Abstract
While the role of dextrorphan and dextromethorphan as N-methyl-D-aspartate (NMDA) receptor antagonists has received considerable research attention, their effects on nicotinic acetylcholine receptors (nAChR) has been less well characterized. Recent in vitro and in vivo research has suggested that these drugs noncompetitively block α3β4*, α4β2, and α7 nAChR subtypes and antagonize nicotine’s antinociceptive and reinforcing effects. Both drugs were most potent at blocking α3β4* AChR. This study investigated the effects of dextrorphan and dextromethorphan on nicotine’s discriminative stimulus effects. Three groups of rats were trained in a two-lever drug discrimination procedure to discriminate 0.4 mg/kg s.c. nicotine from saline. Nicotine dose-dependently substituted for itself in all three groups. In contrast, when dextrorphan (group 1) or dextromethorphan (group 2) were injected i.p., neither substitution for nor antagonism of nicotine was observed for either drug. Since i.p. administration allows substantial metabolism of dextromethorphan to its parent compound dextrorphan, the two drugs were also tested following s.c. administration (group 3). Discrimination results were similar across both routes of administration, in that neither substitution nor antagonism occurred, however, s.c. administration reduced response rates to a much greater extent than did i.p. administration. Previous work suggests that β2 subunits are crucial for mediation of nicotine’s discriminative stimulus effects and may play a role in its reinforcing effects, albeit other research suggests a role for α3β4* nicotinic receptors in the latter. Our results suggest that α3β4* nicotinic receptors do not play a major role in nicotine’s discriminative stimulus effects. Further, they suggest that the role of cholinergic mediation of the behavioral effects of dextrorphan and dextromethorphan related to the abuse properties of nicotine may be minimal.
Keywords: dextromethorphan, dextrorphan, discriminative stimulus, nicotine, receptor subtype
Introduction
Dextromethorphan and dextrorphan share a number of in vivo pharmacological effects in rodents, including phencyclidine-like discriminative stimulus effects (Nicholson et al., 1999), suppression of self-administration of abused substances (Glick et al., 2001), antinociception (France et al., 1989), neuroprotective properties (Steinberg et al., 1993), disruption of prepulse inhibition of acoustic startle (Wiley et al., 2003), and anticonvulsant effects (Tortella & Musacchio, 1986). The degree to which these similar effects are produced by a common mechanism is uncertain, however, because the two drugs have somewhat divergent profiles in receptor binding and functional in vitro assays. For example, dextrorphan displays an affinity for the phencyclidine binding site in the N-methyl-D-aspartate (NMDA) receptor complex that is ten-fold greater than that of dextromethorphan (Ebert et al., 1998; Franklin & Murray, 1992; Murray & Leid, 1984). In contrast, dextrorphan blocks α3β4* nicotinic receptors with only one-third the potency of dextromethorphan (Hernandez et al., 2000). Both drugs also bind with low affinity to sigma-2 (σ2) binding sites and with high affinity to σ1 binding sites (Chou et al., 1999).
Several recent studies have concentrated on investigation of dextromethorphan- and dextrorphan-induced antagonism of nicotinic acetylcholine receptors and its associated effects on nicotine’s in vivo pharmacology. Specifically, Damaj et al. (2005) reported that both dextromethorphan and dextrorphan blocked the antinociceptive effects of nicotine in acute thermal pain assays via antagonism at nicotinic acetylcholine receptors, with dextromethorphan exhibiting approximately 10-fold greater potency than dextrorphan after i.p. administration. Further, this study found that both drugs act as noncompetitive antagonists at α3β4*, α4β2, and α7 nicotinic receptor subtypes expressed in oocytes at micromolar concentrations (IC50 range from 0.7 to 4.3 μM). Although potency for both drugs was greater for α3β4* nicotinic receptors than for the other two nicotinic receptor subtypes, dextromethophan was almost twice as potent as dextrorphan at this receptor subtype. In contrast, potencies at the different nicotinic receptor subtypes were more similar for dextrorphan. Other studies have examined the effects of dextromethorphan and/or dextrorphan in nicotine self-administration and drug discrimination procedures. In a nicotine discrimination paradigm, dextromethorphan (30 mg/kg, s.c.) did not substitute for nicotine nor did it antagonize nicotine’s discriminative stimulus properties (Zakharova et al., 2005). In contrast, both drugs decreased self-administration of nicotine at approximately equal potencies (Glick et al., 2001). Given the aforementioned disparity in relative binding affinities at the phencyclidine site of the NMDA receptor and at α3β4* nicotinic receptors, it seems logical to suggest that if the impact of dextromethorphan and dextrorphan on self-administration of nicotine was mediated by antagonism at the NMDA receptor, dextrorphan would reduce self-administration much more than dextromethorphan (which did not occur). Although dextromethorphan and dextrorphan also differ in their affinities at α3β4* nicotinic receptors (as noted earlier), these disparities are less profound than the differences observed at the NMDA receptor. Hence, Glick et al. (2001) concluded that the similar potency of dextromethorphan and dextrorphan to decrease self-administration of several abused drugs was attributable to antagonism of the α3β4* nicotinic receptor. Unfortunately, a similar comparison could not be made for nicotine discrimination, as Zakharova et al. (2005) performed a probe test with a single dose of dextromethorphan only. In light of the gathering evidence that dextromethorphan and dextrorphan exert a physiologically significant influence on nicotinic acetylcholine receptors, the present series of experiments were undertaken to extend the work of Zakharova and colleagues by conducting a more thorough evaluation of the effects of dextromethorphan and dextrorphan on nicotine discrimination.
Methods
Subjects
Adult, male Long-Evans rats (350–460 g), obtained from Harlan (Dublin, VA), were individually housed in a temperature-controlled (20–22°C) environment with a 12-h light-dark cycle (lights on at 7 a.m.). All experiments were conducted during the animals’ light-cycle. Rats were maintained at 85% of their free-feeding bodyweight by restricting post-session feeding while allowing ad libitum access to water in their home cages. The studies reported in this manuscript were carried out in accordance with guidelines published in “A Guide for the Care and Use of Laboratory Animals” (National Research Council, 1996) and were approved by the Institutional Animal Care and Use Committee at Virginia Commonwealth University. All of the rats used in this study had been used in previous nicotine discrimination studies in which putative nicotine agonists and antagonists were evaluated (Damaj et al., 2005).
Apparatus
Rats were trained and tested in standard operant conditioning chambers (Lafayette Instruments Co., Lafayette, IN) housed in sound-attenuated cubicles. Each chamber had two retractable levers. Pellet dispensers delivered 45-mg BIO SERV (Frenchtown, NJ) food pellets to a food cup on the front wall of the chamber between the two response levers. Fan motors provided ventilation and masking noise for each chamber. House lights located above the food cup were illuminated during training and testing sessions. A personal computer with MED-PC software and associated interface (MED Associates, Georgia, Vermont) was used to control schedule contingencies and to record data.
Procedure
As mentioned above, all of the rats in this study had undergone prior training and testing in a nicotine discrimination study (Damaj et al., 2005). The original training regimen employed in these experiments began with moderate food-restriction and two or three 15-min sessions that featured the non-contingent delivery of a food reinforcer every 30 s. During these “magazine training” sessions, all levers were retracted. Lever-pressing was shaped by successive schedules of reinforcement that began with a fixed-ratio 1 (FR1) schedule and progressed through a fixed-ratio 5 (FR5) schedule to the final fixed-ratio 10 (FR10) schedule. During the shaping period, reinforcement schedules were advanced when response rates reached 0.1 responses/s.
Using these procedures, three groups of rats were trained to press one lever following administration of 0.4 mg/kg nicotine and to press another lever after injection with saline, each according to a fixed-ratio 10 schedule of food reinforcement. Completion of 10 consecutive responses on the injection-appropriate lever resulted in delivery of a food reinforcer. Each response on the incorrect lever reset the ratio requirement on the correct lever. The position of the drug lever was varied among the group of rats. The daily injections for each rat were administered in a double alternation sequence of 0.4 mg/kg nicotine and saline. Rats were injected and returned to their home cages until the start of the experimental session 5 min later. Training occurred during sessions conducted five days a week (Monday–Friday) until the rats had met three criteria during eight of ten consecutive sessions: (1) first completed fixed ratio 10 on the correct lever; (2) percentage of correct-lever responding ≥ 80% for the entire session; and (3) response rate ≥ 0.1 responses/sec. Response rates were calculated using total number of responses emitted on both levers, not just responses on the drug-appropriate lever.
Following successful acquisition of the discrimination, stimulus substitution tests with test compounds were conducted on Tuesdays and Fridays during 15-min test sessions. Training continued on Mondays, Wednesdays, and Thursdays. During test sessions, responses on either lever delivered reinforcement according to a fixed ratio 10 schedule. In order to be tested, rats must have completed the first FR and made at least 80% of all responses on the injection-appropriate lever on the preceding day's training session. In addition, the rat must have met these same criteria during at least one of the training sessions with the alternate training compound (nicotine or saline) earlier in the week.
In order to verify acquisition and provide reference data for previous studies, a nicotine dose-effect curve determination was performed in each group of rats after acquisition criteria were met. Nicotine was injected s.c. 5 min before the beginning of the session. In this study, substitution tests were conducted with dextrorphan (Group 1) and dextromethorphan (Group 2). Intraperitoneal (i.p.) injections were administered 15 or 30 min prior to the start of the session. Pre-session injection time for subcutaneous (s.c.) administration of each drug was 30 min. All doses in dose-effect curves were administered in ascending order. Combination tests with the training dose of nicotine and dextromethorphan or dextrorphan (Groups 1 and 2, respectively) followed. Test drugs were injected i.p. 30 min before the session and nicotine (0.4 mg/kg) was injected s.c. 25 min later. Similar antagonism tests (and associated control points) with nicotine (0.4 mg/kg) and s.c. dextrorphan and dextromethorphan (30 min pre-session) were conducted in a third group of rats. Throughout the study, control tests with saline and 0.4 mg/kg nicotine were conducted during the week before the start of each dose-effect curve determination.
Both s.c and i.p. routes of administration were used in these experiments to control for pharmacokinetic differences. In rats, metabolism of dextromethorphan to dextrorphan is facilitated by an enzyme in the liver that is similar to CYP2D6, one of the isoenzymes that also facilitates this metabolic conversion in most humans (DiMarco et al., 2003). Hence, route of administration (and associated degree of first pass metabolism) is the main determinant of the degree to which dextromethorphan is metabolized to dextrorphan. In rats, three times as much dextrorphan is formed from dextromethorphan after intraperitoneal injection than after s.c. injection (Wu et al., 1995). Indeed, there is evidence that most of the observed effects of dextromethorphan in behavioral paradigms that are sensitive to NMDA receptor antagonism are mediated by dextrorphan (Nicholson et al., 1999; Szekely et al., 1991), although there are also contradictory findings (Holtzman, 1994).
Drugs
(-)-Nicotine (Aldrich Chemical Company, Inc., Milwaukee, WI) was converted to the ditartrate salt as described by Aceto et al. (1979). (-)-Nicotine ditartrate was dissolved in physiological saline and pH-buffered (as needed) with 0.001 M NaOH. Dextrorphan (Sigma/RBI, Natick, MA) and dextromethorphan (Sigma/RBI) were dissolved in physiological saline and the pH was adjusted to neutral levels. Test drug solutions were mixed daily, as needed. Doses of all drugs are expressed as mg/kg of the base. All drugs were injected at a volume of 1 ml/kg, with the exception of 30 mg/kg of dextromethorphan and dextrorphan were injected at a volume of 2 ml/kg. Doses of each drug were chosen based upon our previous research with these drugs (Wiley et al., 2003).
Statistical Analysis
For each test session, the percentages of responses on the drug lever and response rates (responses/s) were calculated for the entire session. When appropriate, ED50s were calculated separately for each drug using least-squares linear regression on the linear part of the dose-effect curves (Tallarida and Murray, 1987) for percentage of drug-lever responding, plotted against log10 transformation of the dose. Since rats that responded less than 10 times during a test session did not press either lever a sufficient number of times to earn a reinforcer, their lever selection data only were excluded from data analysis. Separate repeated measures ANOVA, followed by Tukey post hoc tests as appropriate (α = 0.05), were used to analyze differences in response rates for each dose-effect or drug combination experiment.
Results
Acquisition of the nicotine discrimination occurred prior to the start of the present series of experiments (see Damaj et al., 2005). Following acquisition, nicotine fully and dose-dependently substituted for itself in all three groups of rats. In Group 1 [(n = 7); Fig. 1, top left panel], the ED50 for nicotine was 0.08 mg/kg [confidence interval (CI) = 0.06 – 0.11]. At 0.8 mg/kg, nicotine significantly decreased response rates [F (4, 24) = 53.7, p < 0.05; Fig. 1, bottom left panel]. Dextrorphan, administered i.p. 15 or 30 min pre-session and at doses up to 30 mg/kg, did not substitute for nicotine nor did it antagonize nicotine’s discriminative stimulus effects when injected i.p. 30 min pre-session (Fig. 1, top right panel). Response rates were not affected by dextrorphan alone or by combinations of dextrorphan and 0.4 mg/kg nicotine (Fig. 1, bottom right panel). Fig. 2 shows the results of tests with nicotine and dextromethorphan in Group 2 [n = 7]. Similar to Group 1, Group 2 showed full and dose-dependent substitution for nicotine (Fig. 2, top left panel) [ED50 = 0.10 mg/kg, CI: 0.07 – 0.14]. At 0.8 mg/kg, nicotine significantly decreased response rates [F (4, 24) = 10.868, p < 0.05; Fig. 2, bottom left panel). Dextromethorphan, administered i.p. 15 or 30 min pre-session and at doses up to 30 mg/kg, did not substitute for nicotine nor did it significantly antagonize nicotine’s discriminative stimulus effects when injected i.p. 30 min pre-session (n = 6; Fig. 2, top right panel). Response rates were not significantly affected by dextromethorphan alone; however, the combination of 10 mg/kg dextromethorphan and 0.4 mg/kg nicotine significantly decreased response rates compared to saline or to nicotine alone [F(5,26) = 4.7, p < 0.05; Fig. 2, bottom right panel].
Figure 1.
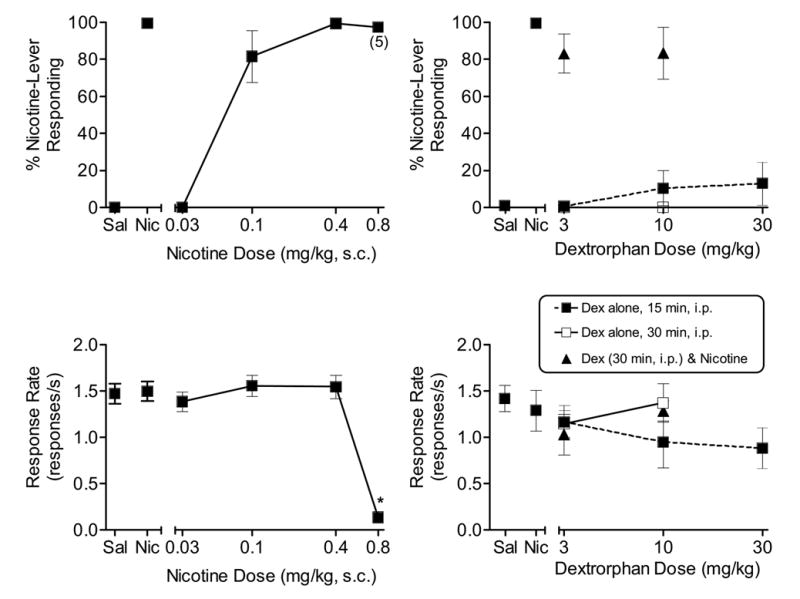
Effects of nicotine (s.c., 5 min pre-session, n=7) [left panels] and dextrorphan (i.p., 15 and 30 min pre-session, n=7) [right panels] on percentage of nicotine-lever responding (upper panels) and response rates (lower panels) in rats trained to discriminate 0.4 mg/kg nicotine from saline. Also shown are results of antagonism tests with dextrorphan (i.p., 30 min, n=7) and 0.4 mg/kg nicotine. Points above Sal and Nic represent the results of control tests with saline and 0.4 mg/kg nicotine conducted before each dose-effect curve determination. For all response rate points, each value represents the mean (±SEM) of the number of rats indicated above for each drug. Number of subjects with responses > 10 is indicated in parentheses for nicotine-lever responding at higher drug doses. * indicates mean is significantly different from saline (p<0.05) based upon Tukey post hoc test.
Figure 2.
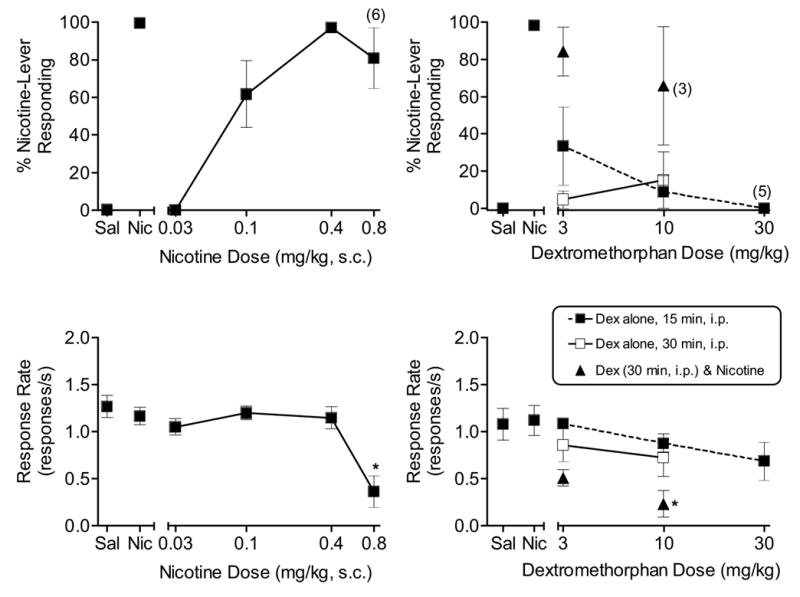
Effects of nicotine (s.c., 5 min pre-session, n=7) [left panels] and dextromethorphan (i.p., 15 and 30 min pre-session, n=6) [right panels] on percentage of nicotine-lever responding (upper panels) and response rates (lower panels) in rats trained to discriminate 0.4 mg/kg nicotine from saline. Also shown are results of antagonism tests with dextromethorphan (i.p., 30 min, n=7) and 0.4 mg/kg nicotine. Points above Sal and Nic represent the results of control tests with saline and 0.4 mg/kg nicotine conducted before each dose-effect curve determination. For all response rate points, each value represents the mean (±SEM) of the number of rats indicated above for each drug. Number of subjects with responses > 10 is indicated in parentheses for nicotine-lever responding at higher drug doses. * indicates mean is significantly different from saline (p<0.05) based upon Tukey post hoc test.
As with Groups 1 and 2, nicotine fully and dose-dependently substituted for itself in Group 3 (n = 6; saline and nicotine training dose data only shown at left of each panel in Fig. 3). Dextrorphan and dextromethorphan failed to substitute for nicotine when administered s.c. 30 min before the start of the session (Fig. 3, top left and right panels, respectively). In contrast with the results for i.p. administration, however, significant dose-dependent decreases in response rates following s.c. injection were observed [F(3, 18) = 3.128, p = 0.05; Fig. 3, bottom left panel and F(3,15) = 6.072, p < 0.05; Fig. 3, bottom right panel] . Dextrorphan (s.c., 30 min), co-administered with 0.4 mg/kg nicotine, did not alter nicotine’s discriminative stimulus effects (Fig. 3, top left panel), but the combination decreased response rates compared to either drug alone [F(2, 8) = 13.946, p < 0.05; Fig. 3, bottom left panel]. The 10 mg/kg dose of dextromethorphan (s.c., 30 min) also did not attenuate the discriminative stimulus effects of the nicotine training dose (Fig. 3, top right panel). Although dextromethorphan (30 mg/kg, s.c.) decreased nicotine-lever responding when co-administered with nicotine, this decrease was associated with substantial variability and was not statistically significant (Fig. 3, top right panel). Response rates of the combination tests were similar to those obtained with dextromethorphan alone (Fig. 3, bottom right panel).
Figure 3.
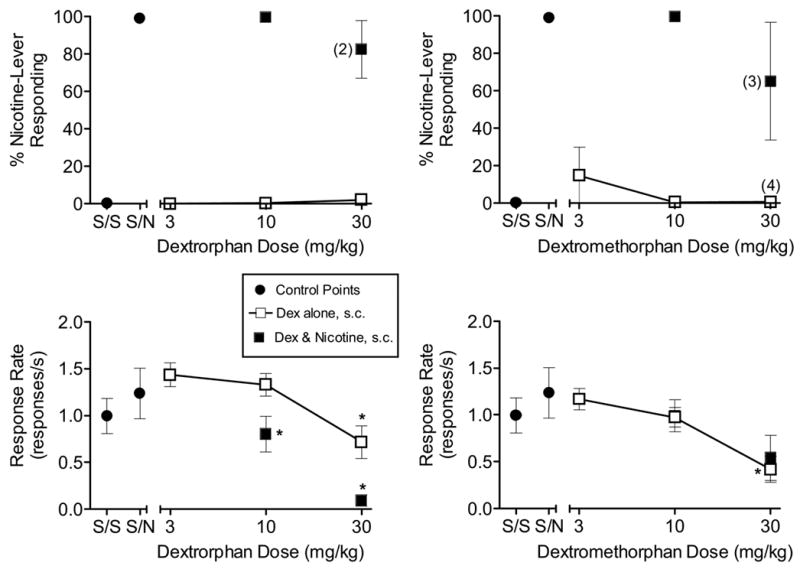
Effects of dextrorphan (s.c., 30 min pre-session) [left panels] and dextromethorphan (s.c., 30 min pre-session) [right panels] on percentage of nicotine-lever responding (upper panels) and response rates (lower panels) in rats trained to discriminate 0.4 mg/kg nicotine from saline. Also shown are results of antagonism tests with 0.4 mg/kg nicotine and dextrorphan (s.c., 30 min) [left panels] or dextromethorphan (s.c., 30 min) [right panels]. Points above S/S and S/N represent the results of control tests with two injections of saline and an injection of saline followed by 0.4 mg/kg nicotine, respectively. For all points, each value represents the mean (±SEM) of 5–7 rats, except data for nicotine-lever responding at higher drug doses (where number of subjects with responses > 10 is indicated in parentheses). * indicates mean is significantly different from saline/saline for drug alone points or is significantly different from saline/nicotine for drug and nicotine combinations (p<0.05 for all comparisons).
Discussion
As expected from the results of numerous studies (for a review, see Wiley et al., 1996), nicotine served as an effective discriminative stimulus in this study, as it fully and dose-dependently substituted for itself in all groups of rats tested. Further, ED50s for nicotine substitution in the dextrorphan- and dextromethorphan-associated groups were similar (0.08 and 0.10 mg/kg, respectively, with overlapping 95% confidence limits). Previous research has shown that nicotine’s discriminative stimulus effects are mediated by its actions as an agonist at nicotinic acetylcholine (ACh) receptors (Mariathasan & Stolerman, 1993; Shoaib et al., 2000).
Neither dextrorphan nor dextromethorphan substituted for nicotine, regardless of whether the route of administration was i.p. or s.c. These results are consistent with and extend the results of a recently published study in which a 30 mg/kg dose of dextromethorphan (s.c.) did not substitute for nicotine and did not antagonize nicotine’s discriminative stimulus effects (Zakharova et al., 2005). Other research has shown that dextromethorphan and dextrorphan, both administered i.p., fully and dose-dependently substituted for the noncompetitive NMDA open channel blocker phencyclidine in rats (Nicholson et al., 1999). With s.c. administration, however, only dextrorphan substituted fully for phencyclidine, with dextromethorphan producing only partial substitution (Nicholson et al., 1999). These results suggest that NMDA antagonism cannot fully account for the stimulus properties of dextromethorphan when metabolism to dextrorphan is attenuated. In rats trained to discriminate dextromethorphan from vehicle, conflicting findings are reported. Gevand et al. (1995) found that cyclazocine, an agonist at σ binding sites, fully substituted for dextromethorphan and that the NMDA antagonist, dizocilpine, substituted only at very high doses that also decreased overall responding. In contrast, Holtzman (1994) reported the opposite results: phencyclidine-like NMDA antagonists substituted for dextromethorphan, but agonists at σ binding sites did not. Hence, mechanism of action for dextromethorphan’s discriminative stimulus effects is uncertain. Nevertheless, they do not appear to be mediated by the same receptors as nicotine (present study; Zakharova et al., 2005).
As mentioned above, neither dextrorphan nor dextromethorphan substituted for nicotine, regardless of route of administration. Interestingly, however, substantial response rate decreases were produced by both dextrorphan and dextromethorphan following s.c. administration. These effects were absent when either drug was injected i.p.; rather, response rates were not significantly different from baseline levels across the entire dose range tested i.p., suggesting significant first-pass metabolism of at least some proportion of each drug to inactive (or less active) metabolites with this route of administration. With s.c. administration, dextromethorphan and dextrorphan reduced response rates with equal potencies, suggesting that the mechanism(s) for this effect are not related to action on receptors at which these two drugs demonstrate differential activities.
The results of antagonism tests with dextromethorphan and dextrorphan may be best appreciated by comparing the effects of these drugs to those of mecamylamine. Mecamylamine, the prototypic noncompetitive nicotinic antagonist, blocks several subtypes of nicotinic receptors, including α7, α4β2 and α3β4, although preferential activity at α3β4* receptors has been noted (Papke et al., 2001). Mecamylamine also dose-dependently antagonizes the discriminative stimulus effects of nicotine without affecting response rates (Mariathasan & Stolerman, 1993), blocks the reinforcing effects of nicotine in i.v. self-administration procedures in rats (Denoble & Mele, 2006), and consistently reverses a number of nicotine’s other pharmacological effects (Damaj et al., 1999, 2005; Grabus et al., 2006). In contrast, mixed results have been reported with dextromethorphan and dextrorphan. While neither drug attenuated the discriminative stimulus effects of nicotine in rats (present study, Zakharova et al., 2005), recent studies have shown that dextromethorphan and dextrorphan decreased self-administration of i.v. nicotine in rats (Glick et al., 2001, 2002) and reversed the antinociceptive effects of nicotine in mice (Damaj et al., 2005). Potency differences between dextromethorphan and dextrorphan in each of these studies, although sometimes statistically significant, were relatively minimal as compared to the 10-fold potency difference noted for their effects as noncompetitive phencyclidine-like NMDA antagonists, suggesting that mediation of their antagonistic effects in these assays does not occur via action at NMDA receptors. A maximum of only about 3-fold difference in potency has been reported for dextromethorphan and dextrorphan as noncompetitive antagonists at α3β4* nicotine receptor subtypes expressed in oocytes (Damaj et al., 2005) and in human embryonic kidney cells (Hernandez et al., 2000); hence, some scientists have suggested that this mechanism may mediate the action of these drugs on nicotine’s reinforcing and antinociceptive effects.
A parsimonious explanation for the variable impact of dextromethorphan and dextrorphan on nicotine pharmacology is variability in the degree to which different nicotinic receptor subtypes contribute to each specific effect of nicotine. The difference in potency of dextromethorphan and dextrorphan for noncompetitive blockade of α3β4* and other nicotinic receptor subtypes is far less than the potency difference between these two drugs at the PCP site of the NMDA receptor complex (Damaj et al., 2005; Hernandez et al., 2000). Consequently, when tested in in vivo pharmacological tests designed to characterize nicotinic agonists and antagonists, the magnitude of their potency differences is dramatically lower than is typically observed in tests designed to characterize in vivo pharmacological action at NMDA receptors.
Shoaib et al. (2002) have reported that the β2 nicotinic receptor subunit is essential for the discriminative stimulus properties of nicotine, as mice that lack this receptor subunit cannot be readily trained to discriminate nicotine. Damaj et al. (2001) found that dextrorphan and dextromethorphan blocked α4β2 nicotinic receptors, but did so only at potencies that were approximately 3 times lower than their potencies for blocking α3β4* nicotinic receptors. Additional work with knockout mice supports the idea that dopamine release and subsequent the reinforcing effects of nicotine, are dependent upon both the α4 and β2 subunits (Picciotto et al., 1998; Tapper et al., 2004; Maskos et al., 2005), although Glick et al. (2001) also hypothesized involvement of α3β4* nicotinic receptor action in the reinforcing actions of dextrorphan and dextromethorphan in a nicotine self-administration procedure. It should be noted, however, that while the reinforcing properties of a drug are related to self-administration, they may not be related to the subjective responses to a drug and the resulting discriminative stimulus properties (Le Foll & Goldberg, 2006).
In summary, the absence of any effect of the noncompetitive α3β4* nicotine receptor antagonists, dextromethorphan and dextrorphan, on nicotine’s discriminative stimulus effects suggests that this receptor subtype plays a minor role in nicotine discrimination. Rather, previous work has implicated a primary role of nicotinic receptors with β2 subunits in nicotine’s discriminative stimulus effects (Shoaib et al., 2002) and for both α4 and β2 subunits in its reinforcing properties, albeit at least one study also posits a role for α3β4* nicotinic receptors (Glick et al., 2001). Together, these results suggest that the role of cholinergic mediation of the behavioral effects of dextrorphan and dextromethorphan related to the abuse properties of nicotine may be minimal.
Acknowledgments
This research was supported by National Institute on Drug Abuse grant DA-05274.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Capon DA, Bochner F, Kerry N, Mikus G, Danz C, Somogyi AA. The influence of CYP2D6 polymorphism and quinidine on the disposition and antitussive effect of dextromethorphan in humans. Clin Pharmacol Ther. 1996;60:295–307. doi: 10.1016/S0009-9236(96)90056-9. [DOI] [PubMed] [Google Scholar]
- Chou YC, Liao JF, Chang WY, Lin MF, Chen CF. Binding of dimemorfan to sigma-1 receptor and its anticonvulsant and locomotor effects in mice, compared with dextromethorphan and dextrorphan. Brain Res. 1999;821:516–9. doi: 10.1016/s0006-8993(99)01125-7. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Flood P, Ho KK, May EL, Martin BR. Effect of dextrometorphan and dextrorphan on nicotine and neuronal nicotinic receptors: In vitro and in vivo selectivity. J Pharmacol Exp Ther. 2005;312:780–5. doi: 10.1124/jpet.104.075093. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Glassco W, Dukat M, Martin BR. Pharmacological characterization of nicotine-induced seizures in mice. J Pharmacol Exp Ther. 1999;291:1284–91. [PubMed] [Google Scholar]
- Denoble VJ, Mele PC. Intravenous nicotine self-administration in rats: effects of mecamylamine, hexamethonium and naloxone. Psychopharmacology. 2006;284:266–72. doi: 10.1007/s00213-005-0054-z. [DOI] [PubMed] [Google Scholar]
- Desai RI, Barber DJ, Terry P. Dopaminergic and cholinergic involvement in the discriminative stimulus effects of nicotine and cocaine in rats. Psychopharmacology. 2003;167:335–43. doi: 10.1007/s00213-003-1426-x. [DOI] [PubMed] [Google Scholar]
- Di Marco A, Yao D, Laufer R. Demethylation of radiolabelled dextromethorphan in rat microsomes and intact hepatocytes. Eur J Biochem. 2003;270:3768–77. doi: 10.1046/j.1432-1033.2003.03763.x. [DOI] [PubMed] [Google Scholar]
- Ebert B, Thorkildsen C, Andersen S, Christrup LL, Hjeds H. Opioid analgesics as noncompetitive N-methyl-D-aspartate (NMDA) antagonists. Biochem Pharmacol. 1998;56:553–9. doi: 10.1016/s0006-2952(98)00088-4. [DOI] [PubMed] [Google Scholar]
- France CP, Snyder AM, Woods JH. Analgesic effects of phencyclidine-like drugs in rhesus monkeys. J Pharmacol Exp Ther. 1989;250:197–201. [PubMed] [Google Scholar]
- Franklin PH, Murray TF. High affinity [3H] dextrorphan binding in rat brain is localized to a noncompetitive antagonist site of the activated N-methyl-D-aspartate receptor-cation channel. Mol Pharmacol. 1992;41:134–46. [PubMed] [Google Scholar]
- Gavend M, Mallaret M, Dematteis M, Baragatti G. Discriminative stimulus properties of dextromethorphan in rats. Biomed & Pharmacother. 1995;49:456–64. doi: 10.1016/0753-3322(96)82690-4. [DOI] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Dickinson HA, Kitchen BA. Comparative effects of dextromethorphan and dextrorphan on morphine, methamphetamine, and nicotine self-administration in rats. Eur J Pharmacol. 2001;422:87–90. doi: 10.1016/s0014-2999(01)01066-4. [DOI] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Kitchen BA. Modulation of nicotine self-administration in rats by combination therapy with agents blocking alpha 3 beta 4 nicotinic receptors. Eur J Pharmacol. 2002;448:185–91. doi: 10.1016/s0014-2999(02)01944-1. [DOI] [PubMed] [Google Scholar]
- Grabus SD, Martin BR, Brown SE, Damaj MI. Nicotine place preference in the mouse: influences of prior handling, dose and strain and attenuation by nicotinic receptor antagonists. Psychopharmacology. 2006;184:456–63. doi: 10.1007/s00213-006-0305-7. [DOI] [PubMed] [Google Scholar]
- Hernandez SC, Bertolino M, Xiao Y, Pringle KE, Caruso FS, Kellar KJ. Dextromethorphan and its metabolite dextrorphan block α3β4 neuronal nicotinic receptors. J Pharmacol Exp Ther. 2000;293:962–7. [PubMed] [Google Scholar]
- Holtzman SG. Discriminative stimulus effects of dextromethorphan in the rat. Psychopharmacology. 1994;116:249–54. doi: 10.1007/BF02245325. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Nicotine as a typical drug of abuse in experimental animals and humans. Psychopharmacology. 2006;184:367–81. doi: 10.1007/s00213-005-0155-8. [DOI] [PubMed] [Google Scholar]
- Levin ED, Mead T, Rezvani AH, Rose JE, Gallivan C, Gross R. The nicotinic antagonist mecamylamine preferentially inhibits cocaine vs. food self-administration in rats. Physiol Behav. 2000;71:565–70. doi: 10.1016/s0031-9384(00)00382-6. [DOI] [PubMed] [Google Scholar]
- Mariathasan EA, Stolerman IP. Discrimination of agonist-antagonist mixtures: experiments with nicotine plus mecamylamine. Behav Pharmacol. 1993;4:555–61. [PubMed] [Google Scholar]
- Marubio LM, Gardier AM, Durier S, David D, Klink R, Arroyo-Jimenez MM, McIntosh JM, Rossi F, Champtiaux N, Zoli M, Changeux JP. Effects of nicotine in the dopaminergic system of mice lacking the alpha4 subunit of neuronal nicotinic acetylcholine receptors. Eur L Neurosci. 2003;17:1329–37. doi: 10.1046/j.1460-9568.2003.02564.x. [DOI] [PubMed] [Google Scholar]
- Murray TF, Leid ME. Interaction of dextrorotary opioids with phencyclidine recognition sites in rat brain membranes. Life Sci. 1984;34:1899–1911. doi: 10.1016/0024-3205(84)90121-8. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the care and use of laboratory animals. 7. Washington, DC: National Academy Press; 1996. [Google Scholar]
- Nicholson KL, Hayes BA, Balster RL. Evaluation of the reinforcing properties and phencyclidine-like discriminative stimulus effects of dextromethorphan and dextrorphan in rats and rhesus monkeys. Psychopharmacology. 1999;146:49–59. doi: 10.1007/s002130051087. [DOI] [PubMed] [Google Scholar]
- Papke RL, Sanberg PR, Shytle RD. Analysis of mecamylamine stereoisomers on human nicotinic receptor subtypes. J Pharmacol Exp Ther. 2001;297:646–56. [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, Fuxe K, Changeux JP. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–7. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Gommans J, Morley A, Stolerman IP, Grailhe R, Changeux JP. The role of nicotinic receptor beta-2 subunits in nicotine discrimination and conditioned taste aversion. Neuropharmacology. 2002;42:530–9. doi: 10.1016/s0028-3908(01)00194-0. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Zubaran C, Stolerman IP. Antagonism of stimulus properties of nicotine by dihydro-beta-erythroidine (DHbetaE) in rats. Psychopharmacology. 2000;149:140–6. doi: 10.1007/s002139900348. [DOI] [PubMed] [Google Scholar]
- Steinberg GK, Kunis D, DeLaPaz R, Poljak A. Neuroprotection following focal cerebral ischemia with the NMDA antagonist dextromethorphan, has a favourable dose response profile. Neurol Res. 1993;15:174–80. doi: 10.1080/01616412.1993.11740131. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Garcha HS, Pratt JA, Kumar R. Role of training dose in discrimination of nicotine and related compounds by rats. Psychopharmacology. 1984;84:413–9. doi: 10.1007/BF00555223. [DOI] [PubMed] [Google Scholar]
- Szekely JI, Sharpe LG, Jaffe JH. Induction of phencyclidine-like behavior in rats by dextrorphan but not by dextromethorphan. Pharmacol Biochem Behav. 1991;40:381–6. doi: 10.1016/0091-3057(91)90569-n. [DOI] [PubMed] [Google Scholar]
- Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C, Whiteaker P, Marks MJ, Collins AC, Lester HA. Nicotine activation of α4* receptors: Sufficient for reward, tolerance, and sensitization. Science. 2004;306(5698):1029–32. doi: 10.1126/science.1099420. [DOI] [PubMed] [Google Scholar]
- Takashima T, Murase S, Iwasaki K, Shimada K. Evaluation of dextromethorphan metabolism using hepatocytes from CYP2D6 poor and extensive metabolizers. Drug Metab Pharmacokinet. 2005;20:177–82. doi: 10.2133/dmpk.20.177. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ, Murray RB. Manual of Pharmacologic Calculations with Computer Programs. 2. New York: Springer-Verlag; 1987. [Google Scholar]
- Tortella FC, Musacchio JM. Dextromethorphan and carbetapentane: centrally acting non-opioid antitussive agents with novel anticonvulsant properties. Brain Res. 1986;383:314–8. doi: 10.1016/0006-8993(86)90031-4. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Harvey SA, Balster RL, Nicholson KL. Affinity and specificity of N-methyl-D-aspartate channel blockers affect their ability to disrupt prepulse inhibition of acoustic startle in rats. Psychopharmacology. 2003;165:378–85. doi: 10.1007/s00213-002-1297-6. [DOI] [PubMed] [Google Scholar]
- Wiley JL, James JR, Rosecrans JA. Discriminative stimulus properties of nicotine: Approaches to evaluating potential nicotinic receptor agonists and antagonists. Drug Dev Res. 1996;38:222–30. [Google Scholar]
- Wu D, Otton SV, Kalow W, Sellers EM. Effects of route of administration on dextromethorphan pharmacokinetics and behavioral response in the rat. J Pharmacol Exp Ther. 1995;274:1431–7. [PubMed] [Google Scholar]
- Zakharova ES, Danysz W, Bespalov AY. Drug discrimination analysis of NMDA receptor channel blockers as nicotinic receptor antagonists in rats. Psychopharmacology. 2005;179:128–35. doi: 10.1007/s00213-004-2067-4. [DOI] [PubMed] [Google Scholar]


