Abstract
In DT40 B lymphocytes, Canonical Transient Receptor Potential 7 (TRPC7) functions as a diacylglycerol-activated non-selective cation channel. However, previous work indicated that the non-store-operated Ca2+ entry in this cell type depends upon inositol trisphosphate receptors (IP3R). With the cell-attached configuration oleyl-acetyl-glycerol (OAG) induced single channel activity (75 pS) that was not observed in TRPC7−/− cells, but was rescued by expression of TRPC7 under conditions expected to produce relatively low levels of expression (LowT7TRPC7−/−). A DT40 cell line lacking IP3R (IP3R−/− cells) showed no OAG-induced single-channel activity, but this activity was rescued by transient expression of an IP3R (IP3RIP3R−/−). Single channel properties inLowT7TRPC7−/− orIP3RIP3R−/− DT40 cells were indistinguishable from one another and from wild-type cells. Thus, TRPC7 forms, or is part of, the channel underlying endogenous diacylglycerol-activated currents in DT40 B lymphocytes, and this activity of native TRPC7 requires IP3R. However, with conditions expected to produce greater expression levels, TRPC7 functioned independently of the presence of IP3R. This finding may serve to resolve previously conflicting reports from expression studies of TRPC channels.
Canonical Transient Receptor Potential (TRPC) channels, which belong to the larger superfamily of mammalian TRP channel forming proteins, are among the most important neurotransmitter and hormone-regulated cation channels in non-excitable cells (1;2). Physiologically, TRPC channels function as multifunctional calcium-permeable cation channels that can be activated through the phospholipase C (PLC) pathway. However, whether individual TRPC members function as store-operated Ca2+ (SOC) channels (whose activation is initiated by depletion of Ca2+ stores), and/or non-store-operated Ca2+ (non-SOC) channels following PLC activation, is still a matter of debate. Some of the confusion stems from the fact that, when heterologously expressed, their mode of regulation seems to be strongly dependent on cell type, expression level, or expression environment (discussed in (3)). TRPC7 for instance, a member of the TRPC3/6/7 subfamily, was originally shown to function as a PLC-regulated channel (4), presumably through PLC-derived diacylglycerol (DAG), but subsequent evidence indicated that it could also function as a SOC channel (5). Recent work from our lab demonstrated that these apparently contradictory findings likely resulted from differences in expression conditions (6). In an attempt to determine which of these two behaviors may correspond to the physiological function of native, endogenously expressed TRPC7, we recently utilized targeted homologous recombination to knock out TRPC7 in DT40 B-lymphocytes. We found that knockout of TRPC7 did not significantly affect the store-operated calcium-selective current Icrac, known to be expressed in these cells (7). Rather, in the avian B-cell line, TRPC7 appears to function as a PLC-regulated, DAG-activated non-selective cation channel. However, previous studies from this laboratory (8) and others (9;10) showed that in DT40 cells non-SOC, receptor-regulated cation entry depends in some manner on inositol trisphosphate (IP3) receptors, but the precise role of IP3 receptors (IP3R) in this pathway has not been defined. Because a Ca2+-impermeable mutant of the IP3R failed to rescue Ca2+ entry in IP3R knockout cells, we speculated that this entry might involve plasma membrane IP3R (8). In the present work, we further explored the role of native TRPC7 and IP3R in DT40 cells by characterizing the single-channel properties of the channels underlying non-store-operated, DAG-activated currents, and evaluating its dependence on IP3R expression.
Materials and methods
Cell culture and transfections
Culture of wild-type and mutant (TRPC7−/− and IP3RKO−/−) DT40 B lymphocytes and transient transfections were carried out essentially as described previously (7;8). When indicated, cells were transfected with either rat type 3 IP3 receptor (10 μg/ml), or the human isoform of TRPC7 (10 or 100 μg/ml; see Results) in pcDNA3 vector, or vector alone (pcDNA3, mock-transfected cells), along with EYFP (5 μg) as marker for transfection. Cells were used for single-channel measurements 20–25 h post-transfection. Fluorimetric evaluation of rate of Ba2+ entry in IP3R−/− andIP3RIP3R−/− cells was performed as in (8). In experiments involving transient transfections, measurements were performed on EYFP-positive cells.
Single-channel recording
Single-channel currents were recorded using the patch-clamp technique in the cell-attached configuration (11). Recording pipettes were pulled from borosilicate glass capillaries (WPI, Sarasota, Fl USA) and heat polished to form high-resistance pipettes (~7–10 M ) when filled with pipette solution. Unless otherwise stated, bath and pipette solution was (mM): 140 KCl, 1 MgCl2, 10 glucose, 2 CaCl2, 10 HEPES pH 7.3 adjusted with KOH. Signals were recorded at room temperature (20–22 °C) with an Axopatch 200B amplifier (Axon Instruments, Foster City, Calif., USA), low pass filtered at 2 kHz, sampled at 20 kHz with a Digidata 1200 series interface using pClamp8 software (Axon), and then analyzed using Clampfit 9.2 program (Axon). Single-channel amplitude at each holding potential was estimated as the averaged mean of the Gaussian fits to amplitude histograms of open events from individual patches obtained using the 50% threshold method with Clampfit 9.2. A correction was made for the rise time of the filter (12), so that events shorter than 250 μs (~1.5X filter rise time) were ignored. Distributions of apparent open and closed dwell times were constructed by plotting the logarithm of duration (in ms, 10 bins per decade) versus the square root of the number of events per bin (13), and time constants were estimated by maximum likelihood fitting with sums of exponential functions using Clampfit 9 (Axon). Only patches in which one channel was apparent were included in this analysis, with the absence of simultaneous, superimposed openings, used as an indicator of channel activity likely derived from the activity of one single channel. This assumption is in line with the observation that single-channel activity and closed time constants were virtually identical between separate patches subject to similar experimental conditions. Additionally, the probability of runs of single openings if there were actually two channels present, was estimated according to the equation: P (r≥no) = πno-1 (14), where π= (1 – PO)/(1 – PO/2), and no is the number of single openings. For the patches here analyzed, the maximum probability of more than one channel present was of the order of ≤10−8. However, although unlikely, the possibility that more than one channel, each opening sporadically, generated single channel activity in these patches, cannot be unequivocally ruled out, thus our estimation of apparent closed time constants should be taken as a lower limit for true values. Channel activity is expressed as NPo, the product of the number of channels in the patch (N) and the channel open probability, and was estimated as the ratio between the areas for closed and open points as derived from the all-points histogram. Data are expressed as mean±SEM. The significance of differences between means was established using Student’s t-test for unpaired samples.
Results
In a recent study (7) we reported responses to the synthetic DAG analogue oleyl-acetyl-glycerol (OAG) in wild-type DT40 B lymphocytes, by using conditions that provide strong inhibition of protein kinase C (PKC). OAG-induced whole cell currents exhibited characteristics similar to those obtained upon PLC stimulation and, of significance, both PLC- and OAG-dependent whole cell currents were absent in TRPC7 knockout (TRPC7−/−) DT40 cells, suggesting that the DAG-sensitive activity is mediated by channels containing native, endogenously expressed TRPC7. A more thorough evaluation of this interpretation requires electrophysiological characterization of the endogenous channels responsible for DAG-sensitive currents. In the present work, we carried out current measurements in the cell-attached configuration to obtain information on OAG-induced currents at the single-channel level. After establishing the cell-attached configuration, current measurements were obtained for several minutes at membrane potentials between −60 and +60 mV. Under these conditions, i.e., in the absence of stimulation, there was no detectable single-channel activity (0 out of 8 cells). Similarly, application of 100 μM OAG to the bath resulted in no channel activity (0 out of 7 cells; Fig. 1A, top trace). However, following inhibition of PKC (3 min pre-incubation with 1 μM Gö6976 and 0.5 μM calphostin C), challenging the cells with 100 μM OAG resulted in appearance of single channel activity (8 out of 12; Fig. 1A, bottom trace). The mean amplitude of open events was 4.5 ±0.13 pA at +60 mV (all-points histogram is shown in Fig. 1B), giving a single channel conductance of 75 ± 2.1 pS. The amplitude of single-channel inward or outward currents was not significantly affected by replacing K+ in the pipette solution by either Cs+ or Na+ (140 mM Cs+ or Na+/10 mM Cl−) and displayed a reversal potential close to 0 mV, consistent with a nonselective cation channel (not shown). Channel activity (NPo) was 0.09 ± 0.01. Single-channel activity was not observed in the absence of PKC inhibitors (0 out of 7), nor following addition of PKC inhibitors alone (0 out of 7), indicating that channel activity was dependent on OAG and sensitive to inhibition by PKC. To obtain information on the kinetics of single-channel behavior, we analyzed the dwell-time distributions for apparent open and closed times. These were well fitted by the sum of two and three exponential components, for open and closed states, respectively, indicating the existence of at least two open (O1 and O2) and three closed (C1–C3) channel states (Fig. 1C). Table 1 summarizes the time constants and corresponding relative areas for these states. Open-time distributions showed a major, highly populated component with a time constant of 1.34 ± 0.18 ms (~81% of events) and a longer component with a time constant of 7.44 ± 0.79 ms (~19% of events). Closed-time distributions showed three components, of which the shortest (C1, mean duration ~0.88 ms) and the longest (C3, mean duration ~170 ms) were relatively equally populated. These estimated parameters will be used to compare single-channel properties under the conditions described below.
Figure 1. OAG-induced single channel activity in Wild-type DT40 B lymphocytes.
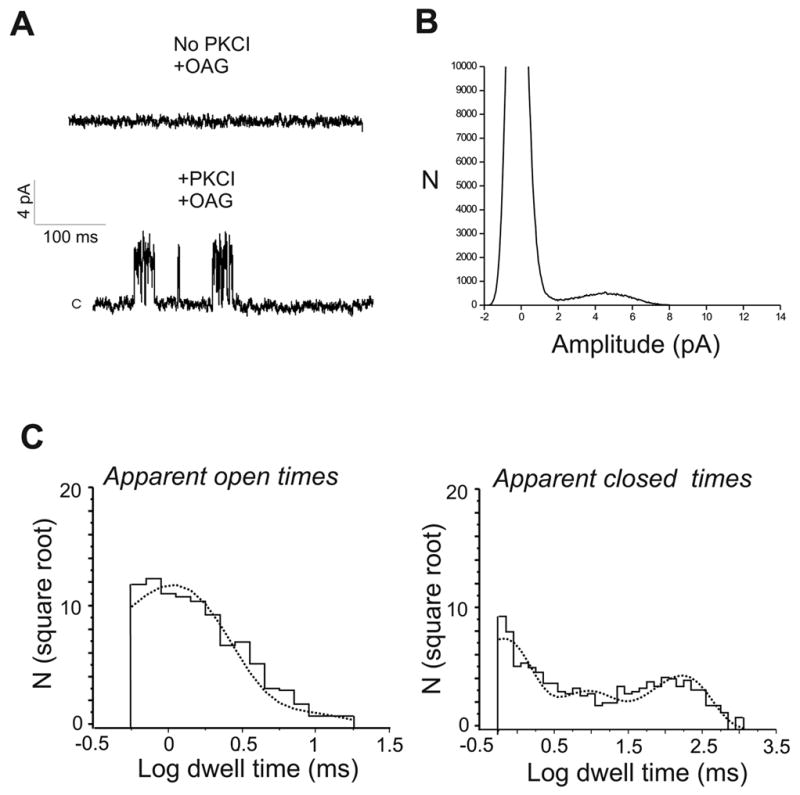
Cells were exposed to 100 μM of the diacylglycerol analog OAG in the absence (top trace in A) or presence (bottom trace in A) of the PKC inhibitors (PKCI) Gö6976 (1 μM) and calphostin C (0.5 μM), which were added 3 min before OAG addition to the bath. Shown are examples of currents recorded in cell-attached patches at a membrane potential of +60 mV. C denotes the closed level. B) All-points amplitude histogram for bottom trace in A. C) Distributions of apparent open and closed times for OAG-induced single-channel activity. Time constants (see Table 1) were estimated by maximum likelihood fitting (dotted trace) with sums of exponential functions using pCLAMP9.
Table 1. Time constants for distributions of apparent open and closed times.
Time constants (in ms) for OAG-induced single channel activity in the different cell lines were obtained by fitting histograms of dwell-time distributions for apparent open and closed times with the sum of two and three exponential components, respectively.
| State | Wild-typea | LowT7TRPC7−/−a | IP3RIP3R−/−a | HighT7IP3R−/−b |
|---|---|---|---|---|
| O1 | 1.33±0.18 (0.81±0.04) | 1.34±0.2 (0.81±0.05) | 1.26±0.21 (0.80±0.05) | 0.58±0.16* (0.94±0.02) |
| O2 | 7.44±0.79 (0.19±0.03) | 8.27±0.77 (0.19±0.06) | 8.47±0.83 (0.20±0.04) | 6.42±1.14 (0.06±0.02) |
| C1 | 0.88±0.12 (0.46±0.04) | 0.72±0.11 (0.42±0.04) | 0.72±0.19 (0.43±0.06) | 0.88±0.22 (0.35±0.02) |
| C2 | 14.37±2.72 (0.14±0.02) | 13.07±2.00 (0.18±0.02) | 14.45±2.85 (0.17±0.06) | 8.99±1.27 (0.46±0.02) |
| C3 | 171.34±23.43 (0.39±0.03) | 179.91±17.88 (0.40±0.04) | 146.33±20.55 (0.39±0.02) | 54.01±9.14** (0.19±0.03) |
Values in parentheses indicate the relative area of distribution of the open or closed time constant. Given are mean ± S.E.M. (n=4).
P<0.01 and **P<0.006, for the difference between these values and the corresponding values in wild-type,LowT7TRPC7−/− orIP3RIP3R−/− cells.
Values correspond to OAG-induced single channel activity in cells pre-treated with PKC inhibitors
Values correspond to OAG-induced single channel activity in cells not treated with PKC inhibitors. O: open; C: closed.
As mentioned above, TRPC7−/− DT40 cells do not exhibit OAG-dependent whole cell currents (7). Consistent with this, OAG-induced single-channel events were not observed in TRPC7−/− cells (0 out of 12 cells), despite the presence of PKC inhibitors, supporting the view that native TRPC7 mediates DAG-sensitive channel activity. We next sought to rescue the effects of TRPC7 deletion by transiently transfecting TRPC7−/− cells with a plasmid encoding for human TRPC7, together with EYFP as transfection marker. In DT40 cells, TRPC3 and 7 expression levels are influenced by simple manipulation of the plasmid concentration used in the transfection reaction. In previous studies (7;15;16) we found that 10 μg/ml of TRPC3 or TRPC7 encoding plasmids under control of the CMV promoter result in comparatively low levels of channel expression. Of importance, for TRPC7, the resulting Ca2+ entry and membrane currents displayed pharmacological and regulatory properties that closely resembled those from wild type cells (7). Transfection of TRPC7−/− cells with TRPC7 encoding plasmid under conditions of presumably low level of expression (LowT7TRPC7−/− cells), did not rescue OAG-induced single channel activity in the absence of PKC inhibitors (0 out of 7 EYFP+ cells; Fig. 2A, top trace). However, when cells were pretreated with PKC inhibitors, OAG-dependent single channel activity was observed (7 out of 12 EYFP+ cells; Fig. 2A, bottom trace). Notably, single channel characteristics closely resembled those from wild-type cells, with a major peak amplitude at + 60 mV of 4.5 ± 0.1 pA (all-points histogram is shown in Fig. 2A), a conductance of 75 ± 1.4 pS, reversal potential near 0 mV and a NPo value of 0.11 ± 0.02 (see also Fig. 4). Distributions of apparent open and closed times were again well fitted by the sum of two and three exponential components, respectively, with open and closed time constants and corresponding frequency probabilities that were not statistically different from those obtained for the wild-type channel (Table 1). TRPC7−/− cells transfected with empty vector did not show OAG-induced channel activity (0 out of 6 EYFP+ cells), despite the presence of PKC inhibitors.
Figure 2. In TRPC7−/− and IP3R−/− DT40 cells, transient expression of human TRPC7 and type 3 rat IP3 receptor, respectively, restores OAG-induced single channel activity.
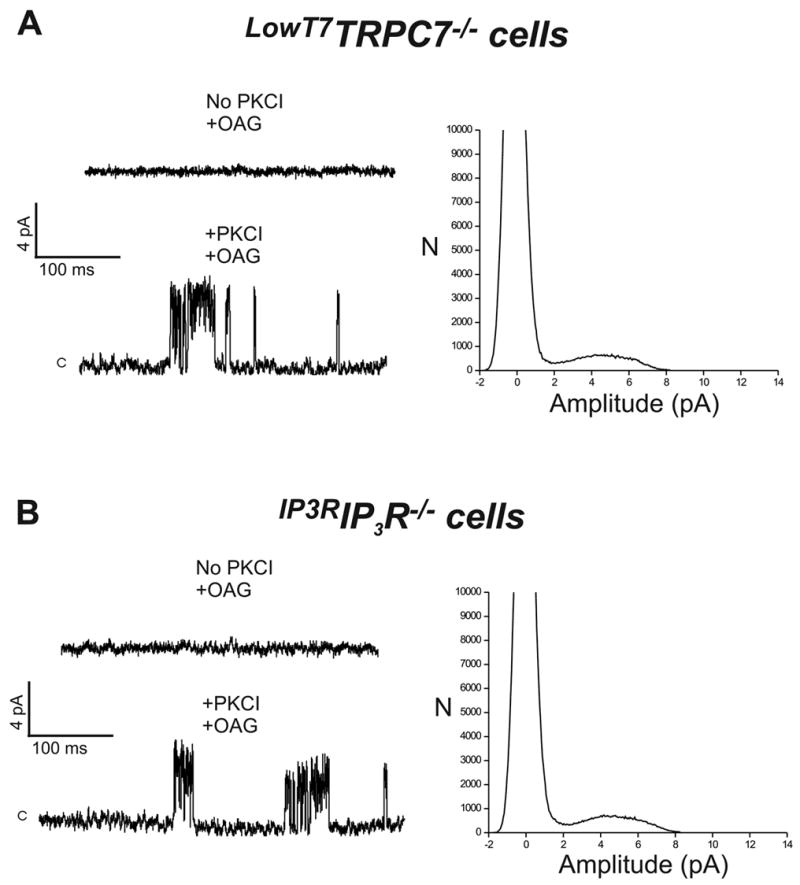
Shown are examples of currents recorded in cell-attached patches (+60 mV) from: A) TRPC7−/− DT40 cells transfected with 10 μg/ml of TRPC7 (designatedLowT7TRPC7−/− cells; see text for details), or B) IP3R−/− DT40 cells transfected with 10 μg/ml of IP3R encoding plasmid (designatedIP3RIP3R−/− cells). Conditions for PKCI and/or OAG treatment were as in Figure 1. All-points amplitude histograms for the traces showing OAG-induced single-channel activity are shown to the right.
Figure 4. Summarized properties of OAG-induced single channel activity.
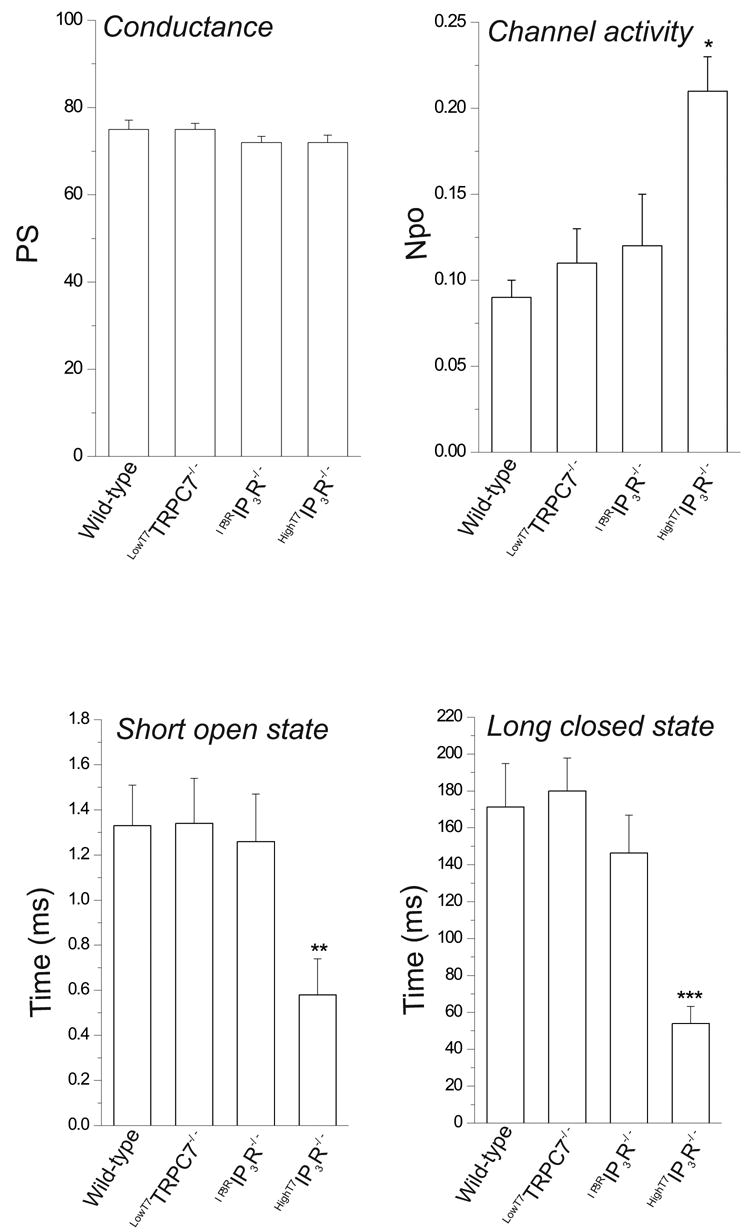
For comparison, values for conductance, activity and time constants for short open and long closed states (O1 and C3 in Table 1) are shown. Values are mean±SEM. n=4–6. *P<0.05; **P<0.01; ***P<0.006.
In a previous study in DT40 cells (see Introduction) we described a non-store-operated, PLC-regulated cation entry that depended in some manner on IP3 receptors (8). We found that a mutant DT40 cell line lacking all three IP3 receptor isoforms (IP3R−/− cells) also lacked this PLC-dependent non-store-operated cation entry, a finding more recently confirmed by others (9;10). If DT40 cells express only a single non-store-operated entry involving TRPC7, one would expect that the lack of non-store-operated cation entry in IP3R−/− cells would be accompanied by absence of the OAG-regulated cation entry pathway. As predicted, neither OAG-induced Ba2+ entry, whole-cell currents or single-channel events were observed in IP3R−/− cells (not shown), regardless of the absence or presence of PKC inhibitors. The simplest interpretation for these findings is that IP3 receptor expression is in some manner required for proper function of native DAG-sensitive TRPC7 channels. To examine this possibility more directly, we attempted to restore OAG-sensitive channel activity in IP3R−/− cells by transient expression of the type 3 isoform of the rat IP3 receptor (designatedIP3RIP3R−/− cells). Expression of the type 3 isoform of the rat IP3 receptor in the IP3R−/− cells restores BCR-dependent, IP3-mediated release of stored Ca2+, an indication not only of appropriate localization and functional expression of this IP3 receptor, but also of proper coupling to the endogenous cell’s signaling machinery ((8) and data not shown). As predicted, in the presence of PKC inhibitors (6 out of 10 EYFP+ cells), but not in their absence (0 out of 7 EYFP+ cells),IP3RIP3R−/− cells showed single channel activity in response to OAG (Fig. 2B), with single channel characteristics indistinguishable from those found inLowT7TRPC7−/− cells as well as in wild-type cells (peak amplitude at + 60 mV of 4.26 ± 0.1 pA, 75 ±1.4 pS, Erev ~ 0 mV, and an NPo value of 0.12 ± 0.03; see also Fig. 4). Time constants derived from distributions of apparent open and closed times, and corresponding frequency probabilities, were comparable to those derived for single-channel activity in wild-type andLowT7TRPC7−/− cells (Table 1). Additionally, inIP3RIP3R−/− cells OAG-induced Ba2+ entry was also restored (initial rate of Ba2+ entry: 0.015 ± 0.005 and 0.047 ± 0.007 ratio units/min, for mock- and IP3R-transfected IP3R−/− cells, respectively; n=6–7 EYFP+ cells, P<0.04). Importantly, expression of the rat IP3 receptor in TRPC7−/− cells did not rescue OAG-dependent channel activity, nor did transient transfection of TRPC7 into IP3R−/− cells (not shown). We have previously shown that in its non-store operated mode, TRPC3, a closely related subfamily member of TRPC7, is able to function independently of IP3R (17). However, we have also shown that for both TRPC3 and TRPC7, channel expression level dramatically influences channel regulation (6;15). Thus, we next examined whether or not the strict requirement on IP3 receptor expression seen here for TRPC7 was related to channel expression conditions. IP3R−/− cells were transfected with TRPC7, but this time using a 10-fold larger quantity (100 μg/ml) of TRPC7 encoding, CMV-driven pcDNA3.1 plasmid, a condition known to result in higher levels of channel expression ((7;15); designatedHighT7IP3R−/− cells). Under these conditions,HighT7IP3R−/− cells exhibited a high level of single channel activity in response to OAG (6 out of 10 EYFP+ cells; Fig. 3A), but now without the necessity of PKC inhibitors. Although single channel conductance (72 ± 1.7 pS) and reversal potential (near 0 mV) were comparable to that estimated for the channel in either wild-type,LowT7TRPC7−/− orIP3RIP3R−/− cells, channel activity was higher (NPo = 0.21 ± 0.02; P<0.04; Fig. 4). Distributions of apparent open and closed times (Fig. 3C and Table 1) showed that the channel inHighT7IP3R−/− cells was characterized by a significant reduction in the duration of the shortest open state, with a time constant of 0.58 ± 0.16 ms, less than half that of wild-type channels (1.33 ± 0.18 ms, P<0.01; Fig. 4); notably, this short lived open state was highly populated (0.94 ± 0.02, P<0.02 compared to the equivalent fractional area in wild-type) and had a relative probability more than twice that of the wild-type channel (P<0.002). Additionally, there was a clear reduction in both duration (−67%, P<0.006) and population (−31%, P<0.03, Table 1) of the long closed state, in keeping with a higher channel activity characterized by short and more frequent openings. Figure 4 summarizes the main properties of OAG-induced single channel activity in the three DT40 cell lines tested and under different expression conditions for TRPC7.
Figure 3. The expression level of TRPC7 influences channel activity and regulation.
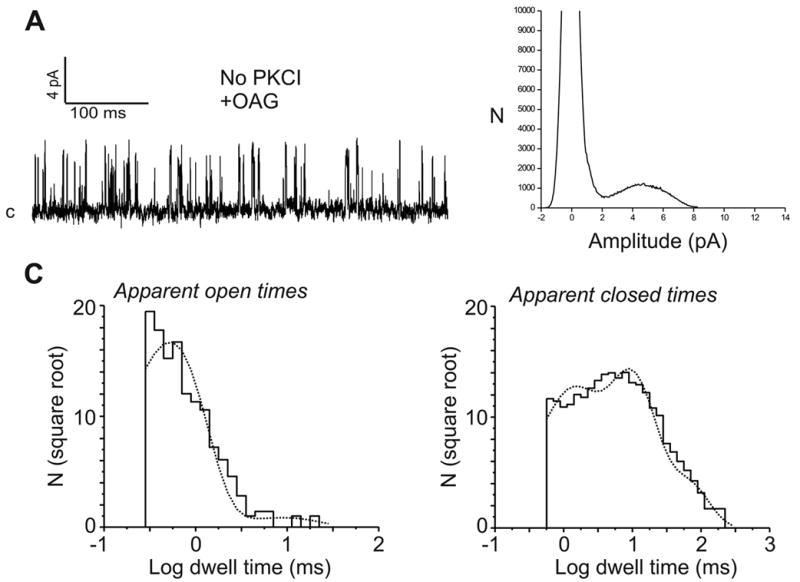
A) Shown is an example of currents recorded in cell-attached patches (+60 mV) from IP3R−/− DT40 cells transfected with 100 μg/ml of TRPC7 encoding plasmid under control of the CMV promoter (designatedHighT7IP3R−/− cells; see text for details). Cells were challenged with OAG (100 μM) without PKCI pre-treatment. B) Corresponding all-points amplitude histogram. C) Distributions of apparent open and closed times (see Table 1).
Discussion
Several novel conclusions can be drawn from the present findings. For the first time, endogenous OAG-sensitive channels in DT40 B lymphocytes have been characterized at the single channel level. There was no evidence of spontaneous channel activity in the absence of stimulation, nor after challenging the cells with OAG; however, OAG-induced single channel activity was observed following inhibition of PKC, suggesting that native channels are negatively regulated through a PKC-dependent mechanism, as was shown for ectopically expressed TRPC3 ((18), and see below). Single channel activity was low, and exhibited characteristics consistent with a nonselective cation channel, whose dwell-time distributions revealed the existence of, at least, two open and three closed states. This channel activity completely disappears after knock-out of the endogenous TRPC7 gene. Importantly, not only is OAG-dependent channel activity fully restored by transfection of TRPC7 into TRPC7−/− cells, but also the biophysical properties of the rescued channel are indistinguishable from those of the wild-type channel. These findings strongly support our hypothesis that TRPC7 forms, or is part of, native DAG-regulated channels in DT40 B lymphocytes.
This is the first demonstration that the activity of native TRPC7 channels has a strict requirement for IP3 receptors. Previous work in DT40 cells already described a dependency of the non-store-operated, PLC-regulated cation entry, on IP3 receptors (8–10). Those studies showed that in a mutant DT40 cell line lacking all three IP3 receptor isoforms (IP3R−/− cells) the PLC-dependent non-store-operated cation entry was deficient, whereas it could be rescued by re-expression of the IP3 receptor. Although the precise role of IP3 receptors in that process was not clear, the simplest interpretation of our findings (8) was that plasma membrane resident IP3 receptors were the channels mediating that process. Here however, we show that the channel rescued by IP3 receptor re-expression in the IP3R−/− cells is indistinguishable from either the channel detected in wild-type cells or the channel rescued by TRPC7 expression in TRPC7−/− cells, suggesting that, rather than plasma membrane IP3 receptors, it is TRPC7 that forms, or is part of, the channel mediating the DAG-sensitive component of the non-store-operated cation entry in DT40 B lymphocytes. Why then is DAG-dependent channel activity deficient in IP3R−/− cells, and what is the basis for its dependency on IP3 receptor expression? Regardless of the controversy derived from functional studies aimed to address the role of IP3 receptors in modulation of TRPC channels (reviewed in (1;19)), all TRPC family members appear capable of associating with the IP3 receptor (20–23), although the physiological significance of the biochemical interactions is uncertain. In our previous study (7), introduction of IP3 into DT40 cells via a patch pipette activated only Icrac, and this current did not depend upon the presence of TRPC7. Therefore, it remains the simplest interpretation of the current findings and data from preceding studies that DAG, and not IP3, is the signal for activation of members of the TRPC3/6/7 subfamily. The requirement for IP3R expression shown here for native TRPC7 channels may therefore reflect a role of the IP3R either in correct targeting of TRPC7 channels, or in correct assembly of TRPC7 channels as part of a signaling complex. It is important to note however, that the present experimental conditions would not likely reveal IP3-induced, IP3 receptor-mediated cationic currents. Thus, we do not rule out the possibility that plasma membrane resident IP3 receptors may also exist as a contributing component of the non-store operated pathway in DT40 cells. If this is the case, a tight reciprocal regulation must exist between TRPC7 and IP3 receptors, so that down-regulation of one path strongly affects the function of the other. Clearly, further studies are required to define not only the exact function and location of the IP3 receptor in the signaling mechanism for TRPC7, but also to define the degree, if any, to which IP3 receptor channels contribute to non-store operated currents in DT40 cells.
In IP3R−/− cells low levels of TRPC7 expression did not rescue OAG-sensitive single-channel currents, but expression of TRPC7 at higher levels (HighT7IP3R−/−) resulted in appearance of OAG-induced channel activity, i.e., independently of IP3 receptor expression. At this higher expression level, channel activation by OAG did not depend on PKC inhibition. Thus, the situation with high expression of TRPC7 recapitulates earlier findings with expression of TRPC3 and TRPC7 in HEK293 cells (4;17), where expression levels are expected to be high (15). How can we interpret these differences in channel behavior and regulation within the context of differences in channel expression level? In the simplest view, one can envision that under low expression conditions channel protein levels more closely correspond to native protein levels, thus allowing TRPC7 to combine with endogenous proteins, limited in number, that either form part of, or act as accessory/regulatory subunits (e.g., IP3 receptor) at the channel complex; the result is a channel complex that more closely matches the native stoichiometry. On the other hand, when higher levels of channel expression are attained, by virtue of the sheer number of TRPC7 molecules, a limited proportion, but significant number of functional channels may be formed. In line with this interpretation, the relationship between OAG-induced whole-cell and single-channel currents gives an estimated number of channels of ~80/cell for wild-type,LowT7TRPC7−/− andIP3RIP3R−/− cells, and ~220/cell forHighT7IP3R−/− cells. As a consequence, high levels of expression of TRPC7 may result in channels whose activity is either uncoupled from or no longer limited by endogenous factors. This interpretation is consistent with the observation that channel activity inHighT7IP3R−/− cells is significantly higher, and characterized by short and more frequent openings, than channel activity in wild-type,LowT7TRPC7−/− orIP3RIP3R−/− cells; this may indicate a role for IP3R in regulating channel gating properties of TRPC7 channels. Also in line with this reasoning is the observation that OAG regulation of single channel activity inHighT7IP3R−/− cells is not affected by PKC, whereas when TRPC7 is expressed at native, low levels (e.g., wild-type,LowT7TRPC7−/−andIP3RIP3R−/− cells) responses to OAG are evident only if PKC is inhibited, suggesting that native channels are negatively regulated by PKC. This may also explain why many cell types are unresponsive to OAG treatment, despite endogenous expression of one or more members of the TRPC3/6/7 group (see (1;19) and references therein), and the use of a PKC inhibitor cocktail may reveal the presence of functional TRPC channels in cell types where they had not been detected previously.
Previous studies on the role of IP3 receptors in modulation of TRPC channels have yielded conflicting results, TRPC3 being an inveterate example (reviewed in (1;19)). Kiselyov et al. (20) reported that in HEK293 cells stably expressing TRPC3, IP3 receptor activated TRPC3 channels in excised patches. However, Trebak et al. (17) reported that in two different clones of TRPC3-expressing HEK293 cells, including the one used in the studies mentioned above, TRPC3 behaved as a DAG-activated channel, whose activity was independent of IP3 or the IP3 receptor. These apparently disparate findings can now be at least somewhat reconciled. Although it appears that when overexpressed in HEK293 cells, TRPC3 functions independently of IP3R, it may, like TRPC7, require IP3R for proper function when natively expressed. Thus, it would not be surprising to find that application of an IP3R to these uncoupled channels would result in an increase in channel activity.
In summary, in the current study we have shown that endogenously expressed TRPC7 channels in DT40 B-lymphocytes are activated by DAG in an IP3R-dependent manner. Increasing the expression of TRPC7 produces a channel with altered kinetic properties and which becomes independent of the IP3 receptor. This underscores the emerging principle that important properties of ion channel function can be lost when the channels are overexpressed at high levels. In addition, these results serve to at least partially reconcile previous disparate findings regarding the relative roles of DAG and IP3R-mediated regulation of TRPC cation channels.
Acknowledgments
The human TRPC7 plasmid was jointly supplied by Christine Murphy and Adrian Wolstenholm of University of Bath, and John Westwick of Novartis, Horsham UK. We thank Drs. David Armstrong and Jerel Yakel for critical reading of the manuscript. This work was supported in part by funds from the intramural program, NIEHS-NIH.
Abbreviations
- TRPC7
canonical transient receptor potential 7
- IP3
inositol 1,4,5-trisphosphate
- IP3R
IP3 receptor
- PLC
phospholipase C
- SOC
store-operated calcium
- DAG
diacylglycerol
- OAG
oleyl-acetyl-glycerol
- Po
open probability
- PKC
protein kinase C
- EYFP
enhanced yellow fluorescent protein
Reference List
- 1.Vazquez G, Wedel BJ, Aziz O, Trebak M, Putney JW., Jr Biochim Biophys Acta. 2004;1742:21–36. doi: 10.1016/j.bbamcr.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 2.Ramsey IS, Delling M, Clapham DE. Annual Review of Physiology. 2006;68:619–647. doi: 10.1146/annurev.physiol.68.040204.100431. [DOI] [PubMed] [Google Scholar]
- 3.Putney JW., Jr Trends Cell Biol. 2004;14:282–286. doi: 10.1016/j.tcb.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Okada T, Inoue R, Yamazaki K, Maeda A, Kurosaki T, Yamakuni T, Tanaka I, Shimizu S, Ikenaka K, Imoto K, Mori Y. J Biol Chem. 1999;274:27359–27370. doi: 10.1074/jbc.274.39.27359. [DOI] [PubMed] [Google Scholar]
- 5.Riccio A, Mattei C, Kelsell RE, Medhurst AD, Calver AR, Randall AD, Davis JB, Benham CD, Pangalos MN. J Biol Chem. 2002;277:12302–12309. doi: 10.1074/jbc.M112313200. [DOI] [PubMed] [Google Scholar]
- 6.Lievremont JP, Bird GS, Putney JW., Jr Am J Physiol Cell Physiol. 2004;287:C1709–C1716. doi: 10.1152/ajpcell.00350.2004. [DOI] [PubMed] [Google Scholar]
- 7.Lievremont JP, Numaga T, Vazquez G, Lemonnier L, Hara Y, Mori E, Trebak M, Moss SE, Bird GS, Mori Y, Putney JW., Jr J Biol Chem. 2005;280:35346–35351. doi: 10.1074/jbc.M507606200. [DOI] [PubMed] [Google Scholar]
- 8.Vazquez G, Wedel BJ, Bird G St J, Joseph SK, Putney JW., Jr EMBO J. 2002;21:4531–4538. doi: 10.1093/emboj/cdf467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morita T, Tanimura A, Nezu A, Kurosaki T, Tojyo Y. Biochem J. 2004;382:793–801. doi: 10.1042/BJ20031970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Rossum DB, Patterson RL, Kiselyov K, Boehning D, Barrow RK, Gill DL, Snyder SH. Proc Natl Acad Sci U S A. 2004;101:2323–2327. doi: 10.1073/pnas.0308565100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Pflüg Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 12.Colquhoun D, Sigworth FJ. Fitting and statistical analysis of single channel records. In: Sakmann B, Neher E, editors. Single-Channel Recording. Plenum Press; New York: 1983. [Google Scholar]
- 13.Sigworth FJ, Sine SM. Biophys J. 1987;52:1047–1054. doi: 10.1016/S0006-3495(87)83298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colquhoun D, Hawkes AG. The principles of the stochastic interpretation of ion-channel mechanism. In: Sakmann B, Neher E, editors. Single-Channel Recording. Plenum Press; New York: 1995. [Google Scholar]
- 15.Vazquez G, Wedel BJ, Trebak M, Bird G St J, Putney JW., Jr J Biol Chem. 2003;278:21649–21654. doi: 10.1074/jbc.M302162200. [DOI] [PubMed] [Google Scholar]
- 16.Trebak M, Bird G St J, McKay RR, Putney JW., Jr J Biol Chem. 2002;277:21617–21623. doi: 10.1074/jbc.M202549200. [DOI] [PubMed] [Google Scholar]
- 17.Trebak M, Bird G St J, McKay RR, Birnbaumer L, Putney JW., Jr J Biol Chem. 2003;278:16244–16252. doi: 10.1074/jbc.M300544200. [DOI] [PubMed] [Google Scholar]
- 18.Trebak M, Hempel N, Wedel BJ, Smyth JT, Bird GS, Putney JW., Jr Mol Pharmacol. 2005;67:558–563. doi: 10.1124/mol.104.007252. [DOI] [PubMed] [Google Scholar]
- 19.Trebak M, Vazquez G, Bird G St J, Putney JW., Jr Cell Calcium. 2003;33:451–461. doi: 10.1016/s0143-4160(03)00056-3. [DOI] [PubMed] [Google Scholar]
- 20.Kiselyov K, Xu X, Mozhayeva G, Kuo T, Pessah I, Mignery G, Zhu X, Birnbaumer L, Muallem S. Nature. 1998;396:478–482. doi: 10.1038/24890. [DOI] [PubMed] [Google Scholar]
- 21.Boulay G, Brown DM, Qin N, Jiang M, Dietrich A, Zhu MX, Chen Z, Birnbaumer M, Mikoshiba K, Birnbaumer L. Proc Nat Acad Sci USA. 1999;96:14955–14960. doi: 10.1073/pnas.96.26.14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mery L, Magnino F, Schmidt K, Krause KH. FEBS Letters. 2001;487:377–383. doi: 10.1016/s0014-5793(00)02362-0. [DOI] [PubMed] [Google Scholar]
- 23.Tang J, Lin Y, Zhang Z, Tikunova S, Birnbaumer L, Zhu MX. J Biol Chem. 2001;276:21303–21310. doi: 10.1074/jbc.M102316200. [DOI] [PMC free article] [PubMed] [Google Scholar]


