Abstract
Ablations of the Axin family genes demonstrated that they modulate Wnt signaling in key processes of mammalian development. The ubiquitously expressed Axin1 plays an important role in formation of the embryonic neural axis, while Axin2 is essential for craniofacial skeletogenesis. Although Axin2 is also highly expressed during early neural development, including the neural tube and neural crest, it is not essential for these processes, apparently due to functional redundancy with Axin1. To further investigate the role of Wnt signaling during early neural development, and its potential regulation by Axins, we developed a mouse model for conditional gene activation in the Axin2-expressing domains. We show that gene expression can be successfully targeted to the Axin2-expressing cells in a spatially and temporally specific fashion. High levels of Axin in this domain induce a region-specific effect on the patterning of neural tube. In the mutant embryos, only the development of midbrain is severely impaired even though the transgene is expressed throughout the neural tube. Axin apparently regulates β -catenin in coordinating cell cycle progression, cell adhesion and survival of neuroepithelial precursors during development of ventricles. Our data support the conclusion that the development of embryonic neural axis is highly sensitive to the level of Wnt signaling.
Keywords: Axin, Wnt signaling, β-Catenin, Cyclin D1, P15, Progenitor cell biology, Neural epithelium, Brain, Developmental abnormalities
1. Introduction
Axin was first identified in a mouse mutant strain, created by random integration of a transgene (Zeng et al., 1997). This transgenic allele (AxinTg1) was shown to be allelic to the classical mouse mutations Fused (AxinFu), Kinky (AxinKi), and Knobby (AxinKb) (Perry et al., 1995; Vasicek et al., 1997). Substantial evidence has established that Axin1 and its homologue Axin2 negatively regulate the canonical Wnt pathway by promoting degradation of β -catenin (Kikuchi, 2000; Miller et al., 1999; Moon et al., 2002; Peifer and Polakis, 2000). Mouse embryos homozygous for several Axin1 alleles exhibited recessive phenotypes, including axis duplication, suggesting that Axin1 regulates embryonic axis formation (Gluecksohn-Schoenheimer, 1949; Jacobs-Cohen et al., 1984; Perry et al., 1995). Unlike the uniform expression of Axin1, Axin2 is expressed in specific regions during embryogenesis (Jho et al., 2002). Axin2 was able to substitute for Axin1 not only in various biological and biochemical assays, but also in genetic experiments where Axin2 was inserted into the Axin1 locus (Behrens et al., 1998; Chia and Costantini, 2005; Yamamoto et al., 1998). Therefore, Axin1 and Axin2 apparently have redundant functions, and the inability of Axin2 to replace Axin1 in Axin1−/ − embryos is due to the restricted expression pattern of Axin2. Despite the high levels of Axin2 in neural crest precursors and migratory neural crest cells (Jho et al., 2002), its disruption in mice did not cause any defects associated with neural crest development (Yu et al., 2005). However, Axin2 is extremely important for skull morphogenesis during early postnatal development (Yu et al., 2005). In the Axin2 mutant mice, premature fusion of cranial sutures induces skeletal abnormalities, resembling craniosynostosis in humans.
Axins serve as scaffold proteins directly associating with several Wnt signaling molecules, including disheveled, the serine/threonine kinase GSK-3, β -catenin, adenomatous polypopsis coli (APC), and the serine/threonine protein phosphatase 2A (PP2A) (Behrens et al., 1998; Fagotto et al., 1999; Hedgepeth et al., 1999; Hsu et al., 1999; Itoh et al., 1998; Julius et al., 2000; Kishida et al., 1998; Sakanaka et al., 1998). In the absence of a Wnt signal, the Axin-dependent complex mediates β -catenin degradation, while Wnt proteins perturb formation of this complex by signaling through frizzled and LRP5/6 receptors (Bhanot et al., 1996; Farr et al., 2000; Li et al., 1999; Smalley et al., 1999; Tamai et al., 2000; Wehrli et al., 2000; Yanagawa et al., 1995; Yang-Snyder et al., 1996). Therefore, β -catenin is accumulated and binds to LEF/Tcf family proteins as a transcriptional co-activator to activate target genes (Behrens et al., 1996; Brannon et al., 1997; Molenaar et al., 1996). In addition to the canonical pathway, certain Wnts (e.g., Wnts 4, 5a and 11) can signal through a planar cell polarity pathway by activating the JNK, or a Ca2+ mediated protein kinase C pathway (Boutros et al., 1998; Heisenberg et al., 2000; Mlodzik, 1999; Sheldahl et al., 1999; Slusarski et al., 1997; Tada and Smith, 2000). However, there is as yet no in vivo evidence that Axin is involved in the alternative Wnt pathways.
Members of the Wnt family are expressed in the developing brain and neural tube (Parr et al., 1993). During neural development, the canonical Wnt pathway is critically involved in cell proliferation, cell fate decisions, morphogenesis of the neural tube, and neuronal differentiation (Castelo-Branco et al., 2003; Chenn and Walsh, 2002; Gunhaga et al., 2003; Hall et al., 2000; Hirabayashi et al., 2004; Krylova et al., 2002; Lee et al., 2004; Megason and McMahon, 2002; Nordstrom et al., 2002; Wilson et al., 2001; Zechner et al., 2003). Previous studies demonstrated that the Wnt signals are required for mammalian head formation. Wnt1 and Wnt3a are both expressed in the dorsolateral region of the neural tube that gives rise to neural crest cells. Wnt1 is required for midbrain patterning (McMahon and Bradley, 1990; McMahon et al., 1992) whereas Wnt3a is essential for the formation of somite, tailbud and hippocampus (Greco et al., 1996; Lee et al., 2000; Takada et al., 1994). While inactivation of either the mouse Wnt1 or Wnt3a gene did not cause defects in craniofacial development, mice in which both the Wnt1 and Wnt3a genes have been eliminated showed a marked deficiency in neural crest derivatives (Ikeya et al., 1997). Furthermore, conditional deletion of β -catenin by Wnt1- Cre causes not only truncation of the midbrain and the entire cerebellum that is reminiscent of the Wnt deficient phenotypes, but also craniofacial deformities (Brault et al., 2001). Wnt signaling appears to regulate the expansion of common precursors for neural crest and neuroepithelium. This was confirmed by lineage tracing/fate mapping analysis using a two component genetic labeling system (Chai et al., 2000; Jiang et al., 2000). In mice carrying both Wnt1-Cre transgene (Danielian et al., 1998) and the Cre-dependent R26RlacZ reporter allele (Soriano, 1999), the lacZ gene is expressed in neural crest derivatives in addition to dorsal neural tube where Wnt1 is normally expressed. These data suggest that Wnt1-expressing cells from the dorsal neural tube migrate to craniofacial regions. Although Wnt regulates induction of the neural precursors, a negative signal might be required for neural crest migration and/or differentiation.
Because of the apparent redundancy of Axin1 and Axin2 in the domains where they are co-expressed, we took a gain of function approach to study their potential functions during early neural development. The level of Axin1 was elevated in the Axin2-expressing cells using a conditional transgenic expression system. In the transgenic embryos, neuroepithelial proliferation, adhesion and survival are reduced by Axin1 through its effects on β -catenin. Although Axin1 is elevated throughout the developing neural tube, midbrain development is particularly sensitive to the transgene expression. Our data support the conclusion that a normal level of Axin1 is important for the development of neuroepithelial precursors, and that development of midbrain is particular sensitive to alterations of the canonical Wnt pathway.
2. Results
2.1. β -Catenin/cyclin D1 signaling in developing ventricular zone
β -Catenin has a significant effect on the decision of neural precursors to proliferate or differentiate (Chenn and Walsh, 2002; Hirabayashi et al., 2004; Lee et al., 2004; Megason and McMahon, 2002; Wilson et al., 2001; Zechner et al., 2003). To gain further insights into the role of the canonical Wnt pathway in neuroepithelial cells, we examined expression of β -catenin, cyclin D1 and its inhibitors P15 and P16 that are important cell cycle regulators during normal development of the ventricles. Immunostaining analyses were performed with a monoclonal antibody α -ABC (van Noort et al., 2002), which recognizes only the non-phosphorylated/activated form of β -catenin at Ser37 and Thr41 residues that stimulate downstream signaling events. In the developing midbrain, β -catenin signaling is highly stimulated in the ventricular zone that consists of neuroepithelial precursors as shown by expression of an early neuroepithelial marker SOX1 (Fig. 1A–D). The activated form of β -catenin is particularly enriched in the cells at inner layers of the ventricle. This differential activation of β -catenin in the ventricular zone also correlated with stimulation of cyclin D1, which is a direct target of the β -catenin and LEF/TCF transcription complex (Fig. 1E and F).
Fig. 1.
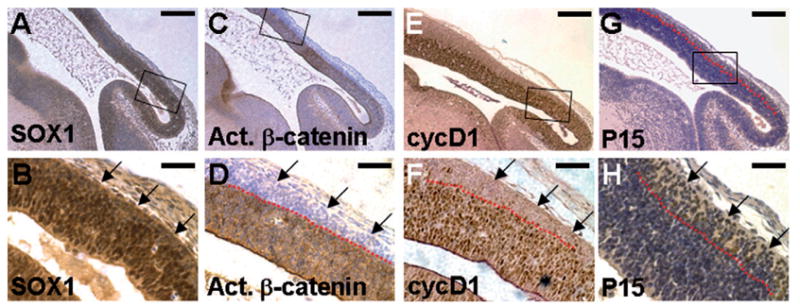
Wnt signaling maintains the mitotic activity of neuroepithelial precursors in developing ventricular zone. Sections of the E13.5 embryos were immunostained with α -SOX1 (A, B), α -ABC (C, D), α -cyclin D1 (E, F) or α -P15 (G, H) antibody (brown staining), and counterstained with hematoxylin (blue staining, A–D and G–H). Enlargements of the insets (A, C, E and G) are shown in B, D, F and H, respectively. Neuroepithelial precursors express SOX1 in the mid/hind brain regions (A, B). Immunohistochemical staining with an antibody that recognizes only the activated form of β -catenin (α -ABC antibody) reveals elevated levels of β -catenin in the neural precursors during brain development (C, D). Nuclear expression of cyclin D1 is evident in the developing ventricular zone (E, F). The red dashed lines represent the boundary of mitotic cells that express activated β -catenin/cyclin D1 and the postmitotic cells that do not (D.F). In contrast, P15 is present only in the post-mitotic cells (G, H). The arrows indicate SOX1-positive neuroepithelial cells, which exit the proliferating zone and express P15, but with no activation of β -catenin and cyclin D1. Scale bars, 0.2 mm (A, C, E and G); 50 μ m (B, D, F and H).
Cells close to the lumen of the ventricle are highly proliferative neural precursors. In contrast, post-mitotic cells are located to the outer layers of the ventricular zone. These cells have no detectable activity for β -catenin and cyclin D1, but express P15, an inhibitor of cyclin D1/cyclin- dependent kinases (Fig. 1G and H). P16 is not involved in the developmental process (data not shown). This distinct signaling pattern suggests that the canonical Wnt pathway might be required for maintaining proliferating activities of the neural precursors in the ventricular zone. It is possible that β -catenin signaling is abolished following cell cycle exit as indicated by the diminished expression of the G1-S regulator cyclin D1 in the developing cortex. The progenitors exit the proliferating zone, and undergo neuronal differentiation during development of the midbrain.
2.2. Development of a transgenic expression system for inducible expression in the Axin2-expressing cells
Using an Axin2lacZ knock-in allele (Lustig et al., 2002; Yu et al., 2005), we examined the expression of Axin2 during early neural development. Axin2 is strongly expressed in the lateral margin of the neural fold at the boundary between surface and neural ectoderms where neural crests arise at E8.5 (Fig. 2A and E). The expression of Axin2 is then detected in the dorsal midline of the neural tube, as well as in migratory neural crest cells and branchial arches at E9.5 and E10.5 (Fig. 2B, C, F and G). In the E12.5 embryo, Axin2 is activated in the central nervous system (CNS) and several facial structures, including external ears, area surrounding the eyes and whisker follicles that are derivatives of the cranial neural crest (Fig. 2D). These results are consistent with previous findings (Jho et al., 2002) and suggest that Axin2 might play a role in early neural development. However, mice with inactivation of Axin2 did not exhibit defects associated with any of these developmental processes. This is likely due to the uniform expression of Axin1 compensating for the loss of Axin2, as mouse embryos homozygous for Axin2 and heterozygous for Axin1 develop severe defects in craniofacial and neural development (W.H. and F.C., unpublished, and B. Jerchow and W. Birchmeier, personal communication).
Fig. 2.
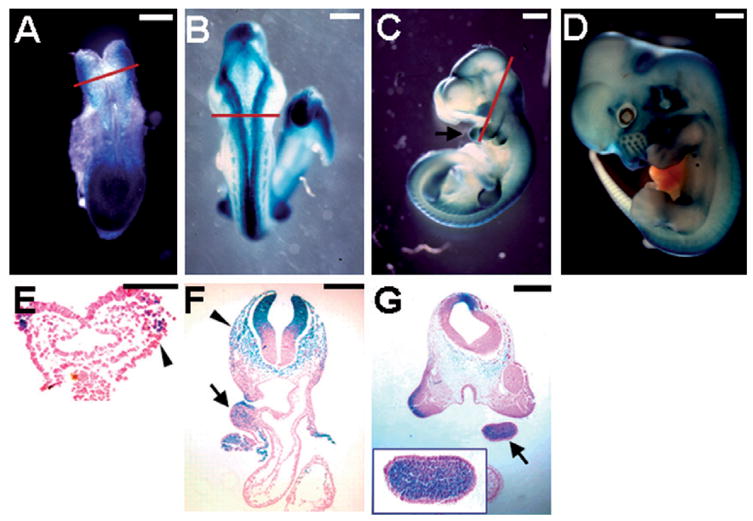
Expression of Axin2 in early neural development. The Axin2 expression pattern was visualized by β -gal staining in whole mounts or sections of the Axin2lacZ heterozygous embryos. At E8.5, Axin2 is expressed in the lateral margin of the neural fold (A) where neural crest cells arise at the boundary between the surface and neural ectoderm (E). By E9.5, strong Axin2 staining is then detected along the dorsal midline of the CNS (B). Axin2 is expressed in the dorsal neural tube, migrating neural crest cells (arrowheads) and branchial arches (arrows) (C, E, F and G). Inset shows an enlargement of the branchial arch of the E10.5 embryo (G). At E12.5, Axin2 is continuously present in the dorsal midline of CNS and many facial structures that are derivatives of CNC (D). First row, the Axin2lacZ knock-in embryos show that Axin2 expression. Second row, cross sections of the β -gal stained embryos. Red lines in A, B and C represent levels of the sections in E, F and G, respectively. Scale bars, 0.2 mm (A, F); 0.3 mm (B, G); 0.5 mm (C); 0.8 mm (D); 0.1 mm (E).
To further investigate the role of Axins in early neural development, we decided to take a gain of function approach by transgenic overexpression. Using the tet-on system (Gossen et al., 1995; Urlinger et al., 2000), we developed a series of mouse strains that permits conditional gene expression in the Axin2-expressing cells. A new Axin2- rtTA mouse strain, expressing an improved version (Urlinger et al., 2000) of rtTA (reverse tetracycline-controlled transactivator) under control of the Axin2 regulatory element, was generated. The Axin2-rtTA mice were crossed to the TRE-lacZ mice (Hennighausen et al., 1995), containing the lacZ reporter under control of TRE (tetracycline response element), to obtain the double transgenic embryos. To induce expression of the lacZ reporter in these embryos, their pregnant mothers were treated with Dox for 5 days. Compared with the endogenous Axin2 expression pattern that is detected by β -gal staining of the E9 and E10.5 Axin2lacZ heterozygous embryos (Fig. 3A and C), our data indicated that this system successfully targeted transgene expression to the Axin2-expressing cells (Fig. 3B and D). The lacZ expression was detected in regions where Axin2 is normally expressed, such as neuroepithelial cells at the dorsal neural tube, migrating neural crest cells, and post-migratory neural crest cells in the branchial arches (Fig. 3E–G). As experimental controls, embryos carrying either both transgenes without Dox treatment or the single TRE-lacZ transgene with Dox treatment did not show any lacZ staining (data not shown). Therefore, we have successfully developed a transgenic system permitting targeted gene expression in the Axin2-expressing cells in a spatially and temporally regulated fashion.
Fig. 3.
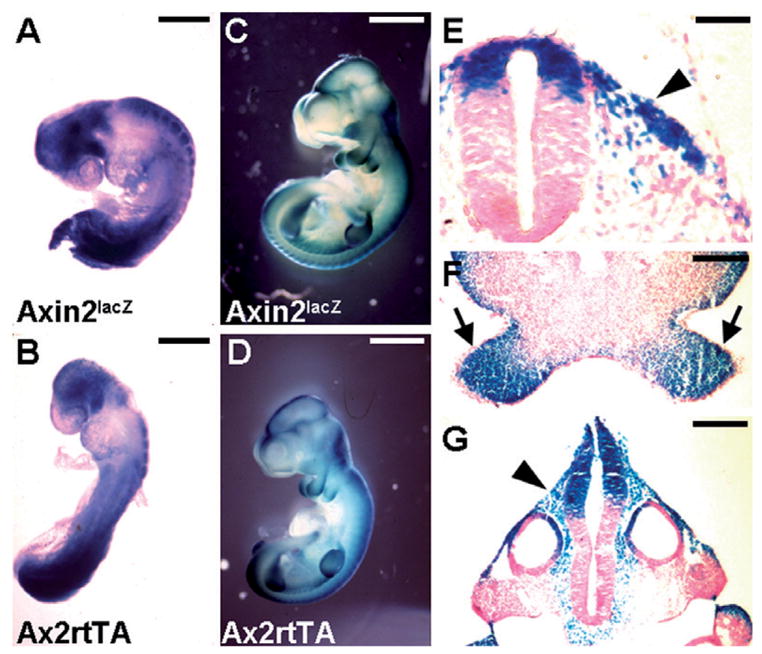
Axin2-rtTA mouse strain permits targeted gene expression in the Axin2-expressing domains. Embryos carrying Axin2-rtTA and TRE-lacZ transgenes enable conditional expression of the lacZ target gene in the Axin2-expressing cells. The transgenic expression was induced by Dox treatment of the pregnant mothers at E4.5/5.5. Embryos were recovered and analyzed by β -gal staining in whole mounts (B, D) and sections (E, F and G). The expression pattern of the lacZ target gene (B, D) is extremely similar to that of endogenous Axin2 gene detected using the Axin2lacZ knock-in allele at E9 (A) and E10.5 (C). The lacZ target gene is highly stimulated in the dorsal part of the neural tube, migrating neural crest cells (arrowheads) and branchial arches (arrows) upon Dox induction (E, F and G). Scale bars, 0.3 mm (A, B); 1 mm (C, D); 50 μ m (E); 0.2 mm (F, G).
2.3. Inducible expression of Axin1 in early neural development
Disruption of Axin2 did not reveal whether it functions in early neural development due to redundancy between Axin2 and the ubiquitously expressed Axin1 (Yu et al., 2005). Therefore, we pursued a gain-of-function strategy to investigate the potential role of Axins in these processes. To test whether inducible expression of Axin1 affects early neural development, we crossed the Axin2-rtTA mice with the TRE-Axin-GFP mouse strains, which we established previously (Hsu et al., 2001). In embryos carrying both the Axin2-rtTA and TRE-Axin-GFP transgenes, the co-expression of Axin1 and GFP in the Axin2-expressing cells can be induced by Dox treatment (Fig. 4). To study the effects of Axin1 in early neural development, the Dox treatment began at E4.5/5.5. The expression of the transgene in the craniofacial regions (Fig. 4A, B and E) and neural tube (Fig. 4C, D and F) was confirmed by whole mount GFP analyses of the E12.5 and E13.5 embryos that permits an easy identification of the transgene-expressed embryos. However, stimulation of Axin1 resulted in obvious structural deformities in brain development interfering with our examination of the transgene expression (Figs. 4F and 5F). We therefore used a TRE-GFP line (Tumbar et al., 2004) without activation of Axin1 to study the transgenic expression domains (Fig. 4A′ –D′ ). Sections of the E13.5 embryos revealed the target gene expression in whisker follicles (Fig. 4A′ ), midbrain (Fig. 4B′ ), hindbrain (Fig. 4C′ ) and dorsal parts of the neural tube (Fig. 4D′ ).
Fig. 4.
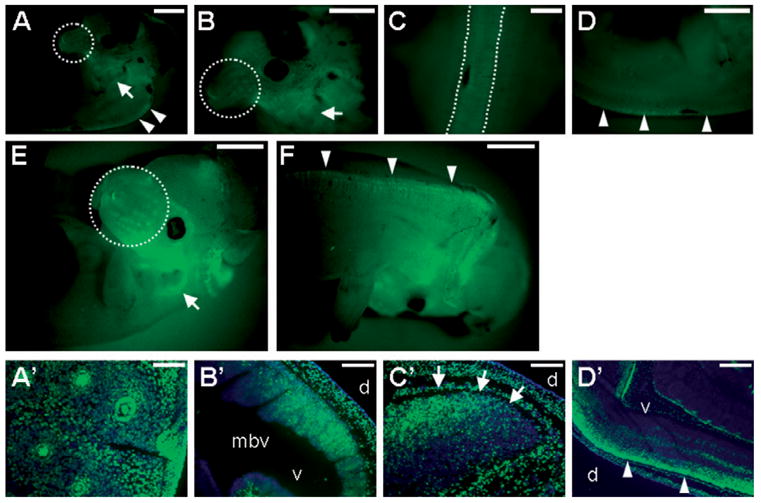
Inducible expression of Axin1 in developing CNS and CNC. Embryos carrying the Axin2-rtTA, and either the TRE-Axin1-GFP or TRE-GFP transgene, display bi-cistronic expression of Axin1 and GFP (A–F) or expression of GFP (A′ –D′ ) in the Axin2-expressing cells. Transgene expression was induced by Dox treatment of the pregnant mothers beginning at E4.5/5.5. Embryos were analyzed by whole mount fluorescence microscopy for GFP at E12.5 (A–D) and E13.5 (E, F). The expression of the transgene was observed in the Axin2-expressing domain, including whisker hair follicles (dashed circle), external ears (arrows) and dorsal midline of CNS (dashed lines and arrowheads). Sections of the E13.5 embryos revealed expression of the target gene (GFP in green, counterstaining in blue) in whisker hair follicles (A′ ), midbrain (B′ ), hindbrain (C′ , arrows) and dorsal neural tube (D′ , arrowheads). mbv, midbrain ventricle; d, dorsal; v, ventral. Scale bars, 1 mm (A–F); 0.5 mm (C, D′ ); 0.1 mm (A′ ); 0.2 mm (B′ , C′ ).
Fig. 5.
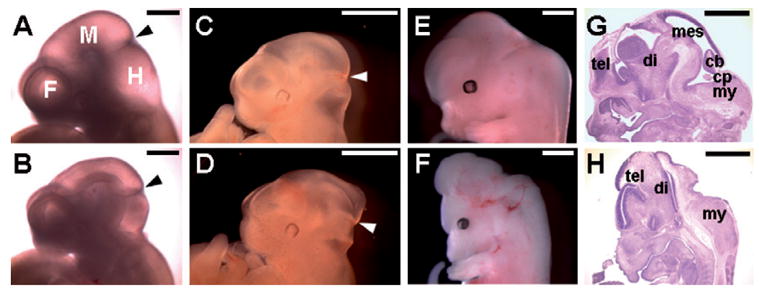
High levels of Axin1 interfere with early brain development. Control embryos (A, C, E and G); Dox-induced transgenic embryos (B, D, F and H). Defects in the midbrain regions were noticeable in the E10.5 (A, B) and E11.5 (C, D) embryos with overexpression of Axin1 in the Axin2-expressing cells. Arrowheads indicate the mid/hindbrain boundary. At E13.5, severe brain abnormalities were detected (E, F). Histological analyses revealed that structures of the mescencephalon (mes), cerebellum (cb) and choroids plexus (cp) were completely missing (G, H). tel, telencephalon; di, diencephalon; my, myelencephalon. Control embryos (A, C, E and G); Dox-induced double transgenic embryos (B, D, F and H). Scale bars, 0.5 mm (A, B); 1 mm (C, D, E, F, G and H).
We next examined the induced expression of Axin1 in the Axin2-expressing domains causes any defects associated with early neural development. Even though the transgene is activated in the Axin2-expressing neural crest derivatives, induced expression of Axin1 did not result in significant craniofacial abnormalities. However, brain development was severely impaired, suggesting that the neural precursors in the dorsal midline of CNS were sensitive to the alteration in Axin1 levels (Fig. 5). The affected embryos showed abnormalities in the mid/hindbrain regions where the ventricles failed to fully extend at E10.5/11.5 (Fig. 5A–D). At E13.5, the affected embryos exhibited brain defects, a phenotype reminiscent of the Wnt deficiency (Fig. 5E and F). Several midbrain structures, including mesencephalon, cerebellum and choroids plexus, were missing in the mutant embryos (Fig. 5G and H). The hindbrain regions were less severely affected.
2.4. Brain development is affected by transgenic expression of Axin1
Because previous studies demonstrated that Axins act as negative regulators for the canonical Wnt pathway (Logan and Nusse, 2004), transgenic expression of Axin1 in the Axin2-expressing cells should interfere with β -catenin signaling in early neural development. Furthermore, mice with inactivation of the engrailed gene En-1 developed brain abnormalities similar to the Wnt1 knockout mice (Wurst et al., 1994). Transgenic expression of En-1 in the Wnt1- expressing regions was able to rescue the developmental defects caused by Wnt1 deficiency (Danielian and McMahon, 1996). Therefore, the engrailed genes, which are down-stream targets of Wnt1, mediate its signaling activity in midbrain patterning. To investigate if the transgenic expression indeed affected the canonical Wnt pathway essential for brain formation, we first examined the expression of Engrailed. The En-expressing domain in the mutant embryos was significantly reduced to a tiny area (Fig. 6A–D). The results support the conclusion that Axins can modulate Wnt/β -catenin signaling during embryonic midbrain development.
Fig. 6.
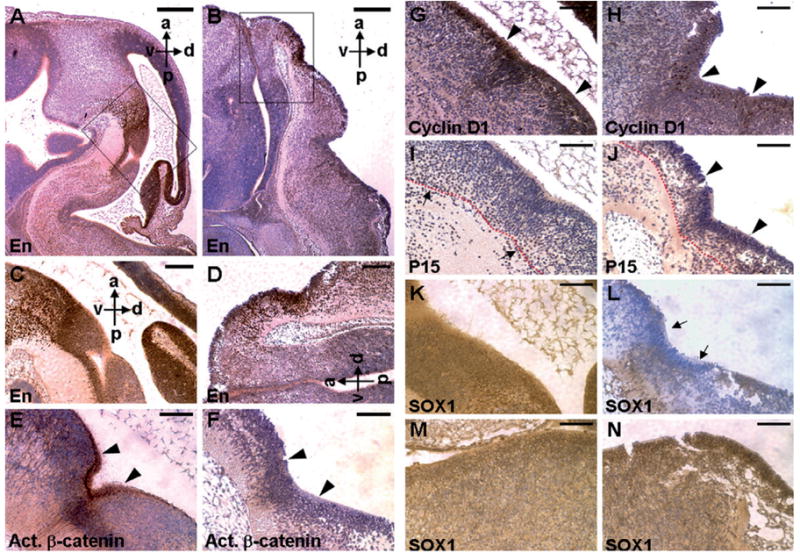
Development of the En-expressing domains is severely affected by Axin1, due to region-specific effects on Wnt mediated brain development. Sagittal sections of embryo at E13.5 were immunostained with specific antibodies (brown staining), and counterstained with hematoxylin (blue staining) as indicated. Immunohistochemical staining identifies the En-expressing domain in the control (A) and transgenic (B) midbrains. Enlargements of the insets (A, B) are shown in C and D, respectively. Panels E–L show the part of brain that is En-positive in adjacent sections (controls, E, G, I and K; transgenics, F, H, J and L). Overexpression of Axin1 affects β-catenin/cyclin D1 signaling in developing midbrain. Expression of the activated form of β-catenin (E, F) and cyclin D1 (G, H) is inhibited in the midbrain of transgenic embryos with elevated levels of Axin1. The arrowheads indicate that neuroepithelial progenitors, which are active mitotic cells with stimulated β-catenin and cyclin D1, are evident in the controls (E, G), but significantly reduced in the transgenics (F, H). P15, normally present in the post-mitotic cells (I, arrows), is detected in the proliferating ventricular zones of the mutants (J, arrowheads). The developing ventricular zone, indicated by the underlying red dashed line, is also reduced in the mutants. Immunohistochemical staining of SOX1 reveals specific inhibition of neural differentiation in the transgenic midbrains with high levels of Axin1. The SOX1-expressing neuroepithelial cells are detected in the control (K, M) and transgenic (L, N) midbrains (K, L) and hindbrains (M, N). High levels of Axin1 interfere with neural differentiation in the transgenic midbrain regions (arrows). a, anterior; p, posterior; d, dorsal; v, ventral. Scale bars, 500 μm (A, B); 400 μm (C, D); 200 μm (E–N).
To elucidate the mechanism by which Axin modulates midbrain development, we tested if transgenic expression of Axin1 interfered with the canonical Wnt pathway during early neural development, especially in the midbrain regions positive for En expression. Immunostaining with α -ABC antibody revealed that transgenic expression of Axin1 prevented activation of β -catenin signaling, in contrast to its stimulation in normal neuroepithelial precursors of the developing midbrain (Fig. 6E and F). This is accompanied by a dramatic reduction of cyclin D1 expression (Fig. 6G and H) and presence of P15 in the proliferating ventricular zones (Fig. 6I and J), suggesting a loss of mitotic neural precursors in the transgenic mutants. The size of developing ventricular zone was also reduced in the transgenic mutants. These data suggest that the canonical Wnt pathway and cell cycle regulation are altered in neural progenitors of the developing ventricle. To investigate if transgenic expression of Axin1 affects neural differentiation, we studied expression of an early neuroepithelial marker SOX1 (Pevny et al., 1998). Expression of SOX1 was highly repressed in the midbrain (Fig. 6K and L), but not in the hindbrain and trunk regions (Fig. 6M and N and data not shown). Thus suggests that the transgenic expression specifically interfered with neuronal differentiation in the mutant midbrains. Although Axin1 was widely stimulated throughout the developing CNS, there were no obvious defects in area posterior to the hindbrain and in the trunk regions. Anterior parts of the CNS seem particularly sensitive to the Axin1 transgenic expression that might disturb Wnt signaling for establishment of the anterior neural axis.
In addition to transducing the Wnt signals (Logan and Nusse, 2004; Moon et al., 2004), β -catenin plays a critical role in cell–cell interaction mediated by adherens junctions (Bienz, 2005; Gumbiner, 2005; Harris and Peifer, 2005). The effect of transgenic Axin1 on β -catenin might also interfere with its function in cell adhesion. We therefore examined the distribution of the actin filaments by immunostaining and confocal microscopy. Similar to the β -catenin distribution (Fig. 7G), actin was co-localized at the adherens junctions of developing midbrain (Fig. 7A and C). However, this prominent staining was depleted in the transgenic mutants (Fig. 7B, E and H). Transgenic expression of Axin1 also caused higher levels of actin in the post-mitotic zones of the developing ventricle (Fig. 7D and F). Furthermore, high levels of Axin induced apoptosis of neural epithelia in the midbrain regions (Fig. 7I and J). No difference in apoptosis was observed in the hindbrain (Fig. 7I and J), nor in the trunk regions where Axin1 was also expressed at elevated levels (data not shown). These data show that the transgenic expression of Axin1 specifically interferes with survival of the neuroepithelial precursors in the midbrain regions. The interference with cell adhesion by Axin1 might cause apoptosis, or might be a consequence of its effects on apoptosis. The data suggest that the precise cellular levels of Axins are critical for modulating the effects of β -catenin on cell proliferation, adhesion and survival during development of the ventricular zone.
Fig. 7.
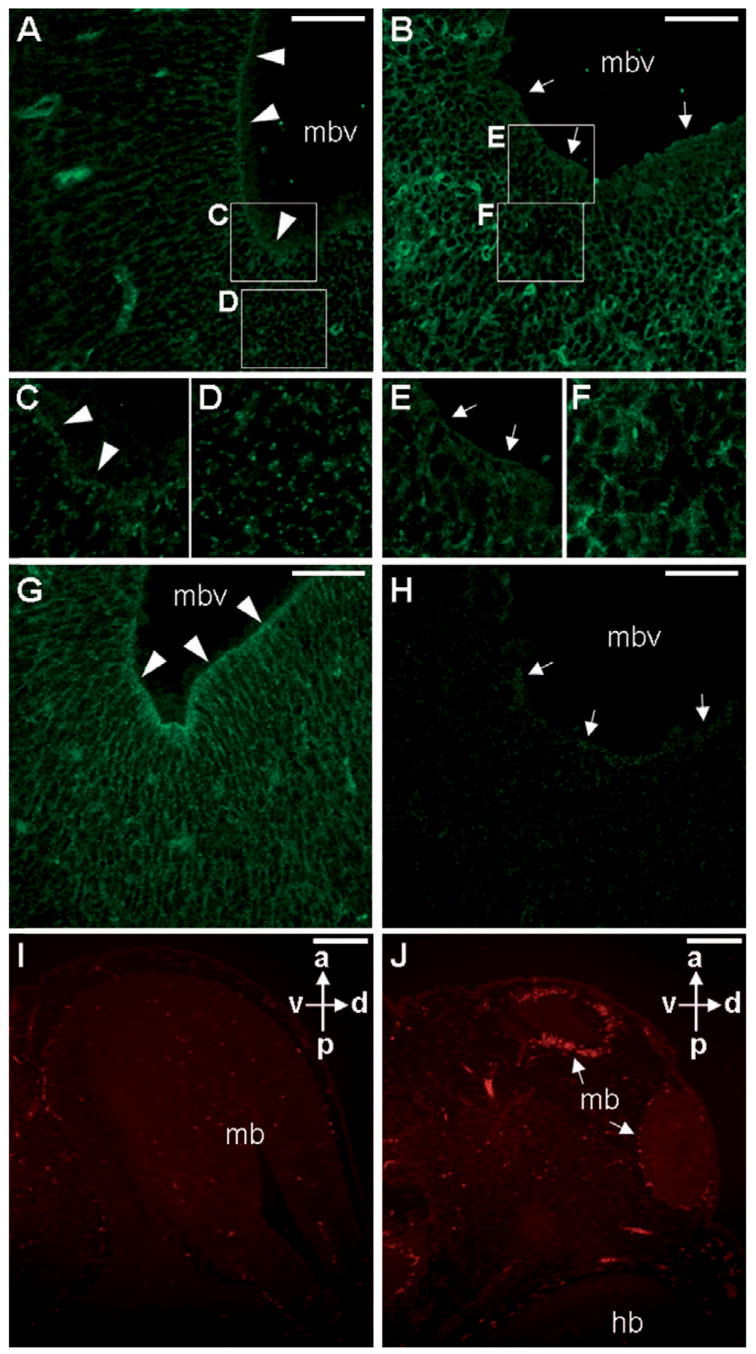
Elevated levels of Axin1 affect formation of the actin microfilaments in the adherens junctions of developing ventricles, and survival of the neuroepithelial cells. Sections of the control (A, C, D and G) and transgenic (B, E, F and H) E13.5 embryos were analyzed by immunostaining of actin (A–F) or activated β-catenin (G, H), followed by confocal microscopy. Actin is localized to the adherens junctions of the control littermates (A, C) along the ventricle lumen of midbrain (arrowheads). In the transgenic embryos (B, E), distribution of the actin microfilaments is drastically reduced (arrows). However, the transgenic post-mitotic zone displays higher levels of actin filaments (D, F). Enlargements of the insets in A and B are shown in C–E and F. The activation of β-catenin is drastically reduced in the transgenic (arrows, H) compared to the control (arrowheads, G). TUNEL staining analyses revealed apoptotic cells in the control (I) and transgenic (J) E12.5 embryos. Increased numbers of apoptotic cells were detected in the transgenic midbrain ventricles (arrows). mb, midbrain; mbv, midbrain ventricle; hb, hindbrain; a, anterior; p, posterior; d, dorsal; v, ventral. Scale bars, 50 μm (A, B, G, H); 200 μm (I, J).
3. Discussion
Although Axin family genes are highly expressed during early neural development (Jho et al., 2002; Zeng et al., 1997), their roles in this developmental process remain elusive. Because of their redundant expression patterns, mice with disruption of one of the Axin genes either die very early in embryogenesis (Axin1) (Zeng et al., 1997) or do not exhibit defects associated with neurogenesis (Axin2) (Yu et al., 2005). Using a gain of function approach, the present study investigates the potential roles of Axin in early neural development. We generated and characterized a new transgenic mouse strain, Axin2-rtTA, which permits inducible expression of a transgene in a spatial and temporal specific manner upon Dox treatment. Our data demonstrated the ability of this system to manipulate gene activity in the Axin2-expressing domains, including neuroepithelial precursors of the dorsal neural tube, migrating neural crest cells and neural crest derivatives, during embryogenesis. Conditional gene expression can also be achieved using this system in developing skin, adrenal gland, kidney, liver, lung, skull and skeleton where Axin2 is also expressed (H.-M.I.Y. and W.H., unpublished data).
Wnt/β-catenin signaling appears to control expansion of the neuroepithelial precursors consistent with previous findings (Chenn and Walsh, 2002; Ikeya et al., 1997; Zechner et al., 2003). Stimulation of β-catenin leads to expression of its transcriptional target cyclin D1 in the proliferating ventricular zones. Upon cell cycle exit, a precise termination of β-catenin/cyclin D1 signaling occurs in the neural precursors where the expression of P15 becomes evident. Transgenic expression of Axin1 in the Axin2-expressing cells results in severe abnormalities in early neurogenesis. In the transgenic mutants, Axin1 appears to interfere with the activity of β-catenin resulting in the inhibition of cyclin D1 during ventricular patterning. This is accompanied by premature activation of P15, a cell cycle inhibitor in the proliferating zones. In addition, the function of β-catenin in cell adhesion also appears to be affected in the Axin1 transgenic mutants. These alterations also lead to apoptosis of the neural precursors, which could also contribute to the defects in cell adhesion.
Transgenic expression of Axin1 was elevated throughout the dorsal midline of the entire neural tube, but only midbrain development was specifically affected. This suggests that development of the anterior neural tube is particularly sensitive to the level of Axin and Wnt signaling. There might be another morphogenetic gradient across the anterior– posterior axis in addition to the dorsal–ventral axis (Megason and McMahon, 2002). According to this model, a gradient of Wnt, higher in anterior regions, might be required for patterning of the neural axis. Although the Axin1 transgene was also induced in the neural crest cells and their derivatives, as well as in the developing limb buds, craniofacial and limb development did not seem to be affected. This might be due to insufficient levels of transgene expression, or a lower sensitivity of these tissues to the high levels of Axin. Mice with the deletion of Axin2 in the Axin1 heterozygous background (Axin2−/−; Axin1+/−) result in severe craniofacial deformities with truncation of the neural crest derived skeletal structures (W.H. and F.C., unpublished; B. Jerchow and W. Birchmeier, personal communication). These data imply that cranial neural crest development might depend on high levels of Axins in the neural precursors. In addition, it remains to be determined if elevated expression of Axin1 in the Axin2-expressing domains of developing cranial sutures causes any skull abnormalities.
Axin has been shown to associate with signaling molecules that are not part of the canonical Wnt pathway in vitro (Furuhashi et al., 2001; Liu et al., 2006; Zhang et al., 1999). However, as yet, there is no in vivo evidence that Axin regulates signaling other than through the canonical Wnt pathway. It is possible that Axin, which has been suggested to stimulate the SAPK/JNK pathway, affects development of the neural tube via the alterative Wnt pathway (PCP, planar cell polarity). Our preliminary studies did not reveal any abnormalities of the JNK pathway in the transgenic embryos (data not shown). Further analysis using mutant mouse strains defective for signaling components of Wnt/PCP might provide important insights into the importance of this pathway during mammalian development (Montcouquiol et al., 2003).
4. Experimental procedures
4.1. Mouse strains
A DNA fragment encoding a modified form of rtTA (rtTA2S-M2) (Urlinger et al., 2000) was inserted into a 5.6 kb DNA expression cassette (Jho et al., 2002) containing the Axin2 promoter to generate the construct for production of the Axin2-rtTA mice using methods as described (Hsu et al., 2001; Shakya et al., 2005). The Axin2 expression cassette, containing the 2883 nucleotides upstream of exon 1, exon 1, intron 1 and part of exon 2, has been defined previously (Jho et al., 2002). Mice were genotyped for the presence of the transgene by PCR using primers 5′-gacaaggaaactcgctcaaaag- 3′ and 5′-ttgctacttgatgctcctgttc-3′, which are located in the rtTA coding sequences. The PCR was performed by denaturation at 94 °C for 5 min and 35 cycles of amplification (94 °C for 30 s, 64 °C for 30 s, and 72 °C for 60 s), followed by a 7-min extension at 72 °C. TRE-Axin- GFP mice were described previously (Hsu et al., 2001), and the TA6 and TA32 lines were used in the current study. Dox (doxycycline, 2 mg/ml plus 50 mg/ml sucrose) was administrated orally in the drinking water, by diluting a freshly prepared 10X Dox/sucrose stock solution. The pregnant female mice were treated with Dox for 2–7 days. Care and use of experimental animals described in this work comply with guidelines and policies of the University Committee on Animal Resources at the University of Rochester.
4.2. Histology, immunostaining and apoptosis analysis
Embryos were fixed in formalin, paraffin embedded, sectioned, and stained with hematoxylin/eosin for histological evaluation as described (Hsu et al., 2001). Tissue sections were subject to immunological staining with avidin:biotinylated enzyme complex as described (Yu et al., 2005), or with fluorescein conjugated avidin (Vector Lab) for fluorescence microscopy. Fluorescence imagines were analyzed using Leica TCS SP spectral confocal microscope. Mouse monoclonal antibodies α-actin (Neo Markers), α-ABC (van Noort et al., 2002), and α-P15 (Neo Markers); rabbit polyclonal antibodies α-Cyclin D1 (Lab Vision), α-EN (a gift of Alex Joyner) and α-Sox1 (a gift of Larysa Pevny) were used as primary antibodies. In situ TUNEL staining of apoptotic cells in tissue sections was performed as described (Hsu et al., 2001). The staining results were evaluated by fluorescence microscopy with appropriate excitation and emission filters.
4.3. β-Gal staining and GFP analyses
Staining for β-galactosidase activity in embryos was performed as described (Yu et al., 2005). In brief, specimens were dissected in phosphate buffered saline (PBS), and prefixed in PBS containing 1% formaldehyde, 0.2% glutaraldehyde, 2 mM magnesium chloride, 5 mM EGTA and 0.02% NP-40 at 4 °C for 30–90 min. Samples were washed three times in PBS containing 0.02% NP-40 at room temperature for 30 min before they were stained in PBS containing 1 mg/ml of X-Gal, 5 mM potassium ferricyanide, 2 mM potassium ferrocyanide, 2 mM magnesium chloride, 0.01% sodium deoxycholate and 0.02% NP-40 at 30 °C for 2–16 h. For analyses in sections, samples were subsequently fixed in formaldehyde and processed for paraffin sections. Whole mount GFP analysis was performed using fluorescence stereomicroscopy to visualize the embryo (Hsu et al., 2001). Embryos were then embedded and processed for frozen sections.
Acknowledgments
We thank Chris Pröschel for fruitful discussion, Alex Joyner and Larysa Pevny for reagents, Zaiqi Wu for technical assistance, and Anthony Mirando for critical reading of the manuscript. This work was supported by grants from the National Institutes of Health to W.H. (CA106308 and DE015654) and to F.C. (HD44265 and DK55388).
References
- Behrens J, Jerchow BA, Wurtele M, Grimm J, Asbrand C, Wirtz R, Kuhl M, Wedlich D, Birchmeier W. Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science. 1998;280:596–599. doi: 10.1126/science.280.5363.596. [DOI] [PubMed] [Google Scholar]
- Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- Bhanot P, Brink M, Samos CH, Hsieh JC, Wang Y, Macke JP, Andrew D, Nathans J, Nusse R. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature. 1996;382:225–330. doi: 10.1038/382225a0. [DOI] [PubMed] [Google Scholar]
- Bienz M. β-Catenin: a pivot between cell adhesion and Wnt signalling. Curr Biol. 2005;15:R64–R67. doi: 10.1016/j.cub.2004.12.058. [DOI] [PubMed] [Google Scholar]
- Boutros M, Paricio N, Strutt DI, Mlodzik M. Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell. 1998;94:109–118. doi: 10.1016/s0092-8674(00)81226-x. [DOI] [PubMed] [Google Scholar]
- Brannon M, Gomperts M, Sumoy L, Moon RT, Kimelman D. A beta-catenin/XTcf-3 complex binds to the siamois promoter to regulate dorsal axis specification in Xenopus. Genes Dev. 1997;11:2359–2370. doi: 10.1101/gad.11.18.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- Castelo-Branco G, Wagner J, Rodriguez FJ, Kele J, Sousa K, Rawal N, Pasolli HA, Fuchs E, Kitajewski J, Arenas E. Differential regulation of midbrain dopaminergic neuron development by Wnt-1, Wnt-3a, and Wnt-5a. Proc Natl Acad Sci USA. 2003;100:12747–12752. doi: 10.1073/pnas.1534900100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai Y, Jiang X, Ito Y, Bringas P, Jr, Han J, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127:1671–1679. doi: 10.1242/dev.127.8.1671. [DOI] [PubMed] [Google Scholar]
- Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297:365–369. doi: 10.1126/science.1074192. [DOI] [PubMed] [Google Scholar]
- Chia IV, Costantini F. Mouse axin and axin2/conductin proteins are functionally equivalent in vivo. Mol Cell Biol. 2005;25:4371–4376. doi: 10.1128/MCB.25.11.4371-4376.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielian PS, McMahon AP. Engrailed-1 as a target of the Wnt- 1 signalling pathway in vertebrate midbrain development. Nature. 1996;383:332–334. doi: 10.1038/383332a0. [DOI] [PubMed] [Google Scholar]
- Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol. 1998;8:1323–1326. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- Fagotto F, Jho E, Zeng L, Kurth T, Joos T, Kaufmann C, Costantini F. Domains of axin involved in protein–protein interactions, Wnt pathway inhibition, and intracellular localization. J Cell Biol. 1999;145:741–756. doi: 10.1083/jcb.145.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr GH, 3rd, Ferkey DM, Yost C, Pierce SB, Weaver C, Kimelman D. Interaction among GSK-3, GBP, axin, and APC in Xenopus axis specification. J Cell Biol. 2000;148:691–702. doi: 10.1083/jcb.148.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuhashi M, Yagi K, Yamamoto H, Furukawa Y, Shimada S, Nakamura Y, Kikuchi A, Miyazono K, Kato M. Axin facilitates Smad3 activation in the transforming growth factor beta signaling pathway. Mol Cell Biol. 2001;21:5132–5141. doi: 10.1128/MCB.21.15.5132-5141.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluecksohn-Schoenheimer S. The effects of a lethal mutation responsible for duplications and twinning in mouse embryos. J Exp Zool. 1949;110:47–76. doi: 10.1002/jez.1401100105. [DOI] [PubMed] [Google Scholar]
- Gossen M, Freundlieb S, Bender G, Muller G, Hillen W, Bujard H. Transcriptional activation by tetracyclines in mammalian cells. Science. 1995;268:1766–1769. doi: 10.1126/science.7792603. [DOI] [PubMed] [Google Scholar]
- Greco TL, Takada S, Newhouse MM, McMahon JA, McMahon AP, Camper SA. Analysis of the vestigial tail mutation demonstrates that Wnt-3a gene dosage regulates mouse axial development. Genes Dev. 1996;10:313–324. doi: 10.1101/gad.10.3.313. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol. 2005;6:622–634. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- Gunhaga L, Marklund M, Sjodal M, Hsieh JC, Jessell TM, Edlund T. Specification of dorsal telencephalic character by sequential Wnt and FGF signaling. Nat Neurosci. 2003;6:701–707. doi: 10.1038/nn1068. [DOI] [PubMed] [Google Scholar]
- Hall AC, Lucas FR, Salinas PC. Axonal remodeling and synaptic differentiation in the cerebellum is regulated by WNT-7a signaling. Cell. 2000;100:525–535. doi: 10.1016/s0092-8674(00)80689-3. [DOI] [PubMed] [Google Scholar]
- Harris TJ, Peifer M. Decisions, decisions: beta-catenin chooses between adhesion and transcription. Trends Cell Biol. 2005;15:234–237. doi: 10.1016/j.tcb.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Hedgepeth CM, Deardor MA, Rankin K, Klein PS. Regulation of glycogen synthase kinase 3beta and downstream Wnt signaling by axin. Mol Cell Biol. 1999;19:7147–7157. doi: 10.1128/mcb.19.10.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisenberg CP, Tada M, Rauch GJ, Saude L, Concha ML, Geisler R, Stemple DL, Smith JC, Wilson SW. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature. 2000;405:76–81. doi: 10.1038/35011068. [DOI] [PubMed] [Google Scholar]
- Hennighausen L, Wall RJ, Tillmann U, Li M, Furth PA. Conditional gene expression in secretory tissues and skin of transgenic mice using the MMTV-LTR and the tetracycline responsive system. J Cell Biochem. 1995;59:463–472. doi: 10.1002/jcb.240590407. [DOI] [PubMed] [Google Scholar]
- Hirabayashi Y, Itoh Y, Tabata H, Nakajima K, Akiyama T, Masuyama N, Gotoh Y. The Wnt/beta-catenin pathway directs neuronal differentiation of cortical neural precursor cells. Development. 2004;131:2791–2801. doi: 10.1242/dev.01165. [DOI] [PubMed] [Google Scholar]
- Hsu W, Shakya R, Costantini F. Impaired mammary gland and lymphoid development caused by inducible expression of Axin in transgenic mice. J Cell Biol. 2001;155:1055–1064. doi: 10.1083/jcb.200107066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu W, Zeng L, Costantini F. Identification of a domain of Axin that binds to the serine/threonine protein phosphatase 2A and a self-binding domain. J Biol Chem. 1999;274:3439–3445. doi: 10.1074/jbc.274.6.3439. [DOI] [PubMed] [Google Scholar]
- Ikeya M, Lee SM, Johnson JE, McMahon AP, Takada S. Wnt signalling required for expansion of neural crest and CNS progenitors. Nature. 1997;389:966–970. doi: 10.1038/40146. [DOI] [PubMed] [Google Scholar]
- Itoh K, Krupnik VE, Sokol SY. Axis determination in Xenopus involves biochemical interactions of axin, glycogen synthase kinase 3 and beta-catenin. Curr Biol. 1998;8:591–594. doi: 10.1016/s0960-9822(98)70229-5. [DOI] [PubMed] [Google Scholar]
- Jacobs-Cohen RJ, Spiegelman M, Cookingham JC, Bennett D. Knobbly, a new dominant mutation in the mouse that affects embryonic ectoderm organization. Genet Res. 1984;43:43–50. doi: 10.1017/s0016672300025702. [DOI] [PubMed] [Google Scholar]
- Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cardiac neural crest. Development. 2000;127:1607–1616. doi: 10.1242/dev.127.8.1607. [DOI] [PubMed] [Google Scholar]
- Julius MA, Schelbert B, Hsu W, Fitzpatrick E, Jho E, Fagotto F, Costantini F, Kitajewski J. Domains of axin and disheveled required for interaction and function in wnt signaling [In Process Citation] Biochem Biophys Res Commun. 2000;276:1162–1169. doi: 10.1006/bbrc.2000.3607. [DOI] [PubMed] [Google Scholar]
- Kikuchi A. Regulation of beta-catenin signaling in the Wnt pathway. Biochem Biophys Res Commun. 2000;268:243–248. doi: 10.1006/bbrc.1999.1860. [DOI] [PubMed] [Google Scholar]
- Kishida S, Yamamoto H, Ikeda S, Kishida M, Sakamoto I, Koyama S, Kikuchi A. Axin, a negative regulator of the wnt signaling pathway, directly interacts with adenomatous polyposis coli and regulates the stabilization of beta-catenin. J Biol Chem. 1998;273:10823–10826. doi: 10.1074/jbc.273.18.10823. [DOI] [PubMed] [Google Scholar]
- Krylova O, Herreros J, Cleverley KE, Ehler E, Henriquez JP, Hughes SM, Salinas PC. WNT-3, expressed by motoneurons, regulates terminal arborization of neurotrophin-3-responsive spinal sensory neurons. Neuron. 2002;35:1043–1056. doi: 10.1016/s0896-6273(02)00860-7. [DOI] [PubMed] [Google Scholar]
- Lee HY, Kleber M, Hari L, Brault V, Suter U, Taketo MM, Kemler R, Sommer L. Instructive role of Wnt/beta-catenin in sensory fate specification in neural crest stem cells. Science. 2004;303:1020– 1023. doi: 10.1126/science.1091611. [DOI] [PubMed] [Google Scholar]
- Lee SM, Tole S, Grove E, McMahon AP. A local Wnt-3a signal is required for development of the mammalian hippocampus. Development. 2000;127:457–467. doi: 10.1242/dev.127.3.457. [DOI] [PubMed] [Google Scholar]
- Li L, Yuan H, Weaver CD, Mao J, Farr GH, 3rd, Sussman DJ, Jonkers J, Kimelman D, Wu D. Axin and Frat1 interact with dvl and GSK, bridging Dvl to GSK in Wnt-mediated regulation of LEF-1. EMBO J. 1999;18:4233–4240. doi: 10.1093/emboj/18.15.4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Rui H, Wang J, Lin S, He Y, Chen M, Li Q, Ye Z, Zhang S, Chan SC, Chen YG, Han J, Lin SC. Axin is a scaffold protein in TGF-beta signaling that promotes degradation of Smad7 by Arkadia. EMBO J. 2006;25:1646–1658. doi: 10.1038/sj.emboj.7601057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, van de Wetering M, Clevers H, Schlag PM, Birchmeier W, Behrens J. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol. 2002;22:1184–1193. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon AP, Bradley A. The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain. Cell. 1990;62:1073–1085. doi: 10.1016/0092-8674(90)90385-r. [DOI] [PubMed] [Google Scholar]
- McMahon AP, Joyner AL, Bradley A, McMahon JA. The midbrain–hindbrain phenotype of Wnt-1-/Wnt-1- mice results from stepwise deletion of engrailed-expressing cells by 9.5 days postcoitum. Cell. 1992;69:581–595. doi: 10.1016/0092-8674(92)90222-x. [DOI] [PubMed] [Google Scholar]
- Megason SG, McMahon AP. A mitogen gradient of dorsal midline Wnts organizes growth in the CNS. Development. 2002;129:2087– 2098. doi: 10.1242/dev.129.9.2087. [DOI] [PubMed] [Google Scholar]
- Miller JR, Hocking AM, Brown JD, Moon RT. Mechanism and function of signal transduction by the Wnt/beta-catenin and Wnt/Ca2+ pathways. Oncogene. 1999;18:7860–7872. doi: 10.1038/sj.onc.1203245. [DOI] [PubMed] [Google Scholar]
- Mlodzik M. Planar polarity in the Drosophila eye: a multifaceted view of signaling specificity and cross-talk. EMBO J. 1999;18:6873–6879. doi: 10.1093/emboj/18.24.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destree O, Clevers H. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86:391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- Montcouquiol M, Rachel RA, Lanford PJ, Copeland NG, Jenkins NA, Kelley MW. Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature. 2003;423:173–177. doi: 10.1038/nature01618. [DOI] [PubMed] [Google Scholar]
- Moon RT, Bowerman B, Boutros M, Perrimon N. The promise and perils of Wnt signaling through beta-catenin. Science. 2002;296:1644–1646. doi: 10.1126/science.1071549. [DOI] [PubMed] [Google Scholar]
- Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- Nordstrom U, Jessell TM, Edlund T. Progressive induction of caudal neural character by graded Wnt signaling. Nat Neurosci. 2002;5:525–532. doi: 10.1038/nn0602-854. [DOI] [PubMed] [Google Scholar]
- Parr BA, Shea MJ, Vassileva G, McMahon AP. Mouse Wnt genes exhibit discrete domains of expression in the early embryonic CNS and limb buds. Development. 1993;119:247–261. doi: 10.1242/dev.119.1.247. [DOI] [PubMed] [Google Scholar]
- Peifer M, Polakis P. Wnt signaling in oncogenesis and embryogenesis – a look outside the nucleus. Science. 2000;287:1606–1609. doi: 10.1126/science.287.5458.1606. [DOI] [PubMed] [Google Scholar]
- Perry WL, 3rd, Vasicek TJ, Lee JJ, Rossi JM, Zeng L, Zhang T, Tilghman SM, Costantini F. Phenotypic and molecular analysis of a transgenic insertional allele of the mouse Fused locus. Genetics. 1995;141:321–332. doi: 10.1093/genetics/141.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pevny LH, Sockanathan S, Placzek M, Lovell-Badge R Division of Developmental Genetics M.R.C.N.I.f.M.R.L.U.K. A role for SOX1 in neural determination. Development (Cambridge, England) 1998;125(10):1967–78. doi: 10.1242/dev.125.10.1967. [DOI] [PubMed] [Google Scholar]
- Sakanaka C, Weiss JB, Williams LT. Bridging of beta-catenin and glycogen synthase kinase-3beta by axin and inhibition of beta-catenin-mediated transcription. Proc Natl Acad Sci USA. 1998;95:3020–3023. doi: 10.1073/pnas.95.6.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakya R, Jho EH, Kotka P, Wu Z, Kholodilov N, Burke R, D’Agati V, Costantini F. The role of GDNF in patterning the excretory system. Dev Biol. 2005;283:70–84. doi: 10.1016/j.ydbio.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Sheldahl LC, Park M, Malbon CC, Moon RT. Protein kinase C is differentially stimulated by Wnt and Frizzled homologs in a G-protein- dependent manner. Curr Biol. 1999;9:695–698. doi: 10.1016/s0960-9822(99)80310-8. [DOI] [PubMed] [Google Scholar]
- Slusarski DC, Yang-Snyder J, Busa WB, Moon RT. Modulation of embryonic intracellular Ca2+ signaling by Wnt-5A. Dev. Biol. 1997;182:114–120. doi: 10.1006/dbio.1996.8463. [DOI] [PubMed] [Google Scholar]
- Smalley MJ, Sara E, Paterson H, Naylor S, Cook D, Jayatilake H, Fryer LG, Hutchinson L, Fry MJ, Dale TC. Interaction of axin and Dvl-2 proteins regulates Dvl-2-stimulated TCF-dependent transcription. EMBO J. 1999;18:2823–2835. doi: 10.1093/emboj/18.10.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Tada M, Smith JC. Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development. 2000;127:2227–2238. doi: 10.1242/dev.127.10.2227. [DOI] [PubMed] [Google Scholar]
- Takada S, Stark KL, Shea MJ, Vassileva G, McMahon JA, McMahon AP. Wnt-3a regulates somite and tailbud formation in the mouse embryo. Genes Dev. 1994;8:174–189. doi: 10.1101/gad.8.2.174. [DOI] [PubMed] [Google Scholar]
- Tamai K, Semenov M, Kato Y, Spokony R, Liu C, Katsuyama Y, Hess F, Saint-Jeannet JP, He X. LDL-receptor-related proteins in Wnt signal transduction. Nature. 2000;407:530–535. doi: 10.1038/35035117. [DOI] [PubMed] [Google Scholar]
- Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urlinger S, Baron U, Thellmann M, Hasan MT, Bujard H, Hillen W. Exploring the sequence space for tetracycline-dependent transcriptional activators: novel mutations yield expanded range and sensitivity. Proc Natl Acad Sci USA. 2000;97:7963–7968. doi: 10.1073/pnas.130192197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Noort M, Meeldijk J, van der Zee R, Destree O, Clevers H. Wnt signaling controls the phosphorylation status of beta-catenin. J Biol Chem. 2002;277:17901–17905. doi: 10.1074/jbc.M111635200. [DOI] [PubMed] [Google Scholar]
- Vasicek TJ, Zeng L, Guan XJ, Zhang T, Costantini F, Tilghman SM. Two dominant mutations in the mouse fused gene are the result of transposon insertions. Genetics. 1997;147:777–786. doi: 10.1093/genetics/147.2.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrli M, Dougan ST, Caldwell K, O’Keefe L, Schwartz S, Vaizel-Ohayon D, Schejter E, Tomlinson A, DiNardo S. arrow encodes an LDL-receptor-related protein essential for Wingless signalling. Nature. 2000;407:527–530. doi: 10.1038/35035110. [DOI] [PubMed] [Google Scholar]
- Wilson SI, Rydstrom A, Trimborn T, Willert K, Nusse R, Jessell TM, Edlund T. The status of Wnt signalling regulates neural and epidermal fates in the chick embryo. Nature. 2001;411:325–330. doi: 10.1038/35077115. [DOI] [PubMed] [Google Scholar]
- Wurst W, Auerbach AB, Joyner AL Division of M and Developmental Biology SLRIMSHTC. Multiple developmental defects in Engrailed-1 mutant mice: an early mid-hindbrain deletion and patterning defects in forelimbs and sternum. Development (Cambridge, England) 1994;120(7):2065–75. doi: 10.1242/dev.120.7.2065. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Kishida S, Uochi T, Ikeda S, Koyama S, Asashima M, Kikuchi A. Axil, a member of the Axin family, interacts with both glycogen synthase kinase 3beta and beta-catenin and inhibits axis formation of Xenopus embryos. Mol Cell Biol. 1998;18:2867–2875. doi: 10.1128/mcb.18.5.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagawa S, van Leeuwen F, Wodarz A, Klingensmith J, Nusse R. The dishevelled protein is modified by wingless signaling in Drosophila. Genes Dev. 1995;9:1087–1097. doi: 10.1101/gad.9.9.1087. [DOI] [PubMed] [Google Scholar]
- Yang-Snyder J, Miller JR, Brown JD, Lai CJ, Moon RT. A frizzled homolog functions in a vertebrate Wnt signaling pathway. Curr Biol. 1996;6:1302–1306. doi: 10.1016/s0960-9822(02)70716-1. [DOI] [PubMed] [Google Scholar]
- Yu HM, Jerchow B, Sheu TJ, Liu B, Costantini F, Puzas JE, Birchmeier W, Hsu W. The role of Axin2 in calvarial morphogenesis and craniosynostosis. Development. 2005;132:1995–2005. doi: 10.1242/dev.01786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechner D, Fujita Y, Hulsken J, Muller T, Walther I, Taketo MM, Crenshaw EB, 3rd, Birchmeier W, Birchmeier C. beta-Catenin signals regulate cell growth and the balance between progenitor cell expansion and differentiation in the nervous system. Dev Biol. 2003;258:406–418. doi: 10.1016/s0012-1606(03)00123-4. [DOI] [PubMed] [Google Scholar]
- Zeng L, Fagotto F, Zhang T, Hsu W, Vasicek TJ, Perry WL, 3rd, Lee JJ, Tilghman SM, Gumbiner BM, Costantini F. The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell. 1997;90:181–192. doi: 10.1016/s0092-8674(00)80324-4. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Neo SY, Wang X, Han J, Lin SC. Axin forms a complex with MEKK1 and activates c-Jun NH(2)-terminal kinase/stress-activated protein kinase through domains distinct from Wnt signaling. J Biol Chem. 1999;274:35247–35254. doi: 10.1074/jbc.274.49.35247. [DOI] [PubMed] [Google Scholar]


