Summary
Behavioral inhibition (BI) is an adaptive defensive response to threat; however, extreme BI is associated with anxiety-related psychopathology. When rats are exposed to a natural predator they display stress- and anxiety-related behavioral alterations and physiological activation. To develop a preclinical rodent model to study mechanisms underlying human BI and anxiety, we examined the extent to which ferret exposure elicits anxiety-related BI and HPA and amygdala activation of the CRF system. In the first experiment, BI and other behaviors were assessed in the presence or absence of a ferret. In the second experiment, ferret-induced corticosterone release and changes in brain c-fos expression were assessed. In the final experiment, gene chip and quantitative real time-PCR analyses were performed on amygdala tissue from control and ferret-exposed rats. Ferret exposure increased BI and submissive posturing, as well as plasma corticosterone and the number of Fos-positive cells in several brain regions including the amygdala. Gene expression analysis revealed increased amygdalar mRNA for CRF-binding protein, but not the CRF1 receptor, CRF2 receptor or CRF. In rodents, ferret exposure can be used to elicit anxiety-related BI, which is associated with HPA and amygdala activation. Since the amygdala and the CRF system have been implicated in adaptive and maladaptive anxiety responses in humans, these data support use of our rodent model to further investigate mechanisms underlying anxiety-related psychopathology in humans.
Keywords: Predator, CRH, Stress, Behavioral inhibition, Freezing, Habenula, Lateral hypothalamus
1. Introduction
While physical stressors are commonly used in rodent stress studies, the most relevant stressors in humans are of a psychological or social nature. Furthermore, psychosocial stressors are known to precipitate stress-induced psychopathology (Dunner et al., 1979; Hammen et al., 1992). Therefore, when performing preclinical studies to understand mechanisms relevant to human psychopathology, it is important to use species-specific psychosocial stressors (Albeck et al., 1997; Kollack-Walker et al., 1997; Blanchard et al., 1998). Exposure of rats to a natural predator such as a ferret represents a potent form of psychosocial stress. Ferret predator stress has been used in recent years to study behavioral and hormonal stress responses, and is a potent stimulus to which rats display high levels of stress-like behaviors (Anisman et al., 1997; Merali et al., 2001; Masini et al., 2006). In addition, ethologically relevant stressors, such as predator exposure, produce long-lasting increases in stress-related behavior and plasma corticosterone. It has also been shown that habituation is less likely to occur with repeated exposure to a predator than with repeated exposure to other stressors such as restraint (Plata-Salaman et al., 2000).
The CRF peptide system is one of the major systems that integrates the response to psychological stress (Dunn and Berridge, 1990). CRF is produced by the hypothalamus in response to stress resulting in the release of adrenocorticotropic hormone (ACTH) from the pituitary and ultimately cortisol (corticosterone in rats) secretion from the adrenal glands (Vale et al., 1981; Berne and Levy, 1993). CRF is also produced in a variety of brain regions including the amygdala where it acts as a neurotransmitter and is thought to participate in the psychological and autonomic response to stressful stimuli (Dunn and Berridge, 1990). The CRF system is composed of at least seven components including the 41-amino acid peptide CRF, and three related urocortin peptides (urocortin 1-3). These peptides can interact with three different proteins, the two CRF receptors (CRF1 and CRF2) and the CRF-binding protein (CRF-BP). The CRF receptors are G protein-linked seven transmembrane domain receptors that are positively coupled to adenylate cyclase as well as other second messenger systems (Chen et al., 1993; Perrin et al., 1995). The CRF-BP binds CRF with an affinity equal to or higher than that of the receptors, and is thought to buffer the action of CRF by preventing interaction with the receptor and possibly targeting the peptide for degradation (Behan et al., 1995). Interestingly, recent work suggests that when CRF is bound to CRF-BP, this complex may act as a cellular signaling molecule (Ungless et al., 2003).
The amygdala is a medial temporal lobe structure that is important in identifying and interpreting cues that are associated with threatening stimuli (Davis and Whalen, 2001; Amaral, 2002; LeDoux, 2003). Considerable evidence implicates the amygdala in conditioned and unconditioned fear responses (Blanchard and Blanchard, 1972; LeDoux, 2000; Davis and Whalen, 2001; Amaral, 2003; Kalin et al., 2004), and human functional imaging studies have demonstrated increased amygdala activation in some patients with anxiety and depressive disorders (Drevets, 2003; Rauch et al., 2003). Furthermore, evidence supports a role for the amygdala CRF system in mediating anxiety and fear. For example, ferret exposure increases release of CRF in the Central amygdala (CeA) (Merali et al., 2001), and administration of a CRF receptor antagonist into the CeA blocks foot shock stress-induced freezing (Swiergiel et al., 1993).
Due to its ethological and psychological relevance, its potency, and the failure of rats to habituate to it after repeated exposure, ferret predator stress provides an excellent method to study the consequences of long-term and short-term psychosocial stress exposure. While this model has been used in rodents, few studies have focused on changes in the amygdala, second messenger systems and the CRF system. In the present study we characterized the behavioral, endocrine and amygdala gene expression changes following 10 min of ferret exposure. We report that brief ferret exposure induced intense fearful responses, caused a 7-fold increase in plasma corticosterone, altered expression of the c-fos gene in numerous brain regions including nuclei within the amygdala, and altered the levels of CRF-BP mRNA as determined by gene chip and quantitative real time-PCR (qRT-PCR) analyses.
2. Methods
2.1. Animals
Male Sprague-Dawley rats (Charles River, Wilmington, MA) weighing 275-300 g were housed in pairs with lights on from 0700 to 1900 h. To control for possible diurnal variability in behavioral or biochemical indices, all testing and sacrificing occurred between 1000 and 1300 h. Food and water were available ad libitum. All rats were handled for several days prior to testing to minimize subsequent handling-related stress. Six ferrets (Marshall Farms, North Rose, NY) were used in these studies and were housed in pairs; food and water were available ad libitum. All animal experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80-23, revised 1978).
3. Experiment 1
3.1. Ferret exposure
Rats (N = 8) were exposed to a ferret for 10 min by being placed within a protective wire cage (7.75 in long×6 in wide×5.5 in high), inside the ferret’s homecage. The bottom and ends of the cage were made of solid black plastic, whereas the top and sides were made of a black metal wire mesh. Control rats (N = 8) were placed inside an identical protective cage within a separate room. All rats were videotaped during the 10 min exposure period for subsequent behavioral scoring.
3.2. Behavioral scoring
All videotapes were analyzed by a single individual who scored the duration of the following behaviors: rearing (raising both front paws off the floor of the test cage), grooming (licking and rubbing with paws of any accessible area of skin and fur), submissive postures (exposing the belly by lying motionless on the back) and behavioral inhibition (BI). BI is defined as the sum of freezing (a period of at least 2 s during which there is an absence of all movement except those required for breathing) and hypervigilance (characterized by no locomotor activity with only minor head movements associated with sniffing and vibrissa movement). It should be noted that hypervigilance is not synonymous with risk-assessment behaviors which involve a greater degree of movement and include such behaviors as stretched attend postures (Kaesermann, 1986).
4. Experiment 2
4.1. Ferret exposure
Rats (N = 8/group) were deeply anesthetized with isoflurane at 0, 15, 30, 60 or 120 min following cessation of the 10 min ferret exposure and blood was collected via syringe directly from the heart for corticosterone measurement. Rats were then perfused with phosphate-buffered saline (PBS) followed by 4% paraformaldehyde in PBS and then decapitated. Control rats were maintained in their home cages prior to anesthesia, blood sampling and perfusion. The onset of ferret exposure was staggered such that the rats were sacrificed at the same time of day, between 1100 h and 1300 h.
4.2. Corticosterone assay
Corticosterone was measured using an enzyme immunoassay kit (Diagnostic Systems Laboratories, Webster, TX), with a detection limit of 5 ng/ml and average intra-assay and inter-assay coefficients of variation of 3.2% and 4.8%, respectively.
4.3. Tissue processing and immunohistochemistry
Brains were post-fixed overnight (∼16 h) in fresh 4% paraformaldehyde and then transferred to 30% sucrose at 4 °C until they sank. Using a cryostat (Leica CM1900, Leica Microsystems, Bannockburn, IL), 40 μm coronal sections were obtained from the frozen brains. Free-floating sections were washed with 0.02 N PBS and incubated with primary c-fos antibody (1:10,000 PC38 Anti-c-fos (Ab-5) (4-17) rabbit pAb, Calbiochem, San Diego, CA) for 72 h on a shaker at 4 °C. The sections were labeled using biotinylated goat anti-rabbit secondary antibody (Vector Laboratories, Burlingame, CA) for 2 h at room temperature and then incubated in avidin-biotin complex (ABC Elite kit, Vector Labs) for 2 h at room temperature. Labeling was visualized using a diaminobenzidine (DAB) substrate kit with nickel chloride enhancement (Vector Labs). Sections were mounted onto chrome-alum-coated slides, air dried, dehydrated, cleared, and cover slipped with Permount.
Brain areas of interest were located under light microscopy using the rat brain atlas (Paxinos and Watson, 1998) and local landmarks in each section. Areas of interest were digitally photographed (Leica DC 300/400 camera) at 5 magnification (Leica DMRX high contrast microscope). The background was adjusted to a similar level across sections and the images were converted to 16-bit grayscale using Image Pro Plus 5.0 (Media Cybernetics, Silver Spring, MD). To set the threshold, an experimenter blind to the treatment condition first manually counted the number of positively stained cells within a defined region of interest that corresponded to the anatomical size and shape of the brain region being examined. Then the threshold was adjusted within the program Scion Image (version 4.0.3.2 Scion Corporation, Frederick, MD) such that the number of cells determined using the auto-analyze for particles function was similar to the manual count. The numbers of stained cells within the regions of interest were then counted using the automated analysis. Using an image of a 1 mm ruler captured at 5×magnification, it was determined that 1 mm equals 475 pixels or 1 mm2 equals 225,625 pixels. Using this conversion factor, the cell count was normalized to the area of the region of interest and expressed as a density (Fos-positive cells/mm2).
5. Experiment 3
5.1. Ferret exposure
Ferret-exposed rats were sacrificed by decapitation (N = 12) 3 h following the onset of the 10 min exposure period, the brain was rapidly removed and briefly chilled in isopentane. Control rats (N = 12) were maintained in their home cages prior to sacrifice and brain extraction. All rats were sacrificed between 1200 and 1300 h. After placing the brain in a block, it was cut into 2 mm thick sections and the entire amygdala was removed with a 2 mm diameter punch tool. The tissue was then frozen on dry ice and stored at 80 °C until used.
5.2. Affymetrix gene chip analysis
Amygdala from 7 control and 7 stressed rats were separately pooled and RNA was extracted with the RNeasy kit (Qiagen, Valencia, CA) according to the manufacturer’s protocol. Because of tissue damage during brain extraction, one rat from each group of 8 had to be excluded from the RNA isolation. Total RNA was quantified spectrophotometrically. RNA labeling, hybridization and expression analysis were done according to the Affymetrix GeneChip Expression Analysis Technical Manual (Affymetrix Inc., Santa Clara, CA). Briefly, an equal mass of total RNA from each pool was converted into first strand cDNA using Superscript II RNAse H-reverse transcriptase (Invitrogen Life Technologies, Carlsbad, CA) followed by second strand cDNA synthesis. The production of biotinylated cRNA from the double stranded cDNA was done with the BioArray High Yield RNA Transcript Labeling Kit (Enzo Life Sciences, Farmingdale, NY) according to the manufacturer’s instructions. Fragmentation of the cRNA was conducted at 0.5 μg/ml final concentration in fragmentation buffer (40 mM Tris-acetate, pH 8.1, 100 mM potassium acetate, 30 mM magnesium acetate). The size range of the cRNA before and after fragmentation was determined by denaturing agarose gel electrophoresis. The quality of the cRNA synthesis was determined from the 3′/5′ ratio of rat housekeeping genes within the array. The pools of RNA from the two conditions were used to prepare three separate cRNA samples and these were hybridized to three independent sets of Affymetrix Rat Genome 230 2.0 Arrays.
Expression levels for each set of probes were determined using the robust multigene average (gcRMA) and were imported into Gene Spring GX software (version 7.2; Agilent Technologies, Palo Alto, CA). Gene lists were generated by determining a ratio of expression levels in the stressed compared to the control condition. Upregulated genes were defined as having a ratio of ≥1.5 and downregulated genes were defined as having a ratio of ≤ 0.67.
5.3. qRT-PCR analysis with TaqMan probes
For genes within the CRF family of proteins that were detectable on the gene chip (CRF1, CRF2, CRF and CRF-BP), qRT-PCR was performed as described below to confirm the gene chip data. Amygdala were separately pooled from an additional 4 control and 4 stressed rats and total RNA was extracted using the RNeasy kit with on-column DNase treatment (Qiagen). The Sequence Detection System 5700 (Applied Biosystems, Foster City, CA) was used for qRT-PCR analysis. TaqMan probes prepared by Applied Biosystems were used to detect and quantify the PCR product. Each TaqMan assay was designed using Primer Express 2.0 software, and the gene-specific primers generated amplicons that averaged 100 bp in length. Each combination of probe and primers was optimized using rat whole brain cDNA. The following primers and probes were used: for CRF, sense primer 5′-CAGCCGTTGAATTTCTTGCA-3′ from nucleotides (NT) 290 to 309 of GenBank accession numberNM_031019, antisense primer 5′-CCCCAGGCGGAGGAAGTAT-3′ from NT 379 to 361 and sense fluorescent probe 5′-CCCCAGCAACCTCAGCCGATTCT-3′ from NT 320 to 342; for CRF1, sense primer 5′-GGCTTCTTTGTGTCTGTGTTCTACTG-3′ from NT 1788 to 1813 of NM_030999, antisense primer 5′-CACCGACGCCACCTCTTC-3′ from NT 1864 to 1847 and sense fluorescent probe 5′-AACAGTGAGGTCCGCTCCGC-TATCC-3′ from NT 1821 to 1845; for CRF2, sense primer 5′-CTCATCAATTTTGTGTTTCTGTTCAA-3′ from NT 1044 to 1069 of NM_022714, antisense primer 5′-CTGTACTGGATGGTCTCG-GATGT-3′ from NT 1132 to 1110, and sense fluorescent probe 5′-CCTGATGACAAAACTGCGAGCCTCCA-3′ from NT 1082 to 1107; for CRF-BP, sense primer 5′-GCCCAGTGAGTTCTCCA-CAGTT-3′ from NT 1092 to 1113 of NM_139183, antisense primer 5′-CATGTGTGCAGGTTTTCAAAGC-3′ from NT 1175 to 1154, and antisense fluorescent probe 5′-ACTG-GAAGGCTTTTCACTCCGTCCAAA-3′ from NT 1142 to 1116.
For the RNA pools from the control and stressed conditions, three reverse transcription reactions were performed and qRT-PCR analysis was done in quadruplicate for each gene of interest on each of the three cDNA reactions. Each PCR reaction contained a gene-specific forward and reverse primer (900 nM final concentration), a gene-specific TaqMan probe (250 nM final concentration) and TaqMan Universal PCR Master Mix with uracil N-glycosylase (UNG; Applied Biosystems). The PCR temperature profile began with 2 min at 50 °C for UNG activation, 10 min at 95 °C for template denaturation and Amplitaq Gold activation. Fluorescence generated by the TaqMan probe was measured during 40 cycles that alternated between 15 s at 95 °C for denaturation and 60 s for annealing and extension at a temperature that ranged between 57 °C to 60 °C depending on the template being amplified. The Ct value, the number of cycles required to reach a preset threshold level of fluorescence, was compared to a standard curve generated by performing qRT-PCR analysis on serial dilutions of rat whole brain cDNA. Relative quantities of PCR product were derived from the equation of the line determined by linear regression of the log of the cDNA amount in the standard curve versus the corresponding Ct value.
The relative quantities of the PCR product for the gene of interest were normalized to the level of the endogenous eukaryotic 18S ribosomal RNA present in each experimental sample. The 18S ribosomal RNA was amplified in different wells on the same plate as the gene of interest with TaqMan ribosomal RNA control reagents (Applied Biosystems). The quantity in each RNA pool was obtained by comparing the Ct value to a standard curve generated by performing qRT-PCR analysis on serial dilutions of rat whole brain cDNA as described above for quantification of the gene of interest. To normalize the data, a ratio was determined of the qRT-PCR signal for the gene of interest divided by the signal for the 18S ribosomal RNA. The normalized signal for the stressed sample was then compared to the normalized signal for the control sample. Because the assay is performed on three separate cDNA syntheses, it is possible to assess assay variability when comparing the normalized mRNA signal of the stressed to the control sample. When averaged across assays performed on 12 different genes, the coefficient of variation (CV), which is the standard deviation expressed as a percentage of the mean, was 13.3%. More specifically, the assay on CRF-BP mRNA that is reported in this study had a CV of 3.6%. Given this level of variability, an array result was considered confirmed by qRT-PCR analysis if the difference in the relative level of expression between the stressed and control sample was greater than 1.2 fold in the same direction as the array data.
5.4. Statistics analyses
Because many of the data sets were not normally distributed, non-parametric analyses were performed. Behavioral data was analyzed with Mann-Whitney U-tests using SPSS 13.0 software (SPSS Inc, Chicago, IL). Corticosterone and c-fos data were analyzed with Kruskal-Wallis H-tests (one-way ANOVA based on rank) followed by comparing each treatment group to the control group using Dunn’s posttest, which adjusts the alpha value for multiple comparisons (Prism 4.03, GraphPad Software Inc, San Diego, CA).
6. Results
6.1. Ferret exposure increases stress-like behaviors in rats
Compared to control rats, exposure to the ferret for 10 min significantly increased the total time during which rats displayed BI (Z = 3.45; p<0.001; Fig. 1A) and submissive posturing (Z = 2.66; p<0.05; Fig. 1B). In addition, ferret exposure significantly decreased total time spent grooming (Z = 3.07; p<0.01; Fig. 1C) and rearing (Z = 3.36; p<0.001; Fig. 1D).
Fig. 1.
Acute ferret exposure increased levels of BI and other anxiety-related behaviors. Rats were placed in test cages for 10 min either in the presence or absence of the ferret and behavior was rated from videotapes. Ferret exposure significantly increased the duration of BI (A) and submissive posturing (B). In contrast, ferret exposure significantly decreased the duration of grooming (C) and rearing (D). Bars represent the mean+SEM for each group from 8 independent determinations, *p<0.05, **p<0.01, ***p<0.001 compared to Control.
6.2. Ferret exposure elevates plasma corticosterone levels
Rats were exposed to a ferret for 10 min and were sacrificed at various time points after cessation of the stress. Measurement of plasma corticosterone levels revealed a significant difference in corticosterone concentration between the treatment groups (χ2 = 37.28, df = 5, p<0.001, Fig. 2). Corticosterone levels were significantly elevated immediately after 10 min of ferret exposure and reached a maximum at 15 min after termination of ferret exposure. By 120 min, the corticosterone values were not significantly different from control levels.
Fig. 2.
Ferret exposure produced a time-dependent increase in corticosterone concentrations. Rats were sacrificed at various time points after 10 min of ferret exposure; control rats remained in their home cages until time of sacrifice. Trunk blood was collected and corticosterone levels were determined. Ferret exposure resulted in a significant increase in corticosterone levels that peaked at 700% above control at 15 min after ferret exposure and declined to baseline by 120 min. Bars represent the mean+SEM for each group from 8 independent determinations, ***p<0.001 compared to Control.
6.3. Ferret exposure increases Fos-positive cells in various brain regions including amygdala nuclei
To identify whether the amygdala was activated following ferret exposure, Fos protein levels were examined by immunohistochemistry in the same rats that were used to measure the corticosterone levels. There was a significant difference in the number of Fos-positive cells within the medial amygdala (MeA) nucleus between treatment groups (χ2 = 39.98; df = 5; p<0.001, Fig. 3A). Compared to home cage controls, the number of Fos-positive cells per mm2 began to rise 30 min after the 10 min ferret exposure and reached a plateau spanning the 60 and 120 min time points. There was also a significant difference in the number of Fos-positive cells between treatment groups within the CeA (χ2 = 21.14; df = 5; p<0.001, Fig. 3A) and basolateral amygdala nucleus (BLA; χ2 = 38.47; df = 5; p<0.001, Fig. 3A). The time course for the CeA and BLA was similarto that seen for the MeA; however, the magnitude of the increase in the CeA was considerably less than that observed in the MeA. Photomicrographs of the Fos-positive cells within the amygdala nuclei show the intense staining of the MeA 60 min following ferret exposure (Fig. 3B).
Fig. 3.
Ferret exposure increased the number of Fos-positive cells in the MeA, CeA and BLA. Immunohistochemistry with an antibody directed against Fos was performed on brains obtained for the same rats that were used for the corticosterone measurements in Fig. 2. (A) The number of Fos-positive cells present per mm2 in the indicated brain region from rats that were sacrificed at various time points after a 10 min exposure to the ferret; control rats remained in their home cages until time of sacrifice. Ferret exposure produced a time-dependent increase in the number of Fos-positive cells/mm2 in each of the brain regions examined. Bars represent the mean+SEM for each group from 8 independent determinations, *p<0.05, **p<0.01, ***p<0.001 compared to Control. (B) Images of the amygdala region from a control and a ferret-exposed rat sacrificed 60 min following ferret exposure. The following regions are identified on the figure: MeA, medial amygdala; CeA, central amygdala; BLA, basolateral amygdala; opt, optic tract; cst, commissural stria terminalis. Scale bar = 500 microns.
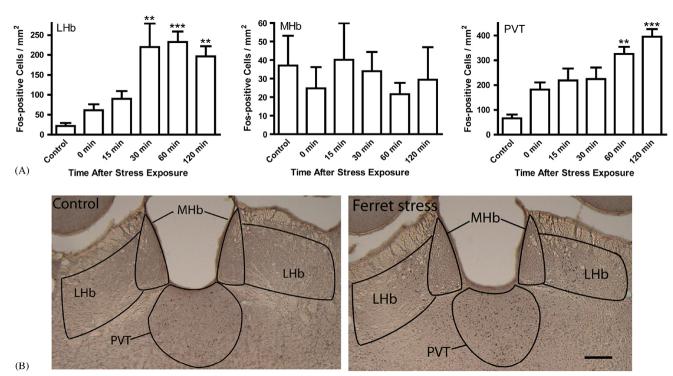
The number of Fos-positive cells also increased with ferret exposure in several other brain regions that are associated with stress and behavior. The lateral habenula (LHb), which has been postulated to modulate the effects of stress on behavior (Lee and Huang, 1988; Klemm, 2004), showed a significant difference in the number of Fos-positive cells between treatment groups (χ2 = 26.74; df = 5; p<0.001, Fig. 4A). This effect was region-specific because there was no difference in the number of Fos-positive cells between treatment groups in the medial habenular nucleus (MHb) (χ2 = 3.15; df = 5; p = 0.677, Fig. 4A). The level of c-fos expression in the LHb was elevated 30 min after ferret exposure and remained at essentially the same level up to the final 120 min time point. The paraventricular nucleus of the thalamus (PVT) was also examined because c-fos expression in the PVT has been shown to increase following a variety of stressors (Fernandes et al., 2002). There was a significant difference between treatment groups in c-fos expression in the PVT (χ2 = 25.10; df = 5; p<0.001, Fig. 4A). There was a stress-induced increase in the number of Fos-positive cells at 60 and 120 min following ferret exposure. The region-specific increase in c-fos expression can be seen in the photomicrographs of the brain region containing the habenula and PVT (Fig. 4B).
Fig. 4.
Ferret exposure increased the number of Fos-positive cells in the paraventricular nucleus of the thalamus and lateral habenular nucleus, but not the medial habenular nucleus. Immunohistochemistry was performed on the same brains used for measurement of c-fos expression in Fig. 3. (A) The number of Fos-positive cells present per mm2 in the indicated brain region from rats that were sacrificed at various time points after a 10 min exposure to the ferret; control rats remained in their home cages until time of sacrifice. Ferret exposure produced a time-dependent increase in the number of Fos-positive cells/mm2 in all of the brain regions examined except the medial habenular nucleus. Bars represent the mean+SEM for each group from 8 independent determinations, **p<0.01, ***p<0.001 compared to Control. (B) Images of the habenula region from a control and a ferret-exposed rat sacrificed 60 min following ferret exposure. The following regions are identified on the figure: LHb, lateral habenula; MHb, medial habenula; PVT, paraventricular nucleus of the thalamus. Scale bar = 200 microns.
The number of Fos-positive cells within the lateral hypothalamus was determined because the lateral hypothalamus has been implicated in mediating the behavioral arousal associated with the stress response (Winsky-Sommerer et al., 2004), and an increase in c-fos expression in the lateral hypothalamus has been shown in response to fox odor (Day et al., 2004). In the present study, there was a significant difference in the number of Fos-positive cells in the lateral hypothalamus between treatment groups (χ2 = 18.07; df = 5; p<0.01). The stress-induced increase was evident at 15 min (control, 6.54 ± 2.51 cells/mm2 vs. stressed, 26.52 ± 2.99 cells/mm2, p<0.05, N 7 and 8, respectively) and remained elevated up to the final = 120 min time point (38.87 ± 8.22 cells/mm2, p<0.01 vs. control, N = 8).
Lastly, previous studies have demonstrated the importance of several medial hypothalamic nuclei in mediating many of the defensive responses rats display in the presence of a cat or cat odor (Canteras et al., 1997; Blanchard et al., 2003). These nuclei also demonstrate increases in c-fos expression following cat exposure, with the dorsal premammillary (PMd) nucleus demonstrating the most robust increase in c-fos expression following cat exposure (Canteras et al., 1997). Because the major focus of the present study was the amygdala, many of the sections that were probed for c-fos did not contain the PMd, which lies posterior to the amygdala. This resulted in a sufficient number of sections to assess only the control group and the 60 and 120 min time points. Analysis revealed a significant and dramatic difference in the number of Fos-positive cells in the PMd between treatment groups (χ2 = 11.84; df = 2; p<0.01). Compared to controls there was a significant increase in the number of Fos-positive cells 60 min following a 10 min ferret exposure (control, 5.30 ± 3.09 cells/mm2 vs. stressed, 196.97 ± 61.04 cells/mm2, p<0.05, N = 6/group). This increase was maintained at the 120 min time point (288.26 ± 790.20 cells/mm2, p<0.01 vs. control, N = 6).
6.4. Affymetrix gene chip analysis
Because the c-fos expression data showed that the amygdala was highly activated following predator exposure, and because previous studies have implicated the amygdala in adaptive and maladaptive stress responses, we were interested in identifying gene expression changes that may occur in the amygdala as a result of predator exposure. As part of a larger study, Affymetrix gene chip analysis showed that out of 31,099 probe sets representing over 30,000 transcripts and variants derived from over 28,000 different genes, on average 59.2% (18,410) of the probe sets were detected as present on each chip. Of these genes, there were a total of 516 that changed by 1.5 fold or more, with 243 showing a stress-induced increase and 273 showing a stress-induced decrease.
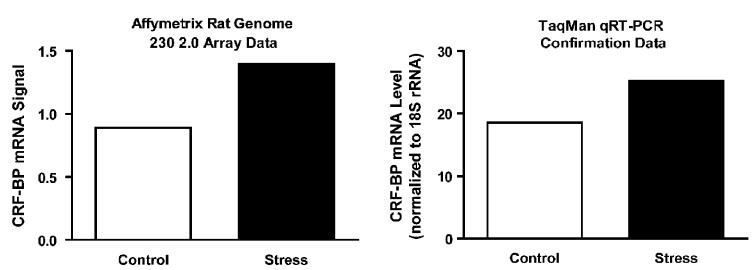
Because of the importance of the CRF system in mediating a variety of responses to psychological stress, for the purposes of the present study we focused on the CRF family of proteins. Based on our previously published work using 1 h of restraint stress (Lombardo et al., 2001; Herringa et al., 2004), we hypothesized that CRF-BP mRNA levels would increase following ferret stress. When examining the CRF family of proteins, the CRF-BP was the only member to demonstrate a greater than 1.5-fold change in expression based on gene chip analysis. Specifically, levels of CRF-BP mRNA were 1.6 fold higher following ferret stress. This result was confirmed as a 1.4-fold stress-induced increase using qRT-PCR performed on a pool of RNA obtained from aseparate set of animals (Fig. 5). It is noteworthy that while it did not meet our fold change criteria, the CRF1 receptor mRNA demonstrated a 1.3-fold increase that was also confirmed by qRT-PCR (data not shown). Additional studies are necessary to confirm the putative change in CRF1 mRNA levels. Additionally, the levels of CRF and CRF2 receptor expression did not change with ferret stress on the array. An oligonucleotide probe set corresponding to urocortin 3 was not present on the gene chip and the expression levels for urocortin and urocortin 2 were below the limit of detection on the gene chip.
Fig. 5.
Ferret exposure induced changes in CRF-BP mRNA from array data validated by qRT-PCR analysis. Gene expression changes were first obtained using the Rat Genome 230 2.0 array and subsequently validated by qRT-PCR performed with TaqMan probes as described in Materials and Methods. Each of the two techniques used a single pool of RNA obtained from independent sets of experimental animals. The graph on the left side of the figure represents normalized data obtained from the arrays and was averaged from three different arrays each hybridized with a separate cDNA synthesis from the same pool of RNA. The graph on the right side of the figure represents qRT-PCR data obtained by averaging results from three separate cDNA syntheses. The numbers represent mRNA levels expressed as a ratio following normalization to eukaryotic 18S ribosomal RNA. Ferret stress produced an increase in CRF-BP mRNA.
7. Discussion
BI is an adaptive defensive response to threat that has been evolutionarily conserved across a large number of species (Porges, 2003). When extreme, BI is a risk factor for the development of anxiety disorders. Understanding the neural and biochemical pathways that underlie BI will provide insight into the neurobiology of fear and anxiety, and anxiety-related psychopathology. The present study demonstrates that rats exposed to a ferret engage in anxiety-related BI which is consistent with previous reports demonstrating that rats exposed to cat or cat odors display an increase in fear-related behavioral responses such as freezing, avoidance and risk-assessment (Blanchard et al., 1989; McGregor et al., 2002). Results with fox odor have been less consistent with some reports of increased freezing when a rat is presented with the synthetic compound 2,5-dihydro-2,4,5-trimethylthiazoline (TMT) derived from the fox anal gland (Wallace and Rosen, 2000; Fendt et al., 2003), whereas other reports fail to demonstrate any fear-related behavioral responses to TMT (McGregor et al., 2002). It is possible that the differences between cat and fox odor result from the type of odor used (odor from fur/skin for cat versus odor from synthetic compound TMT for fox) and variations in the intensity of the odor stimulus that was employed (Takahashi et al., 2005).
In addition to BI, ferret exposure invoked a marked increase in plasma corticosterone concentrations similar to published reports demonstrating elevated plasma corticosterone following exposure of a rat to either a predator or the odor of a predator including the fox odor TMT (File et al., 1993; Adamec et al., 1998; Blanchard et al., 1998;Perrot-Sinal et al., 1999; Morrow et al., 2000). Similarly, exposure to the odor of a ferret has been reported to increase rat corticosterone levels (Masini et al., 2005). In the present study corticosterone concentrations peaked 15 min after cessation of 10 min of ferret exposure and returned to baseline by 120 min.
Since the amygdala mediates adaptive and maladaptive fear and anxiety responses (Blanchard and Blanchard, 1972;LeDoux, 2000; Davis and Whalen, 2001; Amaral, 2003; Kalin et al., 2004), it is of particular importance that ferret exposure increased c-fos expression within various nuclei of the amygdala. Consistent with previous studies using cat odor as a stimulus (Dielenberg et al., 2001; McGregor et al., 2004) we found that 10 min of ferret exposure resulted in a robust increase in the number of Fos-positive cells within the MeA. Similarly, c-fos mRNA was increased in the MeA of rats immediately after a 30 min exposure to ferret odor (Masini et al., 2005). The MeA, which lies medial to the CeA, showed the largest increase in c-fos expression within the amygdala and is of interest because earlier studies associated it with fear and defensive responses (Kemble et al., 1984). The MeA projects to areas adjacent to the PVN and also links to neurons in the bed nucleus of the stria terminalis that project to the PVN (Herman et al., 2002). These linkages with the hypothalamus are consistent with the MeA being the amygdala region that has the greatest modulatory influence on stress-induced HPA activity. Furthermore, recent studies have associated the MeA with fear and anxiety responses induced by the odor of predators (Blanchard et al., 2005; Takahashi et al., 2005; Muller and Fendt, 2006). For example, excitotoxic lesions or inactivation of the MeA blocks freezing behavior in rats exposed to odors associated with a cat or fox (Li et al., 2004; Blanchard et al., 2005).
The expectation for c-fos expression changes in the CeA was less clear. Some studies suggest that the CeA is not involved in unconditioned fear responses as indicated by a lack of an effect of CeA lesions on rat freezing behavior elicited by cat or fox odor (Fendt et al., 2003; Li et al., 2004; Rosen, 2004). In terms of c-fos expression, studies have demonstrated no increase in c-fos expression in the CeA following exposure to cat odor (Dielenberg et al., 2001) or ferret odor (Masini et al., 2005); whereas, another study reported a significant increase in the number of Fos-positive cells in the CeA in rats following fox odor exposure (Day et al., 2004). We demonstrate that acute ferret exposure results in a time-dependent increase in the number of Fos-positive cells in the CeA. Because increases in c-fos expression are used as a marker of neuronal activation, these results indicate that the CeA is activated by ferret exposure.
The role of the BLA in the unconditioned response to predator threat is also unclear. A previous report has shown that lesioning the BLA does not effect fear-related behavioral responses to fox odor (Wallace and Rosen, 2001); whereas, other studies demonstrate that lesioning and/or inactivating the BLA alters freezing in response to cat or fox odors (Vazdarjanova et al., 2001; Takahashi et al., 2005;Muller and Fendt, 2006). In addition, exposure to cat odor failed to increase BLA c-fos expression (Dielenberg et al., 2001), but in another study exposure to red fox odor increased BLA c-fos expression (Funk and Amir, 2000). Lastly, c-fos mRNA levels increased immediately following a 30 min exposure to ferret odor (Masini et al., 2005). Our studies demonstrate an increase in c-fos expression in the BLA in response to ferret exposure, suggesting a role for the BLA in mediating the response to ferret predator threat.
The lateral habenula has been postulated to modulate the effects of stress on behavior as evidenced by habenular lesion-induced alterations in stress-induced open field exploratory behavior (Lee and Huang, 1988; Klemm, 2004). We report that c-fos expression is increased in the lateral, but not the medial habenular nuclei. The selectivity of this response for the lateral habenula is consistent with a previous report showing a restraint stress-induced increase in the number of Fos-positive cells within the lateral but not the medial habenula (Chastrette et al., 1991). Another study showed an increase in c-fos mRNA levels in the lateral habenula following 60 min of restraint stress or after intracerebroventricular administration of 1 μg of CRF (Imaki et al., 1993).
Ferret exposure also increased c-fos expression in the PVT, a region where c-fos expression has been shown to increase following a variety of stressors (Fernandes et al., 2002). The PVT cells that express Fos in response to stress are thought to project to a variety of forebrain structures that mediate the endocrine and behavioral responses to stress such as the medial prefrontal cortex, the CeA and the PVN (Bubser and Deutch, 1999). This is the first report to show an increase in c-fos expression in the PVT following exposure to predator stress and is consistent with the proposed role of the PVT as a modulator of the stress response (Spencer et al., 2004).
We also report a time-dependent increase in the number of Fos-positive cells in the lateral hypothalamus. This is of interest because the lateral hypothalamus is thought to be part of a circuit that contributes to stress-induced arousal (Winsky-Sommerer et al., 2004). Specifically, stress-induced release of CRF is thought to bind to CRF receptor expressing neurons in the lateral hypothalamus resulting in the release of hypocretin peptides known to be important in arousal and alertness. In addition, the number of hypocretinergic-neurons expressing Fos protein in the lateral hypothalamus is increased by foot-shock or restraint stress (Winsky-Sommerer et al., 2004). Few studies have examined c-fos expression in the lateral hypothalamus following predator exposure and none have examined the time course for induction of c-fos expression. One study showed that cat odor failed to increase c-fos expression in the lateral hypothalamus (Dielenberg et al., 2001); whereas, other studies have shown that both fox odor (Day et al., 2004) and ferret odor (Masini et al., 2005) failed to increase c-fos expression in the lateral hypothalamus.
It should be noted that there are a number of other hypothalamic regions that have been reported to show increases in c-fos expression following different types of predator stress. For example, three hypothalamic nuclei, including the PMd nucleus, have been implicated in mediating defensive behaviors in response to cat exposure (Canteras et al., 1997). The major focus of the current study was the amygdala and a detailed analysis of the hypothalamus was beyond the scope of this paper. Because there was an incomplete set of sections covering the PMd, some of the time points could not be assessed. However, it was possible to demonstrate a significant increase in the number of Fos-positive cells in the 60 min and 120 min groups compared to the control group. This is consistent with previous reports using cat exposure (Canteras et al., 1997) and ferret odor exposure (Masini et al., 2005).
Gene expression studies revealed that acute ferret stress increased the level of CRF-BP mRNA in the amygdala. This finding was demonstrated using two different techniques (gene chip and qRT-PCR) performed on separate pools of RNA obtained from separate groups of rats. In addition, this finding confirms and extends our previous reports of restraint stress-induced increases in amygdala CRF-BP mRNA (Lombardo et al., 2001; Herringa et al., 2004). It is possible that stress-induced increases in the expression of CRF-BP play a modulatory role, as increased levels of CRF-BP may serve to buffer the effects of CRF in response to the current or a subsequent stressor. Conversely, because there is some indication that CRF-BP may act as a signaling molecule, increased CRF-BP expression could underlie the sensitization that occurs to some of the effects of stress (Steckler, 2005).
The lack of an effect on CRF2 receptor mRNA is difficult to interpret in part because the levels of CRF2 receptor expression are relatively low in the amygdala. Future studies will determine if CRF2 receptor expression is altered in other brain regions, for example the lateral septum and the bed nucleus of the stria terminalis, that have been implicated in CRF-dependent stress responses (Erb and Stewart, 1999; Bakshi et al., 2002) and also express higher levels of CRF2 receptors.
In the present study, CRF mRNA levels were not increased in the amygdala following ferret exposure. Previous studies by our lab have shown an increase in CRF mRNA in the CeA following restraint stress (Hsu et al., 1998) and work by others have reported a similar increase following rat exposure to a cat (Figueiredo et al., 2003). In addition, exposure of sheep to dogs has been shown to increase CRF release from the CeA by in vivo microdialysis (Cook, 2002). There are several reasons that could account for the lack of a change in amygdala CRF mRNA in the present paradigm. In the published reports, CRF mRNA is shown to increase specifically within the CeA. In the present study, the entire amygdala was used for gene chip and qRT-PCR studies. Thus, a change might have occurred in the CeA but could be obscured by assessing the entire amygdala. Secondly, the CeA CRF mRNA response to cat exposure was only seen in rats that had been sensitized by prior exposure to contextual cues (Figueiredo et al., 2003). In the present study the rats were naïve to the context of ferret exposure. Lastly, studies from a variety of labs have reported variable results when examining acute stress effects on CeA CRF mRNA levels. Our lab and others have shown that restraint stress and psychological stress can increase CeA CRF mRNA levels, whereas other studies have failed to demonstrate a change in CeA CRF mRNA (Helmreich et al., 1999), including a study published from our laboratory (Herringa et al., 2004). These studies suggest that the effects of acute stress on amygdala CRF mRNA are variable and may be dependent on context associated cues and the type of stressor used as well as other factors such as the rat strain used.
In conclusion, in the present study we demonstrate that acute ferret exposure elicits an increase in BI and other anxiety-related behaviors as well as activation of the HPA axis and the amygdala. The amygdala activation is associated with changes in the expression of CRF-BP mRNA that may mediate both the normal and pathological responses to subsequent stressors. Taken together, these data support the use of the ferret-threat paradigm as a means to study molecular mechanisms underlying BI. Findings from these studies should be relevant to understanding human psychopathology, since extreme BI is associated with increased risk for anxiety disorders.
Acknowledgements
The authors thank Mr. James M. Speers (University of Wisconsin, Madison, WI) for performance of the gene chip and qRT-PCR studies, Ms. Kristin R. Evans (University of Wisconsin) for measurement of corticosterone levels, and Mr. Christopher P. Nizzi (University of Wisconsin) and Dr. Ryan J. Herringa (University of Wisconsin) for quantification of immunohistochemical studies. This work was supported by NIH Grant MH40855 (NHK), the University of Wisconsin HealthEmotions Research Institute and Meriter Hospital (Madison, WI). Drs. Ned H. Kalin, Patrick H. Roseboom and Steven A. Nanda have a financial interest in Promoter Neurosciences, LLC (Madison, WI).
References
- Adamec R, Kent P, Anisman H, Shallow T, Merali Z. Neural plasticity, neuropeptides and anxiety in animals—implications for understanding and treating affective disorder following traumatic stress in humans. Neurosci. Biobehav. Rev. 1998;23:301–318. doi: 10.1016/s0149-7634(98)00032-3. [DOI] [PubMed] [Google Scholar]
- Albeck DS, McKittrick CR, Blanchard DC, Blanchard RJ, Nikulina J, McEwen BS, Sakai RR. Chronic social stress alters levels of corticotropin-releasing factor and arginine vasopressin mRNA in rat brain. J. Neurosci. 1997;17:4895–4903. doi: 10.1523/JNEUROSCI.17-12-04895.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG. The primate amygdala and the neurobiology of social behavior: implications for understanding social anxiety. Biol. Psychiatry. 2002;51:11–17. doi: 10.1016/s0006-3223(01)01307-5. [DOI] [PubMed] [Google Scholar]
- Amaral DG. The amygdala, social behavior, and danger detection. Ann. NY Acad. Sci. 2003;1000:337–347. doi: 10.1196/annals.1280.015. [DOI] [PubMed] [Google Scholar]
- Anisman H, Lu ZW, Song C, Kent P, McIntyre DC, Merali Z. Influence of psychogenic and neurogenic stressors on endocrine and immune activity: differential effects in fast and slow seizing rat strains. Brain Behav. Immun. 1997;11:63–74. doi: 10.1006/brbi.1997.0482. [DOI] [PubMed] [Google Scholar]
- Bakshi VP, Smith-Roe S, Newman SM, Grigoriadis DE, Kalin NH. Reduction of stress-induced behavior by antagonism of corticotropin-releasing hormone 2 (CRH2) receptors in lateral septum or CRH1 receptors in amygdala. J. Neurosci. 2002;22:2926–2935. doi: 10.1523/JNEUROSCI.22-07-02926.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behan DP, De Souza EB, Lowry PJ, Potter E, Sawchenko P, Vale WW. Corticotropin releasing factor (CRF) binding protein: a novel regulator of CRF and related peptides. Front. Neuroendocrinol. 1995;16:362–382. doi: 10.1006/frne.1995.1013. [DOI] [PubMed] [Google Scholar]
- Berne RM, Levy MN. Physiology. third ed. Mosby Year Book; St. Louis: 1993. [Google Scholar]
- Blanchard DC, Blanchard RJ. Innate and conditioned reactions to threat in rats with amygdaloid lesions. J. Comp. Physiol. Psychol. 1972;81:281–290. doi: 10.1037/h0033521. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Canteras NS, Markham CM, Pentkowski NS, Blanchard RJ. Lesions of structures showing FOS expression to cat presentation: effects on responsivity to a Cat, Cat odor, and nonpredator threat. Neurosci. Biobehav. Rev. 2005;29:1243–1253. doi: 10.1016/j.neubiorev.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Li CI, Hubbard D, Markham CM, Yang M, Takahashi LK, Blanchard RJ. Dorsal premammillary nucleus differentially modulates defensive behaviors induced by different threat stimuli in rats. Neurosci. Lett. 2003;345:145–148. doi: 10.1016/s0304-3940(03)00415-4. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC, Hori K. Ethoexperimental approaches to the study of defense. In: Blanchard RJ, Brain PF, Blanchard DC, Parmigiani S, editors. Ethoexperimental Approaches to the Study of Behavior. Kluwer Academic Publishers; Boston: 1989. pp. 114–136. [Google Scholar]
- Blanchard RJ, Nikulina JN, Sakai RR, McKittrick C, McEwen B, Blanchard DC. Behavioral and endocrine change following chronic predatory stress. Physiol. Behav. 1998;63:561–569. doi: 10.1016/s0031-9384(97)00508-8. [DOI] [PubMed] [Google Scholar]
- Bubser M, Deutch AY. Stress induces Fos expression in neurons of the thalamic paraventricular nucleus that innervate limbic forebrain sites. Synapse. 1999;32:13–22. doi: 10.1002/(SICI)1098-2396(199904)32:1<13::AID-SYN2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Chiavegatto S, Valle LE, Swanson LW. Severe reduction of rat defensive behavior to a predator by discrete hypothalamic chemical lesions. Brain Res. Bull. 1997;44:297–305. doi: 10.1016/s0361-9230(97)00141-x. [DOI] [PubMed] [Google Scholar]
- Chastrette N, Pfaff DW, Gibbs RB. Effects of daytime and nighttime stress on Fos-like immunoreactivity in the paraventricular nucleus of the hypothalamus, the habenula, and the posterior paraventricular nucleus of the thalamus. Brain Res. 1991;563:339–344. doi: 10.1016/0006-8993(91)91559-j. [DOI] [PubMed] [Google Scholar]
- Chen R, Lewis KA, Perrin MH, Vale WW. Expression cloning of a human corticotropin-releasing-factor receptor. Proc. Natl. Acad. Sci. USA. 1993;90:8967–8971. doi: 10.1073/pnas.90.19.8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook CJ. Glucocorticoid feedback increases the sensitivity of the limbic system to stress. Physiol. Behav. 2002;75:455–464. doi: 10.1016/s0031-9384(02)00650-9. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol. Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Day HE, Masini CV, Campeau S. The pattern of brain c-fos mRNA induced by a component of fox odor, 2,5-di-hydro-2,4,5-trimethylthiazoline (TMT), in rats, suggests both systemic and processive stress characteristics. Brain Res. 2004;1025:139–151. doi: 10.1016/j.brainres.2004.07.079. [DOI] [PubMed] [Google Scholar]
- Dielenberg RA, Hunt GE, McGregor IS. “When a rat smells a cat”: the distribution of Fos immunoreactivity in rat brain following exposure to a predatory odor. Neuroscience. 2001;104:1085–1097. doi: 10.1016/s0306-4522(01)00150-6. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging abnormalities in the amygdala in mood disorders. Ann. NY Acad. Sci. 2003;985:420–444. doi: 10.1111/j.1749-6632.2003.tb07098.x. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Berridge CW. Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res. Rev. 1990;15:71–100. doi: 10.1016/0165-0173(90)90012-d. [DOI] [PubMed] [Google Scholar]
- Dunner DL, Patrick V, Fieve RR. Life events at the onset of bipolar affective illness. Am. J. Psychiatry. 1979;136:508–511. [PubMed] [Google Scholar]
- Erb S, Stewart J. A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. J. Neurosci. 1999;19:RC35. doi: 10.1523/JNEUROSCI.19-20-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendt M, Endres T, Apfelbach R. Temporary inactivation of the bed nucleus of the stria terminalis but not of the amygdala blocks freezing induced by trimethylthiazoline, a component of fox feces. J. Neurosci. 2003;23:23–28. doi: 10.1523/JNEUROSCI.23-01-00023.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes GA, Perks P, Cox NK, Lightman SL, Ingram CD, Shanks N. Habituation and cross-sensitization of stress-induced hypothalamic-pituitary-adrenal activity: effect of lesions in the paraventricular nucleus of the thalamus or bed nuclei of the stria terminalis. J. Neuroendocrinol. 2002;14:593–602. doi: 10.1046/j.1365-2826.2002.00819.x. [DOI] [PubMed] [Google Scholar]
- Figueiredo HF, Bodie BL, Tauchi M, Dolgas CM, Herman JP. Stress integration after acute and chronic predator stress: differential activation of central stress circuitry and sensitization of the hypothalamo-pituitary-adrenocortical axis. Endocrinology. 2003;144:5249–5258. doi: 10.1210/en.2003-0713. [DOI] [PubMed] [Google Scholar]
- File SE, Zangrossi H, Jr., Sanders FL, Mabbutt PS. Dissociation between behavioral and corticosterone responses on repeated exposures to cat odor. Physiol. Behav. 1993;54:1109–1111. doi: 10.1016/0031-9384(93)90333-b. [DOI] [PubMed] [Google Scholar]
- Funk D, Amir S. Circadian modulation of fos responses to odor of the red fox, a rodent predator, in the rat olfactory system. Brain Res. 2000;866:262–267. doi: 10.1016/s0006-8993(00)02249-6. [DOI] [PubMed] [Google Scholar]
- Hammen C, Davila J, Brown G, Ellicott A, Gitlin M. Psychiatric history and stress: predictors of severity of unipolar depression. J. Abnorm. Psychol. 1992;101:45–52. doi: 10.1037//0021-843x.101.1.45. [DOI] [PubMed] [Google Scholar]
- Helmreich DL, Watkins LR, Deak T, Maier SF, Akil H, Watson SJ. The effect of stressor controllability on stress-induced neuropeptide mRNA expression within the para-ventricular nucleus of the hypothalamus. J. Neuroendocrinol. 1999;11:121–128. doi: 10.1046/j.1365-2826.1999.00300.x. [DOI] [PubMed] [Google Scholar]
- Herman JP, Tasker JG, Ziegler DR, Cullinan WE. Local circuit regulation of paraventricular nucleus stress integration: glutamate-GABA connections. Pharmacol. Biochem. Behav. 2002;71:457–468. doi: 10.1016/s0091-3057(01)00681-5. [DOI] [PubMed] [Google Scholar]
- Herringa RJ, Nanda SA, Hsu DT, Roseboom PH, Kalin NH. The effects of acute stress on the regulation of central and basolateral amygdala CRF-binding protein gene expression. Mol. Brain Res. 2004;131:17–25. doi: 10.1016/j.molbrainres.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Hsu DT, Chen FL, Takahashi LK, Kalin NH. Rapid stress-induced elevations in corticotropin-releasing hormone mRNA in rat central amygdala nucleus and hypothalamic paraventricular nucleus: an in situ hybridization analysis. Brain Res. 1998;788:305–310. doi: 10.1016/s0006-8993(98)00032-8. [DOI] [PubMed] [Google Scholar]
- Imaki T, Shibasaki T, Hotta M, Demura H. Intracer-ebroventricular administration of corticotropin-releasing factor Induces c-fos mRNA expression in brain regions related to stress response: comparison with pattern of c-fos mRNA induction after stress. Brain Res. 1993;616:114–125. doi: 10.1016/0006-8993(93)90199-w. [DOI] [PubMed] [Google Scholar]
- Kaesermann HP. Stretched attend posture, a non-social form of ambivalence, is sensitive to a conflict-reducing drug action. Psychopharmacology (Berl) 1986;89:31–37. doi: 10.1007/BF00175185. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. J. Neurosci. 2004;24:5506–5515. doi: 10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemble ED, Blanchard DC, Blanchard RJ, Takushi R. Taming in wild rats following medial amygdaloid lesions. Physiol. Behav. 1984;32:131–134. doi: 10.1016/0031-9384(84)90084-2. [DOI] [PubMed] [Google Scholar]
- Klemm WR. Habenular and interpeduncularis nuclei: shared components in multiple-function networks. Med. Sci. Monit. 2004;10:RA261–RA273. [PubMed] [Google Scholar]
- Kollack-Walker S, Watson SJ, Akil H. Social stress in hamsters: defeat activates specific neurocircuits within the brain. J. Neurosci. 1997;17:8842–8855. doi: 10.1523/JNEUROSCI.17-22-08842.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J. The emotional brain, fear, and the amygdala. Cell. Mol. Neurobiol. 2003;23:727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu. Rev. Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lee EH, Huang SL. Role of lateral habenula in the regulation of exploratory behavior and its relationship to stress in rats. Behav. Brain Res. 1988;30:265–271. doi: 10.1016/0166-4328(88)90169-6. [DOI] [PubMed] [Google Scholar]
- Li CI, Maglinao TL, Takahashi LK. Medial amygdala modulation of predator odor-induced unconditioned fear in the rat. Behav. Neurosci. 2004;118:324–332. doi: 10.1037/0735-7044.118.2.324. [DOI] [PubMed] [Google Scholar]
- Lombardo KA, Herringa RJ, Balachandran JS, Hsu DT, Bakshi VP, Roseboom PH, Kalin NH. Effects of acute and repeated restraint stress on corticotropin-releasing hormone binding protein mRNA in rat amygdala and dorsal hippocampus. Neurosci. Lett. 2001;302:81–84. doi: 10.1016/s0304-3940(01)01680-9. [DOI] [PubMed] [Google Scholar]
- Masini CV, Sauer S, Campeau S. Ferret odor as a processive stress model in rats: neurochemical, behavioral, and endocrine evidence. Behav. Neurosci. 2005;119:280–292. doi: 10.1037/0735-7044.119.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masini CV, Sauer S, White J, Day HE, Campeau S. Non-associative defensive responses of rats to ferret odor. Physiol. Behav. 2006;87:72–81. doi: 10.1016/j.physbeh.2005.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor IS, Hargreaves GA, Apfelbach R, Hunt GE. Neural correlates of cat odor-induced anxiety in rats: region-specific effects of the benzodiazepine midazolam. J. Neurosci. 2004;24:4134–4144. doi: 10.1523/JNEUROSCI.0187-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor IS, Schrama L, Ambermoon P, Dielenberg RA. Not all ‘predator odours’ are equal: cat odour but not 2,4,5 trimethylthiazoline (TMT; fox odour) elicits specific defensive behaviours in rats. Behav. Brain Res. 2002;129:1–16. doi: 10.1016/s0166-4328(01)00324-2. [DOI] [PubMed] [Google Scholar]
- Merali Z, Kent P, Michaud D, McIntyre D, Anisman H. Differential impact of predator or immobilization stressors on central corticotropin-releasing hormone and bombesin-like peptides in fast and slow seizing rat. Brain Res. 2001;906:60–73. doi: 10.1016/s0006-8993(01)02556-2. [DOI] [PubMed] [Google Scholar]
- Morrow BA, Redmond AJ, Roth RH, Elsworth JD. The predator odor, TMT, displays a unique, stress-like pattern of dopaminergic and endocrinological activation in the rat. Brain Res. 2000;864:146–151. doi: 10.1016/s0006-8993(00)02174-0. [DOI] [PubMed] [Google Scholar]
- Muller M, Fendt M. Temporary inactivation of the medial and basolateral amygdala differentially affects TMT-induced fear behavior in rats. Behav. Brain Res. 2006;167:57–62. doi: 10.1016/j.bbr.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. fourth ed. Academic Press; San Diego: 1998. [Google Scholar]
- Perrin M, Donaldson C, Chen R, Blount A, Berggren T, Bilezikjian L, Sawchenko P, Vale W. Identification of a second corticotropin-releasing factor receptor gene and characterization of a cDNA expressed in heart. Proc. Natl. Acad. Sci. USA. 1995;92:2969–2973. doi: 10.1073/pnas.92.7.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrot-Sinal TS, Ossenkopp KP, Kavaliers M. Brief predator odour exposure activates the HPA axis independent of locomotor changes. Neuroreport. 1999;10:775–780. doi: 10.1097/00001756-199903170-00021. [DOI] [PubMed] [Google Scholar]
- Plata-Salaman CR, Ilyin SE, Turrin NP, Gayle D, Flynn MC, Bedard T, Merali Z, Anisman H. Neither acute nor chronic exposure to a naturalistic (predator) stressor influences the interleukin-1beta system, tumor necrosis factor-alpha, transforming growth factor-beta1, and neuropeptide mRNAs in specific brain regions. Brain Res. Bull. 2000;51:187–193. doi: 10.1016/s0361-9230(99)00204-x. [DOI] [PubMed] [Google Scholar]
- Porges SW. Social engagement and attachment: a phylogenetic perspective. Ann. NY Acad. Sci. 2003;1008:31–47. doi: 10.1196/annals.1301.004. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Wright CI. Neuroimaging studies of amygdala function in anxiety disorders. Ann. NY Acad. Sci. 2003;985:389–410. doi: 10.1111/j.1749-6632.2003.tb07096.x. [DOI] [PubMed] [Google Scholar]
- Rosen JB. The neurobiology of conditioned and unconditioned fear: a neurobehavioral system analysis of the amygdala. Behav. Cogn. Neurosci. Rev. 2004;3:23–41. doi: 10.1177/1534582304265945. [DOI] [PubMed] [Google Scholar]
- Spencer SJ, Fox JC, Day TA. Thalamic paraventricular nucleus lesions facilitate central amygdala neuronal responses to acute psychological stress. Brain Res. 2004;997:234–237. doi: 10.1016/j.brainres.2003.10.054. [DOI] [PubMed] [Google Scholar]
- Steckler T. The Neuropsychology of Stress. In: Steckler T, Kalin NH, Reul JMHM, editors. Handbook of Stress and the Brain. Elsevier; New York: 2005. pp. 25–42. [Google Scholar]
- Swiergiel AH, Takahashi LK, Kalin NH. Attenuation of stress-induced behavior by antagonism of corticotropin-releasing factor receptors in the central amygdala in the rat. Brain Res. 1993;623:229–234. doi: 10.1016/0006-8993(93)91432-r. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Nakashima BR, Hong H, Watanabe K. The smell of danger: a behavioral and neural analysis of predator odor-induced fear. Neurosci. Biobehav. Rev. 2005;29:1157–1167. doi: 10.1016/j.neubiorev.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Singh V, Crowder TL, Yaka R, Ron D, Bonci A. Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. Neuron. 2003;39:401–407. doi: 10.1016/s0896-6273(03)00461-6. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Vazdarjanova A, Cahill L, McGaugh JL. Disrupting basolateral amygdala function impairs unconditioned freezing and avoidance in rats. Eur. J. Neurosci. 2001;14:709–718. doi: 10.1046/j.0953-816x.2001.01696.x. [DOI] [PubMed] [Google Scholar]
- Wallace KJ, Rosen JB. Predator odor as an unconditioned fear stimulus in rats: elicitation of freezing by trimethylthiazoline, a component of fox feces. Behav. Neurosci. 2000;114:912–922. doi: 10.1037//0735-7044.114.5.912. [DOI] [PubMed] [Google Scholar]
- Wallace KJ, Rosen JB. Neurotoxic lesions of the lateral nucleus of the amygdala decrease conditioned fear but not unconditioned fear of a predator odor: comparison with electrolytic lesions. J. Neurosci. 2001;21:3619–3627. doi: 10.1523/JNEUROSCI.21-10-03619.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winsky-Sommerer R, Yamanaka A, Diano S, Borok E, Roberts AJ, Sakurai T, Kilduff TS, Horvath TL, de Lecea L. Interaction between the corticotropin-releasing factor system and hypocretins (orexins): a novel circuit mediating stress response. J. Neurosci. 2004;24:11439–11448. doi: 10.1523/JNEUROSCI.3459-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]