Abstract
Phospholemman (PLM) is a small sarcolemmal protein that modulates the activities of Na+/K+-ATPase and the Na+/Ca2+ exchanger (NCX), thus contributing to the maintenance of intracellular Na+ and Ca2+ homeostasis. We characterized the expression and subcellular localization of PLM, NCX and the Na+/K+-ATPase α1-subunit during perinatal development. Western blotting demonstrates that PLM (15 kD), NCX (120 kD) and Na+/K+-ATPase alpha-1 (~100 kD) proteins are all more than 2-fold higher in ventricular membrane fractions from newborn rabbit hearts (1–4 day old) compared to adult hearts. Our immunocytochemistry data demonstrate that PLM, NCX and Na+/K+-ATPase are all expressed at the sarcolemma of newborn ventricular myocytes. Taken together, our data indicate that PLM, NCX and Na+/K+-ATPase alpha-1 proteins have similar developmental expression patterns in rabbit ventricular myocardium. Thus, PLM may have an important regulatory role in maintaining cardiac Na+ and Ca2+ homeostasis during perinatal maturation.
Keywords: phospholemman, Na+/K+-ATPase, Na+/Ca2+ exchanger, development, heart
1. Introduction
Mechanisms underlying developmental changes in cardiac excitation-contraction (EC) coupling and regulation of contractile function remain incompletely defined. The Na+/Ca2+ exchanger (NCX) plays an essential role in EC coupling in the immature heart since Ca2+ movement through NCX is sufficient to sustain contraction and relaxation in neonatal rabbit myocytes [1, 2]. The Na+/K+ pump is responsible for Na+ extrusion from the cells and alterations in Na+/K+ pump function causes changes in the intracellular Na+ activity [3], which in turn affects intracellular Ca2+ and contractility [4]. Since NCX activity depends on transmembrane gradients of Na+ and Ca2+, its activity is strictly linked to that of the Na+/K+ pump. Thus, alterations in the activities of either the Na+/K+ pump or NCX activity will affect the cardiac contractile function in the developing heart (possibly even more so than in the adult, due to the intimate role of NCX in immature Ca2+ homeostasis).
Phospholemman (PLM) is a small (72 amino acids) phosphoprotein with a single transmembrane domain and an apparent molecular mass of approximately 15-kDa. Infrared spectroscopy of proteoliposomes shows that PLM peptide is completely α-helical and inserts into lipid membranes in a trans-bilayer fashion, forming a tetramer [5]. Although identified several years ago [6, 7], the functional role of PLM remains obscure. PLM belongs to the FXYD family of proteins that also includes the Na+/K+-ATPase γ subunit [3, 8–10]. Functionally, PLM modifies the Na+ and K+ affinities of both α1 and α2 subunits of Na+/K+-ATPase when expressed in Xenopus oocytes. In cardiac and skeletal muscle, PLM physically associates with the Na+/K+-ATPase α1 subunit, which is the major Na+/K+-ATPase α isoform transcript expressed during cardiac development [11]. PLM regulates NKA in a manner analogous to phospholamban regulation of SR Ca2+-ATPase (i.e., inhibition that is relieved by PLM phosphorylation) [12, 13]. However, PLM also associates with native NCX1 in rat cardiac myocytes [14, 15] by binding to an intracellular loop of the cardiac NCX1, where it appears to act as a repressor of NCX activity [16, 17]. Thus, two principal proteins involved in regulation of Ca2+ homeostasis (and thus EC coupling) in the immature heart may be under direct control of PLM. It is therefore important to know whether PLM expression parallels that of NCX1 and the Na+/K+-ATPase α1 subunit during perinatal development.
2. Methods
2.1 Isolation of membrane fractions from rabbit hearts
Membrane fractions were isolated from the hearts (left ventricles) of New Zealand White rabbits in the following age groups: 1 to 4-day old (neonatal), 9 to 10-day old (juvenile) or more than 150-day-old (adult). For the neonatal and juvenile age groups either sex was used, whereas male adult rabbits were used. Following pentobarbital overdose (60 mg/kg body weight and 500 IU/kg heparin; intraperitoneally in 1–10 days old rabbits; intravenously in adults), hearts were rapidly excised and washed in ice-cold phosphate buffer saline (PBS) at 4°C. Crude membrane fractions were isolated according to Pond et al. [18] with slight modifications. In brief, the heart was minced and homogenized in 10 volumes of ice cold TE homogenization buffer containing (in mM) 5 Tris-HCl pH 7.4, 1 EDTA and cocktail of protease inhibitors (Sigma) and Phenylmethanesulfonylfluoride (PMSF) using a Polytron homogenizer. The homogenate was centrifuged at 1000g for 10 minutes to remove nuclei and debris, and the supernatant removed. The pellet was rehomogenised in 7 volumes of homogenization buffer using a hand-held motorized Dounce homogenizer and centrifuged again at 1,000g for 10 minutes. Thereafter the supernatants were combined and centrifuged again at 27,000g for 45 minutes. The supernatant was discarded and the pellet was resuspended in TE containing 600mM KI and incubated in ice for 15 minutes. The resuspended pellet was centrifuged at 27,000g for 15 minutes; the resulting pellet was resuspended in TE and re-centrifuged at 27,000g. This step was repeated twice to remove any residual KI. The final pellet was resuspended in TNE containing (in mM) 50 Tris-HCl pH 7.4, 150 NaCl, 1 EDTA and cocktail of protease inhibitors and PMSF and stored at −80°C.
2.2 Western Blotting
Clarified precipitates of membrane fractions were separated by denaturing SDS-PAGE (8% polyacrylamide for NCX and Na+/K+-ATPase, and 15% for PLM) and transferred to PVDF membranes. The PVDF membrane was dried and stained with Sypro Ruby protein gel stain (Molecular Probes) and visualized using a 300 nm UV trans-illuminator to verify equal protein loading in various lanes. The blot was destained (150 mM Tris, 20% methanol, pH 8.8 for 10 minutes) and exposed to a blocking solution (100 mM Tris-HCl at pH 7.5, 150 mM NaCl, 0.5% Tween-20 and 5% non-fat dry milk) overnight. The blot was exposed to primary antibody in the same solution for 1 hour (room temperature) followed by a secondary HRP-conjugated antibody (anti-mouse or anti-rabbit) for 1 hour. Signal was detected by chemiluminescence (Pierce). Multiple exposures were made to ensure that data were obtained within the linear range of the X-ray film. Blots were scanned using a transilluminator and the band intensity was quantified by measuring density of the band using image processing and analysis program Image J.
2.3 Immunocytochemistry
Ventricular myocytes (isolated using a standard enzyme perfusion technique) [19] were allowed to settle on coverslips for 30 minutes. After washing the cells with phosphate-buffered saline (PBS) cells were fixed with cold methanol for 5 minutes at −20°C. The cells were permeabilized with 1% Triton X-100 (15 min at room temperature). Following washing with PBS (2 x 5 minutes), non-specific antigenicity was blocked with 5% serum (goat or donkey) in PBS (2 x 10 minutes) and cells were subsequently incubated with primary antibodies (1 hour at room temperature), washed (3x5 min with PBS) and then incubated with secondary antibodies for 45 minutes. Cells were then washed (4x 10 min in PBS) and mounted on slides. These slides were imaged using a Leica PS2 confocal microscope equipped with an Argon 488 nm gas laser and Helium Neon lasers (543 and 633 lines) Images were obtained using an emission pin hole of 1.5 AE with 63x oil objective.
2.4 Antibodies
Antibodies were raised to a PLM C-terminal peptide (residues 57–70) and affinity purified similar to that previously described [20]. Monoclonal anti-NCX antibodies were from Affinity Bioreagents and the anti-α1 Na+/K+-ATPase subunit antibody was from Upstate cell signaling solutions. Secondary antibodies used were Cy3 conjugated goat anti-rabbit IgG, FITC conjugated goat anti-mouse IgM and Cy2 conjugated donkey anti-mouse IgG. All the secondary antibodies were used in 1:300 dilution (Jackson Immuno Research).
2.5 Statistical Analysis
For comparative purposes, Western blot band intensities of neonatal and juvenile groups were expressed relative to the adult heart sample obtained in the same blot. Results are presented as mean±S.E.M. and were analyzed using one-way ANOVA. Multiple comparisons were made against the adult heart protein values using the Dunnet’s t-test. A value of p<0.05 was considered significant.
3. Results and Discussion
Immunoblotting was performed to determine the relative expression levels of NCX1, PLM and Na+/K+-ATPase α1 subunit during perinatal development in the rabbit heart (Fig. 1). We found strong expression of all three proteins in the newborn (1–2 day) rabbit hearts. Each of the proteins were expressed at lower levels at later states of maturity (protein loading in the different lanes was similar; Fig 1A). Lower levels were observed even at 9–11 days after birth, suggesting that down regulation of protein expression is rapid and occurs within a week after birth in the rabbit. Our data are consistent with previous reports, demonstrating NCX1 (the major NCX isoform expressed in the heart) to be downregulated postnatally in the rabbit, rat, mouse and human heart [21–23]. The α1 Na+/K+-ATPase subunit is the major catalytic subunit during development and is also downregulated after birth in the rat heart [24]. We demonstrate the Na+/K+-ATPase α1 subunit similarly to be downregulated in the developing rabbit heart (we have not been able to examine the expression levels of other Na+/K+-ATPase α subunits since suitable antibodies could not be obtained). A novel aspect of our data is our demonstration that PLM expression parallels that of NCX1 and Na+/K+-ATPase α1 subunits during perinatal development. Since PLM associates with both of these proteins and can regulate their function, [11, 14, 25] our data are in support of the concept that PLM is likely to be an important contributor to Na+ and Ca2+ homeostasis in the developing heart.
Figure 1.
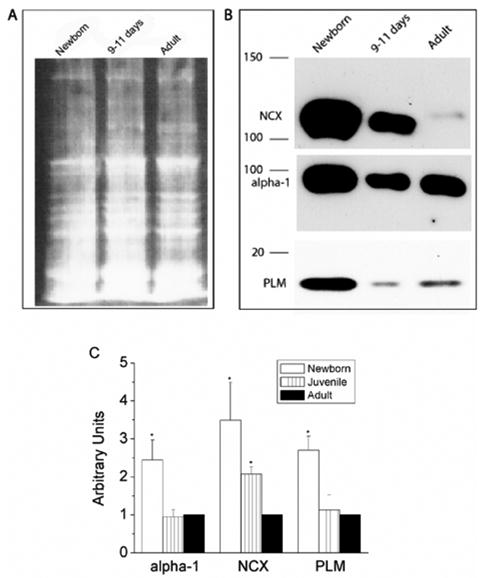
Developmental expression of PLM, NCX and α1 subunit of Na+/K+-ATPase in rabbit heart. Membrane fractions were isolated (see method section for details) from Newborn (1–4 day old), 9–11 day old and adult rabbit hearts and run on an 8% gel for NCX and Na+/K+-ATPase, and on a 15% gel for PLM. (A) Sypro Ruby protein blot stain was used to detect the amount of proteins in each lane. 25 μg of protein was loaded in each lane. (B) Protein expression of PLM, NCX and α1 subunit of Na+/K+-ATPase in newborn rabbit is at least 3 fold higher than in adults. (C) Bar graph showing the protein expression of α1 subunit of Na+/K+-ATPase (n=3), NCX (n=5) and PLM (n=5) during different developmental stages of rabbit heart by using image processing and analysis program Image J. The values were normalized to the respective adult values as 1 in each group.
We next examined the subcellular localization of PLM, NCX1 and Na+/K+-ATPase α1 subunits in ventricular myocytes enzymatically isolated from rabbit hearts. Immunocytochemistry reveals expression of PLM, NCX1 and Na+/K+-ATPase α1 subunits in the sarcolemma of newborn (1–2 days) ventricular myocytes (Fig 2). In adult myocytes, all three proteins have a similar pattern of expression in the sarcolemma as well as in a sarcomeric striated distribution throughout the cell (the latter probably reflects their expression in the t-tubular network). The similarity in expression patterns of two of these proteins (PLM and NCX1) is demonstrated by their co-localization in a co-staining experiment (Fig 2A). The fact that PLM has a subcellular expression pattern similar to that of NCX1 and Na+/K+-ATPase α1 subunits yields morphological support to previous reports demonstrating this phosphoprotein to associate with these two important membrane transporters [11, 14, 15, 25, 26].
Figure 2.
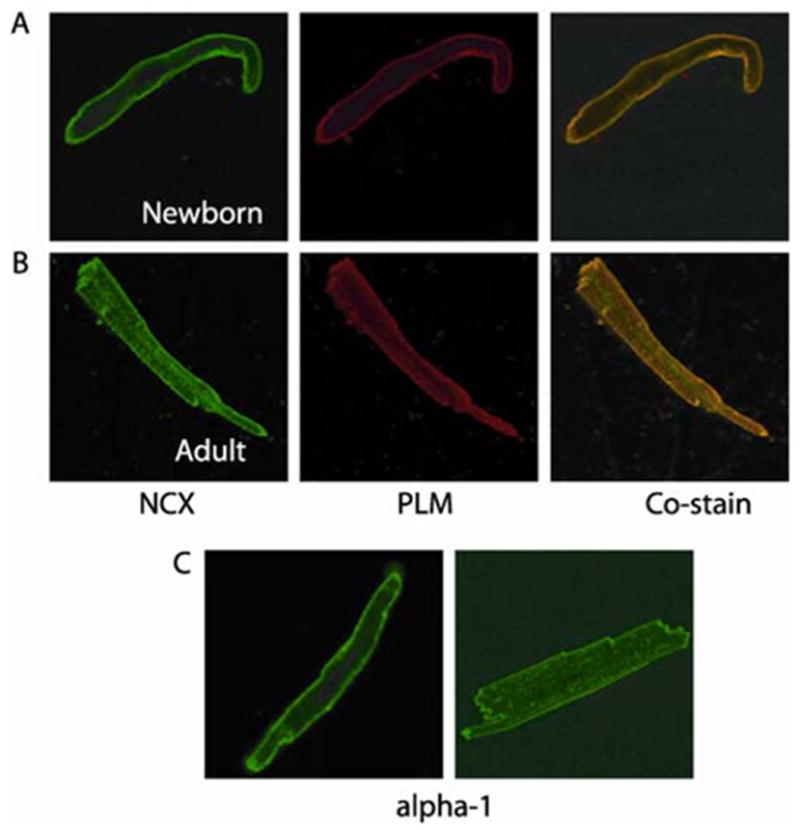
Immunofluorescence localization of PLM, NCX and α1 subunit of Na+/K+-ATPase in newborn and adult rabbit heart. PLM was visualized by using Cy3 conjugated goat anti-rabbit IgG, NCX by using FITC conjugated goat anti-mouse IgM and α1 subunit of Na+/K+-ATPase by using Cy2 conjugated donkey anti-mouse IgG. All the secondary antibodies were used in 1:300 dilution. (A) Newborn and (B) Adult rabbit myocytes stained with NCX (green) and PLM (red) reveal strong expression of these proteins in the sarcolemma in newborns whereas in the sarcolemma and z-lines in the adult. Co-staining demonstrates a co-localization pattern in neonatal as well as adult rabbit heart. (C) Staining with α-1 antibody shows a sarcolemmal staining pattern in newborns whereas in adult rabbit ventricular myocytes, we find sarcolemmal and z-line staining.
Based on the literature and our studies, interaction between PLM and NCX/Na/K pump may have many physiological implications in immature hearts. NCX1 is crucial for triggering contractions and modulating contractile strength in immature rabbit cardiac myocytes [1, 2, 27]. The literature suggests that PLM inhibits NCX1 activity [16]. It is possible that under basal conditions PLM negatively modulates Ca2+ influx and inotropic response. Moreover, PLM has a number of PKA/PKC phosphorylation sites. Phosphorylation of PLM may play an important role in cardiac contractility in immature hearts as it has been demonstrated in cultured neonatal ventricular myocytes, that elevated intracellular Ca2+ and signaling through α- and β-adrenergic receptors coordinately regulates the expression of the α1c-subunit of the L-type calcium channel and the Na/Ca exchanger by activating PKA and PKC pathways [28, 29]. The absence of NKA stimulation in PLM-KO mouse suggests that PKC dependent effects on the pump are mediated primarily by PLM, rather than direct NKA phosphorylation. Additionally, PKC and PKA appear to have additive effects on NKA function [30].
In summary, we demonstrate that PLM expression levels during perinatal development parallel that of NCX1 and Na+/K+-ATPase α1 subunits; two transporter proteins that are known to be regulated by PLM. Our data raises the possibility that PLM is intimately involved in regulation of Na+ and Ca2+ homeostasis (and thus, regulation of contractile activity) and that this regulation could be of particular importance in the immature heart when all three proteins are highly expressed.
Acknowledgments
This work was supported by the Seventh Masonic District Association, Inc. and the National Institutes of Health (HD39988 and HL58899 awarded to MA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Haddock PS, Artman M, Coetzee WA. Influence of postnatal changes in action potential duration on Na-Ca exchange in rabbit ventricular myocytes. Pflugers Arch. 1998;435:789–795. doi: 10.1007/s004240050585. [DOI] [PubMed] [Google Scholar]
- 2.Haddock PS, Coetzee WA, Artman M. Na+/Ca2+ exchange current and contractions measured under Cl(−)-free conditions in developing rabbit hearts. Am J Physiol. 1997;273:H837–846. doi: 10.1152/ajpheart.1997.273.2.H837. [DOI] [PubMed] [Google Scholar]
- 3.Therien AG, Blostein R. Mechanisms of sodium pump regulation. Am J Physiol Cell Physiol. 2000;279:C541–566. doi: 10.1152/ajpcell.2000.279.3.C541. [DOI] [PubMed] [Google Scholar]
- 4.Pieske B, Houser SR. [Na+]i handling in the failing human heart. Cardiovasc Res. 2003;57:874–886. doi: 10.1016/s0008-6363(02)00841-6. [DOI] [PubMed] [Google Scholar]
- 5.Beevers AJ, Kukol A. Secondary structure, orientation, and oligomerization of phospholemman, a cardiac transmembrane protein. Protein Sci. 2006;15:1127–1132. doi: 10.1110/ps.051899406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palmer CJ, Scott BT, Jones LR. Purification and complete sequence determination of the major plasma membrane substrate for cAMP-dependent protein kinase and protein kinase C in myocardium. J Biol Chem. 1991;266:11126–11130. [PubMed] [Google Scholar]
- 7.Jones LR, Besch HR, Jr, Fleming JW, McConnaughey MM, Watanabe AM. Separation of vesicles of cardiac sarcolemma from vesicles of cardiac sarcoplasmic reticulum. Comparative biochemical analysis of component activities. J Biol Chem. 1979;254:530–539. [PubMed] [Google Scholar]
- 8.Delprat B, Bibert S, Geering K. [FXYD proteins: novel regulators of Na,K-ATPase] Med Sci (Paris) 2006;22:633–638. doi: 10.1051/medsci/20062267633. [DOI] [PubMed] [Google Scholar]
- 9.Mercer RW, Biemesderfer D, Bliss DP, Jr, Collins JH, Forbush B., 3rd Molecular cloning and immunological characterization of the gamma polypeptide, a small protein associated with the Na,K-ATPase. J Cell Biol. 1993;121:579–586. doi: 10.1083/jcb.121.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Presti CF, Jones LR, Lindemann JP. Isoproterenol-induced phosphorylation of a 15-kilodalton sarcolemmal protein in intact myocardium. J Biol Chem. 1985;260:3860–3867. [PubMed] [Google Scholar]
- 11.Crambert G, Fuzesi M, Garty H, Karlish S, Geering K. Phospholemman (FXYD1) associates with Na,K-ATPase and regulates its transport properties. Proc Natl Acad Sci U S A. 2002;99:11476–11481. doi: 10.1073/pnas.182267299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bossuyt J, Despa S, Martin JL, Bers DM. Phospholemman phosphorylation alters its fluorescence resonance energy transfer with the Na/K-ATPase pump. J Biol Chem. 2006;281:32765–32773. doi: 10.1074/jbc.M606254200. [DOI] [PubMed] [Google Scholar]
- 13.Bers DM, Despa S, Bossuyt J. Regulation of Ca2+ and na+ in normal and failing cardiac myocytes. Ann N Y Acad Sci. 2006;1080:165–177. doi: 10.1196/annals.1380.015. [DOI] [PubMed] [Google Scholar]
- 14.Mirza MA, Zhang XQ, Ahlers BA, Qureshi A, Carl LL, Song J, Tucker AL, Mounsey JP, Moorman JR, Rothblum LI, Zhang TS, Cheung JY. Effects of phospholemman downregulation on contractility and [Ca(2+)]i transients in adult rat cardiac myocytes. Am J Physiol Heart Circ Physiol. 2004;286:H1322–1330. doi: 10.1152/ajpheart.00997.2003. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Zhang XQ, Ahlers BA, Carl LL, Song J, Rothblum LI, Stahl RC, Carey DJ, Cheung JY. Cytoplasmic tail of phospholemman interacts with the intracellular loop of the cardiac Na+/Ca2+ exchanger. J Biol Chem. 2006;281:32004–32014. doi: 10.1074/jbc.M606876200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang XQ, Ahlers BA, Tucker AL, Song J, Wang J, Moorman JR, Mounsey JP, Carl LL, Rothblum LI, Cheung JY. Phospholemman inhibition of the cardiac Na+/Ca2+ exchanger. Role of phosphorylation. J Biol Chem. 2006;281:7784–7792. doi: 10.1074/jbc.M512092200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang XQ, Qureshi A, Song J, Carl LL, Tian Q, Stahl RC, Carey DJ, Rothblum LI, Cheung JY. Phospholemman modulates Na+/Ca2+ exchange in adult rat cardiac myocytes. Am J Physiol Heart Circ Physiol. 2003;284:H225–233. doi: 10.1152/ajpheart.00698.2002. [DOI] [PubMed] [Google Scholar]
- 18.Pond AL, Scheve BK, Benedict AT, Petrecca K, Van Wagoner DR, Shrier A, Nerbonne JM. Expression of distinct ERG proteins in rat, mouse, and human heart. Relation to functional I(Kr) channels. J Biol Chem. 2000;275:5997–6006. doi: 10.1074/jbc.275.8.5997. [DOI] [PubMed] [Google Scholar]
- 19.Balaguru D, Haddock PS, Puglisi JL, Bers DM, Coetzee WA, Artman M. Role of the sarcoplasmic reticulum in contraction and relaxation of immature rabbit ventricular myocytes. J Mol Cell Cardiol. 1997;29:2747–2757. doi: 10.1006/jmcc.1997.0509. [DOI] [PubMed] [Google Scholar]
- 20.Cala SE, Jones LR. GRP94 resides within cardiac sarcoplasmic reticulum vesicles and is phosphorylated by casein kinase II. J Biol Chem. 1994;269:5926–5931. [PubMed] [Google Scholar]
- 21.Qu Y, Ghatpande A, el-Sherif N, Boutjdir M. Gene expression of Na+/Ca2+ exchanger during development in human heart. Cardiovasc Res. 2000;45:866–873. doi: 10.1016/s0008-6363(99)00402-2. [DOI] [PubMed] [Google Scholar]
- 22.Koban MU, Moorman AF, Holtz J, Yacoub MH, Boheler KR. Expressional analysis of the cardiac Na-Ca exchanger in rat development and senescence. Cardiovasc Res. 1998;37:405–423. doi: 10.1016/s0008-6363(97)00276-9. [DOI] [PubMed] [Google Scholar]
- 23.Boerth SR, Zimmer DB, Artman M. Steady-state mRNA levels of the sarcolemmal Na(+)-Ca2+ exchanger peak near birth in developing rabbit and rat hearts. Circ Res. 1994;74:354–359. doi: 10.1161/01.res.74.2.354. [DOI] [PubMed] [Google Scholar]
- 24.Orlowski J, Lingrel JB. Tissue-specific and developmental regulation of rat Na,K-ATPase catalytic alpha isoform and beta subunit mRNAs. J Biol Chem. 1988;263:10436–10442. [PubMed] [Google Scholar]
- 25.Geering K, Beguin P, Garty H, Karlish S, Fuzesi M, Horisberger JD, Crambert G. FXYD proteins: new tissue- and isoform-specific regulators of Na,K-ATPase. Ann N Y Acad Sci. 2003;986:388–394. doi: 10.1111/j.1749-6632.2003.tb07219.x. [DOI] [PubMed] [Google Scholar]
- 26.Geering K. Function of FXYD proteins, regulators of Na, K-ATPase. J Bioenerg Biomembr. 2005;37:387–392. doi: 10.1007/s10863-005-9476-x. [DOI] [PubMed] [Google Scholar]
- 27.Vetter R, Studer R, Reinecke H, Kolar F, Ostadalova I, Drexler H. Reciprocal changes in the postnatal expression of the sarcolemmal Na+-Ca(2+)-exchanger and SERCA2 in rat heart. J Mol Cell Cardiol. 1995;27:1689–1701. doi: 10.1016/s0022-2828(95)90788-2. [DOI] [PubMed] [Google Scholar]
- 28.Golden KL, Ren J, O’Connor J, Dean A, DiCarlo SE, Marsh JD. In vivo regulation of Na/Ca exchanger expression by adrenergic effectors. Am J Physiol Heart Circ Physiol. 2001;280:H1376–1382. doi: 10.1152/ajpheart.2001.280.3.H1376. [DOI] [PubMed] [Google Scholar]
- 29.Golden KL, Fan QI, Chen B, Ren J, O’Connor J, Marsh JD. Adrenergic stimulation regulates Na(+)/Ca(2+)Exchanger expression in rat cardiac myocytes. J Mol Cell Cardiol. 2000;32:611–620. doi: 10.1006/jmcc.2000.1104. [DOI] [PubMed] [Google Scholar]
- 30.Han F, Bossuyt J, Despa S, Tucker AL, Bers DM. Phospholemman phosphorylation mediates the protein kinase C-dependent effects on Na+/K+ pump function in cardiac myocytes. Circ Res. 2006;99:1376–1383. doi: 10.1161/01.RES.0000251667.73461.fb. [DOI] [PubMed] [Google Scholar]


