Abstract
The costimulatory molecules CD80 and CD86 (B7-1 and B7-2) are upregulated on mature antigen-presenting cells and interact with positive and negative regulators of CD8 T cell function, CD28 and CD152 (CTLA4) respectively. In this study we examined the role of CD80 and CD86 in the immune response to murine gammaherpesvirus-68 (MHV-68) using CD80/86−/− mice. As we had previously shown that CD28 (the only known activating receptor for CD80 and 86) is not essential for long-term control of MHV-68, we predicted that CD80 and 86 would also be dispensable for an effective response to this virus. However, surprisingly, we observed that CD80/86−/− mice failed to maintain effective long-term control of MHV-68 and showed viral reactivation in the lungs. We did not observe viral reactivation in mice deficient in either CD80 or CD86 alone, indicating that these molecules play overlapping roles in the long-term control of MHV-68. Antiviral antibody responses were dramatically reduced in CD80/86−/− mice, while CD8 T cell expansion and recruitment to the lungs were not significantly affected. The unexpected disparity in the requirement for CD28 and CD80/86 in the response to MHV-68 suggests that CD28 is not the only positive regulatory receptor for CD80/86.
Keywords: CD80, CD86, CD28, murine gammaherpesvirus, lung, CD8 T cell, antiviral antibody
Introduction
Murine gammaherpesvirus-68 (MHV-68) is a naturally-occurring rodent pathogen (Blaskovic et al., 1980) which is closely related to Epstein Barr virus (EBV) and the Kaposi’s sarcoma-associated human herpesvirus 8 (KSHV, HHV-8) (Efstathiou et al., 1990; Efstathiou, Ho, and Minson, 1990; Virgin et al., 1997). Intranasal (i.n.) administration of MHV-68 results in acute productive infection of lung alveolar epithelial cells and a latent infection in several cell types including B lymphocytes, dendritic cells, epithelia and macrophages (Flano et al., 2000; Stewart et al., 1998; Sunil-Chandra, Efstathiou, and Nash, 1992; Weck et al., 1999). Infectious virus is cleared from the lungs approximately 10 days after infection by a T cell-mediated process (Ehtisham, Sunil-Chandra, and Nash, 1993; Topham et al., 2001). The antibody response develops several weeks after infection (Stevenson and Doherty, 1998). Following the establishment of latency, viral control can be mediated by either T or B cell-dependent mechanisms (Kim et al., 2002; Stewart et al., 1998). While CD4 T cells are not essential for primary control of lytic MHV-68, they are required for long-term control and the virus reactivates in the lungs of CD4 T cell-deficient mice (Cardin et al., 1996).
As predicted by the two-signal hypothesis, both TCR-mediated and costimulatory signals are important in T cell activation during MHV-68 infection. Thus CD40-CD40L interactions appear to be critical for T cell-mediated control of MHV-68 (Brooks et al., 1999; Lee et al., 2002; Sarawar et al., 2001). CD40 ligation induces upregulation of CD80 and CD86 on antigen presenting cells (Cella et al., 1996). These molecules interact with CD28 resulting in T cell activation and CTLA4 (cytotoxic T lymphocyte antigen-4) resulting in inhibition of T cell function (reviewed in (Chambers et al., 2001; Sharpe and Freeman, 2002)). However, surprisingly, neither CD28 nor its downstream signaling molecule PKCθ appear to be essential for the T cell activation events required for either acute or long-term control of MHV-68 (Giannoni et al., 2005; Kim et al., 2002; Lee et al., 2002). In this study we examined the role of CD80/86 in the immune response to MHV-68 and, interestingly, discovered a CD28-independent role for these molecules in the long-term control of the virus.
Results
Differential requirement for CD80/86 and CD28 in the long-term control of MHV-68
Previous studies have shown that CD28−/− mice maintain effective long-term control of MHV-68, while mice lacking CD4 T cells or CD40 initially control the virus but later show viral reactivation in the lungs (Cardin et al., 1996; Kim et al., 2002; Lee et al., 2002). As CD28 is the only known activating receptor for CD80 and 86, we anticipated that long-term control of MHV-68 would also be independent of CD80 and 86. To test this assumption, wildtype and CD80/86−/− (double-deficient) mice were infected intranasally with MHV-68 and virus titers were determined in the lungs at days 16 and 50 after infection. As expected, no replicating virus was detected in the lungs of CD80/86−/− mice at day 16 after infection (Figure 1), showing that initial control of lytic virus was effective. Latent virus was also assessed in the spleens of wildtype and CD80/86−/− mice using an infectious center assay at day 16 after infection, which is when the peak number of latently-infected cells is observed in this viral model. The frequency of infectious centers in splenocytes from CD80/86−/− mice (856 ± 196, mean ± standard error) was not significantly different from that in wildtype mice (526 ± 73, mean ± standard error).
Figure 1. Differential requirement for CD80/86 and CD28 in the long-term control of MHV-68.

Groups of 3–5 mice were infected intranasally with 105PFU MHV-68. At days 16 or 50 after infection, lungs were harvested and virus titers were determined in lung homogenates by plaque assay. Data are expressed as PFU/0.1g of lung tissue from individual mice and are combined from 2 independent experiments. Horizontal bars represent the mean titer for each group. The detection limit of this assay is 10 PFU/0.1g of lung tissue.
However, surprisingly, CD80/86 −/− showed significant viral reactivation in the lungs at day 50 after infection (Figure 1), whereas no virus was detected in the lungs of wildtype (WT) or CD28−/− mice, confirming our earlier studies (Lee et al., 2002). Comparison with MHC Class II−/− mice suggested that the levels of replicating virus were slightly lower in the lungs of CD80/86−/− than in those of Class II −/− mice (Figure 1). Thus, these data reveal a differential requirement for CD80/86 and CD28 in the long-term control of MHV-68.
CD80 and CD86 play overlapping roles in the long-term control MHV-68
Our next question was whether CD80 and 86 played distinct or overlapping roles in the long-term control of MHV-68. To address this question we compared the long-term control of MHV-68 in mice that were deficient in either CD80 or CD86 or in both molecules. We also compared peak virus titers in the lungs of wildtype, CD80−/−, CD86−/− and CD80/86−/− mice at day 7 post-infection. Groups of wildtype, CD80−/−, CD86−/− or CD80/86−/− mice were infected intranasally with MHV-68 and lung virus titers were determined 7 or 50 days later. Although, on average, lung virus titers at day 7 post-infection appeared to be slightly higher for mice lacking CD80 or 86 than for wildtype mice (Figure 2), the difference was not statistically significant. The results at day 50 post-infection demonstrated that neither CD80−/− or CD86−/− mice showed reactivation of MHV-68 in the lungs (Figure 2), whereas replicating virus was detected in the lungs of CD80/86 −/− mice. Thus CD80 and CD86 appear to play overlapping roles in the long-term control of MHV-68.
Figure 2. CD80 and CD86 play overlapping roles in the long-term control MHV-68.

Groups of 3–4 wildtype (WT), CD80−/−, CD86−/− or CD80/86−/− mice were infected with MHV-68 and lung virus titers were determined at days 7 or 50 after infection as described in the legend to Figure 1.
Unaltered lymphocyte numbers and subset distribution in the lungs and spleen of CD80/86−/− mice
Widtype mice infected with MHV-68 develop splenomegaly and an inflammatory infiltrate in the lungs, comprising mainly T lymphocytes and monocyte/macrophages. To determine whether deficiency of both CD80 and 86 affects the lymphocyte expansion and recruitment that is necessary for these effects, cell numbers and the distribution of lymphocyte subsets were evaluated in the lungs and spleens of MHV-68-infected wildtype and CD80/86−/− mice. These parameters were examined at day 16, when the inflammatory infiltrate and splenomegaly are maximal and at day 50, when the number of inflammatory cells in the lung and splenocyte cell numbers have diminished considerably. Both wildtype and CD80/86−/− mice developed splenomegaly and inflammatory infiltrates in the lungs. There was no significant difference in the cell numbers in the spleen or bronchoalveolar lavage (BAL) of wildtype and CD80/86−/− mice at either day 16 or day 50 (Figure 3). Similarly there was no significant difference in lymphocyte subset distribution in the spleen or BAL between the two groups of mice at either time-point (Figure 4). These data suggest that CD80 and 86 are not essential for the lymphocyte activation events that are required for the induction of splenomegaly or for lymphocyte trafficking to the lung in response to MHV-68 infection.
Figure 3. Cell numbers in the bronchoalveolar lavage (BAL) and spleens of MHV-68-infected wildtype and CD80/86−/− mice.

Cell numbers in the BAL or spleen were determined at day 16 or 50 after infection with MHV-68. Single cell suspensions were prepared from the spleens of individual mice and viable cell counts were determined by Trypan Blue exclusion. Data are means ± standard deviations for groups of 3–5 mice at each time-point and are derived from 2 independent experiments for WT and CD80/86−/− mice and single experiments for the other groups. WT – wildtype; CII−/− MHC Class II −/−
Figure 4. Lymphocyte subsets in the BAL and spleens of MHV-68-infected wildtype and CD80/86−/− mice.
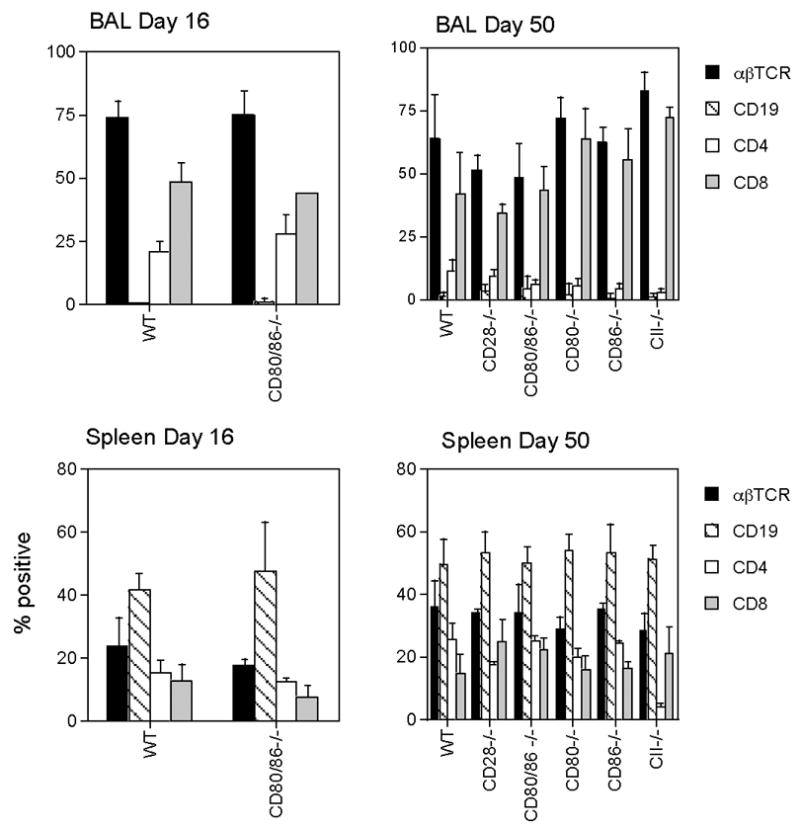
BAL or spleen cells were collected at days 16 and 50 after infection, and stained with PE or FITC-conjugated monoclonal antibodies. The resulting populations were analyzed by flow cytometry using a lymphocyte gate. Data are means ± standard deviations for groups of 3–5 mice at each time-point and are derived from 2 independent experiments for WT and CD80/86−/− mice and single experiments for the other groups. TCR- T cell receptor.
Frequency of virus-specific CD8 T cells is unaffected by lack of CD80/86
Although the total number of CD8 T cells was unaltered in CD80/86−/− mice following infection with MHV-68, it seemed possible that the viral reactivation observed in lungs of these mice reflected a reduction in the number of virus-specific CD8 T cells. To address this point, we compared the frequency of virus-specific CD8 T cells in the lungs and spleen of CD80/86−/− and wildtype mice to determine whether CD80 and CD86 were critical for the expansion and trafficking of virus-specific CD8 T cells in vivo. CD8 T cells were dual-stained with fluorochrome-conjugated anti-CD8 and MHC Class I peptide tetramers H2Kbp56 and H2-Kd p79, which bind to T cells that recognize two major MHV-68 epitopes. Our data showed that there was no significant difference in the frequency of virus-specific CD8 T cells in the lungs or spleens of wildtype and CD80/86−/− mice at either day 16 or day 50 after infection (Figure 5). Thus CD80 and 86 do not appear to be essential for MHV-68-specific CD8 T cell expansion or trafficking to the lung.
Figure 5. Frequency of virus specific CD8 T cells is unaffected by lack of CD80/86.
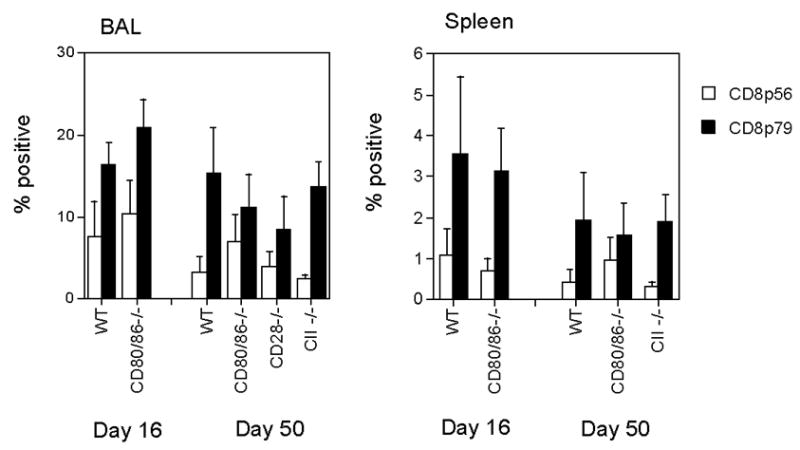
BAL or spleen cells were collected at days 16 and 50 after infection, and stained with PE-conjugated H-2Dbp56 or H-2Kbp79 MHC peptide tetramers and PerCP-conjugated monoclonal antibody to CD8. The resulting populations were analyzed by flow cytometry using a lymphocyte gate. Data are mean percentages of lymphocytes positive for both CD8 and tetramer staining + standard deviations, groups of 3–5 mice at each time-point and are derived from 2 independent experiments for WT and CD80/86−/− mice and single experiments for the other groups.
CD80 and 86 are essential for the MHV-68 specific antibody response
CD28-deficient mice show a significantly reduced antibody response to MHV-68 which is ineffective in preventing viral reactivation (Kim et al., 2002; Lee et al., 2002). To determine whether this was also the case in CD80/86−/− mice, MHV-68-specific serum antibody titers were determined 50 days after infection. There was a drastic reduction in virus-specific antibody titers in CD80/86−/− mice compared with those in wildtype mice (P<0.0001). Levels of virus specific antibody were close to baseline in the CD80/86−/− mice, demonstrating the critical role that these molecules play in antibody responses.
Discussion
Previous studies have shown that, while mice lacking CD4 T cells, CD40 or CD40L can initially clear replicating MHV-68 from the lungs, long-term control is compromised and viral reactivation is observed commencing 20–30 days after infection (Brooks et al., 1999; Cardin et al., 1996; Lee et al., 2002; Sarawar et al., 1997; Sarawar et al., 2001). Our previous studies showed that viral control can be restored in CD4 T cell deficient mice by agonistic antibodies to CD40 (Sarawar et al., 2001). This appears to be mediated by CD8 T cells. A possible explanation for these observations was that stimulation via CD40 upregulates CD80 and 86 on dendritic cells enabling activation of CD8 T cells via CD28. However, our subsequent studies showed that neither CD28 nor its downstream signalling molecule PKCθ are required for the long-term control of MHV-68 (Giannoni et al., 2005; Kim et al., 2002; Lee et al., 2002). As CD28 is the only known receptor for CD80/86 that mediates T cell activation, these studies suggested that CD80/86 would not play an essential role in the long-term control of MHV-68. However, although the present study shows that CD80 and 86 are dispensable for primary clearance of replicating virus, an unexpected role for these molecules in the long-term control of MHV-68 was revealed using CD80/86−/− mice. Our studies with mice deficient in either CD80 or 86 alone showed that these molecules play overlapping roles in the long-term control of MHV-68.
There are two possible explanations for the disparity in the requirement for CD28 and CD80/86 in the long-term control of MHV-68: 1. An unexpected stimulatory function for CTLA4 , which has previously been shown to be a negative regulator of T cell function 2. The existence of a second, currently unidentified stimulatory receptor for CD80/86. There is evidence in the literature from other models supporting both of these hypotheses.
A recent paper (Schneider et al., 2005) showed that CTLA4 engagement by CD80 upregulates lymphocyte-function associated antigen-1 (LFA-1), which is required for antigen-presenting cell function and T cell migration, suggesting a positive regulatory role in T cell function. However, a previous study by Sharpe and colleagues (Mandelbrot 2001) has demonstrated B7 (CD80/86)-dependent T cell costimulation in a model of cardiac allograft rejection in mice lacking both CD28 and CTLA4, suggesting the presence of an alternative receptor. Further work will be necessary to distinguish between these possible explanations for the differential requirement for CD28 and CD80/86 in long-term control of MHV-68.
Studies in other viral models have shown that CD80/86-mediated costimulation is important for the immune response to vesicular stomatitis virus (McAdam et al., 2000), herpes simplex virus (Thebeau and Morrison, 2002) and influenza (Liu et al., 1997; Lumsden et al., 2000), although no disparity in the requirement for CD80/86 and CD28 was reported. Both humoral and cell-mediated immunity was compromised by lack of B7 costimulation in these viral models. In the present study, there was no significant difference in the number or subset distribution of lymphocytes in the spleens or lungs of CD80/86−/− and wildtype mice. Furthermore there was no significant difference in the frequency of virus-specific CD8 T cells in the lungs or spleen. This suggests that CD80/86 is not required for the expansion of CD8 T cells or for lymphocyte trafficking to the lungs. In contrast, serum antiviral antibody titers were dramatically reduced in CD80/86−/− mice, confirming previous findings in other models (Borriello et al., 1997; McAdam et al., 2000; Thebeau and Morrison, 2003). However, MHV-68 reactivation is unlikely to result solely from failure to mount a virus-specific antibody response, as B cell-deficient mice are able to maintain effective long-term control of the virus (Usherwood et al., 1996) and do not show viral reactivation in the lungs. Stewart et al showed that, once latency has been established, either B cells, CD4 or CD8 T cells could prevent reactivation of MHV-68 in the lungs (Stewart et al., 1998). However, the CD8 T cells that develop in the absence of CD4 T cell help appear to be compromised in their ability to mediate long term control of the virus (Cardin et al., 1996). The basis of this defect is currently unknown since CTL activity, IFN-γ production and the frequency of virus specific CD8 T cells are not reduced (Sarawar et al., 2001; Stevenson et al., 1998). Thus it seems likely that viral reactivation that we observed in CD80/86−/− mice reflects defects in both T cell and antibody-mediated immunity.
In summary, our data show an unexpected disparity in the requirement for CD80/86 and CD28 in the long-term control of MHV-68. These data suggest that effective long-term control of this virus may be mediated via a T cell activation pathway that involves a previously unreported stimulatory function of CTLA4 or the interaction of CD80/86 with a novel receptor.
Materials and Methods
Mice
CD80−/−, CD86 −/−, CD80/86 −/− (double-deficient), CD28−/− and wildtype C57BL/6 controls were purchased from Jackson (Bar Harbor, Maine) Age and sex-matched 8–15 week old mice were used in all experiments. Mice were housed under specific pathogen-free conditions in the Animal Resource Center at TPIMS.
Viral infection and sampling
MHV-68 virus (Clone G2.4) (Blaskovic et al., 1980; Sunil-Chandra et al., 1992) was propagated in BHK-21 cells (ATCC CCL-10). Mice were anesthetized with Avertin (2,2,2 tribromoethanol) and infected intranasally with 105 PFU of the virus in PBS. At various intervals after infection, the mice were terminally anesthetized with Avertin and bled from the right axilla or vena cava. The inflammatory cells infiltrating the lung were harvested by bronchoalveolar lavage (BAL) via the trachea and single cell suspensions were prepared from the spleen. Following lavage, the lungs were removed and stored frozen at −80°C prior to virus titration.
Virus titration
Titers of replicating virus were determined by plaque assay on NIH 3T3 cells (ATCC CRL1658) as described previously (Cardin et al., 1996). Briefly, dilutions of stock virus, homogenized mouse tissues or homogenized splenocytes were adsorbed onto NIH 3T3 monolayers for 1hr at 37°C and overlaid with carboxymethyl cellulose (CMC). After 6 days, the CMC overlay was removed, the monolayers fixed with 10% formalin in PBS and stained with 1% Crystal Violet to facilitate determination of the number of plaques. The detection limit of this assay is 10 PFU /0.1g lung tissue or 8 PFU/107 splenocytes based on plaques recovered from homogenates of uninfected tissues spiked with known amounts of virus.
Infectious centers assay
Latent virus was assessed in splenocyte suspensions by an infectious centers assay as previously described (Cardin et al., 1996). Briefly, graded numbers of splenocytes from virus infected wildtype or CD80/86−/− mice were plated on monolayers of NIH 3T3 cells, incubated overnight at 37°C and the overlaid with CMC. 6 days later plaques representing viral reactivation from a single cell were counted after fixation and staining as described above. No replicating virus was detected in preparations of splenocytes disrupted by homogenization to prevent reactivation.
Flow Cytometric Analysis
Cells were stained with phycoerythrin (PE), fluorescein (FITC) or PerCP-conjugated monoclonal antibodies as previously described (Sarawar and Doherty, 1994). Antibodies were purchased from B.D. PharMingen (San Diego, CA.), E-Biosciences (San Diego, CA.) or Biolegend (San Diego, CA.). Isotype controls were included in each assay. In some experiments, cells were also stained with PE-conjugated MHC Class I peptide tetramers (H-2Kbp56 or H-2Dbp79) as previously described (Stevenson et al., 1999). Tetramers were obtained from the NIH tetramer facility (Atlanta, GA).
ELISA for virus-specific antibody
Serum antibody titers were determined by ELISA, as described previously (Lee et al., 2000), using peroxidase-labeled secondary reagents from Southern Biotechnology (Birmingham, Ala). Sera from uninfected mice and positive control sera were included in the assay.
Statistical Analysis
Data were analyzed with SigmaStat software (Jandel Scientific, St Rafael, CA) using Student’s t test or the Mann-Whitney Rank Sum test, depending on whether the data were normally distributed.
Figure 6. CD80 and 86 are essential for the MHV-68 specific antibody response.

Serum was collected from groups of 4 wildtype or CD80/86−/− mice 50 days after infection with MHV-68. Virus specific antibody responses were determined by ELISA. Data are expressed as mean A405 + standard deviation relative to a positive control serum sample (pooled from MHV-68-infected wildtype mice). There was a highly statistically significant difference between the antibody responses for the two groups (P<0.0001, Student’s t test).
Acknowledgments
This work was supported by grants from the NIH (AI50810) and from the Infectious Disease Science Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blaskovic D, Stancekova M, Svobodova J, Mistrikova J. Isolation of five strains of herpesviruses from two species of free living small rodents. Acta Virol. 1980;24(6):468. [PubMed] [Google Scholar]
- Borriello F, Sethna MP, Boyd SD, Schweitzer AN, Tivol EA, Jacoby D, Strom TB, Simpson EM, Freeman GJ, Sharpe AH. B7-1 and B7-2 have overlapping, critical roles in immunoglobulin class switching and germinal center formation. Immunity. 1997;6(3):303–13. doi: 10.1016/s1074-7613(00)80333-7. [DOI] [PubMed] [Google Scholar]
- Brooks JW, Hamilton-Easton AM, Christensen JP, Cardin RD, Hardy CL, Doherty PC. Requirement for CD40 ligand, CD4(+) T cells, and B cells in an infectious mononucleosis-like syndrome. J Virol. 1999;73(11):9650–4. doi: 10.1128/jvi.73.11.9650-9654.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin RD, Brooks JW, Sarawar SR, Doherty PC. Progressive loss of CD8+ T cell-mediated control of a gamma-herpesvirus in the absence of CD4+ T cells. J Exp Med. 1996;184(3):863–71. doi: 10.1084/jem.184.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184(2):747–52. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers CA, Kuhns MS, Egen JG, Allison JP. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annu Rev Immunol. 2001;19:565–94. doi: 10.1146/annurev.immunol.19.1.565. [DOI] [PubMed] [Google Scholar]
- Efstathiou S, Ho YM, Hall S, Styles CJ, Scott SD, Gompels UA. Murine herpesvirus 68 is genetically related to the gammaherpesviruses Epstein-Barr virus and herpesvirus saimiri. J Gen Virol. 1990;71:1365–72. doi: 10.1099/0022-1317-71-6-1365. [DOI] [PubMed] [Google Scholar]
- Efstathiou S, Ho YM, Minson AC. Cloning and molecular characterization of the murine herpesvirus 68 genome. J Gen Virol. 1990;71:1355–64. doi: 10.1099/0022-1317-71-6-1355. Pt 6. [DOI] [PubMed] [Google Scholar]
- Ehtisham S, Sunil-Chandra NP, Nash AA. Pathogenesis of murine gammaherpesvirus infection in mice deficient in CD4 and CD8 T cells. J Virol. 1993;67(9):5247–52. doi: 10.1128/jvi.67.9.5247-5252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flano E, Husain SM, Sample JT, Woodland DL, Blackman MA. Latent murine gamma-herpesvirus infection is established in activated B cells, dendritic cells, and macrophages. J Immunol. 2000;165(2):1074–81. doi: 10.4049/jimmunol.165.2.1074. [DOI] [PubMed] [Google Scholar]
- Giannoni F, Lyon AB, Wareing MD, Dias PB, Sarawar SR. Protein kinase C theta is not essential for T-cell-mediated clearance of murine gammaherpesvirus 68. J Virol. 2005;79(11):6808–13. doi: 10.1128/JVI.79.11.6808-6813.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim IJ, Flano E, Woodland DL, Blackman MA. Antibody-mediated control of persistent gamma-herpesvirus infection. J Immunol. 2002;168(8):3958–64. doi: 10.4049/jimmunol.168.8.3958. [DOI] [PubMed] [Google Scholar]
- Lee BJ, Reiter SK, Anderson M, Sarawar SR. CD28(−/−) mice show defects in cellular and humoral immunity but are able to control infection with murine gammaherpesvirus 68. J Virol. 2002;76(6):3049–53. doi: 10.1128/JVI.76.6.3049-3053.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BJ, Santee S, Von Gesjen S, Ware CF, Sarawar SR. Lymphotoxin-alpha-deficient mice can clear a productive infection with murine gammaherpesvirus 68 but fail to develop splenomegaly or lymphocytosis. J Virol. 2000;74(6):2786–92. doi: 10.1128/jvi.74.6.2786-2792.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wenger RH, Zhao M, Nielsen PJ. Distinct costimulatory molecules are required for the induction of effector and memory cytotoxic T lymphocytes. J Exp Med. 1997;185(2):251–62. doi: 10.1084/jem.185.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumsden JM, Roberts JM, Harris NL, Peach RJ, Ronchese F. Differential requirement for CD80 and CD80/CD86-dependent costimulation in the lung immune response to an influenza virus infection. J Immunol. 2000;164(1):79–85. doi: 10.4049/jimmunol.164.1.79. [DOI] [PubMed] [Google Scholar]
- McAdam AJ, Farkash EA, Gewurz BE, Sharpe AH. B7 costimulation is critical for antibody class switching and CD8(+) cytotoxic T-lymphocyte generation in the host response to vesicular stomatitis virus. J Virol. 2000;74(1):203–8. doi: 10.1128/jvi.74.1.203-208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarawar SR, Cardin RD, Brooks JW, Mehrpooya M, Hamilton-Easton AM, Mo XY, Doherty PC. Gamma interferon is not essential for recovery from acute infection with murine gammaherpesvirus 68. J Virol. 1997;71(5):3916–21. doi: 10.1128/jvi.71.5.3916-3921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarawar SR, Doherty PC. Concurrent production of interleukin-2, interleukin-10, and gamma interferon in the regional lymph nodes of mice with influenza pneumonia. J Virol. 1994;68(5):3112–9. doi: 10.1128/jvi.68.5.3112-3119.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarawar SR, Lee BJ, Reiter SK, Schoenberger SP. Stimulation via CD40 can substitute for CD4 T cell function in preventing reactivation of a latent herpesvirus. Proc Natl Acad Sci U S A. 2001;98(11):6325–9. doi: 10.1073/pnas.101136898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider H, Valk E, da Rocha Dias S, Wei B, Rudd CE. CTLA-4 up-regulation of lymphocyte function-associated antigen 1 adhesion and clustering as an alternate basis for coreceptor function. Proc Natl Acad Sci U S A. 2005;102(36):12861–6. doi: 10.1073/pnas.0505802102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2(2):116–26. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- Stevenson PG, Belz GT, Altman JD, Doherty PC. Virus-specific CD8(+) T cell numbers are maintained during gamma-herpesvirus reactivation in CD4-deficient mice. Proc Natl Acad Sci U S A. 1998;95(26):15565–70. doi: 10.1073/pnas.95.26.15565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson PG, Belz GT, Altman JD, Doherty PC. Changing patterns of dominance in the CD8+ T cell response during acute and persistent murine gamma-herpesvirus infection. Eur J Immunol. 1999;29(4):1059–67. doi: 10.1002/(SICI)1521-4141(199904)29:04<1059::AID-IMMU1059>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Stevenson PG, Doherty PC. Kinetic analysis of the specific host response to a murine gammaherpesvirus. J Virol. 1998;72(2):943–9. doi: 10.1128/jvi.72.2.943-949.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JP, Usherwood EJ, Ross A, Dyson H, Nash T. Lung epithelial cells are a major site of murine gammaherpesvirus persistence. J Exp Med. 1998;187(12):1941–51. doi: 10.1084/jem.187.12.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunil-Chandra NP, Efstathiou S, Arno J, Nash AA. Virological and pathological features of mice infected with murine gamma-herpesvirus 68. J Gen Virol. 1992;73:2347–56. doi: 10.1099/0022-1317-73-9-2347. [DOI] [PubMed] [Google Scholar]
- Sunil-Chandra NP, Efstathiou S, Nash AA. Murine gammaherpesvirus 68 establishes a latent infection in mouse B lymphocytes in vivo. J Gen Virol. 1992;73:3275–9. doi: 10.1099/0022-1317-73-12-3275. [DOI] [PubMed] [Google Scholar]
- Thebeau LG, Morrison LA. B7 costimulation plays an important role in protection from herpes simplex virus type 2-mediated pathology. J Virol. 2002;76(5):2563–6. doi: 10.1128/jvi.76.5.2563-2566.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thebeau LG, Morrison LA. Mechanism of reduced T-cell effector functions and class-switched antibody responses to herpes simplex virus type 2 in the absence of B7 costimulation. J Virol. 2003;77(4):2426–35. doi: 10.1128/JVI.77.4.2426-2435.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topham DJ, Cardin RC, Christensen JP, Brooks JW, Belz GT, Doherty PC. Perforin and Fas in murine gammaherpesvirus-specific CD8(+) T cell control and morbidity. J Gen Virol. 2001;82:1971–81. doi: 10.1099/0022-1317-82-8-1971. [DOI] [PubMed] [Google Scholar]
- Usherwood EJ, Stewart JP, Robertson K, Allen DJ, Nash AA. Absence of splenic latency in murine gammaherpesvirus 68-infected B cell-deficient mice. J Gen Virol. 1996;77:2819–25. doi: 10.1099/0022-1317-77-11-2819. [DOI] [PubMed] [Google Scholar]
- Virgin HWt, Latreille P, Wamsley P, Hallsworth K, Weck KE, Dal Canto AJ, Speck SH. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J Virol. 1997;71(8):5894–904. doi: 10.1128/jvi.71.8.5894-5904.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weck KE, Kim SS, Virgin HI, Speck SH. Macrophages are the major reservoir of latent murine gammaherpesvirus 68 in peritoneal cells. J Virol. 1999;73(4):3273–83. doi: 10.1128/jvi.73.4.3273-3283.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Fournier S, Allison JP, Sharpe AH, Hodes RJ. The role of B7 costimulation in CD4/CD8 T cell homeostasis. J Immunol. 2000;164(7):3543–53. doi: 10.4049/jimmunol.164.7.3543. [DOI] [PubMed] [Google Scholar]


