent mechanisms for Rosi’s anti-inflammatory actions [19,33,34]. In peritoneal macrophages derived from PPARγ conditional knockout mice, Rosi has both PPARγ-dependent and independent effects [35]. There is indirect evidence that some PPARγ-independent effects of Rosi might be mediated by the activation of PPARδ[35]. Further studies will determine roles for PPARγ in mediating the effects of Rosi.
Rosi attenuated the effects of LPS on hepatic genes without reducing expression of the cytokines IL-1β, TNFα and IL6. This was surprising, since PPARγ ligands inhibit production of inflammatory cytokines in monocytes and macrophages in culture, and macrophage-derived liver-resident KCs are the main producers of cytokines in vivo [18,20]. Furthermore, a specific PPARγ agonist inhibited LPS-induced TNFα production in cultured rat KCs, although any effect on mouse KCs remains to be determined [22]. Although the entire array of cytokines was not evaluated, the lack of effect on these critical and sentinel cytokines involved in hepatic APR indicates that the mode of action of Rosi in attenuating the effects of LPS does not appear to be at the level of inhibiting non-parenchymal cell expression of cytokines, nor excess production of anti-inflammatory cytokines. The lack of effect on cytokine levels in LPS-treated mice might, overall, be a positive attribute to Rosi, since cytokine-activated pathways are involved in the hepatic regenerative response, which, in the setting of liver cell damage, should likely be preserved to enhance overall healing from injury.
Recent studies demonstrate that the therapeutic effects of PPARγ ligands are not limited to their use as insulin-sensitizers, as many of these agents have beneficial effects in conditions associated with cardiovascular diseases and inflammation [34]. Animal models of liver cell damage and fibrosis are attenuated with PPARγ agonists [36], while a pilot study in patients with steatohepatitis have shown that Rosi improved the histology & laboratory abnormalities associated with this disease [23,37]. Given the current safety profile of this agent, it is tempting to consider Rosi and other PPARγ-agonists as potential anti-inflammatory agents for clinical trials in liver diseases where inflammation plays a role in pathogenesis.
Overall, we conclude that the PPARγ agonist, Rosi attenuates the effects of inflammation on hepatic gene expression by preventing the nuclear export of the central NR, RXRα. RXRα, as an obligate heterodimer with other class II NRs, regulates the expression of a broad array of genes involved in important physiological processes in the liver, many of which are impaired during the negative hepatic APR. Thus, Rosi may have a role in counteracting the pathophysiology of inflammation in chronic and acute liver disease.
Table 1.
| Gene | Acc. nr. | Forward primer | Reverse primer | Probe | Ref. |
|---|---|---|---|---|---|
| Ntcp | AB003303 | ATGACCACCTGCTCCAGCTT | GCCTTTGTAGGGCACCTTGT | CCTTGGGCATGATGCCTCTCCTC | (51) |
| Bsep | NM_021022 | CTGCCAAGGATGCTAATGCA | CGATGGCTACCCTTTGCTTCT | TGCCACAGCAATTTGACACCCTAGTTGG | (51) |
| Mrp2 | NM_013806 | GCTGGGAGAAATGGAGAATGTC | GACTGCTGAGGGACGTAGGCTA | TGGGCATATCACCATCAAGGGCTCC | (52) |
| Mrp3 | BC048825 | TCCCACTTTTCGGAGACAGTAAC | ACTGAGGACCTTGAAGTCTTGGA | CACCAGTGTCATTCGGGCCTATGGC | (53) |
| Cyp3a11 | X60452 | GGATGAGATCGATGAGGCTCTG | CAGGTATTCCATCTCCATCACAGT | CCAACAAGGCACCTCCCACGTATGA | |
| IL-1β | NM_008361 | CAACCAACAAGTGATATTCTCCATG | GATCCACACTCTCCAGCTGCA | CTGTGTAATGAAAGACGGCACACCCACC | (55) |
| TNFα | NM_013693 | CATCTTCTCAAAATTCGAGTGACAA | TGGGAGTAGACAAGGTACAACCC | CACGTCGTAGCAAACCACCAAGTGGA | (55) |
| sIL-1Ra | M57525 | CTCCTTCTCATCCTTCTGTTTCATT | GCATCTTGCAGGGTCTTTTCC | AGAGGCAGCCTGCCGCCCTT | - |
Acknowledgements
This work was supported by grants from the National Institutes of Health (DK56239 to SJK), the Texas Children’s Hospital Foundation & Public Health Service grant DK56338, which funds the Texas Gulf Coast Digestive Disease Center.
Glossary
The abbreviations used are:
- RXR
retinoid X receptor
- PPAR
peroxisome proliferator-activated receptor
- NR
nuclear receptor
- JNK
c-Jun N-terminal kinase
- LPS
lipopolysaccharide
- AP-1
activator protein-1
- Ntcp
sodium/taurocholate cotransporting polypeptide
- Bsep
Bile salt export pump
- Mrp
multi-drug resistance protein
- Lfabp
liver fatty acid binding protein
- Cyp3a11
cytochrome P450 3a11
- IL-1β
interleukin-1β
- IL-6
interleukin-6
- TNFα
tumor necrosis factor-α
- KC
Kupffer cells
- PCR
polymerase chain reaction
- APR
acute phase response
- DR
Direct Repeat
- IR
Inverted Repeat
- EMSA
electrophoretic mobility shift assay
Footnotes
This work was supported by grants from the National Institutes of Health (DK56239 to SJK), the Texas Children’s Hospital Foundation & Public Health Service grant DK56338, which funds the Texas Gulf Coast Digestive Diseases Center. JM was supported by the Ter Meulen Fund, Royal Netherlands Academy of Arts and Sciences and the European Society for Pediatric Research.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340(6):448–54. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- [2].Karpen SJ. Transcriptional Regulation of Hepatobiliary Transporters. Landes Bioscience; 2003. pp. 96–111. [Google Scholar]
- [3].Schletter J, Heine H, Ulmer AJ, Rietschel ET. Molecular mechanisms of endotoxin activity. Arch Microbiol. 1995;164(6):383–9. doi: 10.1007/BF02529735. [DOI] [PubMed] [Google Scholar]
- [4].Crawford JM, Boyer JL. Clinicopathology conferences: inflammation-induced cholestasis. Hepatology. 1998;28(1):253–60. doi: 10.1002/hep.510280133. [DOI] [PubMed] [Google Scholar]
- [5].Trauner M, Fickert P, Stauber RE. Inflammation-induced cholestasis. J Gastroenterol Hepatol. 1999;14(10):946–59. doi: 10.1046/j.1440-1746.1999.01982.x. [DOI] [PubMed] [Google Scholar]
- [6].Nakamura J, Nishida T, Hayashi K, Kawada N, Ueshima S, Sugiyama Y, Ito T, Sobue K, Matsuda H. Kupffer cell-mediated down regulation of rat hepatic CMOAT/MRP2 gene expression. Biochem Biophys Res Commun. 1999;255(1):143–9. doi: 10.1006/bbrc.1999.0160. [DOI] [PubMed] [Google Scholar]
- [7].Rizzardini M, Zappone M, Villa P, Gnocchi P, Sironi M, Diomede L, Meazza C, Monshouwer M, Cantoni L. Kupffer cell depletion partially prevents hepatic heme oxygenase 1 messenger RNA accumulation in systemic inflammation in mice: role of interleukin 1beta. Hepatology. 1998;27(3):703–10. doi: 10.1002/hep.510270311. [DOI] [PubMed] [Google Scholar]
- [8].Sturm E, Zimmerman TL, Crawford AR, Svetlov SI, Sundaram P, Ferrara JL, Karpen SJ, Crawford JM. Endotoxin-stimulated macrophages decrease bile acid uptake in WIF-B cells, a rat hepatoma hybrid cell line. Hepatology. 2000;31(1):124–30. doi: 10.1002/hep.510310120. [DOI] [PubMed] [Google Scholar]
- [9].Geier A, Dietrich CG, Voigt S, Kim SK, Gerloff T, Kullak-Ublick GA, Lorenzen J, Matern S, Gartung C. Effects of proinflammatory cytokines on rat organic anion transporters during toxic liver injury and cholestasis. Hepatology. 2003;38(2):345–54. doi: 10.1053/jhep.2003.50317. [DOI] [PubMed] [Google Scholar]
- [10].Karpen SJ. Nuclear receptor regulation of hepatic function. J Hepatol. 2002;36(6):832–50. doi: 10.1016/s0168-8278(02)00129-0. [DOI] [PubMed] [Google Scholar]
- [11].Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell. 1995;83(6):841–50. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- [12].Wu Y, Zhang X, Bardag-Gorce F, Robel RC, Aguilo J, Chen L, Zeng Y, Hwang K, French SW, Lu SC, Wan YJ. Retinoid X receptor alpha regulates glutathione homeostasis and xenobiotic detoxification processes in mouse liver. Mol Pharmacol. 2004;65(3):550–7. doi: 10.1124/mol.65.3.550. [DOI] [PubMed] [Google Scholar]
- [13].Wan YJ, An D, Cai Y, Repa JJ, Hung-Po Chen T, Flores M, Postic C, Magnuson MA, Chen J, Chien KR, French S, Mangelsdorf DJ, Sucov HM. Hepatocyte-specific mutation establishes retinoid X receptor alpha as a heterodimeric integrator of multiple physiological processes in the liver. Mol Cell Biol. 2000;20(12):4436–44. doi: 10.1128/mcb.20.12.4436-4444.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zimmerman TL, Thevananther S, Ghose R, Burns AR, Karpen SJ. Nuclear export of RXRalpha in response to IL-1beta-mediated cell signaling: Roles for JNK and SER260. J Biol Chem. 2006 doi: 10.1074/jbc.M508277200. [DOI] [PubMed] [Google Scholar]
- [15].Li D, Zimmerman TL, Thevananther S, Lee HY, Kurie JM, Karpen SJ. Interleukin-1 beta-mediated suppression of RXR:RAR transactivation of the Ntcp promoter is JNK-dependent. J Biol Chem. 2002;277(35):31416–22. doi: 10.1074/jbc.M204818200. [DOI] [PubMed] [Google Scholar]
- [16].Ghose R, Zimmerman TL, Thevananther S, Karpen SJ. Endotoxin leads to rapid subcellular re-localization of hepatic RXRalpha: A novel mechanism for reduced hepatic gene expression in inflammation. Nucl Recept. 2004;2(1):4. doi: 10.1186/1478-1336-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rocchi S, Auwerx J. Peroxisome proliferator-activated receptor-gamma: a versatile metabolic regulator. Ann Med. 1999;31(5):342–51. doi: 10.3109/07853899908995901. [DOI] [PubMed] [Google Scholar]
- [18].Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391(6662):82–6. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- [19].Castrillo A, Tontonoz P. Nuclear receptors in macrophage biology: at the crossroads of lipid metabolism and inflammation. Annu Rev Cell Dev Biol. 2004;20:455–80. doi: 10.1146/annurev.cellbio.20.012103.134432. [DOI] [PubMed] [Google Scholar]
- [20].Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391(6662):79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- [21].Dubuquoy L, Dharancy S, Nutten S, Pettersson S, Auwerx J, Desreumaux P. Role of peroxisome proliferator-activated receptor gamma and retinoid X receptor heterodimer in hepatogastroenterological diseases. Lancet. 2002;360(9343):1410–8. doi: 10.1016/S0140-6736(02)11395-X. [DOI] [PubMed] [Google Scholar]
- [22].Uchimura K, Nakamuta M, Enjoji M, Irie T, Sugimoto R, Muta T, Iwamoto H, Nawata H. Activation of retinoic X receptor and peroxisome proliferator-activated receptor-gamma inhibits nitric oxide and tumor necrosis factor-alpha production in rat Kupffer cells. Hepatology. 2001;33(1):91–9. doi: 10.1053/jhep.2001.21145. [DOI] [PubMed] [Google Scholar]
- [23].Neuschwander-Tetri BA, Brunt EM, Wehmeier KR, Oliver D, Bacon BR. Improved nonalcoholic steatohepatitis after 48 weeks of treatment with the PPAR-gamma ligand rosiglitazone. Hepatology. 2003;38(4):1008–17. doi: 10.1053/jhep.2003.50420. [DOI] [PubMed] [Google Scholar]
- [24].Guo YT, Leng XS, Li T, Zhao JM, Lin XH. Peroxisome proliferator-activated receptor gamma ligands suppress liver carcinogenesis induced by diethylnitrosamine in rats. World J Gastroenterol. 2004;10(23):3419–23. doi: 10.3748/wjg.v10.i23.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Itoh M, Adachi M, Yasui H, Takekawa M, Tanaka H, Imai K. Nuclear export of glucocorticoid receptor is enhanced by c-Jun N-terminal kinase-mediated phosphorylation. Mol Endocrinol. 2002;16(10):2382–92. doi: 10.1210/me.2002-0144. [DOI] [PubMed] [Google Scholar]
- [26].Timchenko NA, Harris TE, Wilde M, Bilyeu TA, Burgess-Beusse BL, Finegold MJ, Darlington GJ. CCAAT/enhancer binding protein alpha regulates p21 protein and hepatocyte proliferation in newborn mice. Mol Cell Biol. 1997;17(12):7353–61. doi: 10.1128/mcb.17.12.7353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Trauner M, Boyer JL. Bile salt transporters: molecular characterization, function, and regulation. Physiol Rev. 2003;83(2):633–71. doi: 10.1152/physrev.00027.2002. [DOI] [PubMed] [Google Scholar]
- [28].Karpen SJ. Transcriptional regulation of sinusoidal transporters. In: Matern S, Boyer J, Keppler D, MeierAbt P, editors. Hepatobiliary Transport: From Bench to Bedside. Kluwer Academic; London: 2001. pp. 22–31. [Google Scholar]
- [29].Sato O, Kuriki C, Fukui Y, Motojima K. Dual promoter structure of mouse and human fatty acid translocase/CD36 genes and unique transcriptional activation by peroxisome proliferator-activated receptor alpha and gamma ligands. J Biol Chem. 2002;277(18):15703–11. doi: 10.1074/jbc.M110158200. [DOI] [PubMed] [Google Scholar]
- [30].Tanaka T, Rodriguez de la Concepcion ML, De Luca LM. Involvement of all-transretinoic acid in the breakdown of retinoic acid receptors alpha and gamma through proteasomes in MCF-7 human breast cancer cells. Biochem Pharmacol. 2001;61(11):1347–55. doi: 10.1016/s0006-2952(01)00600-1. [DOI] [PubMed] [Google Scholar]
- [31].Zhang Y, Sun X, Muraoka K, Ikeda A, Miyamoto S, Shimizu H, Yoshioka K, Yamamoto K. Immunosuppressant FK506 activates NF-kappaB through the proteasome-mediated degradation of IkappaBalpha. Requirement for Ikappabalpha n-terminal phosphorylation but not ubiquitination sites. J Biol Chem. 1999;274(49):34657–62. doi: 10.1074/jbc.274.49.34657. [DOI] [PubMed] [Google Scholar]
- [32].Hazra S, Xiong S, Wang J, Rippe RA, Krishna V, Chatterjee K, Tsukamoto H. Peroxisome proliferator-activated receptor gamma induces a phenotypic switch from activated to quiescent hepatic stellate cells. J Biol Chem. 2004;279(12):11392–401. doi: 10.1074/jbc.M310284200. [DOI] [PubMed] [Google Scholar]
- [33].Chawla A, Barak Y, Nagy L, Liao D, Tontonoz P, Evans RM. PPAR-gamma dependent and independent effects on macrophage-gene expression in lipid metabolism and inflammation. Nat Med. 2001;7(1):48–52. doi: 10.1038/83336. [DOI] [PubMed] [Google Scholar]
- [34].Abdelrahman M, Sivarajah A, Thiemermann C. Beneficial effects of PPAR-gamma ligands in ischemia-reperfusion injury, inflammation and shock. Cardiovasc Res. 2005;65(4):772–81. doi: 10.1016/j.cardiores.2004.12.008. [DOI] [PubMed] [Google Scholar]
- [35].Welch JS, Ricote M, Akiyama TE, Gonzalez FJ, Glass CK. PPARgamma and PPARdelta negatively regulate specific subsets of lipopolysaccharide and IFN-gamma target genes in macrophages. Proc Natl Acad Sci U S A. 2003;100(11):6712–7. doi: 10.1073/pnas.1031789100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Marra F, DeFranco R, Robino G, Novo E, Efsen E, Pastacaldi S, Zamara E, Vercelli A, Lottini B, Spirli C, Strazzabosco M, Pinzani M, Parola M. Thiazolidinedione treatment inhibits bile duct proliferation and fibrosis in a rat model of chronic cholestasis. World J Gastroenterol. 2005;11(32):4931–8. doi: 10.3748/wjg.v11.i32.4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Neuschwander-Tetri BA, Brunt EM, Wehmeier KR, Sponseller CA, Hampton K, Bacon BR. Interim results of a pilot study demonstrating the early effects of the PPAR-gamma ligand rosiglitazone on insulin sensitivity, aminotransferases, hepatic steatosis and body weight in patients with non-alcoholic steatohepatitis. J Hepatol. 2003;38(4):434–40. doi: 10.1016/s0168-8278(03)00027-8. [DOI] [PubMed] [Google Scholar]