Abstract
Objectives
There are several lines of evidence suggesting a link between obesity and heart failure, including chronic inflammation, increased sympathetic tone, and insulin resistance. The goal of this study was to evaluate the changes in systemic metabolism, anthropometrics, and left ventricular contraction as well as geometry in clinically severe obese women after bariatric surgery.
Methods
Enrollment was offered consecutively to 22 women with clinically severe obesity. Participants had abdominal magnetic resonance imaging (MRI) to quantify visceral adipose tissue (VAT) area and tissue Doppler imaging (TDI) echocardiography to measure left ventricular (LV) contractile function. Fasting blood chemistries were drawn to measure inflammatory markers and to calculate insulin sensitivity. All tests were performed before surgery and three months post-operatively.
Results
Three months after surgery there was a significant increase in insulin sensitivity [mean change (+/− SEM): 34.0(10.4), p<0.0001]. VAT significantly decreased [−66.1 cm2(17.8), p=0.002] and was associated with decreases in BMI, serum glucose concentrations, and hsCRP levels (r=0.61, p=0.005, r=0.48, p=0.033, and r=0.53, p=0.016, respectively). Left ventricular mass significantly decreased [−3.8 g/m2.7(1.7), p=0.037] and this decrease was associated with a decrease in glucose concentrations (r=0.46, p=0.041). Left ventricular systolic and diastolic contractile function were normal at baseline and there was no change following surgery.
Conclusions
The early phase of weight loss after bariatric surgery produces favorable changes in left ventricular geometry, and these are associated with normalization in glucose metabolism.
Keywords: Obesity, MRI, Left Ventricular Hypertrophy, Echocardiogram, Insulin Resistance
Introduction
Obesity is an independent risk factor for the development of heart failure in both men and women1. Subclinical heart failure has been demonstrated in young women with obesity2. However, the mechanisms for the development of heart failure in obesity have not been fully elucidated. There are several lines of evidence, in both human and animal models, which suggest that changes in glucose and lipid metabolism have an untoward effect on left ventricular contractile function3, 4.
Abdominal obesity is a central tenet for the metabolic syndrome5, a set of risk factors that predict cardiovascular disease. Increased abdominal visceral adipose tissue (VAT) appears to be a risk factor for hypertension6, insulin resistance7, and alterations in glucose and lipid metabolism8. Weight loss improves many aspects of systemic metabolism and has been shown to change left ventricular (LV) function and geometry9. The goal of this study was to assess the changes in systemic metabolism, anthropometrics, and left ventricular function and geometry in relation to changes in VAT in women with clinically severe obesity, who have undergone bariatric surgery. Specifically, we wanted to test the hypothesis that diastolic function of the left ventricle changes with weight loss three months after bariatric surgery.
Materials and Methods
Subject selection
We offered participation to consecutive patients, of any race/ethnicity, from the University of Texas, Houston Bariatric Surgery Center (UTHBSC), who met the candidacy requirements for bariatric surgery as outlined previously 10. In brief, they include body mass index (BMI) greater than 40 kg/m2 (or 35 kg/m2 with significant obesity related co-morbidities), a normal psychological evaluation, history of multiple failed medically managed weight loss attempts, and absence of any genetic or reversible endocrinologic cause for obesity. Exclusion criteria were known coronary artery disease, ischemic cardiomyopathy, severe peripheral vascular disease, current history of smoking, pregnancy, and age less than 18 years. Patients with a significant risk for coronary artery disease, as defined by their Framingham risk score or clinical symptoms, underwent either perfusion imaging and/or angiography to rule out the presence of coronary artery disease or ischemic cardiomyopathy. The study was approved by the Committee for the Protection of Human Subjects at the University of Texas, Houston. All patients signed an informed consent prior to enrollment in the study.
Study Protocol
We enrolled 22 women, at the University of Texas, Houston Clinical Research Center. The patients represent a subgroup of a larger, longitudinal observational study on the metabolic and cardiovascular effects of bariatric surgery currently underway at our institution. Subject selection was determined by the patient’s eligibility for an abdominal MRI. Patients were subjected to an overnight fast and instructed to take their normal medications with water, if needed. Patients underwent a physical exam, had a resting electrocardiogram, and anthropometric measurements were obtained. Height was measured on a stadiometer to the nearest 0.5 cm. Weight was determined in stocking feet, to the nearest 100 grams, using a either a mechanical scale or an electronic scale if weight was greater than 159kg . Body mass index (BMI) was calculated as the weight in kilograms divided by the square of height in meters. Waist circumference (WC) measurements were taken in the supine position as the distance between the lateral costal border and the iliac crest and made to the nearest 0.5 cm. Blood samples were drawn after an overnight fast and were analyzed at our institution.
Participants had an echocardiogram performed within one hour of their blood draw. Patients also had an MRI of the abdomen to assess the area of VAT by the methods described below.
Insulin was measured using a chemiluminescence assay (Immulite, Los Angeles, CA), and plasma free fatty acids (FFA) were measured spectrophotometrically (Roche/Hitachi 912, Alameda, CA). Markers of systemic inflammation were measured spectrophotmetrically using a multiplex system (Linco Diagnostics, St. Charles, MO). Insulin sensitivity was assessed using the Homeostasis Model of Assessment 2 (HOMA2) 11. Insulin resistance was defined as insulin sensitivity less than 100% based on the HOMA2 computer model 12. Diabetes was determined by the patient’s medical history and/or by a fasting serum glucose concentration of 126 mg/dL or greater, based on the criteria of the American Diabetes Association 13. Dyslipidemia was defined by the National Cholesterol Education Program criteria 14 or by the fact that the patient was on medication for dyslipidemia. Blood pressure was measured at rest. The diagnosis of hypertension was based on the patient’s history and/or treatment with antihypertensive agents. In all other patients, a blood pressure of greater than 140/90 mmHg on three separate resting measurements was used to define hypertension.
Echocardiograms
Two-dimensional echocardiographic, M-mode, and cardiac Doppler echocardiograms were all performed with a commercially available system (Acuson Sequoia, Malvern, PA). All studies were read by a single cardiologist blinded to all patient information to limit interobserver variability and time length bias, respectively. Participants were studied in the left lateral decubitus position, and images were obtained using standard parasternal and apical acoustic windows to record at least ten beats. All echo parameters were measured for three cardiac cycles, and the values were averaged. Myocardial contrast agents were used to improve endocardial resolution.
The echocardiographic measurements of LV internal dimension and interventricular septal and posterior wall thickness were performed according to the recommendations of the American Society of Echocardiography 15. When LV M-mode measurements could not be optimally obtained, LV internal dimensions and wall thickness measurements were made using the leading edge convention as described by the American Society of Echocardiography 16. Measurements from three consecutive cardiac cycles were averaged. End-diastolic left ventricular dimensions were used to calculate LV mass using a previously validated formula 17. End-diastolic and end-systolic LV volumes were calculated by the Teichholz method 18.
The LV ejection fraction (LVEF) was calculated using the following formula: EDV – ESV/ EDV x 100, where ESV and EDV are end systolic volume and end diastolic volume, respectively. The LV percent fractional shortening (%FS) was obtained from the parasternal short-axis view and calculated as %FS = [(Ded –Des)/Ded] x 100, where Ded and Des are the left ventricular mid cavity dimensions at end diastole and end systole, respectively. The ratio of left ventricular mass over height (LVM/ht2.7) was calculated by dividing the LVM by height in meters raised to the 2.7 power. The relative wall thickness was calculated as: RWT = [2 x LVPW]/Ded, where LVPW = LV posterior wall thickness at end diastole.
Pulsed-wave Doppler-derived transmitral inflow measurements
Mitral diastolic inflow velocities were obtained by positioning a pulsed Doppler wave sample volume at the tip of the mitral valve leaflets during diastole in the apical four chamber view. The transmitral peak velocities of the early diastolic wave (E) and late diastolic wave (A) were measured. From these values the early filling velocity to late filling velocity ratio (E/A) was calculated. The deceleration time (DT) was also measured. Isovolumic relaxation time (IVRT) was measured with continuous wave Doppler across the base of the anterior mitral valve leaflet to record simultaneous LV inflow and outflow measurements.
Tissue Doppler imaging
Tissue Doppler imaging was used to determine load-independent myocardial tissue velocities. Measurements were obtained by positioning the sample volume at the junction of the LV wall and mitral annulus in the septal, lateral, anterior, and inferior portion of the annulus. Analyses were performed for the early diastolic velocity (Em), late diastolic velocity (Ea), and mitral annular systolic velocity (Sm). Pulsed Doppler measurements from the mitral inflow and tissue Doppler measurements were recorded from three consecutive cardiac cycles, and the velocities were averaged. The tissue Doppler measurements are presented as the average of the four annular measurements described.
Abdominal/Pelvic MRI
Examination was performed on these patients at River Oaks Imaging and Diagnostics (Houston, TX), both before and three months after patients underwent surgery. MRI technique and protocols have previously been described for estimation of subcutaneous adipose tissuer (SAT) and VAT19, 20. With the patient in supine position, with both arms above head and using body coil, saggital T2-weighted localizer images were obtained to include the abdomen and pelvis using a large field at 10 mm slice thickness and 5 mm interslice gap.
Subsequently using the saggital localizer, axial T1-weighted spin echo images were obtained with 100 ms(millisecond) repetition time (TR), a 6 ms echo time (TE), with a large enough field of view (FOV) to include the patient’s entire body habitus. Utilizing 256 x 256 matrix, 10 mm slice thickness ,10 mm interslice gap, parallel to L4-5 disc, axial images extending from L2 to S1 level were obtained using a breath hold technique. The MRI examinations were performed on a 1.5T system (General Electric, Milwaukee, WI); Picker Eclipse 1.5T (Cleveland, OH); as well as on an open-field magnet, Hitachi Airis II (Hitachi, Twinsburg, OH), in patients that could not be accommodated due to either weight limit or body habitus requiring larger gantry for optimal patient inclusion and comfort.
Cross-sectional images were analyzed for SAT and VAT by a single trained analyst using SliceOmatic image analysis software (TomoVision, Montreal, Canada). The technical error for repeated measurements of the same scan (on four separate occasions) by the same analyst were as follows: 0.7% plus/minus 0.1% for VAT and 1.1% plus/minus 1.2% for SAT.
Intervention
Patients elected to undergo either laparoscopic small pouch gastric bypass with Roux-en-Y (SPGB) or a laparoscopic adjustable gastric banding (LAGB) procedure. Both procedures have been described in detail previously21, 22. Following surgery, patients were placed on a prescribed diet of liquids for one week, soft solids for one week and then a solid diet by week three. This diet was a high protein (>60 grams), low carbohydrate (less than 20 grams) and low fat (less than 20% of daily calories) diet. Patients met with a registered dietician prior to surgery to establish dietary guidelines post-operatively and reconvened at one, two, and three months post-operatively to review nutritional goals and adherence to diet. At three months postoperatively participants returned to the CRC after an overnight fast to have repeat blood chemistries, echocardiogram, MRI and anthropometric measurements.
Statistical Analysis
Statistical analyses were completed with SPSS 13.0 (SPSS Inc., Chicago, IL). Significance levels were set at α =0.05. We evaluated all of the study variables for conformation to normality using Q-Q plots, skewness and kurtosis statistics. Significantly non-normal variables were transformed prior to analysis. Data are expressed as mean values or the change in mean values from baseline to 3 months post-operatively, plus or minus the standard error of the mean. Independent sample t-tests were performed to evaluate differences between the LAGB and the SPGB patients. The effects of the two different types of surgery on the outcomes were analyzed using repeated measures ANOVA. Paired t-tests were performed to determine differences in data from baseline to 3 months post-operatively. Pearson correlation coefficients were prepared to evaluate the univariate relationship.
Results
Baseline Characteristics
Baseline clinical and demographic characteristics are shown in Table 1. The mean age (+/− SEM) and BMI were 44 years (2.1) and 46.8 kg/m2 (1.4), respectively. Mean resting heart rate (HR) and blood pressure were normal. All patients met criteria for abdominal obesity as measured by waist circumference. The abdominal VAT area was 232 cm2 (18.5) at baseline, an area that has been demonstrated to predict significant cardiovascular risk in women23. Despite that only slightly greater than one-third of the cohort had diabetes, the prevalence of insulin resistance was high (Table 1). While left ventricular systolic function was normal in the cohort, there was age adjusted diastolic dysfunction in 31% of the cohort24, as measured by tissue Doppler imaging, and evidence of left ventricular hypertrophy as measured by LVM indexed to height to the 2.7 power25. There were no differences in baseline clinical characteristics, blood chemistries, or left ventricular function or geometry for the type of surgery each patient underwent (Table 1).
Table 1.
Baseline Characteristics (n = 22)
| Mean (SEM) | Mean (SEM) | Mean (SEM) | ||
|---|---|---|---|---|
| Variable | Cohort (n=22) | LAGB (n=6) | SPGB (n=16) | p-value |
| Age (years) | 44 (2.1) | 47 (2.6) | 44 (2.8) | 0.488 |
| Weight (kg) | 126.6 (4.6) | 132.3 (10.9) | 124.5 (5.0) | 0.463 |
| BMI (kg/m2) | 46.8 (1.4) | 49.4 (3.2) | 46.0 (1.5) | 0.286 |
| SBP (mmHg) | 130 (4.3) | 132 (7.3) | 130 (5.4) | 0.876 |
| DBP (mmHg) | 72 (2.8) | 72 (4.7) | 73 (3.5) | 0.898 |
| HR (bpm) | 78 (2.6) | 82 (4.0) | 77 (3.2) | 0.393 |
| VAT (cm2) | 232 (18) | 241 (22) | 228 (22) | 0.755 |
| LVEF (%) | 61 (2.4) | 57 (6.3) | 63 (1.9) | 0.239 |
| Sm (cm/sec) | 9.9 (0.4) | 10.0 (1.0) | 9.9 (0.5) | 0.956 |
| Em (cm/sec) | 11.4 (0.5) | 11.2 (1.2) | 11.6 (0.5) | 0.707 |
| LVM/ht2.7 (g/m2.7) | 50.0 (3.9) | 59.3 (11.3) | 46.1 (2.6) | 0.126 |
| Comorbid Conditions | ||||
| Diabetes | 36% | 17% | 44% | |
| Dyslipidemia | 22% | 17% | 25% | |
| Hypertension | 50% | 67% | 44% | |
| Insulin Resistance | 90% | 100% | 88% | |
BMI, Body Mass Index; SBP, Systolic Blood Pressure; DBP, Diastolic Blood Pressure; HR, Heart rate; VAT, Visceral Adipose Tissue; LVEF, Left Ventricular Ejection Fraction; Sm, Tissue Doppler Imaging Global Systolic Function; Em, Tissue Doppler Imaging Global Diastolic Function; LVM/ht2.7, Left Ventricular Mass Indexed to height to the 2.7 power; SPGB, Small Pouch Gastric Bypass; LAGB, Laparoscopic Adjustable Gastric Banding.
See Methods for definitions of comorbid conditions.
Three months after bariatric surgery there were significant improvements in anthropometric measurements (Table 2) and systemic metabolic parameters (Table 3). The significant increase in insulin sensitivity as measured by HOMA-S % [mean change (+/−SEM): 34 (10.4), p<0.0001] is reflected by the profound decreases in glucose and insulin concentrations. Despite the rapid weight loss there was no change in plasma FFA concentrations three months post-operatively. Leptin levels decreased and correlated with a decrease in weight (r = 0.47, p = 0.037), whereas there was no change in adiponectin concentrations. hsCRP was the only marker of systemic inflammation which significantly decreased three months post-operatively, whereas TNF-α and IL-6 did not change (Table 3).
Table 2.
Anthropometric and Hemodynamic Changes Three Months After Surgery
| Baseline Mean (SEM) | Three Months Mean (SEM) | p value | |
|---|---|---|---|
| BMI (kg/m2) | 46.8 (1.4) | 40.1 (1.5) | <0.0001 |
| Weight (kg) | 126 (4.6) | 107 (4.8) | <0.0001 |
| Waist Circumference (cm) | 128 (3.0) | 113 (2.8) | <0.0001 |
| VAT (cm2) | 232 (18) | 166 (16) | 0.002 |
| SBP (mmHg) | 130 (4.3) | 122 (2.5) | 0.077 |
| DBP (mmHg) | 72 (2.8) | 69 (2.0) | 0.35 |
| HR (bpm) | 78 (2.6) | 65 (3.3) | <0.0001 |
BMI, Body Mass Index; SBP, Systolic Blood Pressure; DBP, Diastolic Blood Pressure; HR, Heart Rate.
P values in bold indicate statistically significant difference between baseline and three months.
Table 3.
Systemic Metabolic and Inflammatory Parameters Three Months After Surgery
| Baseline Mean (SEM) | Three Months Mean (SEM) | p Value | |
|---|---|---|---|
| Glucose (mg/dL) | 120 (13.9) | 82 (3.7) | 0.005 |
| Insulin (μU/ml) | 22.5 (3.8) | 12.6 (1.9) | 0.005 |
| Total Cholesterol (mg/dL) | 180 (6.3) | 160 (5.8) | 0.024 |
| Free Fatty Acids (mmol/dL)* | 0.85 (0.05) | 0.89 (0.06) | 0.51 |
| Triglycerides (mg/dL) | 165 (38) | 106 (11) | 0.13 |
| Adiponectin (μg/mL) | 8.3 (1.3) | 9.0 (1.0) | 0.35 |
| Leptin (ng/mL) | 57.8 (6.7) | 28 (3.7) | <0.0001 |
| hsCRP (μg/mL) | 0.65 (0.07) | 0.46 (0.09) | 0.013 |
| IL-6 (pg/mL) | 15.3 (5.2) | 13.1 (5.0) | 0.37 |
| TNF-α (ng/mL)* | 9.2 (1.2) | 8.5 (0.6) | 0.60 |
hsCRP, High Sensitivity C-Reactive Protein; IL-6, Interleukin 6; TNF-α, Tumor Necrosis Factor Alpha.
P values in bold indicate a statistically significant change from baseline to three months.
Indicates plasma concentrations, whereas all others are serum concentrations.
VAT significantly decreased three months after surgery (Figure 1). The SPGB group lost more VAT at three months compared to the LAGB group (mean difference 78 cm2, p= 0.039). The decrease in VAT correlated with decreases in BMI (r = 0.61, p = 0.005), glucose (r = 0.48, p = 0.033 ), and hsCRP (r = 0.53, p = 0.016). There was a trend for an inverse relation between HOMA and VAT (r = −0.43, p = 0.056).
Figure 1. Abdominal Visceral Adipose Tissue.
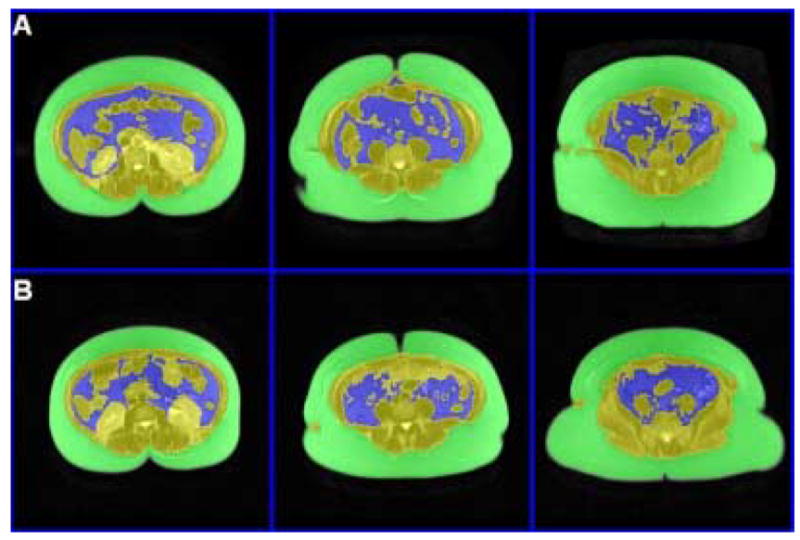
Representative T1-weighted (TR 100 milliseconds, TE 6 milliseconds) of abdominal visceral adipose tissue VAT, (blue) before surgery (A); and three months after Bariatric surgery (B).
There was a trend for the increase in Em (percent increase: 19 %, p = 0.057). Systolic function as measured by either ejection fraction or tissue Doppler imaging did not change three months after surgery (Table 4). However, left ventricular mass, when indexed to height, significantly decreased [−3.8 g/m2.7 (1.7), p = 0.037]. While the change in LVM did not correlate with VAT, the decrease positively correlate with changes in glucose concentration (r = 0.46, p = 0.041).
Table 4.
Left Ventricular Function and Geometry Changes Three Months After Surgery
| Baseline Mean (SEM) | Three Months Mean (SEM) | p Value | |
|---|---|---|---|
| LVEF (%) | 61 (2.4) | 57 (4.0) | 0.43 |
| LVSF (%) | 32 (1.6) | 32 (1.3) | 0.90 |
| Sm (cm/sec) | 9.9 (0.4) | 9.2 (0.4) | 0.18 |
| DT (msec) | 212 (5.0) | 195 (7.5) | 0.074 |
| E (cm/sec) | 89 (3.3) | 92 (3.3) | 0.27 |
| A (cm/sec) | 76 (3.5) | 75 (2.4) | 0.66 |
| E/A ratio | 1.26 (0.06) | 1.22 (0.06) | 0.41 |
| Em (cm/sec) | 11.4 (0.5) | 11.5 (0.6) | 0.92 |
| LVM/ht2.7 (g/m) | 50.0 (3.9) | 46.1 (3.8) | 0.037 |
| RWT (cm) | 0.42 (0.01) | 0.43 (0.02) | 0.67 |
LVEF, Left Ventricular Ejection Fraction; LVSF, Left Ventricular Shortening Fraction; Sm, Tissue Doppler Global Left Ventricular Systolic Function; DT, Deceleration Time; E, Early Mitral Inflow; A, Late Mitral Inflow; E/A, Early to Late Mitral Inflow Ratio; Em, Tissue Doppler Left Ventricular Global Diastolic Function; Left Ventricular Mass Indexed to Height to the 2.7 Power; RWT, Relative Wall Thickness.
P values in bold indicate statistically significant difference between baseline and three months.
There were differences in the decreases in BMI and VAT when comparing the LAGB and SPGB groups (Table 5). Both groups had statistically significant changes but the SPGB had a greater change in BMI and decrease in VAT at three months compared to the LAGB group, whereas none of the other outcome variables were significantly different. However, when the effect of surgery was analyzed using repeated measures ANOVA, the specific type of surgery did not impact the changes observed at this early point in weight loss (Table 5).
Table 5.
Outcome Variables Between Surgery Type and the Effect of Surgery on the Changes
| Variables | LAGB | SPGB | p* | p** |
|---|---|---|---|---|
| BMI(kg/m2) | −5.2 (0.63) | −8.7 (0.43) | 0.001 | 0.10 |
| Weight (kg) | −17.0 (4.15) | −20.0 (3.68) | 0.65 | 0.37 |
| WC (cm) | −17.4 (6.28) | −15.0 (3.00) | 0.71 | 0.44 |
| VAT (cm2) | −11.3 (26.68) | −89.6 (20.19) | 0.041 | 0.127 |
| HR (bpm) | −11.3 (2.93) | −13.7 (3.71) | 0.76 | 0.29 |
| Glucose (mg/dL) | −12.3 (5.70) | −47.6 (16.15) | 0.20 | 0.42 |
| Insulin (μU/mL) | −6.2 (5.03) | −11.4 (4.01) | 0.48 | 0.25 |
| HOMA-S (%) | −7.7 (15.6) | −43.9 (12.4) | 0.12 | 0.36 |
| Leptin (ng/mL) | −22.5 (8.03) | −31.7 (5.67) | 0.39 | 0.53 |
| hsCRP (μg/mL) | −0.018 (0.147) | −0.25 (0.076) | 0.13 | 0.62 |
| LVM/ht2.7 (g/m2.7) | −1.5 (1.52) | −4.9 (2.37) | 0.38 | 0.069 |
p value for the difference in the changes between the LAGB and SPGB groups, independent t-test.
p value for the effect of surgery on the changes in outcomes observed, repeated measures ANVOA
BMI, Body Mass Index; WC, waist circumference; VAT, visceral adipose tissue; HR, heart rate; HOMA-S, Homeostasis Model of Assessment for Insulin Sensitivity; hsCRP, high sensitivity C-reactive protein; LVM/ht2.7, Left ventricular mass indexed to height to the 2.7 power.
P values in bold indicate statistically significant difference between baseline and three months.
Discussion
In a prospective, longitudinal study after bariatric surgery we have demonstrated improvements in systemic metabolism and profound decreases in weight and VAT during the first three months. Concurrent with these changes was a decrease in left ventricular hypertrophy, but no change in left ventricular contractile function three months post-operatively.
Causes of left ventricular hypertrophy include obesity, activation of the sympathetic nervous system26, and derangements in glucose and insulin metabolism27. We have directly demonstrated a decrease in weight and an improvement in insulin sensitivity three months after bariatric surgery, concurrent with a decrease in LVM. Indirect evidence for a decrease in sympathetic tone in our cohort is derived from the significant decrease in heart rate, the improvement in insulin sensitivity28, and the reduction in VAT29.
We found a positive correlation between the change in LVM and the change in glucose concentrations. In the Framingham cohort glucose intolerance was the strongest predictor of the development of left ventricular hypertrophy in women27. A mechanism for the relation between hyperglycemia and increased LVM is not precisely known, but may include alterations in myocardial protein degradation, increases in glycation products, increases in hexose phosphates (personal communication Sharma and Taegtmeyer), or the effects of insulin-like growth factor30.
Obesity has been described as a state of chronic inflammation. The decrease in hsCRP was positively associated with changes in VAT. A recent study in diabetic individuals demonstrated a relation between hsCRP, insulin resistance, and impaired autonomic function31. These findings could have implications in the obese population with regard to inflammation and left ventricular size.
However, TNF-α did not decrease with weight loss. The sustained level of TNF-α is not the result of acute inflammation, rather it is more likely explained by lipolysis of VAT and thus the activation of adipose-related macrophages32. Sustained concentrations of TNF-α in the early phase of weight loss after surgery may be beneficial as TNF-α is the signal responsible for the activation of the ubiquitin-proteosome pathway, an established system in cardiac remodeling33. It is therefore tempting to speculate that decreases in hsCRP and sustained levels of TNF-α contribute to changes in LVM in the setting of weight loss.
While there are conflicting results in the weight loss literature34–37 (behavioral, medical, and surgical) in regards to systemic inflammation and specifically the changes of TNF-α, several recent studies have shown either no change or an increase37 in TNF-α early in the weight loss process. In fact, our data are in line with these most recent studies evaluating TNF-α after surgically induced weight loss up to one year. The differences in the literature may represent different populations being studied, lower baseline BMI and TNF-α concentrations, as well as different modes of weight loss.
We have previously shown that left ventricular diastolic function is negatively associated with plasma free fatty acid levels38 and that this association may be partially related to glucolipotoxicity30. In the rapid phase of weight loss we have demonstrated a significant decrease in VAT which likely contributes to a sustained elevation of FFA concentrations. Interestingly, despite the elevated FFA concentrations, insulin sensitivity improves, although it does not become normal. We therefore speculate that glucose flux is likely still impaired and derangements in calcium homeostasis, an important mechanism in left ventricular relaxation, may not have normalized.
The discrepancy between our findings and those of Willens et al.9, who demonstrated significant changes in diastolic function after SPGB, may be based on when echocardiographic evaluations were performed after bariatric surgery. In the study by Willens et al. left ventricular function was measured when the patient obtained a “significant” amount of weight loss, rather than at a set time point, and occurred a mean of 7.6 months (range 3 to 15 months) after bariatric surgery. While we have shown a non-significant improvement in diastolic function three months after bariatric surgery, we suspect changes in contractile function will become evident at a later stage of weight loss when plasma FFA levels begin to decline.
Interestingly, the type of surgery did not have a significant effect on the changes in the measured variables suggesting that the different types of surgery share a similar mechanism for the early metabolic changes. This mechanism may be as simple as reduced caloric intake, independent of the restriction or the restriction/malabsorption of the LAGB and SPGB procedures, respectively. Long term follow-up data may show a divergence in metabolic and cardiac outcomes between these procedures and possibly differing influences of each procedure on the results.
There are several limitations in this study. This is an observational study and we can only speculate on the mechanisms for the change in LVM. The study is small and limited to women who have a heterogeneous background, from co-morbid conditions to the type of surgery performed. While these differences did not contribute to our findings the results may not be generalizable to all patients with clinically severe obesity who undergo bariatric surgery. Furthermore, the lack of change in some of variables should not be taken as negative outcome as studies in a larger population may demonstrate the benefits of surgery on these variables and different variables may have different time courses.
Conclusions
The early phase of weight loss after bariatric surgery produces favorable changes in left ventricular geometry which is associated with changes in glucose metabolism. Improvement in anthropometrics and decreases in sympathetic tone, as a consequence of weight loss, may also play a role in decreased left ventricular hypertrophy.
Acknowledgments
We would like to thank E Jane Meyers for her administrative support and Mark Punyanitya for his technical assistance with the MRI analysis. We also acknowledge Roxy Tate for her technical and editorial assistance.
Footnotes
Supported in part from the National Institutes of Health, National Heart, Lung, and Blood Institute (5RO1 HL073162-02) to H.T., American Society of Bariatric Surgeons Research Grant to E.B.W., and The University of Texas, Houston General Clinical Research Center, (Grant # M01RR002558)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kenchaiah S, Evans JC, Levy D, et al. Obesity and the risk of heart failure. N Engl J Med1. 2002;347:305–13. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 2.Peterson LR, Waggoner AD, Schechtman KB, et al. Alterations in left ventricular structure and function in young healthy obese women: assessment by echocardiography and tissue Doppler imaging. J Am Coll Cardiol. 2004;43:1399–1404. doi: 10.1016/j.jacc.2003.10.062. [DOI] [PubMed] [Google Scholar]
- 3.Young ME, Guthrie PH, Razeghi P, et al. Impaired long-chain fatty acid oxidation and contractile dysfunction in the obese Zucker rat heart. Diabetes. 2002;51:2587–95. doi: 10.2337/diabetes.51.8.2587. [DOI] [PubMed] [Google Scholar]
- 4.Peterson LR, Herrero P, Schechtman KB, et al. Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circulation. 2004;109:2191–6. doi: 10.1161/01.CIR.0000127959.28627.F8. [DOI] [PubMed] [Google Scholar]
- 5.Worldwide definition for the use in clinical practice. The IDF consensus worldwide definition of the metabolic syndrome. 2004. [Google Scholar]
- 6.Despres J. Health consequences of visceral obesity. Ann Med. 2001;33:534–41. doi: 10.3109/07853890108995963. [DOI] [PubMed] [Google Scholar]
- 7.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106:473–81. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujioka SMY, Tokunaga K, Kawamoto T, et al. Improvement of glucose and lipid metabolism associated with selective reduction of intra-abdominal visceral fat in premenopausal women with visceral fat obesity. Int J Obes. 1991;15:853–9. [PubMed] [Google Scholar]
- 9.Willens HJ, Chakko SC, Byers P, et al. Effects of weight loss after gastric bypass on right and left ventricular function assessed by tissue Doppler imaging. Am J Cardiol. 2005;95:1521–4. doi: 10.1016/j.amjcard.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 10.Gastrointestinal surgery for severe obesity. Consensus Statement: NIH Consensus Development Conference. 1991 March 25–26;9:1–20. [PubMed] [Google Scholar]
- 11.Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21:2191–2. doi: 10.2337/diacare.21.12.2191. [DOI] [PubMed] [Google Scholar]
- 12.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–95. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 13.Genuth S, Alberti KG, Bennett P, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–7. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 14.Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) (Adult Treatment Panel III): Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol Education in Adults; 2001.
- 15.Sahn DJ, DeMaria A, Kisslo J, et al. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–83. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- 16.Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography committee on standards, subcommittee on quantitation of two-dimensional echocardiograms. J Am Soc Echocardiogr. 1989;2:358–67. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 17.Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–8. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 18.Teichholz LE, Kreulen T, Herman MV, et al. Problems in echocardiographic volume determinations: echocardiographic-angiographic correlations in the presence of absence of asynergy. Am J Cardiol. 1976;37:7–11. doi: 10.1016/0002-9149(76)90491-4. [DOI] [PubMed] [Google Scholar]
- 19.Shen W, Punyanitya M, Wang Z, et al. Visceral adipose tissue: relations between single-slice areas and total volume. Am J Clin Nutr. 2004;80:271–8. doi: 10.1093/ajcn/80.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lancaster JL, Ghiatas AA, Alyassin A, et al. Measurement of abdominal fat with T1-weighted MR images. J Magn Reson Imaging. 1991;1:363–9. doi: 10.1002/jmri.1880010315. [DOI] [PubMed] [Google Scholar]
- 21.Higa KD, Boone KB, Ho T, et al. Laparoscopic Roux-en-Y gastric bypass for morbid obesity: technique and preliminary results of our first 400 patients. Arch Surg. 2000;135:1029–33. doi: 10.1001/archsurg.135.9.1029. discussion 1033–1024. [DOI] [PubMed] [Google Scholar]
- 22.Fielding GA, Allen JW. A step-by-step guide to placement of the LAP-BAND adjustable gastric banding system. Am J Surg. 2002;184(6B):26S–30S. doi: 10.1016/s0002-9610(02)01176-5. [DOI] [PubMed] [Google Scholar]
- 23.Williams MJ, Hunter GR, Kekes-Szabo T, et al. Intra-abdominal adipose tissue cut-points related to elevated cardiovascular risk in women. Int J Obes Relat Metab Disord. 1996;20:613–17. [PubMed] [Google Scholar]
- 24.Alam M, Wardell J, Andersson E, et al. Characteristics of mitral and tricuspid annular velocities determined by pulsed wave Doppler tissue imaging in healthy subjects. J Am Soc Echocardiogr. 1999;12:618–28. doi: 10.1053/je.1999.v12.a99246. [DOI] [PubMed] [Google Scholar]
- 25.Liu JE, Roman MJ, Pini R, et al. Cardiac and arterial target organ damage in adults with elevated ambulatory and normal office blood pressure. Ann Intern Med. 1999;131:564–72. doi: 10.7326/0003-4819-131-8-199910190-00003. [DOI] [PubMed] [Google Scholar]
- 26.Schlaich MP, Kaye DM, Lambert E, et al. Relation between cardiac sympathetic activity and hypertensive left ventricular hypertrophy. Circulation. 2003;108:560–5. doi: 10.1161/01.CIR.0000081775.72651.B6. [DOI] [PubMed] [Google Scholar]
- 27.Rutter MK, Parise H, Benjamin EJ, et al. Impact of glucose intolerance and insulin resistance on cardiac structure and function: sex-related differences in the Framingham Heart Study. Circulation. 2003;107:448–54. doi: 10.1161/01.cir.0000045671.62860.98. [DOI] [PubMed] [Google Scholar]
- 28.Landsberg L, Young JB. Insulin-mediated glucose metabolism in the relationship between dietary intake and sympathetic nervous system activity. Int J Obes. 1985;9(Suppl 2):63–8. [PubMed] [Google Scholar]
- 29.Alvarez GE, Beske SD, Ballard TP, et al. Sympathetic neural activation in visceral obesity. Circulation. 2002;106:2533–6. doi: 10.1161/01.cir.0000041244.79165.25. [DOI] [PubMed] [Google Scholar]
- 30.Young ME, McNulty P, Taegtmeyer H. Adaptation and maladaptation of the heart in diabetes: Part II: potential mechanisms. Circulation. 2002;105:1861–70. doi: 10.1161/01.cir.0000012467.61045.87. [DOI] [PubMed] [Google Scholar]
- 31.Anan F, Takahashi N, Nakagawa M, et al. High-sensitivity C-reactive protein is associated with insulin resistance and cardiovascular autonomic dysfunction in type 2 diabetic patients. Metabolism. 2005;54:552–8. doi: 10.1016/j.metabol.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 32.Weisberg SP, McCann D, Desai M, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Razeghi P, Sharma S, Ying J, et al. Atrophic remodeling of the heart in vivo simultaneously activates pathways of protein synthesis and degradation. Circulation. 2003;108:2536–41. doi: 10.1161/01.CIR.0000096481.45105.13. [DOI] [PubMed] [Google Scholar]
- 34.Bruun JM, Verdich C, Toubro S, et al. Association between measures of insulin sensitivity and circulating levels of interleukin-8, interleukin-6 and tumor necrosis factor-alpha. Effect of weight loss in obese men. Eur J Endocrinol. 2003;148:535–42. doi: 10.1530/eje.0.1480535. [DOI] [PubMed] [Google Scholar]
- 35.Dandona P, Weinstock R, Thusu K, et al. Tumor necrosis factor-alpha in sera of obese patients: fall with weight loss. J Clin Endocrinol Metab. 1998;83:2907–10. doi: 10.1210/jcem.83.8.5026. [DOI] [PubMed] [Google Scholar]
- 36.Vazquez LA, Pazos F, Berrazueta JR, et al. Effects of changes in body weight and insulin resistance on inflammation and endothelial function in morbid obesity after bariatric surgery. J Clin Endocrinol Metab. 2005;90:316–22. doi: 10.1210/jc.2003-032059. [DOI] [PubMed] [Google Scholar]
- 37.Molina A, Vendrell J, Gutierrez C, et al. Insulin resistance, leptin and TNF-alpha system in morbidly obese women after gastric bypass. Obes Surg. 2003;13:615–21. doi: 10.1381/096089203322190844. [DOI] [PubMed] [Google Scholar]
- 38.Leichman JG, Aguilar D, King TM, et al. Association of plasma free fatty acids and left ventricular diastolic function in patients with clinically severe obesity. Am J Clin Nutr. 2006;84:336–41. doi: 10.1093/ajcn/84.1.336. [DOI] [PubMed] [Google Scholar]


