Abstract
This study describes a multifunctional envelope-type nano device (MEND) that mimics an envelope-type virus based on a novel packaging strategy. MEND particles contain a DNA core packaged into a lipid envelope modified with an octaarginine peptide. The peptide mediates internalization via macropinocytosis, which avoids lysosomal degradation. MEND-mediated transfection of a luciferase expression plasmid achieved comparable efficiency to adenovirus-mediated transfection, with lower associated cytotoxicity. Furthermore, topical application of MEND particles containing constitutively active bone morphogenetic protein (BMP) type IA receptor (caBmpr1a) gene had a significant impact on hair growth in vivo. These data demonstrate that MEND is a promising non-viral gene delivery system that may provide superior results to existing non-viral gene delivery technologies.
Keywords: non-viral gene delivery system, programmed packaging, octaarginine, multifunctional envelope-type nano device, in vivo topical application, hair growth
Introduction
Several biological barriers limit the efficiency with which non-viral vectors deliver nucleic acids to eukaryotic cells. The most serious intracellular barriers are lysosomal degradation, nucleolytic degradation in the cytosol and inefficient delivery to the nucleus,1–3 whereas extra-cellular barriers include nucleolytic degradation in the serum, recognition by the reticuloendothelial system and nonspecific delivery.4,5 A variety of approaches have been used to overcome these barriers including ligand-mediated targeting of cell surface receptors, pH-sensitive fusogenic peptides that improve cytosolic delivery and nuclear localization signals (NLSs) to enhance nuclear uptake.6–9
Many of the currently available non-viral vectors have some functional devices that enhance their overall efficiency; however, they are still much less efficient than most viral vectors,10 which evolved towards, maximum efficiency through the process of natural selection. Simple mixing of DNA with multiple devices does not guarantee that each device is functional and effective in reducing barriers to efficient gene delivery. In addition, mixing of DNA with multiple devices usually results in the formation of large aggregated complexes,4 which are not efficient vehicles for gene delivery in vivo, which is our ultimate goal. In contrast, smart nanotechnology-based devices offer a promising alternative approach to overcome barriers to effective gene delivery. Such devices can be applied according to a rational strategy, through which nanotechnology is used to assemble and integrate multifunctional devices into a single system.9
For many non-viral gene delivery systems, particles gain entry into the cell through classical endocytosis.2 Unfortunately, classical endocytosis is usually linked to fusion with lysosomes and the ensuing threat of lysosomal degradation.2 Recently, we pointed out that intracellular trafficking can be improved by optimizing the density of a membrane-permeable peptide on the surface of liposomes, which leads to an alternative cellular uptake mechanism.11 In particular, we have shown that a high density of the octaarginine (R8) peptide on the surface of liposomes stimulates macro-pinocytosis, and improves transfection efficiency by avoiding lysosomal degradation.11
Here, we propose a novel packaging approach to assemble multiple devices in a single gene delivery system, so that each device can function at the correct time and correct site according to a program. This program reflects a rational strategy for controlling the intracellular fate of non-viral particles using novel functional devices to avoid biological barriers to efficient gene delivery. The program also considers the topology of the functional devices to achieve maximum activities. We have reported previously the importance of controlling the topology of devices such as the R8 peptide and the fusogenic peptide GALA to exert their functions.4,11–14 Such an approach, called ‘Programmed Packaging’, is expected to develop a non-viral gene delivery system whose efficiency equals that of viral gene delivery systems.
Following this approach, a novel gene delivery system was developed that bears structural similarity to an envelope virus. This system, the multifunctional envelope-type nano device (MEND), includes a polycation-condensed DNA core that facilitates selective delivery to target tissues or organelles15 and a lipid envelope (Figure 1a and b). The envelope can be equipped with various functional devices to improve the system. For example, the R8 peptide was introduced to promote macropinocytosis to avoid lysosomal degradation,11 and the helper lipid dioleoylphosphatidylethanolamine (DOPE) with cholesteryl hemisuccinate (CHEMS) were used to enhance the cytosolic release and nuclear delivery.16,17 Here, we show that MEND particles produced transfection efficiencies equal to adenovirus in cultured cells with minimum cytotoxicity. In addition, topical application of MEND particles containing constitutively active bone morphogenetic protein (BMP) type IA receptor (caBmpr1a) gene altered BMP signaling and hair growth in vivo. This system demonstrates successful use of our proposed approach, namely, to use functional devices in an integrated non-viral gene delivery system to control intracellular trafficking of transfected particles, and to achieve transfection efficiencies equal to viral gene delivery systems.
Figure 1.
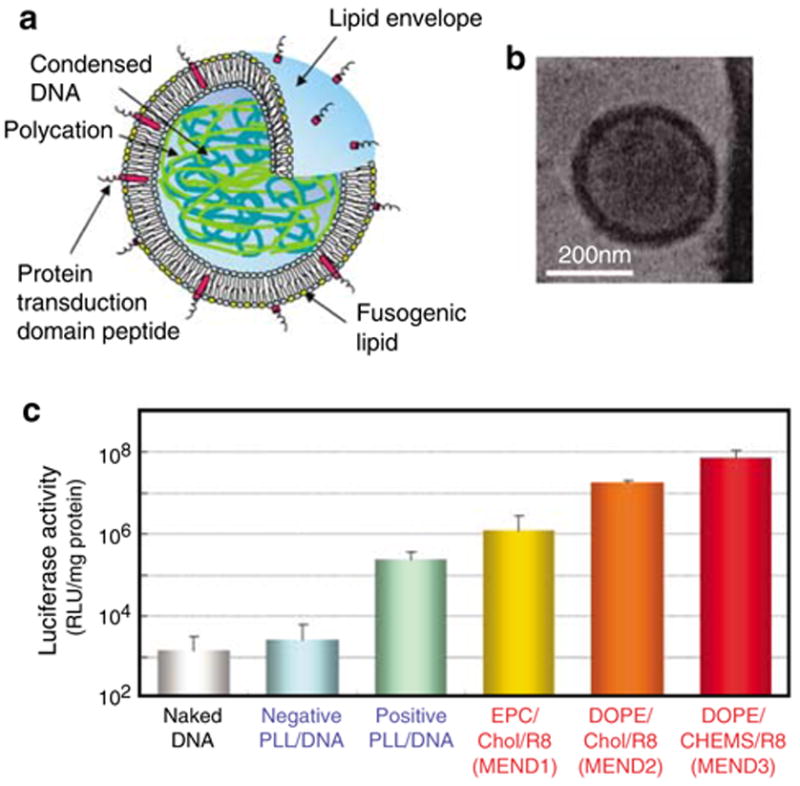
The multifunctional envelope type nano device. (a) Schematic representation of MEND. MEND particles consist of condensed DNA and a lipid envelope containing functional devices including a protein transduction domain peptide that increases intracellular availability, and fusogenic lipids that enhance endosomal escape and facilitate delivery of DNA to the nucleus. (b) Cryo transmission electron microscopic images of MEND particles. DNA-rich MEND3 fractions were isolated by sucrose density gradient centrifugation. (c) Enhanced transfection activities of MEND particles. NIH3T3 cells were transfected with different PLL, DNA-condensed particles or different MEND particles containing luciferase-coding pDNA. Luciferase activity is expressed as relative light units (RLU) per mg of protein. Experiments were performed in triplicate. Error bars show the s.d.
Results
Transfection efficiency of R8-MEND
The following experiments compare the transfection efficiency of several luciferase DNA-containing particles for gene delivery. Particles were prepared with two DNA-condensing protocols and three particle surface coatings as described in Materials and methods. Characterization of different DNA-condensed particles and MENDs is shown in Supplementary Table 1 online. Luciferase activity was measured as an estimate of transfection efficiency. Results are shown in Figure 1c. The results show that positively charged poly-L-lysine (PLL)-condensed DNA produced significantly higher luciferase activity than naked or negatively charged PLL/DNA. However, when negatively charged PLL/ DNA particles were coated with egg phosphatidylcholine (EPC), cholesterol (Chol) and stearyloctaarginine (STR-R8) (MEND1), luciferase activity increased by more than two orders of magnitude. Furthermore, when particles were coated with DOPE/Chol/STR-R8 (MEND2), luciferase activity was approximately four orders of magnitude higher than for uncoated negative PLL/DNA particles. The highest luciferase activity was observed using PLL/DNA particles coated with DOPE/ CHEMS/STR-R8 (MEND3). The high transfection activities of these particles is likely due to efficient cellular uptake of the particles, low frequency of lysosomal degradation, improved cytosolic stability and improved nuclear delivery, all of which may at least in part be mediated by the DOPE/CHEMS/STR-R8 lipid envelope.11,12,16,17
Comparison of MEND3 and adenovirus
Here, the transfection efficiency of MEND3 and adeno-virus were compared using two cell lines that express receptors for adenovirus serotype 5 (i.e., coxsackievirus adenovirus receptor and the integrin receptor).18 In both cell lines, increasing the dose of adenovirus up to 1 × 105 particles/cell resulted in higher transfection efficiency; however, toxicity increased and transfection efficiency decreased at doses higher than 1 × 105 particles/cell. Transfection efficiency with MEND3 equaled the highest transfection efficiency with adenovirus (Figure 2); however, MEND3 produced no detectable cytotoxicity. The low cytotoxicity of MEND3 was confirmed using an MTT assay, which suggested that the cytotoxicity of MEND3 was comparable to that of naked DNA (Supplementary Figure 1 online). In contrast, high doses of Lipofectamine Plus (a commercially available transfection agent) were associated with significant cytotoxicity (Supplementary Figure 1 online). One possible explanation for the low cytotoxicity of MEND3 is that it lacks cationic lipids.19
Figure 2.
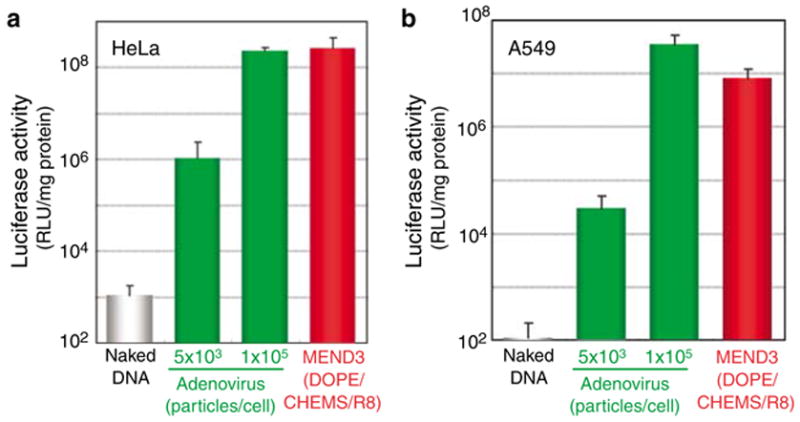
Luciferase activity of MEND3 particles and adenovirus in different cell lines. HeLa (a) or A549 (b) cells were transfected with different doses of adenovirus or with MEND3 particles for a total of 48 h. Luciferase activity is expressed as RLU per mg of protein. Experiments were performed in triplicate. Error bars show the s.d.
Macropinocytosis of R8-modified lipid vesicles
Previous studies suggest that liposomes with a high surface density of R8 enter cells via macropinocytosis.11 Here, the mechanism by which R8-MEND particles are internalized was investigated. First, cells were incubated with R8-MEND1 (EPC/Chol/STR-R8) and transferrin (Tf), a marker for clathrin-mediated endocytosis. The results showed that intracellular DNA only partially colocalized with Tf (Figure 3a), suggesting that classical endocytosis is not the primary mechanism by which R8-MEND is internalized. Next, we examined the uptake of MEND3 (DOPE/CHEMS/STR-R8) in the presence of inhibitors for different endocytic pathways. Hypertonic conditions, which suppress clathrin-mediated endo-cytosis,2,20 only partially inhibited uptake of MEND3 whereas they strongly inhibited uptake of Tf (Figure 3b). Amiloride, which specifically inhibits Na+/H+ exchange required for macropinocytosis,2,20 strongly inhibited the uptake of MEND3, whereas it did not affect the uptake of Tf (Figure 3b). These results confirm that MEND3 particles enter cells primarily via macropinocytosis and that the lipid content of the envelope does not influence the internalization mechanism. After internalization, R8-modified liposomes only partially colocalize with the lysosomal marker LysoSensor,11 indicating that the particles are not highly targeted to lysosomes and thus may escape lysosomal degradation. pH-sensitive fusogenic lipids on MEND3 particles may facilitate delivery of the particle payload to the cytosol or nucleus.16,17
Figure 3.
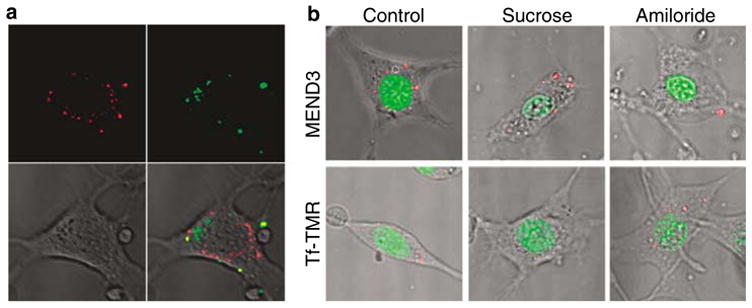
The mechanism of internalization of the R8-MEND. (a) R8-MEND containing FITC-labeled DNA and TMR-Tf were incubated for 30 min and visualized using confocal fluorescence microscopy. Red, Tf; green, DNA. (b) Cells were treated with MEND3 containing rhodamine-labeled pDNA or TMR-Tf (red) in the absence or the presence of sucrose (0.4 M) or amiloride (5 mM) for 1 h. Nuclei were stained with Syto24 (green) and cells were visualized by confocal microscopy.
In vivo applications of MEND3
The topical delivery of genes to hair follicles (HFs) is an attractive approach for the treatment of skin and hair disorders.21–23 Li and Hoffman21 succeeded in delivery of lacZ gene to mouse hair-forming hair matrix cells in the hair follicle bulbs using liposome-based non-viral delivery system, and Saito et al.22 reported gene delivery to hair follicles of mouse skin fragments by adenovirus vector. Domachenko et al.23 also reported delivery of lacZ gene to hair follicle progenitor cells of mouse and human by liposome-based non-viral system. Here, the potential of MEND3 for in vivo gene therapy for hair and skin disorders was tested. MEND3 particles containing lacZ plasmid (MEND3-LacZ) were applied to the dorsal skin of 4-week-old ICR mice, and lacZ activity was scored in tissue sections by a colorimetric assay for β-galactosidase. The results showed β-galactosidase activity in hair follicles 2 weeks following treatment with MEND3-LacZ (Figure 4a, MEND3-LacZ panel). Using this assay, the transfection efficiency was estimated to be approximately 24% (β-galactosidase-positive hair follicles/total hair follicles × 100) (Figure 4b). In contrast, when the same experiment was performed with Lipofectamine-mediated transfection, the transfection efficiency was estimated to be 0.2%. The use of green fluorescence protein (GFP) reporter gene has been established as a marker for visualizing gene expression at different tissues in the whole body by Yang et al.24 MEND3 particles containing a GFP reporter gene produced similar results as MEND3-LacZ, with GFP fluorescence occurring in treated tissue 2 weeks after treatment with MEND3-GFP (Supplementary Figure 2 online). These data demonstrate successful in vivo use of MEND3 particles, and suggest that MEND3 particles may be useful for delivering therapeutic agents to hair follicles.
Figure 4.
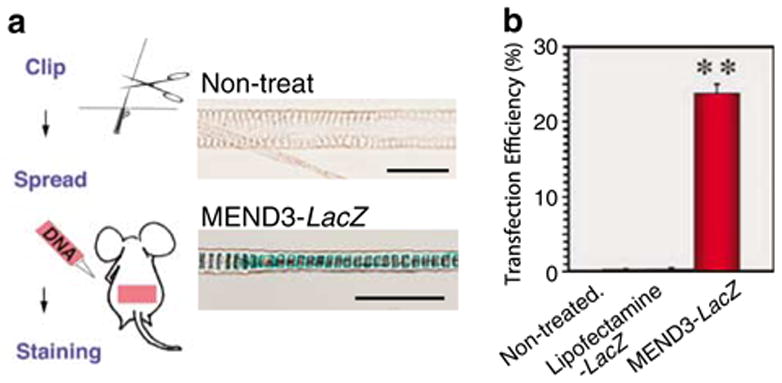
In vivo transfection activity of MEND. (a) Hair form the dorsal skin of 4-week-old ICR mice was clipped and skin was treated with MEND3 containing LacZ-expression vector solution. The X-gal staining of hairs from control or MEND3-LacZ treated skins after 2 weeks is shown. (b) Transfection efficiency of MEND3 versus Lipofectamine. Data are given as the mean ± s.e.m. (The average of four individual experiments are shown and a total of 300 hair shafts were examined for one experiment). **P<0.001; control versus MEND3-LacZ, Lipofectamine versus MEND3-LacZ. Using Student’s t-test, the transfection efficiency of MEND3-LacZ treatment is statistically significant relative to non-treated controls or Lipofectamine-LacZ treatment.
Previous studies show that BMP signals through BMP receptor type IA (BMPR1A) gene play an essential role in regulating hair follicle cycling.25–27 Therefore, MEND particles encoding constitutively active BMPR1A (caBmpr1a) were applied to hair follicles, to examine the role of BMPR1A in hair follicle cycling. For this experiment, it is predicted that ectopic expression of constitutively active BMPR1A will produce ligand-independent BMP signaling in treated hair follicles.
MEND3-ires-GFP (MEND3-control) or MEND3-ca Bmpr1a-ires-GFP (MEND3-caBmpr1a) was applied to the dorsal skin of 4-week-old ICR mice. Two weeks after treatment with MEND3-caBmpr1a, hair follicles were observed in or close to the subcutis space(s); in contrast, hair follicles were restricted to more superficial areas of the dermis (d) in skin treated with MEND3-control (Figure 5a, left panel). This trend was confirmed by analyzing 100 hair follicles per mouse (n = 3 mice per group) (Figure 5b).
Figure 5.
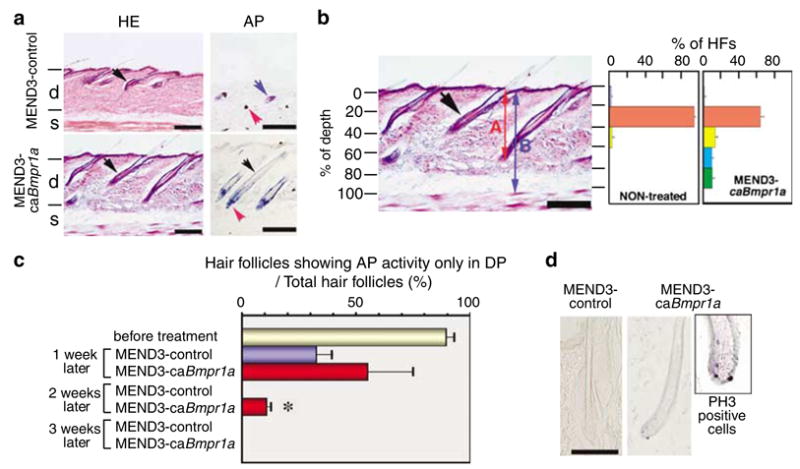
In vivo applications of MEND. (a) Hair follicle formation in mice skin treated with MEND3 containing GFP (MEND3-control) or GFP and a constitutively active form of Bmpr1a (MEND3-caBmpr1a). MEND particles were applied as described in Materials and methods. Tissue sections were stained with HE. Black arrows indicate hair follicles in the dermis. Cells were stained for AP activity to determine hair cycle phase (see text). Blue arrow, sebaceous gland; red arrow, DP; black arrow, ORS; d, dermis; s, subcutis space. Scale bars, 200 μm. (b) A histogram of the number of hair follicles versus depth. Percent depth of hair follicles was calculated by A/B*100 (%). Histograms include averaged data from three controls and three MEND3-caBmpr1a-treated mice. Scale bar, 100 μm. (c) Percent of hair follicles showing AP activity only in DP before and after treatment with MEND3-control or MEND3-caBmpr1a is shown. (d) Cells were stained for PH3 as an indicator of proliferation. Scale bar, 100 μm.
Alkaline phosphatase (AP) activity is a marker that identifies the phase of the hair growth cycle as reported by Handjiski et al.28 In particular, the dermal papilla (DP) display strong AP activity throughout the hair cycle, whereas AP activity occurs in the outer root sheath (ORS) only during late anagen and early catagen phases. In addition, AP activity increases and is higher in the subcutis gland (SG) during late catagen and telogen phases.28 Two weeks after treatment with MEND3 control, AP activity was detected in DP and SG but not in ORS, suggesting that hair follicles were in late catagen or telogen phase in control animals (Figure 5a, right top panel). In contrast, 2 weeks after treatment with MEND3-caBmpr1a, AP activity was detected in DP and the ORS but not in the SG (Figure 5a, right bottom panel). Furthermore, 1 week after treatment with MEND3-caBmpr1a, the fraction of hair follicles with AP activity only in DP, suggesting anagen phase, was significantly greater than in control animals (Figure 5c), and by 2 weeks after treatment with MEND3-caBmpr1a, more than 10% of hair follicles were in anagen to early catagen phase as judged by AP activity and morphology (Figure 5a and c and data not shown). In contrast, none of the hair follicles in control animals showed AP activity only in DP 2 weeks after treatment.
Cell proliferation was also evaluated in hair matrix using the proliferation marker phosphohistone H3 (PH3). Positive staining for PH3 was detected in hair follicles of 4-week-old pretreated mice as well as 2 weeks after treatment with MEND3-caBmpr1a (13.7% PH3-positive hair follicles out of 100 hair follicles per mouse) (Figure 5d and Supplementary Figure 3 online). In contrast, PH3 staining was not detected 2 weeks after treatment with MEND3-control (Figure 5d). These results suggest that MEND3-caBmpr1a delays the growth phase in treated hair follicles, which is consistent with the observation that the fraction of anagen phase hair follicles was higher in MEND3-caBmpr1a-treated animals. However, 7 weeks after treatment with MEND-caBmpr1a or MEND3-control, hair follicles were observed less deep in the dermis, were morphologically intermediate between late catagen and telogen phases and AP activity was higher in SG, suggesting progression to telogen phase (Supplementary Figure 2 online). This result may indicate that expression of caBmpr1a is transient and that hair follicles progress from anagen to catagen stage when expression of caBmpr1a declines.
In summary, these results demonstrate successful MEND3-mediated delivery of caBmpr1a to hair follicles of topically treated mice. Furthermore, the MEND3-delivered gene is expressed in target cells, where it causes an elongation of the growth phase of the hair cycle. These data suggest that MEND-mediated gene delivery may have therapeutic applications for treating hair and skin disorders.
Discussion
This study reports high-efficiency delivery of nucleic acids to eukaryotic cells using MEND particles containing polycation-condensed nucleic acids encapsulated in an R8-DOPE lipid envelope. The R8 peptide was shown to be the most efficient oligoarginine.29 MEND particles are non-cytotoxic and achieve transfection efficiencies as high as adenovirus. The high efficiency of MEND particles is attributed at least in part to the fact that the R8 modification promotes cellular uptake by macropinocytosis, which improved intracellular trafficking towards more efficient gene expression. Thus, MEND-mediated gene delivery provides a novel and efficient mechanism for non-viral transfection of eukaryotic cells, which may be useful for clinical applications including gene therapy.
R8-MEND particles can be prepared with or without fusogenic lipids such as DOPE/CHEMS; however, in experiments described here, fusogenic lipids are required to enhance cytosolic delivery for optimal transfection efficiency. The lipid envelope of MEND particles can also be modified with other functional devices such as polyethyleneglycol, a targeting ligand or a NLS. Thus, MEND can be readily adapted to specific biological systems to achieve desired biological effects.
Recent studies show that TAT–Cre fusion proteins as well as R8 and TAT peptides enter cells primarily via macropinocytosis.30–32 These results are consistent with our observation that R8-MEND particles enter the cell via macropinocytosis. In addition, consistent with reports suggesting that macropinosomes fuse with lysosomes at a low rate in non-phagocytic cells,30,33 liposomes modified with a high surface density of the R8 peptide only partially colocalize with lysosomes.11 Because of these properties, the particles are resistant to lysosomal degradation, and nuclear delivery and subsequent gene expression are enhanced. However, complexes formed between plasmid DNA (pDNA) and R8 or STR-R8 peptides are internalized via classical endocytosis and become trapped in endosomes.12,34 This suggests that the topology of R8 on a particle surface has a significant effect on the mechanism by which the particle enters the cell. Similar observations were made previously regarding the impact of the topology of the fusogenic peptide GALA on endosomal escape.7 Thus, the topology and content of a lipid envelope significantly influence both particle uptake and the function of devices embedded in the envelope. Although the diameters of MENDs1-3 were somewhat different (Supplementary Table 1 online), it is suggested that this difference has no significant effect on the uptake pathway of the particles. We have previously reported that 5% R8-liposomes of around 100 nm was taken up by macropinocytosis.11 Here, we confirmed that the larger R8-MEND3 particles (330 nm) are taken up mainly by the same pathway (Figure 3). Even much smaller arginine-rich peptides and peptide-fusion proteins were taken up by macropinocytosis.30,32 This suggests that the size difference of different MENDs does not affect the uptake pathway. In addition, the uptake via macropinocytosis involves the formation of large macropinosomes (>1 μm), which allows the internalization of small as well as larger MENDs. This is clearly different form the classical clathrin-mediated endocytosis (involves endosomes with a size limit of around 150 nm),2 which may not explain the uptake of MEND3 particles. Regarding the transfection efficiency of different MENDs, we believe that the superiority of MEND3 is mainly because of the enhanced fusiogenic ability of the DOPE/CHEMS combination11 rather than a size effect. However, the possibility that larger particles may sediment more on the cell surface,35 thus allowing more internalization, cannot be completely excluded.
The ultimate goal of our studies is to develop an efficient non-viral delivery system that can be used for in vivo gene therapy. Here, the potential of MEND particles for in vivo gene therapy was tested by applying MEND particles carrying a LacZ reporter gene or a gene encoding constitutively active BMPR1a to the dorsal skin of 4-week-old ICR mice. Four-week-old mice were chosen for this experiment because most hair follicles are at anagen phase in this strain of animals, as judged by histology, AP activity and proliferation ability (Supplementary Figure 3 online). For LacZ delivery, efficient LacZ delivery and expression were observed in hair follicles of MEND3-LacZ-treated animals after 2 weeks (~24% β-galactosidase-positive hair follicles for MEND-mediated delivery vs 0.2% β-galactosidase-positive hair follicles for Lipofectamine-mediated delivery). These data demonstrate that R8-MEND particles have significant potential for efficient in vivo gene therapy and suggest that R8-MEND particles may be superior to Lipofectamine particles for in vivo delivery of LacZ to mouse skin. Although the reasons for superior results with MEND particles are not exactly known, they may include the relatively small diameter of MEND particles and the resistance of MEND particles to lysosomal degradation. Consistent with previous studies by Domashenko et al.,23 transfection of LacZ was poor when MEND3 particles were applied to 8-week-old mice (data not shown), because hair follicles are in late catagen to telogen phase in 8–week-old mice (Supplementary Figure 3 online).
In vivo experiments with MEND3 particles carrying the caBmpr1a gene also gave encouraging results. Thus, delivery of constitutively active BMPR1a resulted in a prolonged anagen phase and extended cycling in treated hair follicles. This conclusion is based on the observations that (i) hair follicles were deeper in the subcutis space in treated mice than in control mice, (ii) a fraction of hair follicles in treated mice were in late anagen or early catagen phase, whereas hair follicles in control mice were in late catagen or telogen phase as judged by AP activity and (iii) proliferating cells were detected 2 weeks after treatment in hair follicles exposed to MEND3-caBmpr1a but not in control hair follicles. Additional studies are necessary to understand fully the molecular consequences of expression of caBmpr1a and subsequent activation of BMP signaling in treated skin. However, it is striking that a single application of MEND3-caBmpr1a had such significant biological effects in vivo.
In summary, this study describes a non-viral gene delivery system called MEND that is based on compacted DNA coated in an R8-DOPE lipid envelope. MEND particles mediate highly efficient non-cytotoxic delivery of nucleic acids in vitro and in vivo. To our knowledge, this is the first virus-like core-shell structure, which can control the topology of functional devices to maximize gene expression. We propose that MEND particles be considered a prototype nanomachine, and we encourage continued research to develop this system for efficient and safe gene therapy.
Materials and methods
Preparation and characterization of MEND
pDNA containing a firefly luciferase gene driven by a cytomegalovirus (CMV) promoter was obtained from Promega (Tokyo, Japan). PLL/DNA-compacted particles were prepared as described previously.15,36 MEND particles were prepared using a hydration method. Briefly, lipids (125 nmol) were dissolved in chloroform and evaporated in a round-bottom glass tube. The lipid film was hydrated for 10 min at room temperature with 0.25 ml HEPES buffer, pH 7.4, containing PLL/DNA-compacted particles. The sample was sonicated for ~1 min in a bath-type sonicator. For MEND1 (EPC/ Chol/STR-R8) and MEND2 (DOPE/Chol/STR-R8), the STR-R8 peptide was mixed with lipids and negatively charged PLL/DNA particles were used for hydration. The stearyl moiety acts as an anchor to the lipid surface. For MEND3 (DOPE/CHEMS/STR-R8), positively charged PLL/DNA particles were used for hydration and the particles were further incubated with STR-R8 for 30 min at room temperature. STR-R8 was prepared as described previously.37 MEND particles were characterized using quasi-elastic light scattering and an electrophoretic light scattering spectrophotometer (ELS8000, Otsuka electronics, Osaka, Japan). Frozen samples of DNA-rich fractions were isolated by sucrose density gradient centrifugation and visualized using cryo transfer and transmission electron microscopy (Hitachi H-7650) at 120 kV and a magnification of 75 000.
Transfection
Samples containing DNA (0.4 μg) in 0.25 ml serum-free Dulbecco’s modified Eagle’s medium were added to NIH3T3, HeLa or A549 cells grown in 24-well plates and incubated for 3 h at 37°C. One milliliter of medium containing 10% FBS was added and the cells were incubated for 45 h. Cells were lysed and luciferase activity was measured using a luminometer (Lumines-cencer-PSN, ATTO, Japan). Protein concentration was determined using a bicinchoninic acid protein assay kit (PIERCE, Rockford, IL, USA).
Adenovirus infection
The luciferase gene was cloned into E1-, E3-, replication-deficient, serotype 5 adenovirus vectors containing an expression cassette with a CMV promoter/enhancer. Viruses were propagated on human embryonic kidney 293 cells, and purified by double centrifugation on cesium-chloride gradients. The virus suspension was mixed with 4 × 104 cells/well in a 24-well plate, and incubated for 3 h at 37°C without serum (total volume 0.25 ml). One milliliter of medium containing 10% FBS was added and the cells were incubated for 45 h.
Confocal laser microscopy
MEND particles were prepared containing fluorescein isothiocyanate (FITC) or rhodamine-labeled pDNA. For the colocalization with Tf, cells were incubated with R8-MEND containing FITC-labeled pDNA and tetra-methylrhodamine-labeled Tf (TMR-Tf) (5 μM) for 30 min. To investigate uptake mechanism, cells were pre-incubated with or without sucrose (0.4 M) for 30 min, or amiloride (5 mM) for 10 min. MEND3 particles containing rhodamine-labeled pDNA or TMR-Tf were added and incubation continued for 1 h. Nuclei were stained with Syto24 for the last 20 min.
MEND delivery to mouse dorsal skin
LacZ expression pDNA was purchased from Clontech Laboratories Inc. (Mountain View, CA, USA) (pCMV-LacZ Vector). Lipofectamine was purchased from Invitrogen Corp. (Carlsbad, CA, USA) and a liposome–plasmid complex was prepared according to the manufacturer’s protocol. MEND suspensions (0.04 mg DNA/ ml) were prepared by condensing DNA with protamine, coating with DOPE/CHEMS and modification with 5 mol% STR-R8. Mice were anesthetized with a ketamine (200 mg/kg body weight, intraperitoneally (i.p.)) plus xylazine (10 mg/kg body weight, i.p.) and were allowed to breath freely. Hair was removed by clipping from the dorsal skin of anesthetized 4-week-old outbreed ICR mice, albino mice strain (Japan SLC). MEND or Lipofectamine preparations were applied to a 1 cm2 area in 0.05 ml aliquots over 60 min. After 2 weeks, hair follicles were stained for β-galactosidase activity. Similar experiments were carried out using MEND3 containing constitutively active BMPR1A gene (MEND3-caBmpr1a). The caBmpr1a expression plasmid was generously donated by Dr Takashi Imamura (Cancer Institute of Japan). A fragment of the caBmpr1a expression plasmid was subcloned into IRES2-EGFP (Clontech). All mouse experiments were performed in accordance with institutional guidelines covering the humane care and use of animals in research.
Histochemistry
Dorsal mouse skin was removed and fixed in 4% paraformaldehyde in phosphate buffer. Frozen cryosections (15 μm) were incubated with blocking buffer (PBS containing 0.01% TritonX and 1.5% normal goat serum). X-gal, hematoxylineosin (HE) and PH3 staining were performed as described previously.25 Endogenous AP activity was measured using a standard AP assay.28
Supplementary Material
Acknowledgments
This work was supported in part by Grants-in-Aid for Scientific Research (B) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan, by the MEXT Grant-in-Aid for Young Scientists (B) and Scientific Research on Priority Areas, and by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences. We thank the Hitachi High-Technologies Corporation for generously performing the cryo TEM observation. We thank Drs H Yoshida, Sarah E Millar, Deborah Lang, Carol Trempus and Mitch Eddy for helpful discussions. We also thank Dr Miriam Sander for helpful advice in writing the English manuscript.
Footnotes
Supplementary Information accompanies the paper on Gene Therapy website (http://www.nature.com/gt)
References
- 1.Kamiya H, Tsuchiya H, Yamazaki J, Harashima H. Intracellular trafficking and transgene expression of viral and non-viral gene vectors. Adv Drug Deliv Rev. 2001;52:153–164. doi: 10.1016/s0169-409x(01)00216-2. [DOI] [PubMed] [Google Scholar]
- 2.Khalil IA, Kogure K, Akita H, Harashima H. Uptake pathways and subsequent intracellular trafficking in non-viral gene delivery. Pharm Rev. 2006;58:32–45. doi: 10.1124/pr.58.1.8. [DOI] [PubMed] [Google Scholar]
- 3.Zabner J, Fasbender AJ, Moninger T, Poellinger KA, Welsh MJ. Cellular and molecular barriers to gene transfer by a cationic lipid. J Biol Chem. 1995;270:18997–19007. doi: 10.1074/jbc.270.32.18997. [DOI] [PubMed] [Google Scholar]
- 4.Bally MB, Harvie P, Wong FM, Kong S, Wasan EK, Reimer DL. Biological barriers to cellular delivery of lipid-based DNA carriers. Adv Drug Deliv Rev. 1999;38:291–315. doi: 10.1016/s0169-409x(99)00034-4. [DOI] [PubMed] [Google Scholar]
- 5.Ochiai H, Harashima H, Kamiya H. Intranuclear disposition of exogenous DNA in vivo: silencing, methylation and fragmentation. FEBS Lett. 2005;580:918–922. doi: 10.1016/j.febslet.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 6.Lee RJ, Huang L. Folate-targeted, anionic liposome-entrapped polylysine-condensed DNA for tumor cell-specific gene transfer. J Biol Chem. 1996;271:8481–8487. doi: 10.1074/jbc.271.14.8481. [DOI] [PubMed] [Google Scholar]
- 7.Kakudo T, Chaki S, Futaki S, Nakase I, Akaji K, Kawakami T, et al. Transferrin-modified liposomes equipped with a pH-sensitive fusogenic peptide: an artificial viral-like delivery system. Biochemistry. 2004;18:5618–5628. doi: 10.1021/bi035802w. [DOI] [PubMed] [Google Scholar]
- 8.Zanta MA, Belguise-Valladier P, Behr JP. Gene delivery: a single nuclear localization signal peptide is sufficient to carry DNA to the cell nucleus. Proc Natl Acad Sci USA. 1999;96:91–96. doi: 10.1073/pnas.96.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamiya H, Akita H, Harashima H. Pharmacokinetic and pharmacodynamic considerations in gene therapy. Drug Discov Today. 2003;8:990–996. doi: 10.1016/s1359-6446(03)02889-7. [DOI] [PubMed] [Google Scholar]
- 10.Hama S, Akita H, Ito R, Mizuguchi H, Hayakawa T, Harashima H. Quantitative comparison of intracellular trafficking and nuclear transcription between adenoviral and lipoplex systems. Mol Ther. 2006;13:786–794. doi: 10.1016/j.ymthe.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Khalil IA, Kogure K, Futaki S, Harashima H. High density of octaarginine stimulates macropinocytosis leading to efficient intracellular trafficking for gene expression. J Biol Chem. 2006;281:3544–3551. doi: 10.1074/jbc.M503202200. [DOI] [PubMed] [Google Scholar]
- 12.Khalil IA, Futaki S, Niwa M, Baba Y, Kaji N, Kamiya H, et al. Mechanism of improved gene transfer by the N-terminal stearylation of octaarginine: enhanced cellular association by hydrophobic core formation. Gene Ther. 2004;11:636–644. doi: 10.1038/sj.gt.3302128. [DOI] [PubMed] [Google Scholar]
- 13.Akita H, Khalil IA, Kogure K, Harashima H. Pharmacokinetic considerations in nonviral gene delivery. In: Taira K, Kataoka K, Niidome T, editors. Non-Viral Gene Delivery: Gene Design and Delivery. Springer-Verlag; Tokyo: 2005. pp. 135–154. [Google Scholar]
- 14.Kogure K, Akita H, Kamiya H, Harashima H. Programmed packaging: a new drug delivery system and its application to gene therapy. In: Knablein J, editor. Modern Biopharmaceuticals. Design, Development and Optimization. Vol. 4. Wiley-VCH; Weinheim: pp. 1521–1536. [Google Scholar]
- 15.Kogure K, Moriguchi R, Sasaki K, Ueno M, Futaki S, Harashima H. Development of a non-viral multifunctional envelope-type nano device by a novel lipid film hydration method. J Control Release. 2004;98:317–323. doi: 10.1016/j.jconrel.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 16.Farhood H, Serbina N, Huang L. The role of dioleoyl phosphatidylethanolamine in cationic liposome mediated gene transfer. Biochim Biophys Acta. 1995;1235:289–295. doi: 10.1016/0005-2736(95)80016-9. [DOI] [PubMed] [Google Scholar]
- 17.Hafez IM, Cullis PR. Cholesteryl hemisuccinate exhibits pH sensitive polymorphic phase behaviour. Biochim Biophys Acta. 2000;1463:107–114. doi: 10.1016/s0005-2736(99)00186-8. [DOI] [PubMed] [Google Scholar]
- 18.Salone B, Martina Y, Piersanti S, Cundari E, Cherubini G, Franqueville L, et al. Integrin alpha3beta1 is an alternative cellular receptor for adenovirus serotype 5. J Virol. 2003;77:13448–13454. doi: 10.1128/JVI.77.24.13448-13454.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plank C, Mechtler K, Szoka FC, Jr, Wagner E. Activation of the complement system by synthetic DNA complexes: a potential barrier for intravenous gene delivery. Hum Gene Ther. 1996;7:1437–1446. doi: 10.1089/hum.1996.7.12-1437. [DOI] [PubMed] [Google Scholar]
- 20.Lamaze C, Schmid SL. The emergence of clathrin-independent pinocytic pathways. Curr Opin Cell Biol. 1995;7:573–580. doi: 10.1016/0955-0674(95)80015-8. [DOI] [PubMed] [Google Scholar]
- 21.Li L, Hoffman RM. The feasibility of targeted selective gene therapy of the hair follicle. Nat Med. 1995;1:705–706. doi: 10.1038/nm0795-705. [DOI] [PubMed] [Google Scholar]
- 22.Saito N, Zhao M, Li L, Baranov E, Yang M, Ohta Y, et al. High efficiency genetic modification of hair follicles and growing hair shafts. Proc Natl Acad Sci USA. 2002;99:13120–13124. doi: 10.1073/pnas.192453799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Domashenko A, Gupta S, Cotsarelis G. Efficient delivery of transgenes to human hair follicle progenitor cells using topical lipoplex. Nat Biotechnol. 2000;18:420–423. doi: 10.1038/74480. [DOI] [PubMed] [Google Scholar]
- 24.Yang M, Baranov E, Moossa AR, Penman S, Hoffman RM. Visualizing gene expression by whole-body fluorescence imaging. Proc Natl Acad Sci USA. 2000;97:12278–12282. doi: 10.1073/pnas.97.22.12278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuhki M, Yamada M, Kawano M, Iwasato T, Itohara S, Yoshida H, et al. BMPR1A signaling is necessary for hair follicle cycling and hair shaft differentiation in mice. Development. 2004;131:1825–1833. doi: 10.1242/dev.01079. [DOI] [PubMed] [Google Scholar]
- 26.Kobielak K, Pasolli HA, Alonso L, Polak L, Fuchs E. Defining BMP functions in the hair follicle by conditional ablation of BMP receptor IA. J Cell Biol. 2003;163:609–623. doi: 10.1083/jcb.200309042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andl T, Ahn K, Kairo A, Chu EY, Wine-Lee L, Reddy ST, et al. Epithelial Bmpr1a regulates differentiation and proliferation in postnatal hair follicles and is essential for tooth development. Development. 2003;131:2257–2268. doi: 10.1242/dev.01125. [DOI] [PubMed] [Google Scholar]
- 28.Handjiski BK, Eichmuller S, Hofmann U, Czarnetzki BM, Paus R. Alkaline phosphatase activity and localization during the murine hair cycle. Br J Dermatol. 1994;131:303–310. doi: 10.1111/j.1365-2133.1994.tb08515.x. [DOI] [PubMed] [Google Scholar]
- 29.Futaki S, Suzuki T, Ohashi W, Yagami T, Tanaka S, Ueda K, et al. Arginine-rich peptides: an abundant source of membrane-permeable peptides having potential as carriers for intracellular protein delivery. J Biol Chem. 2001;276:5836–5840. doi: 10.1074/jbc.M007540200. [DOI] [PubMed] [Google Scholar]
- 30.Wadia JS, Stan RV, Dowdy SF. Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis. Nat Med. 2004;10:310–315. doi: 10.1038/nm996. [DOI] [PubMed] [Google Scholar]
- 31.Nakase I, Niwa M, Takeuchi T, Sonomura K, Kawabata N, Koike Y, et al. Cellular uptake of arginine-rich peptides: roles for macropinocytosis and actin rearrangement. Mol Ther. 2004;10:1011–1022. doi: 10.1016/j.ymthe.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 32.Kaplan IM, Wadia JS, Dowdy SF. Cationic TAT peptide transduction domain enters cells by macropinocytosis. J Control Release. 2005;102:247–253. doi: 10.1016/j.jconrel.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 33.Swanson JA, Watts C. Macropinocytosis. Trends Cell Biol. 1995;5:424–428. doi: 10.1016/s0962-8924(00)89101-1. [DOI] [PubMed] [Google Scholar]
- 34.Akita H, Ito R, Khalil IA, Futaki S, Harashima H. Quantitative three-dimensional analysis of the intracellular trafficking of plasmid DNA transfected by a nonviral gene delivery system using confocal laser scanning microscopy. Mol Ther. 2004;9:443–451. doi: 10.1016/j.ymthe.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 35.Harris SS, Giorgio TD. Convective flow increases lipoplex delivery rate to in vitro cellular monolayers. Gene Ther. 2005;12:512–520. doi: 10.1038/sj.gt.3302397. [DOI] [PubMed] [Google Scholar]
- 36.Moriguchi R, Kogure K, Akita H, Futaki S, Miyagishi M, Taira K, et al. A multifunctional envelope-type nano device for novel gene delivery of siRNA plasmids. Int J Pharm. 2005;301:277–285. doi: 10.1016/j.ijpharm.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 37.Futaki S, Ohashi W, Suzuki T, Niwa M, Tanaka S, Ueda K, et al. Stearylated arginine-rich peptides: a new class of transfection systems. Bioconjug Chem. 2001;12:1005–1011. doi: 10.1021/bc015508l. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


