Abstract
The use of cocaine during adolescent development could alter the normal growth of brain regions affected by cocaine, specifically the reward system, and impact the adult mesolimbic system. However, there is scant literature aimed at determining whether animals are more vulnerable to the adverse effects of drugs during adolescence. The present study investigated whether cocaine pretreatment in either adolescence or adulthood altered the dopaminergic response to a naturally reinforcing substance in adulthood. To evaluate the responsivity of the mesolimbic system after repeated cocaine, sucrose was offered during the dialysis procedure and dialysates were collected. Regardless of age all saline pretreated rats had significant increases in sucrose-induced extracellular dopamine (DA) levels in the nucleus accumbens septi (NAcc) as compared to baseline levels. Rats pretreated with cocaine as adults also had significant increases in DA levels after sucrose. Interestingly, sucrose intake significantly enhanced DA levels in cocaine pretreated adolescent rats as compared to all other conditions. The results from the present study show that in rats pretreated with cocaine during adolescence there is an enhanced response of the dopaminergic system in animals exposed to a naturally reinforcing substance. Therefore, cocaine exposure during adolescence results in long-term functional changes in the mesolimbic pathway. Future studies need to ascertain the underlying mechanisms and their potential role in cocaine addiction.
Keywords: Dopamine, Sucrose, Microdialysis, Neurochemistry, Nucleus accumbens, Development, Adolescence
1. Introduction
Very little is known about how repeated exposure to drugs of abuse during adolescence alters normal brain development. Given that drug use frequently occurs during adolescence it is important to understand the long-term effects of cocaine use (and other drugs of abuse) during the adolescent period given that drug use during adolescence may compromise the circuitry primed for addiction. The present study investigated whether cocaine pretreatment in adolescent and adult rats produced differences in basal tone as well as in the response of the dopaminergic system to a naturally reinforcing substance.
Cocaine is a psychostimulant which acts by blocking the reuptake of dopamine (DA), norepinephrine (NE), and serotonin (5-HT) in the mesolimbic pathway and elsewhere [45]. Research on the neurobiological mechanisms of drugs of abuse has shown that the mesolimbic pathway (reward system) is activated by cocaine and other drugs of abuse. The mesolimbic system consists of dopaminergic (DAergic) inputs from the ventral tegmental area (VTA) that project to several brain regions including the nucleus accumbens septi (NAcc), hippocampus, prefrontal cortex (PFC), amygdala, septum, olfactory bulb and the bed nucleus of the stria terminalis [18].
Natural reinforcers such as food, water, and the opportunity to mate increase activity of the mesolimbic pathway, and these effects are mediated in part by DA in the NAcc [21,22,43,46,67]. DA is also released into the NAcc when a male rat is presented with a receptive female, and remains elevated throughout the display of sexual behavior [7,19,53,54,75]. Microdialysis studies have shown that natural reinforcers like sucrose [29] and water [27] increase DA in the shell region of the NAcc. Furthermore, licking sucrose solutions increases DA in NAcc in a concentration dependent manner and this sucrose-induced increase in DA is greater than observed with water [29]. Laboratory animals can be trained to self-administer drugs of abuse and natural reinforcers, all of which increase activity in the mesolimbic pathway. Moreover, animals trained to lever-press for electrical stimulation directly to the NAcc or for natural reinforcers such as food or water rapidly extinguish responding when a DA antagonist (spiroperidol) is administered [64].
The administration of cocaine (and other psychostimulants) produces an increase in locomotor activity and stereotyped behavior, behaviors that are thought to be mediated by the mesolimbic and nigrostriatal DA pathways [23]. Repeated exposure to cocaine results in an enhanced behavioral response to a subsequent drug challenge [31]. This effect, termed behavioral sensitization has been implicated in the process of addiction and drug craving [63,71] and in the transition from casual drug use to addiction [63]. It is thought that the long lasting nature of behavioral sensitization could be attributed to the persistent enhanced responsiveness of neural inputs to NAcc, such as DAergic neurons from the VTA and glutamatergic (Glu) neurons from the PFC and basolateral amygdala [57,74,76].
Animals sensitized to a particular drug show increases in locomotor activity after the administration of a different drug of the same class. This phenomenon, cross-sensitization, has been shown with many drugs of abuse and more recently with natural reinforcers [4,5,26,32,58,65]. Animals on a high sugar diet show greater behavioral sensitization when administered amphetamine (AMPH) [4] and cocaine [25] than animals on a control diet (i.e., typical rat chow). Furthermore, animals who show behavioral sensitization to AMPH exhibit sugar-induced hyperactivity (AMPH-sugar cross-sensitization) [5].
To date, most studies investigating the effects of psychostimulants on the mesolimbic system have been undertaken using adult rats. However, the initiation of drug use occurs most frequently during adolescence, a time period in which brain maturation and development are substantial (for review see [69]). In humans, adolescence is a period of transition that ranges from childhood to adulthood. In rats, adolescence is thought to be encompassed by the time period from approximately postnatal days (PND) 28 to PND 42 [70]. However, some features of adolescence could emerge as early as PND 20 in female rats and may last as late as PND 55 in males [49,50].
During the adolescent period neuronal circuits continue to change and develop. One important characteristic of the adolescent brain is the extensive overproduction and subsequent pruning of synapses [30,59]. This overproduction and pruning includes cholinergic, DAergic, serotonergic, GABAergic and adrenergic neurons and receptors [38,37]. Additionally, the ontogenetic profile of DA receptor expression has been characterized in the rat striatum. There is a constant increase in DA receptor densities throughout development which peak at about PND 28–30 [47,48,60,78]. Further evidence shows that striatal DA receptors are overexpressed before the onset of puberty, peak at around PND 40 and then decrease to adult levels [3,73]. It has been found that DA transporter (DAT) expression is highest during adolescence in human striatum [44]. However, animal studies revealed that DAT expression in NAcc and striatum increases with age peaking around PND 60 [17,72]. It is clear that significant developmental changes occur during the adolescent period, however, it is presently unknown how drugs of abuse, specially cocaine, a DAT inhibitor, alter this neurodevelopment.
Investigating both the short and long-term effects of adolescent drug use is an issue of utmost importance because the use of cocaine during adolescence may alter subsequent functioning of the mesolimbic pathway. Therefore, the present study investigated whether cocaine pretreatment during adolescence or adulthood in rats produced differences in basal tone and subsequent responsivity of the DAergic system to a natural reinforcer (i.e., sucrose) in adulthood. The purpose of the study was to determine whether rats pretreated with cocaine as adolescents were more or less responsive to a natural reinforcer as a result of the lasting effects of cocaine administration on the developing brain.
2. Materials and methods
2.1. Subjects
Subjects consisted of thirty-two male Sprague–Dawley rats derived from established breeding pairs in the laboratory at the University of South Florida (Tampa, FL). Date of birth was designated as postnatal day (PND) 0. Litters contained 8–10 pups after culling. No more that one pup per litter was placed in a given treatment condition. The pups remained with their dam and littermates until weaning. Animals were weaned on PND 21 and housed with their same sex littermates with free access to food and water and maintained in a temperature and humidity controlled room on a 12-h light/dark cycle with lights on at 7:00 A.M. All rats were housed in similar housing conditions with two rats per cage. Animals were pretreated at one of two ages: adolescent (PND 35–44) or adult (PND 70–79) then tested as adults (PND 65 and PND 100). All National Institutes for Health (NIH) guidelines for the Care and Use of Laboratory Animals were followed (National Institutes of Health, 1986).
2.2. Procedure
Animals were handled for 3 days prior to pretreatment for 5 min per day. Drug pretreatment consisted of the administration of 20.0 mg/kg cocaine (a dose of cocaine (20.0 mg/kg) which enhances locomotor activity in PND 35 rats compared to adults [15]) or saline (i.p.) once daily for a period of 10 days from PND 35–44 and PND 70–79. On PND 58 or 93, a guide cannula was surgically implanted and all animals were allowed 1 week to recover before in vivo microdialysis on either PND 65 or PND 100. In order to train the rats to drink during the microdialysis procedure, 4 days before microdialysis, rats were put on a limited access water schedule described as follows. At 1000 h the rats were transported to the laboratory, and placed in the Raturn apparatus (Bioanalytical Systems, West Lafayette, Indiana) where they were able to access water for a period of 20 min. All Raturn bowls had a cotton ball attached to the top, immediately before the liquid presentation, 0.5 ml of a banana odorant was placed on the cotton ball that was taped to the top of the Raturn bowl. The banana odorant was used to prime drinking when the bottle was presented. After conditioning to drink in the apparatus, rats were placed back in their home cage and returned to the colony room. Animals had no access to water in the home cage. In vivo microdialysis was performed 21 days after the last injection a time point at which all animals were adults (PND 65 or 100).
2.3. Behavior
Locomotor activity was recorded on days 1, 5 and 10 of the pretreatment period. On these days rats were injected and placed in the locomotor activity apparatus for a total of 45 min. After a 15-min habituation period, subjects received a single i.p. injection of 20.0 mg/kg cocaine or saline and behavior recorded for 30 min post-injection. On all other days rats were injected in their home cages and then returned to the colony room. Both total distance moved (TDM) (cm) and stereotypy were recorded during the testing sessions. The stereotypy rating scale for the behavioral effects of psychomotor stimulants in rats used was as follows; 1. Asleep, 2. Inactive, 3. Inplace activities (grooming), 4. Normal, alert, active, 5. Hyperactive, 6. Slow patterned, 7. Fast patterned, 8. Restricted, 9. Dyskinetic-reactive [20].
2.4. Apparatus
The locomotor activity apparatus consisted of a circular table with a black Plexiglas surface on which a white Plexiglas circular barrier (diameter=101 cm, height=45.72 cm) was placed. A camera connected to analysis software located above the apparatus recorded locomotor activity. The apparatus was located in a dimly lit room away from the animal colony and the door remained closed during all testing sessions. Activity was recorded and analyzed using Ethovision Video Tracking System created by Noldus. This software tracked and recorded the TDM of each animal during the testing session.
For the conditioning phase and microdialysis procedure, animals were placed in a Raturn System (see above). The Raturn System consists of a large plastic round bottom bowl (14″ × 16″). For the conditioning phase a cotton ball with banana odor was attached to the top of the Raturn system. For the microdialysis procedure, the animal was placed inside the apparatus the night before microdialysis, and the microdialysis probe was inserted. Rats were then able to habituate to the environment overnight with ad libitum access to food.
2.5. Surgical procedures and in vivo microdialysis
Animals were anesthetized on either PND 58 or 93 using a ketamine/xylazine cocktail (1.0 and 0.15 mg/kg/ip). An incision was made over the skull and the rat was mounted on a stereotaxic instrument for surgery. Four holes were drilled in the skull (three for skull screws and one for the guide cannula). A 10 mm long 21-gauge stainless-steel guide cannula (Plastics One) was inserted aimed above the NAcc shell (Anterior/Posterior: +1.2 mm, Lateral: 0.8 mm, and Ventral: 2.6 mm relative to bregma and the surface of the level skull) and affixed to the skull with cranioplast. Animals were returned to their homecage and singly housed for a 1 week recovery period. The day before dialysis, the microdialysis probe was inserted through the guide cannula. Microdiaysis probes were lab-made with cellulose hollow fiber (MW 13,000) attached to stainless steel tubing (Plastics One) with a 45 cm length fused silica capillary (internal diameter [I.D.] 76 μm; outside diameter [O.D.] 150 μm) inserted into the cellulose tube. The probe body protruded 3.4 mm from the base of the guide cannula shaft and the effective length (membrane) of the dialysis piece was 2 mm so that the probe reached the NAcc shell. Rats were then placed in a BAS Raturn system bowl overnight for habituation. Inlet tubing was attached to a 2.5 ml Hamilton syringe mounted on a WPI syringe pump (sp3201w) set to a flow rate of 0.1 μl/min overnight. In vivo microdialysis probes with 2 mm membrane tips (O.D. 512; MW cutoff 13 kDA) were perfused continuously with artificial cerebrospinal fluid (136 mM NaCl, 3.7 mM KCl, 1.2 mM CaCl2, 1.0 mM MgCl2, 10.0 mM NaHCO3 at pH=7.4) for 12 h prior to the start of sampling. On either PND 65 or 100, dialysates were collected at a flow rate of 1.0 μl/min in 10-min intervals from the probe outlet silica into tubes containing 2.0 μl of 0.1 M hydrochloric acid (HCl) and immediately frozen. Following 4 baseline samples, animals were allowed access to either water or 0.3 M sucrose solution and sampling continued for an additional 120 min. The concentration of sucrose (0.3 M) was selected based on prior evidence showing this concentration results in the highest levels of DA release in the NAcc [39]. Dialysate samples (12 μl) were stored at −80° C until analyzed.
2.6. Neurochemical analysis
Analysis of dialysate samples was performed with a reverse phase high performance liquid chromatography system (BAS) coupled to an electrochemical detector (HPLC-EC) set to oxidize catecholamines (650 mV). An amperometric detector with a LC-4C carbon working electrode referenced to a Ag/ AgCl electrode was used. Neurochemical analyses included the detection of DA. The mobile phase consisted of 0.04 M sodium acetate, 0.01 M citric acid, 0.05 mM sodium octyl sulfate, 20.911 M disodium EDTA, 0.013 M NaCl and 10% v/v methanol (pH=4.5) set at a flow rate of 50 μl/min. Samples (6 μl) were injected onto a C-18 microbore column for peak separation. Data were recorded and quantified by Rainin Dynamax Software on a Power Macintosh 8500/120.
2.7. Histology
Following probe removal, rats were euthanized via CO2 inhalation. Brains were removed and frozen in 2-methylbutane (−40 °C) and stored at −80 °C. Brains were sliced into 40 μm sections, thaw mounted on slides and stained with cresyl violet. Probe placements were verified histologically for placement in the NAcc shell.
2.8. Design and analysis
DA levels were obtained by collecting dialysates for a time period of 160 min. Baseline (BL) samples were collected during the first 40 min of microdialysis. The subsequent samples (2 h) were all taken after the presentation of the natural reinforcer.
Six separate analyses were performed with total distance moved (TDM), stereotypy, BL DA, DA % change from BL, body weight and liquid consumption as dependant measures. Locomotor activity was measured using TDM and stereotypy as dependent measures. Both TDM and stereotypy were analyzed using a 2 (Age of exposure: Adolescent, Adult) X 2 (Drug: saline, cocaine) X 3 (Day: 1, 5, 10) mixed model ANOVA with time as the repeated measure. BL DA levels were analyzed with a 2 (Age of exposure: PND 35, 60) X 2 (Drug: saline, cocaine) ANOVA. Liquid consumption (ml) was analyzed with a 2 (Age: Adolescent, Adult) X 2 (Drug: saline, cocaine) X 2 (Natural reinforcer: water, sucrose) ANOVA. DA % change from baseline was analyzed using a 2 (Age of exposure: Adolescent, Adult) X 2 (Drug: saline, cocaine) X 2 (Natural reinforcer: water, 0.3 M sucrose) X 16 (Time: 0–160 min) mixed model ANOVA with time as a repeated measure. Body weight (g) was measured each day during limited access water and analyzed using a 2 (Age of exposure: Adolescent, Adult) X 2 (Drug: saline, cocaine) X 4 (Day: 1–4) mixed model ANOVAwith time as a repeated measure. Subsequent analyses were performed to isolate simple effects with appropriate post-hoc analyses. A 5% level of significance was set.
3. Results
3.1. Locomotor activity and stereotypy
Fig. 1 illustrates cocaine-induced locomotor activity across days, shown as both TDM and stereotypy. Fig. 1A shows that TDM varied as a result of drug across days [Day by Drug interaction, F(2,56)=4.6, p<0.05]. All rats given i.p. injections of 20.0 mg/kg cocaine engaged in significantly more TDM on day 1 than on days 5, and 10. These data show that over time cocaine treated rats decreased TMD. As can be seen in Fig. 1B, stereotypy also varied as a result of drug over time [Day by Drug interaction, F(2,56)=14.3, p<0.05]. Rats injected with cocaine significantly increased stereotypic behaviors from day 1 to day 5 to day 10. Cocaine-induced locomotor activity progresses from hyperactivity, where rats show very high TDM to restricted stereotypic behaviors where the rat will remain in place (thus have low TDM scores) and engage in repetitive movements such as gnawing, chewing or licking. Overall, these data show that as a result of repeated cocaine administration there is an increase in repetitive stereotypic movements which may compete with the expression of locomotor activity resulting in less TDM. No differences in TDM or stereotypic behaviors were observed across time in saline injected rats.
Fig. 1.

Illustrates the locomotor activity of rats during the pretreatment period. Rats received 20.0 mg/kg of cocaine or saline for 10 days from ages PND 35–44 or PND 70–79. Locomotor activity was recorded on day 1, day 5 and day 10. A) Shows that the total distance moved (cm) in cocaine treated rats was significantly higher on day 1 than on days 5 and 10. B) Average stereotypy scores from rats injected with cocaine significantly increased from day 1 to day 5 to day 10, showing that repeated cocaine enhanced the behavioral response. * indicates significant difference from cocaine pretreated rats on day 1.
3.2. Baseline DA levels
A 2 (Age of exposure: Adolescent, Adult) X 2 (Drug: saline, cocaine) ANOVA on baseline DA levels revealed a significant Age by Drug interaction [F(1,28)=4.4, p <0.05]. Subsequent analysis using the Fisher's LSD test showed that rats pretreated with cocaine during adulthood had significantly lower baseline DA levels than adult saline controls and adolescent cocaine exposed rats (see Fig. 2). In contrast, no differences in baseline DA levels were detected between rats pretreated with saline or cocaine during adolescence (p>0.05). Fig. 3 shows the time course of DA release (nM) in response to water and 0.3 M sucrose solution. Raw data were normalized and analyzed as percent changed from baseline.
Fig. 2.
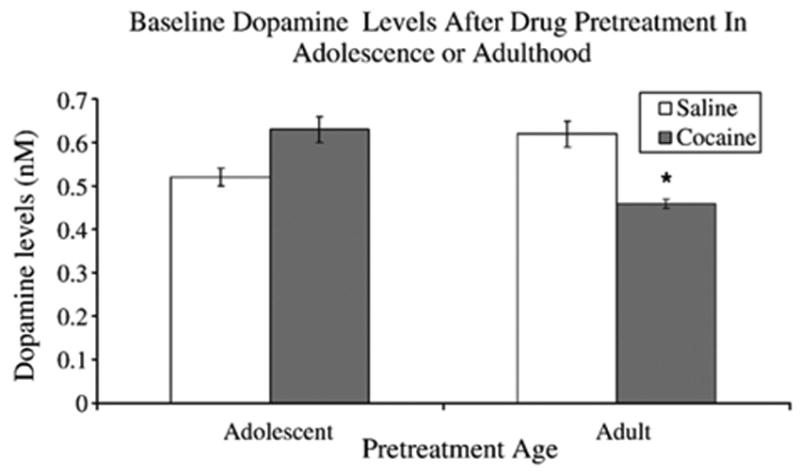
Illustrates baseline DA (nM) levels after drug pretreatment during either adolescence (PND 35–44) or adulthood (PND 70–79) with either saline or 20.0 mg/ kg of cocaine. Rats pretreated with cocaine during adulthood had significantly lower baseline DA levels than saline controls. In addition, cocaine pretreated adults had significantly lower baseline DA levels than rats pretreated with cocaine during adolescence. Interestingly adolescent rats treated with cocaine or saline were not significantly different. Note: 8 rats were used in each condition. * indicates significant difference from adult saline and adolescent cocaine pretreated rats.
Fig. 3.
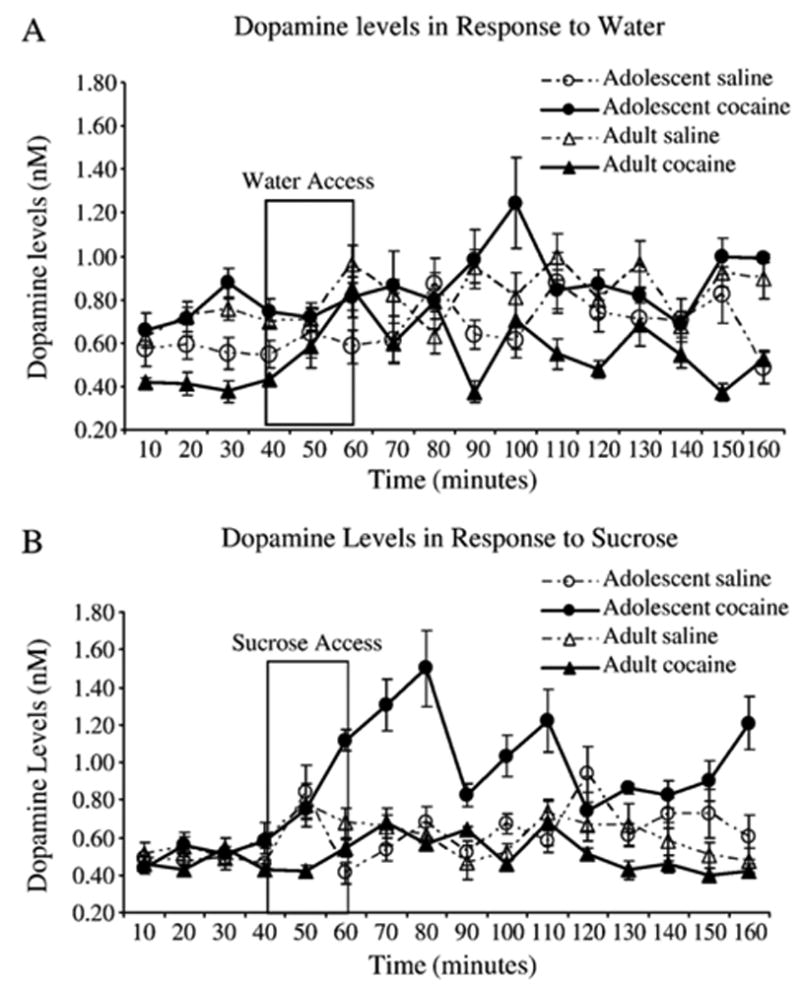
Shows the time course of DA release (nM) in response to A) water and B) 0.3 M sucrose. Water or sucrose was presented from 40–60 min.
3.3. DA percent change from baseline
A 2 (Age: PND 35, 60) X 2 (Drug: saline, cocaine) X 2 (Natural reinforcer: water, 0.3 M sucrose) X 16 (Time: 0–160 min) mixed model ANOVAwith time as a repeated measure revealed a significant interaction between Age and Natural reinforcer [F(1,24)=4.8, p <0.05] with adolescent pretreated rats having significantly higher DA levels in response to sucrose (Mean=150%) than all other groups. In addition, there was a significant interaction of Age, Drug, Natural reinforcer and Time [F(15,360)=1.8, p<0.05]. Dunnett's test revealed that 10 min after access to water or sucrose there was a significant increase in DA from baseline in all rats pretreated as adolescents or adults with saline or cocaine ( p <0.05; see Fig. 4). Interestingly, rats pretreated with cocaine as adolescents had significantly higher DA increases as a result of drinking sucrose compared to all other groups (p<0.05).
Fig. 4.
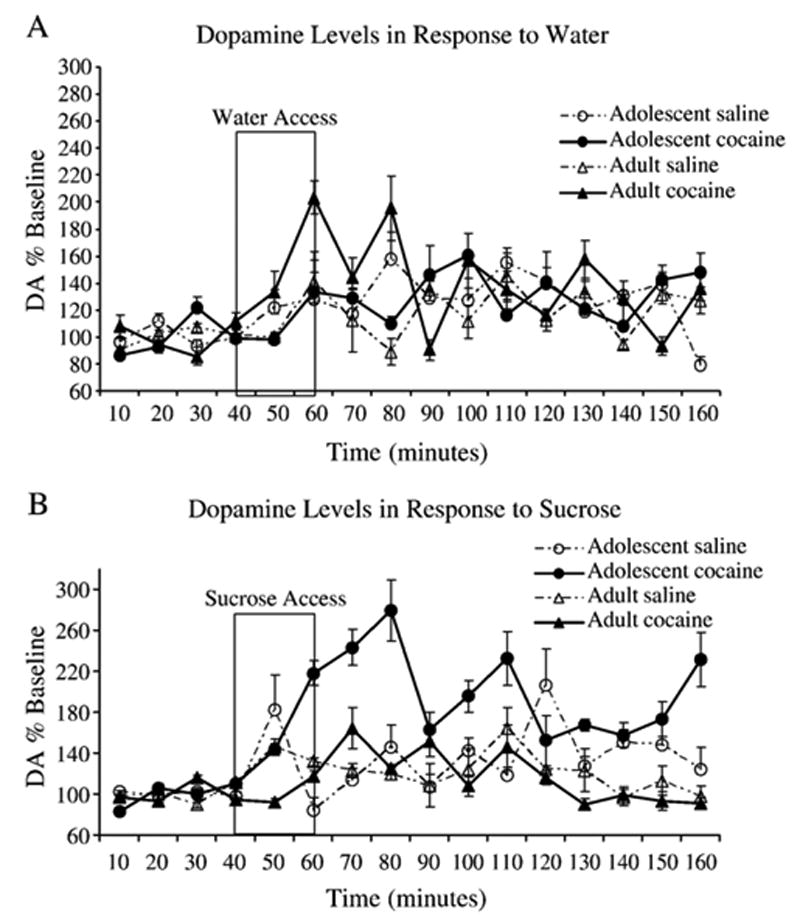
Shows DA levels in response to a natural reinforcer presented by percent change from baseline (y-axis). Figure insert describes pretreatment group as either adolescent saline, adolescent cocaine, adult saline or adult cocaine administration 3 weeks prior to microdialysis. A) DA levels in response to water and B) DA levels in response to 0.3 M sucrose. Adolescent rats pretreated with 20.0 mg/kg of cocaine have significantly higher DA levels in response to sucrose than those pretreated with saline during either adolescence or adulthood and those pretreated with cocaine as adults (p<0.05).
3.4. Liquid consumption
A 2 (Age of exposure: Adolescent, Adult) X 2 (Drug: saline, cocaine) X 2 (Natural reinforcer: water, sucrose) ANOVA on liquid consumption (ml) revealed a significant main effect of natural reinforcer [F(1,24)=145.9, p<0.05]. The Fisher's LSD test showed that independent of age or drug, rats consumed significantly more water (Mean=12.1 ml) than sucrose solution (Mean=3.6 ml) (p<0.05). As shown in Fig. 5, there were no differences in the amount of water, or sucrose consumed based on drug pretreatment or age of pretreatment.
Fig. 5.
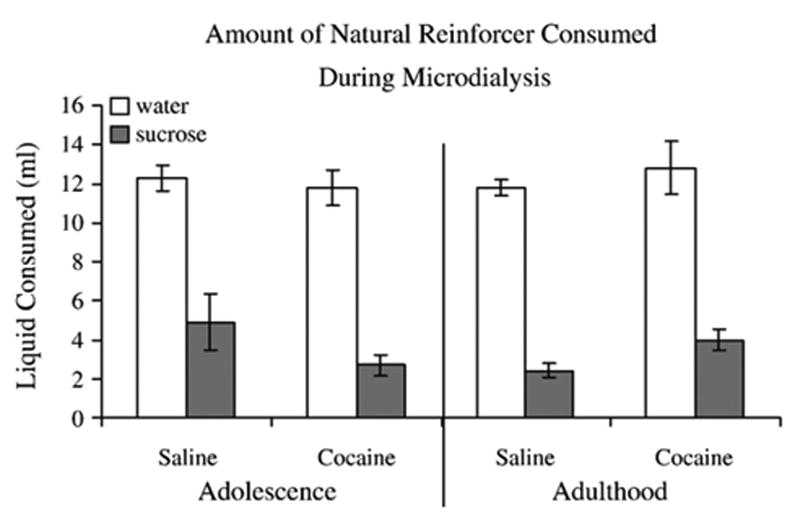
Illustrates the amount of liquid that was consumed by rats for 20 min during microdialysis. Rats consumed significantly more water (Mean=12.1 ml) than sucrose solution (Mean=3.6 ml) ( p<0.05). There were no differences in the amount of water, or sucrose consumed based on drug pretreatment or age of pretreatment.
3.5. Body weight
A 2 (Age of exposure: Adolescent, Adult) X 2 (Drug: saline, cocaine) X 4 (Day: 1–4) mixed model ANOVA with time as a repeated measure revealed a significant main effect of Age [F(1,28) = 191.4, p <0.05] with the adult pretreated rats weighing significantly more (Mean=387.1 g) than the adolescent pretreated rats (Mean=294 g) (p<0.05). In addition, a main effect of day was present with body weight decreasing from day 1 (Mean=346.1 g) to day 4 (Mean=337.9 g) (2.4%) (p<0.05). There was a significant interaction between Day, Age and Drug [F(3,84)=4.5, p<0.05]. The Dunnett's test revealed that cocaine pretreated adults weighed significantly more than saline pretreated adults by day 4 (p<0.05) (see Fig. 6).
Fig. 6.
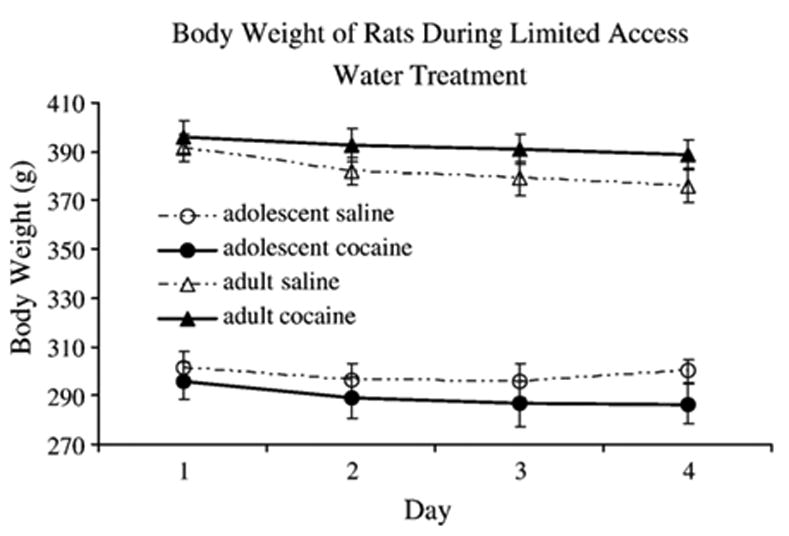
Shows the body weight (g) of rats over the 4 days of limited access water. No differences in body weight were detected as a result of drug pretreatment and rats pretreated as adults weighed significantly more (Mean=387.1 g) than the adolescent pretreated rats (Mean=294 g) (p<0.05).
4. Discussion
The neuroplasticity involved in response to the administration of drugs of abuse has been a major issue for decades. One critical point that we are now confronting is that these studies have primarily been conducted in adult animals without examining the long-term effects that drugs of abuse impose on adolescent neural development. The present study shows that cocaine administration during adolescence alters the response of the reward system to a naturally reinforcing stimulus when exposed animals were tested as adults.
The present study also found that repeated cocaine produced an increase in stereotyped behavior and a decrease in TDM across days an effect that occurred in both adolescent and adult rats. The increase in repetitive stereotypic movements appears to compete with the expression of locomotor activity resulting in less TDM. Therefore we conclude that the administration of 20.0 mg/kg of cocaine for 10 days was effective in inducing behavioral sensitization as indexed in terms of increased stereotypy at both ages.
Basal DA levels in the shell region of the NAcc are affected differentially as a function of age by repeated cocaine use. Specifically, rats pretreated with cocaine as adults had lower baseline DA levels than saline pretreated rats. Basal DA levels (nM concentrations) reported here are consistent with others [6,52,55] however, some report lower levels such as pM [68,77] and fM [13,24,35] concentrations. The decreases observed in basal DA after cocaine administration and withdrawal in the present study are consistent with other studies in the adult literature [39,51,66]. It has been postulated that a decrease in tyrosine hydroxylase levels within the NAcc leads to decreased basal DA levels, and this results in a postsynaptic down-regulation of the DAergic system [39,51,66]. It is interesting that rats pretreated during adolescence with the same dose of cocaine for the same length of time did not have a reduction in basal DA levels. These results suggest that the administration of cocaine differentially produces long-term changes in DA transmission in adolescent and adult rats.
In all conditions there was a significant increase in DA in the NAcc after drinking sucrose solution. These results are consistent with other studies which have shown that sweet solutions (sucrose or saccharine) increase DA the NAcc in a dose dependent manner from 50% up to 300% baseline [8,28,29,42]. The increase in DA as a result of ingesting a sweet solution has been shown to be reduced by conditioned taste aversion to LiCl demonstrating that the release of DA is related to stimulus reward rather than a function of arousal [42]. Therefore, the finding that DA levels increased in response to both water and sucrose in saline pretreated rats was anticipated. Based on previous findings that adult animals on a high sugar diet showed greater behavioral sensitization when administered AMPH [4] and cocaine [25] than animals on a control diet, it was expected that the cocaine pretreated adults in the present study would show a cross-sensitized response. Moreover, it was expected that they would have higher DA levels in response to sucrose than saline pretreated rats, however, this was not the case. DA levels did significantly increase in response to sucrose in the cocaine pretreated adults but this increase was not different from saline pretreated adults, thus there was no enhanced sensitivity of the DAergic system to sucrose.
Interestingly, sucrose intake significantly enhanced DA levels in cocaine pretreated adolescent rats above all other conditions. Other studies have also found that the effects of cocaine during adolescence are long lasting. For example, Brandon and colleagues administered 15.0 mg/kg of cocaine for 5 days and found that adolescent rats pretreated with cocaine showed persistent behavioral sensitization two months after cocaine cessation [11]. In addition, the administration of a high dose of methylphenidate during adolescence, which, like cocaine blocks DATs [34], caused cross-sensitization with cocaine [11]. Adolescent exposure to methylphenidate alters DAergic neurons in the VTA, an effect that is dependent on the length of withdrawal. In early withdrawal from methylphenidate, there was an increase in the excitability of VTA neurons, whereas in late withdrawal when the rats were young adults there was a decrease in DAergic activity [12]. These data suggest that cocaine use or a non-clinical high dose of methylphenidate during adolescence have long lasting effects on the reward system. Furthermore, the present findings demonstrate that this long lasting sensitivity of the mesolimbic system after cocaine use during adolescence extends to palatable substances. Another possibility for the increase in DA in the adolescent rats pretreated with cocaine could be due to the novelty of the sucrose itself since exposure to novelty increases DA in the NAcc shell [61,62]. The fact that adolescent brains are still undergoing development which likely involves the responsiveness of the DAergic system to cocaine could contribute to the enhanced DA levels observed in the present study.
Adolescent neural development is characterized by the extensive overproduction and subsequent pruning of synapses [30,59]. Striatal DA receptor densities peak at PND 40 then decrease to adult levels [3,73]. DAT expression in NAcc and striatum increases with age, peaking at PND 60 [17,72]. Due to increasing numbers of DATs during adolescence, higher reuptake decreases basal DA in the striatum [2] and the NAcc [56] and upregulates cyclic-AMP signaling [1]. The profile of the adolescent mesolimbic system, with lower basal DA levels, increased DA receptors and cAMP levels, could be over-stimulated in the presence of cocaine. Persistent postsynaptic stimulation hyperpolarizes GABAergic neurons and allows for more release of DA into the extracellular space which could in part explain cocaine-induced hyperactivity observed in adolescent rats [15,40,41]. During adolescence glutamatergic projections from PFC to VTA and limbic areas develop (for review see [36]). It is plausible that repeated cocaine during adolescence overstimulates the mesolimbic system causing more synaptic connections of glutamatergic afferents to the VTA. This might explain why there is a stronger response to sucrose in the adolescents pretreated with cocaine. Future studies should examine the dose response relationship of repeated cocaine during adolescence and examine the responsivity of the DAergic system to various natural reinforcers, novel stimuli and drugs of abuse in adulthood. Repeated cocaine during adolescence could result in a circuitry primed for vulnerability to addiction.
The DA response exhibited by cocaine pretreated adults may be explained by the theory of neural sensitization. Repeated cocaine results in plastic changes including reduction of D2-R on Gabaergic efferents [10] and arborizitations of the PFC afferents [14]. During cocaine abstinence a downregulation of activity in the mesolimbic system occurs, resulting in lower basal DA, as is seen in the adult cocaine pretreated condition in the present study. When the system is artificially stimulated by cocaine, DA levels in the NAcc are elevated due to less inhibitory feedback on the VTA from a reduction in D2-R on Gabaergic neurons and the amplification and activation of silent synapses of PFC inputs to VTA, causing sustained stimulation of DA in the NAcc. This is not the case with sucrose as the effects of sucrose on the reward system are more subtle and activate the mesolimbic system indirectly. The activation of the mesolimbic DA system in response to naturally rewarding stimuli provides incentive salience information to the neural correlates of reward-related stimuli and drives wanting or craving [9,33].
Neuronal development is an important factor to consider when investigating the long-term effects of drug abuse. Brain circuits involved with emotion, judgment, and inhibitory control develop during the adolescent period which could increase the risk for substance abuse during development [16]. It is well known that drugs of abuse alter neurotransmitter systems and that the development and fine tuning of these systems occur during adolescence. The results from the present study show that in rats pretreated with cocaine during adolescence there is an enhanced response of the DAergic system to a naturally reinforcing substance therefore; cocaine exposure during adolescence results in long-term functional changes in the mesolimbic pathway. Future studies need to ascertain the underlying mechanisms and their role in the process of addiction.
Acknowledgments
This work was supported by a grant from NIDA: RO1DA14024. The experiments conducted in this manuscript comply with current US law.
References
- 1.Andersen SL. Changes in the second messenger cyclic AMP during development may underlie motoric symptoms in attention deficit/ hyperactivity disorder (ADHD) Behav Brain Res. 2002;130:197–201. doi: 10.1016/s0166-4328(01)00417-x. [DOI] [PubMed] [Google Scholar]
- 2.Andersen SL, Gazzara RA. The ontogeny of apomorphine-induced alterations of neostriatal dopamine release: effects on spontaneous release. J Neurochem. 1993;61:2247–2255. doi: 10.1111/j.1471-4159.1993.tb07466.x. [DOI] [PubMed] [Google Scholar]
- 3.Andersen SL, Thompson AT, Rutstein M, Hostetter JC, Teicher MH. Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse. 2000;37:167–169. doi: 10.1002/1098-2396(200008)37:2<167::AID-SYN11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 4.Avena NM, Hoebel BG. A diet promoting sugar dependency causes behavioral cross-sensitization to a low dose of amphetamine. Neuroscience. 2003;122:17–20. doi: 10.1016/s0306-4522(03)00502-5. [DOI] [PubMed] [Google Scholar]
- 5.Avena NM, Hoebel BG. Amphetamine-sensitized rats show sugar-induced hyperactivity (cross-sensitization) and sugar hyperphagia. Pharmacol Biochem Behav. 2003;74:635–639. doi: 10.1016/s0091-3057(02)01050-x. [DOI] [PubMed] [Google Scholar]
- 6.Badanich KA, Adler KJ, Kirstein CL. Adolescents differ from adults in cocaine conditioned place preference and cocaine-induced dopamine in the nucleus accumbens septi. Eur J Pharmacol. 2006;550:95–106. doi: 10.1016/j.ejphar.2006.08.034. [DOI] [PubMed] [Google Scholar]
- 7.Becker JB, Rudick CN, Jenkins WJ. The role of dopamine in the nucleus accumbens and striatum during sexual behavior in the female rat. J Neurosci. 2001;21:3236–3241. doi: 10.1523/JNEUROSCI.21-09-03236.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bello NT, Hajnal A. Acute methylphenidate treatments reduce sucrose intake in restricted-fed bingeing rats. Brain Res Bull. 2006;70:422–429. doi: 10.1016/j.brainresbull.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 10.Bowers MS, McFarland K, Lake RW, Peterson YK, Lapish CC, Gregory ML, Lanier SM, Kalivas PW. Activator of G protein signaling 3: a gatekeeper of cocaine sensitization and drug seeking. Neuron. 2004;42:269–281. doi: 10.1016/s0896-6273(04)00159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brandon CL, Marinelli M, Baker LK, White FJ. Enhanced reactivity and vulnerability to cocaine following methylphenidate treatment in adolescent rats. Neuropsychopharmacology. 2001;25:651–661. doi: 10.1016/S0893-133X(01)00281-0. [DOI] [PubMed] [Google Scholar]
- 12.Brandon CL, Marinelli M, White FJ. Adolescent exposure to methylphenidate alters the activity of rat midbrain dopamine neurons. Biol Psychiatry. 2003;54:1338–1344. doi: 10.1016/s0006-3223(03)00787-x. [DOI] [PubMed] [Google Scholar]
- 13.Carboni E, Silvagni A, Valentini V, Di CG. Effect of amphetamine, cocaine and depolarization by high potassium on extracellular dopamine in the nucleus accumbens shell of SHR rats. An in vivo microdyalisis study. Neurosci Biobehav Rev. 2003;27:653–659. doi: 10.1016/j.neubiorev.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Carlezon WA, Jr, Nestler EJ. Elevated levels of GluR1 in the midbrain: a trigger for sensitization to drugs of abuse? Trends Neurosci. 2002;25:610–615. doi: 10.1016/s0166-2236(02)02289-0. [DOI] [PubMed] [Google Scholar]
- 15.Catlow BJ, Kirstein CL. Heightened cocaine-induced locomotor activity in adolescent compared to adult female rats. J Psychopharmacol. 2005;19:443–447. doi: 10.1177/0269881105056518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coulter CL, Happe HK, Murrin LC. Dopamine transporter development in postnatal rat striatum: an autoradiographic study with [3H]WIN 35,428. Brain Res Dev Brain Res. 1997;104:55–62. doi: 10.1016/s0165-3806(97)00135-1. [DOI] [PubMed] [Google Scholar]
- 18.Dahlstrom A, Fuxe K. Localization of monoamines in the lower brain stem. Experientia. 1964;20:398–399. doi: 10.1007/BF02147990. [DOI] [PubMed] [Google Scholar]
- 19.Damsma G, Pfaus JG, Wenkstern D, Phillips AG, Fibiger HC. Sexual behavior increases dopamine transmission in the nucleus accumbens and striatum of male rats: comparison with novelty and locomotion. Behav Neurosci. 1992;106:181–191. doi: 10.1037//0735-7044.106.1.181. [DOI] [PubMed] [Google Scholar]
- 20.Ellinwood EH, Jr, Balster RL. Rating the behavioral effects of amphetamine. Eur J Pharmacol. 1974;28:35–41. doi: 10.1016/0014-2999(74)90109-5. [DOI] [PubMed] [Google Scholar]
- 21.Everitt BJ, Wolf ME. Psychomotor stimulant addiction: a neural systems perspective. J Neurosci. 2002;22:3312–3320. doi: 10.1523/JNEUROSCI.22-09-03312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fibiger HC, Nomikos GG, Pfaus JG, Damsma G. Sexual behavior, eating and mesolimbic dopamine. Clin Neuropharmacol. 1992;15(Suppl 1 Pt A):566A–567A. doi: 10.1097/00002826-199201001-00294. [DOI] [PubMed] [Google Scholar]
- 23.Fontana D, Post RM, Weiss SR, Pert A. The role of D1 and D2 dopamine receptors in the acquisition and expression of cocaine-induced conditioned increases in locomotor behavior. Behav Pharmacol. 1993;4:375–387. [PubMed] [Google Scholar]
- 24.Gerrits MA, Petromilli P, Westenberg HG, Di CG, van Ree JM. Decrease in basal dopamine levels in the nucleus accumbens shell during daily drug-seeking behaviour in rats. Brain Res. 2002;924:141–150. doi: 10.1016/s0006-8993(01)03105-5. [DOI] [PubMed] [Google Scholar]
- 25.Gosnell BA. Sucrose intake enhances behavioral sensitization produced by cocaine. Brain Res. 2005;1031:194–201. doi: 10.1016/j.brainres.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 26.Greenberg BD, Segal DS. Acute and chronic behavioral interactions between phencyclidine (PCP) and amphetamine: evidence for a dopaminergic role in some PCP-induced behaviors. Pharmacol Biochem Behav. 1985;23:99–105. doi: 10.1016/0091-3057(85)90137-6. [DOI] [PubMed] [Google Scholar]
- 27.Guion ED, Kirstein CL. The effects of water-odor preference conditioning in the preadolescent nucleus accumbens septi. Dev Psychobiol. 2001;38:46–55. doi: 10.1002/1098-2302(2001)38:1<46::aid-dev4>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 28.Hajnal A, Norgren R. Repeated access to sucrose augments dopamine turnover in the nucleus accumbens. NeuroReport. 2002;13:2213–2216. doi: 10.1097/00001756-200212030-00010. [DOI] [PubMed] [Google Scholar]
- 29.Hajnal A, Smith GP, Norgren R. Oral sucrose stimulation increases accumbens dopamine in the rat. Am J Physiol, Regul Integr Comp Physiol. 2004;286:R31–R37. doi: 10.1152/ajpregu.00282.2003. [DOI] [PubMed] [Google Scholar]
- 30.Huttenlocher PR. Synapse elimination and plasticity in developing human cerebral cortex. Am J Ment Defic. 1984;88:488–496. [PubMed] [Google Scholar]
- 31.Kalivas PW, Duffy P. Similar effects of daily cocaine and stress on mesocorticolimbic dopamine neurotransmission in the rat. Biol Psychiatry. 1989;25:913–928. doi: 10.1016/0006-3223(89)90271-0. [DOI] [PubMed] [Google Scholar]
- 32.Kalivas PW, Weber B. Amphetamine injection into the ventral mesencephalon sensitizes rats to peripheral amphetamine and cocaine. J Pharmacol Exp Ther. 1988;245:1095–1102. [PubMed] [Google Scholar]
- 33.Koob GF. Neurobiological mechanisms in cocaine and opiate dependence. Res Publ Assoc Res Nerv Ment Dis. 1992;70:79–92. [PubMed] [Google Scholar]
- 34.Kuczenski R, Segal DS. Effects of methylphenidate on extracellular dopamine, serotonin, and norepinephrine: comparison with amphetamine. J Neurochem. 1997;68:2032–2037. doi: 10.1046/j.1471-4159.1997.68052032.x. [DOI] [PubMed] [Google Scholar]
- 35.Lecca D, Piras G, Driscoll P, Giorgi O, Corda MG. A differential activation of dopamine output in the shell and core of the nucleus accumbens is associated with the motor responses to addictive drugs: a brain dialysis study in Roman high- and low-avoidance rats. Neuropharmacology. 2004;46:688–699. doi: 10.1016/j.neuropharm.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 36.Lewis DA. Development of the prefrontal cortex during adolescence: insights into vulnerable neural circuits in schizophrenia. Neuropsychopharmacology. 1997;16:385–398. doi: 10.1016/S0893-133X(96)00277-1. [DOI] [PubMed] [Google Scholar]
- 37.Lidow MS, Rakic P. Scheduling of monoaminergic neurotransmitter receptor expression in the primate neocortex during postnatal development. Cereb Cortex. 1992;2:401–416. doi: 10.1093/cercor/2.5.401. [DOI] [PubMed] [Google Scholar]
- 38.Lidow MS, Goldman-Rakic PS, Rakic P. Synchronized overproduction of neurotransmitter receptors in diverse regions of the primate cerebral cortex. Proc Natl Acad Sci U S A. 1991;88:10218–10221. doi: 10.1073/pnas.88.22.10218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maisonneuve IM, Ho A, Kreek MJ. Chronic administration of a cocaine “binge” alters basal extracellular levels in male rats: an in vivo microdialysis study. J Pharmacol Exp Ther. 1995;272:652–657. [PubMed] [Google Scholar]
- 40.Maldonado AM, Kirstein CL. Cocaine-induced locomotor activity is increased by prior handling in adolescent but not adult female rats. Physiol Behav. 2005;86:568–572. doi: 10.1016/j.physbeh.2005.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maldonado AM, Kirstein CL. Handling alters cocaine-induced activity in adolescent but not adult male rats. Physiol Behav. 2005;84:321–326. doi: 10.1016/j.physbeh.2004.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mark GP, Blander DS, Hoebel BG. A conditioned stimulus decreases extracellular dopamine in the nucleus accumbens after the development of a learned taste aversion. Brain Res. 1991;551:308–310. doi: 10.1016/0006-8993(91)90946-s. [DOI] [PubMed] [Google Scholar]
- 43.Mark GP, Rada P, Pothos E, Hoebel BG. Effects of feeding and drinking on acetylcholine release in the nucleus accumbens, striatum, and hippocampus of freely behaving rats. J Neurochem. 1992;58:2269–2274. doi: 10.1111/j.1471-4159.1992.tb10973.x. [DOI] [PubMed] [Google Scholar]
- 44.Meng SZ, Ozawa Y, Itoh M, Takashima S. Developmental and age-related changes of dopamine transporter, and dopamine D1 and D2 receptors in human basal ganglia. Brain Res. 1999;843:136–144. doi: 10.1016/s0006-8993(99)01933-2. [DOI] [PubMed] [Google Scholar]
- 45.Miller NS, Gold MS, Millman RB. Cocaine: general characteristics, abuse, and addiction. N Y State J Med. 1989;89:390–395. [PubMed] [Google Scholar]
- 46.Mitchell JB, Gratton A. Involvement of mesolimbic dopamine neurons in sexual behaviors: implications for the neurobiology of motivation. Rev Neurosci. 1994;5:317–329. doi: 10.1515/revneuro.1994.5.4.317. [DOI] [PubMed] [Google Scholar]
- 47.Murrin LC, Zeng W. Postnatal ontogeny of dopamine D2 receptors in rat striatum. Biochem Pharmacol. 1986;35:1159–1162. doi: 10.1016/0006-2952(86)90154-1. [DOI] [PubMed] [Google Scholar]
- 48.Murrin LC, Zeng WY. Ontogeny of dopamine D1 receptors in rat forebrain: a quantitative autoradiographic study. Brain Res Dev Brain Res. 1990;57:7–13. doi: 10.1016/0165-3806(90)90178-2. [DOI] [PubMed] [Google Scholar]
- 49.Odell WD, Swerdloff RS. Etiologies of sexual maturation: a model system based on the sexually maturing rat. Recent Prog Horm Res. 1976;32:245–288. doi: 10.1016/b978-0-12-571132-6.50017-8. [DOI] [PubMed] [Google Scholar]
- 50.Ojeda SR, Urbanski HF, Ahmed CE. The onset of female puberty: studies in the rat. Recent Prog Horm Res. 1986;42:385–442. doi: 10.1016/b978-0-12-571142-5.50013-6. [DOI] [PubMed] [Google Scholar]
- 51.Parsons LH, Smith AD, Justice JB., Jr Basal extracellular dopamine is decreased in the rat nucleus accumbens during abstinence from chronic cocaine. Synapse. 1991;9:60–65. doi: 10.1002/syn.890090109. [DOI] [PubMed] [Google Scholar]
- 52.Peleg-Raibstein D, Feldon J. Effects of dorsal and ventral hippocampal NMDA stimulation on nucleus accumbens core and shell dopamine release. Neuropharmacology. 2006;51:947–957. doi: 10.1016/j.neuropharm.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 53.Pfaus JG, Phillips AG. Role of dopamine in anticipatory and consummatory aspects of sexual behavior in the male rat. Behav Neurosci. 1991;105:727–743. doi: 10.1037//0735-7044.105.5.727. [DOI] [PubMed] [Google Scholar]
- 54.Pfaus JG, Damsma G, Nomikos GG, Wenkstern DG, Blaha CD, Phillips AG, Fibiger HC. Sexual behavior enhances central dopamine transmission in the male rat. Brain Res. 1990;530:345–348. doi: 10.1016/0006-8993(90)91309-5. [DOI] [PubMed] [Google Scholar]
- 55.Philpot R, Kirstein C. Developmental differences in the accumbal dopaminergic response to repeated ethanol exposure. Ann NY Acad Sci. 2004;1021:422–426. doi: 10.1196/annals.1308.056. [DOI] [PubMed] [Google Scholar]
- 56.Philpot RM, Kirstein CL. Repeated cocaine exposure: effects on catecholamines in the nucleus accumbens septi of periadolescent animals. Pharmacol Biochem Behav. 1999;62:465–472. doi: 10.1016/s0091-3057(98)00198-1. [DOI] [PubMed] [Google Scholar]
- 57.Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Brain Res Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- 58.Pierce RC, Kalivas PW. Amphetamine produces sensitized increases in locomotion and extracellular dopamine preferentially in the nucleus accumbens shell of rats administered repeated cocaine. J Pharmacol Exp Ther. 1995;275:1019–1029. [PubMed] [Google Scholar]
- 59.Rakic P, Bourgeois JP, Goldman-Rakic PS. Synaptic development of the cerebral cortex: implications for learning, memory, and mental illness. Prog Brain Res. 1994;102:227–243. doi: 10.1016/S0079-6123(08)60543-9. [DOI] [PubMed] [Google Scholar]
- 60.Rao PA, Molinoff PB, Joyce JN. Ontogeny of dopamine D1 and D2 receptor subtypes in rat basal ganglia: a quantitative autoradiographic study. Brain Res Dev Brain Res. 1991;60:161–177. doi: 10.1016/0165-3806(91)90045-k. [DOI] [PubMed] [Google Scholar]
- 61.Rebec GV, Christensen JR, Guerra C, Bardo MT. Regional and temporal differences in real-time dopamine efflux in the nucleus accumbens during free-choice novelty. Brain Res. 1997;776:61–67. doi: 10.1016/s0006-8993(97)01004-4. [DOI] [PubMed] [Google Scholar]
- 62.Rebec GV, Grabner CP, Johnson M, Pierce RC, Bardo MT. Transient increases in catecholaminergic activity in medial prefrontal cortex and nucleus accumbens shell during novelty. Neuroscience. 1997;76:707–714. doi: 10.1016/s0306-4522(96)00382-x. [DOI] [PubMed] [Google Scholar]
- 63.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 64.Rolls ET, Rolls BJ, Kelly PH, Shaw SG, Wood RJ, Dale R. The relative attenuation of self-stimulation, eating and drinking produced by dopamine-receptor blockade. Psychopharmacologia. 1974;38:219–230. doi: 10.1007/BF00421374. [DOI] [PubMed] [Google Scholar]
- 65.Schenk S, Snow S, Horger BA. Pre-exposure to amphetamine but not nicotine sensitizes rats to the motor activating effect of cocaine. Psychopharmacology (Berl) 1991;103:62–66. doi: 10.1007/BF02244075. [DOI] [PubMed] [Google Scholar]
- 66.Segal DS, Kuczenski R. Repeated cocaine administration induces behavioral sensitization and corresponding decreased extracellular dopamine responses in caudate and accumbens. Brain Res. 1992;577:351–355. doi: 10.1016/0006-8993(92)90297-m. [DOI] [PubMed] [Google Scholar]
- 67.Smith GP, Schneider LH. Relationships between mesolimbic dopamine function and eating behavior. Ann N Y Acad Sci. 1988;537:254–261. doi: 10.1111/j.1749-6632.1988.tb42111.x. [DOI] [PubMed] [Google Scholar]
- 68.Sorge RE, Stewart J. The effects of long-term chronic buprenorphine treatment on the locomotor and nucleus accumbens dopamine response to acute heroin and cocaine in rats. Pharmacol Biochem Behav. 2006;84:300–305. doi: 10.1016/j.pbb.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 69.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 70.Spear LP, Brake SC. Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Dev Psychobiol. 1983;16:83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- 71.Stewart J, Badiani A. Tolerance and sensitization to the behavioral effects of drugs. Behav Pharmacol. 1993;4:289–312. [PubMed] [Google Scholar]
- 72.Tarazi FI, Tomasini EC, Baldessarini RJ. Postnatal development of dopamine and serotonin transporters in rat caudate–putamen and nucleus accumbens septi. Neurosci Lett. 1998;254:21–24. doi: 10.1016/s0304-3940(98)00644-2. [DOI] [PubMed] [Google Scholar]
- 73.Teicher MH, Andersen SL, Hostetter JC., Jr Evidence for dopamine receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens. Brain Res Dev Brain Res. 1995;89:167–172. doi: 10.1016/0165-3806(95)00109-q. [DOI] [PubMed] [Google Scholar]
- 74.Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- 75.Wenkstern D, Pfaus JG, Fibiger HC. Dopamine transmission increases in the nucleus accumbens of male rats during their first exposure to sexually receptive female rats. Brain Res. 1993;618:41–46. doi: 10.1016/0006-8993(93)90426-n. [DOI] [PubMed] [Google Scholar]
- 76.White FJ, Kalivas PW. Neuroadaptations involved in amphetamine and cocaine addiction. Drug Alcohol Depend. 1998;51:141–153. doi: 10.1016/s0376-8716(98)00072-6. [DOI] [PubMed] [Google Scholar]
- 77.Wu YL, Yoshida M, Emoto H, Tanaka M. Psychological stress selectively increases extracellular dopamine in the ‘shell’, but not in the ‘core’ of the rat nucleus accumbens: a novel dual-needle probe simultaneous microdialysis study. Neurosci Lett. 1999;275:69–72. doi: 10.1016/s0304-3940(99)00747-8. [DOI] [PubMed] [Google Scholar]
- 78.Zeng WY, Hyttel J, Murrin LC. Ontogeny of dopamine D1 receptors in rat striatum. J Neurochem. 1988;50:862–867. doi: 10.1111/j.1471-4159.1988.tb02992.x. [DOI] [PubMed] [Google Scholar]


