Abstract
Reduced levels of brain-derived neurotrophic factor (BDNF) in the hippocampus have been implicated in human affective disorders and behavioral stress responses. The current studies examined the role of BDNF in the behavioral consequences of inescapable stress, or learned helplessness. Inescapable stress decreased BDNF mRNA and protein in the hippocampus of sedentary rats. Rats allowed voluntary access to running wheels for either 3 or 6 weeks prior to exposure to stress were protected against stress-induced reductions of hippocampal BDNF protein. The observed prevention of stress-induced deceases in BDNF, however, occurred in a time course inconsistent with the prevention of learned helplessness by wheel running, which is evident following 6 weeks, but not 3 weeks, of wheel running. BDNF suppression in physically active rats was produced by administering a single injection of the selective serotonin reuptake inhibitor fluoxetine (10 mg/kg) just prior to stress. Despite reduced levels of hippocampal BDNF mRNA following stress, physically active rats given the combination of fluoxetine and stress remained resistant against learned helplessness. Sedentary rats given both fluoxetine and stress still demonstrated typical learned helplessness behaviors. Fluoxetine by itself reduced BDNF mRNA in sedentary rats only, but did not affect freezing or escape learning 24 hours later. Finally, bilateral injections of BDNF (1 μg) into the dentate gyrus prior to stress prevented stress-induced reductions of hippocampal BDNF but did not prevent learned helplessness in sedentary rats.
These data indicate that learned helplessness behaviors are independent of the presence or absence of hippocampal BDNF because blocking inescapable stress-induced BDNF suppression does not always prevent learned helplessness, and learned helplessness does not always occur in the presence of reduced BDNF. Results also suggest that the prevention of stress-induced hippocampal BDNF suppression is not necessary for the protective effect of wheel running against learned helplessness.
Keywords: wheel running, exercise, stress, depression, anxiety, BDNF
Introduction
Brain-derived neurotrophic factor (BDNF), the most highly expressed neurotrophin in the brain, is critically involved in maintenance of neuronal health and plasticity during development and adulthood (Barde, 1994, Lo, 1995). BDNF is highly expressed in the hippocampus, and growing evidence from both the human and animal literatures indicate that hippocampal BDNF is involved in the etiology and treatment of stress-related mood disorders including depression and anxiety (Lang et al., 2005, Duman and Monteggia, 2006). This idea is supported by numerous observations. Exposure to stressful events is a primary causal factor in the development of mood disorders including depression (Kendler et al., 1999, van Praag, 2005), and the hippocampus is particularly sensitive to stress. For example, stressor exposure inhibits dentate gyrus (DG) neurogenesis (Gould et al., 1998, Gould and Tanapat, 1999) and causes hippocampal neuronal atrophy (McEwen, 1999). These stress effects could contribute to the hippocampal volume reduction (Sheline et al., 1996, Sheline et al., 2003, Kitayama et al., 2005), as well as the hippocampal-dependent declarative memory impairments noted in patients with depression and some anxiety disorders (Sternberg and Jarvik, 1976, Burt et al., 1995, Elzinga and Bremner, 2002). Importantly, stressor exposure also reduces BDNF (Smith et al., 1995b, Ueyama et al., 1997) in the hippocampus. Thus, it has been suggested that a reduction in neurotrophic support contributes to stress-induced hippocampal damage (Smith et al., 1995a, Duman, 2004, Duman and Monteggia, 2006).
Further implicating hippocampal BDNF in stress-related mood disorders are reports that depressed suicide victims have reduced levels of BDNF in the hippocampus (Dwivedi et al., 2003) and this reduction may be reversed by chronic antidepressant treatment (Chen et al., 2001). Moreover, chronic administration of antidepressants can increase BDNF in the hippocampus and can prevent stress-induced decreases in hippocampal BDNF (Nibuya et al., 1995) and other damaging effects of stress on the hippocampus (see (Duman, 1998, Duman, 2004) for reviews). Both DG neurogenesis (Santarelli et al., 2003) and BDNF signaling (Saarelainen et al., 2003) are necessary for behavioral effects of antidepressants in animal models. Finally, BDNF injections into the hippocampus can produce antidepressant-like (Shirayama et al., 2002) and anxiolytic (Cirulli et al., 2004) effects in animal models. Together, these data support the hypothesis that reductions in levels of BDNF in the hippocampus could contribute to the pathogenesis of depression, whereas increases in BDNF could relieve depressive symptoms (Duman et al., 1997, Russo-Neustadt and Chen, 2005, Duman and Monteggia, 2006).
An obvious prediction allowed by this hypothesis is that manipulations that prevent or reduce depressive-like behaviors produced by a stressor should also prevent decreases in hippocampal BDNF produced by the same stressor. Moreover, the prevention of stress-induced BDNF suppression should be the primary mechanism by which the treatment reduces the behaviors. One factor that is known to reduce the incidence and severity of depression and anxiety is physical activity. Exercise has many stress-buffering effects (Fleshner, 2005) and it is possible that the antidepressant properties of exercise are mediated through resistance against the effects of stress on hippocampal BDNF. Indeed, voluntary wheel running in rats can prevent the development of depressive-like behaviors in animal models of depression that depend on exposure to stress (Dishman, 1997b, Solberg et al., 1999, Moraska and Fleshner, 2001, Greenwood et al., 2003, Greenwood et al., 2005a, Zheng et al., 2006). For example, we have reported that wheel running prevents behavioral consequences of inescapable tail shock stress (IS), or learned helplessness (LH; (Greenwood et al., 2003). Additionally, compared to sedentary rats, prior access to running wheels increases BDNF in the hippocampus (Neeper et al., 1995, Neeper et al., 1996, Russo-Neustadt et al., 1999, Van Hoomissen et al., 2003, Van Hoomissen et al., 2004) and prevents decreases in hippocampal BDNF produced by immobilization (Adlard and Cotman, 2004), swim stress (Russo-Neustadt et al., 2001), or chronic mild stress (Zheng et al., 2006). Wheel running also has other effects on the hippocampus including stimulating DG neurogenesis (van Praag et al., 1999b) and facilitating hippocampal-dependent learning and memory (van Praag et al., 1999a, Adlard et al., 2004, Van Hoomissen et al., 2004). At least some of the effects of exercise on hippocampal function are mediated through BDNF (Vaynman et al., 2004). Despite the clear benefits of wheel running, the role of BDNF in the behavioral effects of exercise in an animal model of stress-related mood disorders has yet to be determined.
The present set of experiments extend prior work investigating the role of BDNF in LH (Siuciak et al., 1997, Vollmayr et al., 2001, Shirayama et al., 2002, Itoh et al., 2004, Hoshaw et al., 2005, Schulte-Herbruggen et al., 2006) and the protective effect of exercise against stress-related mood disorders. Our approach employed stress, wheel running, acute administration of the selective serotonin (5-HT) reuptake inhibitor (SSRI) fluoxetine, or intra-DG microinjections of BDNF as means to manipulate levels of BDNF in the hippocampus. We hypothesized that 1) IS would decrease hippocampal BDNF in a time course consistent with LH behaviors, which are maximal 24 hours post-IS but are gone 72 hours post-IS (Maier and Watkins, 2005), 2) 6 weeks, but not 3 weeks, of wheel running would prevent the IS-induced suppression of BDNF because 6 weeks, but not 3 weeks, of wheel running prevents LH (Greenwood et al., 2005a), 3) BDNF suppression in the hippocampus of physically active animals would restore LH in these animals following IS, and 4) increasing BDNF levels in the hippocampus of sedentary rats by microinjecting BDNF into the DG would prevent LH. We also examined BDNF levels in the prefrontal cortex (PFC), because this area is known to be involved in behavioral responses to stress (Vermetten and Bremner, 2002, Amat et al., 2005) and to demonstrate stress-induced changes in BDNF opposite to those observed in the hippocampus (Bland et al., 2005, Lee et al., 2006, Bland, in press). Results indicate that LH is independent of levels of BDNF in the hippocampus and suggest that preventing the IS-induced suppression of hippocampal BDNF is not necessary for the protective effect of wheel running against LH.
Experimental Procedures
Animals
Adult, male Fischer F344 rats weighing an average of 258.6 ± 3.7 g just prior to stress or control treatment were housed in a specific pathogen free, temperature (22°C), and humidity-controlled environment and were maintained on a 12:12 hr light/dark cycle (lights on 0600–1800). All rats had ad libitum access to food and water and were weighed and handled weekly. Animals were allowed to acclimatize to these housing conditions for 1 week prior to any experimental manipulation. All animals were individually housed in Nalgene Plexiglas cages (45 × 25.2 × 14.7 cm). Rats in the wheel running groups were housed with running wheels attached to their cages. Wheels were rendered immobile with a metal stake during the acclimation period. Group sizes varied from 4 – 12/group. We used Fischer rats because they are inbred and yield a highly consistent exercise and stress response, and we have previously used Fischer rats to study the effects of wheel running on behavioral and neurochemical consequences of stress (Greenwood et al., 2005a). Care and use of the animals was in accordance with the University of Colorado Animal Care and Use Committee and studies were designed to minimize pain and the number of animals used.
Voluntary Wheel Running
Animals were randomly assigned to either remain sedentary or were allowed voluntary access to running wheels for either 3 weeks (3 wk Run), or 6 weeks (6 wk Run). At the start of a running cycle, the wheels in the cages of physically active rats were unlocked and these rats were allowed voluntary access to their wheels. Daily wheel revolutions were recorded digitally using Vital View software (Mini Mitter, Bend, OR) and distance was calculated by multiplying wheel circumference (1.081 m) by the number of wheel revolutions.
Inescapable Stress Protocol
Rats were randomly assigned to be exposed to either IS or control (no stress) treatment. Stressed rats were given 100, 5 sec tail shocks (1.5 mA) on a 1 min variable-interval schedule while restrained in Plexiglas tubes (23.4 cm long and 7.0 cm in diameter). The entire IS procedure lasted approximately 2 hours. All rats were stressed during their inactive (light) cycle, between 0800 and 1000. This tail shock protocol was used because tail shock is a consistent, quantifiable stressor that is known to produce LH (Maier and Watkins, 2005), and we know that wheel running prevents behavioral consequences of IS (Greenwood et al., 2003).
Behavioral Testing
The behavioral testing procedure was performed as previously described (Maier et al., 1993, Greenwood et al., 2003). A single test session lasted approximately 50 min and was performed by an observer blind to treatment condition of the animals. Both shuttle box escape learning and conditioned fear were tested 24 hr following IS or control treatment. Freezing was measured for the first 5 min after placement in the shuttle boxes (20"W × 10"D × 12"H; Coulbourn Instruments, Allentown, PA), during which time each rat was scored every 8 seconds as either freezing or not freezing. Freezing was defined as the absence of all movement except that required for respiration. Following this initial observation period, rats received 2, 0.6 mA foot shocks that could be terminated by crossing to the other side of the shuttle box once [fixed ratio-1 (FR-1) trials]. Following the two FR-1 trials, rats were observed again for 20 min and scored for freezing as before. Prior work has indicated that this freezing is a measure of fear that has been conditioned to contextual cues in the shuttle box (Fanslow and Lester, 1988). The post FR-1 observation period was followed by 3 more FR-1 escape trials and then by 25, FR-2 escape trials. During FR-2 trials rats were required to cross twice through the shuttle box door in order to terminate foot shock. Shocks occurred with an average intertrial interval of 60 sec and each shock was terminated after 30 sec if an escape response did not occur. The inability to escape within 30 seconds was defined as an escape failure.
Administration of Fluoxetine
The SSRI fluoxetine HCl (Sigma) was dissolved in saline (0.5 mg/ml) and injected i.p. at a dose of 10 mg/kg. Fluoxetine was administered to control rats or 30 minutes prior to commencement of IS in stressed rats. We chose this dose of fluoxetine because a single i.p. injection of 10 mg/kg of fluoxetine has been reported to reduce BDNF mRNA in the rat hippocampus (Coppell et al., 2003), likely due to 5-HT-induced suppression of hippocampal BDNF (Zetterstrom et al., 1999). We chose a non-surgical approach, as apposed to a surgical approach such as intra-hippocampal neurotrophic factor receptor blockade, to reduce BDNF because surgery decreases voluntary running (Morimoto et al., 2000, Greenwood et al., 2005a) and could thus interfere with the protective effects of wheel running.
Assessment of BDNF Protein
Rats were killed by decapitation and the hippocampus and PFC were hand dissected on a frosted glass plate over ice as previously described (Johnson et al., 2004, Johnson et al., 2005). Hippocampus and PFC were placed in microfuge tubes and quickly frozen in liquid nitrogen and stored at −80°C until quantification of BDNF protein. BDNF protein was quantified using E-Max ELISA kits (Promega, WI, USA) according to the manufacturer’s recommendations. Brain tissue was homogenized in lysis buffer containing 137 mM NaCl, 20 mM Tris-HCL (pH 8.0), 1% NP40, 10% glycerol, 1 mM phenylmethylsulfonyl fluoride, leupeptin (1 ug/ml), and sodium vanadate (0.5 mM). Samples containing the hippocampus were diluted 1:2 and frontal cortex samples were run undiluted. Samples were not acid treated. Total protein content of the samples was assessed by Bradford assay (Campisi et al., 2003) and the results are expressed as pg of BDNF per 100 μg of total protein.
Implantation of Cannula
Rats were anesthetized with isoflorane and implanted with bilateral guide cannula into the region of the DG. Twenty-six gauge stainless steel bilateral cannulas (Plastics One, Roanoke, VA) separated by 2.2 mm were implanted stereotaxically based on the atlas of Paxinos and Watson (Paxinos, 1998) and aimed 0.5 mm dorsal to the target region of the DG. Coordinates 0.5 mm dorsal to the target area were as follows: − 3.3 mm anteroposterior; +/− 1.1 mm mediolateral; and − 3.4 mm dorsoventral using Bregma as a reference. The guide cannula was fixed in place by flowing dental acrylic around the protruding exterior of the cannula and 4 anchoring screws (Plastics One). At the completion of surgery a sterile stainless steel bilateral dummy cannula (Plastics One) was inserted into the guide cannula to maintain potency of the cannula lumen. To verify cannula placements at the end of the experiment, rats were decapitated and brains were removed and stored at 4°C in 0.1 M phosphate buffer containing 30% sucrose. Brains were then sectioned on a cryostat and stained with cresyl violet. Rats with misplaced cannula were excluded from statistical analysis.
BDNF Microinjections
Rats received intra-DRN microinjections of human recombinant BDNF (Bachem Bioscience, King of Prussia, PA; 1.0 μg/hemisphere) or sterile saline 30 minutes prior to IS or control treatment (n = 12/group). This dose was chosen because 1.0 μg/hemisphere of BDNF injected into the DG has been reported to reverse deficits in shuttle box escape performance elicited by inescapable foot shocks (Shirayama et al., 2002). BDNF was dissolved in sterile saline and the injection volume was 1 μl/hemisphere. While holding each rat in a soft towel, the dummy cannula was removed and a 33 gauge bilateral microinjector (Plastics One) was inserted through the guide cannula. The microinjector was constructed so the tip protruded 0.5 mm beyond the ventral tip of the guide cannula. The microinjector was connected to two, 25 μl Hamilton syringes attached to Kopf model 5000 microinjector units (David Kopf Instruments, Tujunga, CA) via a length of polyethylene tubing (PE 20). The flow rate of drug was monitored on the microinjector units and was set to 0.5 μl/min. The microinjector was left in place for an additional 2 minutes following drug injection. Upon completion of the procedure a dummy cannula was replaced into the guide cannula. The DG was targeted for BDNF injections because the DG is the sight in the hippocampus where both IS and wheel running produce their greatest effects on BDNF mRNA (current results).
Prior work has shown that 1.0 μg of BDNF injected into the DG remains within the DG for at least 24 hours (Shirayama et al., 2002). Therefore, we chose to inject BDNF prior to IS in this experiment so that high levels of BDNF would be present both during IS and during behavioral testing 24 hours later. To verify that elevated BDNF protein levels were indeed present in the hippocampus 24 hours following IS in BDNF injected rats, a subset of cannulated rats (n = 3 – 4/group) received an injection of either BDNF (1.0 μg/hemisphere) or saline 30 minutes prior to control or IS treatment and were sacrificed 24 hours later. The hippocampus was hand dissected and divided into dorsal (containing the DG) and ventral sections. BDNF protein was quantified using ELISA (Promega), which recognizes both human and rat BDNF protein.
In Situ Hybridization
Following decapitation, brains were removed, frozen rapidly in isopentane and dry ice (−40 to −50°C), and stored at −80°C until sliced into 10 °m coronal sections on a cryostat. Slices through the hippocampus or frontal cortex were thaw-mounted directly onto polylysine-coated slides and stored at −80°C until processed for single-labeled radioactive in situ hybridization as described elsewhere (Day and Akil, 1996, Greenwood et al., 2003, Greenwood et al., 2005b). Briefly, sections were fixed in 4% PF for 1 hr, acetylated in 0.1 M triethenolamine containing 0.25% acetic anhydride (10 min), and dehydrated in graded alcohol. cRNA ribroprobes (courtesy of Dr. Stanley Watson, University of Michigan, Ann Arbor) complementary to BDNF were prepared from cDNA subclones in transcription vectors and labeled with (35S)UTP (AmershamPharmaciaBiotech, Piscataway, NJ), using standard transcription methods. Riboprobes were diluted in 50% hybridization buffer containing 50% formamide, 10% dextran sulfate, 2X saline sodium citrate (SSC), 50 mM PBS (pH = 7.4), 1X Denhardt’s solution, and 0.1 mg/ml yeast tRNA. Brain sections were hybridized with the probe overnight at 55ºC. The next day, sections were washed in 2X SSC, treated with RNase A (200 °g/ml) for 1 hr at 37°C, and washed to a final stringency of 0.1X SSC at 65°C for 1 hr. Dehydrated, air-dried sections were exposed to x-ray film (Biomax-MR; Eastman Kodak, Rochester, NY) for 6 days (hippocampus) or 10 days (PFC).
Image Analysis
Levels of BDNF mRNA were analyzed by computer-assisted optical densitometry. Brain section images were captured digitally (CCD camera, model XC-77; Sony, Tokyo, Japan), and the relative optical density of the x-ray film was determined using scion image version 4.0. A macro was written that enabled signal above background to be automatically determined. For each section, a background sample was taken over an area of white matter, and the signal threshold was calculated as mean gray value of background +3.5 SD. The section was automatically density-sliced at this value, so that only pixels with gray values above these criteria were included in the analysis. Results are expressed as mean integrated density, which reflects both the signal intensity and the number of pixels above assigned background (mean signal above background X number of pixels above background). Quantification of BDNF mRNA in the hippocampus occurred from −3.30mm to −4.52mm posterior to bregma according to Paxinos and Watson (1998). Both left and right DG, CA3, CA2, and CA1 regions were analyzed independently. Quantification of BDNF mRNA in the PFC occurred between 3.2mm to 2.2mm anterior to bregma (Paxinos, 1998). The cingluate cortex, prelimbic cortex, and infralimbic cortex were analyzed independently. Analysis of the subregions of the hippocampus and PFC occurred in 3 – 4 brain sections from each rat. The values obtained from each section were averaged to yield the integrated density value for that subject.
Statistical Analysis
Group sizes for each analysis are depicted in each graph. Group differences in the time course effect of IS on BDNF protein in the hippocampus and PFC were assessed with ANOVA. Two (no stress, IS) by 2 (sedentary, exercise) within subjects ANOVA was used to determine the presence of group differences in BDNF protein levels in the hippocampus and PFC following different durations of wheel running in the presence or absence of stress. BDNF mRNA was analyzed with 2 (sedentary, 6 wk Run) by 2 (saline, fluoxetine) by 2 (no stress, IS) within subjects ANOVA. FR-1 latencies were compared using ANOVA. Freezing data was collapsed into 10, 2 minute blocks and analyzed with repeated measures ANOVA. Freezing data over the entire 20 minutes was also averaged and compared across groups with ANOVA. Similarly, escape latencies were collapsed into 5 blocks of 5 FR-2 trials and analyzed with repeated measures ANOVA. Escape latencies for all 25 trials were also averaged and analyzed with ANOVA. Finally, group differences in the total number of FR-2 failures were assessed with ANOVA. Two (saline, BDNF) by 2 (no stress, IS) ANOVA was used to compare the levels of BDNF protein in the dorsal and ventral hippocampus 24 hours following BDNF or saline microinjection into the DG. Fisher’s protected least significant difference (Fisher’s-PLSD) post hoc analyses were performed when required. Alpha was set at p ≤ .05 for each analysis.
Results
The effects of IS on BDNF protein in the hippocampus and PFC
To determine the effects of IS on BDNF protein, rats were sacrificed 2, 4, 6, 24, or 72 hours following IS or control treatment in a time-matched design. As predicted, IS reduced the levels of BDNF protein in the hippocampus (Figure 1A). ANOVA revealed a significant effect of group (F (5,39) = 2.93; p < .05) on BDNF protein levels. A significant reduction in BDNF protein levels was not observed until 24 hours after IS, although there was a trend for a reduction at 6 hours (p = .08). BDNF protein levels remained suppressed 72 hours following IS. Post hoc analysis (p < .05) revealed that BDNF protein levels in both the 24 and 72 hour groups were significantly lower than the no stress and the 2 hour groups. No other group differences were observed.
Figure 1.
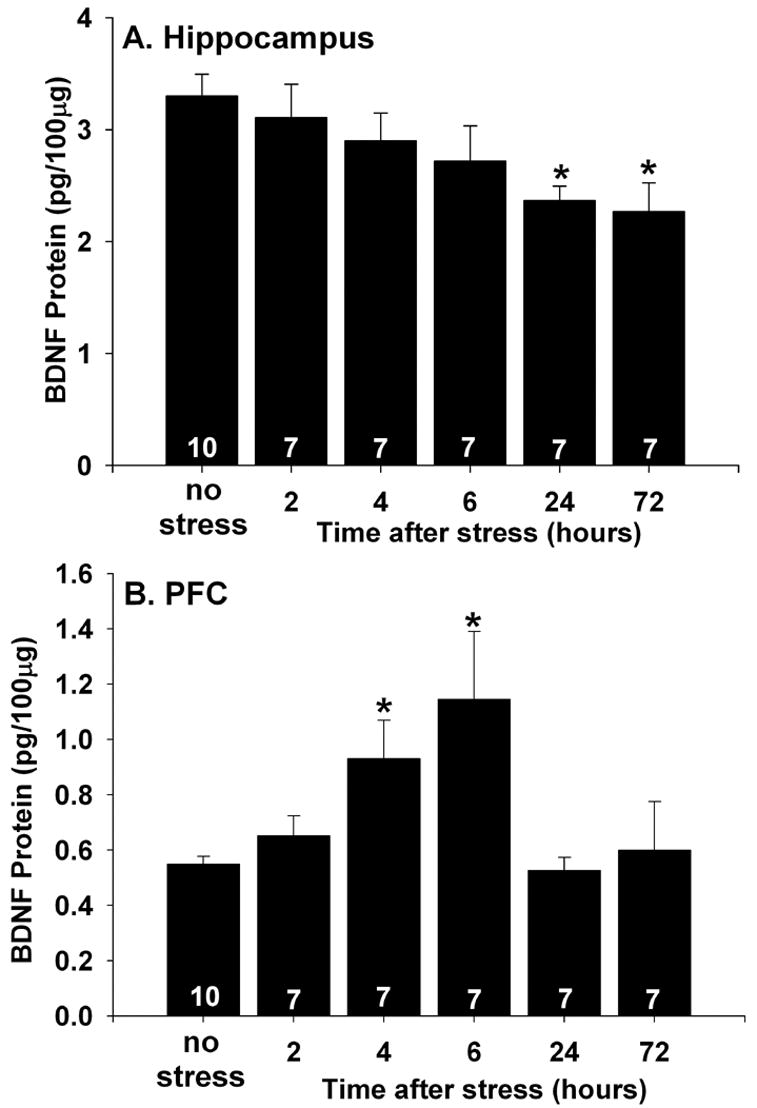
(A and B) Effects of inescapable tail shock stress (stress) on brain-derived neurotrophic factor (BDNF) protein measured by ELISA. Rats (7 – 10/group) were decapitated 2 hours, 4 hours, 6 hours, 24 hours, or 72 hours after exposure to stress or control treatment (no stress) in a time matched design. (A) Stress reduced BDNF protein levels in the hippocampus 24 and 72 hours following stress exposure. (B) Stress increased BDNF protein levels in the prefrontal cortex (PFC) 4 and 6 hours later. Bars represent means ± standard errors. The number in each bar represents the number of rats included in that group. * p < .05 relative to the no stress group.
The effect of IS on BDNF protein levels in the PFC was also examined. In contrast to the effect of IS on hippocampal BDNF, IS increased BDNF protein levels in the PFC (Figure 1B). A significant effect of group on PFC BDNF protein was revealed by ANOVA (F (5,39) = 3.54; p < .05). Subsequent post hoc tests (p < .05) indicated that the 4 hour group differed from the no stress and the 2 hour groups. The 6 hour group differed from all the groups except the 4 hour group. No other group differences were observed.
Wheel running modulates the effect of IS on BDNF protein
Rats in all experiments displayed running behavior similar to what we have previously reported in Fischer rats (Greenwood et al., 2005a, Greenwood et al., 2005b). Rats increased their running distances from 6.3 ± 0.57 km/week during the first week to 18.2 ± 1.9 km/week by the 3rd week of running. Rats maintained this level of running distance between the 3rd and 6th week of running. Rats that ran for 3 weeks ran an average of 1.8 ± 0.19 km/day and rats that ran for 6 weeks ran an average of 2.1 ± 0.22 km/day. Weekly distances run by 3 wk Run and 6 wk Run animals were not significantly different from each other (data not shown).
Wheel running could prevent the behavioral consequences of IS by attenuating the IS-induced suppression of hippocampal BDNF. Sedentary, 3 wk Run, and 6 wk Run rats were either exposed to IS or no stress. Rats were sacrificed 24 hours later (the time point at which we have previously observed the behavioral consequences of IS and the protective effect of wheel running against LH; (Greenwood et al., 2003)) and BDNF protein in the hippocampus and PFC were quantified using ELISA.
Figure 2A indicates that the effect of IS on hippocampal BDNF depends on the history of prior exercise. IS caused a suppression of BDNF protein levels in the hippocampus of sedentary rats measured 24 hours after IS. Both 3 and 6 weeks of wheel running prevented the IS-induced suppression of hippocampal BDNF levels. These results were confirmed with two, 2 (sedentary, 3 wk Run or 6 wk Run) by 2 (no stress, IS) ANOVAs which revealed significant main effects of activity (sedentary, 3 wk Run) F (1,28) = 6.8; p < .05; sedentary, 6 wk Run, F (1,28) = 16.01; p <.001) and a significant interaction between activity (sedentary, 3 wk Run) and stress (F (1,28) = 5.15; p < .05) on hippocampal BDNF protein levels. Results of subsequent post hoc analysis are shown in Figure 2. No significant effects of exercise or IS on BDNF protein levels in the PFC were observed (F (5, 42) = .42; p > .05; Figure 2B).
Figure 2.
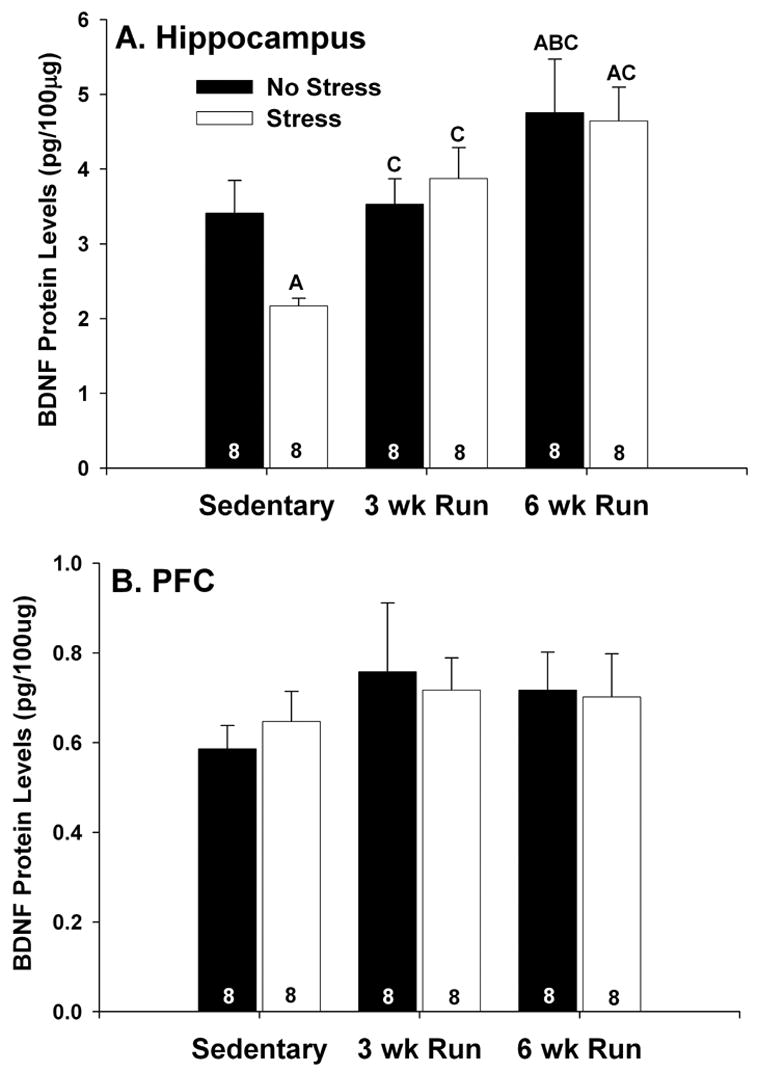
(A and B) Effects of 3 (3 wk Run) and 6 weeks (6 wk Run) of wheel running on stress-induced suppression of brain derived neurotrophic factor (BDNF) protein. Following the assigned duration of sedentary living or wheel running, rats (8/group) were either left undisturbed in their home cages (no stress) or were exposed to inescapable tail shock (stress). Rats were decapitated 24 hours later and BDNF protein was measured with ELISA. (A) Stress reduced BDNF protein in the hippocampus of sedentary rats. Six weeks, but not 3 weeks, of wheel running increased basal levels of BDNF protein in the hippocampus. Both 3 and 6 weeks of wheel running blocked the suppressive effect of stress on hippocampal BDNF protein. (B) Neither wheel running nor stress had any effect on BDNF protein in the prefrontal cortex (PFC). Bars represent means ± standard errors. The number in each bar represents the number of rats included in that group. A p < .05 relative to sedentary/no stress; B p < .05 relative to 3 wk Run/no stress; C p < .05 relative to sedentary/stress.
The effects of fluoxetine and IS on BDNF mRNA in the hippocampus and PFC
Prior work indicates that a single systemic injection of the SSRI fluoxetine can reduce BDNF mRNA levels in the hippocampus (Coppell et al., 2003). We therefore evaluated the possibility that fluoxetine, given alone or in combination with IS; would reduce BDNF levels in the hippocampus of physically active animals. Sedentary and 6 wk Run rats received a single injection of saline or fluoxetine 30 minutes prior to the onset of IS or control (no stress) treatment (8/group). Rats were sacrificed 6 hours after IS termination and BDNF mRNA was quantified in the hippocampus and PFC. We quantified BDNF mRNA in this experiment to allow group comparisons of distinct anatomical subregions of the hippocampus. Actual group sizes vary because of disruptions in tissue integrity incurred during tissue processing.
Autoradiographs showing the effects of IS, fluoxetine, and the combination of IS and fluoxetine on BDNF mRNA in hippocampal subfields of sedentary and 6 wk Run animals are shown in Figure 3. The greatest expression of BDNF mRNA was observed in the dentate gyrus and CA3 regions. Six weeks of wheel running elevated basal levels of BDNF mRNA in the DG, but not other hippocampal subfields. Exposure to IS reduced BDNF mRNA levels in all hippocampal subfields of sedentary rats. 6 wk of wheel running blocked IS-induced BDNF mRNA suppression in the DG, CA3, and CA2, but not CA1. In sedentary rats, fluoxetine, by itself, reduced BDNF mRNA levels in the DG and CA3, but not CA2 or CA1. Fluoxetine, by itself, had no effect on BDNF mRNA in the hippocampus of physically active animals. The combination of IS and fluoxetine reduced BDNF mRNA levels in all hippocampal subfields, regardless of physical activity. These results were confirmed with 2 (sedentary, 6 wk Run), by 2 (no stress, IS), by 2 (saline, fluoxetine) between subjects ANOVA. In the DG (Figure 4A), there were significant main effects of exercise (F (1, 56) = 33.57; p < .0001), stress (F (1, 56) = 7.96; p < .05), and drug treatment (F (1, 56) = 5.63; p < .05) on BDNF mRNA. There was also a significant interaction between exercise, stress, and drug treatment (F (1, 56) = 5.55; p < .05). No other interactions were significant. In CA3 (Figure 4B), there were reliable main effects of exercise (F (1, 53) = 4.01; p < .05), stress (F (1, 53) = 5.42; p < .05), and drug treatment (F (1, 53) = 6.53; p < .05) on BDNF mRNA levels. The interaction between exercise, stress, and drug treatment was also significant (F (1, 53) = 5.3; p < .05). In CA2 (Figure 4C), only the main effect of stress (F (1, 50) = 4.96; p < .05) and the interaction between exercise and stress (F (1,50) = 4.07; p < .05) were significant. Finally, in CA1 (Figure 4D), there was a main effect of stress (F (1, 52) = 11.21; p < .05). No other main effects or interactions were reliable in CA1. Results of post hoc analyses are shown in Figure 4.
Figure 3.
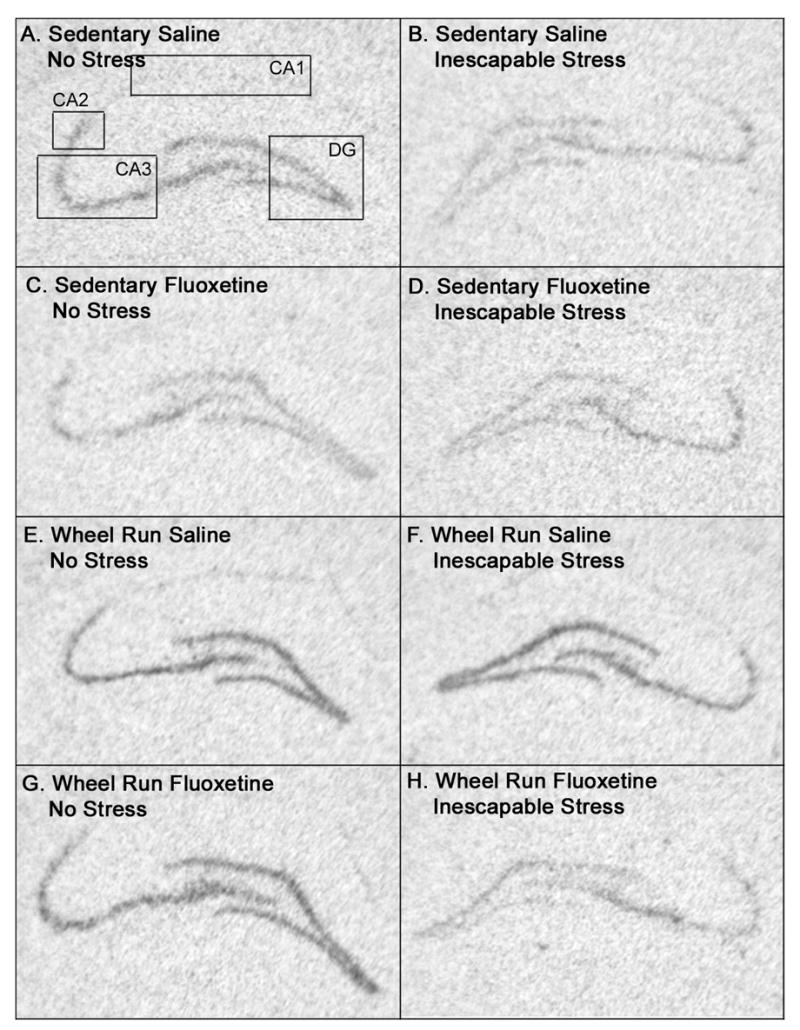
(A–H) Representative autoriadiographic coronal sections through the hippocampus showing the relative levels of brain derived neurotrophic factor (BDNF) mRNA in the dentate gyrus (DG), CA3, CA2, and CA1 regions of the hippocampus betweens groups of sedentary (A–D) or 6 wk wheel run (EH) animals 6 hours after exposure to control (no stress) treatment (left column) or inescapable tail shock stress (right column) in the presence of either saline or fluoxetine (10 mg/kg). Note that, relative to non-stressed sedentary rats treated with saline (A), sedentary rats treated with stress alone (B), fluoxetine in the absence of stress (C), and fluoxetine plus stress (D) all demonstrate lower levels of BDNF mRNA. In contrast, 6 weeks of wheel running alone (E) increased hippocampal BDNF mRNA levels relative to sedentary rats. Neither stress (F) nor fluoxetine (G), by themselves, altered BDNF mRNA levels in physically active rats. The combination of fluoxetine plus stress reduced BNDF mRNA levels in the hippocampus of wheel run animals (H) to a level comparable to that of sedentary counterparts (compare H and D). The boxes in A outline regions of the hippocampus where quantification of BDNF mRNA occurred.
Figure 4.
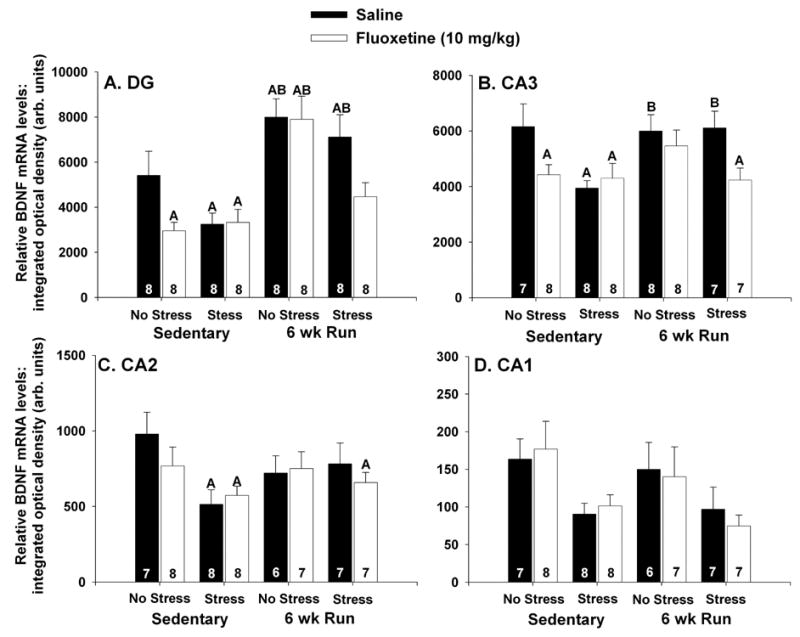
(A–D) Effects of stress and fluoxetine (10 mg/kg) on brain-derived neurotrophic factor (BDNF) mRNA in hippocampal subfields of sedentary rats and rats allowed access to running wheels for 6 weeks (6 wk Run). Rats (8/group) received an i.p. injection of either saline or fluoxetine 30 minutes prior to control treatment (no stress) or inescapable tail shock stress (stress). Rats were decapitated 6 hours later and BDNF mRNA was measured with in situ hybridization. In the dentate gyrus (DG) (A) and CA3 (B), both fluoxetine alone, stress alone, and the combination of fluoxetine and stress reduced BDNF mRNA levels in sedentary rats. Six weeks of wheel running increased basal levels of BDNF mRNA in the DG only, and blocked the effects of fluoxetine and stress, by themselves, on BDNF mRNA in the DG and CA3. The combination of fluoxetine and stress reduced BDNF mRNA in the DG and CA3 of 6 wk Run rats. (C) Fluoxetine by itself had no effect on BDNF mRNA levels in CA2. Stress reduced BDNF mRNA in the CA2 of sedentary rats only. The combination of fluoxetine and stress reduced BDNF mRNA in the CA2 of both sedentary and 6 wk Run animals, relative to sedentary controls. (D) Stress reduced BDNF mRNA levels in CA1 in both sedentary and 6 wk Run rats. Flouxetine had no effect on BDNF mRNA levels in the CA1. Bars represent means ± standard errors. The number in each bar represents the number of rats included in that group. A p < .05 relative to sedentary/saline/no stress; B p < .05 relative to 6 wk Run/fluoxetine/stress.
Figure 5 illustrates subregions of the PFC where analysis of BDNF mRNA took place. We did not observe any group differences in BDNF mRNA in any of the subregions of the PFC analyzed so the 3 subregions were averaged together. Neither wheel running, fluoxetine, nor IS had any effect of BDNF mRNA levels in the PFC (Figure 6).
Figure 5.
Autoradiographic coronal section through the prefrontal cortex (PFC) of a sedentary rat processed with in situ hybridization for brain derived neurotrophic factor (BDNF) mRNA. Outlined in boxes are the subregions of the PFC where quantification of BDNF mRNA occurred. 1, cingulate cortex; 2, prelimbic cortex; 3, infralimbic cortex.
Figure 6.
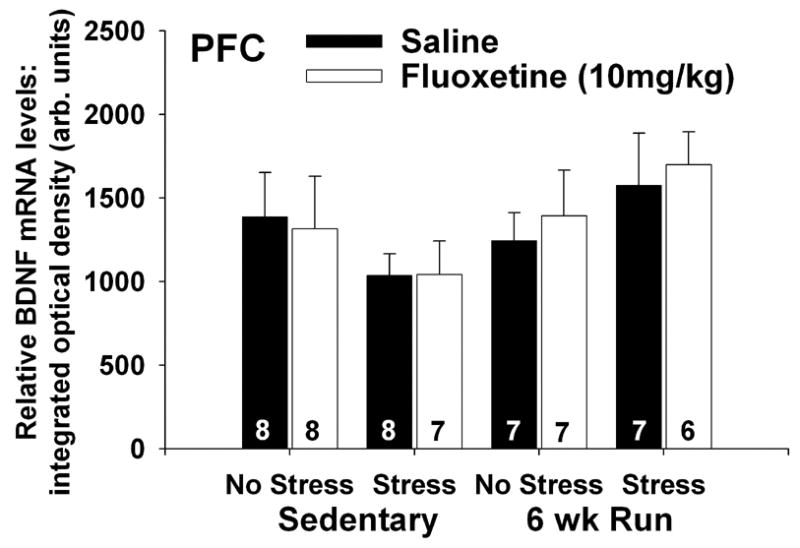
Effects of stress and fluoxetine on brain-derived neurotrophic factor (BDNF) mRNA in the prefrontal cortex (PFC) of sedentary rats and rats allowed access to running wheels for 6 weeks (6 wk Run). Rats (8/group) received either saline or fluoxetine (10 mg/kg i.p.) 30 minutes prior to control treatment (no stress) or inescapable tail shock stress (stress). Rats were decapitated 6 hours later and BDNF mRNA was measured with in situ hybridization. Neither wheel running, fluoxetine, or stress had any effect on BDNF mRNA in the PFC. Bars represent means ± standard errors. The number in each bar represents the number of rats included in that group.
The effects of fluoxetine and IS on LH behaviors
To determine if elevated levels of hippocampal BDNF mRNA following stress are required for the protective effect of wheel running against LH, fluoxetine (10 mg/kg) was administered to sedentary and 6 wk Run rats 30 minutes prior to IS. Freezing and escape behaviors were observed in shuttle boxes 24 hours later. In this experiment we did not include behavioral analysis of several groups (sedentary/saline/IS, 6 wk Run/saline/no stress, 6 wk Run/fluoxetine/no stress, or 6 wk Run/saline/IS) because we have already reported that wheel running, in the absence of IS, does not alter freezing or escape performance in this paradigm (Greenwood et al., 2003, Greenwood et al., 2005a). Furthermore, the effect of IS in sedentary rats is well documented (Greenwood et al., 2003, Greenwood et al., 2005a, Maier and Watkins, 2005) and we already know that prior wheel running prevents the behavioral consequences of IS (Greenwood et al., 2003, Greenwood et al., 2005a). The primary question here was whether 6 weeks of wheel running could prevent LH even despite reduced hippocampal BDNF levels following IS. Therefore, groups of sedentary and 6 wk Run rats exposed to the combination of fluoxetine and IS were included. Another question concerned the effect of fluoxetine alone on freezing and escape behaviors. Since fluoxetine did not alter BDNF in physically active rats (Figure 4), there was no reason to include a 6 wk Run/fluoxetine/no stress group. Fluoxetine by itself, however, did reduce BDNF mRNA in the hippocampus of sedentary rats. Therefore, we included sedentary/no stress rats treated with either fluoxetine or saline in order to examine the effects of fluoxetine, in the absence of IS, on freezing and escape behaviors.
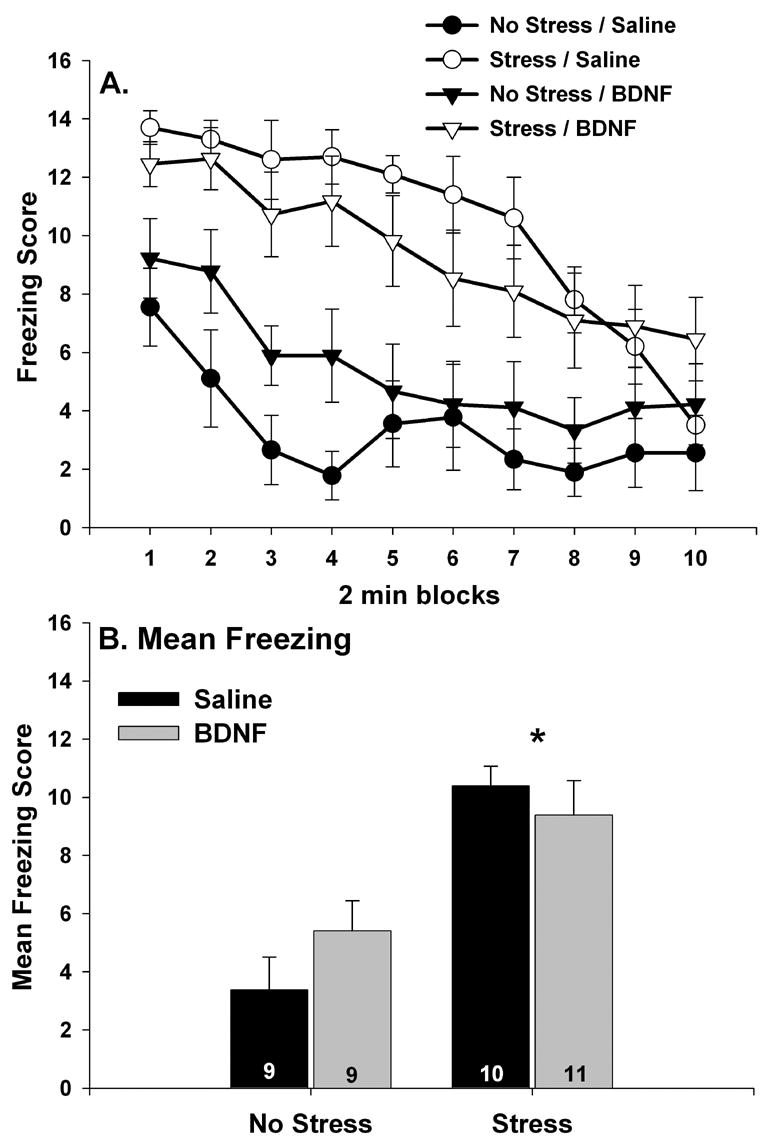
The results were that wheel running prevented LH despite the reduction in BDNF mRNA produced by the combination of fluoxetine and IS. Figure 7A shows freezing scores over 2 min blocks for the 4 groups of rats tested. Freezing was absent in all rats during the first 5 min after placement in the shuttle boxes, prior to the two FR-1 trials (data not shown). After the 2 FR-1 trials, sedentary/no stress rats given fluoxetine 24 hr earlier displayed freezing behavior identical to that of sedentary/no stress rats given saline. Sedentary rats given fluoxetine immediately before IS displayed an exaggerated freezing response to the 2 FR-1 trials. In contrast, 6 wk Run animals who received both fluoxetine and IS were protected against the effect of IS on freezing. Repeated measures ANOVA revealed a reliable main effect of group (F (3, 20) = 3.09; p < .05) and time (F (9, 180) = 12.79; p < .0001) on 2 minute blocks of freezing. Similarly, there was also a significant main effect of group (F (3, 20) = 3.09; p < .05) on mean freezing scores averaged across the 20 minute observation period (Figure 7B). The sedentary/fluoxetine/IS group differed from all other groups, which did not differ from each other.
Figure 7.
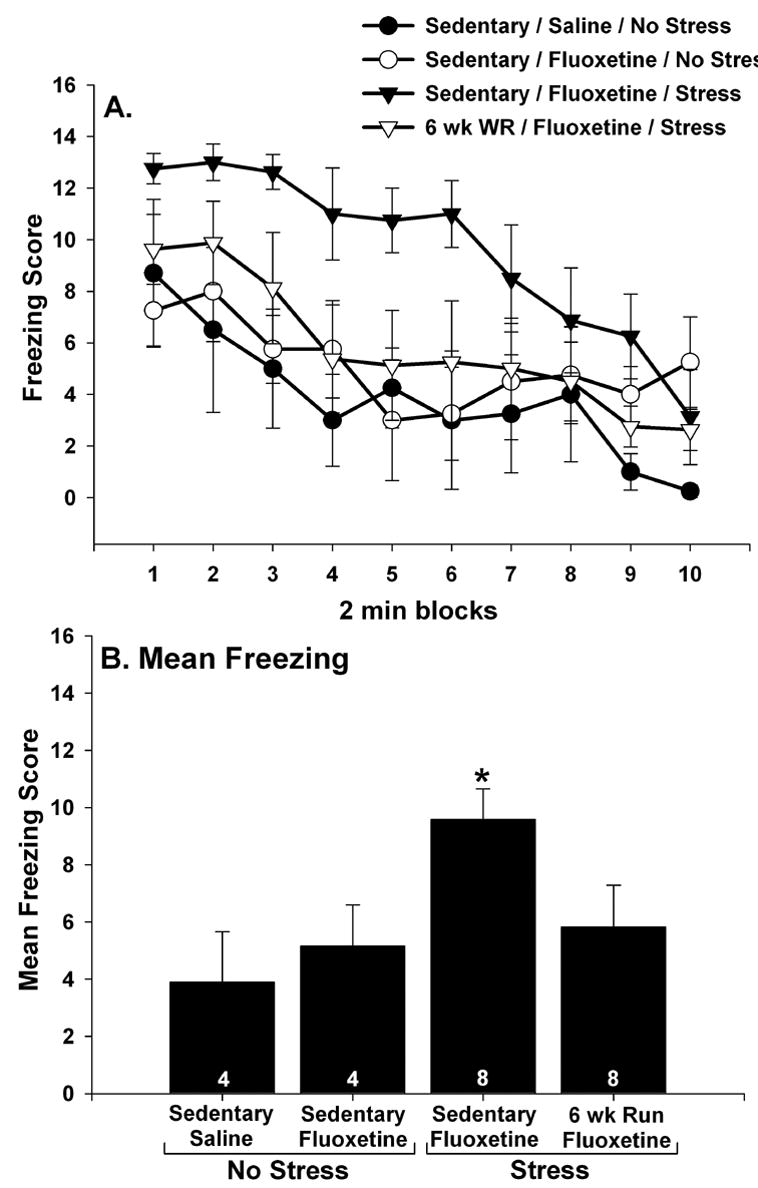
(A and B) The effect of fluoxetine and stress on post-FR-1 freezing behavior. Following sedentary living or 6 weeks of wheel running (6 wk Run), rats received either saline or fluoxetine (10 mg/kg i.p.) 30 minutes before control treatment (no stress) or inescapable tail shock (stress). Twenty four hours later, rats were placed into shuttle boxes and freezing was observed following 2 FR-1 trials. Fluoxetine by itself had no effect on freezing and 6 weeks of wheel running reduced the effect of stress on freezing despite the presence of fluoxetine during stressor exposure. This was the case for both 2 minute blocks of freezing (A) and the mean freezing score for the entire 20 minute observation period (B). Graphs represent means ± standard errors. The number in each bar represents the number of rats included in that group. * p < .05 relative to all other groups.
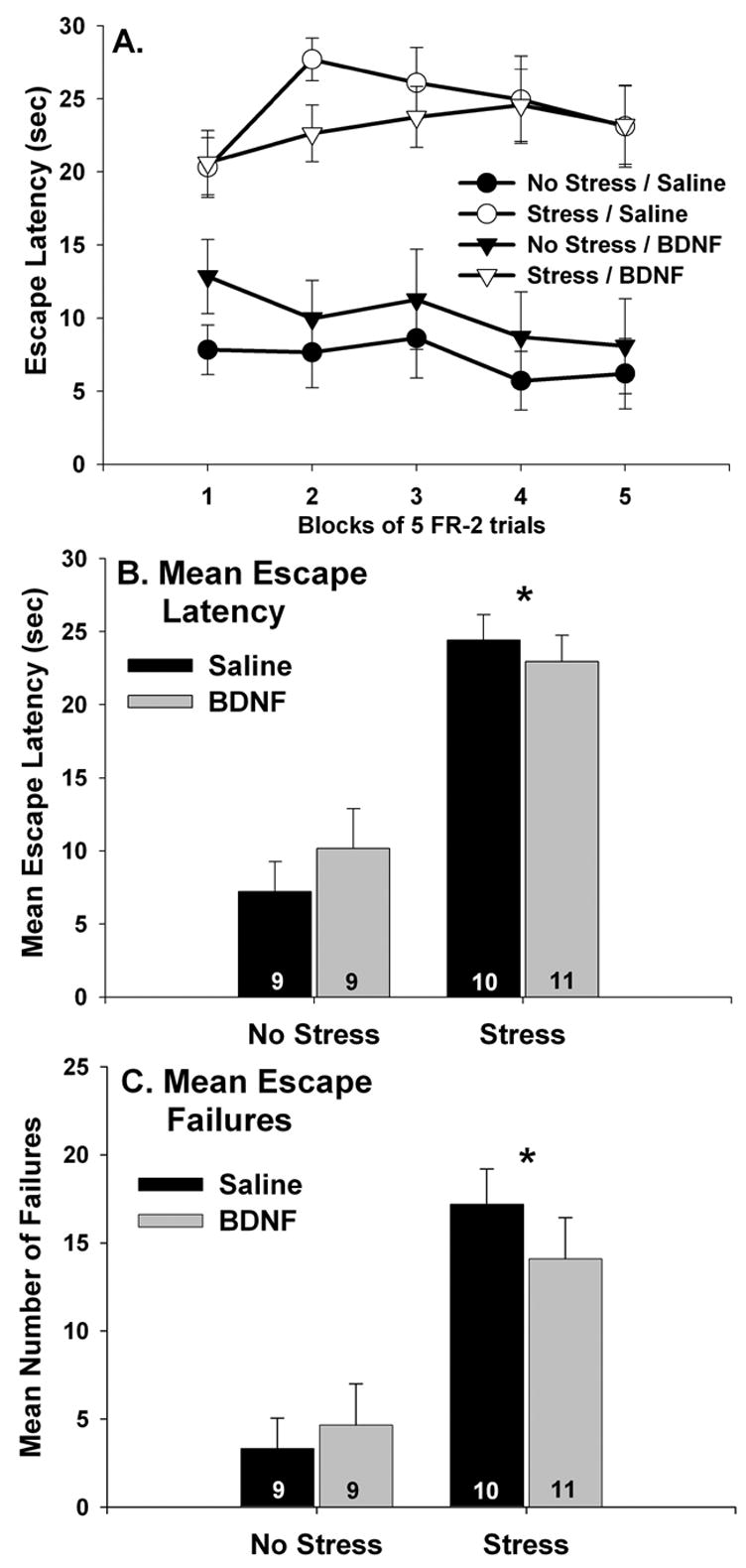
Similar results were obtained for escape performance. There were no group differences in escape latencies during the FR-1 trials (F (3, 20) = 1.05; p > .05; data not shown). Sedentary/no stress rats treated with either saline or fluoxetine all learned to escape rapidly during FR-2 trials. In contrast, sedentary rats given the combination of fluoxetine and IS were slow to escape from FR-2 trials and demonstrated a large number of escape failures. Physically active animals who received IS 24 hours earlier were protected against the behavioral effects of IS despite the presence of fluoxetine during IS. Escape performance over blocks of 5 escape trials is shown in Figure 8A. Repeated measures ANOVA revealed a reliable main effect of group (F (3, 20) = 22.47; p < .0001) and a significant interaction between group and trial blocks (F (12, 80) = 5.12; p < .0001). The sedentary/fluoxetine/IS group differed from all other groups during all 5 blocks of FR-2 trials except the first, when the sedentary/fluoxetine/IS group did not reliably differ from the 6 wk Run/fluoxetine/IS group. The 6 wk Run/fluoxetine/IS group differed from the sedentary/saline/no stress and sedentary/fluoxetine/no stress groups during the 3rd and 4th blocks of escape trials. There were no other group differences. Similar results were obtained when the mean escape latencies across all 25 FR-2 trials were compared between groups (Figure 8B). There was a reliable main effect of group (F (3, 20) = 22.47; p < .0001) on mean escape latency. The sedentary/fluoxetine/IS group differed from all other groups, which did not significantly differ from each other. The mean number of escape failures, defined as the inability to escape within 30 sec, also differed between groups (Figure 8C; F (3, 20) = 23.09; p < .0001). Again, the sedentary/fluoxetine/IS group differed from all other groups, which did not differ from each other.
Figure 8.
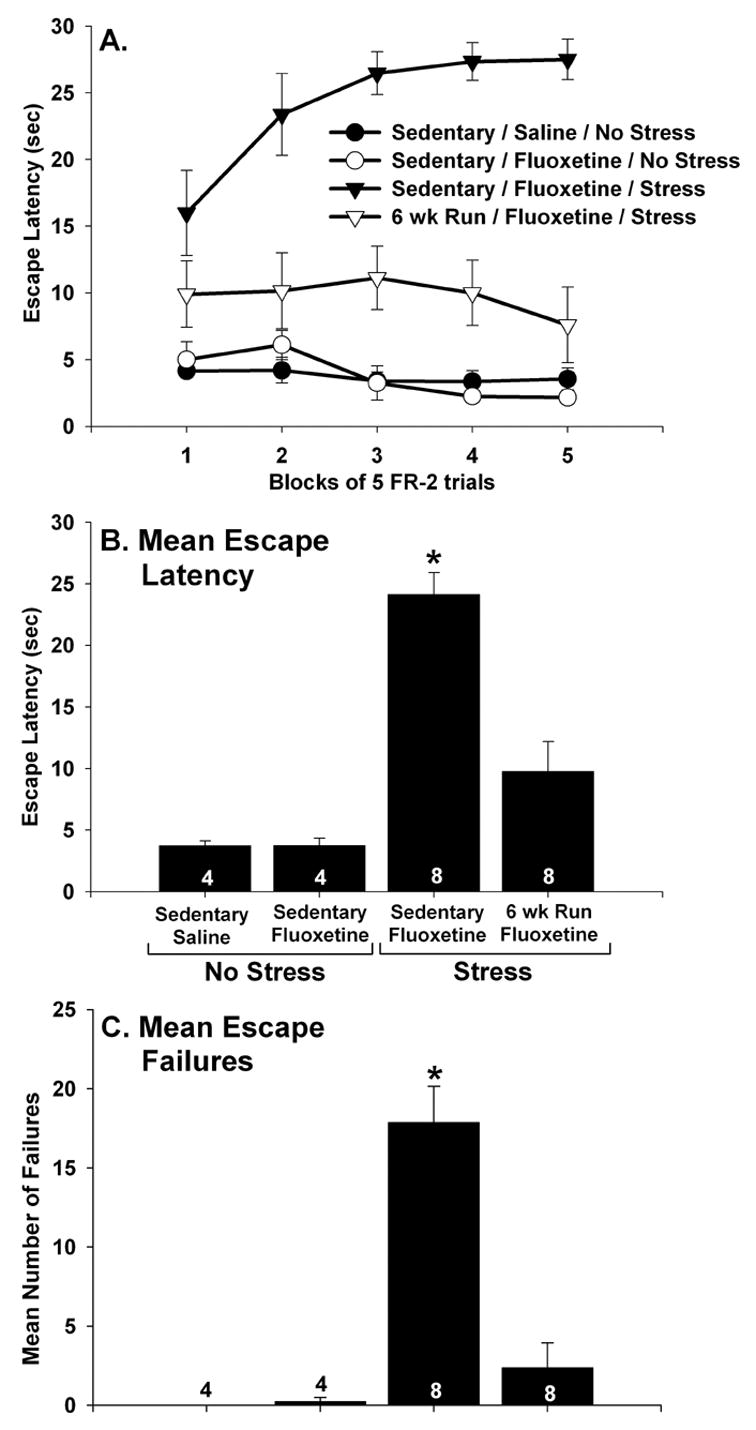
(A–C) The effect of fluoxetine and stress on shuttle box escape performance. Following sedentary living or 6 weeks of wheel running (6 wk Run), rats received either saline or fluoxetine (10 mg/kg i.p.) 30 minutes before control treatment (no stress) or inescapable tail shock (stress). Twenty four hours later, rats were placed into shuttle boxes and FR-2 escape performance was observed. Fluoxetine alone had no effect on latency to escape in sedentary rats. Sedentary rats treated with both fluoxetine and stress failed to learn to escape. In contrast, 6 wk Run animals learned to escape despite the prior combined treatment with fluoxetine and stress. This was the case for blocks of 5 escape trials (A), the average escape latency across all 25 escape trials (B), and for the mean number of escape failures (C). Graphs represent means ± standard errors. The number in each bar represents the number of rats included in that group. * p < .05 relative to all other groups.
BDNF microinjected into the DG has no effect on LH behaviors
Nine of the 48 cannulated rats had misplaced cannula and were excluded from statistical analysis. Injections of BDNF into the DG prior to IS had no effect on LH behaviors 24 hours later. Rats exposed to IS, regardless of drug condition, demonstrated exaggerated freezing and a deficit in shuttle box escape performance 24 hours later. Figure 9A shows freezing scores over 2 min blocks. Freezing was absent in all rats during the first 5 min after placement in the shuttle boxes, prior to the two FR-1 trials (data not shown). Two (no stress, IS) by 2 (saline, BDNF) repeated measures ANOVA revealed reliable main effect of stress (F (1, 35) = 27.7; p < .0001) and trial blocks (F (9, 315) = 24.8; p < .0001) and a significant interaction between stress and trial blocks (F (9,315) = 5.2; p < .0001) on freezing scores. No other main effects or interactions reached significance. BDNF injections also did not alter the mean freezing scores averaged across the 20 minute freezing period (Figure 9B). This was confirmed with 2 (no stress, IS) by 2 (saline, BDNF) ANOVA that revealed a significant main effect of stress (F (1, 35) = 27.7; p < .0001), but not drug, on the collapsed freezing scores. The interaction between stress and drug was not significant.
Figure 9.
(A and B) The effect of brain-derived neurotrophic factor (BDNF) microinjection into the dentate gyrus on post FR-1 freezing behavior. Bilateral cannula were inserted into the dentate gyrus of sedentary rats. Saline or BDNF (1.0 μg/hemisphere) was microinjected into the dentate gyrus 30 minutes before exposure to inescapable tail shock stress (stress) or control (no stress) treatment. Twenty four hours later, rats were placed into shuttle boxes and freezing was observed following 2 FR-1 trials. Stress-induced exaggeration of post FR-1 freezing was not altered by BDNF treatment. This was the case for both 2 minute blocks of freezing (A) and the mean freezing score for the entire 20 minute observation period (B). Graphs represent means ± standard errors. The number in each bar represents the number of rats included in that group. * main effect of stress p < .05.
Figure 10A shows escape performance over blocks of 5 escape trials. There were no group differences in escape latencies during the FR-1 trials (data not shown). Two (no stress, IS) by 2 (saline, BDNF) repeated measures ANOVA revealed a reliable main effect of stress (F (1, 35) = 52.3; p < .0001) and a significant interaction between stress and trial blocks (F (4, 140) = 3.3; p < .05). There were no other main effects or interactions. Similar results were obtained when the mean escape latencies across all 25 FR-2 trials were compared between groups (Figure 10B). There was a reliable main effect of stress (F (1, 35) = 52.3; p < .0001) on mean escape latency. Neither the main effect of drug nor the interaction between stress and drug reached significance. The mean number of escape failures was also reliably altered by stress (Figure 10C; F (1, 35) = 28.9; p < .0001) but not by drug.
Figure 10.
(A–C) The effect of brain-derived neurotrophic factor (BDNF) microinjection into the dentate gyrus on shuttle box escape performance. Bilateral cannula were inserted into the dentate gyrus of sedentary rats. Saline or BDNF (1.0 μg/hemisphere) was microinjected into the dentate gyrus 30 minutes before exposure to inescapable tail shock stress (stress) or control (no stress) treatment. Twenty four hours later, rats were placed into shuttle boxes and FR-2 escape performance was observed. Sedentary rats failed to learn to escape from FR-2 trials regardless of prior drug treatment. This was the case for blocks of 5 escape trials (A), the average escape latency across all 25 escape trials (B), and for the mean number of escape failures (C). Graphs represent means ± standard errors. The number in each bar represents the number of rats included in that group. * main effect of stress p < .05.
BDNF protein levels in the dorsal hippocampus, which contains the target region of the DG, were elevated 24 hours following BDNF microinjections regardless of stress condition (Table 1). There was a significant main effect of drug treatment (F (1, 10) = 22.7; p = .0008). Neither the main effect of stress nor the interaction between drug treatment and stress were significant. Neither stress nor BDNF microinjections altered levels of BDNF protein in the ventral hippocampus (Table 1).
Table 1.
Levels of brain-derived neurotrophic factor (BDNF) protein (pg/100μg) in the dorsal and ventral hippocampus 24 hours after bilateral microinjection of saline or BDNF (1.0 μg/hemisphere) into the dentate gyrus.
| Saline No Stress N = 4 | Saline Stress N = 4 | BDNF No Stress N = 3 | BDNF Stress N = 3 | |
|---|---|---|---|---|
| Dorsal Hippocampus* | 1.73 ± 0.73 | 0.64 ± .09 | 4.93 ± 0.31 | 4.72 ± 1.46 |
| Ventral Hippocampus | 0.97 ± 0.18 | 0.76 ± 0.13 | 0.85 ± 0.13 | 0.59 ± 0.06 |
Values are means ± SEM.
Main effect of drug treatment p < .05.
Discussion
The results presented here confirm and extend prior work on the effects of stress and exercise on BDNF. Exposure to IS produced a delayed but persistent decrease in BDNF protein in the hippocampus and a transient increase in BDNF protein in the PFC. Wheel running, by itself, had no effect on BDNF protein or mRNA in the PFC. Although 3 weeks of wheel running was insufficient to increase hippocampal BDNF protein, 6 weeks of wheel running increased BDNF mRNA and protein in the hippocampus and both 3 and 6 weeks of wheel running blocked the suppressive effects of IS on hippocampal BDNF protein. Additionally, 6 weeks of wheel running blocked the decrease in BDNF mRNA produced by either IS or a single injection of fluoxetine. The protective effect of exercise against reductions in BDNF is overcome by the concurrent administration of fluoxetine and IS. However, physically active rats given the combination of fluoxetine and IS are still resistant to LH, despite the reduced levels of BDNF mRNA in the hippocampus. Finally, contrary to our hypothesis, microinjections of BDNF directly into the DG had no effect on LH behaviors despite the presence of higher levels of BDNF both during IS and during behavioral testing in BDNF injected rats.
The role of BDNF in LH
Consistent with the effects of other stressors, IS reduced levels of BDNF in the hippocampus. The effect of IS on BDNF mRNA preceded that of BDNF protein. IS-induced suppression of BDNF mRNA levels in the DG, CA3, and CA2 of sedentary rats was observed 6 hours after IS. In contrast, levels of BDNF protein in the hippocampus were similar to non-stressed rats 6 hours after IS, but were reduced at 24 and 72 hours post-IS. IS elevates basal levels of circulating corticosterone for at least 48 hours (Fleshner et al., 1995), and corticosterone contributes to stress-induced decreases in hippocampal BDNF (Adlard and Cotman, 2004). It is therefore possible that the enduring suppression of hippocampal BDNF produced by IS is mediated by persistently elevated levels of corticosterone.
Restraint (Molteni et al., 2001), immobilization (Lee et al., 2006), and IS (Bland et al., 2005) all rapidly (within 1 hour) increase BDNF mRNA in the PFC. Here we report that IS can also increase BDNF protein in the PFC. It is not surprising that we did not observe an increase in BDNF mRNA in the PFC 6 hours after IS. Bland et al. (2005) have reported that BDNF mRNA in the PFC is elevated immediately after IS but rapidly returns to basal levels. It is possible, therefore; that we missed the IS-induced increase in PFC BDNF mRNA by several hours. Nonetheless, IS-induced increases in BDNF protein in the PFC were present 4 and 6 hours after IS. These data together with observations in the hippocampus suggest that stress-induced changes in BDNF protein, compared to BDNF mRNA, are slow to develop.
The observations that IS and other stressors decrease hippocampal BDNF are consistent with the hypothesis that reductions in hippocampal BDNF can contribute to stress-related disorders. However, the current data are inconsistent with a role for BDNF in LH. Instead, results support the conclusion that LH is independent of BDNF levels in the hippocampus. Although the duration that shuttle box learning deficits persist following inescapable stress can depend on the novelty of the testing environment (Maier and Watkins, 2005), Maier and Watkins (2005) recently reported that the deficit in shuttle box escape learning produced by the same IS procedure used here and tested in a novel environment only persists for 48 hours. Escape performance of rats exposed to IS 72 hours prior to testing is indistinguishable from non-stressed rats (Maier and Watkins, 2005). Thus, the current observation that BDNF levels in the hippocampus remained reduced 72 hours after IS suggests that BDNF suppression is insufficient to produce a deficit in shuttle box escape learning. Further supporting this conclusion is the observation that a single injection of fluoxetine did nothing to either freezing or escape performance in sedentary rats, despite the clear reduction in hippocampal BDNF mRNA that was present for at least 6 hours post IS. Finally, recent data indicate that inescapable and escapable tail shock stress both reduce BDNF mRNA in the hippocampus measured immediately and 24 hours after stress (Bland et al., in press). By definition, LH effects only occur following inescapable stressors (Maier and Watkins, 2005). Together, these data suggest that a decrease in BDNF levels in the hippocampus, either at the time of stress or at the time of behavioral testing, cannot account for at least some of the behavioral effects of IS. This conclusion is similar to prior work indicating that reduced BDNF levels present in BDNF heterozygous knockout mice is not sufficient to alter behavior in animal models of depression and anxiety (MacQueen et al., 2001, Chourbaji et al., 2004). That normal escape learning occurs in the presence of reduced levels of BDNF is especially important because interference with shuttle box escape learning is the consequence of IS that is most often used to represent depressive-like behaviors and as a screening tool for antidepressant compounds.
The ability of BDNF to prevent LH was directly tested by BDNF microinjections into the DG prior to IS. The injection protocol was sufficient to increase BDNF levels in the dorsal hippocampus (which contains the DG) of both stressed and non stressed rats for at least 24 hours. In fact, the levels of BDNF we observed in the dorsal hippocampus 24 hours after BDNF injection were similar to those observed in 6 wk Run animals, indicating that the BDNF injection did not elevate BDNF protein levels above physiological levels and mimicked the elevated levels produced by wheel running. Because BDNF is highly conserved across species, the assay used to measure BDNF protein does not discriminate between human and rat BDNF. Thus, it is unknown whether the increased levels of BDNF measured in BDNF injected rats 24 hours after the injection represent lingering exogenous BDNF or an increase in endogenous BDNF stimulated by activity of exogenous BDNF on hippocampal TrkB receptors (Saarelainen et al., 2001). Regardless, it is clear that the microinjection procedure increased BDNF levels in the dorsal hippocampus both during IS and during behavioral testing. Despite elevated levels of BDNF during IS and testing, exposure to IS still produced exaggerated freezing and interfered with shuttle box escape performance.
The failure of BDNF injections to alter the effects of IS on escape behaviors is in contrast to prior work indicating that injections of 1μg of BDNF into the hippocampus (DG or CA3; (Shirayama et al., 2002)) or repeated BDNF injections into the midbrain (Siuciak et al., 1997) can reverse uncontrollable stress-induced interference with shuttle box escape learning. The novelty of the testing apparatus (the shuttle box) could be an important factor contributing to the discrepancies between prior work and the current results. Both Shirayama et al. (2002) and Siuciak et al. (1997) administered uncontrollable foot shock to produce escape deficits, whereas we used uncontrollable tail shock. The shuttle box context and/or the foot shocks themselves are not novel to rats who receive prior foot shock. In contrast, both the shuttle box environment and the foot shocks are novel to rats who receive prior tail shock. This is a critical point because both the time course of the escape deficits and the mechanisms underlying the escape deficits are very different depending on the novelty of the testing procedure (Maier and Watkins, 2005). It is possible that hippocampal BDNF may be involved in escape behavior when the context of the testing environment is familiar. This makes sense considering the established role of the hippocampus in contextually-dependent responses (Rudy et al., 2002). On the other hand, the current data clearly indicate that an acute increase in exogenous BDNF is ineffective at protecting against IS-induced exaggerated freezing and escape deficits measured in a novel environment.
The role of BDNF in the protective effect of wheel running against LH
Six weeks of wheel running increased basal levels of BDNF mRNA and protein in the hippocampus and both 3 and 6 weeks of wheel running blocked the suppressive effect of IS on hippocampal BDNF protein. Wheel running had no effect on basal or stress levels of BDNF mRNA or protein in the PFC. Six weeks of wheel running also blocked the effect of IS on BDNF mRNA in the DG and CA3. The effect of 3 weeks of wheel running on BDNF mRNA was not evaluated. These data are similar to previous reports that wheel running for 1 week (Russo-Neustadt, Ha et al. 2001), 3 weeks (Adlard and Cotman 2004), or 4 weeks (Zheng et al., 2006) can prevent decreases in hippocampal BDNF elicited by other stressors. Interestingly, wheel running also prevented the reduction in BDNF mRNA observed in the DG and CA3 following a single injection of fluoxetine. Although unlikely to be involved in LH, the protective effect of wheel running against hippocampal BDNF suppression suggests that exercise could produce resistance against deleterious affective, behavioral, and/or cognitive effects elicited by reductions in BDNF.
It should be noted that 3 weeks of wheel running did not increase basal levels of BDNF protein in the hippocampus. Although elevations in full-length BDNF mRNA in the rat hippocampus have been observed after as little as 6 hours of wheel running (Oliff et al., 1998), our data are consistent with Adlard et al. (2004) who reported that 2 weeks of wheel running is insufficient to increase BDNF protein in the hippocampus of male Sprague-Dawley rats. However, also using Sprague-Dawley rats, Berthold et al. (2005) observed an increase in hippocampal BDNF protein after 2 weeks of wheel running. Clearly, the effect of exercise on BDNF is complex and depends on several factors including time of day BDNF levels are measured (Berchtold et al., 1999), age (Garza et al., 2004, Adlard et al., 2005), gender (Berchtold et al., 2001), and history of prior exercise (Berchtold et al., 2005). BDNF also likely varies across rat strains as a function of activity level. Here we report that running an average of 1.8 ± 0.19 km/day for 3 weeks is insufficient to produce a detectable increase in BDNF protein levels in the hippocampus of adult male, Fischer F344 rats who were sacrificed between 8:00 and 10:00 A.M.
The mechanisms by which wheel running prevents stress-induced reductions in BDNF are currently unknown. The fact that 3 weeks of wheel running did not elevate basal levels of BDNF protein but did prevent IS-induced BDNF suppression suggests that the prevention of the effect of IS on BDNF is independent of elevated BDNF protein levels prior to stress. Many factors can influence the levels of BDNF in the hippocampus. Of these, corticosterone and 5-HT deserve special consideration because of their roles in modulating BDNF in response to stress. IS elevates circulating corticosterone (Fleshner et al., 1995) and 5-HT release in the hippocampus (Amat et al., 1998). Both corticosterone (Schaaf et al., 1998, Vellucci et al., 2001) and 5-HT (Zetterstrom et al., 1999) can decrease BDNF in the hippocampus. Moreover, removal of the adrenal glands blocks the decrease in hippocampal BDNF protein produced by immobilization stress (Adlard and Cotman, 2004), and blockade of 5-HT2A receptors can reduce restraint stress-induced hippocampal BDNF suppression (Vaidya et al., 1997, Vaidya et al., 1999). It is possible that exercise modulates the effects of either corticosterone or 5-HT on BDNF, thereby preventing stress-induced suppression of BDNF. However, wheel running alters neither adrenocorticotropin hormone nor corticosterone responses to severe inescapable shock (Dishman, 1997b, 1997a, Fleshner, 2000). On the other hand, wheel running does produce changes in the 5-HT system that are consistent with constraint of 5-HT activity during IS (Greenwood et al., 2003, Greenwood et al., 2005a, Greenwood et al., 2005b). These data, along with the current observation that wheel running blocked the decrease in BDNF produced by either IS or a single injection of fluoxetine, which rapidly increases 5-HT release in the hippocampus (Hervas et al., 2000), support the hypothesis that wheel running prevents IS-induced BDNF suppression by reducing the inhibitory effects of 5-HT on BDNF.
Regardless of the mechanisms involved, both 3 and 6 weeks of wheel running clearly blocked the suppression of hippocampal BDNF produced by IS. Therefore, our hypothesis that only 6 weeks of wheel running would prevent the IS-induced suppression of hippocampal BDNF was incorrect. Instead, our results suggest that preventing the effect of IS on hippocampal BDNF is not sufficient to prevent LH because both 3 weeks and 6 weeks of wheel running blocked the suppression of BDNF, but only 6 weeks of wheel running prevents LH behaviors (Greenwood et al., 2005a).
Similarly, it is unlikely that preventing IS-induced BDNF suppression is necessary for the protective effect of 6 weeks of wheel running against LH. BDNF mRNA levels in the hippocampus of 6 wk Run animals were reduced with the combined treatment of fluoxetine and IS. Despite the reduced levels of BDNF mRNA, rats allowed to run for 6 weeks prior to treatment with the combination of IS plus fluoxetine remained resistant against the exaggerated freezing and deficit in shuttle box escape learning produced by IS. Importantly, it is unlikely that a potential restorative effect of BDNF reduction on LH behaviors in physically active rats was masked by a compensatory effect of fluoxetine on LH, because sedentary stressed rats treated with fluoxetine demonstrated typical LH behaviors.
Our results confirm those of Zetterstrom and colleagues who have reported rapid decreases in BDNF mRNA in the hippocampus following acute treatment with SSRIs in sedentary rats (Zetterstrom et al., 1999, Coppell et al., 2003). Interestingly, 6 weeks of wheel running blocked the effects of fluoxetine alone on BDNF mRNA. However, the combination of IS plus fluoxetine reduced BDNF mRNA levels in all hippocampal subfields of 6 wk Run animals to that of sedentary stressed rats. Although the effect of the combination of IS and fluoxetine on BDNF protein was not evaluated, changes in BDNF mRNA often precede changes in protein. The effect of the combination of fluoxetine plus stress on BDNF mRNA in the hippocampus of 6 wk Run rats (44% below saline controls in the DG) was similar to that observed in sedentary rats treated only with stress (40% below saline controls in the DG; Figure 4); a decrease we have shown corresponds to a significant reduction in hippocampal BDNF protein levels 24 hours after stress. Indeed, we have preliminary data suggesting that the observed changes in BDNF mRNA produced by the combination of IS plus fluoxetine are followed by similar changes in BDNF protein (Campisi et al., 2005).
Although the data presented here suggest that prevention of stress-induced BDNF suppression is not required for the effect of wheel running in the LH model, a role for BDNF in the prevention of LH cannot be entirely ruled out. Because BDNF mRNA was measured 6 hours after IS and fluoxetine, we do not know if the suppression of BDNF persisted until behavioral testing 24 hours later. However, the current data indicate that the time point following stress at which BDNF levels are assessed is irrelevant in terms of the behavioral consequences of inescapable stress. It is unlikely that restored BDNF levels at the time of testing are critical for protection against the expression of LH because BDNF injections, which increased BDNF levels in the hippocampus of stressed rats during behavioral testing, were ineffective at preventing LH in sedentary rats. Moreover, LH can occur in the presence of maintained levels of BDNF that are present in 3 wk Run rats during behavioral testing 24 hours after IS.
Another possibility that cannot be eliminated is that the chronically elevated BDNF levels in the hippocampus of exercised rats during the weeks preceding exposure to IS could have contributed to changes in neurogenesis (van Praag et al., 1999a, Lee et al., 2002) or synaptic efficacy (McAllister et al., 1999, Poo, 2001, Molteni et al., 2002, Vaynman et al., 2006) in the hippocampus that persist beyond the presence of BDNF and serve to protect the hippocampus against damaging effects of stress. Further research is required to determine the role of chronically elevated levels of hippocampal BDNF in the protective effects of exercise against LH and other stress-related disorders.
In conclusion, the data presented here yield interesting insights into the role of BDNF in LH and in the protective effect of wheel running against LH. Although BDNF may be involved in the etiology and treatment of stress-related mood disorders such as depression and anxiety (Duman and Monteggia, 2006), it seems unlikely that hippocampal BDNF suppression contributes to LH. This is supported by the observations that normal freezing and escape learning occur despite reduced levels of hippocampal BDNF caused by either IS or fluoxetine. In addition, LH can still be present in stressed animals with normal or elevated levels of BDNF protein in the hippocampus, as is the case with 3 wk Run rats and sedentary rats microinjected with BDNF. It is similarly unlikely that the blockade of stress-induced suppression of hippocampal BDNF is involved in the mechanisms by which wheel running prevents LH. Three weeks of wheel running blocks reductions in BDNF in the absence of the ability to prevent LH, and 6 weeks of wheel running prevents LH despite reduced levels of hippocampal BDNF mRNA produced by the combination of fluoxetine plus IS. These data do not preclude an important role of BDNF in other effects of exercise (i.e., hippocampal-dependent learning and memory (Van Hoomissen et al., 2004, Vaynman et al., 2004)). The beneficial effects of exercise-induced increases in BDNF may only be revealed in tasks that are more directly impacted by changes in BDNF. Since the current data raise doubt as to the involvement of BDNF in LH, it is not surprising that hippocampal BDNF may not be involved in the mechanisms by which wheel running prevents LH.
Acknowledgments
The authors would like to thank Dr. Heidi Day and Dr. Serge Campeau for their technical assistance with in situ hybridization and Dr. Steven Maier for his advice on learned helplessness behavioral testing.
Comprehensive list of abbreviations
- BDNF
Brain-derived neurotrophic factor
- DG
dentate gyrus
- IS
inescapable stress
- LH
learned helplessness
- PFC
prefrontal cortex
- SSRI
selective serotonin reuptake inhibitor
- 5-HT
serotonin
Footnotes
Section Editor (Behavioral Neuroscience): Dr. G.J. Quirk, Department of Physiology, Ponce School of Medicine, Ponce, Puerto Rico
These studies were supported by NIMH-068283 & NARSAD awarded to Monika Fleshner.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adlard PA, Cotman CW. Voluntary exercise protects against stress-induced decreases in brain-derived neurotrophic factor protein expression. Neuroscience. 2004;124:985–992. doi: 10.1016/j.neuroscience.2003.12.039. [DOI] [PubMed] [Google Scholar]
- Adlard PA, Perreau VM, Cotman CW. The exercise-induced expression of BDNF within the hippocampus varies across life-span. Neurobiol Aging. 2005;26:511–520. doi: 10.1016/j.neurobiolaging.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Adlard PA, Perreau VM, Engesser-Cesar C, Cotman CW. The timecourse of induction of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus following voluntary exercise. Neurosci Lett. 2004;363:43–48. doi: 10.1016/j.neulet.2004.03.058. [DOI] [PubMed] [Google Scholar]
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Amat J, Matus-Amat P, Watkins LR, Maier SF. Escapable and inescapable stress differentially and selectively alter extracellular levels of 5-HT in the ventral hippocampus and dorsal periaqueductal gray of the rat. Brain Res. 1998;797:12–22. doi: 10.1016/s0006-8993(98)00368-0. [DOI] [PubMed] [Google Scholar]
- Barde YA. Neurotrophins: a family of proteins supporting the survival of neurons. Prog Clin Biol Res. 1994;390:45–56. [PubMed] [Google Scholar]
- Berchtold NC, Chinn G, Chou M, Kesslak JP, Cotman CW. Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience. 2005;133:853–861. doi: 10.1016/j.neuroscience.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Berchtold NC, Kesslak JP, Pike CJ, Adlard PA, Cotman CW. Estrogen and exercise interact to regulate brain-derived neurotrophic factor mRNA and protein expression in the hippocampus. Eur J Neurosci. 2001;14:1992–2002. doi: 10.1046/j.0953-816x.2001.01825.x. [DOI] [PubMed] [Google Scholar]
- Berchtold NC, Oliff HS, Isackson P, Cotman CW. Hippocampal BDNF mRNA shows a diurnal regulation, primarily in the exon III transcript. Brain Res Mol Brain Res. 1999;71:11–22. doi: 10.1016/s0169-328x(99)00137-0. [DOI] [PubMed] [Google Scholar]
- Bland ST, Schmid MJ, Der-Avakian A, Watkins LR, Spencer RL, Maier SF. Expression of c-fos and BDNF mRNA in subregions of the prefrontal cortex of male and female rats after acute uncontrollable stress. Brain Res. 2005;1051:90–99. doi: 10.1016/j.brainres.2005.05.065. [DOI] [PubMed] [Google Scholar]
- Bland ST, Tamblyn J, Berrientos RM, Greenwood BN, Watkins LR, Campeau S, Day HE, Maier SF. Expression of fibroblast growth factor-2 and brain derived neurotrophic factor mRNA in the medial prefrontal cortex and hippocampus after uncontrollable or controllable stress. Neuroscience. 144:1219–1228. doi: 10.1016/j.neuroscience.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt DB, Zembar MJ, Niederehe G. Depression and memory impairment: a meta-analysis of the association, its pattern, and specificity. Psychol Bull. 1995;117:285–305. doi: 10.1037/0033-2909.117.2.285. [DOI] [PubMed] [Google Scholar]
- Campisi J, Greenwood BN, Foley TE, Nickerson M, Thompson R, Fleshner M. Wheel running prevents decreases in BDNF produced by acute fluoxetine. Society for Neuroscience 2005 Abstract Viewer/Itinerary Planner. 2005 Program Number 307.8. [Google Scholar]
- Campisi J, Leem TH, Greenwood BN, Hansen MK, Moraska A, Higgins K, Smith TP, Fleshner M. Habitual physical activity facilitates stress-induced HSP72 induction in brain, peripheral, and immune tissues. Am J Physiol Regul Integr Comp Physiol. 2003;284:R520–530. doi: 10.1152/ajpregu.00513.2002. [DOI] [PubMed] [Google Scholar]
- Chen B, Dowlatshahi D, MacQueen GM, Wang JF, Young LT. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol Psychiatry. 2001;50:260–265. doi: 10.1016/s0006-3223(01)01083-6. [DOI] [PubMed] [Google Scholar]
- Chourbaji S, Hellweg R, Brandis D, Zorner B, Zacher C, Lang UE, Henn FA, Hortnagl H, Gass P. Mice with reduced brain-derived neurotrophic factor expression show decreased choline acetyltransferase activity, but regular brain monoamine levels and unaltered emotional behavior. Brain Res Mol Brain Res. 2004;121:28–36. doi: 10.1016/j.molbrainres.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Cirulli F, Berry A, Chiarotti F, Alleva E. Intrahippocampal administration of BDNF in adult rats affects short-term behavioral plasticity in the Morris water maze and performance in the elevated plus-maze. Hippocampus. 2004;14:802–807. doi: 10.1002/hipo.10220. [DOI] [PubMed] [Google Scholar]
- Coppell AL, Pei Q, Zetterstrom TS. Bi-phasic change in BDNF gene expression following antidepressant drug treatment. Neuropharmacology. 2003;44:903–910. doi: 10.1016/s0028-3908(03)00077-7. [DOI] [PubMed] [Google Scholar]
- Day HE, Akil H. Differential pattern of c-fos mRNA in rat brain following central and systemic administration of interleukin-1-beta: implications for mechanism of action. Neuroendocrinology. 1996;63:207–218. doi: 10.1159/000126959. [DOI] [PubMed] [Google Scholar]
- Dishman RK, Renner KJ, Youngstedt SD, Reigle TG, Bunnell BN, Burke KA, Yoo HS, Mougey EH, Meyerhoff JL. Activity wheel running reduces escape latency and alters brain monoamine levels after footshock. Brain Research Bulletin. 1997a;42:399–406. doi: 10.1016/s0361-9230(96)00329-2. [DOI] [PubMed] [Google Scholar]
- Dishman RK, Warren JM, Youngstedt SD, Yoo H, Bunnell BN, Mougey EH, Meyerhoff JL, Friedmann LJ, Evans DL. Brain monoamines, exercise, and behavioral stress: animal models. Medicine and Science in Sports and Exercise. 1997b;29:63–74. doi: 10.1097/00005768-199701000-00010. [DOI] [PubMed] [Google Scholar]
- Duman RS. Novel therapeutic approaches beyond the serotonin receptor. Biol Psychiatry. 1998;44:324–335. doi: 10.1016/s0006-3223(98)00031-6. [DOI] [PubMed] [Google Scholar]
- Duman RS. Role of neurotrophic factors in the etiology and treatment of mood disorders. Neuromolecular Med. 2004;5:11–25. doi: 10.1385/NMM:5:1:011. [DOI] [PubMed] [Google Scholar]
- Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiatry. 1997;54:597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A Neurotrophic Model for Stress-Related Mood Disorders. Biol Psychiatry. 2006 doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, Pandey GN. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch Gen Psychiatry. 2003;60:804–815. doi: 10.1001/archpsyc.60.8.804. [DOI] [PubMed] [Google Scholar]
- Elzinga BM, Bremner JD. Are the neural substrates of memory the final common pathway in posttraumatic stress disorder (PTSD)? J Affect Disord. 2002;70:1–17. doi: 10.1016/s0165-0327(01)00351-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanslow M, Lester L. A functional behavioristic approach to aversively motivated behavior: predatory imminence as a determinant of the topography of defensive behavior. Evolution and learning. 1988:185–212. [Google Scholar]
- Fleshner M. Exercise and neuroendocrine regulation of antibody production: protective effect of physical activity on stress-induced suppression of the specific antibody response. International Journal of Sports Medicine. 2000;21:S14–S19. doi: 10.1055/s-2000-1454. [DOI] [PubMed] [Google Scholar]
- Fleshner M. Physical activity and stress resistance: sympathetic nervous system adaptations prevent stress-induced immunosuppression. Exerc Sport Sci Rev. 2005;33:120–126. doi: 10.1097/00003677-200507000-00004. [DOI] [PubMed] [Google Scholar]
- Fleshner M, Deak T, Spencer RL, Laudenslager ML, Watkins LR, Maier SF. A long-term increase in basal levels of corticosterone and a decrease in corticosteroid-binding globulin after acute stressor exposure. Endocrinology. 1995;136:5336–5342. doi: 10.1210/endo.136.12.7588279. [DOI] [PubMed] [Google Scholar]
- Garza AA, Ha TG, Garcia C, Chen MJ, Russo-Neustadt AA. Exercise, antidepressant treatment, and BDNF mRNA expression in the aging brain. Pharmacol Biochem Behav. 2004;77:209–220. doi: 10.1016/j.pbb.2003.10.020. [DOI] [PubMed] [Google Scholar]
- Gould E, Tanapat P. Stress and hippocampal neurogenesis. Biol Psychiatry. 1999;46:1472–1479. doi: 10.1016/s0006-3223(99)00247-4. [DOI] [PubMed] [Google Scholar]
- Gould E, Tanapat P, McEwen BS, Flugge G, Fuchs E. Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc Natl Acad Sci U S A. 1998;95:3168–3171. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Burhans D, Maier SF, Fleshner M. The consequences of uncontrollable stress are sensitive to duration of prior wheel running. Brain Res. 2005a;1033:164–178. doi: 10.1016/j.brainres.2004.11.037. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Day HE, Burhans D, Brooks L, Campeau S, Fleshner M. Wheel running alters serotonin (5-HT) transporter, 5-HT(1A), 5-HT(1B), and alpha(1b)-adrenergic receptor mRNA in the rat raphe nuclei. Biol Psychiatry. 2005b;57:559–568. doi: 10.1016/j.biopsych.2004.11.025. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Day HE, Campisi J, Hammack SH, Campeau S, Maier SF, Fleshner M. Freewheel running prevents learned helplessness/behavioral depression: role of dorsal raphe serotonergic neurons. J Neurosci. 2003;23:2889–2898. doi: 10.1523/JNEUROSCI.23-07-02889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervas I, Queiroz CM, Adell A, Artigas F. Role of uptake inhibition and autoreceptor activation in the control of 5-HT release in the frontal cortex and dorsal hippocampus of the rat. Br J Pharmacol. 2000;130:160–166. doi: 10.1038/sj.bjp.0703297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshaw BA, Malberg JE, Lucki I. Central administration of IGF-I and BDNF leads to long-lasting antidepressant-like effects. Brain Res. 2005;1037:204–208. doi: 10.1016/j.brainres.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Itoh T, Tokumura M, Abe K. Effects of rolipram, a phosphodiesterase 4 inhibitor, in combination with imipramine on depressive behavior, CRE-binding activity and BDNF level in learned helplessness rats. Eur J Pharmacol. 2004;498:135–142. doi: 10.1016/j.ejphar.2004.07.084. [DOI] [PubMed] [Google Scholar]
- Johnson JD, Campisi J, Sharkey CM, Kennedy SL, Nickerson M, Greenwood BN, Fleshner M. Catecholamines mediate stress-induced increases in peripheral and central inflammatory cytokines. Neuroscience. 2005;135:1295–1307. doi: 10.1016/j.neuroscience.2005.06.090. [DOI] [PubMed] [Google Scholar]
- Johnson JD, O’Connor KA, Watkins LR, Maier SF. The role of IL-1beta in stress-induced sensitization of proinflammatory cytokine and corticosterone responses. Neuroscience. 2004;127:569–577. doi: 10.1016/j.neuroscience.2004.05.046. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry. 1999;156:837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- Kitayama N, Vaccarino V, Kutner M, Weiss P, Bremner JD. Magnetic resonance imaging (MRI) measurement of hippocampal volume in posttraumatic stress disorder: a meta-analysis. J Affect Disord. 2005;88:79–86. doi: 10.1016/j.jad.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Lang UE, Hellweg R, Kalus P, Bajbouj M, Lenzen KP, Sander T, Kunz D, Gallinat J. Association of a functional BDNF polymorphism and anxiety-related personality traits. Psychopharmacology (Berl) 2005;180:95–99. doi: 10.1007/s00213-004-2137-7. [DOI] [PubMed] [Google Scholar]
- Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem. 2002;82:1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- Lee Y, Duman RS, Marek GJ. The mGlu2/3 receptor agonist LY354740 suppresses immobilization stress-induced increase in rat prefrontal cortical BDNF mRNA expression. Neurosci Lett. 2006;398:328–332. doi: 10.1016/j.neulet.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Lo DC. Neurotrophic factors and synaptic plasticity. Neuron. 1995;15:979–981. doi: 10.1016/0896-6273(95)90085-3. [DOI] [PubMed] [Google Scholar]
- MacQueen GM, Ramakrishnan K, Croll SD, Siuciak JA, Yu G, Young LT, Fahnestock M. Performance of heterozygous brain-derived neurotrophic factor knockout mice on behavioral analogues of anxiety, nociception, and depression. Behav Neurosci. 2001;115:1145–1153. doi: 10.1037//0735-7044.115.5.1145. [DOI] [PubMed] [Google Scholar]
- Maier SF, Grahn RE, Kalman BA, Sutton LC, Wiertelak EP, Watkins LR. The role of the amygdala and dorsal raphe nucleus in mediating the behavioral consequences of inescapable shock. Behav Neurosci. 1993;107:377–388. doi: 10.1037//0735-7044.107.2.377. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Stressor controllability and learned helplessness: The roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev. 2005;29:829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- McAllister K, Katz LC, Lo DC. Neurotrophins and synaptic plasticity. Annu Rev Neurosci. 1999;22:295–318. doi: 10.1146/annurev.neuro.22.1.295. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- Molteni R, Lipska BK, Weinberger DR, Racagni G, Riva MA. Developmental and stress-related changes of neurotrophic factor gene expression in an animal model of schizophrenia. Mol Psychiatry. 2001;6:285–292. doi: 10.1038/sj.mp.4000865. [DOI] [PubMed] [Google Scholar]
- Molteni R, Ying Z, Gomez-Pinilla F. Differential effects of acute and chronic exercise on plasticity- related genes in the rat hippocampus revealed by microarray. Eur J Neurosci. 2002;16:1107–1116. doi: 10.1046/j.1460-9568.2002.02158.x. [DOI] [PubMed] [Google Scholar]
- Moraska A, Fleshner M. Voluntary physical activity prevents stress-induced behavioral depression and anti-KLH antibody suppression. Am J Physiol Regul Integr Comp Physiol. 2001;281:R484–489. doi: 10.1152/ajpregu.2001.281.2.R484. [DOI] [PubMed] [Google Scholar]
- Morimoto K, Tan N, Nishiyasu T, Sone R, Murakami N. Spontaneous wheel running attenuates cardiovascular responses to stress in rats. Pflugers Arch. 2000;440:216–222. doi: 10.1007/s004240000265. [DOI] [PubMed] [Google Scholar]
- Neeper SA, Gomez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature. 1995;373:109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- Neeper SA, Gomez-Pinilla F, Choi J, Cotman CW. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 1996;726:49–56. [PubMed] [Google Scholar]
- Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliff HS, Berchtold NC, Isackson P, Cotman CW. Exercise-induced regulation of brain-derived neurotrophic factor (BDNF) transcripts in the rat hippocampus. Brain Res Mol Brain Res. 1998;61:147–153. doi: 10.1016/s0169-328x(98)00222-8. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; New York: 1998. [Google Scholar]
- Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Barrientos RM, O’Reilly RC. Hippocampal formation supports conditioning to memory of a context. Behav Neurosci. 2002;116:530–538. doi: 10.1037//0735-7044.116.4.530. [DOI] [PubMed] [Google Scholar]
- Russo-Neustadt A, Beard RC, Cotman CW. Exercise, antidepressant medications, and enhanced brain derived neurotrophic factor expression. Neuropsychopharmacology. 1999;21:679–682. doi: 10.1016/S0893-133X(99)00059-7. [DOI] [PubMed] [Google Scholar]
- Russo-Neustadt A, Ha T, Ramirez R, Kesslak JP. Physical activity-antidepressant treatment combination: impact on brain- derived neurotrophic factor and behavior in an animal model. Behav Brain Res. 2001;120:87–95. doi: 10.1016/s0166-4328(00)00364-8. [DOI] [PubMed] [Google Scholar]
- Russo-Neustadt AA, Chen MJ. Brain-derived neurotrophic factor and antidepressant activity. Curr Pharm Des. 2005;11:1495–1510. doi: 10.2174/1381612053764788. [DOI] [PubMed] [Google Scholar]
- Saarelainen T, Hendolin P, Lucas G, Koponen E, Sairanen M, MacDonald E, Agerman K, Haapasalo A, Nawa H, Aloyz R, Ernfors P, Castren E. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J Neurosci. 2003;23:349–357. doi: 10.1523/JNEUROSCI.23-01-00349.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarelainen T, Vaittinen S, Castren E. trkB-receptor activation contributes to the kainate-induced increase in BDNF mRNA synthesis. Cell Mol Neurobiol. 2001;21:429–435. doi: 10.1023/A:1012775808253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Schaaf MJ, de KlER, Vreugdenhil E. Downregulation of BDNF mRNA and protein in the rat hippocampus by corticosterone. Brain Res. 1998;813:112–120. doi: 10.1016/s0006-8993(98)01010-5. [DOI] [PubMed] [Google Scholar]
- Schulte-Herbruggen O, Chourbaji S, Muller H, Danker-Hopfe H, Brandwein C, Gass P, Hellweg R. Differential regulation of nerve growth factor and brain-derived neurotrophic factor in a mouse model of learned helplessness. Exp Neurol. 2006 doi: 10.1016/j.expneurol.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. Am J Psychiatry. 2003;160:1516–1518. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci U S A. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2002;22:3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siuciak JA, Lewis DR, Wiegand SJ, Lindsay RM. Antidepressant-like effect of brain-derived neurotrophic factor (BDNF. Pharmacol Biochem Behav. 1997;56:131–137. doi: 10.1016/S0091-3057(96)00169-4. [DOI] [PubMed] [Google Scholar]
- Smith MA, Makino S, Kvetnansky R, Post RM. Effects of stress on neurotrophic factor expression in the rat brain. Ann N Y Acad Sci. 1995a;771:234–239. doi: 10.1111/j.1749-6632.1995.tb44684.x. [DOI] [PubMed] [Google Scholar]
- Smith MA, Makino S, Kvetnansky R, Post RM. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci. 1995b;15:1768–1777. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solberg LC, Hortaon TH, Turek FW. Circadian rhythms and depression: effects of exercise in an animal model. American Journal of Physiology. 1999;276:R152–R161. doi: 10.1152/ajpregu.1999.276.1.R152. [DOI] [PubMed] [Google Scholar]
- Sternberg DE, Jarvik ME. Memory functions in depression. Arch Gen Psychiatry. 1976;33:219–224. doi: 10.1001/archpsyc.1976.01770020055009. [DOI] [PubMed] [Google Scholar]
- Ueyama T, Kawai Y, Nemoto K, Sekimoto M, Tone S, Senba E. Immobilization stress reduced the expression of neurotrophins and their receptors in the rat brain. Neurosci Res. 1997;28:103–110. doi: 10.1016/s0168-0102(97)00030-8. [DOI] [PubMed] [Google Scholar]
- Vaidya VA, Marek GJ, Aghajanian GK, Duman RS. 5-HT2A receptor-mediated regulation of brain-derived neurotrophic factor mRNA in the hippocampus and the neocortex. J Neurosci. 1997;17:2785–2795. doi: 10.1523/JNEUROSCI.17-08-02785.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya VA, Terwilliger RM, Duman RS. Role of 5-HT2A receptors in the stress-induced down-regulation of brain- derived neurotrophic factor expression in rat hippocampus. Neurosci Lett. 1999;262:1–4. doi: 10.1016/s0304-3940(99)00006-3. [DOI] [PubMed] [Google Scholar]
- Van Hoomissen JD, Chambliss HO, Holmes PV, Dishman RK. Effects of chronic exercise and imipramine on mRNA for BDNF after olfactory bulbectomy in rat. Brain Res. 2003;974:228–235. doi: 10.1016/s0006-8993(03)02584-8. [DOI] [PubMed] [Google Scholar]
- Van Hoomissen JD, Holmes PV, Zellner AS, Poudevigne A, Dishman RK. Effects of beta-adrenoreceptor blockade during chronic exercise on contextual fear conditioning and mRNA for galanin and brain-derived neurotrophic factor. Behav Neurosci. 2004;118:1378–1390. doi: 10.1037/0735-7044.118.6.1378. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999a;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999b;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- van Praag HM. Can stress cause depression? World J Biol Psychiatry. 2005;6(Suppl 2):5–22. doi: 10.1080/15622970510030018. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20:2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- Vaynman SS, Ying Z, Yin D, Gomez-Pinilla F. Exercise differentially regulates synaptic proteins associated to the function of BDNF. Brain Res. 2006;1070:124–130. doi: 10.1016/j.brainres.2005.11.062. [DOI] [PubMed] [Google Scholar]
- Vellucci SV, Parrott RF, Mimmack ML. Down-regulation of BDNF mRNA, with no effect on trkB or glucocorticoid receptor m RNAs, in the porcine hippocampus after acute dexamethasone treatment. Res Vet Sci. 2001;70:157–162. doi: 10.1053/rvsc.2001.0456. [DOI] [PubMed] [Google Scholar]
- Vermetten E, Bremner JD. Circuits and systems in stress. I. Preclinical studies. Depress Anxiety. 2002;15:126–147. doi: 10.1002/da.10016. [DOI] [PubMed] [Google Scholar]
- Vollmayr B, Faust H, Lewicka S, Henn FA. Brain-derived-neurotrophic-factor (BDNF) stress response in rats bred for learned helplessness. Mol Psychiatry. 2001;6:471–474. doi: 10.1038/sj.mp.4000907. 358. [DOI] [PubMed] [Google Scholar]
- Zetterstrom TS, Pei Q, Madhav TR, Coppell AL, Lewis L, Grahame-Smith DG. Manipulations of brain 5-HT levels affect gene expression for BDNF in rat brain. Neuropharmacology. 1999;38:1063–1073. doi: 10.1016/s0028-3908(99)00022-2. [DOI] [PubMed] [Google Scholar]
- Zheng H, Liu Y, Li W, Yang B, Chen D, Wang X, Jiang Z, Wang H, Wang Z, Cornelisson G, Halberg F. Beneficial effects of exercise and its molecular mechanisms on depression in rats. Behav Brain Res. 2006;168:47–55. doi: 10.1016/j.bbr.2005.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]


