Introduction
Cholestasis is a clinical syndrome that results from the disturbances in the formation of bile, a unique and vital property of the liver. The causes of cholestasis are broad and range from rare genetic diseases and disruption of the normal development of the bile excretory anatomy, to progressive, ultimately fatal diseases such as primary biliary cirrhosis and sclerosing cholangitis(1). Cholestasis may also develop from mechanical obstructions in the extrahepatic bile ducts or during the course of viral hepatitis or the administration of certain drugs and hormones. Whatever the cause, the syndrome is manifested by the hepatic retention of products normally excreted into bile, in particular bile salts. As the process progresses with time, jaundice and hypercholesterolemia may follow. The syndrome is almost always characterized by an elevation in serum alkaline phosphatase and if progressive, fibrosis, cirrhosis and clinical signs of liver failure ultimately develop.
During the past decade, as the molecular basis of bile formation has been clarified, the pathogenesis of many cholestatic disorders has also evolved resulting in the following paradigm.
Determinants of bile secretion undergo an adaptive response during cholestasis which tend to minimize hepatic injury. This adaptation occurs by: (a) limiting hepatic uptake of bile acids and other organic solutes, (b) reducing bile acid synthesis, (c) accelerating bile acid detoxification and (d) up-regulating alternative pathways for excretion of bile salts and other solutes in liver, kidney and intestine
Much of this information has resulted from the study of animal models of cholestasis, which with some exceptions have been confirmed in patients with cholestasis (1–3). The molecular basis of this process, which underlies current efforts to intervene clinically, has resulted from 3 fundamental developments: 1) The cloning of transporters that are the determinants of hepatobiliary secretion (greatly advanced by the completion of the human genome project) (1). 2) The discovery that mutations in some of these transporters result in cholestatic liver disease in patients (thereby providing “proof of principle” for their function (4) and 3) The evolving field of nuclear receptors which now enable a partial understanding of how these critical transporter and metabolism genes are transcriptionally regulated (5;6) (Fig 1).
Fig 1.
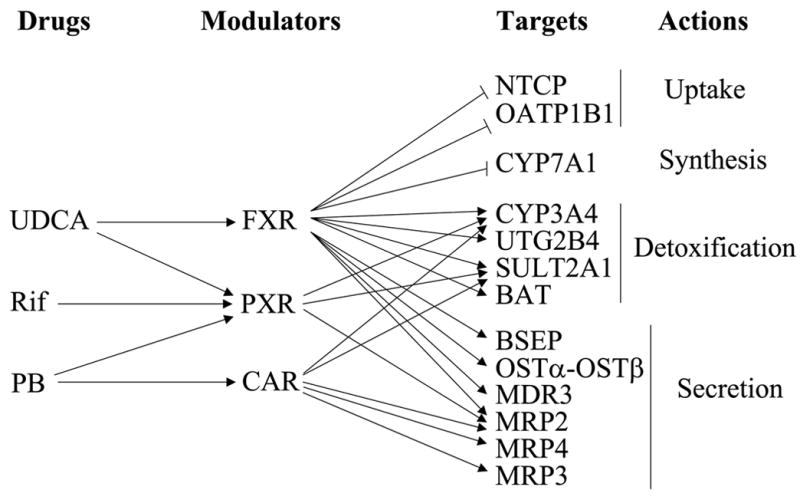
Drugs that regulate hepatic bile acid and other organic solute uptake, synthesis, detoxification and secretion. The figure illustrates their action as nuclear receptor ligands that inhibit or stimulate transcription of key transporters and enzymes in this pathway. UDCA = ursodeoxycholic acid; Rif = Rifampicin; PB = phenobarbital
It is obvious that were these adaptive responses more robust, progressive cholestatic liver diseases might not occur or at least might become more protracted over time. Therefore the central question is whether therapy can be devised that might augment these potentially beneficial adaptive responses in expression of transporters and metabolic pathways? This commentary reviews the molecular basis of bile formation and the adaptive responses that are known to occur in cholestatic liver injury. It follows with a summary of current as well as possible future therapies for jaundice and cholestasis that are mediated by nuclear receptor ligands.
Molecular Basis of Hepatic Bile formation and metabolism and their adaptive responses and regulation during cholestasis
Steps in bile formation and metabolism can be divided into 4 phases: Phase 0 (hepatic uptake); Phase I (hydroxylation ); Phase II (conjugation) and Phase 3 (Excretion) of more water soluble conjugates into blood and bile)
Phase 0
The determinants of hepatic uptake of bile constituents include the sodium taurocholate co-transporting polypeptide (NTCP, SLC10A1) which is largely responsible for the first pass clearance of conjugated bile salts as they are returned to the liver in portal blood. Other unconjugated bile salts as well as bilirubin are variably taken up by one or more of 4 members of the solute carrier organic anion transporter family(SLC21), formally known as organic anion transporting polypeptides (OATPs) (7;8). For bilirubin this appears to be SLC21A6(9). These non-specific carriers also facilitate the uptake of many different organic anions, oligopeptides, and bulky organic cations. The transcription of NTCP is regulated by several different nuclear receptors including hepatocyte nuclear factor 1 (HNF1) and HNF4, the retinoic acid receptor α RARα and the short heterodimeric partner, SHP (NROB2), a nuclear receptor that is regulated by the farnesoid X receptor (FXR, NR1H4), and therefore bile acids, which are specific FXR ligands) (5;6;10). Small organic cations are taken up by another solute carrier, the organic cation transporter 1(OCT1), SLC22A1 (11).
All of these gene products are located on the basolateral membrane of hepatocytes. The expression of all of these uptake systems except OATP1A2 (OATP-A) are down-regulated during cholestasis (12;13). The later may reverse direction and function to facilitate efflux of substrates from the cholestatic hepatocytes. Since OATP1B3 (OATP-8) is directly regulated by FXR, its role as a bile acid efflux mechanisms also remains a possibility (14;15).
Phase l
Once within the hepatocytes, lipid soluble constituents undergo hydroxylation reactions mediated by the cytochrome P-450 (CYP) enzyme system. Many specific CYP enzymes carry out this function in human hepatocytes although the majority of drugs are metabolized by CYP3A4. Bile acids are formed from cholesterol via CYP7A1 and CYP8B1(10) whose activity is inhibited by FXR via SHP. Many CYPs are regulated by the nuclear receptors PXR and CAR whose ligands or activators include many exogenous xenobiotics (16;17). During cholestasis bile acid metabolism is altered in a species specific manner such that less toxic compounds are formed and alternative pathways are selected (18;19).
Phase ll
metabolism consists of conjugation reactions that confer greater aqueous solubility to bile acids and other lipophilic constituents. Newly synthesized bile acids are normally conjugated with taurine or glycine prior to excretion into bile by bile acid-CoA synthetase and bile acid-CoA:amino acid N-acetyltransferase. During cholestasis conjugation reactions favor the formation of bile acid glucuronides and sulfates which are substrates for the multidrug resistance associated protein 2 and 3 (MRP2, ABCC2 and MRP3, ABCC3). These enzymatic reactions are mediated by uridine glucuronyl transferases (UGT2B4) and sulfotransferases (SULT2A1)) respectively. Expression of these phase 11 enzymes increases during cholestasis mediated in part by the bile acid nuclear receptors PXR and FXR and possibly VDR (20;21). Bilirubin is normally conjugated with glucuronides prior to excretion and several steps in the clearance of bilirubin, including its uptake via OATP1B1, intracellular bind to glutathione S- transferase (GSTA1), another phase 11 enzyme), conjugation with UGT1A1 and excretion via MRP2 (and MRP3 during cholestasis (22)) appear to be regulated in part by the nuclear receptor CAR based on studies in the Car null mouse (23;24), whose activator in these circumstances may be bilirubin (25).
Phase 3
Finally these potentially toxic substances are normally excreted from the liver into bile. Bile salt dependent (BSDF) and bile salt independent (BSIF) bile flow are largely generated by the bile salt export pump (BSEP, ABCB11) and MRP2, respectively (1). BSEP and MRP2 are members of the ABC superfamily of ATP dependent transporters. While BSEP is relatively specific for the biliary transport of bile acids, MRP2 exports glutathione and numerous other organic anions into bile usually conjugated with glutathione, glucuronide or sulfate conjugates (26). Both of these transporters are located on the canalicular membrane of the hepatocytes. Mutations in BSEP result in a variable spectrum of cholestatic diseases ranging from Progressive Familial Intrahepatic cholestasis type 2 (PFIC-2) in infants to Benign Recurrent Intrahepatic Cholestasis (BRIC-2) and Intrahepatic Cholestasis of Pregnancy (ICP) in adults (1;4). Mutations in MRP2 result in the Dubin-Johnson Syndrome manifested by direct reacting hyperbilirubinemia while polymorphisms in these transporters may predispose to drug induced cholestasis (27).
A third member of the ABC superfamily, the multidrug resistanace protein 3 (MDR3, ABCB4), is a phospholipid export pump, that functions to translocate phosphatidylcholine to the outer leaflet of the canalicular membrane, facilitating the excretion of this lipid into bile where it associates with bile salts and cholesterol in lipid micelles. Mutations in MDR3 result in deficiencies of phosphatidylcholine in bile, and variable forms of either PFIC type 3 or ICP. Cholestasis occurs presumably because bile salts are no longer able to form micelles in bile are free to exert their detergent properties on lipids lining the canalicular and biliary epithelium (28;29).
The expression of BSEP and MDR3 is highly regulated by the nuclear receptor FXR and thus tends to be maintained if not up-regulated at the canalicular membrane during cholestasis as bile acid concentrations in the liver rise (30;31).
Nevertheless, these adaptive changes are insufficient to prevent cholestasis from developing leading to up-regulation of several other transporters at the sinusoidal surface of the liver. These include MRP3 and MRP4 (ABCC4) and the heteromeric Organic Solute Transporter (OST) composed of two subunits, alpha and beta (OSTα/β) (32;33). Based on experiments in Mrp3 and Mrp4 knockout mice, the export of bilirubin conjugates is thought to be largely mediated by Mrp3 (and possible OATP1A1) whereas bile acid divalent conjugates (Sulfates and glucuronides) may be preferable substrates for Mrp4 (34;35). However in-vitro studies indicate that both human MRP3 and MRP4 transporters are capable of transporting bile acid conjugates (36;37) which can then be excreted via the urine according to animal studies, as a result of a combination of glomerular filtration, down regulation of, the sodium dependent apical bile acid transporter (Asbt) in the proximal tubule, and the up-regulation of renal Mrp2 and Mrp4 (6;38).
In human liver, bile acid efflux across the basolateral membrane of hepatocytes and cholangiocytes is also facilitated by OSTα/β which is up-regulated in human cholestatic disorders, particularly primary biliary cirrhosis (33). Efflux is driven by the rise in bile acid concentrations in the liver. OSTα/β is highly regulated by FXR with 2 identified FXR response elements in the human OSTα promoter and one in OSTβ (39).
Cholangiocytes proliferate in many cholestatic disorders and thus also play a role in the adaptive response to cholestasis by continuing to express ASBT on the luminal surface and MRP3, MRP4 and OSTα/β on their basolateral surface. Thus bile acids that accumulate within obstructed bile ducts may return in part to the circulation and follow similar paths to the urine.
Nuclear receptor medicine in patients with jaundice and cholestatic disorders
Since none of these adaptive responses described briefly above is capable of preventing chronic cholestatic disorders from ultimately progressing, attention is directed to potential mechanisms for augmenting this response. Clinical experience suggests that this is a rational approach.
Constitutive androstane receptor, CAR (NR1I3) activators
Recent studies have demonstrated that Yin Shi Huang, an herbal medicine containing Yin Chin (Artemisia capillairis) and several other plants, and used in Asia to treat neonatal jaundice, is a potent activator of the nuclear receptor CAR (24). Treatment with either Yin Shi Huang or Yin Chin in humanized CAR mice but not in CAR null mice stimulate the expression of all of the critical steps in hepatic bilirubin clearance including SLC1A6 (OATP-C), the presumptive bilirubin uptake transporter, GSTA1 and A2, the cytosolic binding proteins, UGT1A1, the bilirubin conjugating enzyme and MRP2, the bilirubin conjugate export pump. This treatment also enhanced the clearance of bilirubin infusions. 6,7-dimethylesculetin, a compound present in Yin Chin, activated CAR in primary hepatocytes, suggesting that this may be the primary ingredient.
Inchin-ko-to is another herbal medicine also used for treatment of jaundice in Japan and China. Inchin-ko-to is a potent choleretic and a known inducer of Mrp2 (40).
Phenobarbital is also a strong CAR activator, and stimulates the translocation of CAR from the cytoplasm to the nucleus where CAR exerts its broad effects on nuclear transcription (41). Phenobarbital has been used to treat patients with primary biliary cirrhosis and results in a decrease in serum bilirubin and bile acids, a change in the serum chenodeoxycholic:cholic acid ratio and some amelioration of symptoms of pruritus (42). Side effects including mild sedation and formation of inactive vitamin D metabolites, precluded its adoption in clinical practice. One study compared Phenobarbital with Rifampicin in PBC but found the later drug more effective (43)
Pregnane X receptor, PXR (NR1I2) agonists
Rifampicin, a strong PXR ligand, has been used successfully to treat pruritus in children and adults and has also led to improvement in liver function tests in patients with PBC (44–46). Rifampicin is a strong inhibitor of human CYP7A and thus should block bile acid synthesis (47). Previous studies in healthy volunteers noted increased bile acid glucuronide excretion in urine with rifampin and speculated that that bile acid 6-alpha hydroxylation was mediated by stimulating CYP3A4 followed by glucuronidation (48).
Further studies demonstrated that a two week treatment in gallstone patients prior to surgery indeed results in up-regulation of mRNAs for CYP3A4, UGT1A1 and MRP2, all regulated presumably by PXR (5;49). Side effects of rifampicin include drug induced hepatitis, although the drug is well tolerated in most patients.
Ursodoxycholic acid is currently accepted as the standard of care for treatment of primary biliary cirrhosis and is often used in other cholestatic disorders with variable beneficial effects (50;51). Long term studies in PBC indicate that UDCA therapy normalizes life expectancy in patients with early stage 1 or 2 disease on liver biopsies and delays progression of fibrosis (51). While the mechanism of this beneficial effect is undoubtedly complex, UDCA is a relatively strong PXR agonist and a weaker FXR agonist. Animal studies suggest that PXR and CAR deficiency in mice predisposes these null mice to more significant cholestatic liver injury and thus agonists of these nuclear receptors might be therapeutic (52). Thus it seems possible that some of UDCA’s beneficial effects might be mediated via PXR. Indeed studies in FXR null mice indicate that UDCA increases bile acid hydroxylation and Mrp4 expression independently of FXR (53;54). However other in-vitro studies indicate that UDCA can also increase BSEP mRNA as well as FXR protein in human hepatoma cells (55). Treatment of gallstone patients for two weeks with UDCA has led to an increase in expression of BSEP, MDR3, and MRP4 (49) so that it is likely that these effects of UDCA are mediated via FXR for induction of BSEP and MDR3 and via PXR for induction of MRP4. PXR is transcriptionally active in stellate cells which inhibit transformation and proliferation so that it is possible that the antifibrotic effects of UDCA are also mediated via PXR (56). However, in contrast to rifampicin, UDCA induction of CYP3A metabolism has not been demonstrated in patients with Primary Biliary Cirrhosis or healthy volunteers (57).
UDCA also has post-transcriptional effects that may be beneficial in cholestasis including stimulating the apical targeting of Bsep and Mrp2 to the apical membrane from sub-membranous compartments as well as anti-apoptotic effects when induced by more toxic bile acids (58).
Farnesoid X receptor, FXR (NR1H4) agonists
All of the major transport steps in the enterohepatic circulation of bile acids are regulated by FXR (59) This includes 1) down regulation of NTCP indirectly via FXR activation of SHP. SHP suppresses HNF-4 which in turn leads to repression of HNF-1, a major activator of NTCP); 2) FXR activation of expression of BSEP, MDR3, and OSTα/β. FXR ligands should also inhibit bile acid synthesis since CYP7A1 and CYP8B1 are inhibited by SHP via activation of FXR (19). Bile acid conjugation with taurine and glycine is activated by targets of FXR (bile acid-CoA synthetase and bile acid-CoA :amino acid N-acetyltransferase (60). Therefore beneficial adaptive responses in these enzymes and transport steps might be enhanced by drugs designed as FXR ligands. This knowledge has led to the development of several potent FXR ligands, particularly GW4064 (61) and 6-Ethyl chenodeoxycholic acid (6-ECDCA), each of which have binding affinities for the ligand binding domain of FXR that are significantly greater than CDCA, the most potent endogenous FXR ligand (62). Studies in experimental animal models of cholestasis including common bile duct obstruction in the rat and mouse, lithocholic acid administration and ethinyl estradiol administration in rats all demonstrate that co-administration of these synthetic FXR ligands attenuates the cholestatic injury in these short term experiments, thus providing proof of principle (63;64). Other studies also demonstrate an anti-fibrotic role for 6-ECDCA (65;66). Therefore Clinical trials of these novel FXR agonists in cholestatic patients will be of interest.
Vitamin D receptor, VDR (NR1I1) agonists
Lithocholic acid, the most hydrophobic and cholestatic bile acid is a potent ligand for VDR (67). VDR is one of the activators of CYP3A4 and SULT2A1 (68), two enzymes that are needed to detoxify lithocholic acid through hydroxylation (phase 1) and subsequent sulfation (phase 11) reactions.
Conclusions
Based on current knowledge of the diverse effects of nuclear receptor ligands and activators on bile acid metabolism and bile acid transporters in the enterohepatic circulation and kidney summarized here primarily for humans (and recently elsewhere (6;59;69–71), nuclear receptor medicine is already being used to treat certain patients with cholestatic liver disease.
The central role of FXR in the regulation of bile acid transporters and bile acid synthesis suggest that clinical trials with potent FXR ligands should proceed in selected patients with chronic cholestasis. However, they will need to proceed carefully. Recent studies also indicate that FXR is a regulator of lipid and glucose metabolism (72–76). In-vitro studies and findings in FXR null mice suggest that FXR has an anti-atherogenic role (77). Older clinical trials that assessed the effect of CDCA on gallstone dissolution in patients observed parallel declines in plasma triglyceride levels consistent with these more recent findings (78;79). Studies of FXR effects on carbohydrate metabolism are less clear and will require close monitoring once FXR agonists enter clinical practice.
Also, as this field evolves, it is important to recognize that observations of beneficial effects of nuclear receptor ligands, activators and antagonists in animal models of cholestasis may not necessarily extrapolate to humans. In cholestatic mice, bile acids are converted into more hydrophilic and therefore less toxic bile acid metabolites than are seen in man. Thus results concerning therapeutic benefit may lead to inappropriate conclusions. One such example is a recent study in FXR null mice following bile duct obstruction that suggests that FXR antagonists, rather than agonists may be beneficial in obstructive cholestatic disease (80).
Finally, it is likely that combinations of small molecules that act as nuclear receptor ligands or activators may provide more promising results in patients with cholestasis then monotherapy, particularly if administered early in the course of the disease before maximum endogenous adaptive responses have already occurred. Since Rifampicin and UDCA induce different groups of transporters, one might predict that combination therapy with Rifampicin and UDCA and possibly other nuclear receptor ligands, including the more potent FXR agonists might be of even greater clinical benefit. It is an exciting time for this field when discoveries in basic science are shedding light on the mechanisms of old empiric therapies for cholestatic liver disease while at the same time provide novel insights for new treatment opportunities.
Footnotes
Supported in part by USPHS grants DK25636 and The Yale Liver Center DK P30-34989
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Trauner M, Meier PJ, Boyer JL. Molecular pathogenesis of cholestasis. N Eng J Med. 1998;339:1217–27. doi: 10.1056/NEJM199810223391707. [DOI] [PubMed] [Google Scholar]
- 2.Trauner M, Boyer JL. Bile Salt Transporters: Molecular Characterization, Function and Regulation. Physiol Rev. 2003;83:633–71. doi: 10.1152/physrev.00027.2002. [DOI] [PubMed] [Google Scholar]
- 3.Zollner G, Fickert P, Silbert D, Fuchsbichler A, Marschall H-U, Zatloukal K, Denk H, Trauner M. Adaptive changes in hepatobiliary transporter expression in primary biliary cirrhosis. Journal of Hepatology. 2003;38:717–27. doi: 10.1016/s0168-8278(03)00096-5. [DOI] [PubMed] [Google Scholar]
- 4.Jansen PL, Muller M, Sturm E. Genes and cholestasis. Hepatol. 2001;34:1067–74. doi: 10.1053/jhep.2001.29625. [DOI] [PubMed] [Google Scholar]
- 5.Boyer JL. Nuclear receptor ligands: rational and effective therapy for chronic cholestatic liver disease? Gastroenterology. 2005;129:735–40. doi: 10.1016/j.gastro.2005.06.053. [DOI] [PubMed] [Google Scholar]
- 6.Zollner G, Marschall HU, Wagner M, Trauner M. Role of nuclear receptors in the adaptive response to bile acids and cholestasis: pathogenetic and therapeutic considerations. Mol Pharm. 2006;3:231–51. doi: 10.1021/mp060010s. [DOI] [PubMed] [Google Scholar]
- 7.Hagenbuch B, Meier PJ. The superfamily of organic anion transporting polypeptides. Biochim Biophys Acta. 2003;1609:1–18. doi: 10.1016/s0005-2736(02)00633-8. [DOI] [PubMed] [Google Scholar]
- 8.Hagenbuch B, Meier PJ. Organic anion transporting polypeptides of the OATP/SLC21 family: phylogenetic classification as OATP/SLCO superfamily, new nomenclature and molecular/functional properties. Pflugers Arch. 2004;447:653–65. doi: 10.1007/s00424-003-1168-y. [DOI] [PubMed] [Google Scholar]
- 9.Cui Y, Konig J, Leier I, Buchholz U, Keppler D. Hepatic uptake of bilirubin and its conjugates by the human organic anion-transporter SLC21A6. J Biol Chem. 2001;276:9626–30. doi: 10.1074/jbc.M004968200. [DOI] [PubMed] [Google Scholar]
- 10.Kullak-Ublick GA, Stieger B, Meier PJ. Enterohepatic bile salt transporters in normal physiology and liver disease. Gastroenterology. 2004;126:322–42. doi: 10.1053/j.gastro.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Jonker JW, Schinkel AH. Pharmacological and physiological functions of the polyspecific organic cation transporters: OCT1, 2, and 3 (SLC22A1–3) J Pharmacol Exp Ther. 2004;308:2–9. doi: 10.1124/jpet.103.053298. [DOI] [PubMed] [Google Scholar]
- 12.Lee J, Boyer JL. Molecular alterations in hepatocyte transport mechanisms in acquired cholestatic liver disorders. Semin Liv Dis. 2000;20:373–84. doi: 10.1055/s-2000-9390. [DOI] [PubMed] [Google Scholar]
- 13.Kullak-Ublick G-A, Beuers U, Fahney C, Hagenbuch B, Meier PJ, Paumgartner G. Identification and functional characterization of the promoter region of the human organic anion transporting polypeptide gene. Hepatol. 1997;26:991–7. doi: 10.1002/hep.510260429. [DOI] [PubMed] [Google Scholar]
- 14.Jung D, Podvinec M, Meyer UA, Mangelsdorf DJ, Fried M, Meier PJ, Kullak-Ublick GA. Human Organic Anion Transporting Polypeptide 8 Promoter is Transactivated by the Farnesoid X Receptor/Bile Acid Receptor. Gastroenterology. 2002;122:1954–66. doi: 10.1053/gast.2002.33583. [DOI] [PubMed] [Google Scholar]
- 15.Briz O, Romero MR, Martinez-Becerra P, Macias RI, Perez MJ, Jimenez F, San Martin FG, Marin JJ. Oatp8/1B3-mediated cotransport of bile acids and glutathione. An export pathway for organic anions from hepatocytes? J Biol Chem. 2006 doi: 10.1074/jbc.M602048200. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 16.Wei P, Zhang J, Egan-Hafley M, Liang S, Moore DD. The nuclear receptor CAR mediates specific xenobiotic induction of drug metabolism. Nat (Lond ) 2000;407:920–3. doi: 10.1038/35038112. [DOI] [PubMed] [Google Scholar]
- 17.Maglich JM, Stoltz CM, Goodwin B, Hawkins-Brown D, Moore JT, Kliewer SA. Nuclear pregnane x receptor and constitutive androstane receptor regulate overlapping but distinct sets of genes involved in xenobiotic detoxification. Mol Pharmacol. 2002;62:638–46. doi: 10.1124/mol.62.3.638. [DOI] [PubMed] [Google Scholar]
- 18.Staudinger JL, Goodwin B, Jones SA, Hawkins-Brown D, MacKenzie KI, LaTour A, Liu Y, Klaassen CD, Brown KK, Reinhard J, Willson TM, Koller BH, Kliewer SA. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc Natl Acad Sci U S A. 2001;98:3369–74. doi: 10.1073/pnas.051551698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiang JYL. Regulation of bile acid synthesis: pathways, nuclear receptors, and mechanisms. Journal of Hepatology. 2004;40:539–51. doi: 10.1016/j.jhep.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Barbier O, Torra IP, Sirvent A, Claudel T, Blanquart C, Duran-Sandoval D, Kuipers F, Kosykh V, Fruchart J-C, Staels B. FXR induces the UGT2B4 enzyme in hepatocytes: A potential mechanism of negative feedback control of FXR activity. Gastroenterology. 2003;124:1926–40. doi: 10.1016/s0016-5085(03)00388-3. [DOI] [PubMed] [Google Scholar]
- 21.Echchgadda I, Song CS, Roy AK, Chatterjee B. Dehydroepiandrosterone sulfotransferase is a target for transcriptional induction by the vitamin D receptor. Mol Pharmacol. 2004;65:720–9. doi: 10.1124/mol.65.3.720. [DOI] [PubMed] [Google Scholar]
- 22.Soroka CJ, Lee JM, Azzaroli F, Boyer JL. Cellular localization and up-regulation of multidrug resistance-associated protein 3 in hepatocytes and cholangiocytes during obstructive cholestasis in rat liver. Hepatol. 2001;33:783–91. doi: 10.1053/jhep.2001.23501. [DOI] [PubMed] [Google Scholar]
- 23.Huang W, Zhang J, Moore DD. A traditional herbal medicine enhances bilirubin clearance by activating the nuclear receptor CAR. J of Clin Investigation. 2004;113:137–43. doi: 10.1172/JCI200418385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang W, Zhang J, Moore DD. A traditional herbal medicine enhances bilirubin clearance by activating the nuclear receptor CAR. J Clin Invest. 2004;113:137–43. doi: 10.1172/JCI200418385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Assem M, Schuetz EG, Leggas M, Sun D, Yasuda K, Reid G, Zelcer N, Adachi M, Strom S, Evans RM, Moore DD, Borst P, Schuetz JD. Interactions between hepatic Mrp4 and Sult2a as revealed by the constitutive androstane receptor and Mrp4 knockout mice. J Biol Chem. 2004;279:22250–7. doi: 10.1074/jbc.M314111200. [DOI] [PubMed] [Google Scholar]
- 26.Deeley RG, Westlake C, Cole SP. Transmembrane transport of endo- and xenobiotics by mammalian ATP-binding cassette multidrug resistance proteins. Physiol Rev. 2006;86:849–99. doi: 10.1152/physrev.00035.2005. [DOI] [PubMed] [Google Scholar]
- 27.Pauli-Magnus C, Meier PJ. Hepatocellular transporters and cholestasis. J Clin Gastroenterol. 2005;39:S103–S110. doi: 10.1097/01.mcg.0000155550.29643.7b. [DOI] [PubMed] [Google Scholar]
- 28.De Vree JM, Jacquemin E, Sturm E, Cresteil D, Bosma PJ, Aten J, Deleuze JF, Desrochers M, Burdelski M, Bernard O, Elferink RPO, Hadchouel M. Mutations in the MDR3 gene cause progressive familial intrahepatic cholestasis. Proc Natl Acad Sci USA. 1998;95:282–7. doi: 10.1073/pnas.95.1.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosmorduc O, Hermelin B, Poupon R. Mdr3 gene defect in adults with symptomatic intrahepatic and gallbladder cholesterol cholelithiasis. Gastroenterology. 2001;120:1459–67. doi: 10.1053/gast.2001.23947. [DOI] [PubMed] [Google Scholar]
- 30.Ananthanarayanan M, Balasubramanian N, Makishima M, Mangelsdorf DJ, Suchy FJ. Human bile salt export pump promoter is transactivated by the farnesoid X receptor/bile acid receptor. J Biol Chem. 2001;276:28857–65. doi: 10.1074/jbc.M011610200. [DOI] [PubMed] [Google Scholar]
- 31.Huang L, Zhao A, Lew JL, Zhang T, Hrywna Y, Thompson JR, de Pedro N, Royo I, Blevins RA, Pelaez F, Wright SD, Cui J. Farnesoid X receptor activates transcription of the phospholipid pump MDR3. J Biol Chem. 2003;278:51085–90. doi: 10.1074/jbc.M308321200. [DOI] [PubMed] [Google Scholar]
- 32.Keitel V, Burdelski M, Waqrskulat U, Kuhlkamp T, Keppler D, Haussinger D, Kubitz R. Expression and Localization of Hepatobiliary Transport Proteins in Progressive Familial Intrahepatic Cholestasis. Hepatol. 2005;41:1160–72. doi: 10.1002/hep.20682. [DOI] [PubMed] [Google Scholar]
- 33.Boyer JL, Trauner M, Mennone A, Soroka CJ, Cai SY, Moustafa T, Zollner G, Lee JY, Ballatori N. Up-regulation of a Basolateral FXR-dependent Bile Acid Efflux Transporter, OST{alpha}-OST{beta}, in Cholestasis in Humans and Rodents. Am J Physiol Gastrointest Liver Physiol. 2006 doi: 10.1152/ajpgi.00539.2005. [DOI] [PubMed] [Google Scholar]
- 34.Zelcer N, Wetering K, Waart R, Scheffer GL, Marschall HU, Wielinga PR, Kuil A, Kunne C, Smith A, Valk M, Wijnholds J, Elferink RO, Borst P. Mice lacking Mrp3 (Abcc3) have normal bile salt transport, but altered hepatic transport of endogenous glucuronides. J Hepatol. 2006;44:768–75. doi: 10.1016/j.jhep.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 35.Belinsky MG, Dawson Pa, Shchaveleva I, Bain LJ, Wang R, Ling V, Chen ZS, Grinberg A, Westphal H, Klein-Szanto A, Lerro A, Kruh GD. Analysis of the in vivo functions of Mrp3. Mol Pharmacol. 2005;68:160–8. doi: 10.1124/mol.104.010587. [DOI] [PubMed] [Google Scholar]
- 36.Zelcer N, Reid G, Wielinga P, Kuil A, Van Der Heijden I, Schuetz JD, Borst P. Steriod-and bile acid-conjugates are substrates of human MRP4 (ABCC4) Biochem J. 2003;371:361–7. doi: 10.1042/BJ20021886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akita H, Suzuki H, Hirohashi T, Takikawa H, Sugiyama Y. Transport activity of human MRP3 expressed in Sf9 cells: comparative studies with rat MRP3. Pharm Res. 2002;19:34–41. doi: 10.1023/a:1013699130991. [DOI] [PubMed] [Google Scholar]
- 38.Lee J, Azzaroli F, Wang L, Soroka CJ, Gigliozzi A, Setchell KD, Kramer W, Boyer JL. Adaptive regulation of bile salt transporters in kidney and liver in obstructive cholestasis in the rat. Gastroenterology. 2001;121:1473–84. doi: 10.1053/gast.2001.29608. [DOI] [PubMed] [Google Scholar]
- 39.Landrier JF, Eloranta JJ, Vavricka SR, Kullak-Ublick GA. The Nuclear Receptor for Bile Acids, FXR, Transactivates the Human Organic Solute Transporter -{alpha} and -{beta} Genes. AJP - Gastrointestinal and Liver Physiology. 2005;290:G476–G485. doi: 10.1152/ajpgi.00430.2005. [DOI] [PubMed] [Google Scholar]
- 40.Shoda J, Miura T, Utsunomiya H, Oda K, Yamamoto M, Kano M, Ikegami T, Tanaka N, Akita H, Ito K, Suzuki H, Sugiyama Y. Genipin enhances Mrs2 (Abcc2)-mediated bile formation and organic anion transport in rat liver. Hepatol. 2004;39:167–78. doi: 10.1002/hep.20003. [DOI] [PubMed] [Google Scholar]
- 41.Maglich JM, Parks DJ, Moore LB, Collins JL, Goodwin B, Billin AN, Stoltz CM, Kliewer SA, Lambert MH, Willson TM, Moore JT. Identification of a novel human constitutive androstane receptor (CAR) agonist and its use in the identification of CAR target genes. Journal Biol Chem. 2003;19:17277–83. doi: 10.1074/jbc.M300138200. [DOI] [PubMed] [Google Scholar]
- 42.Bloomer J, Boyer JL. Phenobarbital effects in cholestatic liver disease. Annals of Internal Medicine. 1975;82:310–7. doi: 10.7326/0003-4819-82-3-310. [DOI] [PubMed] [Google Scholar]
- 43.Bachs L, Elena M, Pares A, Piera C, Rodes J. Comparison of rifampicin with phenobarbitone for treatment of pruritus in biliary cirrhosis. The Lancet. 1989;1:574–6. doi: 10.1016/s0140-6736(89)91608-5. [DOI] [PubMed] [Google Scholar]
- 44.Cynamon HA, Andres JM, Iafrate RP. Rifampin relieves pruritus in children with cholestatic liver disease. Gastroenterology. 1990;98:1013–6. doi: 10.1016/0016-5085(90)90027-x. [DOI] [PubMed] [Google Scholar]
- 45.Ghent CN, Carruthers SG. Treatment of pruritus in primary biliary cirrhosis with rifampin. Results of a double-blind, crossover, randomized trial. Gastroenterology. 1988;94:488–93. doi: 10.1016/0016-5085(88)90442-8. [DOI] [PubMed] [Google Scholar]
- 46.Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O, Kodama T, Schoonjans K, Bianco AC, Auwerx J. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nat (Lond ) 2006;439:484–9. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- 47.Li T, Chiang JY. Mechanism of rifampicin and pregnane X receptor inhibition of human cholesterol 7 alpha-hydroxylase gene transcription. Am J Physiol Gastrointest Liver Physiol. 2005;288:G74–G84. doi: 10.1152/ajpgi.00258.2004. [DOI] [PubMed] [Google Scholar]
- 48.Wietholtz H, Marschall HU, Sjovall J, Matern S. Stimulation of bile acid 6 alpha-hydroxylation by rifampin. J Hepatol. 1996;24:713–8. doi: 10.1016/s0168-8278(96)80268-6. [DOI] [PubMed] [Google Scholar]
- 49.Marschall HU, Wagner M, Zollner G, Fickert P, Diczfalusy U, Gumhold J, Silbert D, Fuchsbichler A, Benthin L, Grundstrom R, Gustafsson U, Sahlin S, Einarsson C, Trauner M. Complementary stimulation of hepatobiliary transport and detoxification systems by rifampicin and ursodeoxycholic acid in humans. Gastroenterology. 2005;129:476–85. doi: 10.1016/j.gastro.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 50.Pares A, Caballeria L, Rodes J. Excellent long-term survival in patients with primary biliary cirrhosis and biochemical response to ursodeoxycholic. Acid Gastroenterology. 2006;130:715–20. doi: 10.1053/j.gastro.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 51.Corpechot C, Carrat F, Bahr A, Chretien Y, Poupon RE, Poupon R. The effect of ursodeoxycholic acid therapy on the natural course of primary biliary cirrhosis. Gastroenterology. 2005;128:297–303. doi: 10.1053/j.gastro.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 52.Stedman CA, Liddle C, Coulter SA, Sonoda J, Alvarez JG, Moore DD, Evans RM, Downes M. Nuclear receptors constitutive androstane receptor and pregnane X receptor ameliorate cholestatic liver injury. Proc Natl Acad Sci U S A. 2005;102:2063–8. doi: 10.1073/pnas.0409794102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schuetz EG, Strom S, Yasuda K, Lecureur V, Assem M, Brimer C, Lamba J, Kim RB, Ramachandran V, Komoroski BJ, Venkataramanan R, Cai H, Sinal CJ, Gonzalez FJ, Schuetz JD. Disrupted bile acid homeostasis reveals an unexpected interaction among nuclear hormone receptors, transporters, and cytochrome P450. J Biol Chem. 2001;276:39411–8. doi: 10.1074/jbc.M106340200. [DOI] [PubMed] [Google Scholar]
- 54.Zollner G, Wagner M, Moustafa T, Fickert P, Silbert D, Gumhold J, Fuchsbichler A, Halilbasic E, Denk H, Marschall HU, Trauner M. Coordinated induction of bile acid detoxification and alternative elimination in mice: role of FXR-regulated organic solute transporter-alpha/beta in the adaptive response to bile acids. Am J Physiol Gastrointest Liver Physiol. 2006;290:G923–G932. doi: 10.1152/ajpgi.00490.2005. [DOI] [PubMed] [Google Scholar]
- 55.Lew JL, Zhao A, Yu J, Huang L, de Pedro N, Pelaez F, Wright SD, Cui J. The farnesoid X receptor controls gene expression in a ligand- and promoter-selective fashion. J Biol Chem. 2004;279:8856–61. doi: 10.1074/jbc.M306422200. [DOI] [PubMed] [Google Scholar]
- 56.Haughton EL, Tucker SJ, Marek CJ, Durward E, Leel V, Bascal Z, Monaghan T, Koruth M, Collie-Duguid E, Mann DA, Trim JE, Wright MC. Pregnane X receptor activators inhibit human hepatic stellate cell transdifferentiation in vitro. Gastroenterology. 2006;131:194–209. doi: 10.1053/j.gastro.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 57.Dilger K, Denk A, Heeg MH, Beuers U. No relevant effect of ursodeoxycholic acid on cytochrome P450 3A metabolism in primary biliary cirrhosis. Hepatol. 2005;41:595–602. doi: 10.1002/hep.20568. [DOI] [PubMed] [Google Scholar]
- 58.Paumgartner G. Medical treatment of cholestatic liver diseases: From pathobiology to pharmacological targets. World J Gastroenterol. 2006;12:4445–51. doi: 10.3748/wjg.v12.i28.4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cai SY, Boyer JL. FXR: a target for cholestatic syndromes? Expert Opin Ther Targets. 2006;10:409–21. doi: 10.1517/14728222.10.3.409. [DOI] [PubMed] [Google Scholar]
- 60.Pircher PC, Kitto JL, Petrowski ML, Tangirala RK, Bischoff ED, Schulman IG, Westin SK. Farnesoid x receptor regulates bile acid-amino acid conjugation. J Biol Chem. 2003;278:27703–11. doi: 10.1074/jbc.M302128200. [DOI] [PubMed] [Google Scholar]
- 61.Maloney PR, Parks DJ, Haffner CD, Fivush AM, Chandra G, Plunket KD, Creech KL, Moore LB, Wilson JG, Lewis MC, Jones SA, Willson TM. Identification of a chemical tool for the orphan nuclear receptor FXR. J Med Chem. 2000;43:2971–4. doi: 10.1021/jm0002127. [DOI] [PubMed] [Google Scholar]
- 62.Pellicciari R, Fiorucci S, Camaioni E, Clerici C, Costantino G, Maloney PR, Morelli A, Parks DJ, Willson TM. 6alpha-ethyl-chenodeoxycholic acid (6-ECDCA), a potent and selective FXR agonist endowed with anticholestatic activity. J Med Chem. 2002;45:3569–72. doi: 10.1021/jm025529g. [DOI] [PubMed] [Google Scholar]
- 63.Liu Y, Binz J, Numerick MJ, Dennis S, Luo G, Desai B, MacKenzie KI, Mansfield TA, Kliewer SA, Goodwin B, Jones SA. Hepatoprotection by the farnesoid X receptor agonist GW4064 in rat models of intra- and extrahepatic cholestasis. J Clin Invest. 2003;112:1678–87. doi: 10.1172/JCI18945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fiorucci S, Clerici C, Antonelli E, Orlandi S, Goodwin B, Sadeghpour BM, Sabatino G, Russo G, Castellani D, Willson TM, Pruzanski M, Pellicciari R, Morelli A. Protective effects of 6-ethyl chenodeoxycholic Acid, a farnesoid x receptor ligand, in estrogen-induced cholestasis. J Pharmacol Exp Ther. 2005;313:604–12. doi: 10.1124/jpet.104.079665. [DOI] [PubMed] [Google Scholar]
- 65.Fiorucci S, Antonelli E, Rizzo G, Renga B, Mencarelli A, Riccardi L, Orlandi S, Pellicciari R, Morelli A. The nuclear receptor SHP mediates inhibition of hepatic stellate cells by FXR and protects against liver fibrosis. Gastroenterology. 2004;127:1497–512. doi: 10.1053/j.gastro.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 66.Fiorucci S, Rizzo G, Antonelli E, Renga B, Mencarelli A, Riccardi L, Orlandi S, Pruzanski M, Morelli A, Pellicciari R. A farnesoid x receptor-small heterodimer partner regulatory cascade modulates tissue metalloproteinase inhibitor-1 and matrix metalloprotease expression in hepatic stellate cells and promotes resolution of liver fibrosis. J Pharmacol Exp Ther. 2005;314:584–95. doi: 10.1124/jpet.105.084905. [DOI] [PubMed] [Google Scholar]
- 67.Makishima M, Lu TT, Xie W, Whitfield GK, Domoto H, Evans RM, Haussler MR, Mangelsdorf DJ. Vitamin D Receptor As an Intestinal Bile Acid Sensor. Science. 2002;296:1313–6. doi: 10.1126/science.1070477. [DOI] [PubMed] [Google Scholar]
- 68.Chatterjee B, Echchgadda I, Song CS. Vitamin D receptor regulation of the steroid/bile acid sulfotransferase SULT2A1. Methods Enzymol. 2005;400:165–91. doi: 10.1016/S0076-6879(05)00010-8. [DOI] [PubMed] [Google Scholar]
- 69.Zollner G, Wagner M, Moustafa T, Fickert P, Silbert D, Gumhold J, Fuchsbichler A, Halilbasic E, Denk H, Marschall HU, Trauner M. Coordinated induction of bile acid detoxification and alternative elimination in mice: Role of FXR-regulated organic solute transporter {alpha}/{beta} in the adaptive response to bile acids. Am J Physiol Gastrointest Liver Physiol. 2005;290:G923–G932. doi: 10.1152/ajpgi.00490.2005. [DOI] [PubMed] [Google Scholar]
- 70.Eloranta JJ, Meier PJ, Kullak-Ublick GA. Coordinate transcriptional regulation of transport and metabolism. Methods Enzymol. 2005;400:511–30. doi: 10.1016/S0076-6879(05)00028-5. [DOI] [PubMed] [Google Scholar]
- 71.Rizzo G, Renga B, Mencarelli A, Pellicciari R, Fiorucci S. Role of FXR in regulating bile acid homeostasis and relevance for human diseases. Curr Drug Targets Immune Endocr Metabol Disord. 2005;5:289–303. doi: 10.2174/1568008054863781. [DOI] [PubMed] [Google Scholar]
- 72.Lambert G, Amar MJA, Guo G, Brewer HB, Jr, Gonzalez FJ, Sinal CJ. The farnesoid x-receptor is an essential regulator of cholesterol homeostasis. J Biol Chem. 2003:2563–70. doi: 10.1074/jbc.M209525200. [DOI] [PubMed] [Google Scholar]
- 73.Claudel T, Staels B, Kuipers F. The Farnesoid X receptor: a molecular link between bile acid and lipid and glucose metabolism. Arterioscler Thromb Vasc Biol. 2005;25:2020–31. doi: 10.1161/01.ATV.0000178994.21828.a7. [DOI] [PubMed] [Google Scholar]
- 74.Ma K, Saha PK, Chan L, Moore DD. Farnesoid X receptor is essential for normal glucose homeostasis. J Clin Invest. 2006;116:1102–9. doi: 10.1172/JCI25604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kalaany NY, Mangelsdorf DJ. LXRs and FXR: The Yin and Yang of Cholesterol and Fat Metabolism. Annu Rev Physiol. 2005 doi: 10.1146/annurev.physiol.68.033104.152158. [DOI] [PubMed] [Google Scholar]
- 76.Zhang Y, Lee FY, Barrera G, Lee H, Vales C, Gonzalez FJ, Willson TM, Edwards PA. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc Natl Acad Sci U S A. 2006;103:1006–11. doi: 10.1073/pnas.0506982103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102:731–44. doi: 10.1016/s0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
- 78.Carulli N, Ponz dL, Podda M, Zuin M, Strata A, Frigerio G, Digrisolo A. Chenodeoxycholic acid and ursodeoxycholic acid effects in endogenous hypertriglyceridemias. A controlled double-blind trial. J Clin Pharmacol. 1981;21:436–42. doi: 10.1002/j.1552-4604.1981.tb01746.x. [DOI] [PubMed] [Google Scholar]
- 79.Albers JJ, Grundy SM, Cleary PA, Small DM, Lachin JM, Schoenfield LJ. National Cooperative Gallstone Study: the effect of chenodeoxycholic acid on lipoproteins and apolipoproteins. Gastroenterology. 1982;82:638–46. [PubMed] [Google Scholar]
- 80.Stedman C, Liddle C, Coulter S, Sonoda J, Alvarez JG, Evans RM, Downes M. Benefit of farnesoid X receptor inhibition in obstructive cholestasis. Proc Natl Acad Sci U S A. 2006;103:11323–8. doi: 10.1073/pnas.0604772103. [DOI] [PMC free article] [PubMed] [Google Scholar]


