Abstract
Human immunodeficiency virus (HIV-1) exclusively selects tRNALys,3 as the primer for initiation of reverse transcription. How and why HIV-1 selects the tRNA is unresolved. To address this issue, we have generated HIV-1 in which the PBS was changed to be complementary to alternative tRNAs. In this study, we report on HIV-1 that have the PBS mutated to be complementary to tRNAThr, tRNAPhe, tRNASer and tRNATyr. Virus with a PBS complementary to tRNAThr grew slightly slower than the wild type virus and maintained the PBS for an extended culture period before finally reverting back to utilize tRNALys,3. In contrast, viruses with a PBS complementary to tRNAPhe or tRNASer rapidly reverted to utilize tRNALys,3 following limited in vitro replication, while a virus with a PBS complementary to tRNATyr had severely compromised infectivity and did not productively infect a continuous T cell line (SupT1) or human peripheral blood mononuclear cells (PBMC). Modification of the A-loop region to be complementary to tRNAThr with the mutation in the PBS to be complementary to tRNAThr resulted in a virus that could stably utilize this tRNA while the modification of the A-loop to be complementary to the anticodon of tRNASer did not allow the virus to stably utilize tRNASer. Modification of the A-loop region to be complementary to the anticodon of tRNAPhe severely impacted the replication of this virus. Finally, the modification of the A-loop region to be complementary to tRNATyr did not rescue the virus with a PBS complementary to tRNATyr. The results of these studies demonstrate the diverse effects that alteration of the PBS to force selection of alternative primers have on HIV-1 replication and provide a framework to understand the dynamics of primer selection.
Keywords: HIV, tRNA, primer binding site, reverse transcription
Introduction
One of the distinguishing features of retrovirus replication is the process by which the viral RNA genome is converted to a DNA intermediate prior to integration into the host cell chromosome (Temin, 1981). This process is termed reverse transcription and is carried out by a virally encoded enzyme, reverse transcriptase (Baltimore, 1970; Temin and Mizutani, 1970). Reverse transcriptase requires a cellular tRNA as a primer (Panet and Berliner, 1978; Peters and Dahlberg, 1979; Temin, 1981). The initiation of reverse transcription occurs at the 5’ end of the viral RNA genome designated as the primer-binding site (PBS) (Panet and Berliner, 1978). The PBS is an 18-nucleotide sequence that is complementary to the 3’ terminal 18 nucleotides of the cellular tRNA that is used as the primer. During the process of reverse transcription, the RT copies the tRNA primer which facilitates the completion of the proviral genome (Gilboa et al., 1979). Thus, analysis of the PBS from integrated proviruses reveals the tRNA primer used for initiation of reverse transcription.
Although retroviruses can use a variety of cellular tRNAs as primers, only specific tRNAs are preferentially selected for use in reverse transcription. For example, lentiviruses, including human immunodeficiency virus type 1 (HIV-1), select tRNALys,3 as the primer for initiation of reverse transcription (Mak et al., 1997; Marquet et al., 1995). In contrast, human T cell leukemia viruses (HTLV-1) or murine leukemia virus (MuLV) select tRNAPro as the primer for reverse transcription while avian leucosis virus (ALV) selects tRNATrp (Mak et al., 1997; Marquet et al., 1995). How and why retroviruses select specific tRNAs as the primer for reverse transcription is unknown. Studies with HIV, MuLV and ALV have shown that retroviruses have the capacity to select many different tRNAs as the primer for reverse transcription (Das et al., 1995; Li et al., 1994; Lund et al., 1993; Palmer and Morrow, 2004; Wakefield et al., 1995; Whitcomb et al., 1995). The hallmark of all of these studies is that following limited short-term culture of the virus, reversion occurs to select the tRNA used by the wild type virus, indicating that additional features of the virus and/or host cell contribute to the selection of specific tRNA primers.
Previous studies from our laboratory have taken a genetic approach to understand HIV-1 primer selection. For these studies, we have constructed HIV-1 in which the PBS has been altered to be complementary to a variety of different tRNAs, including tRNAPro which is used by MuLV and tRNATrp which is used by avian retroviruses (Kang et al., 1996; Wakefield et al., 1995). Without exception, viruses in which just the PBS was altered to correspond to alternative tRNAs reverted back to utilize tRNALys,3 following short-term in vitro replication. Stabilization of the use of alternative tRNAs has been accomplished for certain tRNAs through additional mutations upstream in a region designated as the A-loop. Previous studies have shown that this region interacts with the anticodon loop of tRNALys,3 to stabilize the tRNA-viral RNA genome interaction necessary for the initiation complex of reverse transcription. Based on this information, we have generated HIV-1 that stably maintains a PBS complementary to tRNALys1,2, tRNAHis, tRNAMet and tRNAGlu (Dupuy et al., 2003; Kang and Morrow, 1999; Kang et al., 1997; Kang et al., 1999; Wakefield et al., 1996; Zhang et al., 1998). Although these viruses stably maintain a PBS complementary to these alternative tRNAs, they possess different replication characteristics from the wild type virus in SupT1 as well as PBMC (Moore et al., 2004; Moore-Rigdon et al., 2005). Interestingly, A-loop and PBS mutations to be complementary to tRNAIle, tRNAPro, tRNATrp or tRNAGln did not result in viruses that could stably utilize these tRNAs for replication ((Kang et al., 1996) Li et al., Submitted).
To develop a thorough understanding of the mechanism of HIV-1 primer selection, it will be necessary to further delineate the tRNAs that can be stably used by the virus for replication. Towards this goal, we have further extended our genetic analysis to include viruses in which the PBS was mutated to a variety of different tRNAs, with the ultimate goal to obtain insights into which tRNAs can stably be used by the virus for replication. In the current study, we report on four of these tRNAs, tRNAThr, tRNASer, tRNATyr, and tRNAPhe because of the diverse effects observed on HIV-1 replication and stability of the PBS. The results of our analysis, when combined with results from our previous studies, provide a better picture as to which tRNAs are available for HIV-1 for primer selection.
Materials and Methods
Construction of NL4-3 proviruses containing modified U5-PBS regions
All the mutations in PBS region were made with pUC119PBS as the template by QuickChange II Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA). The pUC119PBS is the shuttle vector with HIV-1 U5-PBS insertion (Moore et al., 2004). The plasmid pUC119Phe, pUC119Ser, pUC119Thr and pUC119Tyr are mutants with PBS complementary to tRNAPhe, tRNASer, tRNAThr and tRNATyr respectively. The primers for pUC119Phe were 5'-CAGTGGCGCCCGAACCCGGGACTGAAAGCGAA AGGG-3' and 5'-CCCTTTCGCTTTCAGTCCCGGGTTCGGGCGCCACTG-3'. Mutagenesis was done in a single step for pUC119Phe. To construct pUC119Ser, pUC119Thr and pUC119Tyr, a two step mutagenesis was done. For the first step, primers were 5'-GGA AAATCTCTAGCAGTGGCGTAGTCACAGGGACCTGAAAG CGAAAGG-3' and 5'-CCTTTCGCTTTCAGGTCCCTGTGACTACGCCACTGCTAGA GATTTTCC-3' for pUC119Ser; 5'-GAAAATCTCTAGCAGTGGAGGCCCCGCAGGG ACCTGAAAGCGAAAG-3' and 5'-CTTTCGCTTTCAGGTCCCTGCGGGGCCTCCAC TGCTAGAGATTTTC-3' for pUC119Thr; 5'-GAAAATCTCTAGCAGTGGCGCTTCG ACAGGGAACTGAAAGCGAAAGGG-3' and 5'-CCCTTTCGCTTTCAGTTCCCTGTC GAAGCGCCACT GCTAGAGATTTTC-3' for pUC119Tyr. The second step primers are 5'-ATCTCTAGCAGTGGCGTAGTCGGCAGGATCTGAAAGCGAAAGGGAAAC -3' and 5'-GTTTCCCTTTCGCTTTCAGATCCTGCCGACTACGCCACTGCTAGAGA T-3' for UC119Ser; 5'-GTGGAGGCCCCGCTGGGATCTGAAAGCGAAAG-3' and 5'-C TTTCGCTTTCAGATCCCAGCGGGGCCTCCAC-3' for pUC119Thr; 5'-GGAAAATC TCTAGCAGTGGTCCTTCGAGCCGGAACTGAAAGCGAAAGG-3' and 5'-CCTTTC GCTTTCAGTTCCGGCTCGAAGGACCACTGCTAGAGATTTTCC-3' for pUC119Tyr.
To further modify the A-loop region to be complementary to the anti-codon of tRNAPhe, tRNASer, tRNAThr and tRNATyr (pUC119Phe-AC, pUC119Ser-AC, pUC119Tyr-AC and pUC119Thr-AC), the corresponding pUC119PBS mutant was used as template. The primers were 5'-CAGACCCTTTTAGTCAGTGTGGTCTTCTCTCTAGCAGTGGT GCCAAACC-3' and 5'-GGTTTCGGCACCACTGCTAGAGAGAAGACCACACTGAC TAAAAGGGTCTG-3' for pUC119Phe-AC; 5'-TCAGACCCTTTTAGTCAGTGTGGTT CTATCTCTAGCAGTGGCGTAGTC-3' and 5'-GACTACGCCACTGCTAGAGATAG AACCACACTGACTAAAAGGGTCTGA-3' for pUC119Ser-AC; 5'-GACCCTTTTAG TCAGTGTGGTACTATCTCTAGCAGTGGAGGC-3' and 5'-GCCTCCACTGCTAGAG ATAGTACCACACTGACTAAAAGGGTC-3' for pUC119Thr-AC; 5'-GACCCTTTTA GTCAGTGTGGCTACATCTCTAGCAGTGGTCCT-3' and 5'-AGGACCACTGCTAGA GATGTAGCCACACTGACTAAAAGGGTC-3' for pUC119Tyr-AC. Following mutagenesis, the clones were screened by DNA sequencing. To clone the mutations back into the NL4-3 proviruses, HpaI-BssHII fragments of pUC119 mutants were sub-cloned into the SmaI- BssHII sites of NL4-3 HIV-1 proviral plasmid. All resulting NL4-3 constructs were verified by DNA sequencing.
Cell lines and tissue culture
The 293T cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Cellgro, Herndon, VA) plus 10% fetal bovine serum (FBS) (Hyclone, Logan UT) and 1% antibiotic-antimycotic (Gibco BRL, Rockville, MD). SupT1 cells were maintained in RPMI 1640 (Cellgro, Herndon, VA), supplemented with 15% FBS and 1% antibiotic-antimycotic. PBMCs were collected, stimulated using rIL-2 and phytohemagglutinin (PHA) (Sigma, St. Louis, MO) for 72 hours and maintained as described previously (Moore et al., 2004).
DNA transfections
Transfections were performed by Fugene 6 Transfection Reagent (Roche Molecular Biochemicals, Indianapolis, IN). Briefly, 2μg of proviral plasmid DNA and 3μL Fugene reagent were added to 100 μL of DMEM without FBS. This mixture was incubated at room temperature for about 45 minutes then added drop-wise to one well of a 6-well plate containing 50% confluent 293T cells in DMEM with 10% FBS. The transfections were incubated overnight at 37°C, then the medium was replaced with fresh DMEM containing 10% FBS. After 48 hours, all supernatants were collected and centrifuged with 1000 rpm for 10 minutes; the supernatants were aliquoted and stored at −80°C. Supernatants from transfected cells were assayed for HIV-1 p24 antigen by ELISA (Beckman Coulter, Miami, FL) and infectivity was determined by JC53BL assay as described previously (Moore-Rigdon et al., 2005).
Infection of SupT1 and PBMC and virus replication
Under our standard conditions, the viral supernatants containing 250 IU or 1000 IU infectivity were used to infect 1 x 106 SupT1 cells or 1 x 107 PHA and rIL-2 stimulated PBMCs respectively. The mixtures of virus and cells were incubated for 4 hours at 37°C and 5% CO2 with shaking every 30 minutes. The mixtures were then transferred to 25 cm2 tissue culture flasks, and the final volumes were adjusted to 10 mL with RPMI 1640 containing 15% FBS for both SupT1 and PBMC and 30 U/mL rIL-2 for PBMCs.
Every 3 days, the infected SupT1 cells were passaged at 1:4. For PBMC cultures, half of medium was replaced every three days without removing PBMC. Every 7 days, 1 mL of cell suspension was removed from SupT1 or PBMC and centrifuged at 24,000 x g for 1 minute. Supernatant and cell pellets were stored at −80°C for further analysis. For the SupT1 cell cultures, if the cytotoxicity resulted in extensive cell death (and clearing), 1 x 106 SupT1 cells were added to the cultures. For PBMCs, 5 x 106 PHA and rIL-2 stimulated PBMCs were added every two weeks.
DNA sequence analysis of viral U5-PBS region
High-molecular-weight DNA (HMW) was isolated from SupT1 or PBMC cell pellets by Wizard genomic DNA purification kit (Promega, Madison, WI) according to the manufacturer’s protocol. The fragment containing the U5-PBS regions of integrated proviruses was amplified from HMW by PCR with primers EcoRI (5’-CGGAATTCTCTCCTTCTAGCCTCCGCTAGTC-3’) and SphI (5’-CCTTGAGCATGC GATCTACCACA CACAAGGC-3’). The PCR products were run on the 1% agarose gel and the approximate 750bp DNA fragment was excised and extracted by the Qiagen Gel Purification Kit (Qiagen, Valencia, CA). The purified DNA was sequenced directly by automated DNA sequencing using the EcoRI primer.
Results
Construction of HIV-1 proviral mutants with PBS and A-loop complementary to tRNAThr and tRNASer
In a previous study, we examined the effect of altering the PBS of HIV-1 to be complementary to tRNAGln (Li et al., Submitted). From the analysis of the PBS of this virus following in vitro culture, we found that it had converted to be complementary to tRNAThr. Following extended in vitro replication, this virus eventually reverted back to utilize tRNALys,3 as the primer for replication. HIV-1 then, appears to have a preference for the selection of tRNAThr under certain circumstances. The first series of experiments were designed to evaluate HIV-1 with the PBS complementary to tRNAThr. For these studies, we generated HIV-1 in which the PBS was made complementary to tRNAThr as well as the PBS and A-loop sequence made complementary to the 3’ terminal nucleotides of tRNAThr and the anticodon of tRNAThr (NL4-Thr and NL4-Thr-AC, respectively). To pursue our genetic analysis of the preference for HIV-1 for tRNAs, we also constructed an HIV-1 proviral genome in which the PBS was mutated to be complementary to a tRNA not previously tested, tRNASer (NL4-Ser). A second proviral mutant was also generated in which both the A-loop and PBS were complementary to the anticodon and 3’ terminal nucleotides of tRNASer (NL4-Ser-AC) (Figure 1A).
Figure 1. Characterization of HIV-1 U5-PBS complementary to tRNAThr or tRNASer.
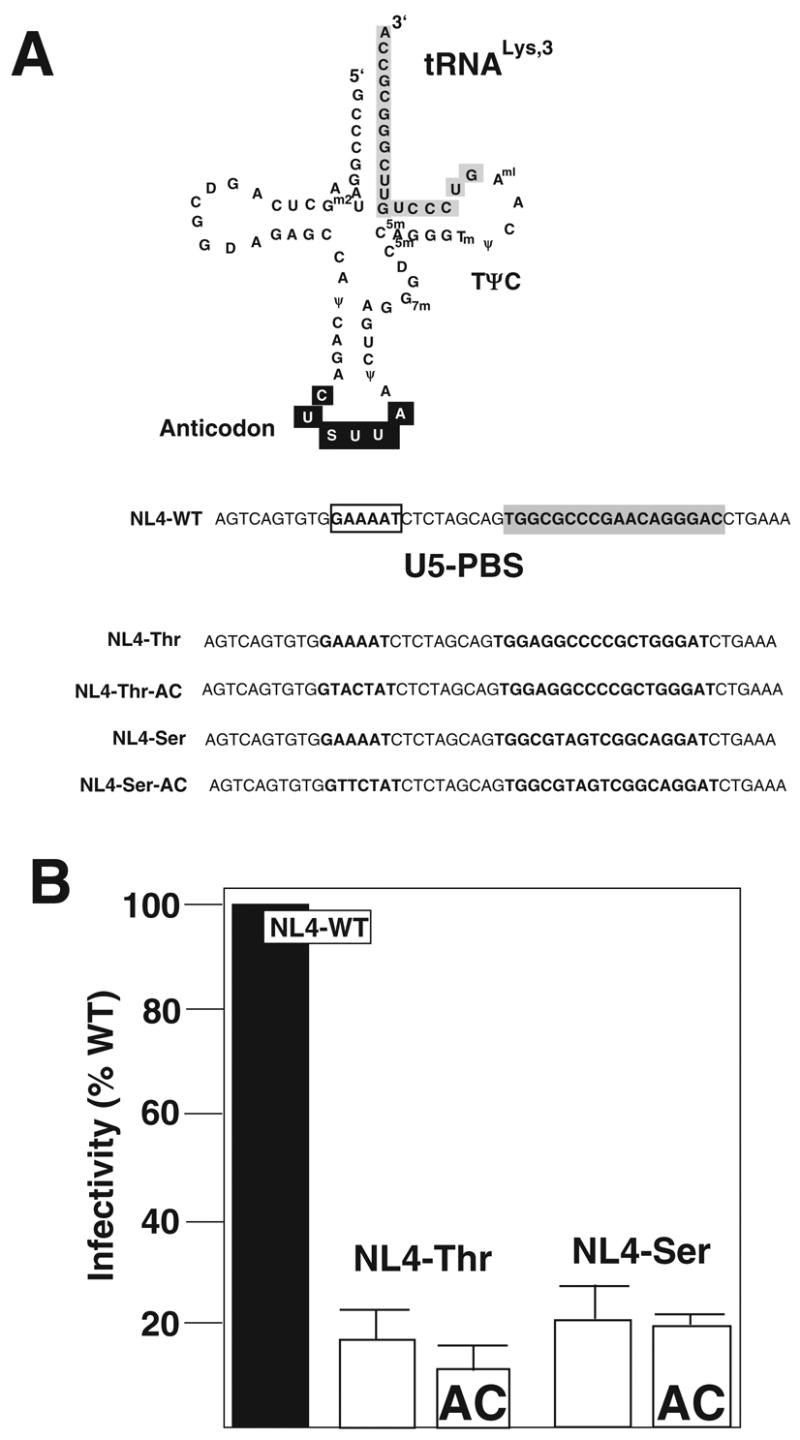
Panel A. tRNALys,3 is depicted with the region complementary to the PBS (shaded) and A-loop (anticodon, in black) highlighted. Post transcriptional modified bases are shown (Sprinzl et al., 1991). The U5-PBS of NL4-WT is shown with PBS (shaded) and A-loop (boxed). The modified nucleotides HIV-1 genomes with A-loop and PBS complementary to tRNAThr and tRNASer. The PBS and A-loop region from the proviral NL4-3 clones were mutated to correspond to the anticodon and 3’ terminal nucleotide of tRNAThr (NL4-Thr, NL4-Thr-AC) or tRNASer (NL4-Ser, NL4-Ser-AC). The mutant proviral genomes were isogenic with NL4 with the exception of the nucleotides mutated in the A-loop and PBS region.
Panel B. Infectivity of HIV-1 with U5-PBS complementary to tRNAThr or tRNASer. Proviral genomes were transfected into 293T cells and the amount of infectious virus (IU) released following transfection was determined using the JC53-BL assay. The total amount of virus was also determined using a p24 antigen ELISA. The infectivity (IU/p24) was determined for each virus and compared to that of the wild type virus (NL4-WT). The data presented is from three independent transfections.
We first analyzed the effects of these mutations have on the infectivity of HIV-1. For these studies, we transfected the proviral genomes into 293T cells and determined the amount of infectious virus released using the JC53-BL assay (Derdeyn et al., 2000). We also determined the levels of p24 antigen produced from the cultures. The infectivity of the virus was then determined by dividing the amount of infectious units by p24 antigen. We found that viruses derived from NL4-Thr, NL4-Thr-AC, NL4-Ser and NL4-Ser-AC had infectivities approximately 20% that of wild type (Figure 1B). We have found a similar reduction in infectivity compared to wild type for other PBS mutant viruses.
Characterization of the replication of HIV-1 with PBS complementary tRNAThr or tRNASer
We next examined the replication of viruses in SupT1. The course of infection was monitored by the amount of p24 antigen in the culture supernatant following infection. Consistent with our previous studies, we noted a rapid rise in the production of p24 antigen from cultures infected with NL4-WT. The amounts of virus peaked at approximately day 21 post initiation of culture and remained at this level for the continuation of the culture period (approximately 60 days). The NL4-Thr virus exhibited a delay in replication compared to the wild type virus, eventually reaching the same level as the wild type virus at 28 days post initiation of culture. In contrast, the NL4-Thr-AC virus grew very slowly in SupT1 cells and only after 35 days in culture began to increase as measured by p24 antigen. From day 35 to 60 post initiation of culture, the virus exhibited a rise in p24 antigen eventually reaching the level similar to that of the wild type. We next characterized the replication of the virus with a PBS complementary to tRNASer. Both NL4-Ser and NL4-Ser-AC had delayed replication compared to the wild type virus. However, by day 21 to 28 post initiation of culture, these viruses grew at levels similar to that of the wild type virus (Figure 2A).
Figure 2. Replication of HIV-1 with A-loop-PBS complementary to tRNAThr and tRNASer.
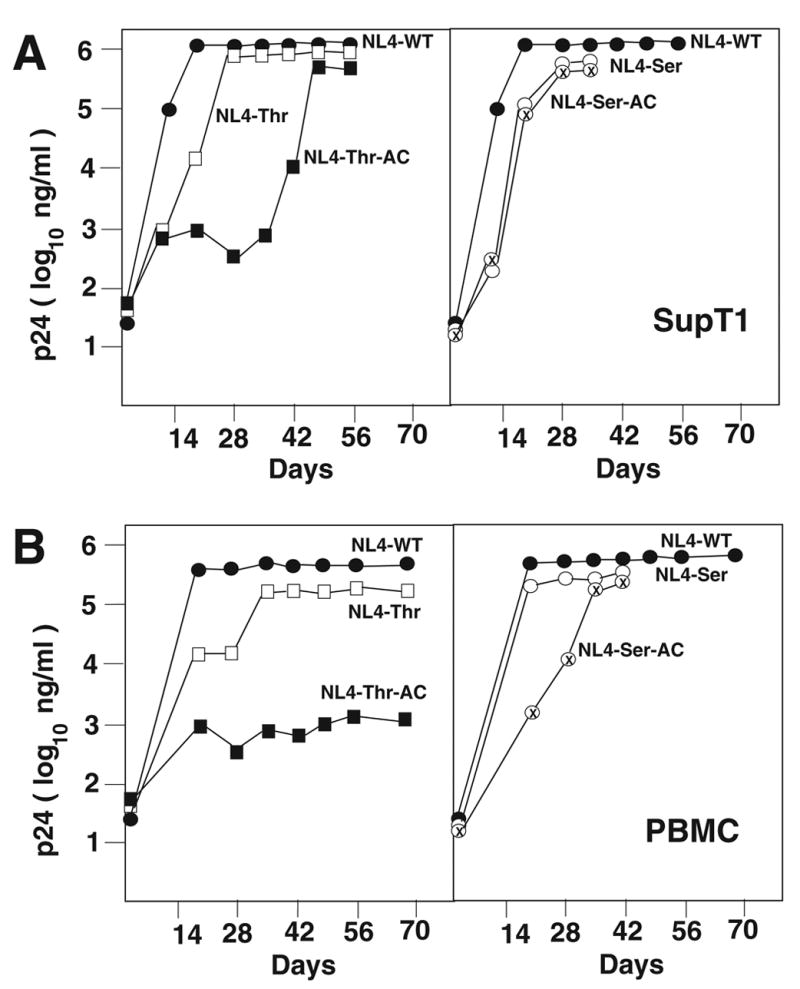
The replication of wild type and mutant viruses was analyzed in SupT1 and PBMC. Virus infection was monitored by analysis of p24 antigen in the cultured supernatant. The replication of viruses is noted in Panel A (SupT1) and Panel B (PBMC).
We then analyzed the replication of these viruses in PBMCs. The wild type virus demonstrated a rapid increase in p24 antigen, peaking at 21 days post initiation of the infection. The NL4-Thr virus also demonstrated a rapid increase in p24 antigen, although slightly delayed compared to the wild type and peaked at approximately day 35 post initiation of culture. In contrast, the virus derived from NL4-Thr-AC grew very slowly in PBMCs, reaching a level of only 1/100 that of the wild type or NL4-Thr virus. The viruses derived from NL4-Ser and NL4-Ser-AC also showed delayed replication compared to the wild type virus but eventually reached levels comparable to the wild type virus by day 35 post initiation of infection (Figure 2B).
The stability of the PBS in these viruses during infection was then evaluated. Although NL4-Thr grown in either SupT1 or PBMCs had reverted to use tRNALys,3 by the end of the culture period, we did find that early in the infection, the virus retained the PBS complementary to tRNAThr. The PBS from viruses derived from NL4-Thr-AC, grown in SupT1 cells, which eventually reached levels comparable to that of the wild type virus by 49 to 60 days post initiation of infection, were still complementary to tRNAThr. Thus, the inclusion of the A-loop region has stabilized this virus to utilize tRNAThr for replication. However, NL4-Thr-AC grew poorly, if at all, in PBMCs; the PBS from these viruses was also still complementary to tRNAThr. In contrast, the PBS from viruses derived from NL4-Ser or NL4-Ser-AC all reverted to utilize tRNALys,3 following short-term of in vitro culture (data not shown).
Characterization of HIV-1 proviral genomes with PBS and A-loop complementary to tRNAPhe and tRNATyr
In a previous study, the PBS of HIV-1 was mutated to be complementary to mammalian tRNAPhe (Li et al., 1994). The results of this study found that the PBS reverted back to be complementary to tRNALys,3 following limited short-term in vitro replication of this virus. To follow-up on this study, we created HIV-1 in which the PBS or PBS and A-loop were complementary to the 3’ terminal 18-nucleotide and anticodon region of tRNAPhe. These viruses were named NL4-Phe and NL4-Phe-AC, respectively. A second virus was constructed in which the PBS was made complementary to tRNATyr and was designated as NL4-Tyr. A virus was also constructed with both PBS and A-loop were mutated to be complementary to 3’ terminal nucleotides and anticodon region of tRNATyr (NL4-Tyr-AC) (Figure 3A). We first analyzed the production of infectious virus following transfection of proviral genomes into 293T cells. Supernatants were used to infect JC53-BL cells and the amounts of infectious virus were determined. Viruses in which the PBS was altered to be complementary to tRNAPhe (NL4-Phe and NL4-Phe-AC) resulted in virus that was approximately 10 – 20% as infective as the wild type. In contrast, alteration of the PBS to be complementary to tRNATyr, as found in NL4-Tyr or NL4-Tyr-AC resulted in viruses with less infectivity ranging from 5 – 8% of the wild type (Figure 3B).
Figure 3. Characterization of HIV-1 U5-PBS complementary to tRNAPhe and tRNATyr.
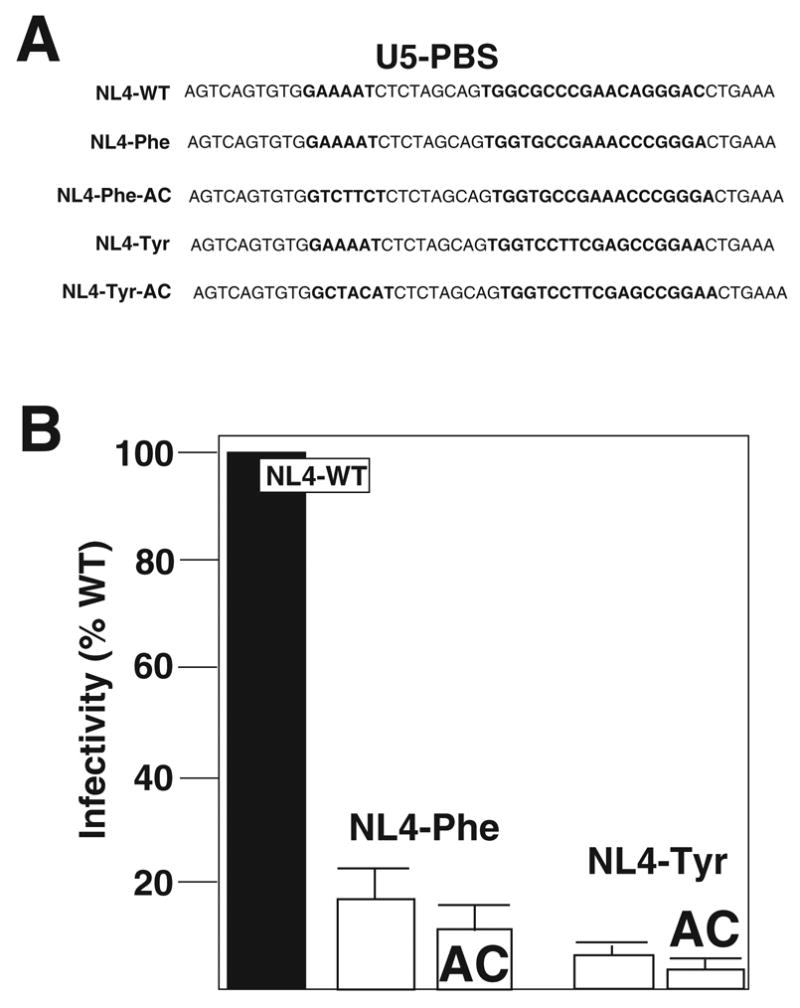
Panel A. HIV-1 was constructed with mutations in the U5-PBS to be complementary to tRNAPhe or tRNATyr. The viruses were isogenic with NL4-WT except for the designated mutations.
Panel B. Infectivity. The viruses with U5-PBS complementary to tRNAPhe or tRNATyr. The infectivity of these viruses following transfection of proviral genomes into 293T cells was determined. The amount of infectious virus released (IUs) was determined using the JC53-BL assay while the levels of total virus produced were measured using the p24 antigen capture ELISA. The infectivity (IU/p24) for each virus was then compared to the wild type virus. Data presented is from three independent transfections.
We next characterized the replication of NL4-Phe and NL4-Phe-AC in SupT1 and PBMCs. Under standard conditions, the wild type virus grew rapidly in SupT1 cells, peaking at day 21 post initiation of culture. The virus derived from NL4-Phe also demonstrated similar kinetics of replication as the wild type virus peaking at day 21 post initiation of culture. The PBS from viruses derived from NL4-Phe grown both in SupT1 and PBMCs at day 21 post initiation of culture was complementary to tRNALys,3, indicating that the virus had rapidly reverted to utilize the wild type primer for replication. In contrast, viruses derived from NL4-Phe-AC did not increase in p24 antigen; by day 21, the p24 levels were essentially at background levels. A similar replication pattern was observed when the virus was grown in PBMC. In this case, again the wild type virus reached maximum levels at day 21 post initiation of culture. The virus derived from NL4-Phe also reached a maximum at day 21 post initiation of culture; while the virus derived from NL4-Phe-AC did not increase in p24 antigen during the culture period but had a gradual decline in p24 reaching a level similar to the background levels by day 35 post initiation of culture (Figure 4). In a second set of experiments, we analyzed the effects of increasing the initial inoculum of NL4-Phe-AC on the capacity of the virus to grow in SupT1 or PBMC. Increasing the amount of infectious virus by 4 to 5 times resulted in growth of NL4-Phe-AC in SupT1s or PBMC. Analysis of the virus following limited in vitro growth revealed this virus reverted to utilize tRNALys,3 during the infection. Thus, the virus derived from NL4-Phe is unstable and reverts quickly to utilize tRNALys,3 for replication. Under standard conditions, the virus NL4-Phe-AC was not infectious, indicating the A-loop has a deleterious effect in virus replication, which could be overcome by increasing the amount of infectious virus in the infection.
Figure 4. Replication of HIV-1 with A-loop PBS complementary to tRNAPhe and tRNATyr.
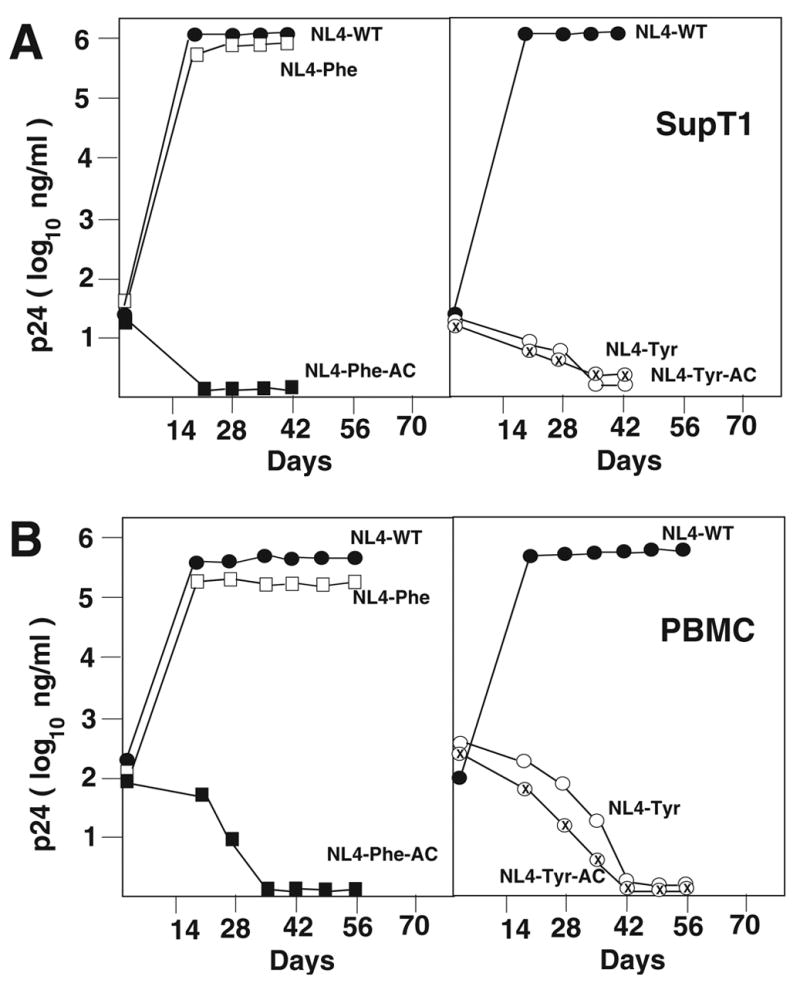
Replication of the wild type and mutant viruses was analyzed in SupT1 (Panel A) or in PBMC (Panel B). The virus replication was monitored by p24 antigen ELISA. A replication of each virus is noted in the figure.
The replication of NL4-Tyr and NL4-Tyr-AC in SupT1 or PBMC was next evaluated. Following infection of SupT1, the wild type virus again replicated efficiently, reaching a peak at day 21 post initiation of culture. In contrast, virus derived from NL4-Tyr or NL4-Tyr-AC did not grow as evidenced by increase in the p24 antigen in culture; at day 35 post initiation of culture the amounts of p24 antigen in the cultures were at background levels (Figure 4A). Analysis of the growth of NL4-Tyr or NL4-Tyr-AC in PBMCs revealed a similar pattern (Figure 4B). Both of these viruses did not increase p24 antigen during the culture period and by day 42 the levels were approximately background levels. By increasing the starting inoculum of viruses by 4 to 5 times that in the initial experiments, as we did for the viruses with a PBS complementary to tRNAPhe, we were able to demonstrate that viruses derived from NL4-Tyr and NL4-Tyr-AC were replication competent in SupT1 cells, although the kinetics of replication were severely delayed, as compared to that of the wild type virus (data not shown). Ultimately, these viruses did revert back to utilize tRNALys,3 for initiation of reverse transcription (data not shown). Analysis of the replication of NL4-Tyr and NL4-Tyr-AC in PBMC using higher amounts of virus to initiate the culture revealed that these viruses were also replication competent, although the levels of p24 antigen increased only 10 to 50 fold during the cultured period (at least 100 fold lower than the wild type virus). However, analysis of the PBS for NL4-Tyr and NL4-Tyr-AC revealed they were still complementary to tRNATyr. Analysis of the infectivity of these viruses following long-term in vitro culture in PBMC revealed that the levels were only 1 to 2% that of the wild type virus, suggesting the alteration of the PBS to be complementary to tRNATyr severely reduced the replication and infectivity of HIV-1 in PBMC.
Discussion
In previous studies, we and others, have investigated the effects that alteration of the PBS to be complementary to alternative tRNAs has on the replication and stability of HIV-1. In general, results from all of these studies have found that although HIV-1 can utilize these alternative tRNAs, the virus rapidly reverts back to utilize tRNALys,3. From our previous studies, we have identified a single virus, with the PBS complementary to tRNAMet, that maintains this PBS following in vitro culture (Moore et al., 2004). Interestingly, this virus was identified from the analysis of a virus in which the PBS was complementary to tRNATrp (Kang et al., 1996). Following in vitro culture of this virus, we noted the presence of a new, unanticipated, virus in which the PBS was complementary to tRNAMet. A similar result was obtained in our identification of the virus that utilizes tRNAThr (Li et al., submitted). In this case, we found that viruses in which the PBS was made complementary to tRNAGln reverted to first utilize tRNAThr then tRNALys,3 following in vitro replication. As shown in the current study, further analysis of the replication of virus with the PBS complementary to tRNAThr revealed that it had a greater inherent stability than viruses with a PBS complementary to tRNASer or tRNAPhe. Thus, similar to tRNAMet, HIV-1 appears to have a preference for the selection of tRNAThr. In contrast, viruses with a PBS complementary to tRNASer or tRNAPhe rapidly revert back to use tRNALys,3, which is similar to our previous studies with viruses with a PBS complementary to tRNAIle, tRNAPro, tRNALys1,2, tRNAHis and tRNAGlu (Dupuy et al., 2003; Kang and Morrow, 1999; Kang et al., 1996; Kang et al., 1999; Wakefield et al., 1996).
A new unexpected result was, the virus with the PBS complementary to tRNATyr was not infectious under our standard culture conditions. We were only able to derive infectious virus if we increased the starting inoculum of this virus; under these conditions, the virus did revert back to utilize tRNALys,3 in SupT1, following extended in vitro replication. The virus derived from NL4-Tyr then represents a unique finding in that alteration of the PBS results in a non-infectious virus under our standard culture conditions. There are two possibilities to explain the differences in the capacity of the viruses with alternative PBS to revert back to utilize tRNALys,3. The first involves potential interactions between tRNALys,3 and the alternative PBS. Based on our previous study, we anticipate that only the first six nucleotides are important for the interaction of tRNALys,3 with the alternative PBS (Rhim et al., 1991; Wakefield et al., 1994). Complementarity within the first six nucleotides is most important for the ability of tRNALys,3 to interact with the alternative PBS. It is clear that the PBS complementary to tRNAPhe or tRNASer show a significant complementarity with the 3’ terminal 6-nucleotides of tRNALys,3 and we would predict that tRNALys,3 should be able to interact with these PBS (Figure 5B). This interaction however, cannot be the sole explanation for the rapid reversion of these viruses, since tRNAMet also has complementarity with the first six nucleotides of tRNALys,3 and previously, viruses with a PBS complementary to tRNAMet are stable (Figure 5A). This complementarity would also not explain the inability of viruses with the PBS complementary to tRNATyr to grow since five of the first six nucleotides are complementary with the 3’ terminal nucleotides of tRNALys,3. However, it is noted that the fifth nucleotide is mismatched with the 3’ nucleotides of tRNALys,3, which could contribute to the lower capacity of the virus to interact with tRNALys,3 needed for reversion (Figure 5C) (Wakefield et al., 1994).
Figure 5. Sequence comparison of PBS and 3’ terminal nucleotides of tRNALys,3.
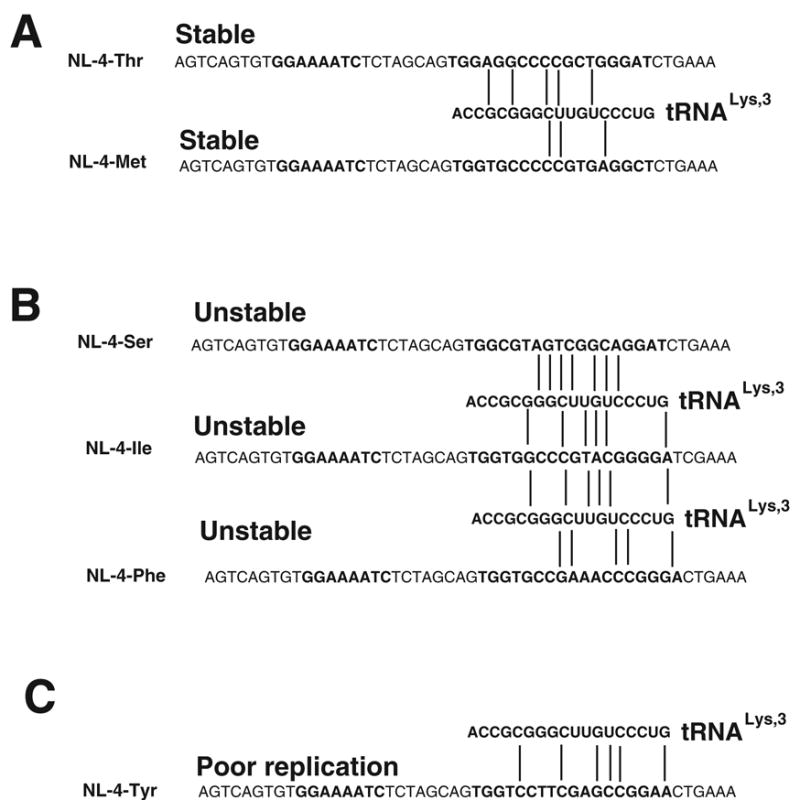
Panel A. Interaction of 3’ terminal 18 nucleotides of tRNALys,3 with viruses that have PBS complementary to tRNAThr (NL4-Thr) and tRNAMet (NL4-Met). Stable refers to PBS region in the absence of A-loop modification.
Panel B. Interaction of 3’ terminal nucleotides of tRNALys,3 with virus that have PBS complementary to tRNASer (NL4-Ser), tRNAIle (NL4-Ile) or tRNAPhe (NL4-Phe). Unstable refers to the PBS region that reverts to be complementary to tRNALys,3 following culture. Note that NL4-Ser and NL4-Ile revert even with A-loop modifications, while NL4-Phe-AC replicates poorly with A-loop modification.
Panel C. Interaction of 3’ terminal nucleotides of tRNALys,3 with PBS complementary to tRNATyr (NL4-Tyr). Poor replication means virus does not grow in SupT1 or PBMC under standard conditions. Note that this virus is not resulted by A-loop modification (NL4-Tyr-AC).
Base pair mismatches between PBS and tRNAs for Panels A, B and C are noted by vertical lines.
During plus-strand synthesis, the reverse transcriptase recopies the attached tRNA primer to provide sequences complementary to the minus-strand PBS that facilitate completion of the proviral genome (Gilboa et al., 1979). Previous studies from our laboratory have shown that complementarity with the plus- and minus- DNA strands of the PBS is important for virus infectivity (Wakefield and Morrow, 1996). In this regard, viruses with a PBS complementary to tRNATyr would not have complementarity with the 3’ nucleotides and tRNALys,3. However, this does not explain the lack of infectivity because viruses with a PBS complementary to tRNAPhe or tRNAIle, which also rapidly reverts back to utilize tRNALys,3, show a similar nucleotide sequence as viruses with the PBS complementary to tRNATyr (Kang et al., 1996) (Figure 5B). Thus, the complementarity between tRNALys,3 and the PBS does not entirely explain the propensity of viruses with certain PBS to revert back to utilize tRNALys,3.
A second possibility to explain the preferences for reversion of certain PBS is that a distinct pool of tRNAs exists for the selection of tRNA primers by HIV-1. Within this pool, tRNALys,3 is most representative with other tRNAs such as tRNAMet and tRNAThr also being highly represented, while tRNAs such as tRNATyr, tRNASer and tRNAPhe are not well represented. HIV-1 genomes in which the PBS is complementary to tRNAs that are not occupied with the cognate tRNA, allowing tRNALys,3 to potentially invade the alternative PBS. It is not clear at the present time what elements define the composition of the tRNA pool used for primer selection. It is possible that during translation of viral proteins, possibly Gag or Gag-pol, that microenvironments are established with pools of certain tRNAs. Previous studies from our laboratory have suggested a coupling of viral translation and primer selection (Kelly et al., 2003). How these primers are linked and to what extent that influences the selection of certain primers will require further study.
The A-loop modifications of the U5 region had range of effects on the replication and stabilities of the alternative PBS. The virus NL4-Thr-AC stably maintained a PBS complementary to tRNAThr following in vitro replication in SupT1s or PBMC. However, analysis of the replication of this virus revealed that it was slower than NL4-Thr, even during the culture times when the virus NL4-Thr maintained the PBS complementary to tRNAThr, the inclusion of the A-loop replication actually slowed the virus replication. Indeed in competition experiments, we found that NL4-Thr-AC grew slower than NL4-Thr (Ni and Morrow, unpublished). We have observed a similar result in viruses in which the PBS was mutated to be complementary to tRNAMet where we noted that the virus with both the PBS and A-loop complementary to tRNAMet replicated slower than the virus with only the PBS complementary to tRNAMet (Moore et al., 2004). It appeared from this analysis then that the inclusion of the A-loop has the effect to slow down virus replication. This in fact was also highlighted upon the analysis of the virus with the PBS and A-loop complementary to tRNAPhe (NL4-Phe-AC), where this virus did not replicate under our standard conditions in SupT1 or PBMC. It was only when the initial virus inoculum was increased 4 to 5 fold that we observed virus replication, where the virus rapidly reverted back to utilize tRNALys,3, similar to the results seen for viruses derived from NL4-Ser-AC. Collectively, the results of these studies suggest that the mutation in the A-loop to be complementary to the anticodon of certain alternative tRNAs does not allow these tRNAs to attract the alternative tRNAs for use in replication, but rather results in the virus slowing down replication, which, in turn, could facilitate the selection of certain alternative tRNAs. It is interesting though that certain A-loop regions function well in this regard to slow down virus replication (i.e., NL4-Phe-AC) while others such as NL4-Ser-AC and the virus with an A-loop region complementary to tRNAIle do not appear to have this effect resulting in a virus that rapidly reverts back to utilize tRNALys,3 for replication (Kang et al., 1996). The reason why certain A-loop regions do not promote the selection of certain alternative tRNAs is unclear. As pointed out previously, it could be due to the lack of these tRNAs in the pool that the virus uses to select the primer tRNA. In this regard, the A-loop could function to attract the tRNA, much like a codon-anti-codon interaction (Puglisi and Puglisi, 1998). Alternatively, it could be due to the fact that certain A-loop modifications maintain an RNA structure impacts on the replication of the virus (Goldschmidt et al., 2002). Additional experiments will be required to further understand the role of the A-loop in primer selection.
Conclusions
The results of these studies demonstrate diverse effects that alteration of the primer specificity for HIV-1 has on virus replication and genome stability. In our studies, we have analyzed the replication of viruses in which the PBS has been altered to be complementary to four tRNAs, tRNAThr, tRNASer, tRNAPhe and tRNATyr. Alteration of the PBS to be complementary to these tRNAs resulted in viruses with different replication characteristics and PBS stability. The inclusion of a second region complementary to the alternative tRNAs, designated as the A-loop region, allowed the stable use of certain tRNAs while for rapidly reverted back to utilize tRNALys,3. Thus, forcing the virus to utilize alternative tRNAs compromises the virus replication and many times results in genome instability that reverts back to utilize the most preferred tRNA, tRNALys,3. Most probably, the exclusive use of tRNALys,3 is due to an adaptation of the virus that allows high-level replication. The results of the current study though, provide new insights into tRNAs that are favored (eg. tRNAThr), those that are not but revert to use tRNALys,3 (tRNASer) or those that result in non-infectious virus (tRNATyr). The development of these preference groups for tRNAs will help to understand the dynamics of the interaction between HIV-1 and the host cell that allows for the selection of this specific tRNA as the primer for initiation of reverse transcription.
Acknowledgments
We thank the members of the Morrow Laboratory for helpful suggestions. Adrienne Ellis is thanked for preparation of the manuscript. The DNA sequencing was carried out by the UAB CFAR DNA Sequencing Core (AI 27767). CDM acknowledges helpful suggestions from MAR. This research was supported by a grant from the NIH (AI34749).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baltimore D. RNA-dependent DNA polymerase in virions of RNA tumour viruses. Nature. 1970;226:1209–1211. doi: 10.1038/2261209a0. [DOI] [PubMed] [Google Scholar]
- Das AT, Klaver B, Berkhout B. Reduced replication of human immunodeficiency virus type 1 mutants that use reverse transcription primers other than the natural tRNALys,3. J Virol. 1995;69(5):3090–3097. doi: 10.1128/jvi.69.5.3090-3097.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derdeyn CA, Decker JM, Sfakianos JN, Wu X, O'Brien WA, Ratner L, Kappes JC, Shaw GM, Hunter E. Sensitivity of human immunodeficiency virus type 1 to the fusion inhibitor T-20 modulated by coreceptor specificity defined by the V3 loop of gp120. J Virol. 2000;74:8358–8367. doi: 10.1128/jvi.74.18.8358-8367.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuy LC, Kelly NJ, Elgavish TE, Harvey SC, Morrow CD. Probing the importance of tRNA anticodon: human immunodeficiency virus type 1 (HIV-1) RNA genome complementarity with an HIV-1 that selects tRNAGlu for replication. J Virol. 2003;77(16):8756–8764. doi: 10.1128/JVI.77.16.8756-8764.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa E, Mitra SW, Goff S, Baltimore D. A detailed model of reverse transcription and tests of crucial aspects. Cell. 1979;18:93–100. doi: 10.1016/0092-8674(79)90357-x. [DOI] [PubMed] [Google Scholar]
- Goldschmidt V, Rigourd M, Ehresmann C, Le Grice SFJ, Ehresmann B, Marquet R. Direct and indirect contributions of RNA secondary structure elements to the initiation of HIV-1 reverse transcription. J Biol Chem. 2002;277(45):43233–43242. doi: 10.1074/jbc.M205295200. [DOI] [PubMed] [Google Scholar]
- Kang SM, Morrow CD. Genetic analysis of a unique human immunodeficiency virus type 1 (HIV-1) with a primer binding site complementary to tRNAMet supports a role for U5-PBS stem-loop RNA structures in initiation of HIV-1 reverse transcription. J Virol. 1999;73:1818–1827. doi: 10.1128/jvi.73.3.1818-1827.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SM, Wakefield JK, Morrow CD. Mutations in both the U5 region and the primer-binding site influence the selection of the tRNA used for the initiation of HIV-1 reverse transcription. Virology. 1996;222:401–414. doi: 10.1006/viro.1996.0437. [DOI] [PubMed] [Google Scholar]
- Kang SM, Zhang Z, Morrow CD. Identification of a sequence within U5 required for human immunodeficiency virus type 1 to stably maintain a primer binding site complementary to tRNAMet. J Virol. 1997;71(1):207–217. doi: 10.1128/jvi.71.1.207-217.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SM, Zhang Z, Morrow CD. Identification of a human immunodeficiency virus type 1 that stably uses tRNALys1,2 rather than tRNALys,3 for initiation of reverse transcription. Virology. 1999;257:95–105. doi: 10.1006/viro.1999.9615. [DOI] [PubMed] [Google Scholar]
- Kelly NJ, Palmer MT, Morrow CD. Selection of retroviral reverse transcription primer is coordinated with tRNA biogenesis. J Virol. 2003;77:8695–8701. doi: 10.1128/JVI.77.16.8695-8701.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Mak J, Arts EJ, Gu Z, Kleiman L, Wainberg MA, Parniak MA. Effects of alterations of primer-binding site sequences on human immunodeficiency virus type 1 replication. J Virol. 1994;68(10):6198–6206. doi: 10.1128/jvi.68.10.6198-6206.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund AH, Duch M, Lovmand J, Jorgensen P, Pedersen FS. Mutated primer binding sites interacting with different tRNAs allow efficient murine leukemia virus replication. J Virol. 1993;67:7125–7130. doi: 10.1128/jvi.67.12.7125-7130.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak J, Khorchid A, Cao Q, Huang Y, Lowy I, Parniak MA, Prasad VR, Wainberg MA, Kleiman L. Effects of mutations in Pr160gag-pol upon tRNALys,3 and Pr160gag-pol incorporation into HIV-1. J Mol Biol. 1997;265:419–431. doi: 10.1006/jmbi.1996.0742. [DOI] [PubMed] [Google Scholar]
- Marquet R, Isel C, Ehresmann C, Ehresmann B. tRNAs as primer of reverse transcriptases. Biochimie. 1995;77:113–124. doi: 10.1016/0300-9084(96)88114-4. [DOI] [PubMed] [Google Scholar]
- Moore KL, Kosloff BR, Kelly NJ, Kirkman RL, Dupuy LC, McPherson S, Morrow CD. HIV type 1 that select tRNAHis or tRNALys1,2 as primers for reverse transcription exhibit different infectivities in peripheral blood mononuclear cells. AIDS Res Hum Retrov. 2004;20(4):373–381. doi: 10.1089/088922204323048122. [DOI] [PubMed] [Google Scholar]
- Moore-Rigdon KL, Kosloff BR, Kirkman RL, Morrow CD. Preferences for the selection of unique tRNA primers revealed from analysis of HIV-1 replication in peripheral blood mononuclear cells. Retrovirology. 2005;2(21) doi: 10.1186/1742-4690-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer MT, Morrow CD. Analysis of murine leukemia virus replication complemented by yeast tRNAPhe reveals inherent preferences for the tRNA primer selected for reverse transcription. Virology. 2004;324(2):430–438. doi: 10.1016/j.virol.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Panet A, Berliner H. Binding of tRNA to reverse transcriptase of RNA tumor viruses. J Virol. 1978;26:214–220. doi: 10.1128/jvi.26.2.214-220.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters G, Dahlberg JE. RNA-directed DNA synthesis in Moloney murine leukemia virus: Interaction between the primer tRNA and the genome RNA. J Virol. 1979;31:398–407. doi: 10.1128/jvi.31.2.398-407.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puglisi EV, Puglisi JD. HIV-1 A-rich RNA loop mimics the tRNA anticodon structure. Nat Struct Biol. 1998;5(12):1033–1036. doi: 10.1038/4141. [DOI] [PubMed] [Google Scholar]
- Rhim H, Park J, Morrow CD. Deletions in the tRNALys primer-binding site of human immunodeficiency virus type 1 identify essential regions for reverse transcription. J Virol. 1991;65:4555–4564. doi: 10.1128/jvi.65.9.4555-4564.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprinzl M, Dank N, Nock S, Schon A. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 1991;19:2127–2171. doi: 10.1093/nar/19.suppl.2127. Suppl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temin HM. Structure, variation and synthesis of retrovirus long terminal repeat. Cell. 1981;27:1–3. doi: 10.1016/0092-8674(81)90353-6. [DOI] [PubMed] [Google Scholar]
- Temin HM, Mizutani S. RNA-dependent DNA polymerase in virions of Rous sarcoma virus. Nature. 1970;226:1211–1213. doi: 10.1038/2261211a0. [DOI] [PubMed] [Google Scholar]
- Wakefield JK, Kang SM, Morrow CD. Construction of a type 1 human immunodeficiency virus that maintains a primer binding site complementary to tRNAHis. J Virol. 1996;70:966–975. doi: 10.1128/jvi.70.2.966-975.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield JK, Morrow CD. Mutations within the primer binding site of the human immunodeficiency virus type 1 define sequence requirements essential for reverse transcription. Virology. 1996;220:290–298. doi: 10.1006/viro.1996.0317. [DOI] [PubMed] [Google Scholar]
- Wakefield JK, Rhim H, Morrow CD. Minimal sequence requirements of a functional human immunodeficiency virus type 1 primer binding site. J Virol. 1994;68:1605–1614. doi: 10.1128/jvi.68.3.1605-1614.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield JK, Wolf AG, Morrow CD. Human immunodeficiency virus type 1 can use different tRNAs as primers for reverse transcription but selectively maintains a primer binding site complementary to tRNALys,3. J Virol. 1995;69:6021–6029. doi: 10.1128/jvi.69.10.6021-6029.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitcomb JM, Ortiz-Conde BA, Hughes SH. Replication of avian leukosis viruses with mutations at the primer binding site: Use of alternative tRNAs as primers. J Virol. 1995;69(10):6228–6238. doi: 10.1128/jvi.69.10.6228-6238.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Kang SM, Li Y, Morrow CD. Genetic analysis of the U5-PBS of a novel HIV-1 reveals multiple interactions between the tRNA and RNA genome required for initiation of reverse transcription. RNA. 1998;4:394–406. [PMC free article] [PubMed] [Google Scholar]


