Abstract
Purpose
To evaluate the impact of dry eye syndrome (DES) on vision-associated quality of life.
Design
Cross-sectional study.
Methods
We identified 450 participants in the Women’s Health Study (WHS) and 240 participants in the Physicians’ Health Study (PHS) and sent a supplementary questionnaire asking how much their everyday activities were limited by symptoms of dry eye and to what degree problems with their eyes limited them in reading, driving, working at the computer, their professional activity, and watching TV. By design, one-third of study subjects had clinically diagnosed DES or severe symptoms and two-thirds did not. We used logistic regression to examine relationships of DES with reported problems with everyday activities in each cohort and pooled estimates using meta-analysis methods.
Results
Of the participants invited, 85% completed the supplementary questionnaire, including 135 WHS and 55 PHS participants with DES, and 250 WHS and 149 PHS participants without DES. Controlling for age, diabetes, hypertension and other factors, those with DES were more likely to report problems with reading (OR=3.64, 95% CI 2.45–5.40, P< 0.0001); carrying out professional work (OR=3.49, 95% CI 1.72–7.09, P= 0.001); using a computer (OR=3.37, 95% CI 2.11–5.38, P< 0.0001); TV watching (OR=2.84, 95% CI 1.05–7.74, P=0.04); driving during the day (OR=2.80, 95% CI 1.58–4.96, P< 0.0001); and driving at night (OR=2.20, 95% CI 1.48–3.28, P<0.0001).
Conclusions
DES is associated with a measurable adverse impact on several common and important tasks of daily living, further implicating this condition as an important public health problem deserving increased attention and resources.
Introduction
Dry eye syndrome (DES) is recognized as a growing public health problem and one of the most frequent reasons for seeking eye care.1–5 The estimated prevalence of DES for people ≥50 years in the US is 7.8% or 3.2 million among women and 4.7% or 1.6 million among men.3 Inclusion of less severe DES cases would add substantially to these overall numbers.4, 6 DES is characterized by a deficiency in the quantity and/or quality of tears, an unstable tear film, ocular surface damage, and bothersome symptoms such as ocular irritation, dryness, fatigue, and fluctuating visual disturbances.
Although some risk factors have been identified, the etiology of DES is still largely unknown.2, 7–9 It is likely that the ever increasing demands of modern living that require prolonged visual tasking may play a role in its development,10, 11 as well as in its consequences for vision and vision-related quality of life.12 As this condition is more frequent in older age3, 13, 14 projections of greater life expectancy in developed countries will predictably result in an even larger burden of DES on society through direct costs for care and treatment and indirect costs associated with decreased visual function15 and quality of life.16
The importance of DES is usually clear to an individual patient and to his/her eye doctor, but there are few published data to describe the impact of DES on quality of life.17, 18 We therefore measured the impact of DES on visual function/quality of life by assessing its effect on several common activities of daily living such as reading, driving, computer work, performing professional work and watching television
Methods
Study population
The Women’s Health Study (WHS) was a randomized, double-masked, placebo-controlled trial among 39,876 US female health professionals (actively working or retired) aged between 39 and 90 years at baseline to assess the benefits and risks of low-dose aspirin and vitamin E in the primary prevention of cardiovascular disease and cancer among healthy women.19, 20 Information on general characteristics of the study population was ascertained on annual questionnaires as has been previously reported.21 The Physicians’ Health Study (PHS) was a randomized, double-masked, placebo-controlled trial to test the effect of low-dose aspirin and beta-carotene in the primary prevention of cardiovascular disease and cancer among 22,071 healthy US male physicians, aged between 40 to 84 at baseline, in 1982. Annual questionnaires were administered during the active treatment phase of the trial,22, 23 as well as during continued observational follow-up of the PHS cohort. Informed Consent was obtained from all study subjects, the study and data accumulation were carried out with approval from the Institutional Review Board of Brigham and Women's Hospital, and the study is in accord with HIPAA regulations.
Assessment of DES
We assessed participants’ history of DES beginning in 1997 using three questions: 1) “How often are your eyes dry (not wet enough)?” and 2) “How often are your eyes irritated?” with possible answers of constantly, often, sometimes, or never; and 3) “Have you ever been diagnosed, by a clinician, as having dry eye syndrome?” and if so requesting the date of this diagnosis. For the present study, we considered participants to have DES if they reported a clinical diagnosis of DES and/or severe symptoms, defined as a response of constantly or often to both the dryness and irritation questions.2, 3 At the time of assessment of DES, WHS participants were aged 49 and older, while PHS participants were 55 years or older.
Selection of subgroup for the present study
For these analyses we selected subgroups of 450 WHS and 240 PHS participants based on their answers to the three DES-related questions such that one-third of them satisfied our definition of DES having reported clinically diagnosed DES and/or both dryness and irritation either constantly or often. The other two-thirds of selected participants who did not meet those criteria were frequency matched on age to the DES cases.
Assessment of vision-related quality of life
We mailed a supplementary DES questionnaire, consisting of eleven questions, to the selected subgroups of WHS and PHS participants. We asked several questions regarding the impact of DES on vision-related quality of life. We included a global question: “How much do dry eye symptoms limit your everyday activities?” and asked participants to grade the impact on a scale of 0 to 10. We also asked the question: “Have problems with your eyes limited you in performing any of the following activities during the last week?” and listed activities including reading, driving during the day, driving at night, computer use, watching TV, and performing professional work.” Possible answers ranged from all of the time, most of the time, half of the time, some of the time, none of the time, or not applicable.
Statistical analysis
We initially examined the frequency distribution of participants’ answers to the questions related to vision-related quality of life, and used the Wilcoxon Rank Sum test to check for an association of each activity score with DES. Individuals who chose a response of not applicable were excluded from analysis of that particular activity. In further analyses, we dichotomized responses to the presence versus absence of any degree of problems with each activity, and used logistic regression models to estimate the odds ratios (OR) and 95% confidence intervals (CI) for the relationship of each activity of daily living with DES while controlling for potential confounding variables. Because the two cohorts are independent, and available data differs in the two cohorts, we fit separate models for women and men. Among women, we controlled for age, educational level, household income, use of hormone replacement therapy, a history of hypertension, diabetes, and connective tissue disease, and for whether or not the participant had an eye exam in previous two years. Among men, we controlled for age, a history of hypertension, diabetes, and benign prostate hypertrophy. Information on history of eye examinations, income levels, and connective tissue diseases were not available in men, who were all physicians and therefore of comparable educational level. We also investigated whether the impact of dry eye on vision-related quality of life was modified by the use of artificial tears by including an interaction term in the models. Finally, we tested for heterogeneity across cohorts and used a random effects method weighted by the sample size for each cohort to obtain pooled OR and 95% CI for the association of DES with each activity, and overall.24
Results
Study Subjects Characteristics
Of the 450 WHS and 240 PHS participants selected, 85% (385 from WHS and 204 from PHS) completed the supplementary DES questionnaire and were included in this analysis. Dry eye was present in 135 women (41 who reported severe symptoms, 62 who had been diagnosed with dry eye, and 31 who had both severe symptoms and a prior diagnosis of dry eye) and 55 men (17 who reported severe symptoms, 25 with diagnosed dry eye, and 13 with both severe symptoms and a prior diagnosis of dry eye). Baseline characteristics of study participants are presented in Table 1. The mean age for both the DES and the control groups was 57 years among women, and 71 years among men. There was no statistically significant difference between participants with DES and controls in regard to the presence of comorbid states such as hypertension, diabetes and, among men, benign prostatic hypertrophy. However, as would be expected, the presence of Sjogren’s syndrome among female participants with DES was significantly greater than that among female controls (p=0.04), but there was not a significant difference between female cases and controls in regard to the presence of any connective tissue disease (p= 0.17).
Table.
Baseline characteristics of study participants, according to presence of severe symptoms or a clinical diagnosis of dry eye syndrome (DES)
| Females* (n=385) | Males† (n=204) | |||
|---|---|---|---|---|
| Baseline Characteristics | DES (n=135) | No DES (n=250) | DES (n=55) | No DES (n=149) |
| Age in years, mean (SD) | 57.4 (6.9) | 57.8 (7.2) | 71.1 (8.1) | 70.7 (8.4) |
| Race, % | ||||
| White | 97.7 | 97.2 | 96.4 | 99.3 |
| Other | 2.3 | 2.8 | 3.6 | 0.7 |
| Highest Educational Level, % | ||||
| LPN/LVN‡ | 11.9 | 11.4 | ||
| 2 year associate RN§ | 10.4 | 9.8 | ||
| 3 year RN degree | 26.9 | 30.6 | ||
| Bachelors degree | 20.9 | 24.9 | ||
| Masters degree | 24.6 | 17.1 | ||
| Doctoral degree | 5.2 | 6.1 | 100. | 100. |
| Household Income, %¶ | ||||
| <$10,000 | 0.8 | 0.9 | ||
| $10,000–19,999 | 3.1 | 2.2 | ||
| $20,000–29,999 | 10.9 | 7.0 | ||
| $30,000–39,999 | 12.5 | 12.2 | ||
| $40,000–49,999 | 11.7 | 17.0 | ||
| $50,000–99,999 | 47.7 | 46.7 | ||
| ≥$100,000 | 13.3 | 14.0 | ||
| Eye exam in the past 2 years, %¶ | 88.0 | 84.8 | ||
| Postmenopausal Hormone Therapy, %# | ||||
| Current | 45.9 | 48.0 | ||
| Past | 9.6 | 4.0 | ||
| Never | 44.4 | 48.0 | ||
| Hypertension, % | 28.1 | 30.0 | 64.1 | 52.8 |
| Diabetes mellitus, % | 2.2 | 3.6 | 5.4 | 9.4 |
| Benign prostate hypertrophy, %# | 40.0 | 30.9 | ||
| Sjogren’s syndrome, % | 4.4 | 1.2 | 0.5 | 0.0 |
| Other connective tissue disease, %¶ | 4.4 | 2.0 | ||
| Use of artificial tears, % | ||||
| Once per day | 16.3 | 4.0 | 5.5 | 2.0 |
| Twice per day | 15.6 | 4.0 | 12.7 | 0.7 |
| ≥Three times per day | 11.1 | 1.2 | 20.0 | 1.3 |
Female participants are a selected subgroup of the Women’s Health Study
Male participants are a selected subgroup of the Physicians’ Health Study
LPN=Licensed practical nurse; LVN=Licensed visiting nurse
RN=Registered nurse
Data on income, having an eye exam in the past 2 years, and history of connective tissue disease were not available for the male participants.
Applies to only one sex
In our initial unadjusted analyses, we observed significant differences between participants with DES and controls in both cohorts in regard to problems with reading, professional work, and computer use (each p<0.001 for both women and men). However, for the activity of driving, differences between subjects with and without DES were significantly different only among women (daytime driving; p<0.001 for women and p=0.07 for men) (driving at night; p<0.001 for women and p=0.06 for men).
Using logistic regression models to control for potential confounding factors, we estimated the impact of DES on the risk of having problems with each activity (Figure 1). The test for heterogeneity across cohorts was not significant for most of the activities (reading, p=0.93; professional work, p=0.21; computer use, p=0.56; driving during the day, p=0.39; driving at night, p=0.70), with the exception of TV watching for which it was of borderline significance (p=0.04). Pooled multivariate logistic regression estimates demonstrated significant associations between the presence of DES and having problems with reading (OR=3.64, 95% CI 2.45–5.40, p< 0.001); carrying out professional work (OR=3.49, 95% CI 1.72–7.09, p= 0.001); using computer (OR=3.37, 95% CI 2.11–5.38, p< 0.001); TV watching (OR=2.84, 95% CI 1.05–7.74, p=0.04); driving during the day (OR=2.80, 95% CI 1.58–4.96, p< 0.001); and driving at night (OR=2.20, 95% CI 1.48–3.28, p<0.001).
Figure 1.
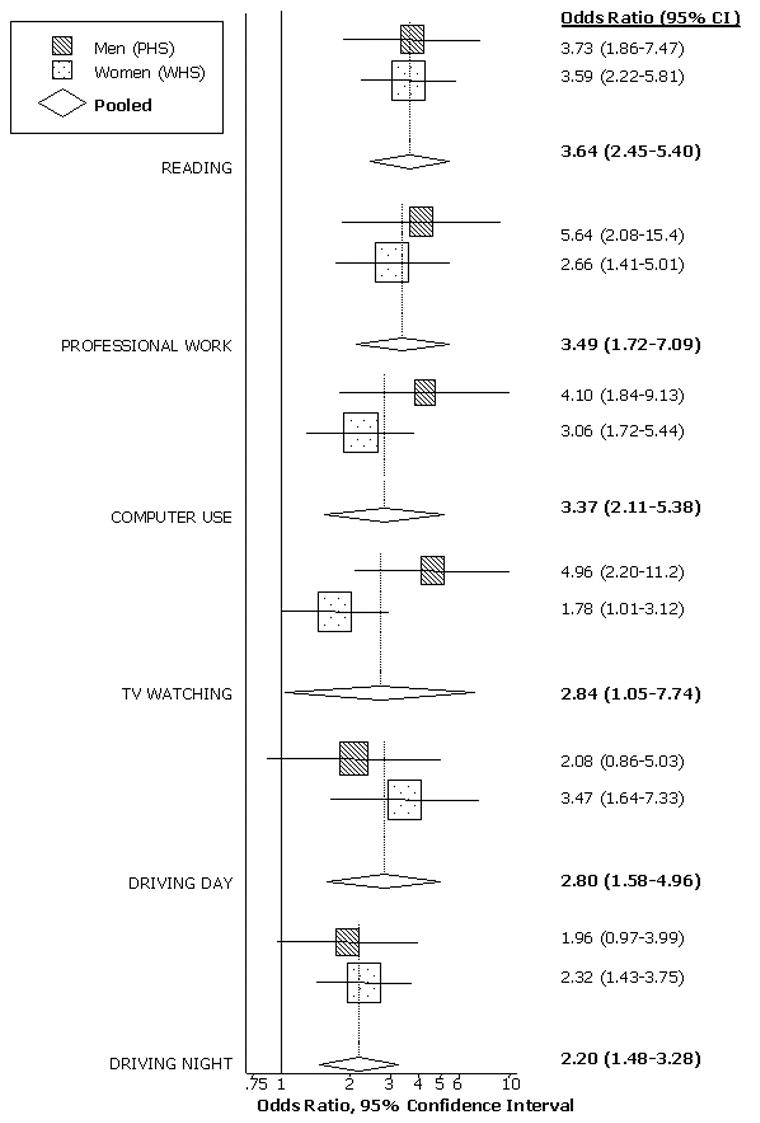
Presented are the separate and pooled results for the association between DES and reported problems with selected activities of daily living, adjusted for potential confounding factors. The center of each square indicates the odds ratio (OR), and the size of the square is proportional to the percent weight each study contributed to the pooled estimate. The horizontal line bisecting each square represents the 95% confidence interval for the OR. The random-effects pooled OR and 95% CI are indicated by the unshaded diamonds. Estimates greater than one signify that subjects with DES were more likely to report having problems with that particular activity.
In addition, in both cohorts, participants with DES reported a significantly greater overall impact of dry eye on problems with everyday activities (pooled OR=3.16, 95% CI 2.14–4.68, p<0.001). Further analyses suggested that the impact of dry eye on everyday activities differed depending on use of artificial tears (P for interaction<0.001). Whereas there was a 2.64-fold increase in problems with everyday activities among those with dry eye who used artificial tears, the increase was 4.80-fold among dry eye subjects who did not use artificial tears, as presented in Figure 2.
Figure 2.
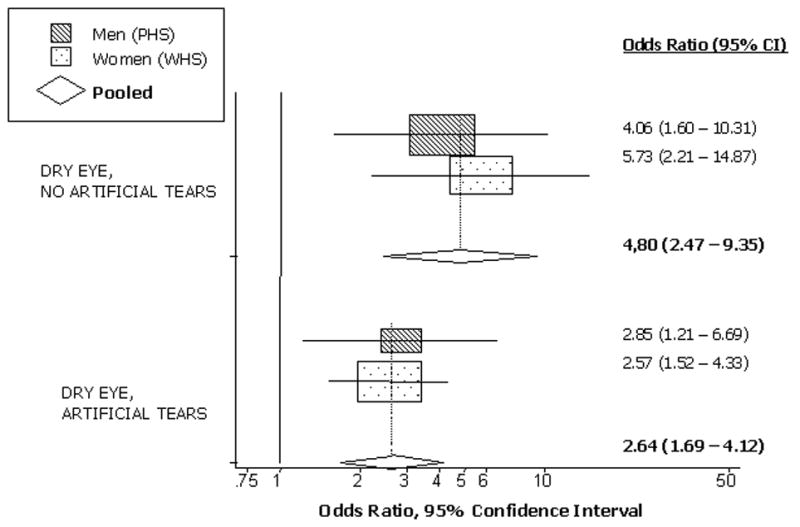
Presented are the separate and pooled findings for the association between DES and overall reported problems with activities of daily living, separately among those who did versus did not report any artificial tear use. The center of each square indicates the odds ratio (OR), and the size of the square is proportional to the percent weight each study contributed to the pooled estimate. The horizontal line bisecting each square represents the 95% confidence interval for the OR. The random-effects pooled OR and 95% CI is indicated by the unshaded diamond. Estimates greater than one signify that subjects with DES were more likely to report problems with vision-related activities of daily living.
Discussion
DES is a common problem that may often be overlooked clinically as it tends not to be a common cause of permanent visual morbidity as traditionally measured.3 However, the present study suggests that DES can have a significant impact on visual function that can diminish a person’s quality of everyday living. More specifically, the present study shows that crucial daily activities of modern living such as reading, computer use, professional work, driving, and TV watching are all negatively impacted by DES.
In general, visual function and quality of life are important outcomes in the evaluation of therapeutic decisions as well as in the assessment of the economic and public health impact of any ocular condition. In addition to our report, there is accumulating evidence of an impact of DES on quality of life measures.17, 25, 26 One such study utilized utility assessment, which yields a single measure of impact of DES. Utility scores that are anchored at perfect health (utility=1) and death (utility=0) can be compared across various health outcomes. In this way, the impact of different health states on patient’s perspective of their quality of life as well as quality-adjusted life years can be compared. Schiffman et al. showed that DES reduced quality of life with mean comorbidity-adjusted utility scores ranging from 0.62 for severe DES to 0.78 for mild DES; which compared to utility scores of 0.75 and 0.72 for moderate and moderate to severe angina pectoris (class III/IV).17
Nichols et al. used the National Eye Institute Visual Function Questionnaire 25 (NEI VFQ-25), a generic vision-related quality of life tool designed to measure the impact of ocular disorders such as glaucoma, cataract and age-related macular degeneration to study vision-related quality of life among predominantly mild to moderate DES patients, particularly in relation to reported ocular pain.26 In these patients, the pain and discomfort subscale of this instrument had the lowest score of all the subscales (83.8%). Others have observed statistically significant differences in both the generic SF-36 quality of life scale as well as a DES-specific quality of life instrument, the Impact of Dry Eye on Everyday Life (IDEEL) questionnaire, across varying levels of DES severity.18
Others have devised measures of functional visual acuity (FVA) that may better simulate vision requirements for daily acts of gazing with unconscious blink suppression, such as that which occurs during activities of modern living including computer use, reading, and driving.12, 27, 28 People with DES blink twice as often as normal controls under relaxed conditions10 and this compensatory action probably stimulates tear secretion and spreading on the ocular surface and might result in a normal VA using standard testing. However, DES is associated with an unstable tear film as compared to the normal state29 and consequently a measure such as FVA might still be reduced.12, 27, 28 In one such study, the reported average FVA of DES patients was 20/71,28 which is just less than the acuity generally required for licensure for nighttime driving in most US states.30 There is currently little if any information available on the potential impact of conditions such as DES which may not affect VA as measured in the clinic, but which may nevertheless impact vision while driving.12, 27, 28, 30–32 It is estimated that the proportion of the population >65 years of age will double from its current levels to 20% of the total population (or 71.5 million people) by the year 2030.33 Studies have demonstrated a reduction in the rate of blinking with increased driving speed, which can result in further tear film instability in subjects with DES.31, 34 Our data in conjunction with those from studies of FVA12, 27, 28 and tear instability in subjects with DES29 suggest that consideration may need to be given to the impact of conditions like DES that may result in fluctuating vision, reduced contrast sensitivity, or increased glare disability.32, 35, 36
The present study was limited by our classification of DES based on participant responses to our short dry eye questionnaire. However, our prior work on validation of this questionnaire-based assessment of DES showed a good balance of sensitivity and specificity versus commonly used clinical diagnostic tests for DES.37 Moreover, misclassification of DES status would tend to bias results toward the null and therefore is unlikely to explain the positive findings of the present study. Nonetheless, we do not know the severity of DES in our population and could not differentiate possible differences in visual function between mild and more severe cases. Additionally, our subjects are more educated and somewhat healthier than the general population and therefore results should be generalized only cautiously. However, by studying this group of health care professionals, we achieved a high participation rate and the higher level of health-related education and awareness of our study population may have aided participants’ ability to respond accurately to these questions. The questionnaire-based assessment of vision-related quality of life is also subject to possible bias or confounding if patients with versus without dry eye would, for some reason other than dry eye, tend to answer these questions differently. In this regard, however, our finding of a significant impact on activities for which sustained visual attention is important is consistent with those of other groups who have used alternative methodologies such as functional visual acuity measurement.
The interface between the tear film and the surrounding air represents the largest refractive index differential in the human optical system and is consequently of critical importance for clear vision. DES patients with an unstable tear film can usually clear a blurred image temporarily by blinking frequently to redistribute the tear film over the ocular surface. However, this may not be sustainable during activities requiring prolonged gazing, and those with more severe symptoms may experience difficulty keeping their eyes open. Our findings of nearly 3 and 5-fold increased risks of having problems with activities such as reading, computer use and professional work among both women and men with DES who did and did not use artificial tears, respectively, support and extend those of prior studies by pointing to specific areas of functioning that are problematic among people with DES. In future studies, it would be interesting to examine whether environmental conditions such as a patients proximity to air conditioning or heating vents, ambient humidity, and similar factors might modify the association between dry eye and visual functioning.
In summary, findings of the present study indicate that DES is associated with an adverse impact on vision-related quality of life. Several common tasks of daily living such as reading and driving are adversely affected by this condition. These data add further weight to the consideration of DES as a significant public health problem that deserves further study.
Acknowledgments
A. Funding/Support. Supported primarily by an unrestricted grant from Pfizer Consumer Healthcare Inc., Morris Plains, NJ, to Dr. Schaumberg. Follow-up of the Physicians’ Health Study cohort is supported by NIH grant CA40360, and the Women’s Health Study by NIH grants CA47988 and HL43851. The study also received funding from the Joint Clinical Research Center, Massachusetts Eye & Ear Infirmary, Schepens Eye Research Institute, Department of Ophthalmology, Boston MA. None of the funding organizations participated in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
B. Financial Disclosures now or in the previous two years that might relate to this manuscript (including none); Dr Schaumberg reports that she currently or in the past two years has received research funding support from not-for-profit entities including the National Eye Institute, the National Institute for Diabetes, and Digestive, and Kidney Diseases, and the Juvenile Diabetes Foundation. She has also received investigator-initiated research support from for-profit entities including Pfizer Consumer Healthcare and DSM Pharmaceuticals. Dr Schaumberg has also served as a consultant to Novartis and Inspire Pharmaceuticals, and on the Scientific Advisory Board of OcuSense.
Dr. Sullivan reports that he currently and in the past two years has received research funding from the National Eye Institute and Allergan. Dr. Sullivan has also acted as a consultant to Allergan, Novartis, Alcon and SIFI, and served on the Scientific Advisory Board of OcuSense. Dr. Dana and Dr. Miljanovic have no financial disclosures that are related to this manuscript.
C. Contributions of Authors: Design of the study (RD, DASullivan, DASchaumberg); Conduct of the study (BM, RD, DASchaumberg); Collection and management of the data (DASchaumberg), Analysis and interpretation of the data (BM, RD, DASullivan, DASchaumberg); Preparation of the manuscript (BM, DASchaumberg), Review and approval of the manuscript (BM, RD, DASullivan, DASchaumberg).
Biographies
 Biljana Miljanović, MD, MPH, MSc is a Division of Aging, Brigham and Women's Hospital in Boston. She is intrigued by questions of the consequences of modern living, e.g. with industrialized food production and dietary changes, manmade environments, medication use, and how these impact chronic diseases of aging and their consequences.
Biljana Miljanović, MD, MPH, MSc is a Division of Aging, Brigham and Women's Hospital in Boston. She is intrigued by questions of the consequences of modern living, e.g. with industrialized food production and dietary changes, manmade environments, medication use, and how these impact chronic diseases of aging and their consequences.
 Debra A. Schaumberg, ScD, OD, MPH is Associate Professor of Medicine at Harvard Medical School, Clinical Associate Scientist at the Schepens Eye Research Institute, and the Director of Ophthalmic Epidemiology, Division of Preventive Medicine, Brigham and Women's Hospital, Boston. Her principal research interests regard the roles of both environmental and genetic risk factors, and their interactions, in common eye diseases including dry eye syndrome, cataract, macular degeneration, and diabetic retinopathy.
Debra A. Schaumberg, ScD, OD, MPH is Associate Professor of Medicine at Harvard Medical School, Clinical Associate Scientist at the Schepens Eye Research Institute, and the Director of Ophthalmic Epidemiology, Division of Preventive Medicine, Brigham and Women's Hospital, Boston. Her principal research interests regard the roles of both environmental and genetic risk factors, and their interactions, in common eye diseases including dry eye syndrome, cataract, macular degeneration, and diabetic retinopathy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Saaddine JB, Narayan KM, Vinicor F. Vision loss: a public health problem? Ophthalmology. 2003;110:253–4. doi: 10.1016/s0161-6420(02)01839-0. [DOI] [PubMed] [Google Scholar]
- 2.Schaumberg DA, Buring JE, Sullivan DA, Dana MR. Hormone replacement therapy and dry eye syndrome. JAMA. 2001;286:2114–9. doi: 10.1001/jama.286.17.2114. [DOI] [PubMed] [Google Scholar]
- 3.Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003;136:318–26. doi: 10.1016/s0002-9394(03)00218-6. [DOI] [PubMed] [Google Scholar]
- 4.Schein OD, Munoz B, Tielsch JM, Bandeen-Roche K, West S. Prevalence of dry eye among the elderly. Am J Ophthalmol. 1997;124:723–8. doi: 10.1016/s0002-9394(14)71688-5. [DOI] [PubMed] [Google Scholar]
- 5.Lemp MA. Report of the National Eye Institute/Industry workshop on Clinical Trials in Dry Eyes. CLAO J. 1995;21:221–32. [PubMed] [Google Scholar]
- 6.Dalzell MD. Dry eye: prevalence, utilization, and economic implications. Manag Care. 2003;12:9–13. [PubMed] [Google Scholar]
- 7.Sullivan DA, Yamagami H, Liu M, et al. Sex steroids, the meibomian gland and evaporative dry eye. Adv Exp Med Biol. 2002;506:389–99. doi: 10.1007/978-1-4615-0717-8_56. [DOI] [PubMed] [Google Scholar]
- 8.Miljanovic B, Trivedi KA, Dana MR, Gilbard JP, Buring JE, Schaumberg DA. Relation between dietary n-3 and n-6 fatty acids and clinically diagnosed dry eye syndrome in women. Am J Clin Nutr. 2005;82:887–93. doi: 10.1093/ajcn/82.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albietz JM, Lenton LM, McLennan SG. Dry eye after LASIK: comparison of outcomes for Asian and Caucasian eyes. Clin Exp Optom. 2005;88:89–96. doi: 10.1111/j.1444-0938.2005.tb06673.x. [DOI] [PubMed] [Google Scholar]
- 10.Tsubota K, Nakamori K. Dry eyes and video display terminals. N Engl J Med. 1993;328:584. doi: 10.1056/NEJM199302253280817. [DOI] [PubMed] [Google Scholar]
- 11.Wolkoff P, Nojgaard JK, Troiano P, Piccoli B. Eye complaints in the office environment: precorneal tear film integrity influenced by eye blinking efficiency. Occup Environ Med. 2005;62:4–12. doi: 10.1136/oem.2004.016030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goto E, Yagi Y, Matsumoto Y, Tsubota K. Impaired functional visual acuity of dry eye patients. Am J Ophthalmol. 2002;133:181–6. doi: 10.1016/s0002-9394(01)01365-4. [DOI] [PubMed] [Google Scholar]
- 13.Moss SE, Klein R, Klein BE. Prevalence of and risk factors for dry eye syndrome. Arch Ophthalmol. 2000;118:1264–8. doi: 10.1001/archopht.118.9.1264. [DOI] [PubMed] [Google Scholar]
- 14.Moss SE, Klein R, Klein BE. Incidence of dry eye in an older population. Arch Ophthalmol. 2004;122:369–73. doi: 10.1001/archopht.122.3.369. [DOI] [PubMed] [Google Scholar]
- 15.Reddy P, Grad O, Rajagopalan K. The economic burden of dry eye: a conceptual framework and preliminary assessment. Cornea. 2004;23:751–61. doi: 10.1097/01.ico.0000134183.47687.75. [DOI] [PubMed] [Google Scholar]
- 16.Mertzanis P, Abetz L, Rajagopalan K, et al. The relative burden of dry eye in patients' lives: comparisons to a U.S. normative sample. Invest Ophthalmol Vis Sci. 2005;46:46–50. doi: 10.1167/iovs.03-0915. [DOI] [PubMed] [Google Scholar]
- 17.Schiffman RM, Walt JG, Jacobsen G, Doyle JJ, Lebovics G, Sumner W. Utility assessment among patients with dry eye disease. Ophthalmology. 2003;110:1412–9. doi: 10.1016/S0161-6420(03)00462-7. [DOI] [PubMed] [Google Scholar]
- 18.Rajagopalan K, Abetz L, Mertzanis P, et al. Comparing the discriminative validity of two generic and one disease-specific health-related quality of life measures in a sample of patients with dry eye. Value Health. 2005;8:168–74. doi: 10.1111/j.1524-4733.2005.03074.x. [DOI] [PubMed] [Google Scholar]
- 19.Cook NR, Lee IM, Gaziano JM, et al. Low-dose aspirin in the primary prevention of cancer: the Women's Health Study: a randomized controlled trial. JAMA. 2005;294:47–55. doi: 10.1001/jama.294.1.47. [DOI] [PubMed] [Google Scholar]
- 20.Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 21.Rexrode KM, Lee IM, Cook NR, Hennekens CH, Buring JE. Baseline characteristics of participants in the Women's Health Study. J Womens Health Gend Based Med. 2000;9:19–27. doi: 10.1089/152460900318911. [DOI] [PubMed] [Google Scholar]
- 22.Final report on the aspirin component of the ongoing Physicians' Health Study. Steering Committee of the Physicians' Health Study Research Group. N Engl J Med. 1989;321:129–35. doi: 10.1056/NEJM198907203210301. [DOI] [PubMed] [Google Scholar]
- 23.Hennekens CH, Buring JE, Manson JE, et al. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med. 1996;334:1145–9. doi: 10.1056/NEJM199605023341801. [DOI] [PubMed] [Google Scholar]
- 24.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 25.Sullivan RM, Cermak JM, Papas AS, Dana MR, Sullivan DA. Economic and quality of life impact of dry eye symptoms in women with Sjogren's syndrome. Adv Exp Med Biol. 2002;506:1183–8. doi: 10.1007/978-1-4615-0717-8_167. [DOI] [PubMed] [Google Scholar]
- 26.Nichols KK, Mitchell GL, Zadnik K. Performance and repeatability of the NEI-VFQ-25 in patients with dry eye. Cornea. 2002;21:578–83. doi: 10.1097/00003226-200208000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Ishida R, Kojima T, Dogru M, et al. The application of a new continuous functional visual acuity measurement system in dry eye syndromes. Am J Ophthalmol. 2005;139:253–8. doi: 10.1016/j.ajo.2004.08.075. [DOI] [PubMed] [Google Scholar]
- 28.Goto E, Yagi Y, Kaido M, Matsumoto Y, Konomi K, Tsubota K. Improved functional visual acuity after punctal occlusion in dry eye patients. Am J Ophthalmol. 2003;135:704–5. doi: 10.1016/s0002-9394(02)02147-5. [DOI] [PubMed] [Google Scholar]
- 29.Begley CG, Himebaugh N, Renner D, et al. Tear breakup dynamics: a technique for quantifying tear film instability. Optom Vis Sci. 2006;83:15–21. doi: 10.1097/01.opx.0000195569.36185.fd. [DOI] [PubMed] [Google Scholar]
- 30.American Academy of Ophthalmology. Policy Statement: Vision Requirements for Driving. c2001 [cited 11/20/2006]. Available from: http://www.aao.org/aao/member/policy/driving.cfm.
- 31.Singh S, Perel M. Drivers’ perceptions of headlight glare from oncoming and following vehicles (DOT HS 809 669) c2004 [cited 11/20/2006]. Available from: http://www-nrd.nhtsa.dot.gov/pdf/nrd-30/NCSA/Rpts/2003/809-669/images/GLARE_PERCEP.pdf.
- 32.Huang FC, Tseng SH, Shih MH, Chen FK. Effect of artificial tears on corneal surface regularity, contrast sensitivity, and glare disability in dry eyes. Ophthalmology. 2002;109:1934–40. doi: 10.1016/s0161-6420(02)01136-3. [DOI] [PubMed] [Google Scholar]
- 33.Statistics on the Aging Population [database on the Internet] Department of Health and Human Services, Administration on Aging. [Updated 05/02/2005; cited 11/20/2006]. Available from: http://www.aoa.gov/prof/Statistics/statistics.asp.
- 34.Bullough JD, Derlofske JV, Dee P, Chen J, Akashi Y. An Investigation of Headlamp Glare: Intensity, Spectrum and Size. National Highway Traffic Safety Administration NHTSA, Report No. DOT HS 809 692. Washington, DC: 2003:48. [cited 11/20/2006]. Available from: http://www-nrd.nhtsa.dot.gov/departments/nrd-01/GlareSpectrum/pages/TOC.html.
- 35.Rolando M, Iester M, Macri A, Calabria G. Low spatial-contrast sensitivity in dry eyes. Cornea. 1998;17:376–9. doi: 10.1097/00003226-199807000-00006. [DOI] [PubMed] [Google Scholar]
- 36.Fan-Paul NI, Li J, Miller JS, Florakis GJ. Night vision disturbances after corneal refractive surgery. Surv Ophthalmol. 2002;47:533–46. doi: 10.1016/s0039-6257(02)00350-8. [DOI] [PubMed] [Google Scholar]
- 37.Gulati A, Sullivan R, Buring JE, Sullivan DA, Dana R, Schaumberg DA. Validation and repeatability of a short questionnaire for dry eye syndrome. Am J Ophthalmol. 2006;142:125–131. doi: 10.1016/j.ajo.2006.02.038. [DOI] [PubMed] [Google Scholar]


