Abstract
Enterostatin injected into the amygdala selectively reduces dietary fat intake by an action that involves a serotonergic component in the paraventricular nucleus. We have investigated the role of melanocortin signaling in the response to enterostatin by studies in melanocortin 4 receptor (MC4R) knockout mice and by the use of the MC4R and MC3R antagonist SHU9119, and by neurochemical phenotyping of enterostatin activated cells. We also determined the effect of enterostatin in vivo on the expression of AgRP in the hypothalamus and amygdala of rats and in culture on a GT1-7 neuronal cell line. Enterostatin had no effect on food intake in MC4R knock out mice. SHU9119 icv blocked the feeding response to amygdala enterostatin in rats. Amygdala enterostatin induced fos activation in α-melanocyte stimulating hormone (α-MSH) neurons in the arcuate nucleus. Enterostatin also reduced the expression of AgRP in the hypothalamus and amygdala and in GT1-7 cells. These data suggest enterostatin inhibits dietary fat intake through a melanocortin signaling pathway.
Keywords: Melanocortin receptors, Melanocyte stimulating hormone, dietary fat, amygdala
INTRODUCTION
Enterostatin (APGPR) is the aminoterminal pentapeptide of procolipase and is known to induce satiation in rats. Specifically, it suppresses intake of a high fat diet, but not a high carbohydrate diet when administrated centrally or peripherally to overnight fasted rats (26,28). Enterostatin is released from pancreatic procolipase by proteolytic activity in the small intestine after the ingestion of dietary fat (5). More recently, procolipase and enterostatin have also been localized to the gastric mucosa and to certain brain regions (amygdala, hypothalamus, cortex) by immunohistochemistry (38,44) and by the presence of procolipase mRNA (27,44) suggesting that the gene is expressed in both the GI tract and the CNS, like many other feeding related peptides.
A number of neuropeptides and aminergic compounds have been suggested to have selective effects on the ingestion of macronutrients. Growth hormone releasing hormone (GHRH) will promote protein ingestion (2), epinephrine will stimulate carbohydrate intake (13), NPY may promote carbohydrate and fat ingestion (34,39) and agouti-related protein (AgRP) will enhance dietary fat intake (6). In contrast, enterostatin has been shown to have selective effects to reduce dietary fat intake when rats have a choice of macronutrients or diets (16, 26, 28). However, when rats are fed a single choice diet, enterostatin is only effective in reducing food intake if the diet has a high fat content. Rats fed a high carbohydrate/low fat diet are unresponsive to enterostatin (26).
The neuronal pathway through which enterostatin inhibits dietary fat intake has not been fully mapped. Stereotaxic injections of enterostatin suggest that the amygdala is a site of action (17). Immunohistochemical approaches (44) have also identified both the precursor protein procolipase and enterostatin peptide in this region and shown that amygdala enterostatin induces neuronal activation (fos expression) in regions of the hypothalamus that innervate the paraventricular nucleus (PVN) (22). The anorectic response to amygdala enterostatin is prevented by a 5HT1B receptor antagonist injected into the paraventricular nucleus (43), the site of a serotonergic system that inhibits dietary fat intake (35, 37). Likewise, the inhibition of food intake in response to enterostatin in 5HT2CReceptor −/− mice is also blocked by a 5HT1B Receptor antagonist (43). Since enterostatin increases 5HT release (12,42), it appears likely that this PVN serotonergic pathway dependent upon the activity of 5HT1B receptors modulates the enterostatin effect on dietary fat intake. It is also dependent upon the presence of CCKA receptors although the location of these receptors is not yet known (21). A role for endogenously produced enterostatin in feeding behavior is suggested by the ability to increase intake of high fat diets by the enterostatin antagonist β-casomorphin1–7 (18) and by the increase in 24 hour food intake after intracerebroventricular injection of an antibody to enterostatin (unpublished data). At a high dose, enterostatin also increased intake of a high fat diet (15), possibly reflecting activity as a partial antagonist or suggesting that enterostatin and β-casomorphin1–7 have separate binding sites on its receptor (30).
In the experiments reported in this manuscript, we provide evidence that the response to enterostatin is also dependent upon melanocortin 4 receptors (MC4R) and might be affected partly at least through the regulation of agouti-related protein (AgRP).
MATERIALS AND METHODS
Peptides and Drugs
Enterostatin (APGPR) was synthesized by solid-phase chemistry, purified by HPLC, and estimated to be of greater than 99% purity by American Peptide Company (Sunnyvale, CA). SHU9119 was purchased from Phoenix Pharmaceuticals (Belmont, CA). Peptides and drugs were dissolved in 0.9 % (w/v) sterile saline.
Feeding and Gene Expression studies in mice and rats
Female C57Bl/6J mice and C57Bl/6J MC4R−/− mice were kindly provided by Dr. Andrew Butler (Pennington Biomedical Research Center) and maintained on a 45% calorie high fat diet (D12451, Research Diets, New Brunswick, NJ) for 4 weeks prior to study. They were 5 months of age at the time of the study. After an overnight fast, mice were injected free hand into the third ventricle while under isoflurane anesthesia using a Hamilton 10 mu;L syringe with either saline vehicle (2 mu;L) or enterostatin (1nmole) and food intake was recorded hourly for 4 hours and again after 24 hours.
Male Osborne –Mendel (OM) bred at the Pennington Biomedical Research Center or Sprague-Dawley rats (Harlan Sprague Dawley, Indianapolis IN), 10 weeks of age, were individually housed in hanging wire mesh cages in a room at 20–22°C with a 12hr light dark cycle. They were adapted to a high fat diet (HF, 4.78 kcal/g, 56% fat energy) prepared from individual macro and micronutrients as previously described (19) for 14 days. Lateral intracerebroventricular (icv)(feeding studies) or third ventricular (gene expression studies) stainless steel 22g cannulas (12mm length), or cannulas directed at the amygdala were implanted in rats anesthetized by subcutaneous injections of 1.25ml/kg body weight of the mixture of ketamine (80mg/ml), acepromazine (1.6mg/ml) and xylazine (5mg/ml). Rats were placed in a stereotaxic frame with incisor bar 3.3mm below the ear bar. The following coordinates were used: Lateral ventricular cannulas, 1.4mm lateral to midline, 0.8mm posterior to bregma and 3.5mm ventral to the dura; third ventricular, midline, 2.5mm caudal to bregma and 7.7mm subdural; amygdala AP -2.4mm, L -3.8mm and 6mm subdural according to the atlas of Paxinos and Watson (31). The cannulas were secured in place with 3 anchor screws and dental acrylic and occluded with a 26 gauge wire stylet. The infusion cannulas for the amygdala projected 2mm beyond the tip of the implanted guide cannulas. The animals were allowed at least 7 days to recover from surgery before testing.
For feeding study, rats were fasted overnight, injected with the MC3/MC4 R antagonist SHU9119 (1.0nmole) or drug vehicle (5 mu;L saline) into the lateral ventricle. After 90 minutes rats then received a second injection of either enterostatin (1nmole) or vehicle (5 mu;L saline) after which food was replaced into their cages and food intake measured, allowing for all spillage, at 0.5, 1.0 and 2 hours. Injections were made through a 26g stainless steel injector that projected 0.5mm beyond the guide cannula.
For in vivo gene expression studies, rats were fasted overnight and injected with either enterostatin (1nmole) or saline vehicle (1.0 mu;L into ventricle or 0.5 mu;L onto amygdala) and sacrificed 1 hour later by guillotine. Brains were rapidly dissected and tissues frozen at −80°C until further processing.
Cell Culture
GT1-7 neuronal cells were cultured and maintained in Dulbecco’s modified Eagle’s medium (Gibco, Carlsbad, CA) containing 10% fetal bovine serum, penicillin (100U/mL) and streptomycin (100ug/mL) at 37°C in 5% CO2 atmosphere. Cells below passage 20 were used. When the cells reached approximately 80% confluency, they were incubated in the presence or absence of enterostatin (10–1000nM) for one hour at which time cells were harvested for RNA isolation.
RNA Isolation and quantitative RT-PCR
RNA was extracted from hypothalamus, amygdala or GT1-7 cells using Tri-Reagent (Molecular Research Center, Cincinnati, OH) following the manufacturer’s instructions. RNA was treated with RNase-free DNase (Qiagen, Valencia, CA) and cleaned by RNeasy kit (Qiagen). For semiquantitative RT-PCR, five micrograms of total RNA from individual samples was reverse-transcribed with Moloney Murine Leukemia Virus (M-MLV) reverse transcriptase (Promega, Madison, WI) using oligo(dT)12–18. The cycles were used on the exponential portion of the response for each gene. The PCR product was electrophoresed on 2% agarose gels and the intensities of the bands were quantified using Quantity One 4.2.1 Gel Doc Software (BioRad Lab, Hercules, CA). For real-time quantitative RT-PCR, one microgram of RNA was reverse transcribed using SuperscriptTM First-Strand Synthesis System for RT-PCR (Invitrogen, Carisbad, CA) and the two step PCR protocol in an ABI prism 7700 with SYBR® Green master mix (Applied Byosystems, Foster city, CA). The expression level for agouti-related protein (AgRP) and POMC were determined by using the following primers and cyclophilin B was used as a reference. Primers were designed using Primer Express version 2.1 (Applied Biosystems). For AgRP expression, 5′-AGCTTTGGCGGAGGTGCTA-3′ and 5′-AGGACTCGTGCAGCCTTACAC-3’ for GT1-7 cells; 5’-GCACCACTGAAGGGCATCAG-3’ and 5’-CGGTCTGCTGCTGTCTTCTT-3’ were used for rat samples. For POMC expression, 5′-AGAGGCCACTGAACATCTTCGT-3′ and 5′-TGTAGCAGAATCTCGGCATCTTC-3′ were used. For mouse cyclophilin B, 5’-GCTGGATGGCAAGCATGTG-3’ and 5’TGTCTTGGTGCTCTCCACCTT-3’ for GT1-7 and 5’-ACAGTGGATAATTTTGTAGCCTTAGCT-3’ and for rat 5’-AGTCCTTGATGACACGATGGAA-3’ were used.
Immunohistochemistry
Male Sprague-Dawley rats (~300g) adapted to a high fat diet (56% energy as fat) were anesthetized with a mixture of ketamine, acepromazine and xylazine. A stainless steel guide cannula (24G) was implanted towards the central nucleus of amygdala. The coordinates were as previously described above. Ten days after surgery, rats were divided into two groups for saline control and enterostatin treatment. Enterostatin (0.1nmole) or saline(0.1 mu;L) was injected into the amygdala through an injector cannula that projected 2mm beyond the indwelling guide cannula tip. Rats were anesthetized 2 hours later and transcardially perfused with 4% phosphate-buffered paraformaldehyde solution. The tissue blocks were embedded in O.C.T. compound (Miles Elkhart, IN). Coronal (30 mu;m) sections were cut on a cryostat and collected serially in five sets in multiwell culture plates with cryoprotectant (5 mM phosphate-buffered saline (PBS), pH 7.3, 30% ethylene glycol and 20% glycerol) and stored at −20°C until further processing. Sections were removed from cryoprotectant and rinsed in 0.01 M PBS, pH 7.3 prior to immunocytochemical procedures. The sections were pre-treated with 1% NaBH4 for 30 min to reduce any remaining fixative, and a solution of 1.5% hydrogen peroxide, 20% methanol and 0.5 % Triton X-100 for 30 min to inactivate endogenous peroxidase. Tissue sections were preincubated for 2 hours in 5% normal goat serum plus 1% of bovine serum albumin, 0.5% Triton X in PBS to block non-specific binding of the primary antibody, then successive sections were incubated with a rabbit anti c-fos (1:30,000) (Oncogene Research Products, San Diego, CA) overnight at room temperature with gentle agitation. After four rinses, sections were incubated with a biotinylated secondary antibodies (1:500, goat anti-rabbit) immunoglobulin G, (Vector Lab, Burlingham, CA), followed by reaction with an avidin-biotin complex (Vectastain Elite ABC kit, Vector Lab). The antibody peroxidase complex was visualized with a metal-enhanced DAB substrate kit (0.5% 3.3V-diaminobenzidine tetrahydrochloride, 1% cobalt chloride and nickel chloride with stable hydrogen peroxide; Pierce Chemical, Rockford, IL) for 5–10 minutes to generate a blue-black c-fos nuclear product. The c-fos-labeled sections were subsequently processed for localization of α-MSH after blocking with a 5% normal donkey serum solution, using a sheep anti α-MSH (1:50,000) antibody and a donkey anti-sheep (1:200) immunoglobulin G, (Vector Lab, Burlingham, CA). The remainder of the process was similar to that described above. The DAB without metal was used to produce a brown staining MSH product, which was present in the cytoplasm of the neurons. Brain sections were mounted on microscope glass slides, air-dried, dehydrated, cleared in xylene and cover slipped with mounting medium. Slides were observed under a Zeiss Axiophot microscope (Carl Zeiss, Thornwood, NY). Sections of the arcuate nucleus were viewed; images were taken by a digital camera using the computer software Spot Advance program (Diagnostic Instruments, Sterling Heights, MI). Stained neurons were counted using Image-Pro Plus software (version 4.1, Media Cybernetics, Silver Spring, MD). Sections from four enterostatin-treated and three saline vehicle rats were viewed and counts were performed on two representative sections from each animal. Counts from individual animals are the average count of two sections. Data are expressed as means ± standard error by each treatment group. The difference of treatment groups was compared using two tailed Student’s t-test.
Data Analysis
The food intake data are expressed as Means ± SEM. Statistical analysis was performed using a 2-way analysis of variance (ANOVA) with repeated measures and Neumann-Keuls tests were used for post hoc analyses between individual groups.
RESULTS
Effect of enterostatin in MC4R knock out mice
After an overnight fast, intracerebroventricular enterostatin inhibited the food intake of wild type, but not MC4R −/− mice, the effect lasting for the initial 2 hours after refeeding (Figure 1). The food intake of the knockout mice was however reduced compared to the wild type mice for the initial 4 hours but had recovered to control levels after 24 hours.
Figure 1.
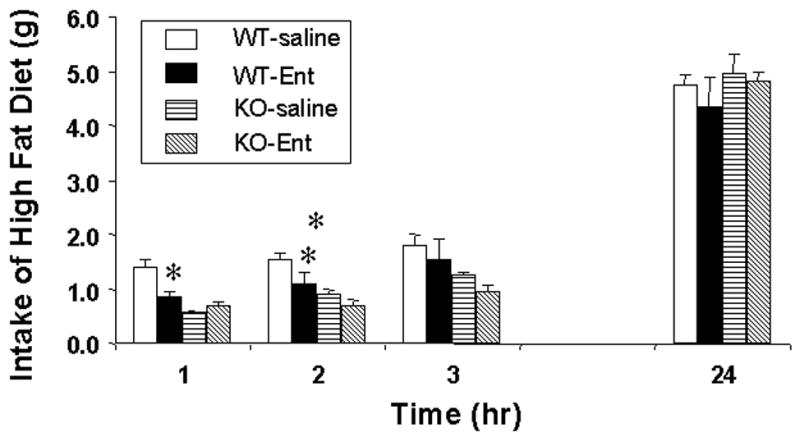
The effect of icv enterostatin (1nmole) on food intake in wild-type and MC4R −/− mice. Data represent Means ± SEM for 5–8 animals per group. * p<0.01 compared to saline group.
Effect of the MC3/MC4 Receptor antagonist SHU9119 on the enterostatin inhibition of food intake
Intracerebroventricular enterostatin (1nmole) reduced food intake by approximately 25% compared to control vehicle injected rats, the majority of this effect being observed in the first 30 minutes of feeding. While SHU9119, at the dose used (1nmole), had no effect alone on food intake, it blocked the inhibitory effect of enterostatin (Figure 2).
Figure 2.
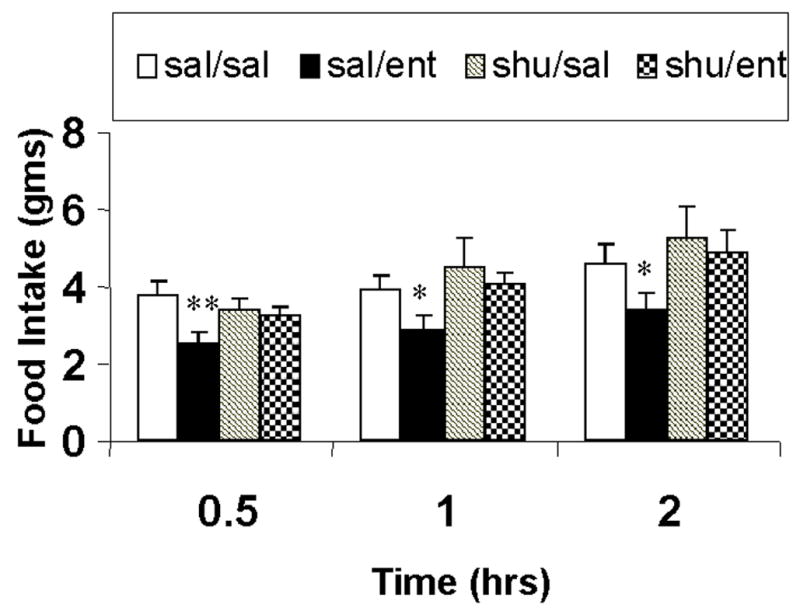
The effect of SHU9119 on the feeding response to enterostatin in rats adapted to high fat diets. Data represent Means ± SEM for 2 hour food intake for 5–8 animals per group. * p<0.01 compared to saline group.
Enterostatin activation of α-MSH neurons in the arcuate nucleus
We have previously shown that amygdala enterostatin activates neurons in the arcuate nucleus using c-fos immunohistochemistry (22). We now have used immunohistochemistry to identify the neurochemical phenotype of neurons in the arcuate that are activated when enterostatin is injected into the amygdala. The amygdala was chosen as the site of enterostatin injection since this is the most sensitive and responsive site for the enterostatin suppression of dietary fat intake (17). Enterostatin increased the number of fos expressing neurons in the arcuate nearly 3 fold. Relatively few arcuate α-MSH neurons (12.7%) showed any c-fos activity in the control saline-injected rats but over 68% of α-MSH neurons showed c-fos activation in the enterostatin-treated rats. Expressed in a different manner 82% of fos activated neurons were α-MSH neurons after enterostatin injections onto the amygdala compared to only 42% in the vehicle control animals (Figure 3 and Table 1).
Figure 3.
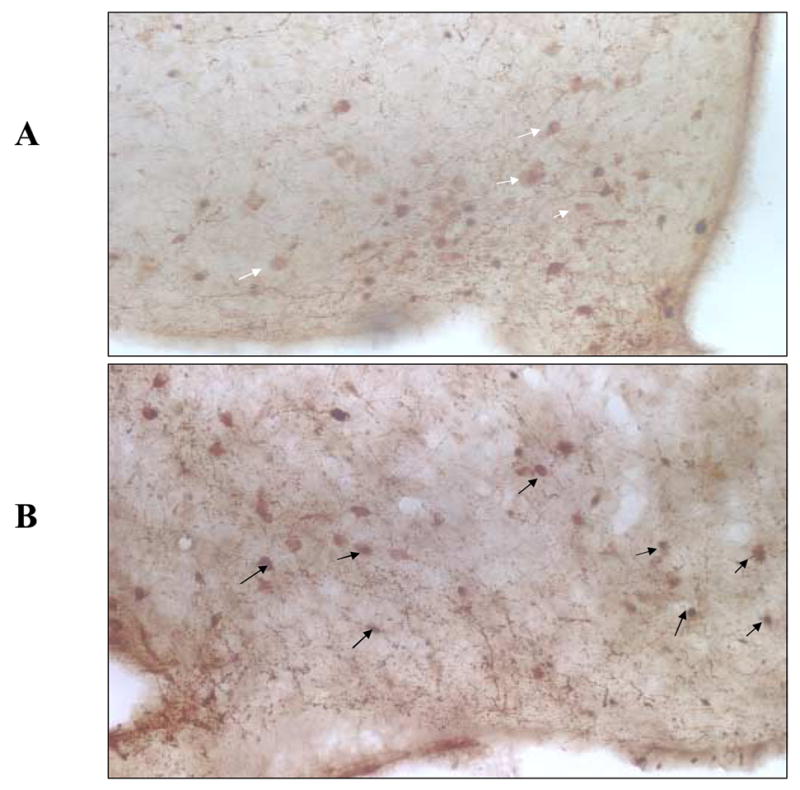
Colocalization of c-fos and αMSH immunoreactivity in the arcuate nucleus after enterostatin injections into the amygdala. A. After saline injection, white arrows sshowing α-MSH positive cells; B after enterostatin injection into the amygdala, black arrows showing fos nuclear staining in α-MSH positive cells.
Table 1.
c-Fos expression in αMSH neurons of the Arcuate nucleus in rats receiving enterostatin into the Amygdala.
| Treatment | c-fos neurons | αMSH neurons | c-fos/αMSH Neurons | Colocalization % fos/MSH | Colocalization % fos/MSH |
|---|---|---|---|---|---|
| Saline | 12 ± 4 | 36 ± 4 | 5 ± 2 | 12.7 ± 3.5 | 29.0 ± 4.2 |
| Enterostatin | 28 ± 4** | 33 ± 2 | 23 ± 4** | 68.8 ± 8.0** | 45.4 ± 1.1** |
Values represent Means ± SEM of identified neurons in the Arcuate nucleus for 4 rats in each group. Two represenative sections from each rat were counted.
p<0.01 compared to saline group.
Enterostatin effects on AgRP and POMC gene expression in vivo and in GT1-7 cells
Figure 4 shows the effect of intracerebroventricular enterostatin (1nmole) on the expression of AgRP and POMC in amygdala and hypothalamus. It is evident that one hour after enterostatin administration, the expression of AgRP had been greatly reduced in amygdala or hypothalamus. In contrast, POMC expression was increased in amygdala but unaltered in the hypothalamus (data not shown) with 1nmole enterostatin. Likewise, in GT1-7 murine hypothalamic neuronal cells in culture, one hour exposure to enterostatin significantly reduced the expression of AgRP in a dose responsive manner but POMC expression was increased only in the incubation with high dose (1uM) of enterostatin.
Figure 4.
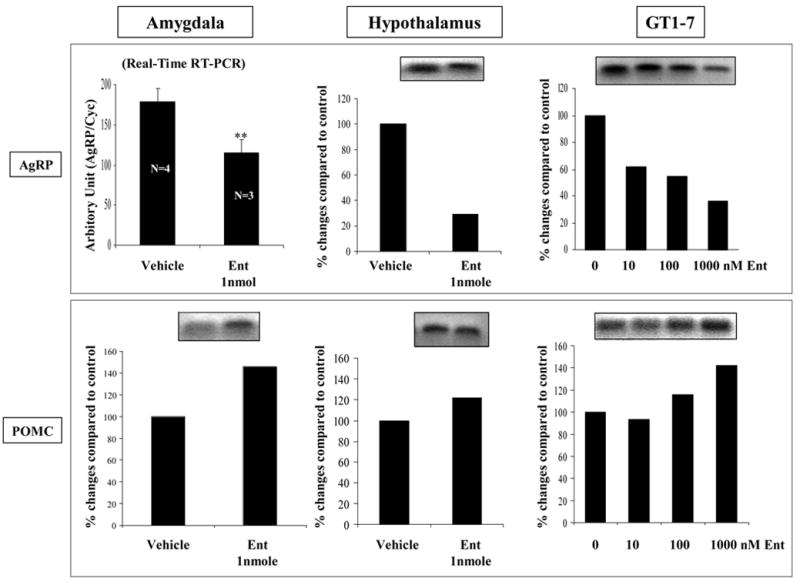
The effect of enterostatin on gene expression of AgRP and POMC in amygdala (1nmole enterostatin onto amygdala) and hypothalamus (1nmole enterostatin icv) of rats adapted to high fat diets and in GT1-7 cells in culture (10–1000nM enterostatin).
*p<0.01 compared to vehicle group.
DISCUSSION
Much is known about the biological effects of enterostatin. Its ability to inhibit the intake of dietary fat, to activate sympathetically mediated brown adipose tissue thermogenesis and inhibit insulin secretion has been described (5, 25, 28, 29). However, less is known about the neural network and the target effectors of these responses.
Enterostatin has preferential effects on the intake of dietary fat when rats are given a choice of diets or macronutrients (26) and its feeding response can also be observed in rats that are adapted to a high fat, but not a high carbohydrate, diet (15,19). MC4R activation has been shown to be essential for the melanocortin inhibition of dietary fat intake (33), whereas antagonism of the melanocortin receptor with either the natural antagonist AgRP or by ectopic expression of Agouti is associated with a selective increase in the intake of dietary fat in rodents given a choice of diets (6,11).
Neural mapping approaches have shown that enterostatin activates (fos induction) neurons in the amygdala, the arcuate nucleus and the paraventricular nucleus (19). Further, by use of retrograde tracing, the neurons activated in the arcuate nucleus by amygdala enterostatin were shown to have projections to the Paraventricular nucleus (22). In this manuscript we have shown that nearly 70% of arcuate α-MSH (POMC/CART) neurons were activated by enterostatin and that 82% of the neurons activated by enterostatin (fos positive neurons) were α-MSH (POMC/CART) neurons. This, together with the observations that MC4R receptor activity is needed for the response to enterostatin, suggests that enterostatin might enhance α-MSH release in the PVN (1). The AgRP/NPY neurons in the arcuate nucleus are known also to be important regulators of feeding through their axonal projections to the PVN and other brain regions (4). The inability to show an inhibitory feeding response to icv enterostatin in MC4R knockout mice, and the demonstration that SHU9119, an MC3/MC4 receptor antagonist, blocked the inhibitory effect of enterostatin on intake of a high fat diet, suggests that the enterostatin inhibition on intake of dietary fat is mediated, at least in part, through the MC4Rs. Suppression of AgRP signaling in response to enterostatin, shown here in vivo both in the hypothalamus and amygdala and in the GT1-7 hypothalamic neuronal cell line, would further enhance the activity of the MC4R pathway to suppress dietary fat intake. While it is not possible to show neurons inhibited by enterostatin using the fos technique, the evidence on inhibition of AgRP expression would be consistent with enterostatin inhibition of this cell type.
The studies in the MC4R knockout mice, however, are a little difficult to interpret since the food intake of the knockout mice was already suppressed compared to the wild type animals. The reason for this is unclear. In our hands, these mice overeat at the start of the dark cycle but appear to have an impaired response to an overnight fast. However, taken with the other data, the evidence suggests a role for AgRP and the MC4Receptor in the response to enterostatin. Whether this is the sole mechanism requires to be seen.
The enterostatin effect on AgRP gene expression was seen rapidly, within one hour, but it is unlikely to explain the immediate suppression of feeding when enterostatin is given either centrally or into the near celiac artery of an overnight fasted rat given access to food (5, 19, 20, 28). It is possible that the immediate response to enterostatin is modulated through its effects on serotonergic pathways. A serotonergic action to suppress dietary fat intake has been localized to the PVN (35,37). Enterostatin increases serotonin release (12,42) and the enterostatin inhibition of dietary fat intake is dependent upon 5HT1BR activity (43). The serotonergic inhibition of feeding appears to be upstream of the melanocortin receptors as the serotonergic inhibition of food intake is absent when MC3R and MC4R are genetically absent or pharmacologically blocked (7,45). Further, 5HT drugs activate the α-MSH (POMC/CART) neurons in the arcuate nucleus although this has been related more to 5HT2C activity (7,45) rather than 5HT1BR which appears to be important for the enterostatin responses (43). Serotonin also stimulates the release of α-MSH in superfused slices of rat hypothalamus (40). In contrast, 5HT did not influence AgRP release from perfused hypothalamic slices (14).
The anorectic effect of fenfluramine is absent in both 5HT1BR and 5HT2CR knock out mice (23,47) suggesting both receptor subtypes are important for the response to 5HT. Both 5HT1B (43) and MC4R activities are required for the feeding response to enterostatin. 5HT1BRs have been localized to the arcuate nucleus and paraventricular nucleus (24) and are thought to modulate the 5HT inhibitory influence on NPY secretion (3). Whether enterostatin inhibits the release of AgRP from neuronal terminals in addition to its genomic effects, is not known at this time. However, the coordinate activation of α-MSH neurons in the arcuate with the down regulation of AgRP gene expression suggests that activation of MC4Rs is an important component of the enterostatin inhibition of dietary fat intake. Indeed, MC4R activation has been shown to be essential for the melanocortin inhibition of dietary fat intake (33), an effect that is opposed by the natural antagonist AgRP (6). Whether all of the enterostatin effects on POMC neurons and AgRP expression are mediated through 5HT remains to be confirmed.
Enterostatin’s main site of action appears to be the central bed nucleus of the amygdala. Hence it is of interest that enterostatin downregulated AgRP expression in the amygdala as well as the hypothalamus. The role of AgRP in feeding behavior has been focused on the arcuate/paraventricular nucleus axis and its role in the amygdala is unknown. However, the amygdala is known from lesion (10 ) and other studies (8) to have an important role in food selection and food intake. Both opioids and galanin enhance feeding after injection onto the central bed nucleus of the amygdala (9,36 ). Additionally, we have recently shown that NPY injected onto the amygdala promotes carbohydrate and reduces fat intake without altering total caloric intake (32). It is possible that AgRP has an opposite effect to NPY on food selection in the amygdala.
Acknowledgments
This work was supported by funding from NIH: NIDDK 4527.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adan RA, Cone RD, Burbach JP, Gispen WH. Differential effects of melanocortin peptides on neural melanocortin receptors. Mol Pharmacol. 1994;46:1182–1190. [PubMed] [Google Scholar]
- 2.Dickson PR, Feifel D, Vaccarino FJ. Blockade of endogenous GRF at dark onset selectively suppresses protein intake. Peptides. 1995;16:7–9. doi: 10.1016/0196-9781(94)00153-w. [DOI] [PubMed] [Google Scholar]
- 3.Dryden S, Frankish HM, Wang Q, Williams G. increased feeding and neuropeptide Y (NPY) but not NPY mRNA levels in the hypothalamus of the rat following central administration of the serotonin synthesis inhibitor p-chlorophenyalanine. Brain Res. 1996;724:232–237. doi: 10.1016/0006-8993(96)00329-0. [DOI] [PubMed] [Google Scholar]
- 4.Elmquist JK, Maratos-Flier E, Saper CB, Flier JS. Unravelling the central nervous system pathways underlying responses to leptin. Nat Neurosci. 1998;1:445–450. doi: 10.1038/2164. [DOI] [PubMed] [Google Scholar]
- 5.Erlanson-Albertsson C, York DA. Enterostatin - A peptide regulating fat intake. Obesity Research. 1997;5:360–372. doi: 10.1002/j.1550-8528.1997.tb00565.x. [DOI] [PubMed] [Google Scholar]
- 6.Hagan MM, Rushing PA, Benoit SC, Woods SC, Seeley RJ. Opioid receptor involvement in the effect of AgRP- (83-132) on food intake and food selection. Am J Physiol. 2001;280:R814–21. doi: 10.1152/ajpregu.2001.280.3.R814. [DOI] [PubMed] [Google Scholar]
- 7.Heisler LK, Cowley MA, Kishi T, Tecott LH, Fan W, Low MJ, Smart JL, Rubinstein M, Tatro JB, Zigman JB, Cone RD, Elmquist JK. Central serotonin melanocortin pathways regulating energy homeostasis. Ann NY Acad Sci. 2003;994:169–174. doi: 10.1111/j.1749-6632.2003.tb03177.x. [DOI] [PubMed] [Google Scholar]
- 8.Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward related learning. Neurosci Biobehav Rev. 2004;27:765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 9.Kim E-M, Quinn JC, Levine AS, O’Hare E. A bi-directional mu;-opioid connection between the nucleus of the accumbens shell the central nucleus of the amygdala in the rat. Brain Res. 2004;1029:135–139. doi: 10.1016/j.brainres.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 10.King BM, Rossiter KN, Stines SG, Zaharan GM, Cook JT, Humphries MD, York DA. Amygdaloid-lesion hyperphagia: impaired response to caloric challenges and altered macronutrient selection. Am J Physiol. 1998;275:R485–R493. doi: 10.1152/ajpregu.1998.275.2.R485. [DOI] [PubMed] [Google Scholar]
- 11.Koegler F, Schaffhauser AO, Mynatt RL, York DA, Bray GA. Macronutrient diet intake of the lethal yellow agouti (Ay/a) mouse. Physiol & Behav. 1999;67:809–812. doi: 10.1016/s0031-9384(99)00104-3. [DOI] [PubMed] [Google Scholar]
- 12.Koizumi M, Kimura S. Enterostatin increases extracellular serotonin and dopamine in the lateral hypothalamic area in rats measured by microdialysis. Neurosci Letts. 2002;320:96–98. doi: 10.1016/s0304-3940(01)02580-0. [DOI] [PubMed] [Google Scholar]
- 13.Leibowitz SF. Hypothalamic paraventricular nucleus: Interaction between _2-noradrenergic system and circulating hormones and nutrients in relation to energy balance. Neurosci Biobehav Rev. 1998;12:101–109. doi: 10.1016/s0149-7634(88)80002-2. [DOI] [PubMed] [Google Scholar]
- 14.Li JY, Finniss S, Yang YK, Zeng Q, Qu SY, Barsh B, Dickinson C, Gantz I. Agouti-related protein-like immunoreactivity: Characterization of release from hypothalamic tissue presence in serum. Endocrinology. 2000;141:1942–1950. doi: 10.1210/endo.141.6.7462. [DOI] [PubMed] [Google Scholar]
- 15.Lin L, S Okada, York DA, Bray GA. Structural requirements for the biological activity of enterostatin. Peptides. 1994;15:849–854. doi: 10.1016/0196-9781(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 16.Lin L, Chen J, York DA. Chronic icv enterostatin preferentially reduced fat intake and lowered body weight. Peptides. 1997;18:657–661. doi: 10.1016/s0196-9781(97)00128-9. [DOI] [PubMed] [Google Scholar]
- 17.Lin L, York DA. Enterostatin actions in the amygdala and PVN to suppress feeding in the rat. Peptides. 1997;18:1341–1347. doi: 10.1016/s0196-9781(97)00191-5. [DOI] [PubMed] [Google Scholar]
- 18.Lin L, Umahara M, York DA, Bray GA. β-casomorphins stimulate and enterostatin inhibits the intake of dietary fat in rats. Peptides. 1998;19:325–331. doi: 10.1016/s0196-9781(97)00307-0. [DOI] [PubMed] [Google Scholar]
- 19.Lin L, York DA. Chronic ingestion of dietary fat is a prerequisite for inhibition of feeding by enterostatin. Am J Physiol. 1998;275:R619–R623. doi: 10.1152/ajpregu.1998.275.2.R619. [DOI] [PubMed] [Google Scholar]
- 20.Lin L, Bray G, York DA. Enterostatin suppresses food intake in rats after near celiac and intracarotid arterial injection. Am J Physiol. 2000;278:R1346–R1351. doi: 10.1152/ajpregu.2000.278.5.R1346. [DOI] [PubMed] [Google Scholar]
- 21.Lin L, Thomas SR, Kilroy G, Schwartz GJ, York DA. The enterostatin inhibition of dietary fat intake is dependent upon CCKA receptors. Am J Physiology. 2003;285:R321–328. doi: 10.1152/ajpregu.00147.2003. [DOI] [PubMed] [Google Scholar]
- 22.Lin L, York DA. Amygdala enterostatin induces c-fos expression in regions of hypothalamus that innervate the PVN. Brain Res. 2004;1020:147–153. doi: 10.1016/j.brainres.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 23.Lucas JL, Yamamoto A, Scearce-Levie K, Saudou F, Hen R. Absence of fenfluramine-induced anorexia reduced c-fos induction in the hypothalamus central amygdaloid complex of serotonin 1B receptor knock-out mice. J Neurosci. 1998;18:5537–5544. doi: 10.1523/JNEUROSCI.18-14-05537.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Makarenko IG, Meguid MM, Ugrumov MV. Distribution of serotonin 5-hydroxytryptamine 1B (5HT1B) receptors in the normal rat hypothalamus. Neurosci letts. 2002;328:155–159. doi: 10.1016/s0304-3940(02)00345-2. [DOI] [PubMed] [Google Scholar]
- 25.Nagase H, Nakajimia S, Sekihara H, York DA, Bray GA. Regulation of feeding behavior, gastric emptying, and sympathetic nerve activity to interscapular brown adipose tissue by galanin and enterostatin: the involvement of vagal-central nervous system interactions. J Gastroenterol. 2002;14(Suppl):118–27. doi: 10.1007/BF03326430. [DOI] [PubMed] [Google Scholar]
- 26.Okada S, York DA, Bray GA, Erlanson-Albertsson C. Enterostatin, (VAL-PRO-ASP-PRO-ARG), the activation peptide of procolipase selectively reduces fat intake. Physiol & Behav. 1991;49:1185–1189. doi: 10.1016/0031-9384(91)90349-s. [DOI] [PubMed] [Google Scholar]
- 27.Okada S, York DA, Bray GA. Procolipase mRNA: Tissue localization and effects of diet and adrenalectomy. Biochem J. 1993;292:787–789. doi: 10.1042/bj2920787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ookuma K, Barton C, York DA, Bray GA. Effect of enterostatin and Kappa-opioids on macronutrient selection and consumption. Peptides. 1997;18:785–791. doi: 10.1016/s0196-9781(97)00029-6. [DOI] [PubMed] [Google Scholar]
- 29.Ookuma M, York DA. Inhibition of insulin release by enterostatin. Int J Obesity. 1998;22:800–805. doi: 10.1038/sj.ijo.0800663. [DOI] [PubMed] [Google Scholar]
- 30.Park M, Lin L, Thomas S, Braymer HD, Smith PM, Harrison DH, York DA. The F(1)-ATPase beta-subunit is the putative enterostatin receptor. Peptides. 2005;25:2127–33. doi: 10.1016/j.peptides.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 31.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 2. Academic Press; Sydney: 1982. [DOI] [PubMed] [Google Scholar]
- 32.Primeaux SD, Bray GA, York DA. Neuropeptide Y administration into the amygdala alters high fat food intake. Peptides. 2006;27:1644–1651. doi: 10.1016/j.peptides.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 33.Samama P, Rumennik L, Grippo JF. The melanocortin receptor MCR4 controls fat consumption. Peptides. 2003;113:85–88. doi: 10.1016/s0167-0115(02)00299-9. [DOI] [PubMed] [Google Scholar]
- 34.Smith BK, Berthoud H, York DA, Bray GA. Differential effects of baseline macronutrient selection after galanin, NPY, and an overnight fast. Peptides. 1997;18:207–211. doi: 10.1016/s0196-9781(96)00318-x. [DOI] [PubMed] [Google Scholar]
- 35.Smith BK, York DA, Bray GA. Chronic d-fenfluramine treatment reduces fat intake independent of macronutrient preference. Pharmacology Biochem & Behavior. 1998;60:105–114. doi: 10.1016/s0091-3057(97)00549-2. [DOI] [PubMed] [Google Scholar]
- 36.Smith BK, York DA, Bray GA. Effects of dietary preference and galanin administration in the paraventricular and amygdaloid nucleus on diet self selection. Brain Res Bull. 1996;39:149–154. doi: 10.1016/0361-9230(95)02086-1. [DOI] [PubMed] [Google Scholar]
- 37.Smith BK, York DA, Bray GA. Activation of hypothalamic serotonin receptors reduced intake of dietary fat and protein but not carbohydrate. Am J Physiol. 1999:R802–R811. doi: 10.1152/ajpregu.1999.277.3.R802. [DOI] [PubMed] [Google Scholar]
- 38.Sorhede M, Mulder H, Mei J, Sundler F, Erlanson-Albertsson C. Procolipase is produced in the rat stomach--a novel source of enterostatin. Biochim Biophys Acta. 1996;1301:207–12. doi: 10.1016/0005-2760(96)00034-3. [DOI] [PubMed] [Google Scholar]
- 39.Stanley BG, Daniel DR, Chin AS, Leibowitz SF. Paraventricular nucleus injection of peptide YY and neuropeptide Y preferentially enhance carbohydrate ingestion. Peptides. 1985;6:1205–11. doi: 10.1016/0196-9781(85)90452-8. [DOI] [PubMed] [Google Scholar]
- 40.Tiligada E, Wilson IF. Regulation of alpha-melanocyte stimulating hormone release from superfused slices of rat hypothalamus by serotonin and interaction of serotonin and dopaminergic system inhibiting peptide release. Brain Res. 1989;503:225–228. doi: 10.1016/0006-8993(89)91668-5. [DOI] [PubMed] [Google Scholar]
- 41.Vickers SP, Clifton PG, Dourish CT, Tecott LH. Reduced satiating effect of d-fenfluramine in serotonin 5-HT(2C) receptor mutant mice. Psychopharmacology (Berl) 1999;143:309–314. doi: 10.1007/s002130050952. [DOI] [PubMed] [Google Scholar]
- 42.York DA, Waggoner J, Bray GA. Brain amine responses to peripheral enterostatin. Int J Obesity. 1994;18 (Suppl 2):102. [Google Scholar]
- 43.York DA, Lin L. 5HT1B receptors modulate the feeding inhibitory effects of enterostatin. Brain Res. 2005;1062:26–31. doi: 10.1016/j.brainres.2005.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.York DA, Lin L, Thomas SR, Braymer HD, Park M. Procolipase gene expression in the rat brain: Source of endogenous enterostatin production in the brain. Brain Res. 2006;1087:52, 59. doi: 10.1016/j.brainres.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 45.Zhou L, Williams T, Lachey JL, Kishi T, Cowley MA, Heisler LK. Serotonergic pathways converge upon central melanocortin systems to regulate energy balance. Peptides. 2005;26:1728–1732. doi: 10.1016/j.peptides.2004.12.028. [DOI] [PubMed] [Google Scholar]


