Abstract
The generation of lymphoid cells in mice depends on the function of the Ikaros protein. Ikaros has been characterized as a lymphoid-restricted, zinc-finger transcription factor that is derived from an alternatively spliced message. Ikaros knockout mice have defects in multiple cell lineages, raising the question of whether the protein regulates multiple committed progenitors and/or multipotent stem cells. To address this issue, we examined Ikaros expression in purified populations of multipotent cells and more committed progenitors. We found that the DNA-binding isoforms of Ikaros were localized in the nucleus of the most primitive hematopoietic stem cell subset. Changes in the RNA splicing pattern of Ikaros occurred at two stages: (i) as long-term self-renewing stem cells differentiated into short-term self-renewing stem cells and (ii) as non-self-renewing multipotent progenitors differentiated into lymphoid-committed progenitors. Unexpectedly, we found Ikaros localized to heterochromatin in Abelson-transformed pre-B lymphocytes by using immunogold electron microscopy. These observations suggest a complex role for Ikaros in lymphoid development.
The hematopoietic system in mammals is continuously regenerated by a rare population of self-renewing, multipotent stem cells resident within the adult bone marrow (1, 2). Hematopoietic stem cells (HSC) can be divided into long-term self-renewing (LT-HSC) and short-term self-renewing (ST-HSC) subsets based on the differential expression of Mac-1 surface antigen. A third, non-self-renewing population of multipotent progenitor cells (MPP), which expresses low levels of CD4 surface marker, can also be purified (3). These three subpopulations form a lineage from the most primitive long-term HSC to the most mature multipotent progenitor prior to, or in the process of, lymphoid commitment (4).
Refined definitions of cellular intermediates in the differentiation of HSC into progenitor B and T lymphocytes provide a framework for understanding the roles different genes play in regulating developmental transitions (4–7). Insights into the genetic programs that regulate these developmental fate decisions have come from gene knockout experiments in the mouse (8–10). One mutation that has a profound effect on the development of the lymphoid system, including B, T, NK, and dendritic cells, is that seen in Ikaros knockout mice (11, 12).
Ikaros was first characterized as a lymphoid-restricted, zinc-finger transcription factor that bound an important regulatory element within the CD3δ enhancer (13). The same factor (referred to as LyF-1) was also identified as an activity in nuclear extracts that bound a functionally important site within the terminal deoxynucleotide transferase promoter (14). Subsequent experiments by two groups defined at least eight splice variants of the gene in different lymphoid cell lines (15, 16). All of the variants have common N-terminal and C-terminal domains, the latter of which contains two zinc fingers that mediate interactions between the different Ikaros isoforms (17) and at least two other related proteins (ref. 18; K. Hahm and S. Smale, unpublished results). Splice variants that do not include at least three of the four internal zinc fingers do not bind single Ikaros binding sites and functionally interfere with the activity of the longer isoforms (17).
Mice homozygous for an Ikaros null allele, created by deletion of the common C-terminal zinc fingers, have no B or NK cells and severely reduced numbers of γδ T cells and thymic dendritic cells (12). αβ T cells develop abnormally, with skewing of mature T cells toward the CD4 lineage. In addition to the lymphoid defects, there was also a striking defect in the maturation of neutrophils, with an approximate 10-fold reduction in bone marrow Gr-1+Mac-1+ cells. Given the impairments in lymphoid and myeloid differentiation, Ikaros may regulate important sets of genes for both of these pathways in hematopoietic stem cells. Alternatively, Ikaros may be required at independent stages in the development of both neutrophils and lymphoid cells. To address this issue, we have looked for expression of Ikaros RNA and protein in populations of multipotent and lymphoid-committed progenitor cells purified by fluorescence-activated cell sorting (FACS).
MATERIALS AND METHODS
HSC Isolation and Sorting.
Bone marrow cells were isolated from 4- to 8-week-old C57BL/Ka-Thy-1.1 mice, which were bred and maintained at Stanford University Medical Center. HSC were isolated as described (4). The LT-HSC phenotype was sorted as Thy-1loSca-1+Lineage−Mac-1−CD4−c-kit+. ST-HSC were sorted as Thy-1loSca-1+Lineage−Mac-1loCD4−. The MPP population was sorted as Thy-1loSca-1+Mac-1loCD4lo.
Pro-B, Pro-T, and Neutrophil Isolation.
Progenitor B cells were isolated from the bone marrow by sorting Thy-1.1loB220+ CD43+IgM− cells. Progenitor T cells were isolated from the thymus as Thy-1.1+(CD3, CD4, CD8)-/loc-kit+HSAhi. Myeloid cells, including neutrophils, were isolated from the bone marrow by sorting Gr-1+Mac-1+ cells.
Reverse Transcriptase (RT)-PCR of Ikaros Isoforms.
Cells were sorted once and then clone-sorted directly into 0.2-ml tubes containing 20 μl of RT lysis buffer [5 × first strand buffer (GIBCO/BRL)/10 mM DTT/2% Triton X-100/0.01% BSA/0.2 mM spermidine/0.4 units RNasin (Promega)/100 ng of RT primer/0.5 mM each dNTP] to which 1 μl of Moloney murine leukemia virus reverse transcriptase was added. Ten percent of the cDNA reaction was amplified for 30 cycles by using the outside primers listed below. Five percent of the first PCR reaction was used for a second round of 30 cycles by using the inside primers. Southern analysis was done with an oligonucleotide (Ik-probe) complementary to the common N-terminal exon.
Ikaros Oligonucleotides for RT-PCR and Southern Analysis.
The following oligonucleotides are written in 5′-to-3′ orientation: RT primer, GGGGGGCTTGTGCAGCTGGTA; outside primers, TGGATGTCGATGAGGGTCAAG (5′-end) and GGGACTCAGCCCCCAGGTAGT (3′-end); inside primers, ACTCCAGATGAAGGGGATGAG (5′-end) and GGCATGTCTGACAGGCACTTG (3′-end); Ik probe, TCCAAGAGTGATCGAGGCATG.
Immunohistochemistry.
Sorted cell populations were fixed in −20°C acetone for 5 min, dried, and blocked with 5% nonfat dry milk. Cells were incubated with an anti-Ikaros antibody or preimmune sera, washed, and then stained with a goat anti-rabbitFITC antibody for an additional hour at 25°C. For neutrophils, a 50% glycerol in PBS solution containing 0.15 μg/ml Hoeschst 33342 was used.
Immunogold Electron Microscopy.
Samples of the Abelson-transformed pre-B cell lines, bcl2–3 and 2M3, were fixed in a 2% paraformaldehyde/2.5% glutaraldehyde solution buffered with 0.1 M sodium cacodylate (pH 7.4) for 10 min, dehydrated, embedded in LX resin, and then cured at 50°C. Ultrathin sections (approximately 70 nm) were picked up on nickel grids and then processed for staining with the primary antibody (preimmune serum, anti-Elf-1, N-and C-terminal-specific Ikaros) for 2 h. Sections were washed, incubated with goat anti-rabbit antibody conjugated to 10-nm gold particles for 2 h, and then fixed by using 2% glutaraldehyde in PBS for 10 min. Dried sections were counterstained by using uranyl acetate and lead citrate.
RESULTS
Ikaros Is Nuclearly Localized in Long-Term Self-Renewing HSC.
To investigate the expression of Ikaros within the multipotent progenitor subsets, we used an affinity-purified polyclonal antibody raised against the C-terminal domain of Ikaros (15). This antibody specifically stains all Ikaros isoforms from a number of cell lines by Western blot and does not cross-react with other Ikaros homologs that have been identified (ref. 15 and unpublished observations). Each population was sorted twice consecutively to ensure that >98% of the sorted cells would be of the defined phenotype.
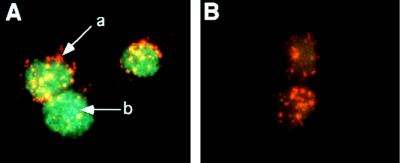
Fixed LT-HSC stained brightly with the anti-Ikaros antibody and showed a punctate pattern that was localized exclusively in the nucleus of the cells (Fig. 1). In addition to the punctate pattern, there was a general, more diffuse staining pattern throughout the nucleus. Cells stained with preimmune serum had undetectable levels of FITC stain. The punctate staining pattern was also observed in the ST-HSC and MPP populations (data not shown). The observation that all LT-HSC stained positively, of which approximately 4% are in S-G2-M phase of the cell cycle, suggests that Ikaros is expressed and nuclearly localized in both resting and cycling LT-HSC (3).
Figure 1.
Ikaros expression in long-term self-renewing hematopoietic stem cells. (A) LT-HSC purified from adult bone marrow with the cell surface phenotype Sca-1+Lin−Thy-1.1loc-kit+ were sorted twice consecutively and then fixed on glass slides. Cells were stained with an affinity-purified, rabbit polyclonal antibody to the C-terminal portion of Ikaros. The secondary staining was done by using an FITC-conjugated, goat anti-rabbit antibody. Red staining (a) represents the Texas Red-conjugated, anti-Sca-1 antibody used in the sort. The punctate staining pattern (b) was seen in all stem cell and lymphoid cell populations analyzed with either the C-terminal antibody or with an affinity-purified antibody to the N-terminal portion of Ikaros. (B) LT-HSC were stained with rabbit preimmune serum followed by secondary staining with goat anti-rabbitFITC antibody.
Ikaros Transcripts Present in Multipotent Progenitor Subsets and Progenitor B and T Lymphocytes.
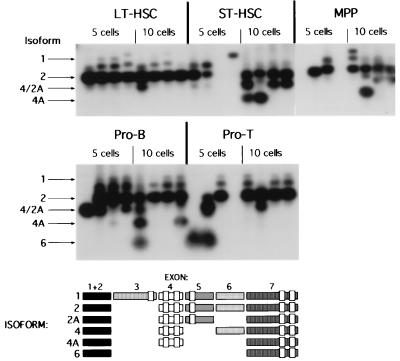
We next wanted to test which splice variants were present in multipotent and lymphoid-committed progenitors and whether splicing patterns change during lymphocyte differentiation. Cells of defined phenotypes were sorted once and then resorted directly into tubes for RT-PCR. Only five or ten cells of each population were sorted in each tube to make it very unlikely that a contaminating cell would affect the data. After two rounds of PCR with nested primers, products were resolved on a gel and then blotted for Southern analysis by using an internal oligonucleotide probe complementary to a portion of the common N-terminal exon. In the LT-HSC subset, only those isoforms (Ik-1 and Ik-2; isoform VI and isoform V in the nomenclature of ref. 15) that bind DNA were present with rare exceptions (Fig. 2). In 24 independent, five-cell reactions of the LT-HSC phenotype, Ik-6 was never detected, and Ik-4A was seen once. In eight independent reactions using ten cells, Ik-2A/Ik-4 was seen twice. Ik-2 was the predominant transcript in every sample, although biases in the PCR reactions may skew the representation of the individual messages. The amplified products were sequenced to ascertain the exon organization of each transcript. We were unsuccessful in our effort to sequence the band that migrated between Ik-1 and Ik-2. This band probably represents a PCR artifact that has been seen by others (16).
Figure 2.
Ikaros RNA splicing isoforms in stem cells and progenitor B and T cell populations. HSC and pro-B cells from adult bone marrow and pro-T cells from adult thymus were double-sorted based on cell surface marker expression (See Materials and Methods). Five or ten cells were cloned by FACS into tubes for RT-PCR. The RT reaction was done by using an Ikaros-specific primer complementary to a portion of exon 7. Two sequential rounds of nested PCR were performed with an aliquot of cDNA using primers localized to exons 1/2 and exon 7. The Southern blot was probed with an internal oligonucleotide complementary to sequence found in exon 1/2. The vertical rectangles on the isoform diagram denote the zinc finger domains within Ikaros.
As LT-HSC differentiate into multipotential cells with short-term self-renewal capacity (ST-HSC), two new isoforms were induced (Ik-2A/Ik-4 and Ik-4A; Fig. 2). These messages represent alternative usage of exons 5 and 6, which are present in the largest Ikaros isoforms. Because isoforms Ik-2A and Ik-4 comigrate, they are described as Ik-2A/Ik-4 in samples that were not sequenced. Ik-2A was seen in sequencing reactions of bands from the MPP population but did not appear in lymphocyte sequencing reactions. Ik-4 was seen in lymphocyte sequencing reactions. Ikaros isoform expression in the non-self-renewing MPP population did not differ from that seen in ST-HSC (Fig. 2).
Differentiation of multipotent cells into a rare class of IL-7R+Lin−Sca-1loc-kitloThy-1.1− clonogenic bone marrow lymphoid progenitors, which only give rise to B, T, and NK cells (19), gave a similar distribution of isoforms as that seen in the ST-HSC and MPP populations, with the exception that Ik-6 appeared in one out of eight samples at the five-cell level (unpublished observation). In pro-B and pro-T cells, the shortest of the isoforms, Ik-6, was again found (Fig. 2). This isoform lacks the internal zinc finger exons that encode the DNA binding domain of Ikaros. Interestingly, this isoform was not seen in all of the samples and therefore may be present in only a subset of pro-B and pro-T cells. These subsets might include cells that are actively cycling or cells that are undergoing developmental transitions within given populations defined by surface marker expression.
Ikaros knockout mice have defects in fetal liver B cells and γδ T cells (11, 12), suggesting that Ikaros is expressed in fetal liver progenitor cells. We double-sorted the long-term self-renewing, fetal liver HSC from 14 dpc fetal liver (20) and analyzed Ikaros expression by RT-PCR. The Ikaros isoforms present in fetal liver LT-HSC were similar to those expressed in bone marrow ST-HSC and MPP populations (Ik-1 in 4/11 reactions, Ik-2 in 8/11, Ik-2A/Ik-4 in 4/11, and Ik-4A in 2/11). The similarities in Ikaros isoform expression between fetal liver LT-HSC and adult ST-HSC suggests that the induced Ikaros isoforms may not be involved in the decision of HSCs to self-renew for short versus long intervals. The differences presumably reflect other activities of these cells.
Ikaros Is Expressed and Nuclearly Localized in Mature Neutrophils.
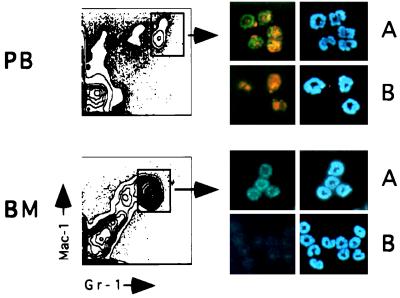
Cells in the bone marrow and peripheral blood that express high levels of Gr-1 and Mac-1 on their surface represent an essentially pure population of mature neutrophils (21). Neutrophils were purified by FACS, fixed on glass slides, and stained with the anti-C-terminal Ikaros antibody. Prior to staining, cells were incubated with an anti-FcR antibody (2.4G2) to block binding of antibody to Fc receptor on the surface of neutrophils. In three independent experiments, all cells stained positively in the nucleus for Ikaros expression (Fig. 3A). Cells stained with preimmune serum had no detectable staining (Fig. 3B). Neutrophils stained with an independent antibody raised to the common N-terminal portion of Ikaros gave similar results (unpublished observations).
Figure 3.
Ikaros expression in neutrophil populations from bone marrow and peripheral blood. Pure populations of neutrophils were double-sorted by using antibodies to myeloid lineage determinants. Gr-1hiMac-1hi cells from peripheral blood (PB) and bone marrow (BM) were fixed and stained with (A) the C-terminal, anti-Ikaros antibody (FITC stain) or (B) preimmune serum. Bone marrow neutrophils were photographed using an FITC filter so the red staining from the Gr-1PE (phycoerythrin) antibody used in the sort is not visible (in contrast with peripheral blood neutrophils). The right column shows the same cells photographed with a UV filter to highlight the Hoechst-stained nuclei.
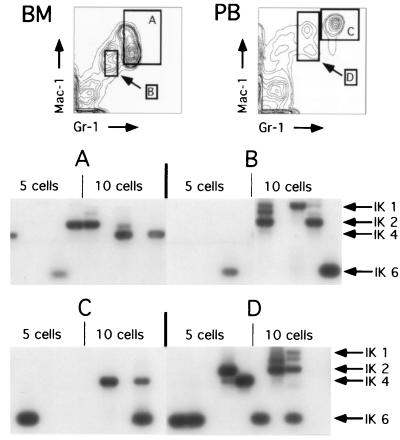
Similar sorts were done to assess the Ikaros transcripts present in neutrophils and in myeloid cells with lower levels of Gr-1 and Mac-1 staining from both peripheral blood and bone marrow. The latter population of cells in bone marrow largely represent immature cells of the granulocytic and monocytic cell lineages. In peripheral blood, Mac-1hiGr-1lo cells principally contain monocytes (ref. 21; Fig. 4). When cells were assayed by RT-PCR, there was a reproducible amplification of single isoforms in samples of five or ten cells in almost every case (Fig. 4). This is in contrast to the multiple Ikaros isoforms, which were detected in stem cells or lymphocytes (Fig. 2 and Table 1). The predominant transcript, especially in peripheral blood myeloid cells, was isoform 6. Interestingly, only about 50% of the samples yielded any amplification products. This could be due to a lower level of Ikaros transcripts in myeloid cells, higher ribonuclease activity in the cells, or the presence of reverse transcriptase inhibitors, which make the reaction inefficient. We did not detect the novel isoforms Ik-2A and Ik-4A in the myeloid lineages.
Figure 4.
Ikaros RNA splicing variants in myeloid lineage cells. Four populations representing different classes of myeloid lineage cells were purified based on differential levels of Mac-1 and Gr-1 staining. Populations A and C represent bone marrow and peripheral blood neutrophils, respectively. Populations B and D would represent more heterogeneous groups of immature myeloid cells and monocytes, respectively. RT-PCR conditions were identical to those described in Fig. 2.
Table 1.
Summary of Ikaros RNA expression in hematopoietic subsets
| IK-1 | IK-2 | IK-2A/ IK-4* | IK-4A | IK-6 | |
|---|---|---|---|---|---|
| LT-HSC | X | X | |||
| ST-HSC | X | X | X | X | |
| MPP | X | X | 2A | X | |
| Lymphoid progenitor | X | X | X | X | X |
| pro-B | X | X | X(4) | X | X |
| pro-T | X | X | X | X | X |
| Neutrophil† | X | X | 4 | X |
For the IK-2A/IK-4 comigrating bands. “X” represents the presence of a band, and 2A or 4 indicates a positive identification by sequence. The IK-4 identified as pro-B was sequenced in an Abelson-transformed pre-B cell line and has been identified in a T cell line by Molnar and Georgopoulos (16). All other X designations come from RT-PCR analyses of normal cells sorted by FACS according to their distinctive phenotype.
Neutrophils generally contained only one of the indicated isoforms in five-cell PCRs.
Ikaros Is Principally Localized to Heterochromatin in Pre-B Cell Lines.
Given the punctate staining pattern of Ikaros in the nucleus of lymphoid cells, we examined the nuclear localization pattern more closely by using immunogold electron microscopy. We analyzed two Abelson-transformed pre-B cell lines because of the pronounced punctate staining in these cells. We were unable to obtain enough purified LT-HSC for electron microscopy.
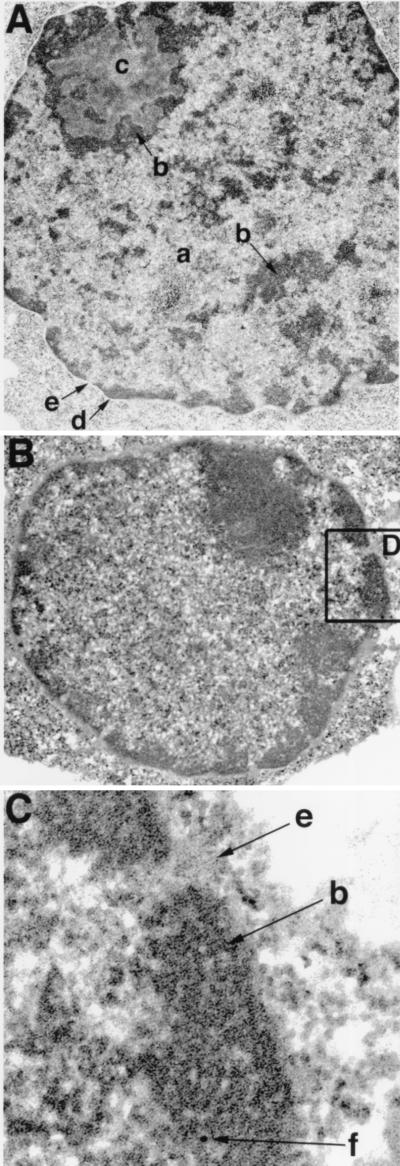
Unexpectedly, we found Ikaros localized predominantly to the electron-dense or heterochromatic regions of the nucleus (Fig. 5 and Table 2). Gold particles were specifically detected in the nucleus and were distributed throughout the heterochromatic regions. By using ultrathin sections (approximately 70 nm, which would represent about 1% of the thickness of the nucleus), the frequency of positively stained sections relative to the total number of sections examined was about 50%. This observation is consistent with results using confocal microscopy, which showed that pre-B cells contain a limited number (8–20) of Ikaros-stained, punctate foci within the nucleus (22). One would therefore not expect to find Ikaros in all planes of the nucleus even though immunofluorescence studies show that all cells stain positively for Ikaros expression in pre-B cell lines (unpublished observation). Within the positively stained sections, an average of one to two particles were observed. Staining with preimmune sera showed five randomly distributed gold particles in a survey of 65 sections. Statistics on gold particle localization were compiled based on examination of the first 50–60 stained sections. Localization was determined by two independent persons based on examination of unlabeled photographs to eliminate bias in assigning location of the gold particles. The ratio of particles found in heterochromatin versus euchromatin was 2.8 or 2.3 when using Ikaros antibodies directed against N-terminal and C-terminal epitopes, respectively (Table 2). The identical staining pattern was seen by using either Ikaros antibody. The biased localization of Ikaros to heterochromatin was particularly significant in that a large percentage of the nuclear area consisted of euchromatin (Fig. 5A). Ikaros staining in heterochromatin associated with the nuclear envelope was approximately 2-fold higher than in other heterochromatic regions within the nucleus. However, this may have more to do with the total surface area of the various regions than any distinct localization pattern. Depending on the plane of sectioning through the nucleus, it is possible that heterochromatin, which in two dimensions appears to be centrally localized, may actually be associated with the nuclear envelope. Approximately 5% of the gold particles were found within the nucleolus. Cytoplasmic staining and localization to the perinuclear space was seen in rare instances.
Figure 5.
Ikaros localization in heterochromatin of pre-B lymphocytes. (A) One representative nucleus from the Abelson-transformed pre-B cell line, bcl2–3, is shown at a magnification of 15,000×. The euchromatic (a), heterochromatic (b), and nucleolar (c) regions are indicated along with the nuclear envelope (d) and a nuclear pore (e). (B, C) Low (15,000×) and high power (31,000×) images of a nucleus stained with the anti-N-terminal Ikaros antibody. Localization of one 10-nm gold particle to heterochromatin associated with the nuclear envelope is shown (f).
Table 2.
Nuclear localization patterns of Ikaros and Elf-1 in pre-B cells
| Antibody | # nuclei analyzed | Heterochromatin* | Euchromatin | Het:Eu ratio |
|---|---|---|---|---|
| Ikaros (N-terminal) | 52 | 55 | 20 | 2.75 |
| Ikaros (C-terminal) | 61 | 72 | 32 | 2.25 |
| Elf-1 | 55 | 35 | 60 | 0.58 |
The proportion of gold particles localized to heterochromatin did not differ significantly between the two Ikaros antibodies. There was a highly significant difference (P < 0.001) between either Ikaros antibody and Elf-1 by pairwise z-tests.
To confirm the specificity of heterochromatin staining, we used a control anti-Elf-1 antibody (23). Elf-1 is a member of the ETS family of transcriptional activating proteins and is believed to positively activate transcription of genes like TdT in immature lymphocytes (23). In contrast with Ikaros, Elf-1 was predominantly localized to the lighter, euchromatic regions of the nucleus (Table 2). The ratio of gold particles in heterochromatin versus euchromatin was reciprocally related to that observed in Ikaros-stained samples, with Elf-1 being found 2-fold more frequently in euchromatin than in heterochromatin. Staining was again specific for the nucleus and could be found in approximately 50% of the total number of nuclei examined. Only 3% of the particles were found within the nucleolus.
DISCUSSION
One hypothesis to explain the lack of lymphoid cells in Ikaros-deficient animals is that Ikaros may function as a master regulator of lymphoid commitment from multipotent progenitor cells. A simple prediction would be that Ikaros is transcriptionally activated in cells that are undergoing the transition from a multipotent cell to a lymphoid-restricted progenitor. Our results show that the DNA-binding isoforms of Ikaros (principally Ik-2 but also Ik-1) are nuclearly localized in all LT-HSC (Figs. 1 and 2). These isoforms of Ikaros are therefore not sufficient to induce lymphoid commitment from multipotential cells unless their activity is being post-transcriptionally regulated. A recently published paper (18) also concluded that Ikaros is present in stem cells, although the cell surface phenotype of the sorted cells would suggest that the population analyzed contained mainly non-HSC (3, 24).
As multipotent cells differentiated into lymphoid-committed progenitors, the smallest Ikaros isoform, Ik-6, was seen in one out of eight PCR reactions using five cells. The other isoforms in lymphoid-committed progenitors (Ik-1 in 5/8 reactions, Ik-2 in 5/8 reactions, and Ik-2A/Ik-4 in 2/8 reactions) are similar to those seen in the ST-HSC and non-self-renewing MPP populations. Committed pro-B and pro-T cell populations both expressed Ik-6 in a fraction of the samples along with Ik-4 and at least one of the other induced isoforms (Ik-4A, Fig. 2 and Table 1). Isoform 6 was the only splice variant that was associated with lymphoid commitment, albeit in only a fraction of the samples that were analyzed. Ik-6 was also found in a number of myeloid cell populations (Fig. 4), so its expression correlates more with differentiation of multipotential cells into both myeloid and lymphoid-committed progenitors. The expression of Ik-4 may be correlated with lymphoid commitment, although its comigration with Ik-2A made this difficult to evaluate. At all stages in the differentiation process, high levels of Ik-2 were expressed in almost every sample.
What then might be the role of alternative splicing in the differentiation of LT-HSC into ST-HSC and in the transition between multipotent progenitor and lymphoid-committed progenitor? Purification of Ikaros from lymphocyte extracts using DNA affinity chromatography demonstrates that Ik-6 is tightly associated with other Ikaros isoforms and at least two additional zinc-finger proteins, one of which is the recently described Aiolos protein (18), in a multi-subunit complex that can bind DNA (K. Hahm and S. Smale, unpublished results). In contrast, gel shift experiments and transient cotransfection studies suggest that Ik-6 inhibits the DNA-binding and functional activity of the larger Ikaros isoforms (17). Both of these observations suggest that induced isoforms, like Ik-2A, Ik-4A, Ik-4, and Ik-6, have the potential of altering the composition of the complex and perhaps the specificity of the complex for certain DNA binding sites. The induced isoforms may also function to modulate the ability of Ikaros to interact with other proteins found in the Ikaros complex, which themselves may be the key regulators of lymphocyte development.
To understand how Ikaros might influence neutrophil development, we examined Ikaros expression in neutrophils and myeloid progenitors. We found that neutrophils and other myeloid cell populations expressed Ikaros isoforms that were similar to those found in lymphocytes, with the exception that Ik-6 was more frequently seen in myeloid cells (Figs. 3 and 4). Neutrophils also did not express the multipotent progenitor isoforms Ik-2A and Ik-4A. Curiously, only 50% of the samples had what was usually a single amplified band (Fig. 4). A similar amplification pattern was seen in stem cell and lymphocyte reactions when one cell was analyzed, which supports the notion that limited amounts of Ikaros mRNA may lead to biased PCR reactions and the preferential amplification of single bands (unpublished observation). We cannot rule out the possibility that only one of the various splice junctions is utilized at any given time within a single cell. At another time in the life history of that same cell, another splice form might predominate. The expression of Ikaros in neutrophils suggests that the lack of Gr-1+ cells in the mutant mice is probably a deficit in the regulation of genes necessary for differentiation of neutrophils from myeloid progenitor cells. In Ikaros knockout mice, these progenitors retain the capacity to differentiate along erythroid and monocytic pathways (11, 12).
The punctate nuclear staining pattern of Ikaros in HSC and lymphocytes (Fig. 1) resembles that seen in the putative mammalian homolog of the Drosophila polycomb complex (25, 26). The polycomb proteins (reviewed in refs. 27–29) function by maintaining the repressed state of different clusters of homeotic genes. Mice with a targeted disruption of the bmi-1 proto-oncogene show severe skeletal and hematopoietic abnormalities (25), which may be a consequence of derepressed homeotic genes. Although Ikaros null animals are viable (12), they do display a severe hematopoietic phenotype that may be a consequence of derepressed genes regulating developmental decisions in hematopoietic stem cells.
In support of the notion that Ikaros might function as a negative regulator of transcription, we found a surprising localization of Ikaros predominantly to heterochromatin in Abelson-transformed pre-B cell lines by using immunogold electron microscopy (Fig. 5 and Table 2). Brown et al. (22) recently made similar observations by using confocal microscopy. It is possible that the largely heterochromatic localization of Ikaros proteins in Abelson-transformed pre-B cells is only representative of leukemic cells and not the variety of normal hematolymphoid stages studied here. However, the contrasting localization of Elf-1 predominantly to euchromatin and Ikaros to heterochromatin in the same cell line suggests that these localizations are specific and not simply the reflection of relative DNA content in heterochromatin versus euchromatin (Table 2).
A number of possible models should be considered. Ikaros may act as a classical repressor and bind important regulatory cis-acting sequences in specific promoter or enhancer regions. This may block access of basal transcription machinery to the promoter or block binding of key activators to their sites in lymphoid or myeloid genes. If this occurs in euchromatin, it could play a role in inducing heterochromatin formation (see below). Interestingly, a recent paper has shown that Ikaros and Ets family members compete for binding to an important cis-regulatory element within the TdT promoter (23). From that study, it seems that Ets family members, in particular Elf-1, may positively regulate TdT transcription in vivo. Consistent with this, the chromatin localization of Elf-1 in pre-B cells is largely restricted to euchromatic regions (Table 2). Ikaros may also inhibit expression of target genes by associating with proteins that regulate the condensed state of chromatin. For example, Ikaros could facilitate the formation of heterochromatin around different sets of genes, and these events could require particular isoforms of Ikaros. In that view, different Ikaros isoforms could act as targets for proteins that aid in the formation and/or maintenance of heterochromatin like those of the mammalian polycomb group. Ikaros could also function by targeting multiprotein complexes involved in the heterochromatin to euchromatin switch, like SWI/SNF (30), to particular genes for activation. Finally, Ikaros may be localized to heterochromatin without being necessary for protein targeting, the maintenance of chromatin structure, or for gene repression. That is to say, Ikaros may be localized to heterochromatin as a structural protein or have a role other than those possibilities enumerated above.
The observation that Ikaros can function as a transcriptional activator when transiently cotransfected into a fibroblast cell line along with a reporter gene containing multiple copies of an Ikaros binding site seems inconsistent with our findings of heterochromatin localization (16, 17). Many possible explanations might address both observations. Ikaros function may be dependent on the context of its binding site, with some genes being actively repressed while other genes with different Ikaros binding site configurations (which might bind different Ikaros isoform assemblies) are actively transcribed. This would be consistent with our observation that some gold particles in Ikaros-stained nuclei localize to euchromatin as well as heterochromatin in pre-B cells (Table 2). Another possibility is that Ikaros may only function as a repressor when genes are stably integrated in chromatin, which would not be addressed by the transient transfection approach.
From our data, a simple working model for the role of Ikaros in stem cells might be to sequester genes in transcriptionally inactive chromatin. Gene- and developmental stage-specific activation of transcription would then occur, as specific activators of a given gene like Elf-1, Aiolos (18), p70 (K. Hahm and S. Smale, unpublished work), and EBF (31, 32) would displace Ikaros through direct binding to the cis sequence of a repressed gene (23) or by direct association with the Ikaros complex (Aiolos and p70). Other regulators of chromatin structure, like SWI/SNF proteins, may also be involved in the activation process. Expression of the induced Ikaros isoforms (Ik-2A, Ik-4, Ik-4A, and Ik-6) could also change the function of the Ikaros complex during lymphoid development. In any case, the observations that Ikaros is expressed in HSC, that different isoforms are expressed in different hematopoietic progenitors, and that Ikaros is localized to heterochromatin both further complicate and inform models of Ikaros function. Future studies may reveal the significance of these observations.
Acknowledgments
We express our appreciation to Amanda Fisher, Brad Cobb, Hewson Swift, and members of the Weissman lab for stimulating discussion. We thank Koichi Akashi and Motonari Kondo for communicating unpublished data and Doug Wright, David Traver, and Eric Lagasse for critical reading of the manuscript. Thanks to Libuse Jerabek for laboratory management, Veronica Braunstein for antibody preparation, Tim Knaak for FACS operation, Phil Verzola for photography, and L. Hidalgo for animal care. Sean Morrison was a predoctoral fellow with the Howard Hughes Medical Institute and Christopher Klug is a fellow of the Irvington Institute. This work was supported by National Cancer Institute Grant CA 42551 and a grant from SyStemix/Sandoz.
ABBREVIATIONS
- LT-HSC
long-term self-renewing hematopoietic stem cell
- ST-HSC
short-term self-renewing HSC
- MPP
multipotent progenitor
- FITC
fluorescein isothiocyanate
- FACS
fluorescence-activated cell sorting
- RT
reverse transcriptase
References
- 1.Siminovitch L, McCulloch E, Till J. J Cell Comp Physiol. 1963;62:327–336. doi: 10.1002/jcp.1030620313. [DOI] [PubMed] [Google Scholar]
- 2.Morrison S J, Uchida N, Weissman I L. Annu Rev Cell Dev Biol. 1995;11:35–71. doi: 10.1146/annurev.cb.11.110195.000343. [DOI] [PubMed] [Google Scholar]
- 3.Morrison S J, Weissman I L. Immunity. 1994;1:661–673. doi: 10.1016/1074-7613(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 4.Morrison S J, Wandycz A M, Hemmati H D, Wright D E, Weissman I L. Development (Cambridge, UK) 1997;124:1929–1939. doi: 10.1242/dev.124.10.1929. [DOI] [PubMed] [Google Scholar]
- 5.Hardy R R, Carmack C E, Shinton S A, Kemp J D, Hayakawa K. J Exp Med. 1991;173:1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu L, Li C L, Shortman K. J Exp Med. 1996;184:903–911. doi: 10.1084/jem.184.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shortman K, Wu L. Annu Rev Immunol. 1996;14:29–47. doi: 10.1146/annurev.immunol.14.1.29. [DOI] [PubMed] [Google Scholar]
- 8.Clevers H C, Grosschedl R. Immunol Today. 1996;17:336–343. doi: 10.1016/0167-5699(96)10019-0. [DOI] [PubMed] [Google Scholar]
- 9.Shivdasani R A, Orkin S H. Blood. 1996;87:4025–4039. [PubMed] [Google Scholar]
- 10.Singh H. Curr Opin Immunol. 1996;8:160–165. doi: 10.1016/s0952-7915(96)80053-7. [DOI] [PubMed] [Google Scholar]
- 11.Georgopoulos K, Bigby M, Wang J H, Molnar A, Wu P, Winandy S, Sharpe A. Cell. 1994;79:143–156. doi: 10.1016/0092-8674(94)90407-3. [DOI] [PubMed] [Google Scholar]
- 12.Wang J H, Nichogiannopoulou A, Wu L, Sun L, Sharpe A H, Bigby M, Georgopoulos K. Immunity. 1996;5:537–549. doi: 10.1016/s1074-7613(00)80269-1. [DOI] [PubMed] [Google Scholar]
- 13.Georgopoulos K, Moore D D, Derfler B. Science. 1992;258:808–812. doi: 10.1126/science.1439790. [DOI] [PubMed] [Google Scholar]
- 14.Lo K, Landau N R, Smale S T. Mol Cell Biol. 1991;11:5229–5243. doi: 10.1128/mcb.11.10.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hahm K, Ernst P, Lo K, Kim G S, Turck C, Smale S T. Mol Cell Biol. 1994;14:7111–7123. doi: 10.1128/mcb.14.11.7111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molnar A, Georgopoulos K. Mol Cell Biol. 1994;14:8292–8303. doi: 10.1128/mcb.14.12.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun L, Liu A, Georgopoulos K. EMBO J. 1996;15:5358–5369. [PMC free article] [PubMed] [Google Scholar]
- 18.Morgan B, Sun L, Avitahl N, Andrikopoulos K, Ikeda T, Gonzales E, Wu P, Neben S, Georgopoulos K. EMBO J. 1997;16:2004–2013. doi: 10.1093/emboj/16.8.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kondo M, Weissman I L, Akashi K. Cell. 1997;91:661–672. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 20.Morrison S J, Hemmati H D, Wandycz A M, Weissman I L. Proc Natl Acad Sci USA. 1995;92:10302–10306. doi: 10.1073/pnas.92.22.10302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lagasse E, Weissman I L. J Immunol Methods. 1996;197:139–150. doi: 10.1016/0022-1759(96)00138-x. [DOI] [PubMed] [Google Scholar]
- 22.Brown, K. E., Guest, S. S., Smale, S. T., Hahm, K., Merkenschlager, M. & Fisher, A. (1997) Cell, in press. [DOI] [PubMed]
- 23.Ernst P, Hahm K, Trinh L, Davis J N, Roussel M F, Turck C W, Smale S T. Mol Cell Biol. 1996;16:6121–6131. doi: 10.1128/mcb.16.11.6121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okada S, Nakauchi H, Nagayoshi K, Nishikawa S, Miura Y, Suda T. Blood. 1992;80:3044–3050. [PubMed] [Google Scholar]
- 25.van der Lugt N M T, Domen J, Linders K, van Roon M, Robanus-Maandag E, te Riele H, van der Valk M, Deschamps J, Sofroniew M, van Lohuizen M, Berns A. Genes Dev. 1994;8:757–769. doi: 10.1101/gad.8.7.757. [DOI] [PubMed] [Google Scholar]
- 26.Alkema M J, Bronk M, Verhoeven E, Otte A, van’t Veer L J, Berns A, van Lohuizen M. Genes Dev. 1997;11:226–240. doi: 10.1101/gad.11.2.226. [DOI] [PubMed] [Google Scholar]
- 27.Orlando V, Paro R. Curr Opin Genet Dev. 1995;5:174–179. doi: 10.1016/0959-437x(95)80005-0. [DOI] [PubMed] [Google Scholar]
- 28.Pirrotta V. Curr Opin Genet Dev. 1995;5:466–472. doi: 10.1016/0959-437x(95)90050-q. [DOI] [PubMed] [Google Scholar]
- 29.Kingston R E, Bunker C A, Imbalzano A N. Genes Dev. 1996;10:905–920. doi: 10.1101/gad.10.8.905. [DOI] [PubMed] [Google Scholar]
- 30.Struhl K. Cell. 1996;84:179–182. doi: 10.1016/s0092-8674(00)80970-8. [DOI] [PubMed] [Google Scholar]
- 31.Feldhaus A L, Mbangkollo D, Arvin K L, Klug C A, Singh H. Mol Cell Biol. 1992;12:1126–1133. doi: 10.1128/mcb.12.3.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Travis A, Hagman J, Hwang L, Grosschedl R. Mol Cell Biol. 1993;13:3392–3400. doi: 10.1128/mcb.13.6.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]