Abstract
The ability to reliably detect aberrant glycosylation of human chorionic gonadotropin (hCG) may have profound implications for the diagnosis and monitoring of malignant gestational trophoblastic neoplasia, germ cell tumors, other malignancies, and pregnancy complications. To become a clinically useful assay, however, this discrimination of glycoforms should be possible on minimally treated biological specimens. Towards this end, we have developed a lectin-based sandwich-type immunoassay to compare the glycosylation patterns of hCG among urine specimens from patients presenting with a normal pregnancy, invasive mole, choriocarcinoma, and male germ cell tumors using carbohydrate-free antibody fragments as capture reagents and a panel of eight lectins, five recognizing neutral sugars and three recognizing sialic acid. There was no significant difference in the binding of any of the lectins to hCG in the urine of women over the gestational range of 6 – 38 weeks. Three lectins, however, exhibited differential binding to urinary hCG derived from these normal pregnant controls and that from patients with malignant forms of gestational trophoblastic disease and male germ cell tumors. Galanthus nivalis agglutinin and Maackia amurensis lectin, which bind terminal mannose and α(2–3)sialic acid, respectively, preferentially bound pregnancy-derived hCG, whereas the lectin, wheat germ agglutinin, which binds sialic acid and β(1–4)N-acetylglucosamine, exhibited decreased binding to pregnancy-derived hCG compared to that from patients with male germ cell tumors and malignant gestational trophoblastic neoplasia. The differential binding observed with these three promising lectins is most encouraging and warrants further examination. The experimental paradigm also holds promise for the development of comparable assays for other glycosylated tumor markers.
Keywords: gestational trophoblastic disease, male germ cell tumors, choriocarcinoma, invasive mole, human chorionic gonadotropin, hyperglycosylation, lectins, surface plasmon resonance
Introduction
Human chorionic gonadotropin (hCG) is a glycoprotein hormone, and, like all members of this hormone family, contains a common α-subunit noncovalently associated with a unique hormone-specific β-subunit. Each subunit contains two N-linked oligosaccharides, and the hCGβ-subunit additionally bears four O-linked oligosaccharides on the carboxyterminal peptide (Hearn & Gomme 2000). During pregnancy, high levels of hCG and metabolic derivatives circulate through the bloodstream; the heterodimer, free subunits, and a metabolic breakdown product of the β-subunit, termed the β-core fragment (hCGβcf), are also secreted in the urine (Birken et. al. 1988, Cole 1998). Missing from this core fragment are the O-linked oligosaccharides, but truncated N-linked oligosaccharides remain (Birken et al. 1988, Blithe et al. 1989).
The glycosylation profile of hCG changes throughout gestation. Early pregnancy isoforms are more acidic (Wide et al. 1994) and hyperglycosylated (Kovalevskaya et al. 1999) than those produced later in pregnancy, becoming the minority as pregnancy progresses. This shift in the glycosylation pattern results from production initially by cytotrophoblasts gradually shifting to that by syncyciotrophoblasts (Kovalevskaya et al. 2002a). Aberrant levels of the early glycoforms may forecast pregnancy loss (Kovalevskaya et al.. 2002b) or suggest a Down syndrome pregnancy (Cole et al. 1999). The term hyperglycosylation as used for hCG is typically understood to mean enhanced branching into triantennary and unusual biantennary N-linked oligosaccharides, and a preponderance of the more complex Core 2, as opposed to Core 1, O-linked sugars, (Kobata 1988, Elliott et al. 1997, Birken et al. 2003). However, since the development of the only antibody which can specifically recognize forms of hyperglycosylated hCG which contain Core 2 O-linked sugars on the hCGβ COOH-terminal region (B152), the term hyperglycosylated is now frequently used to mean forms of hCG recognized by the particular antibody (Cole et al. 1999, Birken 2005).
Outside of pregnancy, production of hyperglycosylated hCG is associated with malignant forms of gestational trophoblastic disease such as invasive mole and choriocarcinoma (Mizuochi et al. 1983, Amano et al. 1988), as well as male and female germ cell tumors (Endo et al. 1991). Additionally, it can be detected in the serum or urine of patients suffering from a variety of malignant conditions including ovarian and cervical (Cole et al. 1988), colon (Lundin et al. 2001), bladder (Mora et al. 1996, Hotakainen et al. 1999), and lung (Yokotani et al. 1997) cancer. Although only germ cell tumors, choriocarcinoma, invasive mole, and placental site trophoblastic tumors secrete the prodigious quantities of hormone traditionally required for structural determination of the oligosaccharide chains, hCG produced by transformed cells differs from pregnancy-derived hCG in its retention or exclusion on lectin affinity columns (Yoshimoto et al. 1979, Mann & Karl 1983, Endo et al. 1988, Sakai et al. 1994), implying that perhaps all malignancy-derived hCG carries altered carbohydrate structures. There is evidence that the hyperglycosylated forms of hCG in pregnancy differ somewhat from those produced by various malignancies (Kovalevskaya et al. 2002b, Birken 2005,).
Recently, we described a method to prepare carbohydrate-depleted capture antibodies with greatly reduced interference in lectin-binding assays for studying hCG glycosylation (Kelly et al. 2005). Using a biosensor format, these fragments were used to capture hCG from choriocarcinoma cell media for comparison with pregnancy-derived hCG. Strong binding differences were found for lectins recognizing mannose (Galanthus nivalis agglutinin, GNA), galactose (Ricinus communis agglutinin, RCA), and α(2–6)sialic acid (Sambucis nigra agglutinin, SNA). In this report, the biosensor assay was extended to screen the binding of eight lectins to hCG and derivatives captured from urine specimens from pregnant women, women with invasive mole and choriocarcinoma, and male germ cell tumors.
Materials and Methods
Antibody Preparation
Antibody B108 is a type of antibody commonly produced since it is directed to a highly antigenic site of hCG which is available on hCG, the free β-subunit, the βcore fragment of hCGC and hCGβcf). (O’Connor et al. 1994). This antibody is used to pair with other hCG antibodies for development of sensitive assays for hCG such as the widely used B109-B108 IRMA for measurement of heterodimeric hCG (O’Connor et al. 1988). Its binding site has been mapped to the region of β-77 (Rao & Moyle 1994). In order to remove the one site of N-linked glycosylation on each heavy chain and thus eliminate undesired lectin binding to the antibody, F(ab′) fragments were prepared as described elsewhere (Kelly et al. 2005). Briefly, 50 μg of B108 IgG were incubated with 1 mg of immobilized ficin (Sigma) in 0.1 M citrate buffer, pH 6.0, containing 1 mM cysteine and 5 mM EDTA, to produce F(ab′)2. After digestion, the reaction mixture was passed over a 0.22 μm centrifuge filter to remove the agarose-bound enzyme. Then the fragmented antibody was dialyzed (10 kD MW cut-off) into PBS and partially reduced by incubation with 5 mM mercaptoethanolamine at 37º C for 1 h. The resulting F(ab′) fragments were dialyzed into immobilization buffer (10 mM sodium acetate, pH 4.0).
Antibody fragment immobilization
The F(ab′) fragment of B108 was immobilized to a CM5 chip (BIAcore) through its free hinge thiol groups using a maleimide coupling protocol adapted from one provided by BIAcore. B108 was diluted to an estimated concentration of 250 μg F(ab′)/ml of immobilization buffer. During immobilization, flow over the chip was set at 5 μl/min with running buffer (HBS-10 mM HEPES, pH 7.5, 150 mM NaCl, 2 mM CaCl2, 2 mM MgCl2, and 0.005% Tween 20). The chip was activated with 25 μl of 200 mM EDC (1-ethyl-3-[3-dimethylaminopropyl] carbodimide hydrochloride)/50 mM NHS (N-hydroxysuccinimide), followed by 20 μl of 1 M ethylenediamine, pH 8.5. Sulfo-MBS (40 μl of 50 mM m-maleimidobenzoyl-Nhydroxysulfo-succinimide ester, Pierce Chemical Company) was used to cross-link the B108 fragments (60 μl) to the chip. Any remaining active sites were quenched with 50 μl of 100 mM cysteine in 10 mM sodium acetate buffer, pH 4.5. In this fashion, approximately 10,000 resonance units (RU) of F(ab′) were affixed to a channel.
Human samples
The urine concentrates from patients with choriocarcinoma (n=3), invasive mole (n=3), and male germ cell tumor (n=2) were generously provided by Drs. Hugh Mitchell and Edwards Newlands of Charing Cross Hospital, London, U.K. The EB pregnancy urine sample was collected at Columbia University under approved institutional protocols. These urine samples were frozen and stored at −20°C. Each urine was then thawed, filtered on Whatman GF/A, diafiltered against distilled water (dilution factor of 1,000), and concentrated using an Amicon spiral S1Y3 filter (MW cutoff of 3,000). The samples were then lyophilized and later resuspended in water. Other pregnancy urine and non-pregnant control urine samples were collected in Athens, Georgia with permission of the University of Georgia Internal Review Board. These urine samples, stored at −20 °C, were from different patients, and a gestational range of 6–38 weeks was sampled.
Western blot
As a survey of the forms of immunoreactive hCGβ present in each sample, urine samples were electrophoresed in 10% polyacrylamide, non-reducing gels at ambient temperature, followed by qualitative visualization with B108 probing on a western blot. Results from a total hCGβ radioimmunoassay (ICN Pharmaceuticals) were used to dilute the urine to contain approximately equivalent levels of hormone in each sample. However, this radioimmunoassay does not detect hCGβcf, which is bound by B108, and the radioimmunoassay and Western blot were not used as quantitative tools for calculating dilutions for subsequent biosensor analysis. Non-hormone-containing samples were not diluted before gel loading.
BIAcore assay
Surface plasmon resonance uses technology based on mass detection of reagents-bound or covalently attached to biosensor chips, thus obviating the need for radiolabeling or fluorescence probes. The sandwich assay used herein utilizes antibody fragments (devoid of or low in carbohydrates) covalently attached to the chip (see above for protocol), followed by addition of control or hCG-containing samples and then individual lectins. Real-time binding is then detected by deflections of the sensorgrams. Flow rates for all injections but the regeneration pulse were 5 μl/min. Each hCG-containing sample was diluted into HBS to give an empirically determined binding response to the antibody of 150 ± 5 RU, corresponding to about 150 ng protein bound to the flow cell surface. Non-pregnant control patients were diluted to equal the most concentrated solution. The hormone-containing samples were injected in random order for 5 min, followed by a 4 min injection of each of the lectins (Table 1) at 250 μg/ml with 100 μg/ml bovine serum albumin in HBS. The biosensor chip was regenerated to the antibody layer between samples with 15 μl of 10 mM glycine, pH 2.0, flowing at 30 μl/min. Deflections on the sensorgrams caused by injection noise or residual lectin binding to the antibody fragments were removed by subtracting the response of a blank injection of running buffer followed by the lectin from each experimental sensorgram using BIAevaluation software. Similarly, deflections due to mass transfer effects, air bubbles, and other random events were removed by subtracting the sensorgram simultaneously generated on a non-derivatized reference channel from each experimental sensorgram. Samples were grouped according to their condition for comparison of lectin binding.
Table 1.
Lectins used in binding experiments.
| Lectin | Abbreviation | Nominal Specificity |
|---|---|---|
| Recognizing neutral sugars: | ||
| Aleuria aurantia lectin | AAL | fucose |
| Galanthus nivalis agglutinin | GNA | terminal mannose |
| Phaseolus vulgaris erythroagglutinin | PHA-E | Galβ(1–4)GlcNAcβ(1–2)Man |
| Pisum satvium agglutinin | PSA | glucose/mannose |
| Ricinus communis agglutinin | RCA-I | terminal Galβ(1–4)GlcNac |
| Recognizing sialic acid: | ||
| Maackia amurensis lectin II | MAL-II | NeuNacα(2–3)Galβ(1–4)GlcNAc |
| Sambucis nigra agglutinin | SNA | NeuNAcα(2–6)Gal/GalNAc |
| Wheat germ agglutinin | WGA | sialic acid; β(1–4)GlcNAc |
Gal-galactose, GalNAc-N-acetylgalactosamine, GlcNAc-N-acetylglucosamine, NeuNAc-N- acetylneuraminic acid, Man-mannose
Results
There was no detectable B108 immunoreactivity to urine from non-pregnant females. All hCGpositive samples contained immunoreactive forms with approximate molecular weights less than 25 kD, presumably representing hCGβcf and forms at sizes corresponding to free hCGβ, the intact heterodimer, or both (Fig. 1). The differential treatment and storage of the samples seemed to have an effect on the distribution of the forms of the β-subunit. For example, the pregnancy samples collected specifically for these experiments contained predominantly heterodimeric hCG, whereas EB, a urine concentrate from late first trimester pregnancy that was collected for previous projects and stored for some time, apparently contained only free hCGβ as suggested by the SDS gel blot analyses (Fig. 1). Dissociation of the heterodimer into subunits may explain the prevalence of hCGβ in this sample and the other stored and lyophilized samples. Those samples that had been concentrated showed slower-migrating proteins, perhaps indicating aggregation of hCG or its subunits during or after the concentration procedures. Nonetheless, all of these forms should be captured in the biosensor assay.
Figure 1.
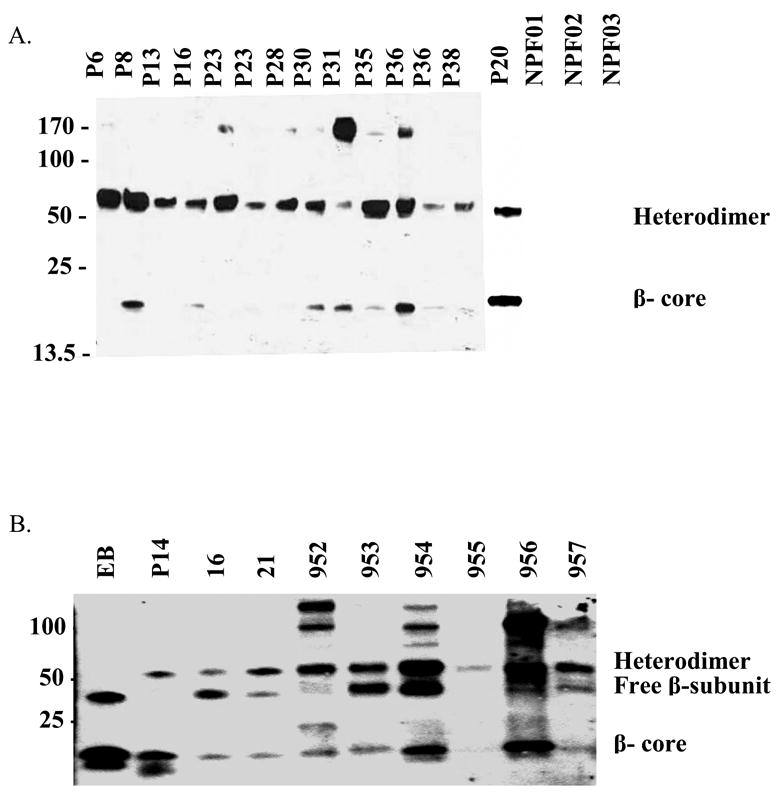
Immunoreactive forms of hCGβ in urine samples as determined by Western analysis. Samples EB, 16, 21, and 952–957 had been previously concentrated; the others are raw urine samples. Diluted samples were probed with monoclonal antibody B108, which recognizes an epitope common to hCG, hCGβ, and the hCGβ-core fragment, and serves as the capture antibody for the biosensor assay. A. Pregnancy samples are designated P#, with the number indicating the week of pregnancy at time of sampling. The three non-pregnant controls are designated NPF01-NPF03 B. Pregnancy (EB = 11 weeks and P14 =14 weeks) and patient disease samples. 16, 21, 954 = choriocarcinoma; 952, 955, 956 = invasive mole; 953, 957 = male germ cell tumor
In representative sensorgrams (Fig. 2), the baseline corresponds to the concentration of thiol-linked B108 F(ab′) fragments. Hormone from the injected urine samples binds to the antibody fragments and remains associated. Although a separate wash step was not included, running buffer continues to flow over the chip as the lectin injection is being prepared, a process that takes about 2 min. A jump in resonance units upon injection signifies lectin binding. Because equivalent levels of hCG and hCG-derived molecules were bound from all hCG-containing samples, plot overlays quickly become visually complicated and only the independent shift accumulated at the end of the lectin binding phase is presented for comparisons of lectin binding.
Figure 2.
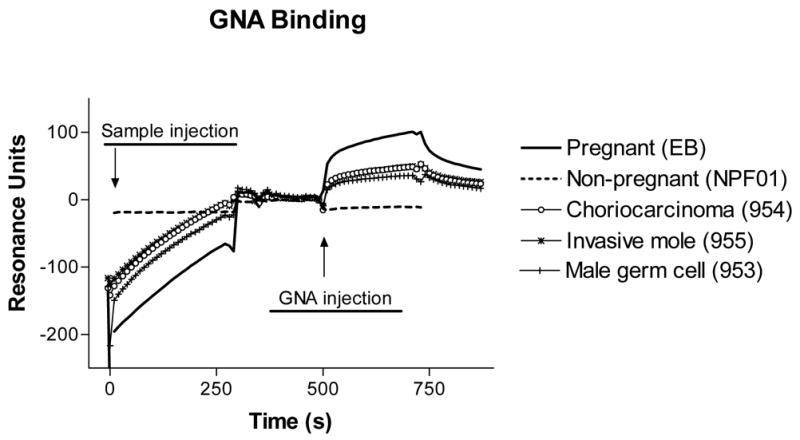
Representative sensorgrams of lectin binding to hCG captured from urine specimens. Samples were diluted to equivalent amounts (150 ± 5 RU) of hormone so that their binding of lectins could be directly compared. The binding of GNA to hCG captured from one sample of each patient condition is shown.
An examination of lectin binding to hCG and derivatives from pregnancy urine revealed no dramatic changes throughout the gestation period examined (6–38 weeks), but some trends were suggested. There appeared to be increased binding of MAL II, PHA-E, and WGA to samples collected at six and eight weeks gestation relative to later periods; however, additional studies are required to address this possibility. Since no significant differences were discerned in lectin binding to urinary hCG between 6–38 weeks gestation, the results from all pregnancy samples were averaged to show “pregnancy” values in the comparative studies.
Among the five lectins exclusively recognizing neutral sugars, AAL, GNA, PHA-E, PSA, and RCA-I, only the mannose-binding lectin GNA discriminated pregnancy from the malignant gestational trophoblastic neoplasia and male germ cell tumor samples, with binding 2–4-fold higher to the pregnancy samples (Fig. 3). Three lectins were tested that recognize sialic acid, MAL-II, SNA, and WGA. Differential binding was observed with MAL-II (3-6-fold higher in the pregnancy samples), indicating decreased α(2–3)sialic acid content in the samples from women with malignant forms of gestational trophoblastic disease and from men with germ cell tumors (Fig. 4). The binding of SNA, which recognizes α(2–6) sialic acid, was highly variable, and no significant differences were observed between samples. Grouped with the sialic acid binding lectins, WGA exhibited 2–3-fold lower binding to pregnancy hCG; however, its complicated pattern of recognition makes it difficult to conclude which carbohydrates are actually less prevalent.
Figure 3.
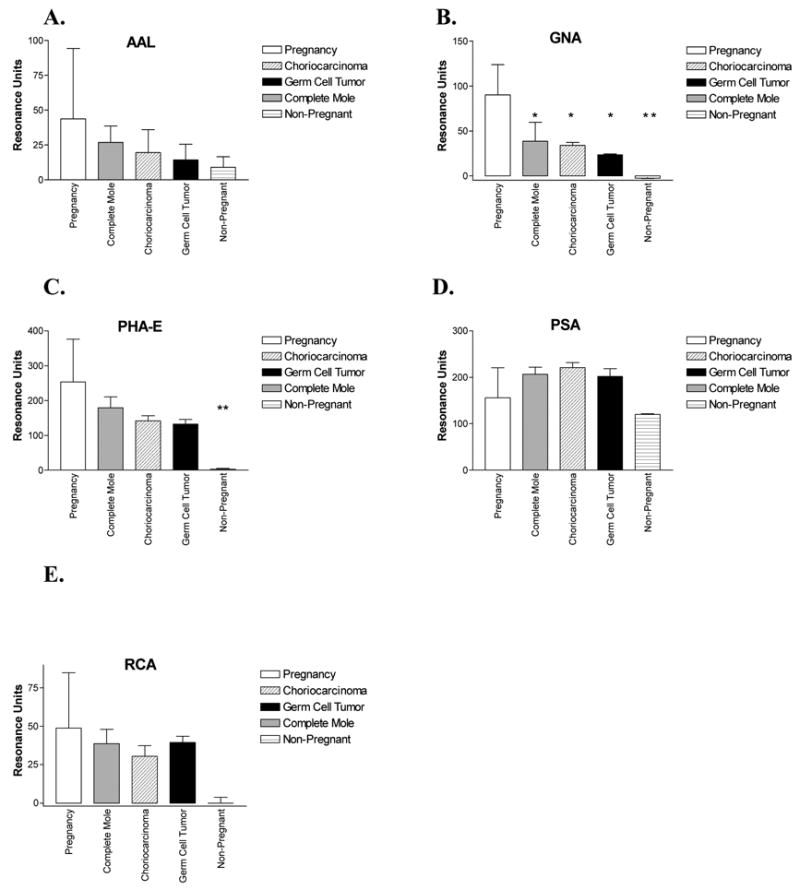
Binding of lectins (mean ± SD) recognizing neutral sugars to 150 RU hCG captured from human urine samples, grouped according to condition and compared to non-pregnant controls with ANOVA. A. AAL. B. GNA. C. PHA-E. D. PSA. E. RCA-I. * = p< 0.05, ** = p<0.01
Figure 4.
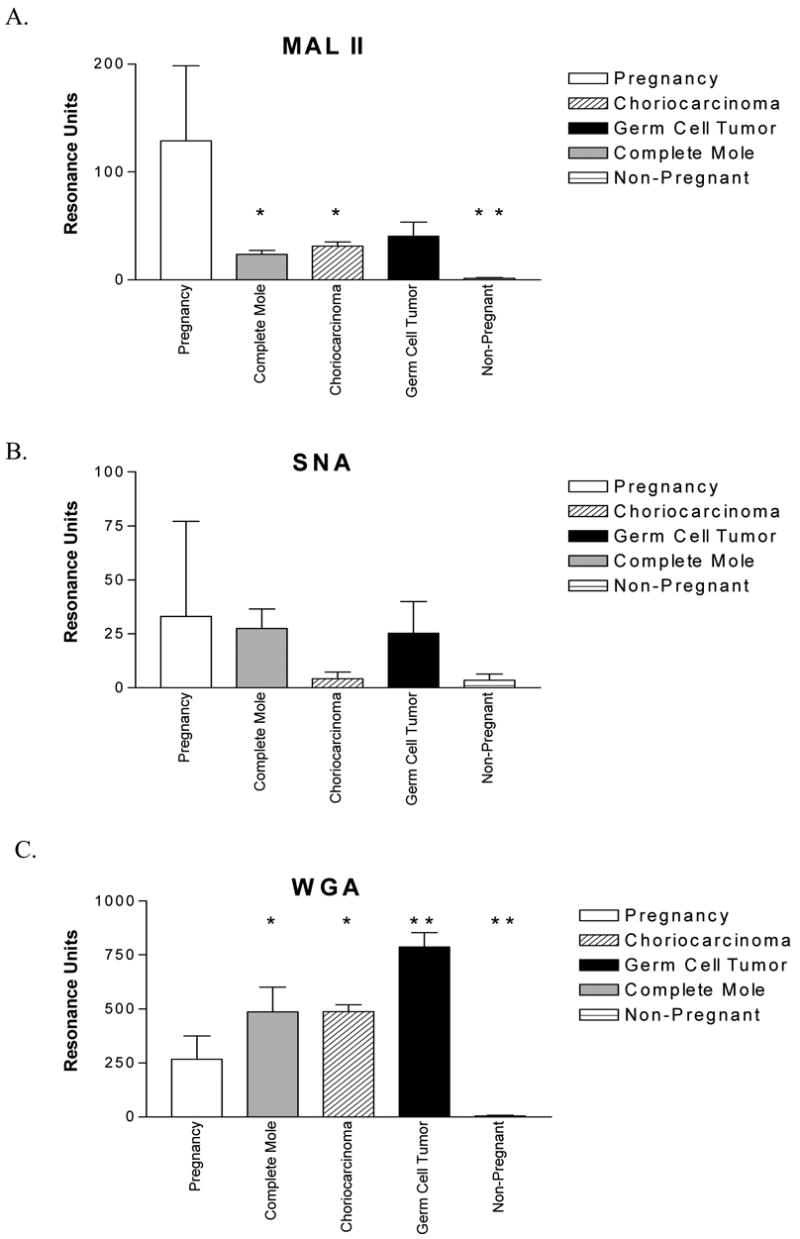
Binding of lectins (mean ± SD) recognizing sialic acid to 150 RU hCG captured from human urine samples, grouped according to condition and compared to nonpregnant controls with ANOVA. A. MAL II. B. SNA. C. WGA.
* = p< 0.05, ** = p<0.01
Discussion
These results show, for the first time, the use of a surface plasmon resonance-based sandwich assay to distinguish urinary hyperglycosylated hCG, produced by malignant gestational trophoblastic neoplasia and male germ cell tumors, from urinary hCG produced in normal pregnancy. Using the F(ab′) fragment of a monoclonal antibody to capture hCG, a panel of eight lectins was screened for their ability to bind the carbohydrate moieties of hCG on the hCG-F(ab′) complex. Of these lectins, three, GNA, MAL-II, and WGA, were capable of binding differentially to urinary hCG from patients with germ cell tumors, choriocarcinoma, and invasive mole compared to pregnant individuals. While we do not yet have a large number of patients in each of the three disease states, i.e. choriocarcinoma, complete mole, and male germ cell tumor, the results indicate that these lectins represent useful tools in distinguishing different hCG-secreting populations. It is somewhat surprising that AAL failed to distinguish hyperglycoslylated hCG, but this may be due to the steric restrictions by the oligosaccharide and protein in the vicinity of fucose. There was no significant difference in the binding of the eight lectins to hCG in the urine of pregnant women between 6–38 weeks of gestation. One would expect that earlier in gestation some hyperglycosylated hCG would be detected (Kovalevskaya et al. 2002b).
In an earlier study (Kelly et al. 2005), we reported the results of an ELISA-based assay and a biosensor assay (as described herein) to distinguish urinary hCG from pregnant individuals from that secreted by three human choriocarcinoma cell lines, JAR, JEG-3, and BeWo. The hCG captured from choriocarcinoma cell supernatant bound more RCA-I and SNA, and less GNA, among other differences. Some lectin binding patterns using urine samples from patients with choriocarcinoma, invasive mole, and male germ cell tumors were consistent with those described for the choriocarcinoma cell lines; for example, GNA and MAL-II binding were decreased in both cases relative to urinary pregnancy hCG. Very striking, however, were the different binding profiles of SNA, which recognizes α(2–6)sialic acid. SNA bound extremely well to hCG from the three choriocarcinoma cell lines, indeed at levels far above that on pregnancy-derived hCG, but it bound roughly the same to urinary hCG from pregnant individuals and patients with the malignancies investigated herein. These differences may arise from the fact that urinary hCG has been partially degraded during renal secretion, a process that depends in part on the sialylation status of the hormone (Liu et al. 1989) and generates different populations of hCG in serum versus urine (Fein et al.. 1980, Kovalevskaya et al. 1999, Jeschke et al. 2003, Sutton 2004). The binding of SNA to hCG from choriocarcinoma serum needs to be examined and may more closely correlate with that seen on hCG secreted immediately into the cell culture supernatant.
A previous study using RCA-I as the capture agent described increased lectin binding to hCG partially purified from choriocarcinoma patient urine (Imamura et al. 1987), which we also found true for hCG secreted by choriocarcinoma cell lines (Kelly et al. 2005). Measuring binding levels directly on captured hormone, however, we found no difference in mean levels of RCA-I binding to hCG from urine specimens of patients with choriocarcinoma, invasive mole, and male germ cell tumors above pregnancy hCG, even despite indications of lower levels of sialylation of urinary hCG from the disease groups (i.e. lower MAL-II binding). The presence in urine of substantial amounts of hCGβcf, which contains minimal (Birken et al. 1988, de Medeiros et al. 1993) or no (Blithe et al. 1989, Jacoby et al. 2000) galactose, may explain the overall lower levels of RCA-I binding for hCG from patient urine specimens than from choriocarcinoma cell culture supernatant (36 RU RCA-I per 150 RU immunoreactive urinary hCG compared to 180 RU RCA-I per 200 RU immunoreactive cellular hCG (Kelly et al. 2005).
The core fragment is unsuitable for studies on differences in sialic acid or terminal galactose residues, but its glycosylation profile may explain the higher binding of GNA, a terminal mannose-binding lectin, on pregnancy hCG. Some isoforms of the full-length oligosaccharides of the heterodimer do include an exposed terminal α(1–6)mannose, but these are not seen in excess in pregnancy samples (Elliott et al. 1997). Although one binding site of GNA accommodates single terminal residues (Shibuya et al. 1988), a higher affinity site binds the branched tri-mannose as found at the core of N-linked oligosaccharide chains (Wright & Hester 1996), but only if no residues extend beyond that core. Both binding modes of GNA could be exploited on the abbreviated carbohydrates of hCGβcf. Although many hCGβcf oligosaccharides are highly truncated, not all of the glycoforms contain terminal mannose (de Medeiros et al. 1993). Almost 20% contain GlcNAc moieties distal to the mannose core (Jacoby et al. 2000), and these were postulated to derive from the low percentage of choriocarcinoma-like bi- and triantennary hCG glycoforms found in normal pregnancy. These isoforms will not bind GNA, but could potentially bind WGA.
The structures of the glycoforms of hCGβcf derived from germ cell tumor patients have yet to be determined, but it is expected that variations found on the full-length carbohydrates will persist in their remnants (Endo et al. 1992, Jacoby et al. 2000). The carbohydrates of the core fragment from two Down syndrome pregnancies were composed of more GlcNAc and fucose than that from normal pregnancy (Cole et al. 1997). The core fragment may be an especially useful marker because it is often observable in urine even when its precursor is undetectable in the serum (Cole et al. 1988, O'Connor et al. 1988, Rinne et al. 1999); moreover, it is exceptionally stable upon storage (de Medeiros et al.. 1991, Cole 1997, Mulder et al. 1997). Our hypothesis that differential binding of GNA mainly reflects differences in glycosylation on the core fragment needs to be examined by using a hCGβcf-specific capture antibody (O'Connor et al. 1994).
Because these three lectins probe the N-linked oligosaccharides on hCG, they could complement the use of B152, which recognizes a linear epitope encompassing a branched (Core 2) O-linked oligosaccharide (Kovalevskaya et al. 2002b), independent of sialylation status (Birken et al. 1999), on Serine 132 in the CTP-region of hCG (Birken et al. 2003). This antibody was raised against a choriocarcinoma-derived hCG, and its increased binding may indicate trophoblastic disease (Birken et al. 2001), but it may also be useful in detecting risk of pregnancy complications such as early pregnancy loss (Kovalevskaya et al. 2002b) and Down syndrome (Cole et al. 1999, Pandian et al. 2003). A lectin immunoassay using WGA binding on hCG in serum (Abushoufa et al. 2000) was not an effective additional marker in screening for Down syndrome in the first trimester (Spencer et al. 2002), but it was not tested with urine specimens. It would be interesting to ascertain if differences in GNA binding could supplement differences in B152 binding as part of a multiple-marker screening for Down syndrome.
In conclusion, the lectins, GNA, MAL-II, and WGA, discriminated immunoreactive urinary hCG from a limited number of individuals with choriocarcinoma, invasive mole, and male germ cell tumors from those at various stages of a normal pregnancy. Differential binding of lectins among hCG sources has been previously described, but this report is the first to examine the discriminatory power of GNA binding and the first to use a surface plasmon resonance-based assay on human urine samples. By using deglycosylated antibody fragments as capture reagents, the clinical potential of lectin discrimination of condition-specific glycoproteins can now be explored.
Acknowledgments
We thank Ms. Kristen Scarbrough and Dr. Margaret Cramer for their assistance in obtaining the pregnancy urine samples. This research was supported by NIH DK33973, the University of Georgia Research Foundation, Inc., the Georgia Research Alliance, and Oncose, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abushoufa RA, Talbot JA, Brownbill K, Rafferty B, Kane JW, Robertson WR. The development of a sialic acid specific lectin-immunoassay for the measurement of human chorionic gonadotrophin glycoforms in serum and its application in normal and Down's syndrome pregnancies. Clinical Endocrinology. 2000;52:499–508. doi: 10.1046/j.1365-2265.2000.00968.x. [DOI] [PubMed] [Google Scholar]
- Amano J, Nishimura R, Mochizuki M, Kobata A. Comparative study of the mucin-type sugar chains of human chorionic gonadotropin present in the urine of patients with trophoblastic diseases and healthy pregnant women. The Journal of Biological Chemistry. 1988;263:1157–1165. [PubMed] [Google Scholar]
- Birken S. Specific measurements of O-linked Core 2 sugar-containing isoforms of hyperglycosylated human chorionic gonadotropin by Antobody B152. Tumour Biol. 2005;26:131–141. doi: 10.1159/000086484. [DOI] [PubMed] [Google Scholar]
- Birken S, Armstrong EG, Kolk MA, Cole LA, Agosto GM, Krichevsky A, Vaitukaitis JL, Canfield RE. Structure of the human chorionic gonadotropin beta-subunit fragment from pregnancy urine. Endocrinology. 1988;123:572–583. doi: 10.1210/endo-123-1-572. [DOI] [PubMed] [Google Scholar]
- Birken S, Krichevsky A, O'Connor J, Schlatterer J, Cole L, Kardana A, Canfield R. Development and characterization of antibodies to a nicked and hyperglycosylated form of hCG from a choriocarcinoma patient. Endocrine. 1999;10:137–144. doi: 10.1385/ENDO:10:2:137. [DOI] [PubMed] [Google Scholar]
- Birken S, Kovalevskaya G, O'Connor J. Immunochemical measurement of early pregnancy isoforms of HCG: potential applications to fertility research, prenatal diagnosis, and cancer. Archives of Medical Research. 2001;32:635–643. doi: 10.1016/s0188-4409(01)00329-0. [DOI] [PubMed] [Google Scholar]
- Birken S, Yershova O, Myers RV, Bernard MP, Moyle W. Analysis of human choriogonadotropin core 2 o-glycan isoforms. Molecular and Cellular Endocrinology. 2003;204:21–30. doi: 10.1016/s0303-7207(03)00153-9. [DOI] [PubMed] [Google Scholar]
- Blithe DL, Wehmann RE, Nisula BC. Carbohydrate composition of β-core. Endocrinology. 1989;125:2267–2272. doi: 10.1210/endo-125-5-2267. [DOI] [PubMed] [Google Scholar]
- Cole LA. Stability of hCG free β-subunit and β-core fragment in urine. Prenatal Diagnosis. 1997;17:185–189. [PubMed] [Google Scholar]
- Cole LA. hCG, its free subunits and metabolites: roles in pregnancy and trophoblastic disease. Journal of Reproductive Medicine. 1998;43:3–10. [PubMed] [Google Scholar]
- Cole LA, Wang YX, Elliott M, Latif M, Chambers JT, Chambers SK, Schwartz PE. Urinary human chorionic gonadotropin free beta-subunit and beta-core fragment: a new marker of gynecological cancers. Cancer Research. 1988;48:13561360. [PubMed] [Google Scholar]
- Cole LA, Cermik D, Bahado-Singh R. Oligosaccharide variants of hCG-related molecules: potential screening markers for Down syndrome. Prenatal Diagnosis. 1997;17:1187–1190. [PubMed] [Google Scholar]
- Cole LA, Shahabi S, Oz UA, Bahado-Singh RO, Mahoney MJ. Hyperglycosylated human chorionic gonadotropin (invasive trophoblast antigen) immunoassay: A new basis for gestational Down syndrome screening. Clinical Chemistry. 1999;45:2109–2119. [PubMed] [Google Scholar]
- de Medeiros SF, Amato F, Matthews CD, Norman RJ. Molecular heterogeneity of the ß-core fragment of human chorionic gonadotropin. Journal of Endocrinology. 1993;139:519–532. doi: 10.1677/joe.0.1390519. [DOI] [PubMed] [Google Scholar]
- de Medeiros SF, Amato F, Norman RJ. Stability of immunoreactive beta-core fragment of hCG. Obstetrics and Gynecology. 1991;1:53–59. [PubMed] [Google Scholar]
- Elliott MM, Kardana A, Lustbader JW, Cole LA. Carbohydrate and peptide structure of the α- and β- subunits of human chorionic gonadotropin from normal and aberrant pregnancy and choriocarcinoma. Endocrine. 1997;7:15–32. doi: 10.1007/BF02778058. [DOI] [PubMed] [Google Scholar]
- Endo T, Iino K, Nozawa S, Iizuka R, Kobata A. Immobilized Datura stramonium agglutinin column chromatography, a novel method to discriminate the urinary hCGs of patients with invasive mole and choriocarcinoma from those of normal pregnant women and patients with hydatidiform mole. Japanese Journal of Cancer Research (Gann) 1988;79:160–164. doi: 10.1111/j.1349-7006.1988.tb01572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo T, Nishimura R, Teshima S, Ohkura H, Baba S, Kobata A. Structures of the asparagine-linked sugar chains of human chorionic gonadotropin from a patient with extragonadal germ cell tumour. European Journal of Cancer. 1991;27:277–280. doi: 10.1016/0277-5379(91)90515-f. [DOI] [PubMed] [Google Scholar]
- Endo T, Nishimura R, Saito S, Kanazawa K, Nomura K, Katsuno M, Shii K, Mukhopadhyay K, Baba S, Kobata A. Carbohydrate structures of β-core fragment of human chorionic gonadotropin isolated from a pregnant individual. Endocrinology. 1992;130:2052–2058. doi: 10.1210/endo.130.4.1547728. [DOI] [PubMed] [Google Scholar]
- Fein HG, Rosen SW, Weintraub BD. Increased glycosylation of serum human chorionic gonadotropin and subunits from eutopic and ectopic sources: comparison with placental and urinary forms. Journal of Clinical Endocrinology and Metabolism. 1980;50:1111–1120. doi: 10.1210/jcem-50-6-1111. [DOI] [PubMed] [Google Scholar]
- Hearn MTW, Gomme PT. Molecular architecture and biorecognition processes of the cystine knot protein superfamily: Part I. The glycoprotein hormones. Journal of Molecular Recognition. 2000;13:223–278. doi: 10.1002/1099-1352(200009/10)13:5<223::AID-JMR501>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Hotakainen K, Lintula S, Stenman J, Rintala E, Lindell O, Stenman UH. Detection of messenger RNA for the β-subunit of chorionic gonadotropin in urinary cells from patients with transitional cell carcinoma of the bladder by reverse transcription-polymerase chain reaction. International Journal of Cancer. 1999;84:304–308. doi: 10.1002/(sici)1097-0215(19990621)84:3<304::aid-ijc18>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Imamura S, Armstrong EG, Birken S, Cole LA, Canfield RE. Detection of desialylated forms of human chorionic gonadotropin. Clinica Chimica Acta. 1987;163:339–349. doi: 10.1016/0009-8981(87)90252-x. [DOI] [PubMed] [Google Scholar]
- Jacoby ES, Kicman AT, Laidler P, Iles RK. Determination of the glycoforms of human chorionic gonaotropin ß-core fragment by matrix-assisted laser desorption/ionization time-of-flight mass spectroscopy. Clinical Chemistry. 2000;46:1796–1803. [PubMed] [Google Scholar]
- Jeschke U, Stahn R, Goletz C, Wang X, Briese V, Friese K. hCG in trophoblast tumour cells of the cell line Jeg3 and hCG isolated from amniotic fluid and serum of pregnant women carry oligosaccharides of the sialyl Lewis X and sialyl Lewis a type. Anticancer Research. 2003;23:1087–1092. [PubMed] [Google Scholar]
- Kelly LS, Kozak M, Walker T, Pierce M, Puett D. Lectin immunoassays to detect varied glycosylation patterns on human chorionic gonadotropin secreted by normal and transformed cells. Analytical Biochemistry. 2005;338:253–262. doi: 10.1016/j.ab.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Kobata A. Structural changes induced in the sugar chains of glycoproteins by malignant transformation of producing cells and their clinical application. Biochemie. 1988;70:1575–1585. doi: 10.1016/0300-9084(88)90293-3. [DOI] [PubMed] [Google Scholar]
- Kovalevskaya G, Birken S, Kakuma T, O'Connor JF. Early pregnancy human chorionic gonadotropin (hCG) isoforms measured by an immunometric assay for choriocarcinoma-like hCG. Journal of Endocrinology. 1999;161:99–106. doi: 10.1677/joe.0.1610099. [DOI] [PubMed] [Google Scholar]
- Kovalevskaya G, Genbacev O, Fisher SJ, Caceres E, O'Connor JF. Trophoblast origin of hCG isoforms: cytotrophoblasts are the primary source of choriocarcinoma-like hCG. Molecular and Cellular Endocrinology. 2002a;194:147–155. doi: 10.1016/s0303-7207(02)00135-1. [DOI] [PubMed] [Google Scholar]
- Kovalevskaya G, Birken S, Kakuma T, Ozaki N, Sauer M, Lindheim S, Cohen M, Kelly A, Schlatterer J, O'Connor JF. Differential expression of human chorionic gonadotropin (hCG) glycosylation isoforms in failing and continuing pregnancies: preliminary characterization of the hyperglycosylated hCG epitope. Journal of Endocrinology. 2002b;172:497–506. doi: 10.1677/joe.0.1720497. [DOI] [PubMed] [Google Scholar]
- Liu L, Southers JL, Cassels JW, Jr, Banks SM, Wehmann RE, Blithe DL, Chen HC, Nisula BC. Structure-kinetic relationships of choriogonadotropin and related molecules. The American Journal of Physiology. 1989;256:E721–724. doi: 10.1152/ajpendo.1989.256.6.E721. [DOI] [PubMed] [Google Scholar]
- Lundin M, Nordling S, Lundin J, Alfthan H, Stenman UH, Haglund C. Tissue expression of human chorionic gonadotropin β predicts outcome in colorectal cancer: a comparison with serum expression. International Journal of Cancer. 2001;95:18–22. doi: 10.1002/1097-0215(20010120)95:1<18::aid-ijc1003>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Mann K, Karl H-J. Molecular heterogeneity of human chorionic gonadotropin and its subunits in testicular cancer. Cancer. 1983;52:654–660. doi: 10.1002/1097-0142(19830815)52:4<654::aid-cncr2820520415>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Mizuochi T, Nishimura R, Derappe C, Taniguchi T, Hamamoto T, Mochizuki M, Kobata A. Structures of the asparagine-linked sugar chains of human chorionic gonadotropin produced in choriocarcinoma: appearance of triantennary sugar chains and unique biantennary sugar chains. The Journal of Biological Chemistry. 1983;258:14126–14129. [PubMed] [Google Scholar]
- Mora J, Gascon N, Tabernero JM, Rodriguez-Espinosa J, Gonzalez-Sastre F. Different hCG assays to measure ectopic hCG secretion in bladder carcinoma patients. British Journal of Cancer. 1996;74:1081–1084. doi: 10.1038/bjc.1996.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder C, Schutter EM, van Uxem WI, van Kamp GJ. Stability of human urinary gonadotropin peptide. Tumour Biology. 1997;18:274–277. doi: 10.1159/000218040. [DOI] [PubMed] [Google Scholar]
- O'Connor JF, Schlatterer JP, Birken S, Krichevsky A, Armstrong EG, McMahon D, Canfield RE. Development of highly sensitive immunoassays to measure human chorionic gonadotropin, its β-subunit, and β core fragment in the urine: application to malignancies. Cancer Research. 1988;48:1361–1366. [PubMed] [Google Scholar]
- O'Connor JF, Birken S, Lustbader JW, Krichevsky A, Chen Y, Canfield RE. Recent advances in the chemistry and immunochemistry of human chorionic gonadotropin: impact on clinical measurements. Endocrine Reviews. 1994;15:650–683. doi: 10.1210/edrv-15-5-650. [DOI] [PubMed] [Google Scholar]
- Pandian R, Lu J, Ossolinska-Plewnia J. Fully automated chemiluminometric assay for hyperglycosylated human chorionic gonadotropin (invasive trophoblast antigen) Clinical Chemistry. 2003;49:808–810. doi: 10.1373/49.5.808. [DOI] [PubMed] [Google Scholar]
- Rinne K, Shahabi S, Cole L. Following metastatic placental site trophoblastic tumor with urine β -core fragment. Gynecologic Oncology. 1999;74:302–303. doi: 10.1006/gyno.1999.5438. [DOI] [PubMed] [Google Scholar]
- Rao SNV, Moyle WR. Modeling human chorionic gonadotropin using distance geometry and immunological constraints. In: Carbb JW, editor. Techniques in Protein Chemistry. Academic Press; New York: 1994. pp. 413–420. [Google Scholar]
- Sakai H, Yamagishi F, Miura M, Hata K, Koyama I, Sakagishi Y, Komoda T. Sugar chain heterogeneity of human urinary chorionic gonadotropin determined by serial lectin affinity chromatography: difference between benign and malignant disease. Tumour Biology. 1994;15:230–235. doi: 10.1159/000217896. [DOI] [PubMed] [Google Scholar]
- Shibuya N, Goldstein IJ, Van Damme EJ, Peumans WJ. Binding properties of a mannose-specific lectin from the snowdrop (Galanthus nivalis) bulb. The Journal of Biological Chemistry. 1988;263:728–734. [PubMed] [Google Scholar]
- Spencer K, Talbot JA, Abushoufa RA. Maternal serum hyperglycosylated human chorionic gonadotrophin (HhCG) in the first trimester of pregnancies affected by Down syndrome, using a sialic acid-specific lectin immunoassay. Prenatal Diagnosis. 2002;22:656–662. doi: 10.1002/pd.351. [DOI] [PubMed] [Google Scholar]
- Sutton JM. Charge variants in serum and urine hCG. Clinica Chimica Acta. 2004;341:199–203. doi: 10.1016/j.cccn.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Wide L, Lee J-Y, Rasmussen C. A change in the isoforms of human chorionic gonadotropin occurs around the 13th week of gestation. Journal of Clinical Endocrinology and Metabolism. 1994;78:1419–1423. doi: 10.1210/jcem.78.6.7515388. [DOI] [PubMed] [Google Scholar]
- Wright CS, Hester G. The 2.0Å structure of a cross-linked complex between snowdrop lectin and a branched mannopentaose: evidence for two unique binding modes. Structure. 1996;4:1339–1352. doi: 10.1016/s0969-2126(96)00141-4. [DOI] [PubMed] [Google Scholar]
- Yokotani T, et al. Expression of α and β genes of human chorionic gonadotropin in lung cancer. International Journal of Cancer. 1997;71:539–544. doi: 10.1002/(sici)1097-0215(19970516)71:4<539::aid-ijc6>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Yoshimoto Y, Wolfsen AR, Odell WD. Glycosylation, a variable in the production of hCG by cancers. The American Journal of Medicine. 1979;67:414–419. doi: 10.1016/0002-9343(79)90787-3. [DOI] [PubMed] [Google Scholar]


