Abstract
Evidence suggests that vascular function is strongly regulated by extracellular matrix (ECM) proteins via integrin-mediated signaling. To determine whether integrin expression on cerebral blood vessels is altered during chronic neuroinflammation, we examined β1 and β4 integrin expression in transgenic mice with astrocyte production of the pro-inflammatory cytokines interleukin-6 (IL-6) or interferon-α (IFN-α). Chronic production of IL-6 or IFN-α in the CNS promoted vascular expression of the β4 and α5 integrin subunits, and this was contributed mostly by astrocytes. Vascular expression of the ECM ligands laminin and fibronectin was also increased. Cell culture studies showed that astrocyte expression of the β4 and α5 integrins was significantly upregulated by IL-6 and IFN-α respectively, while endothelial expression of these integrins was unchanged. These results show that astrocytes respond to IL-6 and IFN-α by upregulating integrin expression. We propose that during neuroinflammation, astrocytes attempt to increase adhesive interactions at the blood-brain barrier (BBB), in order to increase barrier integrity.
INTRODUCTION
In the CNS, the vascular system is highly specialized because blood vessels have high electrical resistance and are relatively impermeable, compared to vessels in other organs (Fischer et al. 2002; Wolburg and Lippoldt 2002; Pardridge 2003; Ballabh et al. 2004). This high resistance is thought to be the result of very strong cell-cell adhesion between adjacent endothelial cells, which is further promoted by the influence of astrocyte foot processes (Risau et al. 1986; Janzer and Raff 1987; Risau et al. 1998; Abbruscato and Davis 1999). This high resistance constitutes the blood-brain barrier (BBB), which effectively separates the vascular and CNS compartments, thus shielding the sensitive neuronal population from the potentially harmful effects of some of the components of blood (Rubin and Staddon 1999; Pardridge 2003; Ballabh et al. 2004).
The ECM and integrins are essential for blood vessel formation and function (Stromblad and Cheresh 1996; Eliceiri and Cheresh 1999; Hynes et al. 1999; Hynes et al. 2002). Null mutations in fibronectin (George et al. 1993) or the α4 (Yang et al. 1995), α5 (Yang et al. 1993), αv (Bader et al. 1998) or β8 (Zhu et al. 2002) integrin subunits all result in defective vascular development. Cerebral blood vessels show strong expression of β1 integrins, and this is matched with high levels of the ECM protein laminin within the vascular basal lamina (Grooms et al. 1993; Paulus et al. 1993; Kloss et al. 1999). In a previous study, we showed that maturation of cerebral blood vessels during development is associated with a marked upregulation of β1 integrin and laminin expression, and a switch in specific β1 integrins, from fibronectin-binding integrins (α4β1 and α5β1) during angiogenesis to laminin-binding ones (α1β1 and α6β1) in the adult CNS (Milner and Campbell 2002b).
During chronic inflammation, many aspects of blood vessel function are disturbed, including changes in vascular permeability and growth of new vessels (Dvorak et al. 1995; Jackson et al. 1997; Majno 1998; Walsh and Pearson 2001). Investigation of the underlying mechanisms have revealed that pro-inflammatory cytokines regulate several aspects of vascular cell behavior, including cell proliferation, migration, differentiation, and vascular permeability (Grau et al. 1989; Stanimirovic and Satoh 2000). Significantly, the expression and function of vascular cell integrins is regulated both during chronic inflammation in vivo (Previtali et al. 1997; Sobel et al. 1998; Kloss et al. 1999), and by individual cytokines in vitro (Defillipi et al. 1992; Frank et al. 1996). Specifically, integrin expression on cerebral blood vessels is decreased during focal cerebral ischemia (Tagaya et al. 1997; Wagner et al. 1997; Tagaya et al. 2001) and acute demyelination events (Sobel et al. 1998), but is increased during chronic inflammatory events in the demyelinating animal model, experimental autoimmune encephalomyelitis (EAE) (Previtali et al. 1997), and in the facial motor nucleus lesion model (Kloss et al. 1999). When taken together with the role of the ECM in regulating vascular function (Eliceiri and Cheresh 1999; Hynes et al. 1999; Kim et al. 2000; Milner and Campbell 2002b), this suggests that dynamic alterations in integrin expression might contribute to some of the vascular changes observed during these conditions. In light of this, it is important to examine the influence of individual cytokines on vascular cell integrin expression and function in vivo, during chronic inflammation.
Interleukin 6 (IL-6) and interferon alpha (IFN-α) are two pro-inflammatory cytokines that play important roles during chronic inflammation (Sehgal 1990; Ershler 1993; Feghali and Wright 1997; Cacquevel et al. 2004). Interestingly, IL-6 and IFN-α exert opposite effects on blood vessel formation; IL-6 is angiogenic (Campbell et al. 1993), while IFN-α is angiostatic (Sidky and Borden 1987; Folkman and Ingber 1992). To investigate the roles of these cytokines during CNS inflammation, we previously generated transgenic mice that chronically produce IL-6 or IFN-α specifically within the CNS, under the control of the glial fibrillary acidic protein (GFAP) promoter. Both transgenic mouse models (GFAP-IL6 and GFAP-IFNα) display chronic inflammatory lesions specifically within the CNS, though the timing and pathological features of each model are distinct and unique to each cytokine (Campbell et al. 1993; Akwa et al. 1998). In the current study we used these transgenic mouse models to examine the influence of chronic exposure of IL-6 or IFN-α on integrin expression on cerebral blood vessels. We then characterized integrin expression on pure cell cultures of astrocytes and endothelial cells, and investigated the influence of recombinant IL-6 or IFN-α on integrin expression in these cell types.
RESULTS
Cerebral blood vessels in transgenic mice expressing IL-6 or IFN-α show altered β1 integrin expression
To examine the influence of the pro-inflammatory cytokines IL-6 and IFN-α on integrin expression within the CNS, immunohistochemistry was performed on frozen sections of brain obtained from age-matched wild-type mice and compared with that of transgenic mice chronically producing either IL-6 (GFAP-IL6) or IFN-α (GFAP-IFNα). These transgenic mice were engineered to constitutively express IL-6 or IFN-α specifically within the CNS, under the control of the astrocyte-specific GFAP promoter. Both transgenic mouse models have been extensively described previously (Campbell et al. 1993; Akwa et al. 1998). Both models display chronic inflammatory lesions specifically within the CNS, though the timing of pathological progression, pathological features and predominant immune effector cell types are unique to each cytokine. Specifically, GFAP-IL6 transgenic mice show a severe neurologic phenotype with a postnatal onset, characterized by runting, tremor, ataxia and seizure. At the neuropathological level, these mice exhibit neurodegeneration, gliosis and a marked proliferative angiopathy (Campbell et al. 1993). GFAP-IFNα transgenic mice display an adult-onset inflammatory encephalopathy, with marked calcium mineralization, meningoencephalomyelitis, gliosis and neurodegeneration. Calcium mineralization is prominently observed in blood vessels, and some blood vessels dilate and exhibit endothelial hypertrophy (Akwa et al. 1998). Previous studies addressed whether chronic production of a single specific cytokine in these transgenic mouse strains might influence the expression of other cytokines. This was performed by RNAse protection assay using a multi-probe set which included probes for IL-1, IL-2, IL-3, IL-4, IL-5, IL-6, TNF-α, TNF-β and IFN-γ (Campbell 1998). This showed that in the GFAP-IL6 strain, only mRNA for IL-6 was detected. In the GFAP-IFNα strain, induction of mRNA for TNF and IL-1 was detected (I.L.C., unpublished observations). In both the GFAP-IL6 and GFAP-IFNα transgenic mice, the neuropathological manifestations are most pronounced in the cerebellum, pons and medulla (Campbell et al. 1993; Akwa et al. 1998). Therefore, for the purpose of this study of integrin expression, we compared these areas in the wild-type brain with corresponding areas taken from transgenic mice displaying chronic inflammation in these areas of the CNS.
In keeping with studies performed by us and others (Grooms et al. 1993; Paulus et al. 1993; Kloss et al. 1999; Milner and Campbell 2002b), the highest level of expression of the integrin subunits β1, α1 and α6 in wild-type brain was found on blood vessels (Figure 1). Interestingly, cerebral blood vessels in both the GFAP-IL6 and GFAP-IFNα transgenic mice appeared to show increased expression of the α6 and β1 integrin subunits, but the level of expression of the α1 integrin subunit remained unchanged. In addition, expression of the α5 integrin subunit, which is barely detectable in the wild-type brain, was markedly upregulated in both transgenic models of chronic neuroinflammation, particularly in the GFAP-IFNα transgenic. This pattern of alteration in vascular integrin expression was consistently observed across several experiments (n=4 different animals) and throughout all regions of the CNS examined. Vascular expression of the different integrin subunits was then quantified by counting the number of blood vessels positive for each integrin subunit per field of view. As shown in Figure 1B, this showed that the number of α5 integrin positive blood vessels in the GFAP-IL6 and GFAP-IFNα transgenic models was significantly increased compared to the wild-type (GFAP-IL6: 106±12.3 vessels/field and GFAP-IFNα: 69±10.5 vessels/field compared to 9±4.1 vessels/field in wild-type mice, p<0.002 and p<0.005 respectively). In contrast, although the intensity of staining per blood vessel for the β1 and α6 integrin subunits appeared stronger in both transgenic models, the absolute number of β1 or α6 integrin-positive blood vessels was not significantly increased over the wild-type.
Fig. 1.

Integrin expression in the CNS of transgenic mice constitutively producing IL-6 or IFN-α in the CNS. A. Frozen sections of brainstem from wild-type, GFAP-IL6 and GFAP-IFNα transgenic mice were immunostained with monoclonal antibodies specific for the α1, α5, α6 and β1 integrin subunits as described in Experimental Methods. Scale bar = 50μm. Note that in the CNS of the transgenic GFAP-IL6 and GFAP-IFNα mice, blood vessels appeared to show higher expression levels of the α5, α6 and β1 integrin subunits per blood vessel, but expression levels of the α1 integrin subunit were unchanged. B. Quantification of the number of blood vessels expressing the α1, α5, α6 and β1 integrin subunits in the wild-type, GFAP-IL6 and GFAP-IFNα CNS. The number of vessels positive for each of the integrin subunits was counted in four separate fields of view in the brainstem within each animal per experiment. All points represent the mean ± SD of blood vessels positive for the integrin subunit in four separate experiments. * indicates statistically significant difference. Note that the number of α5 integrin-positive blood vessels was significantly increased in the brainstem of GFAP-IL6 and GFAP-IFNα mice compared to the wild-type control.
The number of α1 integrin positive blood vessels was not significantly altered in either model. In order to quantify the total levels of protein expression of the α5 and α6 integrin subunits in the CNS, western blots were performed on hindbrain lysates obtained from wild-type, GFAP-IL6 or GFAP-IFNα transgenic mice. This revealed that expression of the α5 integrin subunit was markedly increased in the CNS of the GFAP-IL6 and GFAP-IFNα transgenic mice (Figure 2). Western blot analysis showed that expression of the α6 integrin subunit was increased in the CNS of GFAP-IL6 transgenic mice, but not in the GFAP-IFNα transgenic mice. That the western blotting did not confirm the increased vascular expression of α6 integrins we observed in immunohistochemistry of the GFAP-IFNα transgenic mice was at first unexpected. However, we noticed that blood vessel density in the brain of GFAP-IFNα transgenic mice was reduced compared to wild-type. To quantify alterations in blood vessel density in these transgenic mice, we identified blood vessels using the endothelial-specific marker endoglin, and quantified vascular density in the cerebellum and the medulla. This showed that in the cerebellum, blood vessel density was reduced in the GFAP-IFNα transgenic strain (to 72±9.5% of the density in wild-type brain, p<0.05), but increased in the GFAP-IL6 transgenic strain (to 136±12.5% of the density in wild-type brain, p<0.05). Similar changes were seen in the medulla (blood vessel density decreased to 78±4.8% of wild-type brain in GFAP-IFNα mice (p<0.05) and increased to 132±8.9% of wild-type brain in GFAP-IL6 transgenic mice (p<0.05)). These changes in vascular density are consistent with previous observations in these transgenic mice strains, detailing vascular degeneration and dilatation in the brain of GFAP-IFNα mice (Akwa et al. 1998), and proliferative angiopathy in the brains of GFAP-IL6 mice (Campbell et al. 1993; Brett et al. 1995). With this information in hand, we conclude that while immunohistochemistry shows that cerebral blood vessels in the GFAP-IFNα mice express increased levels of α6 integrin per blood vessel (Figure 1), the reduced blood vessel density in the brain of these mice will tend to mask this effect, so that the total level of α6 integrin present in the brain shows no apparent difference from the wild-type.
Fig. 2.

Western blot analysis of expression of the α5 and α6 integrin subunits in the CNS of GFAP-IL6 and GFAP-IFNα transgenic mice. Protein lysates obtained from the hindbrain of wild-type, GFAP-IL6 and GFAP-IFNα mice were resolved by SDS-PAGE on 8% non-reducing gels, transferred to nitrocellulose membranes, and the expression of the α5 and α6 integrin subunits detected as described in Experimental Methods. Note that total brain expression of the α5 integrin subunit was markedly increased in the CNS of the GFAP-IL6 and GFAP-IFNα transgenic mice relative to wild-type mice. Total brain expression of the α6 integrin subunit was increased in the CNS of GFAP-IL6 transgenic mice, but not in the GFAP-IFNα transgenic mice, relative to wild-type mice.
The β4 integrin is induced on cerebral blood vessels in GFAP-IL6 and GFAP-IFNα transgenic mice
The α6 integrin subunit dimerizes with either the β1 or β4 subunit to form the α6β1 and α6β4 integrin heterodimers (Hynes 1992; Hemler 1999; Hynes 2002). Because our findings show that cerebral blood vessels in both GFAP-IL6 and GFAP-IFNα transgenic mice express higher levels of the α6 integrin subunit, we next investigated whether expression of the other potential partner of α6, β4 was affected in these transgenic models. Consistent with previous observations in the wild-type CNS (Haring et al. 1996; Previtali et al. 1997), we found that in contrast to the high levels of α6 and β1 integrin expression, β4 was expressed at barely detectable levels, and only on a small number of blood vessels (Figure 3). In contrast, cerebral blood vessels in the GFAP-IL6 or GFAP-IFNα transgenic mice expressed greater numbers of β4 integrin-positive blood vessels, though β4 expression in GFAP-IL6 transgenic mice was much more extensive and showed higher staining intensity per blood vessel than GFAP-IFNα transgenic mice. In the CNS of GFAP-IL6 transgenic mice, β4 reactivity was detected most strongly within the cerebellum and at comparatively weaker levels throughout the brainstem. In contrast, in the GFAP-IFNα transgenic mice, β4 reactivity showed a more restricted pattern, being expressed in only small areas within the cerebellum. The observation that the β4 integrin subunit shows highest expression within the cerebellum is probably a reflection of the fact that this is the site of maximal neuroinflammation within both transgenic models of chronic neuroinflammation, as a result of the GFAP-IL6 and GFAP-IFNα transgenes showing highest levels of expression in this area (Campbell et al. 1993; Akwa et al. 1998). Quantification of β4 or α6 integrin-positive blood vessels within the cerebellum revealed that in both the GFAP-IL6 and GFAP-IFNα transgenic mice, the number of blood vessels positive for the β4 integrin subunit was significantly increased compared to wild-type mice (GFAP-IL6: 70±9.7 vessels/field and GFAP-IFNα: 35.5±7.0 vessels/field compared to 3.8±3.0 vessels in the wild-type, p<0.002 and p<0.01 respectively). In addition, the number of blood vessels positive for the α6 integrin subunit was significantly increased in the cerebellum of the GFAP-IL6 mice, but not in the GFAP-IFNα mice (130.5±9.1 vessels/field in GFAP-IL6 mice compared to 87.5±11.5 vessels/field in the wild-type, p<0.05) (Figure 3B).
Fig. 3.

Expression of α6 and β4 integrin subunits in the CNS of GFAP-IL6 and GFAP-IFNα transgenic mice. A. Frozen sections of cerebellum were taken from wild-type, GFAP-IL6 and GFAP-IFNα transgenic mice and immunostained with monoclonal antibodies specific for the α6 and β4 integrin subunits as described in Experimental Methods. Scale bar = 50μm. Note that in the cerebellum of wild-type mice, only very few blood vessels label with the β4 antibody. In contrast, many blood vessels in the cerebellum of the transgenic GFAP-IL6 stained positive for the β4 integrin, and this was matched with a concomitant increase in the expression of the α6 integrin subunit, relative to wild-type mice. Blood vessels in the cerebellum of the GFAP-IFNα mice also showed more β4 positive blood vessels than wild-type mice, though the number of β4 positive levels and staining intensity in the GFAP-IFNα mice was always at a lower level than the GFAP-IL6 mice. B. Quantification of the number of blood vessels expressing the α6 and β4 integrin subunits in the wild-type, GFAP-IL6 and GFAP-IFNα cerebellum. The number of vessels positive for each of the integrin subunits was counted in four separate fields of view in the cerebellum within each animal per experiment. All points represent the mean ± SD of blood vessels positive for the α6 and β4 integrin subunits in four separate experiments. * indicates statistically significant difference. Note that the number of β4 integrin-positive blood vessels was significantly increased in the cerebellum of GFAP-IL6 and GFAP-IFNα mice compared to the wild-type control.
In order to identify the cellular source of the observed induction of α5 and β4 integrins we observe in the GFAP-IL6 and GFAP-IFNα transgenic models of chronic inflammation, we next performed dual-color immunohistochemistry with antibodies specific for the integrin subunits or the astrocyte marker GFAP. As shown in Figure 4, in the GFAP-IL6 mouse brain, this revealed that expression of the α5 or β4 integrin subunits strongly colocalized with GFAP, suggesting that it is astrocytes that respond to the inflammatory cytokine by upregulating their cell surface expression of the α5 and β4 integrin subunits. Similar results were observed in the GFAP-IFN-α brain. This cell-type specific localization is entirely consistent with previous reports demonstrating expression of the α6β4 integrin on astrocyte foot processes that contact the vascular basal lamina (Wagner et al. 1997), and with the finding that the β4 integrin subunit is induced specifically on astrocytes in EAE (Previtali et al. 1997).
Figure 4.
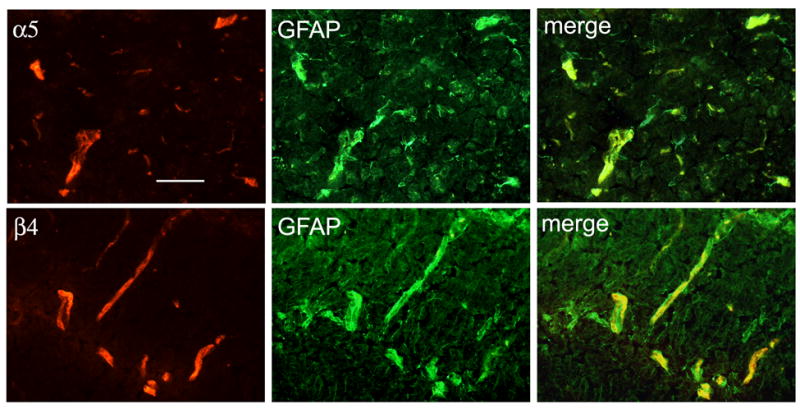
Co-localization of the α5 and β4 integrins with GFAP. Dual-color immunofluorescence was performed on frozen sections of brainstem from GFAP-IL6 transgenic mice, using monoclonal antibodies specific for the α5 or β4 integrin subunits (Cy-3) and polyclonal antibodies specific for GFAP (FITC), as described in Experimental Methods. Scale bar = 25μm. Note that vascular expression of the α5 and β4 integrin subunits colocalized with the astrocyte-marker GFAP, suggesting that astrocytes are the major contributor of these integrins.
The ECM ligands fibronectin and laminin are also upregulated in cerebral blood vessels in GFAP-IL6 and GFAP-IFNα transgenic mice
This study has shown that chronic production of the pro-inflammatory cytokines IL-6 and IFN-α in the CNS leads to elevated expression of the integrin subunits α5, α6 and β4 on cerebral blood vessels. To investigate whether there was a corresponding alteration in the expression of ECM ligands for these specific integrins, immunohistochemistry was performed to examine expression of fibronectin (the α5β1 ligand) and laminin (the α6β1/α6β4 ligand) within the brain of these transgenic models. As shown in Figure 5, both fibronectin and laminin expression was markedly increased in the basal lamina of cerebral vessels in both GFAP-IL6 and GFAP-IFNα transgenic mice. This resulted in an increased thickness of the vascular basal lamina within cerebral blood vessels.
Figure 5.

Expression of fibronectin and laminin in the vascular basal lamina in the CNS of transgenic mice constitutively producing IL-6 or IFN-α in the CNS. Frozen sections of brainstem from wild-type, GFAP-IL6 and GFAP-IFNα transgenic mice were immunostained with polyclonal antibodies specific for fibronectin (Fn) and laminin (Ln), as described in Experimental Methods. Scale bar = 50μm. Note that expression of laminin and fibronectin were both concentrated in the basal lamina of blood vessels, and that both were expressed at higher levels in the transgenic GFAP-IL6 and GFAP-IFNα mice. The increased expression of ECM proteins led to a generalised thickening of the vascular basal lamina in both transgenic mice strains. These results were consistently observed in four different experiments using brain sections from individual animals each time.
Integrin expression on astrocytes and brain capillary endothelial cells in vitro
The two main cellular components of cerebral blood vessels are endothelial cells, which line the blood vessel, and astrocytes, which elaborate foot processes that cover the entire surface of the vascular basal lamina (Risau et al. 1986; Janzer and Raff 1987; Risau et al. 1998; Abbruscato and Davis 1999; Ballabh et al. 2004). Although the dual-color immunohistochemistry showed that the astrocyte is the major contributor to the change in vascular integrin expression in vivo, it is still important to examine the influence of IL-6 and IFN-α on integrin expression on endothelial cells or astrocytes in vitro, to determine if the influence of the cytokines is direct or indirect. Primary cultures of brain capillary endothelial cells were derived from adult wild-type mice and found to be greater than 99% endothelial cells as determined by CD31 (PECAM-1). In parallel, astrocyte cultures were obtained from postnatal mice and determined as greater than 98% pure by GFAP. As laminin represents the most abundant ECM protein present in the cerebrovascular basal lamina, astrocytes and endothelial cells were cultured on laminin for 2 days, and were then analysed by flow cytometry for expression of the vascular-associated integrin subunits: α1, α5, α5, β1 and β4. As shown in Figure 6, this showed that astrocytes and endothelial cells in vitro both express the α1, α5 and α6 integrin subunits. Analysis of β4 integrin expression showed a key difference between the two cell types. Astrocytes did express the β4 integrin, but only in a small proportion of cells (about 5%). In contrast, brain capillary endothelial cells did not express the β4 integrin. Interestingly, the observation that the β4 integrin is expressed in only a limited percentage of astrocytes in vitro correlates with the data from in vivo studies showing that β4 is only found in a small proportion of blood vessels within the brain (Haring et al. 1996; Previtali et al. 1997). This finding is also consistent with a previous study showing that the β4 integrin subunit is expressed on only a small proportion of astrocytes in vitro (Previtali et al. 1997).
Figure 6.

Expression of β1 and β4 integrins by astrocytes and brain capillary endothelial cells in culture. Astrocytes and brain capillary endothelial cells were isolated as described in Experimental Methods and then cultured on laminin. After 2 days in culture, expression of the different β1 integrin-associated α subunits (A) and the β4 integrin subunit (B) was analyzed by flow cytometry as described in Experimental Methods. Note that astrocytes and brain capillary endothelial cells both expressed the α1, α5 and α6 integrin subunits (panel A). However, astrocytes and brain capillary endothelial cells differed in their expression of the β4 integrin. While endothelial cells expressed no β4 integrin, astrocytes did express β4, but only in a small proportion of cells (panel B).
IL-6 and IFN-α modulate astrocyte integrin expression in vitro
One question raised by these studies is whether the influence of the pro-inflammatory cytokines IL-6 and IFN-α to increase integrin expression on cerebral blood vessels is a direct or indirect effect of the cytokine. This is an important issue because of the chronic nature of exposure to IL-6 or IFN-α in the transgenic mice, it is possible that other secondary effects such as induction of other cytokines, or induction of basal lamina proteins like fibronectin and laminin, may then induce integrin expression in the vascular cell types. To address this issue, we examined whether integrin expression by astrocyte or brain capillary endothelial cells in vitro could be regulated by short-term (2 days) exposure to IL-6 or IFN-α. Pure cultures of astrocytes or brain capillary endothelial cells were incubated with two different concentrations of IL-6 (0.2 and 1.0 ng/ml) or IFN-α (0.2 and 1.0 x 103 U/ml). These concentrations of cytokine were based on the levels defined in the original studies describing the GFAP-IL6 (Campbell et al. 1993) and GFAP-IFN-α (Akwa et al. 1998) transgenic mice (IL-6, 0.3ng/ml and IFN-α, 0.05U/ml). After 2 days incubation with cytokine, cells were analysed for expression of the integrin subunits α5, α6, β1 and β4 by flow cytometry. Two significant changes were observed, as shown in Figure 7. First, astrocytes exposed to IFN-α showed a significantly increased α5 integrin expression compared with the untreated control cells (136±7.6% at 0.2 x103U/ml, p<0.02 and 147±11.1% at 103 U/ml, p<0.02) (Figure 7A). In contrast, IFN-α had no significant influence on brain capillary endothelial expression of the α5 integrin subunit. IL-6 did not significantly alter α5 integrin expression on either cell type. Second, the number of astrocytes that were positive for the β4 integrin was significantly increased by IL-6 (7.17±0.78% at 0.2ng/ml and 8.56±0.82% at 1.0ng/ml, compared with 5.17±0.17% under control conditions, p<0.05 and p<0.01 respectively) (Figure 7B). IFN-α did not influence β4 expression on astrocytes. IL-6 or IFN-α did not induce β4 integrin expression on brain capillary endothelial cells (not shown). In addition, neither IL-6 nor IFN-α had any significant influence on the expression of astrocyte or endothelial expression of the β1 or α6 integrin subunits (data not shown). To exclude the possibility that longer-term exposure to IL-6 or IFN-α may alter expression of the α6 or β1 integrin subunits on endothelial cells or astrocytes in vitro, we performed longer-term experiments, increasing the incubation time to 7 days. In these experiments, no significant changes in endothelial or astrocyte expression of the α6 or β1 integrin subunits were observed (not shown). Taken together, these in vitro studies support the notion that it is the expression of astrocyte, but not endothelial integrins that is directly altered by the pro-inflammatory cytokines IL-6 and IFN-α.
Figure 7.
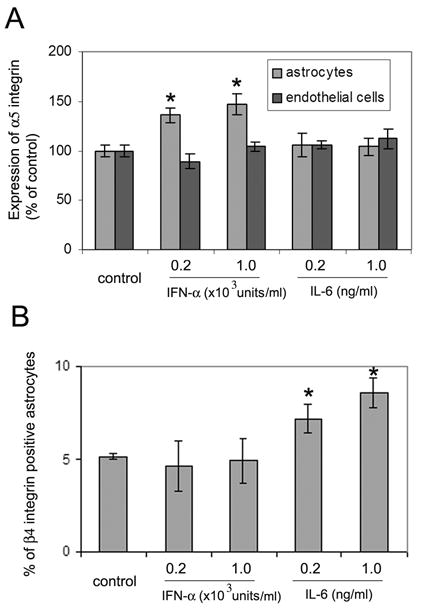
Recombinant IL-6 and IFN-α modulate astrocyte expression of the β4 and α5 integrin subunits respectively. Pure cultures of astrocytes or endothelial cells were incubated with two different concentrations of IL-6 (0.2 and 1.0 ng/ml) or IFN-α (0.2 and 1.0 x 103 U/ml). After 2 days, cells were analyzed for expression of the integrin subunits α5 and β4 by flow cytometry. A. Expression of the α5 integrin subunit is presented as the percentage expression compared to control conditions (no cytokine); all points represent the mean ± SD of four separate experiments. * indicates statistically significant difference. B. Astrocyte expression of the β4 integrin subunit is presented as the percentage of astrocytes that express the β4 integrin subunit; all points represent the mean ± SD of four separate experiments. * indicates statistically significant difference. Note that two significant changes were observed in the expression of astrocyte integrins. First, IFN-α significantly increased astrocyte α5 integrin expression compared with the untreated control cells (panel A). In contrast, IL-6 had no significant effect on astrocyte α5 expression. Neither cytokine altered endothelial expression of the α5 integrin. Second, IL-6 increased the number of β4 integrin positive astrocytes, but IFN-α had no effect on β4 integrin expression by astrocytes (panel B).
DISCUSSION
In this study we examined the influence of two pro-inflammatory cytokines, IL-6 and IFN-α on vascular expression of integrins in the CNS. Our in vivo studies showed that chronic astrocyte production of IL-6 or IFN-α led to increased vascular expression of the β4 and α5 integrin subunits, and that this expression was contributed mainly by astrocytes. Subsequent in vitro studies using pure cell populations of astrocytes and brain capillary endothelial cells showed that these cytokines directly influenced astrocyte, but not endothelial integrin expression. In these short term in vitro experiments, expression of astrocyte α6β4 and α5β1 integrins was increased by IL-6 and IFN-α respectively.
Regulation of integrin expression on cerebral blood vessels
Within the CNS, β1 and β4 integrins are expressed at the highest levels by cells within blood vessels, and the ECM ligands for these integrins (fibronectin, laminin and collagen IV) are concentrated within the basal lamina of blood vessels (Grooms et al. 1993; Paulus et al. 1993; Kloss et al. 1999; Milner and Campbell 2002b). This expression is tightly regulated both during development and in pathological conditions. In postnatal development we have shown previously that maturation of the blood-brain barrier is associated with marked upregulation of β1 integrin expression, and with a developmental switch from fibronectin-binding integrins to laminin-binding ones (Milner and Campbell 2002b).
Vascular integrin expression is altered in a number of different pathological conditions. In multiple sclerosis (MS), endothelial expression of the α6 and β1 integrins is reduced during the acute phase of disease, but then returned to normal levels in the chronic active phase (Sobel et al. 1998). In focal cerebral ischemia, expression of the β1 and α6 integrins (Tagaya et al. 1997; Tagaya et al. 2001), and astrocyte expression of the α6β4 integrin is lost (Wagner et al. 1997). In experimental autoimmune encephalomyelitis (EAE), a demyelinating model of MS, astrocyte expression of the α6β4 integrin is upregulated (Previtali et al. 1997). In addition, neuroinflammation associated with lesions in the facial motor nucleus stimulates increased vascular expression of the α5, α6 and β1 integrin subunits (Kloss et al. 1999). When viewed together, an important point emerges from these studies: acute events (such as focal ischemia and acute demyelination) lead to reduced integrin expression on cerebral blood vessels, while chronic events (such as chronic demyelination in MS and EAE and chronic inflammation of the facial motor nucleus) lead to restitution or elevated level of vascular integrin expression. Our findings are consistent with this pattern; chronic exposure to the pro-inflammatory cytokines IL-6 or IFN-α promoted increased vascular expression of the α5 and β4 integrin subunits. In addition, our observation that chronic inflammation in the CNS led to elevated levels of basal lamina ECM proteins is consistent with previous reports, which describe increased expression of fibronectin and laminin in cerebral blood vessels after chronic exposure to IL-12 (Maier et al. 2002) and increased synthesis of laminin and collagen IV and thickening of the glomerular basement membrane following intravenous administration of IFN-α in newborn mice (Moss et al. 1988).
Potential functions of integrins in blood vessels
Blood vessels in the CNS are unique in having very high electrical resistance, as a result of tight cell-cell adhesion between adjacent endothelial cells (Fischer et al. 2002; Wolburg and Lippoldt 2002; Pardridge 2003; Ballabh et al. 2004). Astrocytes, which elaborate foot processes along the blood vessel basal lamina, play an important instructive role in promoting this tight resistance (Risau et al. 1986; Janzer and Raff 1987; Risau et al. 1998; Abbruscato and Davis 1999). This high vascular resistance constitutes the blood-brain barrier (BBB), which effectively separates the blood and brain compartments, thus protecting the sensitive neurons of the CNS from potentially harmful agents within blood (Rubin and Staddon 1999). The high level of integrin expression on vascular structures and the specific localization of integrins on endothelial cells and astrocyte foot processes argues for an important role for ECM-integrin interactions in maintaining the architecture and stability of cerebral blood vessels. This idea is supported by the strong upregulation of integin expression that coincides with maturation of the BBB during development (Milner and Campbell 2002b). Taken together, we propose that during inflammation, vascular cells ramp up their adhesive mechanisms in an attempt to increase the stability of the blood vessels.
Our studies have shown that short-term exposure to the pro-inflammatory cytokines IL-6 and IFN-α induces increased integrin expression on astrocytes. This suggests that the influence observed in vivo is a direct effect of cytokine on astrocyte integrin expression. However, we cannot exclude the possibility that during chronic inflammation in vivo, other secondary effects may also contribute to increased integrin expression by vascular cells.
The β4 integrin
One of the most striking changes we observed was the strong induction of β4 integrin expression by astrocytes, which differed from being barely detectable in wild-type brain to a high level of expression in the transgenic GFAP-IL6 and GFAP-IFNα mice. Interestingly, we found both in vitro and in vivo that only a small proportion of astrocytes expressed the β4 integrin, and that this was upregulated following exposure to IL-6. Our findings are consistent with those of Previtali et al (Previtali et al. 1997) who showed a marked upregulation of β4 integrin expression on astrocytes during EAE, and described β4 expression on only a small proportion of astrocytes in vitro, which was increased by the pro-inflammatory cytokines IL-3 and TNF. Outside the CNS, β4 is expressed at high levels in hemi-desmosomes of epithelial cells, and is essential for the maintenance of epidermal integrity (Kennel et al. 1989; Stepp et al. 1990). This is well illustrated in β4 integrin null mice, which show pronounced skin blistering, analogous to the human disease junctional epidermolysis bullosa (van der Neut et al. 1996). We propose therefore, that the function of astrocyte α6β4 is to anchor astrocytes to the vascular basal lamina to increase the mechanical stability of the neurovascular unit and thereby increase the resistance of the BBB. As β4 is also strongly upregulated in neoplastic and reactive astrocytes (Previtali et al. 1996b; Previtali et al. 1996a; Fasen et al. 2003), this raises alternative potential functions for the β4 integrin, which include regulation of cell migration and proliferation (Clarke et al. 1995; Mercurio and Rabinovitz 2001; Mercurio et al. 2001).
In conclusion, we have shown that chronic astrocyte production of IL-6 or IFN-α in transgenic mice led to increased vascular expression of the β4 and α5 integrin subunits, with the expression being contributed mainly by astrocytes. In vitro studies confirmed this regulation of astrocyte integrin expression, with astrocyte expression of the β4 and α5 integrin subunits being increased by IL-6 and IFN-α respectively. Taken together with previous data describing upregulation of cerebrovascular integrin expression, both during development of the BBB and in a wide range of chronic inflammatory states, we propose that during inflammation, vascular cells ramp up their adhesive mechanisms in an attempt to increase the stability of blood vessels.
EXPERIMENTAL METHODS
Animals
The generation and characterization of the GFAP-IL6 and GFAP-IFNα transgenic mice was described previously (Campbell et al. 1993; Akwa et al. 1998). For the present studies animals of age-matched (6 months) from the G369 (GFAP-IL6) or GIFN-39 (GFAP-IFNα) transgenic lines were used, and compared to age-matched non-transgenic littermates. For these experiments, brain sections were obtained from four independent animals of each genotype, and four brain sections from each animal processed for each integrin subunit. Brain sections were also incubated with isotype control rat and hamster monoclonal antibodies and normal rabbit serum to control for non-specific binding of secondary antibodies.
Immunohistochemistry
Immunohistochemistry was performed as described previously (Pagenstecher et al. 2000) on frozen sections of cold saline-perfused brains taken from either C57/Bl6J wild-type mice or from transgenic littermates expressing the cytokines IL-6 (GFAP-IL6 or G369 strain) or IFN-α (GFAP-IFNα or GIFN39 strain). The following antibodies were obtained from BD Pharmingen (La Jolla, CA): rat monoclonal antibodies reactive for the integrin subunits α5 (5H10-27 (MFR5)), α6 (GoH3), β1 (9EG7), and β4 (346-11A), endoglin (CD105, clone MJ7/18) and CD31 (PECAM-1) (MEC13.3) and the hamster monoclonal antibody reactive for the integrin subunits α1 (Ha31/8), and the isotype control antibodies, rat anti-KLH (A110-2) and hamster (Ha4/8). The rabbit polyclonal antibodies reactive for fibronectin and laminin were obtained from Sigma Chemical Co. (St. Louis, MO), reactive for the α5 integrin subunit from Chemicon (Temecula, CA), reactive for the α6 integrin subunit from Dr. Vito Quaranta (The Scripps Research Institute), reactive for beta-actin from Neo-marker (Fremont, CA) and reactive for GFAP from Sigma. Biotinylated secondary antibodies used in immunohistochemistry were obtained from Zymed (San Francisco, CA) and BD Pharmingen. Fluorescent-conjugated anti-rat and anti-rabbit secondary antibodies were obtained from Jackson Immunologicals. Quantification of the number of blood vessels positive for the different integrin subunits was performed by counting the number of events within a given field of view per brain section of four different sections per animal. Each experiment was performed four separate times with different animals each time, and the results expressed as the mean ± SD of the number of integrin positive vessels per field of view. Blood vessel density in the cerebellum and medulla regions of the brain was quantified by counting the number of endoglin-positive vessels per field of view in four different areas of each brain section. Each experiment was performed four separate times with different animals each time, and the results expressed as the percentage of the vascular density in wild-type brain. Statistical significance was assessed by using the Student’s paired t test, in which p < 0.05 was defined as statistically significant.
Western blotting
Brains were removed from perfused mice, separated into forebrain and hindbrain, and then homogenized in lysis buffer, consisting of PBS containing 1% NP40 (Sigma) and a cocktail of protease inhibitors (Roche). After 30 minutes on ice, the homogenate was centrifuged to remove the insoluble fraction and the protein concentration of the brain lysate quantified (Bio-rad Laboratories, Hercules, CA). Equal amounts of protein (10μg) were then mixed with non-reducing sample buffer, boiled for 5 minutes and analysed on 8% SDS-PAGE resolving gels (Invitrogen, Carlsbad, CA) under non-reducing conditions. Proteins were then electro-blotted for 3 hours onto nitrocellulose membranes (Invitrogen) and blocked for one hour in 5% non-fat milk in PBS containing 0.1% Tween-20 (Sigma). The membranes were then probed with rabbit polyclonal antibodies directed against the α5 (Chemicon) or α6 (antibody 6845, a gift of Dr. Vito Quaranta) integrin subunits for one hour, washed, and then incubated with anti-rabbit HRP conjugate (Sigma) for one hour, before being extensively washed. Finally protein bands were visualised with the ECL detection system (Amersham Pharmacia Biotech, Piscataway, NJ) according to the manufacturers instructions.
Cell culture
Primary cultures of brain capillary endothelial cells were prepared as previously described (Wang and Milner 2006), according to the method of Sapatino et al (Sapatino et al. 1993), with some modifications. Briefly, forebrains of young mice (2-3 months old) were removed, cleaned of meninges and external blood vessels, then finely chopped, and dissociated for one hour in an enzymatic solution containing 30U/ml papain (Worthington, Lakewood, NJ), 0.24mg/ml L-cysteine (Sigma, St. Louis, MO) and 40μg/ml DNAse I type IV (Sigma) in 1 ml MEM-HEPES, as previously described for mixed glial cultures (Milner and ffrench-Constant 1994). After incubation, the disrupted brain tissue was triturated and then added to a universal 25ml tube containing 22% bovine serum albumin (BSA) (Sigma), and centrifuged at 1000g for 15 minutes, in order to separate out the myelin from the vascular tubes. The vascular tubes were resuspended and filtered through a 40μm cell strainer (Falcon) to separate tubes from single cells. The tubes were then washed and centrifuged in MEM-HEPES before being resuspended in endothelial cell growth media (ECGM) consisting of Hams F12 (Sigma), supplemented with 10% FCS, Heparin, ascorbic acid, L-glutamine (all from Sigma) and endothelial cell growth supplement (ECGS) (Upstate cell signaling solutions, Lake Placid, NY). The vascular tubes were then added to T25 flasks (Falcon, Franklin Lakes, NJ) coated with type I collagen (Sigma) and cultured at 37º C and 5% CO2. Cell culture media was replaced every 3 days and confluent cultures were obtained after approximately 7 days. Cells were passaged at a ratio of 1:3 and cells used only for the first 2 passages. The purity of these brain capillary endothelial cultures was assessed by live immunostaining of endothelial cells grown on glass coverslips, using antibodies for the cell surface marker CD31 (PECAM-1) and determined as greater than 99% pure endothelial cells.
Pure cultures of mouse astrocytes were obtained from postnatal (less than 2 days old) forebrains, and cultured on poly-D-lysine coated T75 flasks in DMEM supplemented with 10% fetal bovine serum as described previously (Milner and ffrench-Constant 1994). Astrocytes were used only for the first two passages. The purity of these astrocyte cultures was assessed by immunostaining with antibodies for GFAP and determined as greater than 98% pure.
Immunocytochemistry
For assessment of endothelial purity, mouse brain capillary endothelial cells were cultured on laminin coated glass coverslips in ECGM. Cells were blocked in 5% normal goat serum (NGS) in PBS for 30 minutes then live-labeled with an anti-CD31 (PECAM-1) rat monoclonal antibody for 1 hour at room temperature. Cells were then washed in PBS and incubated with a FITC-conjugated anti-rat secondary antibody for 30 minutes at room temperature and washed again. The cells were then fixed in acid/alcohol (95:5) at -20ºC for 30 minutes, before being washed and incubated with Hoechst stain (Sigma) for 10 minutes at room temperature to label cell nuclei. Coverslips were washed again before being mounted in aquamount (Polysciences, Warrington, PA). Astrocyte cultures were analysed in a similar way, with the exception that these cultures were fixed in acid/alcohol (5%/95%) at -20°C for 20 minutes, before being blocked, and labeled with a rabbit polyclonal anti-GFAP antibody, followed by washing and incubation with a FITC-conjugated anti-rabbit secondary antibody.
Flow cytometry
Mouse brain capillary endothelial cells or astrocytes were cultured in laminin-coated six-well plates (Nunc) in ECGM or DMEM containing 10% fetal bovine serum respectively. The laminin substrate was prepared by coating six-well plates with a 10μg/ml laminin (Sigma) solution for 2 hours at 37°C, before being washed in PBS. After reaching confluency, the cells were incubated for 2 days with: no cytokine, or IL-6 (R&D systems, Minneapolis, MN; 0.2 or 1.0ng/ml) or IFN-α (Life Technologies, Grand Island, NY; 0.2 or 1.0 x103U/ml). After 2 days in culture, the cells were removed from the plates and integrin expression analyzed by flow cytometry as described previously (Milner and Campbell 2002a). The fluorescent intensity of the labeled cells was analyzed with a Becton Dickinson (San Diego, CA) FACScan machine, with 10,000 events recorded for each condition. For each experimental condition, the mean fluorescent intensity was compared with the control state (no cytokines) and expressed as the percentage change relative to the control condition. For astrocyte expression of the β4 integrin, the influence of cytokines was expressed in terms of the percentage of numbers of astrocytes that were positive for the β4 integrin. Each experiment was repeated a minimum number of four times and the data expressed as mean ± SD. Statistical significance was assessed by using the Student’s paired t test, in which p < 0.05 was defined as statistically significant.
Acknowledgments
This work was supported by NIH grants MH62231, MH62261 and DA12444. RM was the recipient of a Wellcome Prize Travelling Research Fellowship. This is manuscript # 15497-NP from the Scripps Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbruscato T, Davis T. Protein expression of brain endothelial cell E-cadherin after hypoxia/aglycemia: influence of astrocyte contact. Brain Res. 1999;842:277–286. doi: 10.1016/s0006-8993(99)01778-3. [DOI] [PubMed] [Google Scholar]
- Akwa Y, Hassett D, Eloranta M, Sandberg K, Masliah E, Powell H, Whitton J, Bloom F, Campbell I. Transgenic expression of IFN-alpha in the central nervous system of mice protects against lethal neurotropic viral infection but induces inflammation and neurodegeneration. J Immunol. 1998;161:5016–5026. [PubMed] [Google Scholar]
- Bader B, Rayburn H, Crowley D, Hynes R. Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all alpha v integrins. Cell. 1998;95:507–519. doi: 10.1016/s0092-8674(00)81618-9. [DOI] [PubMed] [Google Scholar]
- Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview. Structure, regulation and clinical implications. Neurobiology of Disease. 2004;16:1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Brett FM, Mizisin AP, Powell HC, Campbell IL. Evolution of neuropathologic abnormalities associated with blood-brain barrier breakdown in transgenic mice expressing interleukin-6 in astrocytes. J Neuropath Exp Neurol. 1995;54:766–775. doi: 10.1097/00005072-199511000-00003. [DOI] [PubMed] [Google Scholar]
- Cacquevel M, Lebeurrier N, Cheenne S, Vivien D. Cytokines in neuroinflammation and Alzheimer's disease. Curr Drug Targets. 2004;5:529–534. doi: 10.2174/1389450043345308. [DOI] [PubMed] [Google Scholar]
- Campbell I, Abraham C, Masliah E, Kemper P, Inglis J, Oldstone M, Mucke L. Neurologic disease induced in transgenic mice by the cerebral overexpression of interleukin 6. Proc Natl Acad Sci USA. 1993;90:10061–10065. doi: 10.1073/pnas.90.21.10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell IL. Structural and functional impact of the transgenic expression of cytokines in the CNS. Ann N Y Acad Sci. 1998;840:83–96. doi: 10.1111/j.1749-6632.1998.tb09552.x. [DOI] [PubMed] [Google Scholar]
- Clarke AS, Lotz MM, Chao CC, Mercurio AM. Activation of the p21 pathway of growth arrest and apoptosis by the beat 4 integrin cytoplasmic domain. J Biol Chem. 1995;270:22673–22676. doi: 10.1074/jbc.270.39.22673. [DOI] [PubMed] [Google Scholar]
- Defillipi P, Silengo L, Tarone G. Alpha 6 beta 1 integrin (laminin receptor) is down-regulated by tumor necrosis factor alpha and interleukin-1 beta in human endothelial cells. J Biol Chem. 1992;267:18303–18307. [PubMed] [Google Scholar]
- Dvorak HF, Detmar M, Claffey KP, Nagy JA, van De Water L, Senger DR. Vascular permeability factor/vascular endothelial growth factor: an important mediator of angiogenesis in malignancy and inflammation. Int Arch Allergy Immunol. 1995;107:233–235. doi: 10.1159/000236988. [DOI] [PubMed] [Google Scholar]
- Eliceiri BP, Cheresh DA. The role of av integrins during angiogenesis: insights into potential mechanisms of action and clinical development. J Clin Invest. 1999;103:1227–1230. doi: 10.1172/JCI6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ershler WB. Interleukin-6: a cytokines for gerontologists. J Am Geriatr Soc. 1993;41:176–181. doi: 10.1111/j.1532-5415.1993.tb02054.x. [DOI] [PubMed] [Google Scholar]
- Fasen K, Elger CE, Lie AA. Distribution of alpha and beta integrin subunits in the adult rat hippocampus after pilocarpine-induced neuronal loss, axonal reorganization and reactive gliosis. Acta Neuropath. 2003;106:319–322. doi: 10.1007/s00401-003-0733-y. [DOI] [PubMed] [Google Scholar]
- Feghali CA, Wright TM. Cytokines in acute and chronic inflammation. Front Biosci. 1997;2:d12–26. doi: 10.2741/a171. [DOI] [PubMed] [Google Scholar]
- Fischer S, Wobben M, Marti HH, Renz D, Schaper W. Hypoxia-induced hyperpermeability in brain microvessel endothelial cells involves VEGF-mediated changes in the expression of zonula occludens-1. Microvasc Res. 2002;63:70–80. doi: 10.1006/mvre.2001.2367. [DOI] [PubMed] [Google Scholar]
- Folkman J, Ingber D. Inhibition of angiogenesis. Semin Cancer Biol. 1992;3:89–96. [PubMed] [Google Scholar]
- Frank R, Adelmann-Grill BC, Herrmann K, Haustein UF, Petri JB, Heckmann M. Transforming growth factor-β controls cell-matrix interaction of microvascular dermal endothelial cells by downregulation of integrin expression. J Invest Dermatol. 1996;106:36–41. doi: 10.1111/1523-1747.ep12327182. [DOI] [PubMed] [Google Scholar]
- George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- Grau GE, Piguet PF, Vassalli P, Lambert PH. Involvement of tumour necrosis factor and other cytokines in immune-mediated vascular pathology. Int Arch Allergy Appl Immunol. 1989;88:34–39. doi: 10.1159/000234744. [DOI] [PubMed] [Google Scholar]
- Grooms SY, Terracio L, Jones LS. Anatomical localisation of beta1 integrin-like immunoreactivity in rat brain. Exp Neurol. 1993;122:253–259. doi: 10.1006/exnr.1993.1125. [DOI] [PubMed] [Google Scholar]
- Haring H-P, Akamine P, Habermann R, Koziol J, del Zoppo G. Distribution of integrin-like immunoreactivity on primate brain microvasculature. J Neuropath Exp Neurol. 1996;55:236–245. doi: 10.1097/00005072-199602000-00012. [DOI] [PubMed] [Google Scholar]
- Hemler ME. Integrins. In: Kreis T, Vale R, editors. Guidebook to the extracellular matrix, anchor and adhesion proteins. New York: Oxford University Press; 1999. pp. 196–212. [Google Scholar]
- Hynes RO. Integrins: Versatility, modulation and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Hynes RO, Bader BL, Hodivala-Dilke K. Integrins in vascular development. Braz J Med Biol Res. 1999;32:501–510. doi: 10.1590/s0100-879x1999000500002. [DOI] [PubMed] [Google Scholar]
- Hynes RO, Lively JC, McCarty JH, Taverna D, Francis SE, Hodivala-Dilke K, Xiao Q. The diverse roles of integrins and their ligands in angiogenesis. Cold Spring Harb Symp Quant Biol. 2002;67:143–153. doi: 10.1101/sqb.2002.67.143. [DOI] [PubMed] [Google Scholar]
- Jackson JR, Seed MP, Kircher CH, Willoughby DA, Winkler JD. The codependence of angiogenesis and chronic inflammation. FASEB J. 1997;11:457–465. [PubMed] [Google Scholar]
- Janzer RC, Raff MC. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature. 1987;325:253–257. doi: 10.1038/325253a0. [DOI] [PubMed] [Google Scholar]
- Kennel SJ, Foute LJ, Falcioni R, Sonnenberg A, Stringer CD, Crouse C, Hemler ME. Analysis of the tumour associated antigen TSP-180. Identity with α6β4 in the integrin superfamily. J Biol Chem. 1989;264:15515–15521. [PubMed] [Google Scholar]
- Kim S, Bell K, Mousa SA, Varner JA. Regulation of angiogenesis in vivo by ligation of integrin a5b1 with the central cell-binding domain of fibronectin. Am J Pathol. 2000;156:1345–1362. doi: 10.1016/s0002-9440(10)65005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloss CU, Werner A, Klein MA, Shen J, Menuz K, Probst JC, Kreutzberg GW, Raivich G. Integrin family of cell adhesion molecules in the injured brain: regulation and cellular localization in the normal and regenerating mouse facial motor nucleus. J Comp Neurol. 1999;411:162–178. doi: 10.1002/(sici)1096-9861(19990816)411:1<162::aid-cne12>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Maier J, Kincaid CL, Pagenstecher A, Campbell IL. Regulation of signal transducer and activator of transcription (STAT) and suppressor of cytokine signaling (SOCS) gene expression in the brain of mice with astrocyte-targeted production of IL-12 and experimental autoimmune encephalomyelitis. Am J Pathol. 2002;160:271–288. doi: 10.1016/S0002-9440(10)64371-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majno G. Chronic inflammation: links with angiogenesis and wound healing. Am J Pathol. 1998;153:1035–1039. doi: 10.1016/S0002-9440(10)65648-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercurio AM, Rabinovitz I. Towards a mechanistic understanding of tumor invasion- lessons from the alpha 6 beta 4 integrin. Semin Cancer Biol. 2001;11:129–141. doi: 10.1006/scbi.2000.0364. [DOI] [PubMed] [Google Scholar]
- Mercurio AM, Rabinovitz I, Shaw LM. The alpha 6 beta 4 integrin and epithelial cell migration. Curr Opin Cell Biol. 2001;13:541–545. doi: 10.1016/s0955-0674(00)00249-0. [DOI] [PubMed] [Google Scholar]
- Milner R, ffrench-Constant C. A developmental analysis of oligodendroglial integrins in primary cells: changes in αv-associated β subunits during differentiation. Development. 1994;120:3497–3506. doi: 10.1242/dev.120.12.3497. [DOI] [PubMed] [Google Scholar]
- Milner R, Campbell IL. Cytokines regulate microglial adhesion to laminin and astrocyte extracellular matrix via protein kinase C-dependent activation of the α6β1 integrin. J Neurosci. 2002a;22:1562–1572. doi: 10.1523/JNEUROSCI.22-05-01562.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner R, Campbell IL. Developmental regulation of β1 integrins during angiogenesis in the central nervous system. Mol Cell Neurosci. 2002b;20:616–626. doi: 10.1006/mcne.2002.1151. [DOI] [PubMed] [Google Scholar]
- Moss J, Shore I, wooodrow D, Gresser I. Interferon-induced glomerular basement membrane and endothelial cell lesions in mice. An immunogold ultrastructural study of basement membrane components. Am J Pathol. 1988;133:557–563. [PMC free article] [PubMed] [Google Scholar]
- Pagenstecher A, Lassmann S, Carson MJ, Kincaid CL, Stalder AK, Campbell IL. Astrocyte-targeted expression of IL-12 induces active cellular immune responses in the central nervous system and modulates experimental allergic encephalomyelitis. J Immunol. 2000;164:4481–4492. doi: 10.4049/jimmunol.164.9.4481. [DOI] [PubMed] [Google Scholar]
- Pardridge WM. Blood-brain barrier drug targetting: the future of brain drug development. Mol Med. 2003;3:90–105. doi: 10.1124/mi.3.2.90. [DOI] [PubMed] [Google Scholar]
- Paulus W, Baur I, Schuppan D, Roggendorf W. Characterisation of integrin receptors in normal and neoplastic brain. Am J Pathol. 1993;143:154–163. [PMC free article] [PubMed] [Google Scholar]
- Previtali S, Archelos J, hartung HP. Modulation of the expression of integrins on glial cells during experimental autoimmune encephalomyelitis. Am J Pathol. 1997;151:1425–1435. [PMC free article] [PubMed] [Google Scholar]
- Previtali SC, Quattrini A, Nemni R, Truci G, Wrabetz L, Canal N. α6β4 integrin expression in ENU-induced gliomas. J Neurooncol. 1996a;30s:148. [Google Scholar]
- Previtali SC, Quattrini A, Nemni R, Truci G, Ducati A, Wrabetz L, Canal N. α6β4 and α6β1 integrins in astrocytomas, and other CNS tumours. J Neuropathol Exp Neurol. 1996b;55:456–465. doi: 10.1097/00005072-199604000-00008. [DOI] [PubMed] [Google Scholar]
- Risau W, Esser S, Engelhardt B. Differentiation of blood-brain barrier endothelial cells. Pathologie et Biologie. 1998;46:171–175. [PubMed] [Google Scholar]
- Risau W, Hallman R, Albrecht U, Henke-Fahle S. Brain astrocytes induce the expression of an early cell surface marker for blood-brain barrier specific endothelium. EMBO. 1986;5:3179–3183. doi: 10.1002/j.1460-2075.1986.tb04627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LL, Staddon JM. The cell biology of the blood-brain barrier. Annu Rev Neurosci. 1999;22:11–28. doi: 10.1146/annurev.neuro.22.1.11. [DOI] [PubMed] [Google Scholar]
- Sapatino BV, Welsh CJ, Smith CA, Bebo BF, Linthicum DS. Cloned mouse cerebrovascular endothelial cells that maintain their differentiation markers for factor VIII, low density lipoprotein, and angiotensin-converting enzyme. In Vitro Cell Dev Biol Anim. 1993;29:923–928. doi: 10.1007/BF02634230. [DOI] [PubMed] [Google Scholar]
- Sehgal PB. Interleukin-6: molecular pathophysiology. J invest Dermatol. 1990;94:2S–6S. doi: 10.1111/1523-1747.ep12874963. [DOI] [PubMed] [Google Scholar]
- Sidky YA, Borden EC. Inhibition of angiogenesis by interferons: effects on tumor- and lymphocyte-induced vascular responses. Cancer Res. 1987;47:5155–5161. [PubMed] [Google Scholar]
- Sobel R, Hinojoza J, Maeda A, Chen M. Endothelial cell integrin laminin receptor expression in multiple sclerosis lesions. Am J Pathol. 1998;153:405–415. doi: 10.1016/S0002-9440(10)65584-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanimirovic D, Satoh K. Inflammatory mediators of cerebral endothelium: a role in ischemic brain inflammation. Brain Pathol. 2000;10:113–126. doi: 10.1111/j.1750-3639.2000.tb00248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepp MA, Spurr-Michaud S, Tisdale A, Elwell J, Gipson IK. a6b4 integrin heterodimer is a component of hemidesosomes. Proc Nat Acad Sci USA. 1990;87:8970–8974. doi: 10.1073/pnas.87.22.8970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromblad S, Cheresh DA. Integrins, angiogenesis and vascular cell survival. Chem Biol. 1996;3:881–885. doi: 10.1016/s1074-5521(96)90176-3. [DOI] [PubMed] [Google Scholar]
- Tagaya M, Liu KF, Copeland BR, Seiffert D, Engler R, Garcia JH, del Zoppo GJ. DNA scission after focal brain ischemia. Temporal differences in two species. Stroke. 1997;28:1245–1254. doi: 10.1161/01.str.28.6.1245. [DOI] [PubMed] [Google Scholar]
- Tagaya M, Haring H-P, Stuiver J, Wagner S, Abumiya T, Lucero J, Lee P, Copeland B, Seiffert D, del Zoppo G. Rapid loss of microvascular integrin expression during focal brain ischemia reflects neuron injury. J Cereb Blood Flow Metab. 2001;21:835–846. doi: 10.1097/00004647-200107000-00009. [DOI] [PubMed] [Google Scholar]
- van der Neut R, Krimpenfort P, Calafat J, Niessen CM, Sonnenberg A. Epithelial detachment due to absence of hemidesmosomes in integrin beta 4 null mice. Nature Genetics. 1996;13:366–369. doi: 10.1038/ng0796-366. [DOI] [PubMed] [Google Scholar]
- Wagner S, Tagaya M, Koziol J, Quaranta V, del Zoppo GJ. Rapid disruption of an astrocyte interaction with the extracellular matrix mediated by integrin α6β4 during focal cerebral ischemia/reperfusion. Stroke. 1997;28:858–865. doi: 10.1161/01.str.28.4.858. [DOI] [PubMed] [Google Scholar]
- Walsh DA, Pearson CI. Angiogenesis in the pathogenesis of inflammatory joint and lung diseases. Arthritis Res. 2001;3:147–153. doi: 10.1186/ar292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Milner R. Fibronectin promotes brain capillary endothelial cell survival and proliferation through a5b1 and avb3 integrins via MAP kinase signaling. J Neurochem. 2006;96:148–159. doi: 10.1111/j.1471-4159.2005.03521.x. [DOI] [PubMed] [Google Scholar]
- Wolburg H, Lippoldt A. Tight junctions of the blood-brain barrier; development, composition and regulation. Vascular Pharmacology. 2002;38:323–337. doi: 10.1016/s1537-1891(02)00200-8. [DOI] [PubMed] [Google Scholar]
- Yang JT, Rayburn H, Hynes RO. Embryonic mesodermal defects in α5 integrin-deficient mice. Development. 1993;119:1093–1105. doi: 10.1242/dev.119.4.1093. [DOI] [PubMed] [Google Scholar]
- Yang JT, Rayburn H, Hynes RO. Cell adhesion events mediated by α4 integrins are essential in placental and cardiac development. Development. 1995;121:549–560. doi: 10.1242/dev.121.2.549. [DOI] [PubMed] [Google Scholar]
- Zhu J, Motejlek K, Wang D, Zang K, Scmidt A, Reichardt LF. β8 integrins are required for vascular morphogenesis in mouse embryos. Development. 2002;129:2891–2903. doi: 10.1242/dev.129.12.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]


