The C1′-oxidized abasic DNA lesion 2-deoxyribonolactone (dL, 1) has attracted significant attention in recent years owing to its potential mutagenicity, which is associated with the ability of lesion 1 to misincorporate nucleotides during DNA synthesis, its resistance to base-excision-repair (BER) enzymes, and its ability to form cross-links with BER enzymes efficiently.[1] Lesion 1 is produced in model systems by the action of a number of DNA-damaging agents such as copper(I)phenanthroline,[2] other metal complexes,[3] enediyne antibiotics,[4] as well as by UV light,[5] photosensitization,[6] and ionizing radiation.[7] However, the formation of 1 in vivo and its biological effects have been poorly documented. Until recently, lesion 1 in model systems has been identified primarily by detection of its low-molecular decomposition product 5-methylene-2-furanone (5-MF, 3).[2b,8] Only recently has a method for the selective detection of 1 been developed based on its reactivity with biotinylated cysteine.[9]
Lesion 1 is relatively unstable; decomposition of 1, which is significantly accelerated by heating or alkaline treatment, results in strand scission and release of 3 (Scheme 1).[10–12] Decomposition of 1 is a two-step process: β elimination of the 3′-phosphate fragment forms butenolide 2, and δ elimination of the 5′-phosphate fragment produces 3 as the final product. The product 3 is unstable at basic pH values and hence cannot be detected upon alkaline decomposition of 1.
Scheme 1.
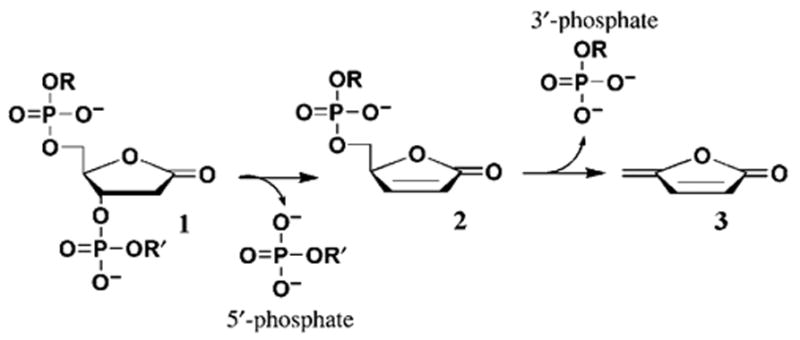
Decomposition of 1.
In our recent paper[8] we demonstrated that 3, a characteristic decomposition product of 1, is released from X-irradiated calf thymus DNA upon postirradiation treatment with DNA-phosphate-binding catalysts such as spermine. We hypothesized that 1 is a precursor to 3 in irradiated DNA and suggested that the yield of 1 in DNA can be quantified by using the yield of released 3.
Herein we report the isolation of dL-containing tetramers from X-irradiated d(CGCG) and d(pCGCG) films, with the dL lesions corresponding to all four positions of the d-(C1G2C3G4) tetramer. We demonstrate that these dL-containing tetramers undergo catalytic decomposition with quantitative release of 3. These results provide proof that lesion 1 is the major, perhaps only, precursor to 3 in irradiated highly polymerized DNA.
Figure 1 shows the HPLC profiles of d(CGCG), X-irradiated as a “dry” film and then dissolved in water for analysis. In the absence of heat, a group of products that do not contain strand breaks but presumably contain modified nucleotides were observed with a retention time of 16–23 min (Figure 1b). Furthermore, peaks are observed for the free bases C and G and for phosphate fragments produced as a result of immediate strand scission.
Figure 1.
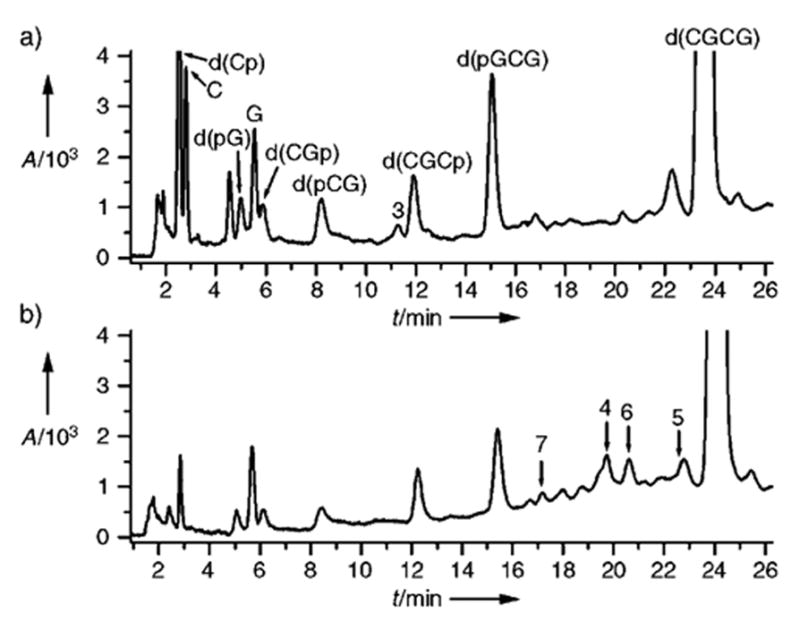
HPLC profiles of the solution of X-irradiated d(CGCG) film (ammonium acetate (40 mM), pH 6.8, spermine (10 mM)). a) 20 min at 90°C with spermine; b) no heat. Peaks for products 4–7 are shown with arrows. HPLC conditions: C18 μ-Bondapack 8 mm 0 100 mm Radial Pak cartridge (Waters) washed with ammonium acetate (40 mM) as running phase and methanol as eluent (1–16.5% methanol over 30 min, linear gradient, 2 mLmin−1).
Upon heating of the same solution in the presence of spermine (10 MM), a number of peaks corresponding to non-strand-break d(CGCG) lesions disappear. These changes are accompanied by the growth of peaks corresponding to products of strand scission, d(pGCG), d(CGCp), d(CGp), d(pCG), d(pG), and d(Cp), as well as free bases C and G (Figure 1a). This observation indicates that at least some of the non-strand-break products are latent lesions, which undergo catalytic decomposition to give strand scission. Also, the release of 3 was observed (Figure 1a), and is shown below to be a result of the decomposition of dL-containing products.
Each of the four products 4–7 (see peaks in Figure 1b), have been identified as a tetramer containing dL located at one of the four numbered positions in d(C1G2C3G4) (see Scheme 2). These products were isolated as individual compounds by semipreparative HPLC and purified through several rounds of HPLC fractionation. MALDI-TOF analysis of the isolated compounds supports the assignments to dL structures 4–7. Similarly, a 5′-phosphorylated tetramer 8, which contains the terminal dL at position 1 of d(pC1G2C3G4) (see Scheme 2), was isolated from the X-irradiated d-(pCGCG) film and identified by using MALDI-TOF and decomposition patterns. Additional evidence is provided by the characteristic decomposition patterns of the dL-containing tetramers 4–8 (see Figures 2 and 3).
Scheme 2.
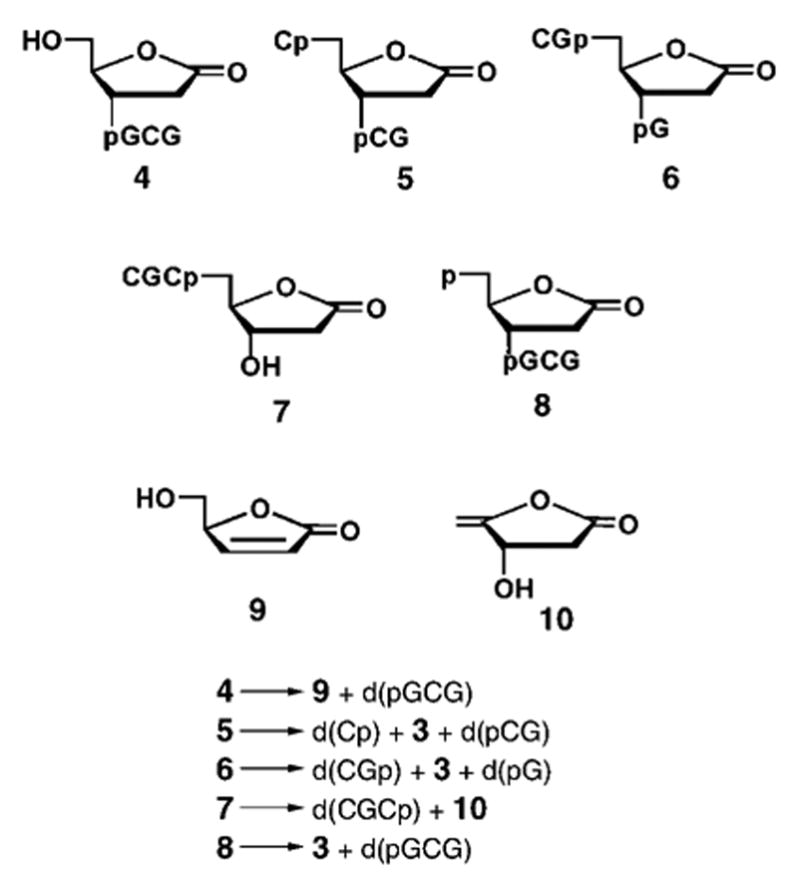
Structures and decomposition patterns of dL-containing oligonucleotides isolated from X-irradiated d(CGCG) and d(pCGCGp) films.
Figure 2.
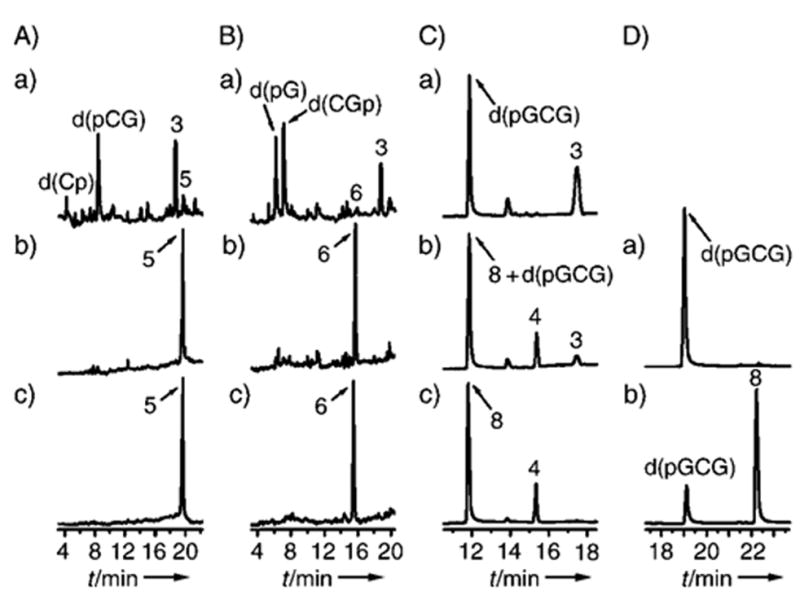
Thermal decomposition of 5, 6, and 8. Reaction mixtures contained 50 mM sodium acetate buffer, pH 5.2, 10 mM spermine (added before or after heating). A–C: RP HPLC; a) 20 min at 90°C with spermine; b) as a) but heated without spermine; c) no heat. HPLC conditions: Gemini C18 4.6 mm 0 250 mm analytical column (Phenomenex) washed with ammonium acetate (40 mM) as running phase and acetonitrile as eluent (1–8% acetonitrile over 20 min, nonlinear gradient type 5 in the original Water’s definition). D: Ion-exchange HPLC; a) 20 min at 90°C with spermine; b) no heat. HPLC conditions: Dionex DNAPac PA-100 column washed with ammonium acetate-(40 mM)/acetonitrile (10%) running phase and a solution of NaCl as eluent (5–250 mM NaCl over 20 min, linear gradient, 1 mLmin−1).
Figure 3.
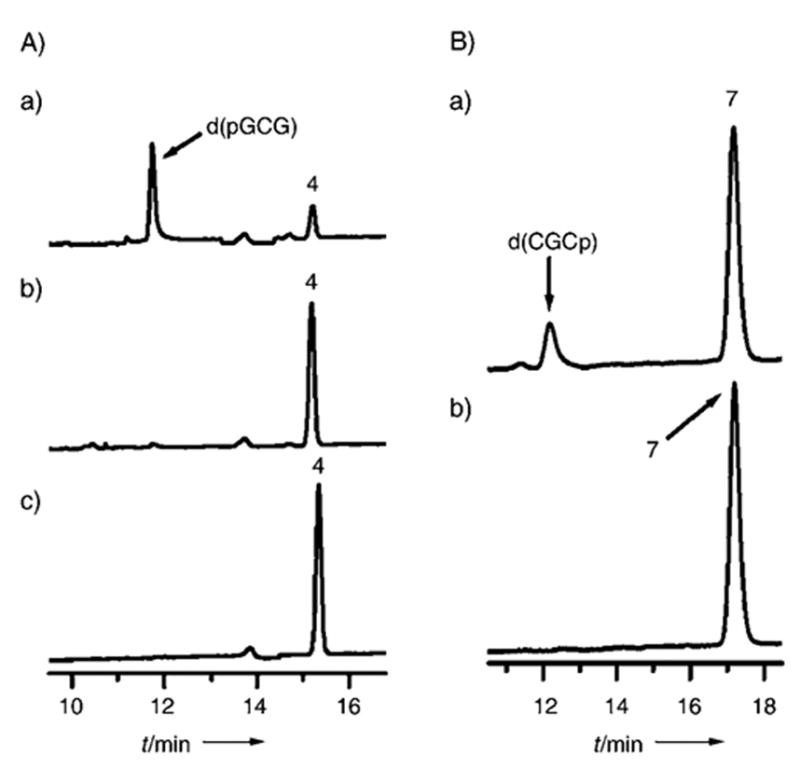
Thermal decomposition of 4 (A) and 7 (B). A) sodium acetate buffer (50 mM), pH 5.2, spermine (10 mM); a) 20 min at 90 °C with spermine; b) as a) but heated without spermine; c) no heat; HPLC conditions: as in Figure 2A–C. B) ammonium acetate (40 mM) buffer, pH 6.8, spermine (50 mM) a) 30 min at 90°C with spermine; b) no heat; HPLC conditions: Luna C18 4.6 mm 0 250 mm analytical column (Phenomenex) washed with ammonium acetate (40 mM) and acetonitrile as eluent (1–9.6% acetonitrile over 20 min, linear gradient, 1 mLmin−1).
The patterns of the thermal decomposition of 4–8 are shown in Scheme 2. Thermal decomposition of 5, 6, and 8 under conditions analogous to those used for the release of 3 from X-irradiated highly polymerized DNA[8] is illustrated in Figure 2. It can be seen from Figure 2C that 8 and its product d(pGCG) have overlapping retention times in the reversed-phase (RP) HPLC chromatogram; therefore, ion-exchange chromatography was applied to resolve the peaks for 8 and d(pGCG) (Figure 2D).
All dLs shown in Figure 2 (5, 6, and 8) are heat-resistant at pH 5.2 in the absence of a catalyst (see panels b of Figure 2A–C). Furthermore, 5, 6, and 8 readily decompose with release of 3 upon heating under the same conditions but with spermine (10 mM) added. Thus these dLs show decomposition patterns analogous to irradiated calf thymus DNA.[8] All dLs in Figure 2 undergo quantitative decomposition; 3 and its corresponding phosphate fragments are formed in nearly stoichiometric ratios (with the exception of d(Cp) for 5). These results show that the yield of 3, released upon catalytic treatment of dL-containing oligonucleotides, correlates well with the yield of its dL precursor.
The 5′-terminal dL 4 undergoes about 80% conversion upon heating for 20 min at 90°C with spermine (10 MM) at pH 5.2 (Figure 3Aa), while its 3′-terminal analogue 7 is stable under these conditions (results not shown). Significant decomposition of 7 required more prolonged heating at pH 6.8 (30 min or more, Figure 3B,a. The optical absorption of the butenolides 9 and 10 (see Scheme 2) supposedly formed upon decomposition of 4 and 7 respectively, is apparently too weak at 254 nm to be detected under the conditions of this study.
The total yield of dL was estimated for the X-irradiated films of d(pCGCGp) (Figure 4). All four types of dL formed from d(pCGCGp) release 3 upon thermal catalytic decomposition; the yield of 3 therefore, is assumed to correlate with the total yield of dL. This assumption is justified by the quantitative release of 3 upon decomposition of phosphorylated dL-containing tetramers and by the stability of 3 under the experimental conditions employed (at slightly acidic pH values).[8] The inset in Figure 4 shows the dose dependence for the formation of 3; the radiation yield of 3, 0.0103 ± 0.0004 μmolJ−1, was obtained from the slope of the linear fit to the experimental data. This yield is 3.4-fold lower than the yield of 3 obtained for the X-irradiated films of calf thymus DNA[8] subjected to analogous post-irradiation treatment. This degree of variation is not surprising given the large differences in the primary and tertiary structures of d-(pCGCGp) when compared with genomic DNA.
Figure 4.
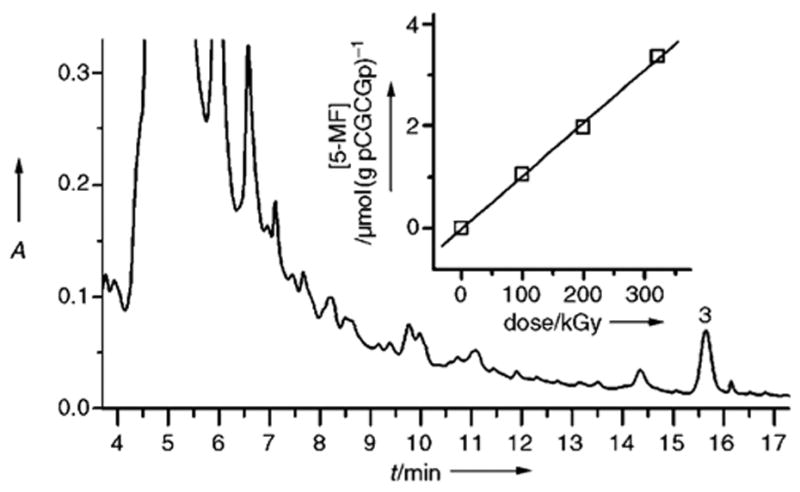
Release of 3 from the solution of the X-irradiated d-(pCGCGp) film. The reaction mixture containing ammonium acetate buffer (40 mM), pH 6.8, and spermine (10 mM) was heated 25 min at 90°C. The inset shows the dose dependence of the release of 3. HPLC conditions: Gemini C18 4.6 mm 0 250 mm analytical column (Phenomenex) washed with 40 mM ammonium acetate as a running phase, with acetonitrile as eluent (4–9.6% acetonitrile over 20 min, gradient type 5, 1 mLmin−1).
In conclusion, we have demonstrated that catalytic decomposition of dL isolated from X-irradiated d(CGCG) and d(pCGCG) films quantitatively produces 3. This finding supports our earlier hypothesis that 1 is the major, perhaps only, precursor of 3 released from X-irradiated highly polymerized DNA.[8] Our data suggest that quantification of the yield of 3 released upon catalytic decomposition of dL can be applied as a method for selective quantitative detection of lesion 1 in DNA.
Experimental Section
Details of the isolation and characterization of dL-containing oligonucleotides are included in the Supporting Information. The product 3 used as a reference compound in HPLC experiments was synthesized according to a published procedure.[13] All the DNA oligonucleotides used in this study were purified by semipreparative RP HPLC. The “dry” films containing the oligonucleotides (≈500 μg) were prepared from aqueous solution (200 μL) dried over saturated NaOH (5% relative humidity) on a microscope glass slide and then subjected to vacuum overnight.
X-irradiation of the films: A Phillips tube (tungsten anode) operated at 55 kV and 20 mA, giving a dose rate of 163 kGyh−1, was used as the X-ray source. The dose rate was measured with radiochromic films (Far West Technology, Inc.). Typically, the DNA films were irradiated to a dose of ≈330 kGy. For the measurements of the radiation yield of 3 released from d(pCGCGp), the d-(pCGCGp) films were irradiated to doses from 100 to 326 kGy. Immediately upon irradiation, the films were dissolved in H2O (200 μL), transferred into microcentrifuge tubes, and stored at −20°C until used.
HPLC analysis: Typically, the reaction mixture (200 μL) containing the oligonucleotide (50–200 μg) was injected. All HPLC experiments were performed at 30°C and pH 6.8, with UV detection at λ = 254 nm. The retention times of the products were determined by coinjection of a corresponding reference compound. The yields of released products were quantified from HPLC peak areas as previously described.[14] For the measurements of the radiation yield of 3 released from d(pCGCGp), the yield of 3 was quantified by co-injection of a known amount of authentic 3.
Thermal decomposition of 2-deoxyribonolactones: Typically, the reaction mixture (200 μL) of the dL-containing tetramer (50 μL), (≈20–50 μM final concentration), sodium acetate (50 mM), and spermine (10 mM) at pH 5.2 was heated for 20 min (unless otherwise stated) at 90°C and then cooled to 4°C. For thermal decomposition without spermine, the spermine was added after the reaction mixture was heated and cooled. These conditions have been optimized in our previous work for the maximum release of 3.[8] As shown herein, the loss of 3 owing to its decomposition and/or side reactions does not exceed 10% under the experimental conditions employed.
Supplementary Material
Footnotes
We thank Prof. David M. Close for helpful discussions. We are grateful for support from PHS Grant 2-RO1-CA32546, awarded by NCI of DHHS, and from Grant CC5956 awarded by Research Corporation.
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- 1.a) Berthet N, Roupioz Y, Constant JF, Kotera M, Lhomme J. Nucleic Acids Res. 2001;29:2725–2732. doi: 10.1093/nar/29.13.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Kroeger KM, Jiang YL, Kow YW, Goodman MF, Greenberg MM. Biochemistry. 2004;43:6723–6733. doi: 10.1021/bi049813g. [DOI] [PubMed] [Google Scholar]; c) Faure V, Constant JF, Dumy P, Saparbaev M. Nucleic Acids Res. 2004;32:2937–2946. doi: 10.1093/nar/gkh622. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Greenberg MM, Weledji YN, Kim J, Bales BC. Biochemistry. 2004;43:8178–8183. doi: 10.1021/bi0496236. [DOI] [PubMed] [Google Scholar]
- 2.a) Kuwabara M, Yoon C, Goyne T, Thederahn T, Sigman DS. Biochemistry. 1986;25:7401–7408. doi: 10.1021/bi00371a023. [DOI] [PubMed] [Google Scholar]; b) Goyne TE, Sigman DS. J Am Chem Soc. 1987;109:2846–2848. [Google Scholar]; c) Meijler MM, Zelenko O, Sigman DS. J Am Chem Soc. 1997;119:1135–1136. [Google Scholar]; d) Chen T, Greenberg MM. J Am Chem Soc. 1998;120:3815–3816. [Google Scholar]; e) Bales BC, Pitie M, Meunier B, Greenberg MM. J Am Chem Soc. 2002;124:9062–9063. doi: 10.1021/ja026970z. [DOI] [PubMed] [Google Scholar]
- 3.a) Bose RN, Moghaddas S, Mazzer PA, Dudones LP, Joudah L, Stroup D. Nucleic Acids Res. 1999;27:2219–2226. doi: 10.1093/nar/27.10.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Pitie M, Bernadou J, Meunier B. J Am Chem Soc. 1995;117:2935–2936. [Google Scholar]; c) Neyhart GA, Cheng CC, Thorp HH. J Am Chem Soc. 1995;117:1463–1471. doi: 10.1021/ja00110a001. [DOI] [PubMed] [Google Scholar]
- 4.Kappen LS, Goldberg IH. Proc Natl Acad Sci USA. 1992;89:6706–6710. doi: 10.1073/pnas.89.15.6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Urata H, Yamamoto K, Akagi M, Hiroaki H, Uesugi S. Biochemistry. 1989;28:9566–9569. doi: 10.1021/bi00451a002. [DOI] [PubMed] [Google Scholar]
- 6.a) Decarroz C, Wagner JR, Cadet J. Free Radical Res Commun. 1987;2:295–301. doi: 10.3109/10715768709065295. [DOI] [PubMed] [Google Scholar]; b) Buchko GW, Cadet J. Can J Chem. 1992;70:1827–1832. [Google Scholar]
- 7.a) Cadet J, Teoule R. Bull Soc Chim Fr. 1975:891–895. [PubMed] [Google Scholar]; b) Dizdaroglu M, Schulte-Frohlinde D, von Sonntag C. Int J Radiat Biol Relat Stud Phys Chem Med. 1977;32:481–483. doi: 10.1080/09553007714551241. [DOI] [PubMed] [Google Scholar]; c) von Sonntag C. The Chemical Basis of Radiation Biology. Taylor and Francis; New York: 1987. [Google Scholar]; d) Shaw AA, Cadet J. Int J Radiat Biol. 1996;70:1–6. doi: 10.1080/095530096145265. [DOI] [PubMed] [Google Scholar]
- 8.Roginskaya M, Bernhard WA, Marion RT, Razskazovskiy Y. Radiat Res. 2005;163:85–89. doi: 10.1667/rr3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sato K, Greenberg MM. J Am Chem Soc. 2005;127:2806–2807. doi: 10.1021/ja0426185. [DOI] [PubMed] [Google Scholar]
- 10.Roupioz Y, Lhomme J, Kotera M. J Am Chem Soc. 2002;124:9129–9135. doi: 10.1021/ja025688p. [DOI] [PubMed] [Google Scholar]
- 11.Oyoshi T, Sugiyama H. J Am Chem Soc. 2000;122:6313–6314. [Google Scholar]
- 12.Zheng Y, Sheppard TL. Chem Res Toxicol. 2004;17:197–207. doi: 10.1021/tx034197v. [DOI] [PubMed] [Google Scholar]
- 13.Grundmann C, Kober E. J Am Chem Soc. 1955;77:2332–2333. [Google Scholar]
- 14.Razskazovskiy Y. Radiat Res. 2003;159:543–549. doi: 10.1667/0033-7587(2003)159[0543:ranaoo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


