Abstract
Naturally occurring electric fields (EFs) have been implicated in cell guidance during embryonic development and adult wound healing. Embryonic Xenopus laevis neurons sprout preferentially towards the cathode, turn towards the cathode, and migrate faster towards the cathode in the presence of an external EF in vitro. A recent Phase 1 clinical trial has investigated the effects of oscillating EFs on human spinal cord regeneration. The purpose of this study was to investigate whether embryonic zebrafish neurons respond to an applied EF, and thus extend this research into another vertebrate system. Neural tubes of zebrafish embryos (16–17 somites) were dissected and dissociated neuroblasts were plated onto laminin-coated glass. A 100 mV/mm EF was applied to cell cultures for 4 or 20 hours and the responses of neurons to the applied EFs were investigated. After 4 hours in an EF neurites were significantly shorter than control neurites. No other statistically significant effects were observed. After 20 hours, control and EF-exposed neurites were no different in length. No length difference was seen between cathodally- and anodally-sprouted neurites. Application of an EF did not affect the average number of neurons in a chamber. Growth cones did not migrate preferentially towards either pole of the EF and no asymmetry was seen in neurite sprout sites. We conclude that zebrafish neurons do not respond to a 100 mV/mm applied EF in vitro. This suggests that neurons of other vertebrate species may not respond to applied EFs in the same ways as Xenopus laevis neurons.
Keywords: Zebrafish neurons, Growth cone guidance, Electric field
In an early paper unfortunately lacking in experimental detail, S. Ingvar [6] reported that nerve fiber outgrowth from electric field (EF)-exposed chick brain explants occurred “almost entirely along the lines of force in the galvanic field” (Ingvar’s emphasis). He also suggested that there were “morphological differences” between fibers growing towards the anode (positive electrode) and those growing towards the cathode (negative electrode). These findings were challenged by Weiss [16], and Williams [17], who saw no effect of an applied EF on nerve fibers growing out from chick CNS explants. Indeed, Williams states that “in every case where any change was seen in the culture, this change was degenerative and in no case was the direction of growth of fibers changed” [17]. Marsh and Beams [8] appeared to have resolved the principal questions in showing that outgrowth of nerve fibers from chick medulla fragments in an applied EF was suppressed on the anodal side, and that a majority of nerve fibers growing at an angle to the field vector turned towards the cathode. Despite these seemingly conclusive results, D. Ingvar [5] reported that “the current had no influence on the direction of the outgrowing nerve cell processes.” Later, Jaffe and Poo [7] showed that the growth rate of cathodal neurites was “several times faster” than anodal neurites in cultured chick dorsal root ganglia exposed to 70–140 mV/mm EFs, though they saw no turning of neurites towards either pole of the EF. In recent years, the Xenopus laevis dissociated embryonic spinal neuron culture has emerged as the best established system for the study of nerve growth in an EF.
The growth cones of neurons isolated from embryonic Xenopus laevis neural tubes migrate directionally towards the cathode of an applied EF of physiological magnitude in vitro, often executing substantial turns in order to do so. The neurite conveniently records where the growth cone has been, allowing the turning to be measured. Neurite initiation sites are more numerous towards the cathode than the anode [13]. Rates of growth are enhanced towards the cathode as compared to anodal growth rates, and anodal neurites are often retracted [10]. Importantly, when the EF polarity is reversed, previously cathodal-facing neurites (now anodal) are retracted at a slower rate than the rate of advance of previously anodal neurites (now cathodal) [9]. This means that a degree of cathodal advancement will have occurred before the anodal neurites begin to retract. If an oscillating EF is employed, when the EF polarity is reversed the previously anodal neurite will advance a little before the previously cathodal neurite begins retracting. Thusly, net stimulation of growth of neurites in both directions is achieved by precisely controlling the rate of oscillation. This forms the basis of a recently completed Phase 1 clinical trial aimed at improving neuronal recovery from traumatic spinal cord injury [14]. Very little is known about how growth cones sense the EF and respond to it, but recent work has begun to reveal the intracellular signaling mechanisms involved [11, 12].
Since the effects of applied EFs on growing neurons are well established only in a single experimental system, and given the possible medical importance of this line of research, we felt it important to extend the research into another vertebrate system. Therefore we have utilized an in vitro culture system for embryonic zebrafish (Danio rerio) neurons [1] to investigate neuronal responses to applied EFs in a model system that offers more opportunity for genetic manipulation than Xenopus laevis. We are only interested in responses to EFs of physiological magnitude. By physiological, we refer to the magnitude of EFs a growing neurite may encounter in the developing embryo. These EFs are distinct from the EFs to which a cell residing in an ion-pumping epithelium would be exposed. Electrical potential differences of tens of millivolts are typically measured across a 20–30 μm epithelial layer, resulting in EFs of hundreds of mV/mm across the epithelial layer [2–4]. Cells existing outside the epithelial layer will never be exposed to EFs of this magnitude in the intact organism. Actual measured voltage gradients in the tissues of Xenopus laevis embryos are around 10 mV/mm along the neural folds and approximately 40 mV/mm on the ventral side [4]. In the somite regions of chicken embryos, voltage gradients are measured up to a maximum of around 25 mV/mm [3]. We used a 100 mV/mm EF because we must allow for the possibility that voltage gradients larger than those previously measured may exist in developing embryos. Xenopus laevis neurites in culture respond maximally at this EF magnitude [2].
We report that embryonic zebrafish spinal neurons in culture showed almost no response to a 100 mV/mm applied EF. Neurites exposed to an EF for 4 hours were significantly shorter than control neurites of the same age. No asymmetry was seen in neurite initiation site or final direction of growth cone migration. No difference was observed between lengths of neurites sprouted towards the cathode and those sprouted towards the anode.
Four different chambers were utilized: a glass bottomed EF-application chamber coated overnight with laminin (Sigma, L-2020; 1 mg/mL laminin in Tris-buffered NaCl), a glass-bottomed EF-application chamber coated overnight with zebrafish fibronectin (2 μg/μL fibronectin stock solution; a gift from our Purdue colleague, Dr. Paul Collodi), an uncoated glass-bottomed EF-application chamber, and an uncoated Falcon tissue culture plastic EF-application chamber. Each of these chambers allowed us to pass electrical current if required. Laminin and fibronectin coating were carried out overnight at 4°C. An aliquot (25 μL) of laminin solution was dissolved in 500 μL phosphate buffered saline (PBS) and 200 μL of this was added to the central portion of a glass-bottomed EF-application chamber. The chamber was transferred to 4°C overnight. One aliquot (1 μL) of fibronectin was dissolved in 200 μL PBS. This was added to the central portion of a glass-bottomed EF-application chamber, and the chamber kept at 4°C overnight.
Cultures were prepared essentially as described by Andersen [1]. Zebrafish embryos were provided by Dr. Paul Collodi. Adult zebrafish were maintained in conditions designed to minimize pain and discomfort. Embryos were aged at 24–25°C to the 16–17 somite stage in 10% Hank’s balanced saline (13.7 mM NaCl, 0.54 mM KCl, 0.13 mM CaCl2, 0.1 mM MgSO4, 0.044 mM KH2PO4, 0.025 mM Na2HPO4, 0.42 mM NaHCO3). At this stage a substantial amount of neural tube closure had occurred. Embryos were washed for 5 seconds in 70% ethanol, then washed for 5 seconds in Marc’s Modified Ringer’s (MMR: 1M NaCl, 20 mM KCl, 20 mM CaCl2, 10 mM MgSO4, 50 mM HEPES, 0.1 mM EDTA, pH 7.5), and transferred to MMR for dissection. The outer chorion was removed using forceps (Dumont #5) and the neural tube region was dissected from the rest of the embryo using micro-dissection scissors. Neural tubes and associated somites were rolled out of the closely apposed vitelline envelopes using forceps, and then transferred to Ca2+/Mg2+-free solution (136.8 mM NaCl, 5.5 mM Na2CO3, 5.4 mM KCl, 5.5 mM glucose, 0.6 mM EDTA, 0.05% trypsin) for 20 minutes to facilitate cell dissociation. Most Ca2+/Mg2+-free solution was then removed and the neural tubes suspended in zebrafish neuron culture medium (50% L-15 (Sigma), 49% 0.1 x MMR, 1% fetal bovine serum (FBS; Sigma); 200 μL medium per neural tube). Each experimental chamber received 200 μL of the cell suspension thus created. Therefore, each chamber contained the neuroblasts from a single neural tube.
Electrical contact with the culture medium was made via agar bridges constructed from 100 mm lengths of 2 mm inside diameter hollow glass tubing. The bridges were filled with zebrafish neuron medium gelled with 1.5% agarose (Sigma, A-6560) and inserted into holes in either end of the chambers. The other end of each bridge rested in a beaker of zebrafish neuron medium containing Ag/AgCl electrodes which were, in turn, connected to a constant current power supply. The magnitude of the EF (mV/mm) being applied was measured directly at the edges of the coverslip roof of the chamber via Ag/AgCl electrodes connected to a multimeter. A 100 mV/mm EF was applied from hours 2–6 or from hours 2–22.
The first 30 neurons observed in each chamber were photographed using a Nikon D70 digital camera mounted on a Nikon microscope equipped with phase contrast optics. Neurons were photographed 6 or 22 hours after plating using a 20 X objective. Only neurons whose cell bodies, neurite sprout sites, neurites, and growth cones could be unambiguously identified were included. Digital photographs were printed and all measurements made manually. Neurite length was measured using a ruler. Neurite sprout point and the growth cone trajectory at the time of photography were measured as described in the legend to Fig. 1. Comparisons were made of neurite length, sprout site and growth cone final trajectory in chambers exposed to an EF and those that were not. Additionally, the lengths of neurites that were sprouted cathodally and those that were sprouted anodally were compared in field-exposed cultures. For control cultures, comparison was made of neurites that were sprouted to the left of the cell body and those sprouted to the right. Average neurite lengths, neurite sprout site average cosines and growth cone trajectory average cosines were compared using a two-tailed Student’s t-test. Significance level was set at P ≤ 0.05. An average cosine of 0 indicates randomness; approaching 1 or −1 indicates a bias towards one side of the chamber.
Fig.1.
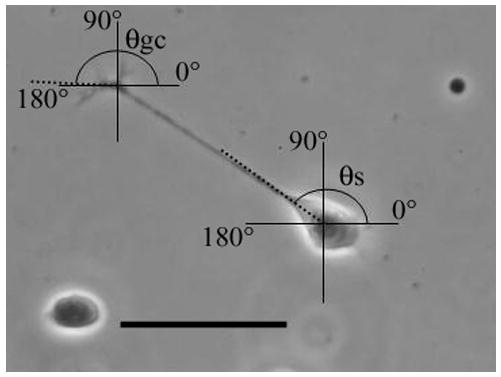
Measurement of neurite sprout point angle and the angle of growth cone migration. A vertical line and a horizontal line were drawn and intersected at the centre of the neuron cell body. A third line (dotted) was drawn from the centre of the cell body through the neurite sprout site. The angle θs was the angle of sprouting. This angle was converted to a cosine and the cosines of all the sprout points of a data set were averaged. A vertical line and a horizontal line were drawn which intersected at the center of the growth cone. A line (dotted), originating at the point of intersection, was drawn tangent to the final direction of growth cone migration. The angle, θgc, was the angle of growth cone migration. The cosines of all the growth cone migration angles of a data set were averaged. For EF-exposed cultures 0° always represented the anode and 180° the cathode. Scale bar represents 50 μm.
In agreement with Andersen [1] we found that neuron outgrowth and rate of growth was very much enhanced on laminin compared to any other substrate. On laminin-coated glass, 172 neurons were seen, on average, per chamber. An average of 34 neurons was seen on fibronectin-coated glass; 25 on uncoated glass; and 15 on uncoated Falcon tissue culture plastic. Neurons were also many times longer on laminin-coated glass than on any of the other substrates, though we did not quantify this. For this reason, we investigated the effects of applied EFs on neurons cultured on laminin-coated glass only. Neurite sprouting occurred approximately 2 hours after plating, so EF application was initiated at hour 2 and continued until hour 6 or hour 22 after plating.
Application of an EF had no effect on the number of neurons per chamber. In chambers not exposed to an EF, 172 neurons were seen 6 hours after plating and an average of 152 were seen in chambers exposed to 100 mV/mm from hours 2–6 (P = 0.17; Table 1).
Table 1.
Effects of a 100 mV/mm applied electric field on zebrafish neurites
| Hours after Plating | EF | Neurons/chamber | n | Neurite Length (μm)
|
Sprout Site | Growth Cone | ||
|---|---|---|---|---|---|---|---|---|
| (Hours in EF) | (mV/mm) | (# chambers) | (# chambers) | Mean | Cathodal | Anodal | Mean Cosine | Mean Cosine |
| 6 | 0 | 172±9.5 (4) | 315 (6) | 70.1±2.7* | 71.8±3.8 | 68.3±3.9 | −0.007±0.04 | 0.005±0.04 |
| 6 (4) | 100 | 152±8.8 (4) | 310 (6) | 61.3±2.5* | 57.4±3.1 | 65.4±3.9 | −0.02±0.04 | −0.04±0.04 |
| 22 | 0 | n/d | 156 (3) | 73.7±5.1 | 75.9±7.6 | 71.2±6.8 | 0.01±0.06 | −0.04±0.05 |
| 22 (20) | 100 | n/d | 147 (3) | 76.6±5.7 | 65.7±6.2 | 87±9.4 | −0.003±0.06 | 0.03±0.06 |
The angle of neurite sprouting was measured for each neurite with respect to the center of the cell body and the polarity of the EF. The angle of growth cone growth at the time of photography was measured for each growth cone with respect to the polarity of the EF. These angles were converted to cosines and averaged. An average cosine of 0 indicates randomness; approaching 1 indicates a bias towards the anode; and approaching −1 indicates a cathodal bias. For all directional measures in control cells, cathodal was assigned to the left side of the cell body. Where appropriate, numbers are expressed as mean ± standard error of the mean. Unless noted, there are no statistically significant differences between comparable data sets.
P = 0.016; n/d = not determined.
It has been suggested that the cathodal bias of neurite initiation sites in Xenopus laevis neuron cultures exposed to an EF is the result of retraction of anodally sprouted neurites, and not preferential sprouting on the cathodal side of the soma [9, 13]. If this is the case in zebrafish neuron cultures exposed to an EF, a similar bias should be seen. This bias should be evident even when an EF is applied after sprouting has occurred. Neurite sprout site with reference to the center of the neuronal cell body was investigated in zebrafish neuron cultures exposed to a 100 mV/mm EF and those that were not. The mean cosine of the sprout sites in chambers not exposed to an EF was −0.007 at 6 hours after plating, and 0.01 at 22 hours after plating (Table 1). The mean cosine of sprout sites in cells exposed to a 100 mV/mm EF from hours 2–6 was −0.02. For cultures exposed to an EF from hours 2–22, the mean cosine of neurite sprout sites was −0.003. These were not significantly different from control cultures at the same times (6 hours, P = 0.77; 22 hours, P = 0.83). Therefore, exposure to an EF did not lead to an asymmetry in neurite sprout sites.
Neurite lengths were measured in cells exposed to an EF and in control chambers (Table 1). Six hours after plating, average neurite length was 70.1 μm (n = 315). The average length of neurites exposed to a 100 mV/mm EF from hours 2–6 was 61.3 μm (n = 310). This was significantly different from neurites not exposed to an EF (P = 0.016). Neurites sprouted towards the cathode were 57.4 μm long on average, and anodal neurites were 65.4 μm long. This difference in average length was not significant (P = 0.11). After 4 hours, in chambers not exposed to an EF, the average lengths of neurites sprouted towards the left were no different from neurites sprouted to the right (P = 0.52). Neurites which have not been exposed to an EF were, on average, 73.7 μm long after 22 hours, and those sprouted to the left were no different in length to those sprouted to the right (P = 0.65). After 22 hours (in a 100 mV/mm EF from hours 2–22), neurites were on average 76.6 μm long (n = 147). This was not significantly different from neurites not exposed to an EF (P = 0.35). Cathodal neurites at 22 hours were 65.7 μm long on average and the length of anodal neurites was not significantly different, an average of 87 μm long (P = 0.06). Anodal neurite lengths were no different from the average 22 hour neurite not exposed to an EF (P = 0.17) and lengths of cathodal neurites did not differ from the average control neurite (P = 0.35). Therefore cathodally-sprouted neurite length was not significantly different from anodally-sprouted neurite length in neurites exposed to an EF from hours 2–6 or 2–22
Finally, the direction of growth cone migration was measured at 6 hours and 22 hours after plating (Table 1; Fig. 1). At 6 hours after plating, in cells not exposed to an EF the average cosine of growth cone trajectories was 0.005 (n = 315). In neurites exposed to a 100 mV/mm EF from hours 2–6, the average cosine was −0.04 (n = 310). This was not significantly different from control (P = 0.41) At 22 hours after plating, neurites exposed to a 100 mV/mm EF from hours 2–22 had an average growth cone mean cosine of 0.03, and the average cosine of those which had not been exposed to an EF was −0.04. These were not significantly different from one another (P = 0.35). Therefore, EF-exposure had no effect on the direction of growth cone migration.
We here present evidence that zebrafish neurites exposed to a 100 mV/mm EF in culture exhibit no overall turning of growth cones towards either electrode. Neurites exposed to a 100 mV/mm EF are significantly shorter after 4 hours EF exposure than neurites not exposed to an EF. Lengths of neurites sprouted towards the anode are no different from those towards the cathode after 4 hours or 20 hours exposure to an EF (Table 1). No asymmetry is seen in neurite sprout angle, and the number of neurons per chamber is the same in control and experimental chambers. Contrast this with cultured Xenopus laevis embryonic spinal neurons, in which 70% of growth cones turn towards the cathode, the growth rates of cathodally-directed neurites are increased, anodal neurites are slowed or even retracted [10], and neurites are sprouted preferentially towards the cathode [13]. Taken as a whole, our results show that the only effect of an applied EF on cultures of zebrafish embryonic spinal neurons is a reduction in average neurite length after 4 hours exposure to the EF. We return to the words of Williams [17]: “in every case where any change was seen in the culture, this change was degenerative”.
Our cultures almost certainly contain multiple types of neurons. We have not tried to identify individual neuronal cell types and thereby find out if one type responds in any way to the applied EF, and others do not. Cultures of spinal neurons from Xenopus laevis also contain a mixed neuronal population. To our knowledge, no attempt has been made in that system to identify the responses of specific types of neurons, though 67% of neurons in this culture are cholinergic [15], suggesting that they are motor neurons. Most of the growth cones in Xenopus laevis embryonic neuronal cultures turn to grow towards the cathode at EF magnitudes similar to, or considerably smaller than, those employed here. This is immediately apparent upon inspection of diagrams of traced neurite paths [10]. We intentionally chose to study zebrafish neurons from a comparable stage of development as those used previously in Xenopus laevis cultures to aid in comparison between the two species. Had zebrafish spinal neurons turned in response to an applied EF in our cultures this would have been evident both from neurite-trace diagrams (Fig. 2) and from the growth cone average cosine, which would approach 1 had they turned towards the cathode and −1 if they had turned towards the anode. In fact, the average cosine in all of our control and experimental data sets was around zero, indicating random orientation of growth cones at the time of photography (Table 1). We therefore feel confident that zebrafish neuron growth cones in culture do not migrate directionally towards either pole of an applied EF.
Fig. 2.
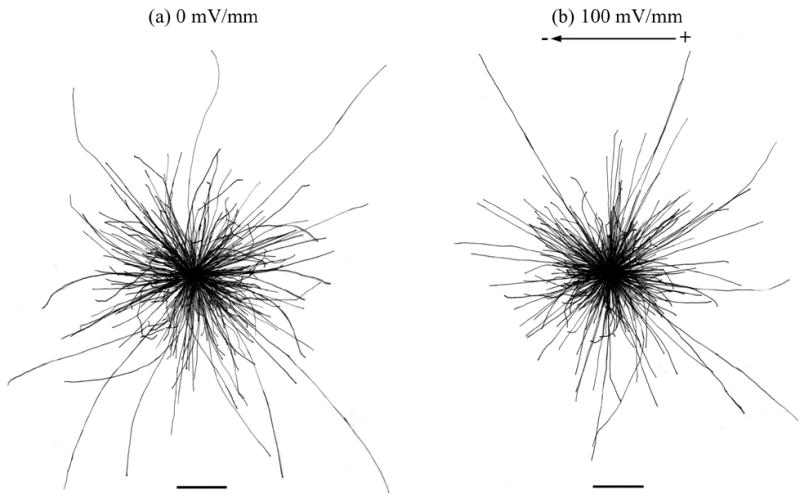
Zebrafish neurons exposed to an EF. Zebrafish neuron cell bodies were overlaid 6 hours after plating and the neurites traced. Neurites not exposed to an EF (a) were sprouted from random positions around the cell bodies and grow in all directions. Application of a 100 mV/mm EF from hours 2–6 (b) had no effect on neurite sprout point, and the direction of growth was not affected. Scale bars represent 50 μm.
We only studied the responses of embryonic zebrafish neurons to applied EFs on laminin-coated glass. We found that neuron growth was not well supported on the other substrates we tested (fibronectin-coated glass, uncoated glass, and uncoated Falcon tissue culture plastic), and that the number of neurite-bearing cells was much lower on these substrates than on laminin. This agrees well with the findings of Andersen [1]. We felt that the amount of growth seen on the other substrates was insufficient to allow for meaningful data to be gathered, and so did not pursue this avenue of study. Xenopus laevis neurons grow well on plastic, laminin and polylysine, but their response to an applied EF is substrate-dependent. On Falcon tissue culture plastic, 70% of neurites turn towards the cathode. On laminin coated glass this is reduced to 56%. On polylysine the effect is reversed, with 61% of neurites turning towards the anode. The rate of growth towards the cathode is increased on plastic and laminin, but not on polylysine [13]. These findings suggest that the response of a Xenopus laevis neuronal growth cone to an in vivo EF would be dependent upon the substrate over which it was migrating.
Our results lead us to the conclusion that zebrafish neurons do not respond to an applied EF in vitro. Thus the directional growth and cathodal growth stimulation seen in Xenopus laevis neurites exposed to an EF in vitro cannot be assumed to be a universal feature of vertebrate neurons.
Acknowledgments
We thank the lab of Dr. Paul Collodi for providing zebrafish embryos. This work was supported by NIH, grant R21 GM71768.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Andersen SSL. Preparation of dissociated zebrafish spinal neuron cultures. Methods Cell Sci. 2002;23:205–209. doi: 10.1023/a:1016349232389. [DOI] [PubMed] [Google Scholar]
- 2.Hinkle L, McCaig CD, Robinson KR. The direction of growth of differentiating neurones and myoblasts from frog embryos in an applied electric field. J Physiol. 1981;314:121–135. doi: 10.1113/jphysiol.1981.sp013695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hotary KB, Robinson KR. Endogenous electrical currents and the resultant voltage gradients in the chick embryo. Dev Biol. 1990;140:149–160. doi: 10.1016/0012-1606(90)90062-n. [DOI] [PubMed] [Google Scholar]
- 4.Hotary KB, Robinson KR. Endogenous electrical currents and voltage gradients in Xenopus embryos and the consequences of their disruption. Dev Biol. 1994;166:789–800. doi: 10.1006/dbio.1994.1357. [DOI] [PubMed] [Google Scholar]
- 5.Ingvar D. Experiments on the influence of electric current upon growing nerve cell processes in vitro. Acta Physiol Scand. 1947;13:150–154. doi: 10.1111/j.1748-1716.1947.tb00413.x. [DOI] [PubMed] [Google Scholar]
- 6.Ingvar S. Reaction of cells to the galvanic current in tissue cultures. Proc Soc Exp Biol Med. 1920;17:198–199. [Google Scholar]
- 7.Jaffe LF, Poo M-m. Neurites grow faster towards the cathode than the anode in a steady field. J Exp Zool. 1979;209:115–128. doi: 10.1002/jez.1402090114. [DOI] [PubMed] [Google Scholar]
- 8.Marsh G, Beams HW. In vitro control of growing chick nerve fibers by applied electric currents. J Cell Comp Physiol. 1946;27:139–157. doi: 10.1002/jcp.1030270303. [DOI] [PubMed] [Google Scholar]
- 9.McCaig CD. Spinal neurite reabsorption and regrowth in vitro depend on the polarity of an applied electric field. Development. 1987;100:31–41. doi: 10.1242/dev.100.1.31. [DOI] [PubMed] [Google Scholar]
- 10.McCaig CD, Rajnicek AM, Song B, Zhao M. Controlling cell behaviour electrically: Current views and future potential. Physiol Rev. 2005;85:943–978. doi: 10.1152/physrev.00020.2004. [DOI] [PubMed] [Google Scholar]
- 11.Rajnicek AM, Foubister LE, McCaig CD. Growth cone steering by a physiological electric field requires dynamic microtubules, microfilaments and rac-mediated filopodial asymmetry. J Cell Sci. 2006;119:1736–1745. doi: 10.1242/jcs.02897. [DOI] [PubMed] [Google Scholar]
- 12.Rajnicek AM, Foubister LE, McCaig CD. Temporally and spatially coordinated roles for Rho, Rac, and Cdc42 and their effectors in growth cone guidance by a physiological electric field. J Cell Sci. 2006;119:1723–1735. doi: 10.1242/jcs.02896. [DOI] [PubMed] [Google Scholar]
- 13.Rajnicek AM, Robinson KR, McCaig CD. The direction of neurite growth in a weak DC electric field depends on the substratum: contributions of adhesivity and net surface charge. Dev Biol. 1998;203:412–423. doi: 10.1006/dbio.1998.9039. [DOI] [PubMed] [Google Scholar]
- 14.Shapiro S, Borgens R, Pascuzzi R, Roos K, Groff M, Purvines S, Rodgers RB, Hagy S, Nelson P. Oscillating field stimulation for complete spinal cord injury in humans: a Phase 1 trial. J Neurosurg Spine. 2005;2:3–10. doi: 10.3171/spi.2005.2.1.0003. [DOI] [PubMed] [Google Scholar]
- 15.Sun Y-a, Poo M-m. Evoked release of acetylcholine from the growing embryonic neuron. Proc Natl Acad Sci USA. 1987;84:2540–2544. doi: 10.1073/pnas.84.8.2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weiss P. In vitro experiments on the factors determining the course of the outgrowing nerve fiber. J Exp Zool. 1934;68:393–448. [Google Scholar]
- 17.Williams SC. A study of the reactions of growing embryonic nerve fibers to the passage of direct electric current through the surrounding medium. Anat Rec. 1936;64:56–57. Suppl 3. [Google Scholar]


