Abstract
Demyelination of the human peripheral nervous system (PNS) can be caused by diverse mechanisms including viral infection. Despite association of several viruses with the development of peripheral demyelination, animal models of the condition have been limited to disease that is either autoimmune or genetic in origin. We describe here a model of PNS demyelination based on direct injection of sciatic nerves of mice with the cardiovirus, Theiler's murine encephalomyelitis virus (TMEV). Sciatic nerves of FVB mice develop inflammatory cell infiltration following TMEV injection. Schwann cells and macrophages are infected with TMEV. Viral replication is observed initially in the sciatic nerves and subsequently the spinal cord. Sciatic nerves are demyelinated by day 5 post-inoculation (p.i.). Injecting sciatic nerves of scid mice resulted in increased levels of virus recovered from the sciatic nerve and spinal cord relative to FVB mice. Demyelination also occurred in scid mice and by 12 days p.i., hind limbs were paralyzed. This new model of virus-induced peripheral demyelination may be used to dissect processes involved in protection of the PNS from viral insult and to study the early phases of lesion development.
Keywords: TMEV, peripheral demyelination, sciatic nerve
Introduction
Infectious agents are implicated in the development of human peripheral demyelinating diseases such as Guillain-Barre syndrome (Bitan, Or et al., 2004; Takahashi, Kunishige et al., 2005; Tababella & Nowzari, 2005; Ponticelli & Campise, 2005; Koga, Gilbert et al., 2005; Izurieta, Haber et al., 2005). Diverse viral infections have been associated with peripheral demyelinating disease including those caused by cytomegalovirus (Tababella & Nowzari, 2005), Epstein-Barr virus (Bitan, Or et al., 2004; Takahashi, Kunishige et al., 2005; Koga, Gilbert et al., 2005), human immunodeficiency virus-1 (Brannagan & Zhou, 2003), and varicella zoster virus (Roccatagliata, Uccelli et al., 2001). Some cases of peripheral demyelination have also been associated with viral vaccinations (Izurieta, Haber et al., 2005; Khamaisi, Shoenfeld et al., 2004). These reports cumulatively suggest that infection and/or the resulting immune response to infection may be responsible for inducing peripheral demyelinating disease in humans but despite this strong inference, models used to study this condition involve only autoimmune or genetic etiologies (van Rijk, Sweers et al., 2003; Moon, Kim et al., 2006; Zhu, Ljunggren et al., 2001; Bour-Jordan, Thompson et al., 2005; Meekins, Emery et al., 2004). Although current animal models recapitulate certain forms of peripheral nervous system (PNS) myelin loss (van Rijk, Sweers et al., 2003; Moon, Kim et al., 2006; Zhu, Ljunggren et al., 2001; Bour-Jordan, Thompson et al., 2005; Meekins, Emery et al., 2004), none of them describe virus-induced demyelination of PNS. This prompted us to explore the possibility of establishing a murine model to mimic this human condition.
Intracerebral (i.c.) inoculation of mice with Theiler’s murine encephalomyelitis virus (TMEV), is commonly used to study viral demyelination and remyelination of the central nervous system (CNS) (Drescher, Nguyen et al., 1998; Drescher, Zoecklein et al., 2000; Dal Canto & Lipton, 1975; Lipton, 1975; Rodriguez, 1993; Pena Rossi, Delcroix et al., 1997; Rodriguez, Pavelko et al., 1995; Rodriguez, Nabozny et al., 1994; Rodriguez & David, 1985). Because the pathologic changes and subsequent clinical signs of the disease in mice are similar to those observed in humans with multiple sclerosis (MS) TMEV infection of mice is also used to model a hypothetical viral etiology of MS (Dal Canto & Lipton, 1975; Lipton, 1975). Demyelination is induced by TMEV-mediated damage to CNS white matter (Lipton, 1975); chronic demyelination is attributed to the development of autoimmune responses to several myelin protein epitopes (Miller, Vanderlugt et al., 1997a; Miller, Vanderlugt et al., 1997b). Strains of TMEV belonging to the TO subgroup (BeAn and Daniel’s), induce acute encephalitis followed by chronic demyelinating disease in susceptible strains of mice (Lipton, 1975). The primary host determinant of demyelination by TMEV is the murine haplotype: H-2b, H-2d mice are resistant to TMEV-induced demyelination (Rodriguez, Dunkel et al., 1993). Resistance or susceptibility to demyelination can also be altered by deletion or depletion of CD8+ T cells (Rodriguez, Rivera-Quinones et al., 1997) or CD4+ T cells (Rodriguez, Rivera-Quinones et al., 1997; Rodriguez & Sriram, 1988), or by modulating a number of immune system components (Rodriguez, Pavelko, & Coffman, 1995; Fiette, Aubert et al., 1995; Murray, McGavern et al., 1998; Paya, Leibson et al., 1990).
Natural TMEV infections of mice are spread via an oral /fecal route of transmission (Theiler, 1940; Theiler & Gard, 1940). A likely route of infection following ingestion of the virus involves the peripheral nerves of the gut (Ren & Racaniello, 1992) whereby transport of virus to the CNS could then establish a chronic demyelinating disease characterized by inflammation and destruction of the myelin sheaths surrounding the axons. We therefore used the sciatic nerve in the leg (a relatively accessible peripheral nerve) as an example of the PNS to determine whether TMEV injection directly into the nerve would induce peripheral demyelination. Following direct inoculation of sciatic nerves of female FVB mice with TMEV, we observed demyelination coincident with viral replication within the nerve. Virus subsequently spread into the CNS. In immunodeficient scid mice, increased viral replication and subsequent paralysis indicated a role for the adaptive immune response in controlling PNS virus infection. Because the precise site of initial virus inoculation is known, this model can be used to study the earliest stages of lesion development.
Results
TMEV is detected in sciatic nerves following direct injection with virus
Initial demyelination is due to virus-induced damage in the model of TMEV-triggered CNS demyelination (Dal Canto & Lipton, 1975). To test whether TMEV could be detectable in sciatic nerves following inoculation, FVB mice were inoculated with 1 x 104 pfu/sciatic nerve (described in Methods). Briefly, the thigh musculature of the mouse was separated to expose the sciatic nerve, into which virus was injected directly using a 30 ga needle. Injection did not occur through the muscle itself. Mice were killed, the nerve dissected, and then immunostaining of OCT-embedded sciatic nerves was performed using a polyclonal TMEV antiserum. At 3 days post-inoculation (p.i.), viral protein was detected in sciatic nerves of virus-injected (Figure 1A), but not HBSS-injected (control) mice (Figure 1B). Earlier in infection, virus positive cells were rarely detected in the sciatic nerve (data not shown). To test whether we were detecting residual protein from the virus inoculum, an equivalent amount of UV-inactivated TMEV was prepared and injected as before into sciatic nerves of FVB mice. UV-inactivation of virus was confirmed by the inability of the virus to form plaques on an L2 cell monolayer (data not shown). Examination of the injected sciatic nerve by immunohistochemistry for the presence of virus 3 days p.i. showed viral protein was not detected in the sciatic nerve of mice inoculated with UV-inactivated virus (Figure 1C).
Figure 1. Immunolocalization of TMEV antigens in the sciatic nerve of FVB mice.
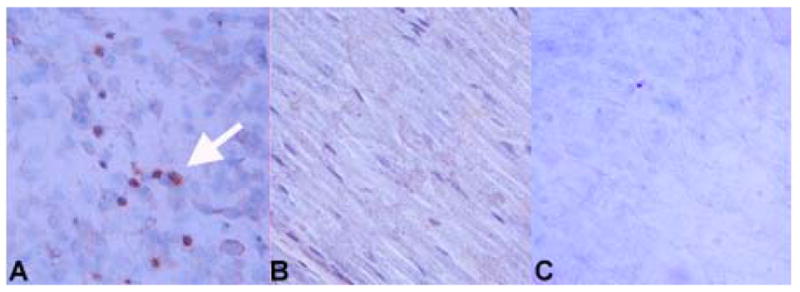
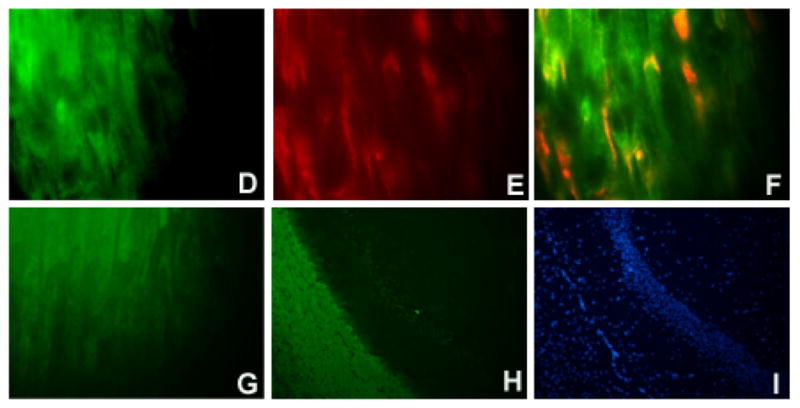
Virus antigen positive cells are apparent in sciatic nerves at 3 days post-infection of the sciatic nerve with TMEV; staining of a TMEV-injected sciatic nerve is shown in (A). Dark reaction product indicates positive staining (examples shown by white arrowhead). No immunoreactivity was observed in the sciatic nerve of an HBSS-injected mouse (B). Following injection of UV-inactivated virus into the sciatic nerve, viral proteins were not detected by immunohistochemistry (C). Slides were stained using the ABC Methodology as described previously (Drescher, Nguyen et al., 1998). Development was carried out using DAB. Double immunofluorescent staining was performed using markers specific for myelinating cells (D, F, G) combined with identification of TMEV (E) as described in the Methods. Single channel images are shown (D, E) as well as merged images (F, G). Staining is shown for sciatic nerves from FVB mice at 5 days post-inoculation (D–F). A merge image of the uninfected contralateral sciatic nerve is negative for TMEV (G). Controls included staining a normal mouse brain with the antibody to myelinating cells to demonstrate specificity for white matter areas of the brain (H). A DAPI image of the brain is shown for comparison purposes (I). Original magnification X480 (A–C), X1000 (D–G), X80 (H, I).
To identify the cells associated with TMEV in the sciatic nerve, we performed immunofluorescent staining on TMEV-infected sciatic nerves from female FVB mice. A polyclonal antisera to TMEV was used, in combination with antibodies specific for either macrophages or myelinating (Schwann) cells. Markers of these cell types were chosen as macrophages have been reported as a reservoir of TMEV in the CNS model of demyelination (Clatch, Miller et al., 1990), and direct infection of Schwann cells would support the ability of TMEV to induce demyelination of the PNS. At 5 days post-infection, the time-point where we observed maximal immunoreactivity for virus, virus co-localized with markers for myelinated cells (Figure 1D–F), indicating that Schwann cells are infected following intrasciatic nerve injection. This was not observed in control mice; only myelin immunoreactivity was observed in the uninfected (contralateral) sciatic nerves (Figure 1G). To demonstrate the specificity of the anti-myelin antibody, brain tissue from a normal mouse was used as a control (Figure 1H–I). The cortex, an area of the brain rich in myelin (that is, a white matter area) showed intense immunofluorescence (Figure 1H) An adjactent gray matter region which contains minimal numbers of myelin-producing cells (the hippocampus) was devoid of staining (Figure 1H). Macrophages in the sciatic nerve of virus-injected animals were also observed to be infected (data not shown). Together, the immunostaining data suggest that TMEV can productively infect the sciatic nerve of mice by this route of infection.
Replicating virus can be isolated from sciatic nerves
We isolated sciatic nerve tissue from FVB mice 5 days p.i. and assayed for infectious virus. At 5 days p.i., the adaptive antiviral immune system has been triggered in mice when inoculated i.c. with TMEV (Lindsley, Thiemann et al., 1991; Lindsay & Rodriguez, 1989). Nerve tissue was homogenized, frozen and thawed, then titered on L2 cell monolayers. Titers of 5.0 ± 0.7 x 104 TCID50 (n=3) per mg nerve tissue were detected at this time-point. At an average weight of 31 mg per dissected sciatic nerve, the total infectious viral load in the dissected nerves at 5 days p.i. averaged 1.55 x 106 ± 2.2 x 101, an approximate 100-fold increase in titer over that which was inoculated. Earlier in the infection (day 3 p.i.), sciatic nerve titers were lower (9.1 x 104 ± 0.5 x 101; n = 3), supporting the conclusion that replication was occurring in the sciatic nerve. Prior to day 3, virus was rarely isolated from the sciatic nerve. By days 7–9 post-injection, viral load in the sciatic nerve was below the level of detection of the assay, which is about 3x103 per mg. As similar results were obtained in SJL/J mice (data not shown), the results observed are unlikely to be solely a function of the genetic background of the mice.
It has been established that TMEV replicates to higher titers in the CNS of scid mice, which lack functional B and T cells (Bosma, Custer et al., 1983), than in immunocompetent mice following i.c. inoculation (Drescher, Murray et al., 1999; Njenga, Marques et al., 2004). We hypothesized that TMEV would similarly generate higher titers in sciatic nerve tissue of scid mice if the adaptive immune response were involved in protection of the sciatic nerve. Scid mice were inoculated with 1x104 pfu per sciatic nerve, then killed 5 days later. We observed an increase of more than 4 logs in viral titer in sciatic nerves of the scid mice (3.2±5.1 x 109 TCID50/mg nerve tissue) relative to immunocompetent FVB mice. These results indicated that the adaptive immune response plays a critical role in protection of mice inoculated with TMEV via the sciatic nerve.
Inflammatory cells infiltrate TMEV-injected sciatic nerves
Inflammatory cells are not detected in normal PNS or CNS (Male, Pryce et al., 1992; Male, Pryce et al., 1990b; Male, Pryce et al., 1990a; Wekerle, Linington et al., 1986) but damage to either the PNS or CNS can culminate in the recruitment of inflammatory and/or immune cells to the site of injury (Male, Pryce et al., 1990a; Male, Pryce et al.,1992). Lymphocytic infiltration of the brain in the TMEV model of CNS demyelination is apparent by 3–5 days p.i. (Lindsay & Rodriguez, 1989; Lindsley, Thiemann et al., 1988); the tissue is infiltrated by T and B cells as well as macrophages (Lindsley, Thiemann, & Rodriguez, 1988).
Immunohistochemical staining was used to examine whether immune cells were recruited during TMEV replication in the sciatic nerve. Sciatic nerves were dissected from FVB mice 5 days after TMEV injection were embedded in OCT as described previously (Drescher, Kono et al., 2004). Inflammatory cells were not observed in uninjected sciatic nerves from control, unmanipulated mice (data not shown) but macrophages (Figure 2A) and T cells (Figure 2B) were detectable in TMEV-inoculated nerve tissue. HBSS injected (mock-inoculated) sciatic nerve tissue was also negative for inflammatory cells at the same time point (Figure 2C, D). B cells were not detected in any sample (data not shown). The absence of immune cells in HBSS-inoculated sciatic nerves indicates that the recruitment of inflammatory cells to virus-containing sciatic nerve tissue was not attributable to the physical insult of the injection process but occurred in response to a specific, sustained viral insult to the sciatic nerve.
Figure 2. Immunostaining for immune cells in the sciatic nerves of virus (A, B) and control (C, D) injected mice.
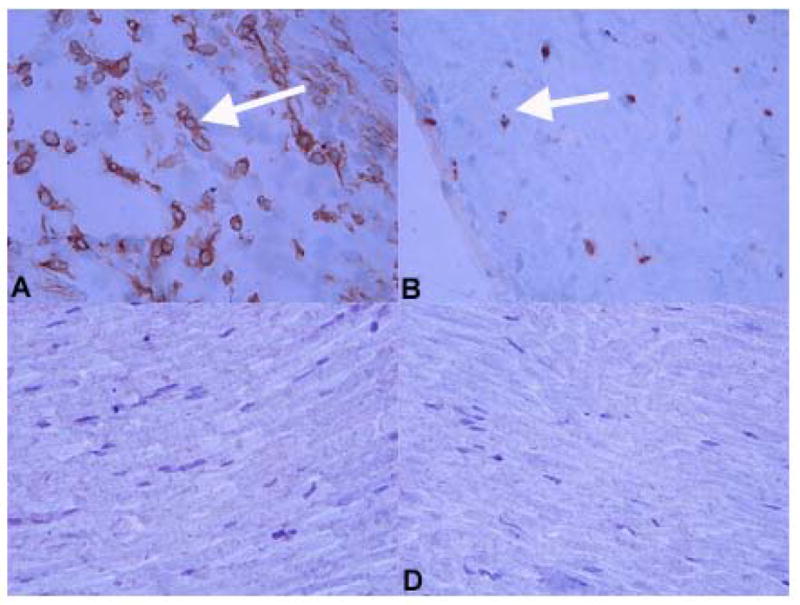
At day 4 p.i., macrophages (A) and T cells (B) were detected in sciatic nerves of virus-injected FVB mice (examples shown by white arrowhead). Neither macrophages (C) or T cells (D) were identified in the sciatic nerve of control, HBSS-injected female FVB mice. Markers used to identify these cell types were F4/80 (macrophages) and CD3 (T cells). Slides were stained using the ABC immunoperoxidase technique as described (Methods). Development was carried out using DAB. Dark colored reaction product indicates positive staining. Original magnification X480.
TMEV spreads from the sciatic nerve (PNS) to the spinal cord (CNS)
Studies utilizing poliovirus (Solomon & Willison, 2003; Sabin & Ward, 1941; Sabin, 1956), herpes simplex virus (Hemachudha, Wacharapluesadee et al., 2005; Faber, Pulmanausahakul et al., 2004; Song & Jia, 1999; Fujiki & Tashiro, 1997), and rabies virus (Hemachudha, Wacharapluesadee et al., 2005; Faber, Pulmanausahakul, Nagao et al., 2004) have shown that virus can be transported to the CNS during viral replication following infection of the PNS. We therefore asked whether TMEV was also capable of spreading from the PNS to the CNS following direct injection of the sciatic nerve. At day 3 post-injection, no virus was detected in the spinal cords of either FVB or scid mice. (sensitivity of plaque assay, 200 pfus/g tissue). Seven days following sciatic nerve injection of scid mice with TMEV, we detected 2.4 + 0.8 x 106 pfu/g (n = 3) TMEV in the spinal cord, while a hundred fold lower viral titers detected in the spinal cords (6.4±1.7 x 103 pfu/g; n=3) of FVB mice again indicated a role of the adaptive immune system in suppressing TMEV replication in this system. The differences in viral titers between FVB and scid mice are similar to those that have been reported in the CNS following intracerebral injection with TMEV (Njenga, Asakura et al., 1997).
We also determined whether mice were functionally impaired following sciatic nerve injection with virus. Scid mice first demonstrated hind limb paralysis 12 days after sciatic nerve injection with TMEV, although FVB mice appeared showed no altered movements or activity levels at the same time-point relative to mock-infected control mice. Neither scid nor FVB mice were maintained longer than 16 days p.i. These results indicate that virus is transported from the PNS to the CNS following injection of TMEV into the sciatic nerve and that in the absence of a functional adaptive immune response, the virus induces sufficient damage to the nervous system which can result in functional alterations and eventually death.
PLP levels are altered and demyelination is induced following TMEV infection of the sciatic nerves
Injury to myelin-producing cells results in alterations in mRNA levels of genes involved in myelination (Gupta, Pringle et al., 1991; Yaghootfam, Gieselman et al., 2005; Kawczak, Mathisen et al., 1998; Ozden, Aubert et al., 1993). If remyelination processes were invoked following virus insult, an increase in transcription of genes encoding myelin components should be detectable. However, if demyelination occurred in the absence of remyelination, a decrease in transcripts associated with the production of myelin proteins might be the result of either death and/or damage to myelin-producing cells. In the CNS model of TMEV-induced demyelination, myelin proteolipid protein (PLP) transcripts in spinal cord have been shown to be altered relatively within hours following TMEV-induced injury (Rodriguez, Prayoonwiwat et al., 1994). We injected sciatic nerves of FVB mice with virus to test whether injection of TMEV into the sciatic nerve altered levels of PLP gene transcripts in the sciatic nerve. RNA was isolated from dissected sciatic nerves at various time-points after injection, and real-time RT-PCR performed utilizing primers and a dual-labeled fluorogenic probe for PLP. Real-time PCR was standardized to a housekeeping gene (glyceraldehyde-3-phosphate-dehydrogenase, GAPDH) (Medhurst, Harrison et al., 2000). Data are expressed as the fold-increase in PLP mRNA level relative to the levels of PLP mRNA in uninjected (control) sciatic nerves. A significant increase in the level of PLP mRNA was observed within 2 hours post-injection compared to uninfected controls (4.9 ± 0.5 –fold-increase over control; p = 0.02). However, although there was an increase in PLP mRNA abundance over the following 24 hours, the differences between the points were not significant (1.8 ± 0.4 fold over baseline; p < 0.2) (Figure 3). By 72 hours p.i., a 15.7 ± 2.7-fold increase (p<0.032) in transcript levels had occurred in virus-injected sciatic nerve tissue relative to the level in uninjected control mice (Figure 3). PLP mRNA levels then decreased to a 3.1 ± 0.3-fold increase over control by day 5 p.i (p = 0.02). To determine whether sustained inflammation was required for alterations in PLP mRNA levels, PLP mRNA abundance was also measured in sciatic nerves of mock-infected female FVB mice (injected with HBSS). No significant alterations in PLP mRNA levels were detected relative to the uninjected sciatic nerve over the course of the experiment (5 days; [Figure 3]), results that indicated putative transient inflammatory responses which may be induced by injecting the nerve with physiologic salt solution, are inadequate for PLP transcript levels to be altered in the sciatic nerve. Together, these results are consistent with the induction of myelin repair processes in the virus-injected sciatic nerves within hours of virus infection.
Figure 3. Real-time RT- PCR demonstrates that PLP mRNA levels peak at day 3 post-injection of the sciatic nerve with TMEV (solid circles).
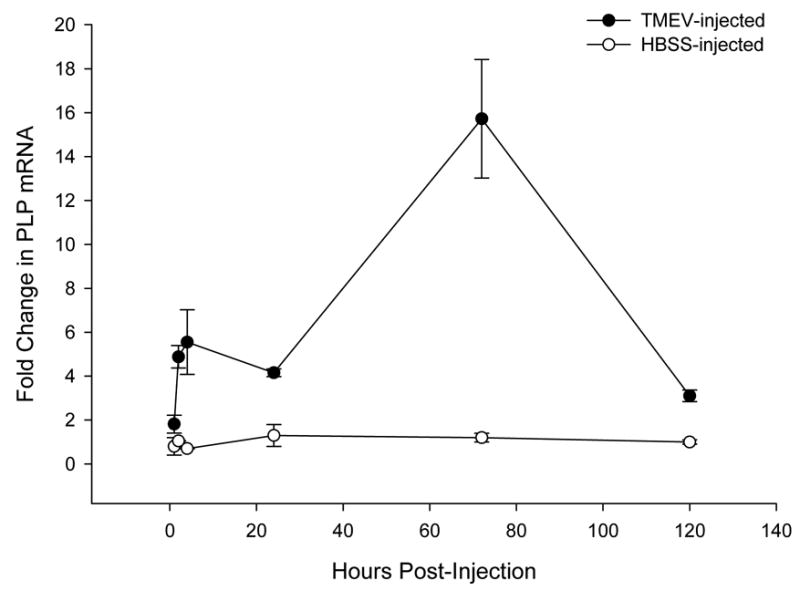
A 4.9 ± 0.5 -fold relative increase in PLP mRNA levels is evident as early as 2 hours after sciatic nerve injection with TMEV (solid circles; p = 0.02). By day 3 p.i. PLP transcript levels peak, with a 15.7 ± 2.7-fold increase in mRNA levels as compared to uninjected control sciatic nerves (p < 0.032). This increase in PLP mRNA levels begins to subside by day 5 p.i. with virus, but still remain 3.1 ± 0.3-fold higher than baseline levels (p = 0.02). Additional control mice were injected with HBSS (open circles) into the sciatic nerve. No significant alterations in PLP mRNA levels were observed over 5 days. Standardization was performed using GAPDH as a housekeeping gene. Real-time PCR was performed using TaqMan methodology (Methods) on an ABI 7000 SDS Sequence Detection System (Applied Biosystems).
Demyelination of the sciatic nerve is observed following injection with TMEV
As the observation that PLP mRNA levels were increased in TMEV-inoculated sciatic nerves was consistent with activation of a myelin repair mechanism in response to the viral insult, we performed histopathological analyses of sciatic nerves from virus-injected mice for evidence of demyelination. Osmicated plastic sections of sciatic nerves inoculated with TMEV 5 days previously were stained with para-phenylenediamene (PPD), then examined for evidence of myelin loss; HBSS-injected sciatic nerves served as controls. Sciatic nerves injected with HBSS appeared histologically normal, characterized by uniform, well-preserved myelin around the axons (Figure 4A). However, extensive myelin loss was observed in sciatic nerves of TMEV-inoculated mice (Figure 4B, C). Demyelination of sciatic nerves of TMEV-inoculated mice was distinguished by loss of myelin around the axons (thick, white arrows, Figure 4B, C), by the presence of cellular debris (thin black arrows, Figure 4C) and by cells that appear to be foamy macrophages, indicating that the macrophages are engaged in cleaning cellular debris (thin, white arrows, Figure 4C). These results demonstrate that demyelination occurs as a result of the productive TMEV infection occurring in sciatic nerves following direct inoculation with virus.
Figure 4. Pathology of the sciatic nerve of virus-injected (B,C) and HBSS-injected (A) mice.
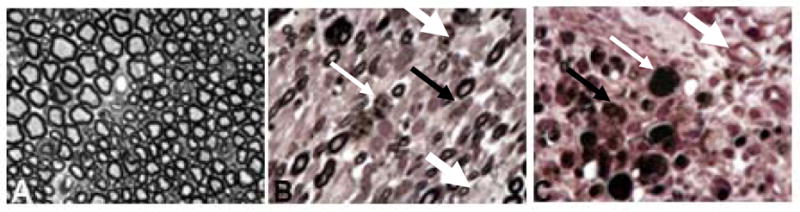
HBSS-injected sciatic nerves are characterized by uniform, well-preserved myelin around the axons; infiltrating cells are absent (A). Mice injected with TMEV (B, C) show demyelination of the axons (demyelination presents as paler circles versus the dark well-myelinated axons; thick white arrow). In addition, the sciatic nerve contains cells which, due to their foamy appearance, appear to be macrophages (thin, white arrow) as well as myelin debris (thin black arrow). Sciatic nerves were osmicated, embedded in plastic and 1 micron sections prepared. Slides were stained with 4% para-phenylenediamene (PPD) and examined by light microscopy. Original magnification X1200.
Discussion
Results presented here describe a novel model of virus-induced peripheral demyelinating disease. Following inoculation of TMEV directly into the mouse sciatic nerve, infectious virus transits to the CNS. Detection of increased virus loads demonstrate productive virus replication occurs in inoculated nerves. Demyelination of the sciatic nerve was related to the presence of replicating virus for no demyelination was observed in mock-infected nerves. Demyelination could be the result of direct infection of myelinating cells and/or due the in resulting immune response. This model should have relevance for testing putative infectious etiologies of human nervous system diseases such as Guillain-Barre syndrome and multiple sclerosis, as well as for studies of PNS remyelination. In our model of virus-induced peripheral demyelinating disease, only virus-injected (not mock-infected) sciatic nerves were infiltrated by immune cells, indicating that immune cell recruitment to the sciatic nerve requires sustained, specific stimuli. Observations in scid mice demonstrated TMEV achieved higher viral titers than in immunocompetent FVB mice, suggesting a role for the adaptive immune system in virus suppression and likely, virus clearance. These results may be relevant to the human condition in instances of severely immunosuppressed individuals experiencing peripheral demyelinating diseases (Rao & Thomas, 2005; Hulgan, Haas et al., 2005; Ferrari, Vento et al., 2006). An advantage of the model presented herein, is that the site of the initial damage is known, thus allowing for the study of the beginning phases (that is, minutes or hours after insult) of lesion development and repair – processes that are typically difficult to study in vivo. One can speculate that this model may also be applicable for studies of viruses that may not replicate well when injected into mice: direct injection of infectious virions into the sciatic nerve may induce a host response of interest and might also provide a productive microenvironment for the virus to replicate in an otherwise refractory host.
Early work (Rustigian & Pappenheimer, 1949) demonstrated that injection of TMEV into the leg muscle resulted in flaccid paralysis, initially observed in the injected leg and subsequently in the uninfected leg. Naturally occurring TMEV infections occur via the fecal-oral route; in some cases virus transits to the CNS with subsequent paralysis (Theiler, 1940; Theiler & Gard, 1940). Such spread of virus from the alimentary tract to the CNS could be via peripheral nerves and/or blood (Ren & Racaniello, 1992). Although the receptor for TMEV remains unknown, one potential receptor for TMEV is P0, a protein structurally consistent with other picornavirus receptors (Libbey, McCright et al., 2001; Mendelsohn, Wimmer et al., 1989; Bernhardt, Bibb et al., 1994) and thought to be confined to the PNS (Libbey, McCright et al., 2001) . Schwann cells, the cell responsible for myelination of the PNS, are susceptible to infection by diverse viruses including TMEV (Watanabe, Ohtori et al., 2006; Jin, Perng et al., 2004; Tanimura, Imada et al., 2004; Levine, Buchman et al., 2003; Nien, Schmidt et al., 1998; Libbey, McCright et al., 2001; Frankel, Friedmann et al., 1986). Based on the literature there was no a priori reason to doubt that such peripheral infections indeed can and do occur naturally and could be modeled in experimental systems. Indeed, polio has been well-studied with regards to its transport from the periphery to the CNS (Ren & Racaniello, 1992; Ohka, Yang et al., 1998).
We hypothesize that following TMEV injection of the sciatic nerve, Schwann cells are infected and demyelination occurs through death of Schwann cells or impairment of their ability to produce myelin. Once Schwann cells are infected, replicating virus can be transported to the spinal cord. The mechanism of transport to the spinal cord has not been elucidated, but based on the literature axonal transport is one potential mechanism (Martinat, Jarousse et al., 1999). In contrast to the situation observed by others (Martinat, Jarousse et al., 1999) using the neurovirulent GDVII strain, immunocompetent mice in our studies did not experience overt clinical deficits (i.e, slowed movement, paralysis, death) associated with virus infection. The differences in clinical presentation between our studies and the previous studies could be attributed to either different cell types becoming infected in the spinal cord, or to altered viral loads. Despite the high degree of genetic identity between the GDVII and DA strains of TMEV (Pevear, Borkowski et al., 1988; Pevear, Calendoff et al., 1987), these TMEV strains preferentially infect different cell types in the CNS. GDVII localizes primarily in gray matter, while DA establishes infection primarily in white matter. Several reports ascribe the differences in virus localization in the mouse CNS to differences in receptor usage (Shah & Lipton, 2002; Jnaoui, Minet et al., 2002 Tsunoda, Wada et al., 2001; McCright, Tsunoda et al., 1999; Zhou, Luo et al., 2000; Jnaoui & Michiels, 1999).
Previous work has shown that TMEV, strain GDVII, can be transported from the periphery to the CNS via fast axonal transport within 48 hours of footpad inoculation (Martinat, Jarousse et al., 1999). Although the impact of the virus infection on myelin integrity was not explicitly addressed in this study (Martinat, Jarousse et al., 1999), it was noted that pathologic changes were reported in the gray matter of the spinal cord once virus was in the CNS, implying minimal involvement of white matter areas of the CNS and PNS. Strain GDVII is considered highly neurovirulent and has not been shown to infected white matter areas of the CNS in vivo; a characteristic attributable to differences in receptor usage by the GDVII and TO viruses (Tsunoda, Wada et al., 2001; Jnaoui, Minet, & Michiels, 2002).
The majority of studies involving virus infection of the PNS have studied cases of human peripheral dysfunction (Ferrari, Vento et al., 2006; Koga, Gilbert, Li et al., 2005; Pavone, Maccarrone et al., 2002; Roccatagliata, Uccelli, & Murialdo, 2001; Khamaisi, Shoenfeld, & Orbach, 2004; Ponticelli & Campise, 2005; Izurieta, Haber et al., 2005). The PNS is extremely efficient at remyelination: our results showed increases in myelin PLP mRNA levels beginning within 2 hours of infection with a peak increase in PLP mRNA at 72 hours p.i.. These results indicate that a rapid stimulation of the processes involved in protection of the myelin sheaths occurs following TMEV injection. Thus, we hypothesize that demyelination may well have occurred in human cases of peripheral neuropathy attributed to infection with particular viruses but was unable to be observed as myelin repair mechanisms were rapidly invoked.
In summary, the results presented herein introduce a new murine model for studying pathogenic mechanisms of virus-mediated peripheral nerve demyelination. The ability to study lesion dynamics in the context of both myelin damage and repair is a unique character of this model. The model may additionally be utilized to study the roles of the immune system in limiting demyelination as well as repairing damage to the PNS. A greater understanding of these processes could permit better treatment of conditions associated with viral infections of the PNS in humans.
Materials and methods
Virus
TMEV, strain DA, is a prototypic demyelinating strain of the TO subgroup; the disease observed in the CNS following infection with TMEV/DA has been extensively described (Drescher, Nguyen et al., 1998; Drescher, Zoecklein et al., 2000; Drescher, Murray et al., 2000; Dal Canto & Lipton, 1975; Lipton, 1975; Lipton & Dal Canto, 1976; Pena Rossi, Delcroix et al., 1997; Lipton & Dal Canto, 1977; Rodriguez & David, 1985; Pena Rossi, McAllister et al., 1991; Lipton & Dal Canto, 1979). TMEV was propagated in BHK cells as previously described (Rodriguez, Leibowitz et al., 1986), collected by freeze-thaw lysis. Following removal of cellular debris by centrifugation (Beckman SW28.1, 25,000xRPM, 8°C, 30 min), the virus was collected by overnight ultracentrifugation through 30% w/v sucrose, 100mM NaCl, 20mM Tris-HCl pH 7.5, into a glycerol pad. Virus was suspended in Hank’s Balanced Salt solution (HBSS), titered on BHK monolayers (pfu/mL) and stored frozen in aliquots at −80C. Titers are measured either by pfu/mL or TCID50/mL. In some experiments, UV-inactivated TMEV was used. Undiluted virus stock was placed under a UV light for 10 minutes. An aliquot of the virus was assayed on an L2 cell monolayer to ensure inactivation; lack of cytopathic effect was used as an indicator of inactivation. The same stock of virus was used for all experiments.
Mice
Female mice (FVB or B6.CB17-Prkdcscid/SzJ) were purchased from Jackson Laboratories (Bar Harbor ME) at 6 weeks of age. Mice were housed at 5 per cage in Thoren Maxi-Miser System cages (Hazelton, PA) and provided food and water ad libitum. The entire cage set including food and water was steam sterilized prior to use. Cages were changed weekly.
Injection of the sciatic nerve
Mice were anesthetized using IsoFlo (Abbott Laboratories, North Chicago, IL), the hindleg wetted with 95% ethanol, skin cut, and muscle moved to expose the sciatic nerve, per published methodology (Palmer, Branston et al., 2000). Mice were kept on a warming pad which aids in reduction of stress by helping the mouse to maintain proper body temperature during the procedure. A 30 gauge needle attached to a Hamilton syringe (Fisher Scientific, St. Louis, MO) was used to inject the sciatic nerve with 10 μL of virus diluent (Hanks’ Balanced Salt Solution, HBSS) containing 2x104 pfu of TMEV. Mock-infected, control animals were injected with 10 μL HBSS. Wounds were closed using surgical staples (MikRon® AUTOCLIP® 9mm, Becton Dickinson, Sparks, MD) and the wound site treated with triple anti-bacterial ointment . Actetaminophen was added to the drinking water (80 mg/5ml) as a post-operative analgesia.
All animal experiments were performed in accordance with guidelines of the Association for Accreditation and Assessment of Laboratory Animal Care (AAALAC), the Creighton University Institutional Animal Care and Use Committee (IACUC), and Federal regulations.
Tissue collection
Mice were killed with an injection of 100 μL sodium pentobarbital (Sleepaway, Fort Dodge Animal Health, Fort Dodge, IA) i.p. Mice were then perfused with 4% paraformaldehyde in phosphate buffered saline (PBS) (Drescher, Murray et al., 2000). Sciatic nerves and spinal columns were dissected and placed in 4% paraformaldehyde in PBS at room temperature. In some cases, tissues were snap frozen under dry ice for RNA extraction or virus titering, or embedded in OCT Frozen Embedding Media (Miles, Inc., Elkhart, IN) for immunostaining.
Immunostaining
Six micron thick sections of tissue embedded in OCT Frozen Embedding Media (Miles, Inc.) were cut and fixed in chilled acetone. Sections were stained with monoclonal antibodies to CD4 (BD Pharmingen, San Diego, CA), CD8 (BD Pharmingen), F4/80 (Chemicon, Temecula CA), or a polyclonal antisera to TMEV using the avidin-biotin complex (ABC) immunoperoxidase technique (Vector Laboratories, Inc., Burlingame, CA) as described previously (Drescher, Murray et al., 1999). Development was performed using the DAB Substrate Kit (Vector Laboratories, Inc.). Slides were lightly counterstained with Mayer’s hematoxylin (Sigma), dehydrated, and mounted with Permount (Fisher Scientific).
Additional slides were stained using using immunofluorescence. OCT-embedded sections were stained with primary antibodies to TMEV, and a monoclonal antibody to either F4/80 (Chemicon) or myelin (Clone HPC-7; Biosource, Saco, ME). Visualization was performed using Alexa-Fluor-labeled secondary antibodies (Molecular Probes/Invitrogen, Carlsbad, CA) which were visualized at a wavelength of either 488 or 568. Coverslips were applied using Vectashield Mounting Media with DAPI (Vector Labs).
Viral infectivity
Sciatic nerves and spinal cords were dissected from mice at various times post-infection and tissue stored at −80°C until used. Plaque assays were performed on spinal cord tissue samples as described previously (Patick, Pease et al., 1990) while titers in sciatic nerve tissue were expressed as TCDI50/g nerve tissue (Tracy, Drescher et al., 2002). In each case, a minimum of three samples were assayed per time point.
Real-time PCR
RNA was isolated using Trizol (Invitrogen, Carlsbad, CA) from snap frozen sciatic nerves at various times p.i. per the manufacturer’s instructions. Real-time PCR were performed using dual-labeled fluorogenic TaqMan probes (Medhurst, Harrison et al., 2000; Khademi, Illes et al., 2004). The reporter dye 6-carboxy-fluorescein (FAM) is attached to the 5’ end of the probe, and 6-carboxy-tetramethyl-rhodamine (TAMRA) to the 3’ end of the probe. Primers and probes were obtained / produced from Applied Biosystem’s Assays-by-Design Service. The primers/probes used are as follows: PLP: forward (5'→3'): CGC TGT CAG GCA GAT CTT TG; PLP: reverse (5'→3'): TGC GCT CAG GCC CTT G; PLP probe (5'→3'): ACT ACA AGA CCA CCA TCT GC. Assays and data analysis were performed using the ABI 7000 SDS Sequence Detection System (Applied Biosystems); five replicates of each sample were run. All data were reported as fold-increase (or decrease) compared to control. Relative changes in mRNA levels were determined using the difference in the cycle threshold value (ΔCT method) (Medhurst, Harrison et al., 2000; Khademi, Illes et al., 2004).
Sciatic nerve morphology
Sciatic nerves were stored in 4% paraformaldehyde for at least one week after perfusion prior to processing the samples for plastic embedding. Samples were osmicated and embedded in Epon 812 plastic (Electron Microscopy Sciences, Hatfield, PA) as described previously (Drescher, Zoecklein et al., 2000). One micron sections were cut, stained with 4% PPD and cover-slipped. Sections were examined by light microscopy.
Acknowledgments
This work was supported in part by grants from the NIH (1 P20 RR018788-01) (KMD), the Nebraska Tobacco Settlement, LB 692 (KMD), the Juvenile Diabetes Research Foundation (ST) and the American Diabetes Association (ST). This investigation was conducted in a facility constructed with support from Research Facilities Improvement Program (1 C06 RR17417-01) from the National Center for Research Resources, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bernhardt G, Bibb JA, Bradley J, Wimmer E. Molecular characterization of the cellular receptor for poliovirus. Virol. 1994;199:105–113. doi: 10.1006/viro.1994.1102. [DOI] [PubMed] [Google Scholar]
- Bitan M, Or R, Shapira MY, Mador N, Resnick IB, Saleh N, Weinberger KM, Samuel S, Schechter E, Slavin S, Wolf DG. Early-onset Guillain-Barre syndrome associated with reactivation of Epstein-Barr virus infection after nonmyeloablative stem cell transplantation. Clin Infect Dis. 2004;39:1076–1078. doi: 10.1086/424015. [DOI] [PubMed] [Google Scholar]
- Bosma GC, Custer RP, Bosma MJ. A severe combined immunodeficiency mutation in the mouse. Nature. 1983;301:527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- Bour-Jordan H, Thompson HL, Bluestone JA. Distinct effector mechanisms in the development of autoimmune neuropathy versus diabetes in nonobese diabetic mice. J Immunol. 2005;175:5649–5655. doi: 10.4049/jimmunol.175.9.5649. [DOI] [PubMed] [Google Scholar]
- Brannagan TH, 3, Zhou Y. HIV-associated Guillain-Barre syndrome. J Neurol Sci. 2003;208:39–42. doi: 10.1016/s0022-510x(02)00418-5. [DOI] [PubMed] [Google Scholar]
- Clatch RJ, Miller SD, Metzner R, Dal Canto MC, Lipton HL. Monocytes/macrophages isolated from the mouse central nervous system contain infectious Theiler's murine encephalomyelitis virus (TMEV) Virology. 1990;176:244–254. doi: 10.1016/0042-6822(90)90249-q. [DOI] [PubMed] [Google Scholar]
- Dal Canto MC, Lipton HL. Primary demyelination in Theiler's virus infection. An ultrastructural study. Lab Invest. 1975;33:626–637. [PubMed] [Google Scholar]
- Drescher KM, Kono K, Bopegamage S, Carson SD, Tracy S. Coxsackievirus B3 infection and type 1 diabetes development in NOD mice: insulitis determines susceptibility of pancreatic islets to virus infection. Virology. 2004;329:381–394. doi: 10.1016/j.virol.2004.06.049. [DOI] [PubMed] [Google Scholar]
- Drescher KM, Murray PD, David CS, Pease LR, Rodriguez M. CNS cell populations are protected from virus-induced pathology by distinct arms of the immune system. Brain Pathol. 1999;9:21–31. doi: 10.1111/j.1750-3639.1999.tb00206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drescher KM, Murray PD, Lin X, Carlino JA, Rodriguez M. TGF-β2 reduces demyelination, virus antigen expression, and macrophage recruitment in a viral model of multiple sclerosis. J Immunol. 2000;164:3207–3213. doi: 10.4049/jimmunol.164.6.3207. [DOI] [PubMed] [Google Scholar]
- Drescher KM, Nguyen LT, Taneja V, Coenen MJ, Leibowitz JL, Strauss G, Hammerling GJ, David CS, Rodriguez M. Expression of the human histocompatibility leukocyte antigen DR3 transgene reduces the severity of demyelination in a murine model of multiple sclerosis. J Clin Invest. 1998;101:1765–1774. doi: 10.1172/JCI167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drescher KM, Zoecklein L, Pavelko KD, Rivera-Quinones C, Hollenbaugh D, Rodriguez M. CD40L is critical for protection from demyelinating disease and development of spontaneous remyelination in a mouse model of multiple sclerosis. Brain Pathol. 2000;10:1–15. doi: 10.1111/j.1750-3639.2000.tb00238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber M, Pulmanausahakul R, Nagao K, Prosniak M, Rice AB, Koprowski H, Schnell MJ, Dietzschold B. Identification of viral genomic elements responsible for rabies virus neuroinvasiveness. Proc Nat Acad Sci USA. 2004;101:16328–16332. doi: 10.1073/pnas.0407289101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S, Vento S, Monaco S, Cavallaro T, Cainelli F, Rizzuto N, Temesgen Z. Human immunodeficiency virus-associated peripheral neuropathies. Mayo Clin Proc. 2006;81:213–219. doi: 10.4065/81.2.213. [DOI] [PubMed] [Google Scholar]
- Fiette L, Aubert C, Muller U, Huang S, Auget M, Brahic M, Bureau JF. Theiler's virus infection of 129 Sv mice that lack the interferon α/β or interferon γ receptors. J Exp Med. 1995;181:2069–2076. doi: 10.1084/jem.181.6.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel G, Friedmann A, Amir A, David Y, Shahar A. Theiler's virus replication in isolated Schwann cell cultures. J Neurosci Res. 1986;15:127–136. doi: 10.1002/jnr.490150202. [DOI] [PubMed] [Google Scholar]
- Fujiki N, Tashiro K. Herpes viruses--herpes simplex virus, varicella-zoster virus, EB virus, cytomegalovirus. Nippon Rinsho. 1997;55:855–860. [PubMed] [Google Scholar]
- Gupta SK, Pringle J, Poduslo JF, Mezei C. Levels of proteolipid protein mRNAs in peripheral nerve are not under stringent axonal control. J Neurochem. 1991;56:1754–1762. doi: 10.1111/j.1471-4159.1991.tb02077.x. [DOI] [PubMed] [Google Scholar]
- Hemachudha T, Wacharapluesadee S, Mitrabhakdi E, Wilde H, Morimoto K, Lewis RA. Pathophysiology of human paralytic rabies. J Neurovirol. 2005;11:93–100. doi: 10.1080/13550280590900409. [DOI] [PubMed] [Google Scholar]
- Hulgan T, Haas DW, Haines JL, Ritchie MD, Robbins GK, Shafer RW, Clifford DB, Kallianpur AR, Summar M, Canter JA. Mitochondrial haplogroups and peripheral neuropathy during antiretroviral therapy: an adult AIDS clinical trails group study. AIDS. 2005;19:1341–1349. doi: 10.1097/01.aids.0000180786.02930.a1. [DOI] [PubMed] [Google Scholar]
- Izurieta HS, Haber P, Wise RP, Iskander J, Pratt D, Mink C, Chang S, Braun MM, Ball R. Adverse events reported following live, cold-adapted, intranasal influenza vaccine. JAMA. 2005;294:2720–2725. doi: 10.1001/jama.294.21.2720. [DOI] [PubMed] [Google Scholar]
- Jin L, Perng GC, Brick DJ, Naito J, Nesburn AB, Jones C, Weschler SJ. Methods for detecting the HSV-1 LAT anti-apoptosis activity in virus infected tissue culture cells. J Virol Methods. 2004;118:9–13. doi: 10.1016/j.jviromet.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Jnaoui K, Michiels T. Analysis of cellular mutants resistant to Theiler's virus infection: differential infection of L929 cells by persistent and neurovirulent strains. J Virol. 1999;73:7248–7254. doi: 10.1128/jvi.73.9.7248-7254.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jnaoui K, Minet M, Michiels T. Mutations that affect the trophism of DA and GDVII strains of Theiler's virus in vitro influence sialic acid binding and pathogenicity. J Virol. 2002;76:8138–8147. doi: 10.1128/JVI.76.16.8138-8147.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawczak JA, Mathisen PM, Drazba JA, Fuss B, Macklin WB, Tuohy VK. Digitized image analysis reveals diffuse abnormalities in nomal-appearing white matter during acute experimental autoimmune encephalomyelitis. J Neurosci Res. 1998;54:364–372. doi: 10.1002/(SICI)1097-4547(19981101)54:3<364::AID-JNR7>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Khademi M, Illes Z, Gielen AW, Marta M, Takazawa N, Baecher-Allan C, Brundin L, Hannerz J, Martin C, Harris RA, Hafler DA, Kuchroo VK, Olsson T, Piehl F, Wallstrom E. T cell Ig- and mucin-domain-containing molecule-3 (TIM-3) and TIM-1 molecules are differentially expressed on human Th1 and Th2 cells and in cerebrospinal fluid-derived mononuclear cells in multiple sclerosis. J Immunol. 2004;172:7169–7176. doi: 10.4049/jimmunol.172.11.7169. [DOI] [PubMed] [Google Scholar]
- Khamaisi M, Shoenfeld Y, Orbach H. Guillain-Barre syndrome following hepatitis B vaccination. Clin Exp Rheumatol. 2004;22:767–770. [PubMed] [Google Scholar]
- Koga M, Gilbert M, Li J, Koike S, Takahashi M, Furukawa K, Hirata K, Yuki N. Antecedent infections in Fisher syndrome: a common pathogenesis of molecular mimicry. Neurol. 2005;64:1605–1611. doi: 10.1212/01.WNL.0000160399.08456.7C. [DOI] [PubMed] [Google Scholar]
- Levine J, Buchman CA, Fregien N. Influenza A virus infection of human Schwann cells in vitro. Acta Otolaryngol. 2003;123:41–45. doi: 10.1080/0036554021000028092. [DOI] [PubMed] [Google Scholar]
- Libbey JE, McCright IJ, Tsunoda I, Wada Y, Fujinami RS. Peripheral nerve protein, PO, as a potential receptor for Theiler's murine encephalomyelitis virus. J Neurovirol. 2001;7:97–104. doi: 10.1080/13550280152058753. [DOI] [PubMed] [Google Scholar]
- Lindsay MD, Rodriguez M. Characterization of the inflammatory response in the central nervous system of mice susceptible or resistant to demyelination by Theiler's virus. J Immunol. 1989;142:2677–2682. [PubMed] [Google Scholar]
- Lindsley MD, Thiemann R, Rodriguez M. Cytotoxic T cells isolated from the central nervous systems of mice infected with Theiler's virus. J Virol. 1991;65:6612–6620. doi: 10.1128/jvi.65.12.6612-6620.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley MD, Thiemann RL, Rodriguez M. Enumeration and distribution of T-cell subsets, macrophages, and IgG positive cells in the CNS of SJL/J mice infected with Theiler's virus. Ann NY Acad Sci. 1988;540:657–660. doi: 10.1111/j.1749-6632.1988.tb27203.x. [DOI] [PubMed] [Google Scholar]
- Lipton HL. Theiler's virus infection in mice: an unusual biphasic disease process leading to demyelination. Infect Immun. 1975;11:1147–1155. doi: 10.1128/iai.11.5.1147-1155.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton HL, Dal Canto MC. Contrasting effects of immunosuppression on Theiler's virus infection in mice. Infect Immun. 1977;15:903–909. doi: 10.1128/iai.15.3.903-909.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton HL, Dal Canto MC. Theiler's virus-induced demyelination: prevention by immunosuppression. Science. 1976;192:62–64. doi: 10.1126/science.176726. [DOI] [PubMed] [Google Scholar]
- Lipton HL, Dal Canto MC. The TO strains of Theiler's viruses cause 'slow-virus like' infection in mice. Ann Neurol. 1979;6:25–28. doi: 10.1002/ana.410060106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Male D, Pryce G, Hughes C, Lantos P. Lymphocyte migration into brain modelled in vitro: control by lymphocyte activation, cytokines, and antigen. Cell Immunol. 1990a;127:1–11. doi: 10.1016/0008-8749(90)90109-5. [DOI] [PubMed] [Google Scholar]
- Male D, Pryce G, Linke A, Rahman J. Lymphocyte migration into the CNS modelled in vitro. J Neuroimmunol. 1992;40:167–171. doi: 10.1016/0165-5728(92)90130-d. [DOI] [PubMed] [Google Scholar]
- Male D, Pryce G, Rahman J. Comparison of the immunological properties of rat cerebral and aortic endothelium. J Neuroimmunol. 1990b;30:161–168. doi: 10.1016/0165-5728(90)90100-2. [DOI] [PubMed] [Google Scholar]
- Martinat C, Jarousse N, Prevost MC, Brahic M. The GDVII strain of Theiler's virus spreads via axonal transport. J Virol. 1999;73:6093–6098. doi: 10.1128/jvi.73.7.6093-6098.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCright IJ, Tsunoda I, Whitby FG, Fujinami RS. Theiler's viruses with mutations in loop I of VP1 lead to altered trophism and pathogenesis. J Virol. 1999;73:2814–2824. doi: 10.1128/jvi.73.4.2814-2824.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medhurst AD, Harrison DC, Read SJ, Campbell CA, Robbins MJ, Pangalos MN. The use of TaqMan RT-PCR assays for semiquantitative analysis of gene expression in CNS tissues and disease models. J Neurosci Methods. 2000;98:9–20. doi: 10.1016/s0165-0270(00)00178-3. [DOI] [PubMed] [Google Scholar]
- Meekins GD, Emery MJ, Weiss MD. Nerve conduction abnormalities in the trembler-j mouse: a model for Charcot-Marie-Tooth disease type 1A? J Peripher Nerv Sys. 2004;9:177–182. doi: 10.1111/j.1085-9489.2004.09310.x. [DOI] [PubMed] [Google Scholar]
- Mendelsohn CL, Wimmer E, Racaniello VR. Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of new member of the immunoglobulin superfamily. Cell. 1989;56:855–865. doi: 10.1016/0092-8674(89)90690-9. [DOI] [PubMed] [Google Scholar]
- Miller SD, Vanderlugt CL, Begolka WS, Pao W, Neville KL, Yauch RL, Kim BS. Epitope spreading leads to myelin-specific autoimmune responses in SJL mice chronically infected with Theiler's virus. J Neurovirol. 1997a;3:S62–S65. [PubMed] [Google Scholar]
- Miller SD, Vanderlugt CL, Begolka WS, Pao W, Yauch RL, Neville KL, Katz-Levy Y, Carrizosa A, Kim BS. Persistent infection with Theiler's virus leads to CNS autoimmunity via epitope spreading. Nat Med. 1997b;3:1133–1136. doi: 10.1038/nm1097-1133. [DOI] [PubMed] [Google Scholar]
- Moon C, Kim H, Ahn M, Jin JK, Wang H, Matsumoto Y, Shin T. Enhanced expression of netrin-1 protein in the sciatic nerves of Lewis rats with experimental autoimmune neuritis: possible role of the netrin-1/DCC binding pathway in an autoimmune disorder. J Neuroimmunol. 2006;172:66–72. doi: 10.1016/j.jneuroim.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Murray PD, McGavern DB, Lin X, Njenga MK, Leibowitz J, Pease LR, Rodriguez M. Perforin-dependent neurologic injury in a viral model of multiple sclerosis. J Neurosci. 1998;18:7306–7314. doi: 10.1523/JNEUROSCI.18-18-07306.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nien JK, Schmidt J, Cartier L, Alverez J. Cerebrospinal fluid of HTLV-1 associated myelopathy patients induces axonal sproutings and Schwann cell proliferation in the rat sciatic nerve. J Neurol Sci. 1998;159:17–24. doi: 10.1016/s0022-510x(98)00145-2. [DOI] [PubMed] [Google Scholar]
- Njenga MK, Asakura K, Wettstein P, Pease LR, Rodriguez M. Immune system preferentially clears Theiler's virus from the gray matter of the central nervous system. J Virol. 1997;71:8592–8601. doi: 10.1128/jvi.71.11.8592-8601.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njenga MK, Marques C, Rodriguez M. The role of cellular immune response in Theiler's virus-induced central nervous system demyelination. J Neuroimmunol. 2004;147:73–77. doi: 10.1016/j.jneuroim.2003.10.042. [DOI] [PubMed] [Google Scholar]
- Ohka S, Yang WX, Terada E, Iwasaki K, Nomoto A. Retrograde transport of intact poliovirus through the axon via the fast transport system. Virology. 1998;250:67–75. doi: 10.1006/viro.1998.9360. [DOI] [PubMed] [Google Scholar]
- Ozden S, Aubert C, Bureau JF, Gonzalez-Dunia D, Brahic M. Analysis of proteolipid protein and Po transcripts in mice infected with Theiler's virus. Microb Pathog. 1993;14:123–131. doi: 10.1006/mpat.1993.1013. [DOI] [PubMed] [Google Scholar]
- Palmer JA, Branston RH, Lilley CE, Robinson MJ, Groutsi F, Smith J, Latchman DS, Coffin RS. Development and optimization of herpes simplex virus vectors for multiple long-term gene delivery to the peripheral nervous system. J Virol. 2000;74:5604–5618. doi: 10.1128/jvi.74.12.5604-5618.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patick AK, Pease LR, David CS, Rodriguez M. Major histocompatibility complex-conferred resistance to Theiler's virus-induced demyelinating disease is inherited as a dominant trait in B10 congenic mice. J Virol. 1990;64:5570–5576. doi: 10.1128/jvi.64.11.5570-5576.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavone P, Maccarrone F, Sorge A, Piccolo G, Greco F, Caruson P, Sorge G. Guillain-Barre syndrome after varicella zoster virus infections. A case report. Minerva Ped. 2002;54:259–262. [PubMed] [Google Scholar]
- Paya CV, Leibson PJ, Patick AK, Rodriguez M. Inhibition of Theiler's virus-induced demyelination in vivo by tumor necrosis factor alpha. Internat Immunol. 1990;2:909–913. doi: 10.1093/intimm/2.9.909. [DOI] [PubMed] [Google Scholar]
- Pena Rossi C, Delcroix M, Huitinga I, McAllister A, Van Rooijen N, Claassen E, Brahic M. Role of macrophages during Theiler's virus infection. J Virol. 1997;71:3336–3340. doi: 10.1128/jvi.71.4.3336-3340.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena Rossi C, McAllister A, Fiette L, Brahic M. Theiler's virus infection induces a specific cytotoxic T lymphocyte response. Cell Immunol. 1991;138:341–348. doi: 10.1016/0008-8749(91)90158-8. [DOI] [PubMed] [Google Scholar]
- Pevear DC, Borkowski J, Calendoff M, Oh CK, Ostrowski B, Lipton HL. Insights into Theiler's virus neurovirulence based on genomic comparison of the neurovirulent GDVII and less virulent BeAn strains. Virology. 1988;165:1–12. doi: 10.1016/0042-6822(88)90652-6. [DOI] [PubMed] [Google Scholar]
- Pevear DC, Calendoff M, Rozhon E, Lipton HL. Analysis of the complete nucleotide sequences of the picornavirus Theiler's murine encephalomyelitis virus indicates that it is closely related to cardioviruses. J Virol. 1987;61:1507–1516. doi: 10.1128/jvi.61.5.1507-1516.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponticelli C, Campise MR. Neurological complications in kidney transplant recipients. J Nephrol. 2005;18:521–528. [PubMed] [Google Scholar]
- Rao VK, Thomas FP. Neurological complications of HIV/AIDS. BETA. 2005;17:37–46. [PubMed] [Google Scholar]
- Ren R, Racaniello VR. Poliovirus spreads from muscle to the central nervous system by neural pathways. J Infect Dis. 1992;166:747–752. doi: 10.1093/infdis/166.4.747. [DOI] [PubMed] [Google Scholar]
- Roccatagliata L, Uccelli A, Murialdo A. Guillain-Barre syndrome after reactivation of varicella-zoster virus. N Eng J Med. 2001;344:65–66. doi: 10.1056/nejm200101043440117. [DOI] [PubMed] [Google Scholar]
- Rodriguez M. The cellular immune response and pathogenesis of Theiler's virus-induced demyelination. In: Thomas BD, editor. Viruses and the cellular immune response. Marcel Dekker, Inc.; New York: 1993. pp. 473–489. [Google Scholar]
- Rodriguez M, David CS. Demyelination induced by Theiler's virus: influence of the H-2 haplotype. J Immunol. 1985;135:2145–2148. [PubMed] [Google Scholar]
- Rodriguez M, Dunkel AJ, Thiemann RL, Leibowitz J, Zijlstra M, Jaenisch R. Abrogation of resistance to Theiler's virus-induced demyelination in H-2b mice deficient in β2-microglobulin. J Immunol. 1993;151:266–276. [PubMed] [Google Scholar]
- Rodriguez M, Leibowitz J, David CS. Susceptibility to Theiler's virus-Induced demyelination: mapping of the gene within the H-2D region. J Exp Med. 1986;163:620–631. doi: 10.1084/jem.163.3.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M, Nabozny GH, Thiemann RL, David CS. Influence of deletion of T cell receptor Vβ genes on the Theiler's virus model of multiple sclerosis. Autoimmunity. 1994;19:221–230. doi: 10.3109/08916939409071347. [DOI] [PubMed] [Google Scholar]
- Rodriguez M, Pavelko KD, Coffman RL. Gamma interferon is critical for resistance to Theiler's virus-induced demyelination. J Virol. 1995;69:7286–7290. doi: 10.1128/jvi.69.11.7286-7290.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M, Prayoonwiwat N, Howe C, Sanborn K. Proteolipid protein gene expression in demyelination and remyelination of the central nervous system: a model for multiple sclerosis. J Neuropath Exp Neurol. 1994;53:136–143. doi: 10.1097/00005072-199403000-00004. [DOI] [PubMed] [Google Scholar]
- Rodriguez M, Rivera-Quinones C, Murray PD, Njenga MK, Wettstein PJ, Mak T. The role of CD4+ and CD8+ T cells in demyelinating disease following Theiler's virus infection: a model for multiple sclerosis. J Neurovirol. 1997;3:S43–S45. [PubMed] [Google Scholar]
- Rodriguez M, Sriram S. Successful therapy of TMEV-induced demyelination (DA strain) with monoclonal anti lyt2.2 antibody. J Immunol. 1988;140:2950–2955. [PubMed] [Google Scholar]
- Rustigian R, Pappenheimer AM. Myositis in mice following intramuscular injection of viruses of the mouse encephalomyelitis group and of certain other neurotrophic viruses. J Exp Med. 1949;89:69–92. doi: 10.1084/jem.89.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabin AB. Pathogenesis of poliomyelitis. Science. 1956;123:1151–1157. doi: 10.1126/science.123.3209.1151. [DOI] [PubMed] [Google Scholar]
- Sabin AB, Ward R. The natural history of human poliomyelitis. I. Distribution of virus in nervous and non-nervous system tissue. J Exp Med. 1941;73:771–793. doi: 10.1084/jem.73.6.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah AH, Lipton HL. Low-neurovirulence Theiler's viruses use sialic acid moieties on N-linked oligosaccharide structures for attachment. Virology. 2002;304:443–450. doi: 10.1006/viro.2002.1735. [DOI] [PubMed] [Google Scholar]
- Solomon T, Willison H. Infectious causes of acute flaccid paralysis. Curr Opin Infect Dis. 2003;16:375–381. doi: 10.1097/00001432-200310000-00002. [DOI] [PubMed] [Google Scholar]
- Song GY, Jia W. The heterogeneity in the immune response and efficiency of viral dissemination in brain infected with herpes simplex virus type 1 through peripheral or central route. Acta Neuropathol (Berl) 1999;97:649–656. doi: 10.1007/s004010051042. [DOI] [PubMed] [Google Scholar]
- Tababella G, Nowzari H. Cytomegalovirus-associated peridontitis and Guillain-Barre syndrome. J Peridontol. 2005;76:2306–2311. doi: 10.1902/jop.2005.76.12.2306. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Kunishige M, Shinohara M, Kubo K, Inoue H, Yoshino H, Asano A, Honda S, Matsumoto T, Mitsui T. Guillain-Barre syndrome and hemophagocytic lymphohistocytosis in a patient with severe chronic active Epstein-Barr virus infection syndrome. Clin Neurol Neurosurg. 2005;108:80–83. doi: 10.1016/j.clineuro.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Tanimura N, Imada T, Kashiwazaki Y, Shahirudin S, Sharifah SH, Aziz AJ. Monoclonal antibody-based immunohistochemical diagnosis of Malaysian Nipah virus infection in pigs. J Comp Pathol. 2004;131:199–206. doi: 10.1016/j.jcpa.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Theiler M. Spontaneous encephalomyelitis of mice, a new virus disease. J Exp Med. 1940;65:705–719. doi: 10.1084/jem.65.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theiler M, Gard S. Encephalomyelitis of mice. III. epidemiology. J Exp Med. 1940;72:79–90. doi: 10.1084/jem.72.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy S, Drescher KM, Chapman NM, Kim KS, Carson SD, Pirruccello S, Lane PH, Romero JR, Leser JS. Toward testing the hypothesis that group B coxsackieviruses (CVB) trigger insulin-dependent diabetes: inoculating nonobese diabetic mice with CVB markedly lowers diabetes incidence. J Virol. 2002;76:12097–12111. doi: 10.1128/JVI.76.23.12097-12111.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda I, Wada Y, Libbey JE, Cannon TS, Whitby FG, Fujinami RS. Prolonged gray matter disease without demyelination caused by Theiler's murine encephalomyelitis virus with a mutation in VP2 puff B. J Virol. 2001;75:7494–7505. doi: 10.1128/JVI.75.16.7494-7505.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijk AF, Sweers MAM, Merkx GFM, Lammens M, Bloemendal H. Pathogenesis of axonal dystrophy and demyelination in α A-crystillin-expressing transgenic mice. Int J Exp Pathol. 2003;84:91–99. doi: 10.1046/j.1365-2613.2003.00340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe TS, Ohtori S, Koda M, Aoki Y, Doya H, Shirasawa H, Yamazaki M, Moriya H, Takahashi K, Yamahita T. Adenoviral gene transfer in the peripheral nervous system. J Orthop Sci. 2006;11:64–69. doi: 10.1007/s00776-005-0971-z. [DOI] [PubMed] [Google Scholar]
- Wekerle H, Linington C, Lassmann H, Meyermann R. Cellular immune reactivity within the CNS. TINS. 1986;8:271–277. [Google Scholar]
- Yaghootfam A, Gieselman V, Eckhardt M. Delay of myelin formation in arylsulphatase A-deficient mice. Eur J Neurosci. 2005;21:711–720. doi: 10.1111/j.1460-9568.2005.03891.x. [DOI] [PubMed] [Google Scholar]
- Zhou L, Luo Y, Wu Y, Tsao J, Luo M. Sialylation of the host receptor may modulate entry of the demyelinating persistent Theiler's virus. J Virol. 2000;74:1929–1937. doi: 10.1128/jvi.74.3.1477-1485.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Ljunggren HG, Mix E, Li HL, van der Meide P, Elhassan AM, Winblad B, Zhu J. Suppression of autoimmune neuritis in IFN-γ receptor-deficient mice. Exp Neurol. 2001;169:472–478. doi: 10.1006/exnr.2001.7662. [DOI] [PubMed] [Google Scholar]


