Abstract
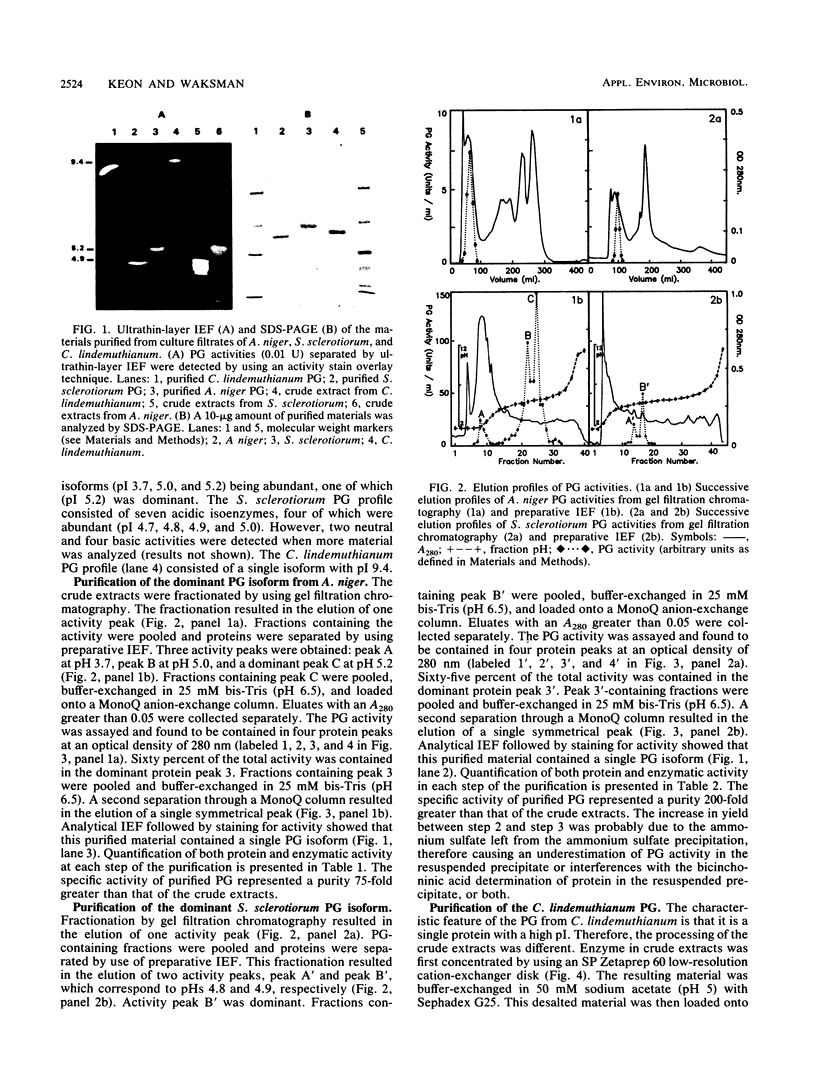
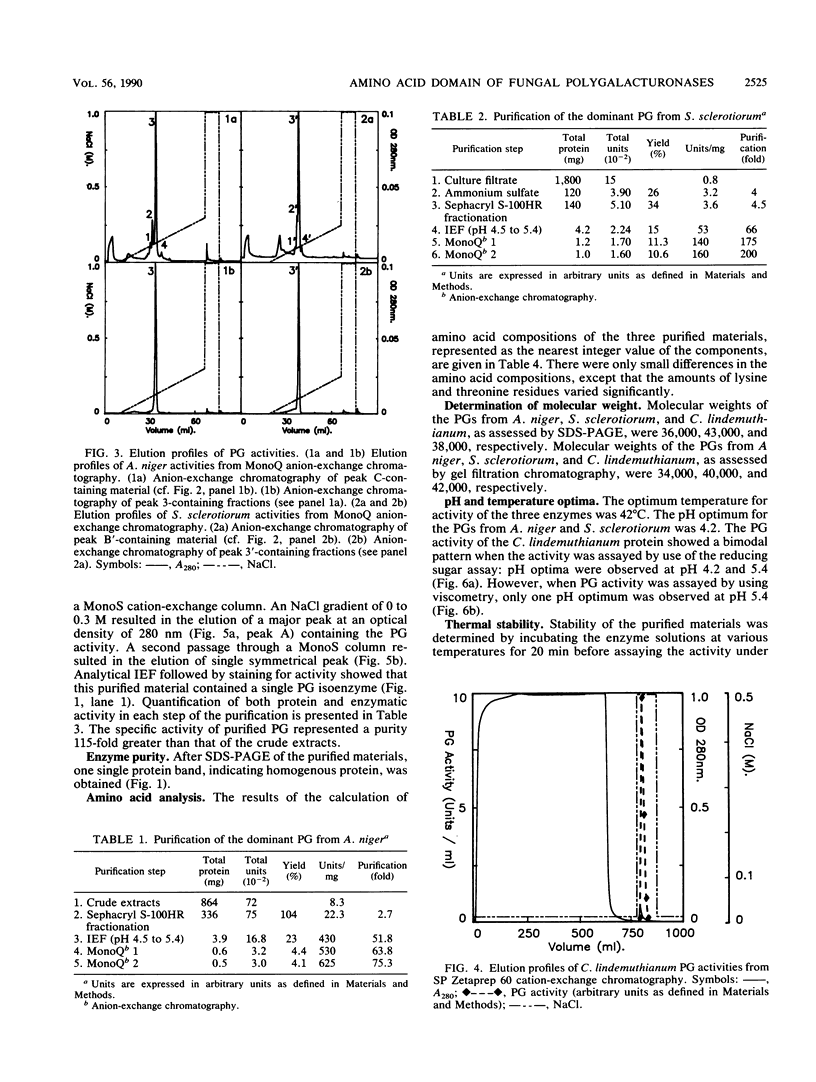
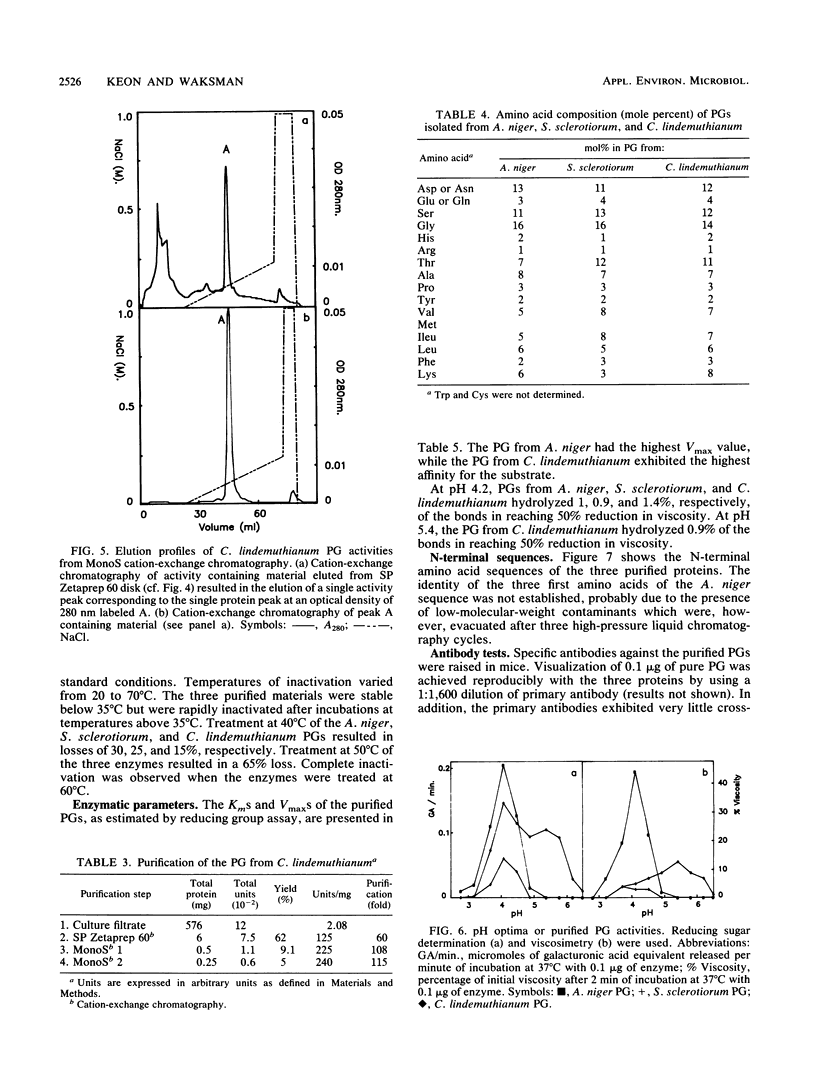
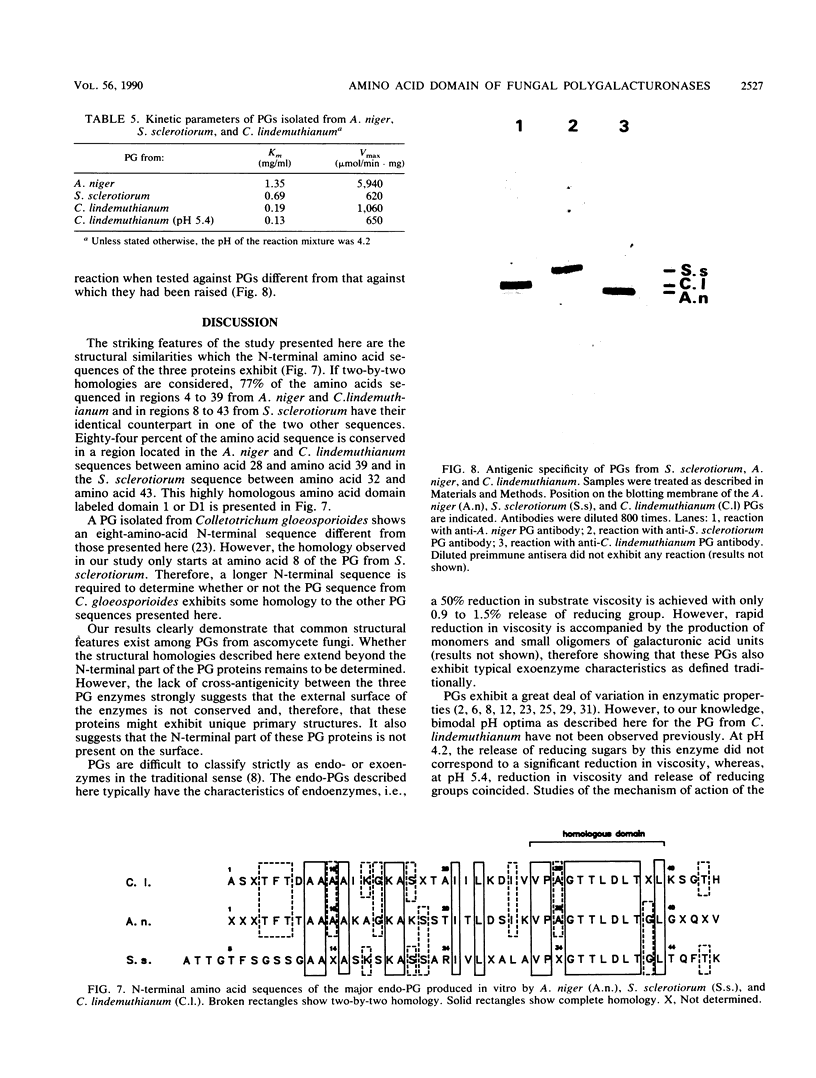
The endopolygalacturonase (EC 3.2.1.15) enzymes produced in vitro by three ascomycete fungi, Aspergillus niger, Sclerotinia sclerotiorum, and Colletotrichum lindemuthianum were studied by using thin-layer isoelectric focusing and activity stain overlay techniques. The polygalacturonases from A. niger and S. sclerotiorum consisted of numerous isoforms, whereas the endopolygalacturonase from C. lindemuthianum consisted of a single protein species. The most abundant endopolygalacturonase isoform produced by each of these organisms was purified and characterized. Biochemical parameters, including molecular weight, isoelectric point, kinetic parameters, temperature and pH optima, and thermal stability, were determined. Considerable differences in physical and chemical properties were demonstrated among these fungal polygalacturonases. Antibodies raised against individual proteins exhibited little cross-reaction, suggesting that these enzymes differ structurally as well as biochemically. In contrast, the analysis of the N-terminal amino acid sequences of the three proteins showed extensive homology, particularly in a region labeled domain 1 in which 84% of the amino acids were conserved.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bidlingmeyer B. A., Cohen S. A., Tarvin T. L. Rapid analysis of amino acids using pre-column derivatization. J Chromatogr. 1984 Dec 7;336(1):93–104. doi: 10.1016/s0378-4347(00)85133-6. [DOI] [PubMed] [Google Scholar]
- DEMAIN A. L., PHAFF H. J. Hydrolysis of the oligogalacturonides and pectic acid by yeast polygalacturonase. J Biol Chem. 1954 Sep;210(1):381–393. [PubMed] [Google Scholar]
- English P. D., Maglothin A., Keegstra K., Albersheim P. A Cell Wall-degrading Endopolygalacturonase Secreted by Colletotrichum lindemuthianum. Plant Physiol. 1972 Mar;49(3):293–298. doi: 10.1104/pp.49.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karr A. L., Albersheim P. Polysaccharide-degrading Enzymes are Unable to Attack Plant Cell Walls without Prior Action by a "Wall-modifying Enzyme". Plant Physiol. 1970 Jul;46(1):69–80. doi: 10.1104/pp.46.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller A., Neukom H. Untersuchungen über den Abbaumechanismus einer gereinigten Polygalakturonase aus Aspergillus niger. Eur J Biochem. 1969 Feb;7(4):485–489. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Rexová-Benková L. The size of the substrate-binding site of an Aspergillus niger extracellular endopolygalacturonase. Eur J Biochem. 1973 Nov 1;39(1):109–115. doi: 10.1111/j.1432-1033.1973.tb03109.x. [DOI] [PubMed] [Google Scholar]
- Ried J. L., Collmer A. Activity stain for rapid characterization of pectic enzymes in isoelectric focusing and sodium dodecyl sulfate-polyacrylamide gels. Appl Environ Microbiol. 1985 Sep;50(3):615–622. doi: 10.1128/aem.50.3.615-622.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand L. L., Corden M. E., MacDonald D. L. Characterization of two endopolygalacturonase isozymes produced by Fusarium oxysporum f. sp. lycopersici. Biochim Biophys Acta. 1976 May 13;429(3):870–883. doi: 10.1016/0005-2744(76)90333-8. [DOI] [PubMed] [Google Scholar]
- Waksman G. Purification of the beta-glucosidase from Sclerotinia sclerotiorum. Biochim Biophys Acta. 1988 Oct 13;967(1):82–86. doi: 10.1016/0304-4165(88)90191-2. [DOI] [PubMed] [Google Scholar]
- Wang M. C., Keen N. T. Purification and characterization of endopolygalacturonase from Verticillium albo-atrum. Arch Biochem Biophys. 1970 Dec;141(2):749–757. doi: 10.1016/0003-9861(70)90193-1. [DOI] [PubMed] [Google Scholar]