Abstract
The microtubule binding protein gephyrin plays a prominent role in establishing and maintaining a high concentration of inhibitory glycine receptors juxtaposed to presynaptic releasing sites. Here, we show that endogenous gephyrin undergoes proline-directed phosphorylation, which is followed by the recruitment of the peptidyl-prolyl isomerase Pin1. The interaction between gephyrin and Pin1 is strictly dependent on gephyrin phosphorylation and requires serine–proline consensus sites encompassing the gephyrin proline-rich domain. Upon binding, Pin1 triggers conformational changes in the gephyrin molecule, thus enhancing its ability to bind the beta subunit of GlyRs. Consistently, a downregulation of GlyR clusters was detected in hippocampal neurons derived from Pin1 knockout mice, which was paralleled by a reduction in the amplitude of glycine-evoked currents. Our results suggest that phosphorylation-dependent prolyl isomerisation of gephyrin represents a mechanism for regulating GlyRs function.
Keywords: gephyrin, glycine inhibitory receptors, Pin1, proline directed phosphorylation
Introduction
The reversible phosphorylation of proteins on serine and threonine residues preceding proline represents a key signalling pathway for the control of various cellular processes. Extensively characterised as pivotal mechanism controlling cell proliferation and differentiation (Blume-Jensen and Hunter, 2001; Lu et al, 2002), this signalling cascade has been recently involved in the regulation of synaptic structure and function. At excitatory synapses, mass spectrometric analysis performed on isolated postsynaptic density proteins (PSD) has led to the identification of a number of novel serine/proline phosphorylation sites (Jaffe et al, 2004). Interestingly, three scaffolding molecules, namely PSD-95, PSD-93 and Shank3, are shown to undergo proline-directed phosphorylation and to share a similar phosphorylation motif. Moreover, PSD-95, the central organising element of the PSD that links NMDA receptors to the cytoskeleton, has been shown to be phosphorylated by the serine/threonine kinase Cdk-5, and this event appears to negatively regulate the clustering of NMDA receptors (Morabito et al, 2004).
At inhibitory synapses, antisense (Kirsch et al, 1993) and knockout (Feng et al, 1998) experiments have clearly highlighted the involvement of the scaffolding molecule gephyrin in the proper localisation of glycine receptors (GlyRs) and selected gamma-aminobutyric acid A receptors (GABAARs) (Essrich et al, 1998; Kneussel et al, 1999a, 2001). Gephyrin has been shown to bind with high affinity to an amphipathic amino-acid sequence in the large cytoplasmic loop of the β subunit of GlyR (Meyer et al, 1995; Kneussel et al, 1999b), whereas it only functionally associates with individual GABAA receptor subtypes (Meyer et al, 1995). Gephyrin interacts with the actin- and microtubule-based cytoskeleton (Mammoto et al, 1998; Giesemann et al, 2003) and these interactions are thought not only to provide the physical constraints required to maintain receptors at synapses, but also to regulate the constant flux of receptor and scaffolding elements in and out postsynaptic sites (Choquet and Triller, 2003; Hanus et al, 2006). Receptor accumulation at synapses has been proposed to rely on the ability of gephyrin to reversibly multimerise into a submembraneous hexagonal protein lattice, which accommodates a high number of receptor binding sites (Schwarz et al, 2001; Sola et al, 2001, 2004; Xiang et al, 2001). Finally, an early association of gephyrin with GlyR clusters along the biosynthetic pathway has been documented, which seems to modify receptor trafficking and delivery to synapses (Hanus et al, 2004; Maas et al, 2006). However, the precise molecular mechanisms underlying these dynamic processes are still largely unknown.
Given the emerging role of proline-directed phosphorylation in the regulation of glutamatergic synapses (Jaffe et al, 2004), in the present study we have investigated whether a similar type of modulation occurs at inhibitory synapses. Compelling evidence has shown that proline-directed phosphorylation acts through the induction of conformational changes onto the target proteins (Yaffe et al, 1997; Zhou et al, 1999). These modifications are catalysed by a peptidyl-prolyl isomerase, Pin1 (Peptidyl-prolyl Isomerase NIMA interacting protein 1) (Lu et al, 1996), which specifically binds phosphorylated serine or threonine residues immediately preceeding proline (pSer/Thr-Pro motifs) and promotes the cis/trans isomerisation of the peptide bond (Ranganathan et al, 1997; Shen et al, 1998). Such conformational changes have been shown to have profound effects on the function of Pin1 substrates as they can modulate catalytic activity, phosphorylation status, protein–protein interactions, subcellular localisation and protein stability (Lu, 2004; Wulf et al, 2005). Interestingly, gephyrin purified from GlyR preparations has been found to be phosphorylated at the serine and threonine residues (Langosch et al, 1992). Moreover, from the analysis of gephyrin amino-acid sequence, several putative Pin1 consensus sites have been identified, suggesting an involvement of this post-phosphorylation regulatory mechanism in the modulation of gephyrin scaffolding functions.
Here, we provide evidence that endogenous gephyrin undergoes proline-directed phosphorylation. Gephyrin, upon phosphorylation, interacts with the peptidyl-prolyl cis/trans isomerase Pin1, which in turn induces a conformational change in gephyrin. Interestingly, gephyrin binding to the large cytoplasmic loop of the GlyR β subunit (GlyR β loop), a functional surrogate for full-length GlyRs (Meier and Grantyn, 2004), is strongly reduced in Pin1−/− cells. Moreover, hippocampal neurons isolated from Pin1 knockout mice show a reduced number of GlyR clusters, which is associated with a significant decrease in the peak amplitude of glycine-evoked currents.
Results
Recombinant and endogenous gephyrin undergo proline-directed phosphorylation
Previous experiments have identified a kinase activity tightly associated with affinity-purified GlyR preparations that phosphorylates gephyrin mainly on serine and, to a lesser extent, on threonine residues (Langosch et al, 1992). In order to test whether some of these serine and threonine residues precede a proline (pSer/Thr-Pro), we took advantage of the phospho-dependent antibody mitotic phosphoprotein monoclonal 2 (MPM-2) (Davis et al, 1983). To this aim, FLAG-tagged gephyrin was overexpressed in HEK 293 cells and half of the cell lysate was treated with calf intestine phosphatase (CIP) before performing immunoprecipitation experiments with a monoclonal antibody specific for the FLAG epitope or with anti-myc 9E10 monoclonal antibody as negative control. Western blotting using the MPM-2 antibody revealed that a protein of about 100 kDa molecular weight was recognised only in the anti-FLAG immunoprecipitate obtained from CIP-untreated cell lysate (Figure 1A, lane 4). When the same membrane was stripped and reprobed with a polyclonal anti-gephyrin antibody, it was found that gephyrin-FLAG was efficiently immunoprecipitated from both cell lysates and, most importantly, that the MPM-2 reactive band was also specifically recognised by the anti-gephyrin antibody (Figure 1A, lane 10). Altogether, these findings indicate that gephyrin is indeed an MPM-2 antigen.
Figure 1.
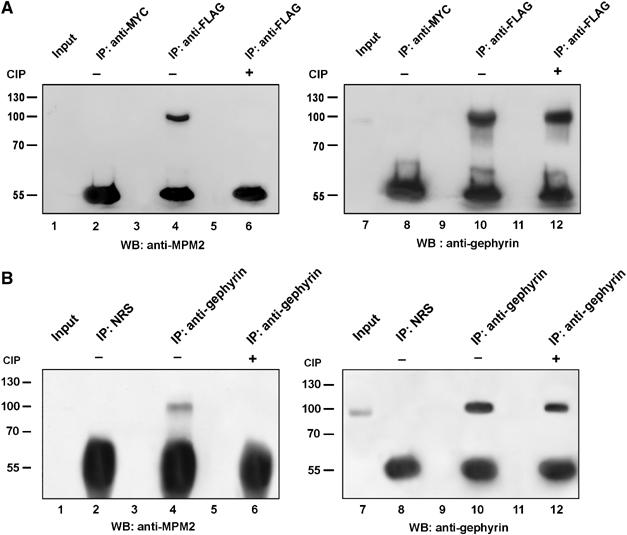
Recombinant and endogenous gephyrin contains consensus sequences for proline-directed phosphorylation. (A) HEK 293 cells transfected with gephyrin-FLAG were lysed, half of the lysate was treated with CIP and immunoprecipitated with anti-FLAG monoclonal antibody (lanes 4 and 6) and with a non-related antibody (anti-MYC) as negative control (lane 2). Immunoprecipitates were analysed by Western blotting using the MPM-2 antibody (left panel). The same membrane was stripped and reprobed with the anti-gephyrin antibody (right panel). (B) Endogenous gephyrin was immunoprecipitated from mouse brain lysate with an anti-gephyrin polyclonal antibody (lanes 4 and 6) and with normal rabbit serum (NRS) as negative control (lane 2). Half of the cell homogenate was treated with CIP before immunoprecipitation. Immunoprecipitates were analysed as described in (A).
To see whether endogenous gephyrin is phosphorylated on Ser/Thr-Pro residues, we immunoprecipitated gephyrin from mouse brain homogenates using the affinity-purified polyclonal antibody raised against the full-length protein or with preimmune serum as negative control and immunoblotted with the MPM-2 antibody (Figure 1B, left panel). Also in this case, half of the homogenate was dephosphorylated as described above. As shown in Figure 1B (left panel), the MPM-2 reactive band was observed only in the anti-gephyrin immunoprecipitate from CIP-untreated brain lysate (lane 4). Upon stripping and reprobing the membrane with a monoclonal anti-gephyrin antibody, it was found that endogenous gephyrin was efficiently immunoprecipitated from both CIP-treated and -untreated homogenates (Figure 1B, lanes 10 and 12).
These data indicate that the scaffolding protein gephyrin undergoes proline-directed phosphorylation in mouse brain, thus representing a newly identified MPM-2 antigen.
Gephyrin interacts in vitro with the peptidyl-prolyl isomerase Pin1 in a phosphorylation-dependent manner
The significance of proline-directed phosphorylation as a signalling mechanism relies on the ability of phosphorylated Ser/Thr-Pro motifs to recruit the prolyl isomerase Pin1 (Lu, 2004). Pin1's phosphoserine- and phosphotreonine-binding activity is mediated by its N-terminal WW domain, a compact protein-interacting module characterised by the presence of two highly conserved tryptophan (W) residues (Lu et al, 1999). As the MPM-2 antibody recognises phosphorylated Ser/Thr-Pro epitopes on several important Pin1 substrates, we investigated whether gephyrin also represents a Pin1 target. To this aim, lysates of HEK 293 cells transfected with gephyrin-FLAG were subjected to pull-down assay with beads loaded with GST-Pin1 or with GST alone as negative control. Proteins bound to beads were separated on SDS-containing gels and immunoblotted using the anti-FLAG antibody. As shown in Figure 2A, only GST-Pin1 beads precipitated with high efficiency the ectopically expressed gephyrin-FLAG. Similar pull-down experiments were then performed to assay the ability of endogenous gephyrin present on neuroblastoma SH-SY5Y cells to interact with Pin1 (Figure 2B). Also in this case, gephyrin was detected only when associated with GST-Pin1 fusion protein. In this case, immunoblot analysis was performed using the monoclonal antibody raised against the C-terminal domain of gephyrin protein. Finally, to examine whether the WW domain of Pin1 exhibited a phosphorylation-dependent binding activity on endogenous gephyrin from SH-SY5Y, as shown for many other Pin1 interactors, we employed the Tyr23 to Ala Pin1 (Pin1Y23A), a mutant that contains a single alanine substitution at the critical Tyr23 in the WW domain resulting in a loss of the phosphoserine/threonine-binding activity (Lu et al, 1999). As shown in Figure 2C, the mutant expressed and purified as GST fusion protein completely abrogated the interaction between Pin1 and gephyrin. In contrast, inactivation of the prolyl isomerase activity in the mutant Cys113 to Ala (Pin1C113A) (Winkler et al, 2000) did not affect the binding of Pin1 to endogenous gephyrin.
Figure 2.
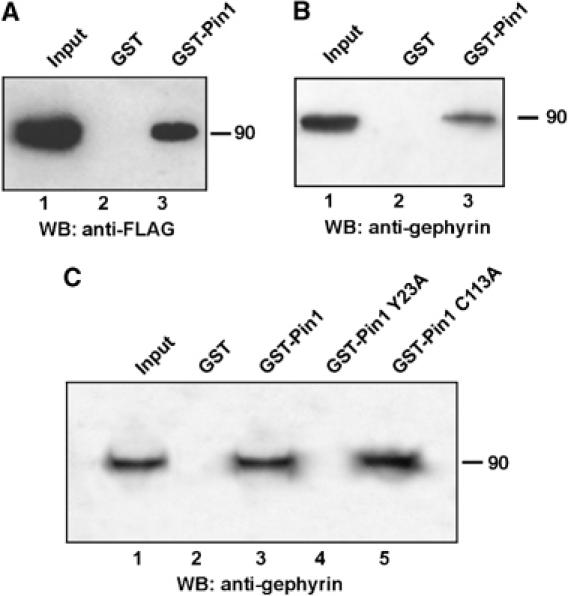
Gephyrin interacts with Pin1 in a phosphorylation-dependent manner in vitro. (A) Lysates of HEK 293 cells transfected with gephyrin FLAG were subjected to GST (lane 2) or GST-Pin1 (lane 3) pull-down followed by Western blot with anti-FLAG antibody. (B) Endogenous gephyrin from lysates of a neuroblastoma cell line (SH-SY5Y) was subjected to GST pull-down as described in (A). Western blot analysis was performed with anti-gephyrin antibody. (C) Endogenous gephyrin from SH-SY5Y cells was subjected to pull-down using GST (lane 2), GST-Pin1 (lane 3), GST-Pin1 Y23A (lane 4) and GST-Pin1 C113A (lane 5). Western blot analysis was performed with anti-gephyrin antibody.
These results demonstrate that the WW domain of Pin1 is responsible for binding the phosphorylated form of gephyrin.
Pin1 binding to gephyrin requires Ser-Pro epitopes contained within the proline-rich domain
Phosphoproteins known to recruit Pin1 in a phosphorylation-dependent manner are commonly phosphorylated on multiple Ser/Thr residues clustered at critical regulatory domains. In this context, the intervening region of gephyrin, which contains several potential protein interaction domains, harbors the majority of Ser/Thr-Pro epitopes that are organised in two clusters. Interestingly, one of these clusters containing three serine–proline epitopes encompasses a proline-rich region of gephyrin, making it an attractive candidate for Pin1 interaction. To explore this possibility, we initially constructed a gephyrin mutant devoid of the proline-rich region (Δ174–243) encoded by exon 8 and tested it using the previously described GST-Pin1 pull-down assay. As shown in Figure 3A, gephyrin mutant displayed a greatly reduced binding to Pin1 as compared with full length gephyrin, suggesting that Ser188, Ser194 and Ser200-Pro epitopes are involved in Pin1 recruitment. It is possible that this difference reflects major structural changes owing to the loss of the entire gephyrin proline-rich region. Therefore, we performed a serine to alanine scan mutagenesis throughout gephyrin exon 8 and assayed the mutant proteins for Pin1 binding. As shown in Figure 3B, sequential disruption of Ser-Pro sites almost completely abolished the interaction of gephyrin to Pin1, as the triple mutant (Mut-C) retained a very low residual binding similar to the deletion mutant initially tested. These findings prompted us to expand our site-directed mutagenesis to all the remaining Pin1 putative consensus sites. The following gephyrin mutants were therefore generated and their contributions studied in GST-Pin1 pull-down assays: Thr96Ala–Thr123Ala (Mut-E); Thr266Ala–Ser270Ala–Thr286Ala (Mut-F) and Ser319Ala–Thr337Ala (Mut-G). Moreover, a Ser/Thr-Ala gephyrin mutant comprehensive of all seven consensus sites, namely Thr96Ala–Thr132Ala–Thr266Ala–Ser270Ala–Thr286Ala–Ser319Ala–Thr337Ala (Mut-D), was also included. As shown in Figure 3C, all gephyrin variants tested showed similar levels of binding as the gephyrin wild-type (WT) protein to GST-Pin1 beads, thus strongly confirming the importance of serine residues present within the proline-rich domain of gephyrin for Pin1 interaction.
Figure 3.
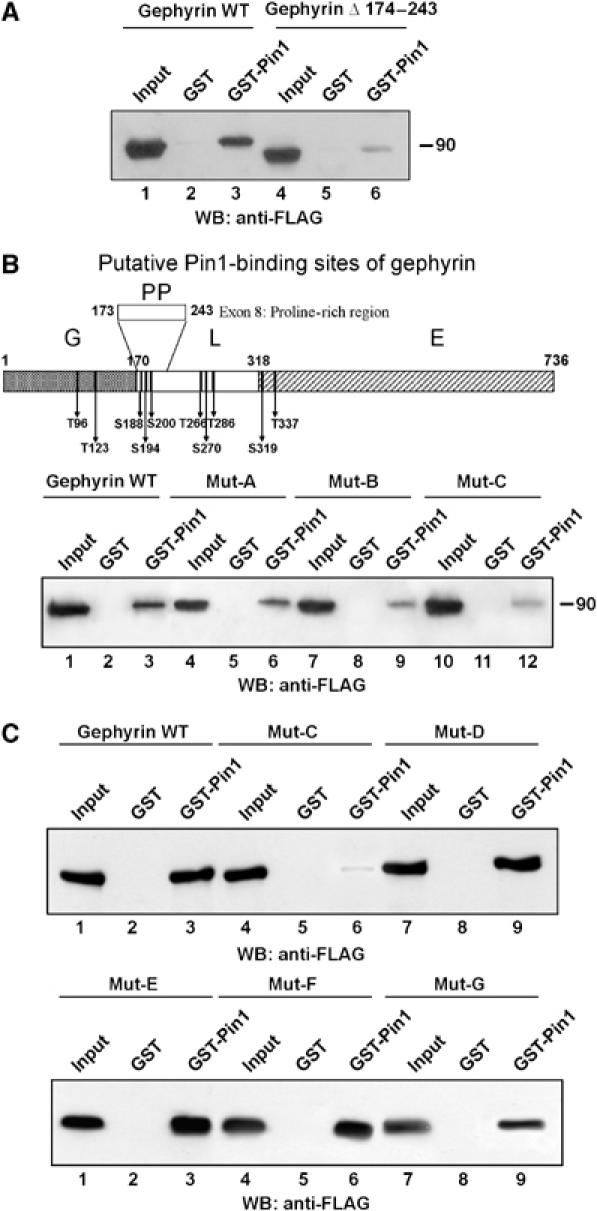
Pin1 binding requires Ser-Pro epitopes contained within the proline-rich domain of gephyrin. (A) GST-Pin1 pull-down assay using lysates of HEK 293 cells transfected with gephyrin WT (lanes 1–3) compared with gephyrin depleted of exon 8 (lanes 4–6). (B) (upper panel) Schematic representation of gephyrin domains (G, N-terminal domain; L, linker or intervening region; E, C-terminal domain) together with the positions of putative Pin1 consensus motifs. The proline-rich domain (PP) contains three Pin1 consensus sites at residues 188, 194 and 200. Mut-A, Mut-B and Mut-C refer to gephyrin S188A, S188A-S194A and S188A-S194A-S200A mutants, respectively. (Lower panel) GST-Pin1 pull-down assay using gephyrin WT (lanes 1–3), Mut-A (lanes 4–6), Mut-B (lanes 7–9) and Mut-C (lanes 10–12). The triple mutant retained a low residual binding similar to the deletion mutant initially tested in (A). (C) (upper panel) GST-Pin1 pull-down assay performed on gephyrin WT (lanes 1–3), Mut-C (lanes 4–6) and Mut-D (T96A-T132A-T266A-S270A-T286A-S319A-T337A) (lanes 7–9). (Lower panel) GST-Pin1 pull-down on Mut-E (T96A-T123A) (lanes 1–3), Mut-F (T266A-S270A-T286A) (lanes 4–6) and Mut-G (S319A-T337A) (lanes 7–9).
Gephyrin associates with Pin1 in HEK 293 cells and in the mouse brain
To characterise the potential interaction between Pin1 and gephyrin in intact mammalian cells, we initially expressed both proteins in HEK 293 cells and examined their subcellular distribution. Under these conditions, ectopically expressed gephryin produces large cytoplasmic aggregates characterised by their ability to actively sequester several gephyrin interaction partners (Figure 4A, left panel). In single transfections, Pin1-FLAG showed a diffuse distribution both in the nucleus and the cytoplasm (Figure 4A, middle panel). When gephyrin-GFP and Pin1-FLAG were cotransfected, a fraction of Pin1-FLAG was clearly relocalised to intracytoplasmic gephyrin aggregates (Figure 4B), thus indicating colocalisation of the two proteins. We also expressed gephyrin Mut C-GFP in HEK 293 cells. This triple mutant formed cytosolic aggregates in a manner indistinguishable from that generated by WT gephyrin (Figure 4A, right panel). Consistent with the pull-down data, this gephyrin mutant was strongly impaired in recruiting Pin1-FLAG immunoreactivity upon cotransfection (Figure 4C).
Figure 4.

Gephyrin interacts with Pin1 in vitro. (A) Immunofluorescence assay to determine the subcellular distribution of gephyrin WT, Mut-C (see above) and Pin1-FLAG ectopically expressed in HEK 293 cells. In single transfection experiments, gephyrin-GFP and Mut-C-GFP were revealed by the intrinsic green fluorescence of GFP. Pin1-FLAG was visualised by anti-FLAG antibody, followed by TRITC-conjugated secondary antibody. Scale bar, 10 μm. (B) Cotransfection experiments with gephyrin-GFP and Pin1-FLAG. (C) Cotransfection experiments with gephyrin Mut-C-GFP and Pin1-FLAG. (D) Lysates of HEK 293 cells transfected with Pin1WT in the presence of gephyrin FLAG or with the vector alone (as a negative control) were immunoprecipitated with monoclonal antibodies anti-FLAG (lanes 4 and 5). Immunoprecipitates were analysed by Western blotting using anti-gephyrin and anti-Pin1 antibodies. Efficient dephosphorylation of gephyrin upon CIP treatment was verified by Western blot on total lysates and on immunoprecipitated gephyrin-FLAG with MPM-2 antibody (7–8 and 10–11, respectively.) (E) Co-immunoprecipitation experiment on mouse brain lysates using a polyclonal anti-gephyrin antibody and NRS as negative control (lanes 2 and 3, respectively). Immunoprecipitates were analysed by Western blotting using a monoclonal antibody anti-gephyrin and a polyclonal antibody against Pin1.
We then performed immunoprecipitation experiments to investigate the presence of Pin1/gephyrin complexes in vitro. HEK 293 cells were cotransfected with plasmids encoding for Pin1WT and gephyrin-FLAG, or Pin1WT and vector alone as negative control, and cell lysates were immunoprecipitated with the anti-FLAG monoclonal antibody. The bound protein complexes were analysed by Western blotting using anti-gephyrin and anti-Pin1 polyclonal antibodies for gephyrin and Pin1 detection, respectively. As shown in Figure 4D, not only Pin1 was specifically immunoprecipitated from cells expressing gephyrin-FLAG (lane 5), but also its gephyrin-dependent immunoprecipitation was completely abolished upon dephosphorylation of gephyrin-FLAG by phosphatase treatment (lane 6). The efficient dephosphorylation of Pin1 binding sites upon CIP addition was confirmed by the lack of MPM-2 immunoreactivity on immunoprecipitated gephyrin-FLAG (Figure 4D, right panel, lane 10). This latter result is in agreement with our findings with the Pin1-binding-defective mutant (Pin1Y23A) and further supports the phosphorylation-dependent interaction of Pin1 with gephyrin.
In addition, endogenous Pin1 and gephyrin were found in complex upon co-immunoprecipitation from mouse brain homogenates (Figure 4E), indicating that gephyrin is phosphorylated on Pin1 consensus sites and it interacts with the prolyl isomerase in neuronal cells.
Pin1 elicits conformational changes in gephyrin
To test whether Pin1 can induce a conformational change in gephyrin, a partial proteolysis assay was carried out. To this aim, His-tagged gephyrin full length was overexpressed in fibroblasts obtained from the Pin1 knockout mouse embryo (Pin1−/− mouse embryo fibroblasts, MEFs). This allows phosphorylation of ectopically expressed gephyrin in the absence of Pin1-mediated rearrangement. After transfection (48 h), His-tagged gephyrin was efficiently purified from cell extracts on nickel column and incubated with either GST-Pin1, the catalytically inactive mutants GST-Pin1C113A and GST-Pin1S67E (Zhou et al, 2000) or GST alone. Finally, all the reactions were incubated with the protease subtilisin under identical conditions. After limited digestion, the corresponding protease fragments were visualised by SDS–PAGE followed by Western blot analysis using a pool of anti-gephyrin antibodies. The different pattern of digestion products obtained highlights a more efficient gephyrin protection from subtilisin cleavage promoted by GST-Pin1 as compared with GST or GST-Pin1C113A and GST-Pin1S67E (Figure 5). This effect is not due to steric hindrance exerted by GST or Pin1 binding, but is dependent only on Pin1 isomerase activity as both Pin1 mutants catalytically impaired but fully competent to bind their substrate were completely ineffective.
Figure 5.
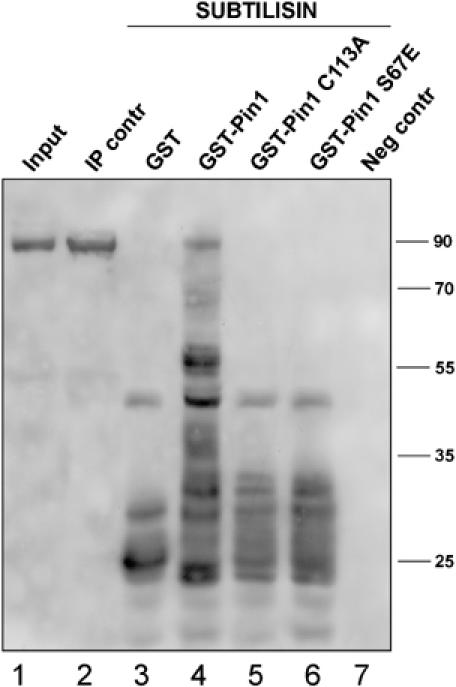
Pin1 induces conformational changes of gephyrin. HIS-tagged gephyrin was overexpressed in Pin1−/− MEFs and purified from cell extracts on a nickel column (lane 2). The purified protein was incubated with GST (lane 3), GST-Pin1 (lane 4), GST-Pin1 C113A (lane 5) and GST-Pin1 S67E (lane 6). Each sample was incubated with subtilisin and the corresponding protease fragments were visualised by SDS–PAGE followed by Western blot analysis using a pool of anti-gephyrin antibodies. Lane 7 represents an experiment performed in the absence of HIS-tagged gephyrin to exclude the possibility that the pool of anti-gephyrin antibodies may recognise some other antigen absorbed aspecifically on a nickel column.
Pin1 regulates gephyrin ability to interact with the β subunit of GlyRs
Pin1-dependent conformational rearrangement of gephyrin may affect the ability of this protein to bind the β subunit of GlyRs. To address this question, MEFs derived from Pin1 knockout and WT mice were cotransfected with gephyrin-FLAG and GFP-tagged intracellular loop of the β subunit of GlyRs (GFP-β loop). After transfection (48 h), gephyrin-FLAG solubilised from both cell lines was immunoprecipitated using either the anti-FLAG monoclonal antibody or, as negative control, the anti-myc 9E10 monoclonal antibody. The bound protein complexes were analysed by Western blotting using the anti-gephyrin polyclonal antibody, for gephyrin detection, or anti-GFP polyclonal antibody for the GFP-β loop. Regardless of Pin1 expression, the anti-FLAG antibody immunoprecipitated comparable amounts of gephyrin-FLAG (Figure 6). However, in the absence of endogenous Pin1, the amount of GFP-β loop co-immunoprecipitated by gephyrin-FLAG was drastically reduced (compare lanes 7–5). Interestingly, the impairment of binding of GFP-β loop to gephyrin was fully rescued when Pin1−/− MEFs were cotransfected with Pin1WT (lane 9). These results provide evidence that Pin1-induced conformational changes of gephyrin influence the ability of this protein to interact with the functional surrogate of GlyR β subunit.
Figure 6.
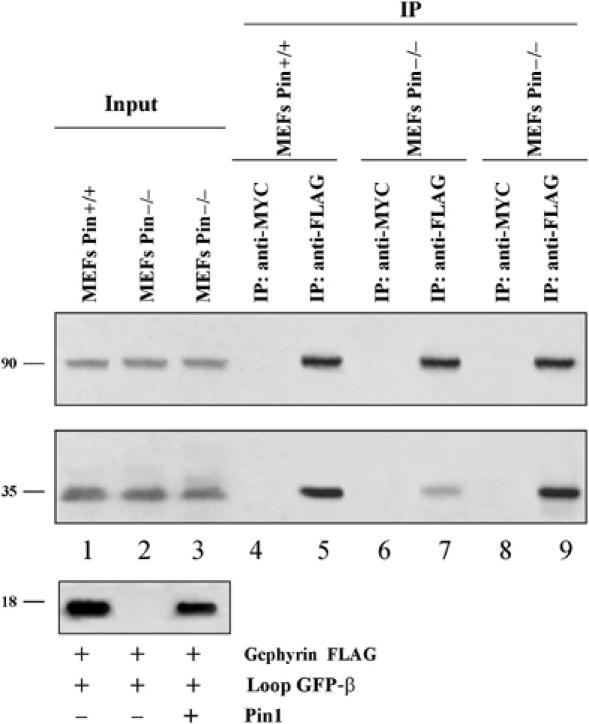
Lack of Pin1 impairs the ability of gephyrin to interact efficiently with GlyRs. Gephyrin-FLAG and GFP-β loop were transfected in MEFs Pin1+/+ (1), MEFs Pin1−/− (2) and MEFs Pin1−/− plus Pin1 (3). Lysates were immunoprecipitated with anti-FLAG (lanes 5, 7 and 9) or anti-MYC as negative control (lanes 4, 6 and 8). Immunoprecipitates were analysed by Western blotting using polyclonal antibodies against gephyrin, GFP and Pin1.
GlyRs punctae and the amplitude of glycine-evoked currents are reduced in hippocampal neurons from Pin1 knockout mice
To further assess the involvement of Pin1 in gephyrin-dependent GlyRs function(s), we initially investigated the cellular distribution of GlyRs in primary hippocampal neurons derived from WT and Pin1 knockout mice (Atchison et al, 2003). In several staining experiments, we consistently found a loss of GlyRs immunoreactive punctae on the membranes of Pin1−/− neurons as compared with WT neurons (Figure 7A and B). By contrast, immunocytochemical staining for intracellular gephyrin revealed a similar distribution of its immunoreactive clusters. As a consequence, a reduced level of colocalisation between GlyRs and gephyrin immunoreactivities was detected.
Figure 7.
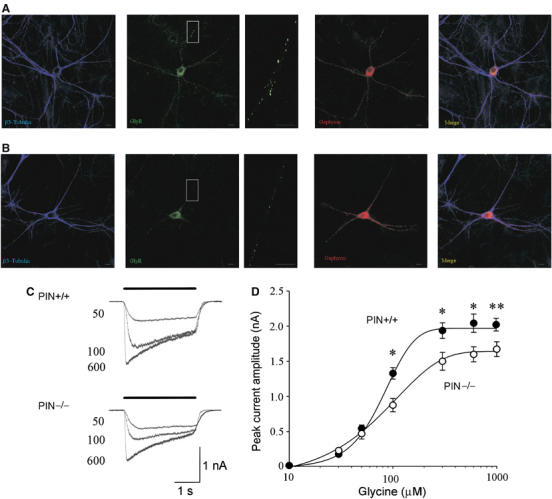
Hippocampal neurons from Pin−/− mice express a reduced number of GlyRs punctae associated with decreased peak amplitude of glycine-evoked currents. (A) Hippocampal neurons derived from Pin1 WT and (B) knockout mice were labelled with monoclonal antibody (mAb4a) recognising the GlyRs (green), polyclonal anti-gephyrin antibody (red) and the neuronal marker β3-tubulin (blue). Side panels are magnification of the boxed areas. Scale bars, 10 μm. (C) Superimposed current responses evoked at −40 mV by different concentrations of glycine (bar) in WT (PIN+/+, upper traces) and knockout (PIN−/−, lower traces) mice. The numbers near the current traces refer to the concentrations of glycine used (in μM). (D) Dose–response curve for glycine-evoked currents obtained in WT (filled symbols) and knockout mice (open symbols). Each point is the average of 8–16 individual responses (*P<0.05 and **P<0.001).
To examine whether the reduced number of GlyR clusters observed in mice lacking Pin1 expression induces modifications in GlyR function, whole-cell patch-clamp recordings (in voltage clamp configuration) were performed at −40 mV from WT and Pin1−/− neurons. Recordings were routinely performed in the presence of the GABAA antagonist picrotoxin (50 μM), the GABAB receptor antagonist CGP 55485 (1 μM) and the broad-spectrum ionotropic glutamate receptor antagonist kynurenic acid (1 mM). All cells tested (n=84) responded to glycine application with an inward current of variable amplitude. It is worth noting that glycine responses were mediated by heteromeric GlyRs as picrotoxin at the concentration of 50 μM is known to completely block homomeric GlyR types (Pribilla et al, 1992; Burzomato et al, 2003). Glycine-mediated currents were readily blocked by the selective glycine receptor antagonist strychnine (0.5 μM; n=5), indicating that they were mediated by glycine receptors (data not shown). As shown in Figure 7C, glycine-evoked currents from Pin1−/− hippocampal neurons were significantly reduced in amplitude as compared with WT. A saturating concentration of glycine (1 mM) induced current responses whose peak amplitudes were 2.0±0.09 nA (n=16) and 1.6±0.1 nA (n=14) in WT and Pin1−/− mice, respectively. These two values were significantly different (P<0.001). Concentration–response curves revealed similar EC50 values (91 μM and 85 μM in WT and Pin1−/− mice, respectively; Figure 7D). These data suggest a reduced number of heteromeric GlyRs in hippocampal neurons from Pin1−/− mice.
Discussion
The present results clearly show that endogenous gephyrin undergoes proline-directed phosphorylation. This event allows the recruitment of the peptidyl-prolyl cis/trans isomerase Pin1, which binds gephyrin in a phosphorylation-dependent manner and isomerises specific pSer/Thr-Pro motifs. Hippocampal neurons derived from Pin1 knockout mice showed a decreased number of GlyRs clusters at cell surface, which was paralleled by a reduction in the amplitude of glycine-evoked currents. These findings suggest the existence of a post-phosphorylation regulatory mechanism that is crucial for the proper functioning of glycinergic synapses.
Gephyrin is the substrate of proline-directed phosphorylation signalling cascade
Proline-directed phosphorylation is one of the major signalling mechanisms whose involvement in the functional organisation of the postsynaptic membrane has just started to be investigated (Jaffe et al, 2004). This signalling pathway regulates protein function by phosphorylation-induced conformational changes. In fact, in folded proteins, proline residues possess the unique property of existing in two distinct isomers, cis and trans, which provides a potential backbone switch in the polypeptide chain that is controlled by cis/trans isomerisation. Pin1 is the only known peptidyl-prolyl cis/trans isomerase that can efficiently isomerise phosphorylated Ser/Thr-Pro motifs. Initially identified as a mitotic regulator (Lu et al, 1996; Lu, 2000), Pin1 has been subsequently involved in the regulation of several cellular processes such as apoptosis, endocytosis, translation, maintenance of the cytoskeleton and neuronal functions (Gerez et al, 2000; Lu, 2004). In particular, Pin1 has been found to be highly expressed in neurons, where it plays a critical role in protecting against age-dependent tauopathy and neurodegeneration (Liou et al, 2003; Ramakrishnan et al, 2003; Thorpe et al, 2004; Lim and Lu, 2005; Pastorino et al, 2006).
Interestingly, Pin1 consensus motifs are present within gephyrin amino-acid sequence, and some phosphorylated serine and threonine residues have previously been detected on gephyrin in vivo (Langosch et al, 1992). Several lines of evidence suggest that gephyrin undergoes proline-directed phosphorylation followed by Pin1-dependent prolyl isomerisation. First, MPM-2 immunoreactive epitopes are present on gephyrin overexpressed in HEK 293 cells and on endogenous gephyrin immunoprecipitated from mouse brain homogenates. Second, pull-down and co-immunoprecipitaton analyses performed on ectopically expressed gephyrin as well as endogenous protein from neuronal cell lines and mouse brain homogenates demonstrate the existence of Pin1/gephyrin complexes. It is worth noting that only a small fraction of Pin1 has been found in complex with endogenous gephyrin. This result is in line with the notion that Pin1 is an enzyme that transiently interacts with its substrates. Therefore, once recruited upon gephyrin phosphorylation, it catalyses conformational changes on it, as revealed by partial proteolysis assay, and it is rapidly released for another cycle of catalysis. Third, the binding of Pin1 to gephyrin pSer/Thr-Pro motifs relies entirely on gephyrin phosphorylation. A Pin1 mutant known to be impaired in phosphoproteins binding, Pin1Y23A, totally failed to interact with gephyrin. Moreover, Pin1/gephyrin co-immunoprecipitation was completely abolished when gephyrin was stripped of all its phosphate groups by phosphatase treatment.
What are the consensus sites whose phosphorylation promotes Pin1 recruitment? The mammalian brain contains a mosaic expression of metabolic non-neuronal and heterogeneous neuronal isoforms of gephyrin (Ramming et al, 2000; David-Watine, 2001; Rees et al, 2003). At least 11 different splice variants have been identified whose spatial and temporal expression is still largely unknown. Despite this complex scenario, all gephyrin isoforms share at least 10 identical Pin1 putative consensus sites, which are organised into four clusters. The Ser-Pro epitopes encompassing the proline-rich region of gephyrin represent three major consensus motifs for Pin1 recruitment. In support of this view is the observation that deletion of the entire proline-rich domain or point mutations at Ser188, Ser194 and Ser200 residues almost completely abated Pin1 binding to gephyrin. Mutagenesis analysis extended to all Ser-Pro and Thr-Pro residues located outside the proline-rich region of gephyrin did not reveal any major contribution by other Pin1 consensus sites, as all gephyrin variants showed similar levels of binding as the gephyrin WT. It is interesting to note that these findings are in good agreement with the initial phosphoamino-acid analysis showing that gephyrin copurified with GlyRs from rat spinal cord preparation is mainly phosphorylated at serine residues (Langosch et al, 1992). However, the observation that gephyrin Ser188Ala–Ser194Ala–Ser200Ala mutant (Mut-C) still maintains a residual binding activity leaves open the possibility that other Ser/Thr-Pro consensus sites located outside the proline-rich domain may participate in Pin1 recruitment in vivo. Overall, it can be concluded that Ser-Pro epitopes lying within the proline-rich domain of gephyrin are critical for Pin1–gephyrin interaction. The fact that this domain is constantly present in all gephyrin isoforms identified so far further underlines the critical role played by proline-directed phosphorylation signalling cascade in regulating gephyrin functions.
Post-phoshorylation conformational changes of gephyrin affect GlyRs function
Despite the apparent stability of postsynaptic membrane specialisations, it has been clearly demonstrated that both receptors and scaffolding elements are constantly renewed both at rest as well as during plasticity events (Meier et al, 2001; Choquet and Triller, 2003; Hanus et al, 2006). Several mechanisms can account for this dynamic turnover of postsynaptic elements, namely (a) insertion or removal of receptors from the plasma membrane via endo/exocytosis processes, (b) exchange between pools of synaptic and extrasynaptic receptors through lateral diffusion within the plane of the membrane and (c) affinity of the receptor for scaffolding molecules and interactions between scaffolding molecules. The present experiments add an additional layer of complexity on this already multifaceted scenario by showing that phosphorylation-dependent prolyl isomerisation signalling cascade targeting the scaffolding molecule gephyrin impinges upon GlyRs function. Several pieces of evidence support this notion. First, lack of Pin1 expression strikingly reduces gephyrin binding to the GlyR β loop. Second, cultured hippocampal neurons derived from Pin1−/− mice are characterised by a marked loss in the number of GlyR immunoreactive punctae at the cell surface. Third, patch-clamp recordings performed using Pin1 knockout neurons have unveiled a significant reduction in the mean amplitude of glycine-evoked currents. This latter effect correlates with a decrease in the number of the available receptors and not with changes in receptor affinity as WT and Pin1 knockout neurons show similar values of EC50.
Although the present observations indicate that Pin1 is involved in modulating GlyRs function, the molecular mechanisms underlying this process still remain to be elucidated. The activity of Pin1 could be required to increase the level of GlyRs binding to the underlying gephyrin scaffold, leading to a more prolonged stabilisation of GlyRs within a cluster. It is well known that the oligomerisation state of gephyrin has been shown to influence its affinity for GlyRs (Sola et al, 2004; Kim et al, 2006). Structural analysis of gephyrin functional domains has clearly established that the N-terminal G-domain folds into a trimeric conformation (Schwarz et al, 2001; Sola et al, 2001), whereas the C-terminal E-domain forms dimers (Xiang et al, 2001). Each C-terminal E-domain of gephyrin, present as a monomer within cytosolic trimers, is able to bind the β subunit of GlyRs, but is not competent to promote gephyrin dimerisation. Therefore, an appropriate stimulus able to trigger a conformational change of trimeric gephyrin has been proposed as the event initiating gephyrin multimerisation (Sola et al, 2004). E-domain dimers display both high-affinity and low-affinity binding sites for GlyR β subunit and this feature has been regarded as the molecular mechanism that renders gephyrin scaffold dynamic enough to allow rapid exchange of receptors in and out of the postsynaptic specialisation. These findings raise the intriguing possibility that post-phosphorylation Pin1-dependent prolyl isomerisation of gephyrin may represent the molecular mechanism responsible for the regulated removal of the so-called ‘trimerisation constraint' for gephyrin E-domain interaction. However, the fact that two differentially tagged forms of gephyrin (gephyrin-FLAG and gephyrin-GFP) distinguishable by electromobility were equally co-immunoprecipitated regardless of Pin1 expression (data not shown) allows us to exclude this possibility. In this context, the conformational changes induced by Pin1 onto gephyrin lattice may convert some of the low-affinity binding sites for GlyR β subunit into high-affinity sites, ultimately increasing both the stability of the lattice and the strength of GlyRs anchoring. It is interesting to note that a recent structural study aimed at deciphering the structural framework of GlyR anchoring by gephyrin suggests the possibility that phosphorylation events could target gephyrin lattice, thus influencing its stability (Kim et al, 2006).
It is also conceivable that Pin1-induced conformational changes on gephyrin promotes its binding to components of motor protein complexes, facilitating GlyRs delivery to the cell surface. Evidence that gephyrin binds components of motor protein complexes has been obtained (Fuhrmann et al, 2002), although its involvement in GlyRs trafficking has been recently unveiled (Hanus et al, 2004). Unravelling all these issues will certainly require a combination of biochemical and imaging approaches.
In conclusion, our findings have clearly established that proline-directed phosphorylation targeting the scaffolding protein gephyrin plays an important role in the organisation of glycinergic clusters. In addition, a novel neuronal function has been assigned to Pin1. Extensively studied in proliferating cells and in the field of cancer, most studies addressing the role of Pin1 in a neuronal context have focused on unravelling its contribution to the pathogenesis of Alzheimer's disease and other tauopathies, where spurious cell-cycle events are observed. The existence of other Pin1 neuronal functions has been suggested based on the fact that Pin1 promoter contains a number of consensus sequences related to brain and/or neuronal differentiation (Quandt et al, 1995). Our studies therefore support this notion and allow us to extend the roles of Pin1 from neuronal survival to functional modulation of inhibitory receptors.
Materials and methods
Plasmids and mutagenesis
The cDNA encompassing the open reading frame of the gephyrin splice variant p1 (Prior et al, 1992) was kindly provided by H Betz (Max-Planck-Institute, Germany). Complementary DNAs encoding gephyrin WT FLAG-tagged, gephyrin WT GFP-tagged and gephyrin devoid of the exon 8 together with the various gephyrin point mutants and Pin1S67E were generated by polymerase chain reaction (PCR) and cloned into pcDNA3 or pGEX-4T1. All PCR-amplified products were fully sequenced to exclude the possibility of second site mutations. pGEX4T1 plasmids containing Pin1WT, Pin1Y23A and Pin1C113A, as well as pcDNA3 plasmids containing Pin1 WT and PinC113A, were previously described (Zacchi et al, 2002). The cDNAs encoding the β subunit of the GlyR were kindly provided by Sivilotti L (University College London, UK). Amino acids 378–426 of the β-subunit containing the gephyrin binding motif were fused to recombinant GFP by cloning a 150-bp fragment generated by PCR into the pEGFP-C2 vector (Clontech, Germany).
Cell culture and treatment
Primary hippocampal neurons from Pin1 WT and knockout mice (Atchison et al, 2003) were prepared as previously described (Andjus et al, 1997) and cultured in 500 ml MEM (Gibco), 3 g D-(+)-glucose, 1.8 g HEPES (14 mM final concentration), 50 mg apo-transferrin (0.1 mg/ml final concentration), 15 mg insulin (dissolve in 10 ml H2O and add 1–2 drops of 1 N HCl), 0.05 mg d-biotin (dissolve 1 mg biotin in 10 ml H2O and add 500 μl to the medium) solution, 0.75 mg vitamin B12 (final concentration 1 mM), 1.12 ml of gentamicin 1 mg/ml (final concentration 2 μg/ml), pH 7.4, and 5% FBS. Experiments were performed on cells cultured for at least 7 days.
All the other cell lines used were cultured at 37°C in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), penicillin (100 U/ml) and streptomycin (100 μg/ml). HEK 293 is a human embryonic kidney cell line, and SH-SY5Y is a human neuroblastoma cell line. Pin1−/− and Pin1+/+ MEFs have been described previously (Zacchi et al, 2002).
Antibodies
Anti-gephyrin rabbit polyclonal antiserum was raised against the full-length protein expressed in bacteria and purified by standard procedures. Anti-Pin1 rabbit polyclonal antiserum has been previously described (Zacchi et al, 2002). The following primary antibodies were used: mouse monoclonal anti-gephyrin (BD Transduction Laboratories), pSer/pThr-Pro (MPM-2, Upstate Biotechnology), anti-FLAG (Sigma), mAb4a anti-GlyR alpha subunit (Synaptic System), rabbit polyclonal anti-GFP (gift from K Ainger and F Paoletti) and anti-β-tubulin 3 chicken polyclonal antibody mixture (AvesLab).
In vitro binding, immunoprecipitation and Western blot analysis
Transfections were performed with the calcium phosphate method. MEFs were transfected with Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. GST pull-down assays were performed as previously described (Zacchi et al, 2002). CIP phosphatase (20 U/ml, New England Biolabs) was added to protein extracts for 30 min at 30°C. Immunoprecipitation experiments of gephyrin ectopically expressed in HEK 293 cells and from mouse brain were performed using a lysis buffer containing 50 mM Tris–HCl, pH 7.4, 1% NP-40, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EGTA, 1 mM Na3VO4, 1 mM NaF and protease inhibitor cocktail. For gephyrin and Pin1 co-immunoprecipitation, HEK 293 cells overexpressing gephyrin-FLAG and Pin1WT were lysed in 50 mM Tris–HCl, pH 7.5, 100 mM NaCl, 0.1% Tween20, 10% glycerol, 10 mM EDTA and 2 mM MgCl2, and immunoprecipitated by the anti-FLAG antibody. For the analysis of Pin1–gephyrin interaction, whole brains were used after homogenising by passing through a 26G needle and subsequently lysing in a buffer containing 0.1% Tween 20, as previously described. After incubation overnight with rabbit polyclonal anti-gephyrin antibody, immunoprecipitation experiment was performed according to standard procedures. Primary antibodies were revealed by HRPO-conjugated secondary antibodies (Sigma) followed by ECL (Amersham).
Subtilisin proteolysis
Ectopically expressed His-gephyrin was purified from MEFs Pin1−/− on a nickel column and incubated with 100 ng of either GST-Pin1WT, GST-Pin1C113A, GST-Pin1S67E or GST in the following buffer: 50 mM Tris–HCl, pH 7.5, 100 mM NaCl and 1 mM MgCl2, supplemented with phosphatase inhibitors. After 30 min incubation at 20°C, subtilisin was added for a further 20 min at 20°C. The reaction was stopped by the addition of boiling sample buffer and the proteolytic fragments were resolved by 10% SDS–PAGE and revealed by Western blotting using a pool of gephyrin antibody.
Immunofluorescence staining
HEK 293 cells were fixed with 4% (w/v) paraformaldehyde/4% sucrose for 15 min, permeabilised with 0.1% (v/v) NP-40 for 5 min and then blocked with 10% (w/v) FBS in PBS for 30 min. Hippocampal neurons were fixed for 10 min with methanol at −20°C and processed as described above. Antibody staining was performed by standard procedures. GFP was visualised by autofluorescence. Immunostainings were analysed using a Leica TCS-SP confocal laser scanning microscope (Bensheim, Germany).
Electrophysiological recordings
Glycine-evoked currents were recorded at the holding potential of −40 mV in the whole-cell configuration of the patch-clamp technique using a Multiclamp 700A amplifier (Axon Instruments, Foster City, CA). Patch electrodes were pulled from borosilicate glass capillaries (Hilgenberg, Germany). They had a resistance of 4–6 MΩ when filled with an intracellular solution containing: 137 mM CsCl, 10 mM HEPES, 11 mM BAPTA, 2 mM MgCl2, 2 mM Mg ATP and 1 mM CaCl2 (pH was adjusted to 7.3 with CsOH). The composition of the external solution was (in mM): 137 mM NaCl, 5 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 20 mM glucose and 10 mM HEPES hemisodium. All experiments were performed at room temperature (22–24°C).
The stability of the patch was checked by repetitively monitoring the input and series resistance during the experiments. Cells exhibiting 15–20% changes were excluded from the analysis. Different concentrations of glycine (0.03–1 mM) were applied through a multibarrel RSC-200 perfusion system (Bio-logic, Grenoble, France). With this system, a complete exchange of the solution around the cell was obtained in less than 30 ms. Data were transferred to a computer after digitisation with an A/D converter (Digidata 1200, Axon Instruments, CA). Data acquisition was achieved with pClamp 8.2 (Axon Instruments, CA). Data were sampled at 20–100 kHz and filtered with a cutoff frequency of 1 kHz. Data are expressed as mean±s.e.m. Statistical comparisons were made by unpaired t-test. Differences were considered significant at P<0.05.
Acknowledgments
We acknowledge H Betz (Department of Neurochemistry, Max-Planck-Institute for Brain Research, Frankfurt, Germany) for gephyrin P1 cDNA. We are grateful to AR Means (Department of Pharmacology and Cancer Biology, Duke University, NC, USA) for providing the Pin1 null mice developed with funds from an R01 NCI grant (CA082845). We thank K Ainger and F Paoletti for providing reagents and B Pastore and L Masten for their technical support in neuronal culture. We are extremely grateful to E Dreosti for her help and for critical discussion during experiments and to Cristina Degrassi for the animalhouse facility. This work was supported by a grant from Ministero Istruzione, Universita', Ricerca (MIUR, PRIN 2005) to E Cherubini.
References
- Andjus PR, Stevic-Marinkovic Z, Cherubini E (1997) Immunoglobulins from motoneurone disease patients enhance glutamate release from rat hippocampal neurones in culture. J Physiol 504: 103–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atchison FW, Capel B, Means AR (2003) Pin1 regulates the timing of mammalian primordial germ cell proliferation. Development 130: 3579–3586 [DOI] [PubMed] [Google Scholar]
- Blume-Jensen P, Hunter T (2001) Oncogenic kinase signalling. Nature 411: 355–365 [DOI] [PubMed] [Google Scholar]
- Burzomato V, Groot-Kormelink PJ, Sivilotti LG, Beato M (2003) Stoichiometry of recombinant heteromeric glicine receptors revealed by a pore-lining region point mutation. Receptor Channels 9: 353–361 [DOI] [PubMed] [Google Scholar]
- Choquet D, Triller A (2003) The role of receptor diffusion in the organization of the postsynaptic membrane. Nat Rev Neurosci 4: 251–265 [DOI] [PubMed] [Google Scholar]
- David-Watine B (2001) The human gephyrin (GPHN) gene: structure, chromosome localization and expression in non-neuronal cells. Gene 271: 239–245 [DOI] [PubMed] [Google Scholar]
- Davis FM, Tsao TY, Fowler SK, Rao PN (1983) Monoclonal antibodies to mitotic cells. Proc Natl Acad Sci USA 80: 2926–2930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essrich C, Lorez M, Benson JA, Fritschy JM, Luscher B (1998) Postsynaptic clustering of major GABAA receptor subtypes requires the gamma 2 subunit and gephyrin. Nat Neurosci 1: 563–571 [DOI] [PubMed] [Google Scholar]
- Feng G, Tintrup H, Kirsch J, Nichol MC, Kuhse J, Betz H, Sanes JR (1998) Dual requirement for gephyrin in glycine receptor clustering and molybdoenzyme activity. Science 282: 1321–1324 [DOI] [PubMed] [Google Scholar]
- Fuhrmann JC, Kins S, Rostaing P, El Far O, Kirsch J, Sheng M, Triller A, Betz H, Kneussel M (2002) Gephyrin interacts with Dynein light chains 1 and 2, components of motor protein complexes. J Neurosci 22: 5393–5402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerez L, Mohrmann K, van Raak M, Jongeneelen M, Zhou XZ, Lu KP, van Der SP (2000) Accumulation of rab4GTP in the cytoplasm and association with the peptidyl-prolyl isomerase pin1 during mitosis. Mol Biol Cell 11: 2201–2211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesemann T, Schwarz G, Nawrotzki R, Berhorster K, Rothkegel M, Schluter K, Schrader N, Schindelin H, Mendel RR, Kirsch J, Jockusch BM (2003) Complex formation between the postsynaptic scaffolding protein gephyrin, profilin, and Mena: a possible link to the microfilament system. J Neurosci 23: 8330–8339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanus C, Ehrensperger MV, Triller A (2006) Activity-dependent movements of postsynaptic scaffolds at inhibitory synapses. J Neurosci 26: 4586–4595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanus C, Vannier C, Triller A (2004) Intracellular association of glycine receptor with gephyrin increases its plasma membrane accumulation rate. J Neurosci 24: 1119–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe H, Vinade L, Dosemeci A (2004) Identification of novel phosphorylation sites on postsynaptic density proteins. Biochem Biophys Res Commun 321: 210–218 [DOI] [PubMed] [Google Scholar]
- Kim EY, Schrader N, Smolinsky B, Bedet C, Vannier C, Schwarz G, Schindelin H (2006) Deciphering the structural framework of glycine receptor anchoring by gephyrin. EMBO J 25: 1385–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch J, Wolters I, Triller A, Betz H (1993) Gephyrin antisense oligonucleotides prevent glycine receptor clustering in spinal neurons. Nature 366: 745–748 [DOI] [PubMed] [Google Scholar]
- Kneussel M, Brandstatter JH, Gasnier B, Feng G, Sanes JR, Betz H (2001) Gephyrin-independent clustering of postsynaptic GABA(A) receptor subtypes. Mol Cell Neurosci 17: 973–982 [DOI] [PubMed] [Google Scholar]
- Kneussel M, Brandstatter JH, Laube B, Stahl S, Muller U, Betz H (1999a) Loss of postsynaptic GABA(A) receptor clustering in gephyrin-deficient mice. J Neurosci 19: 9289–9297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneussel M, Hermann A, Kirsch J, Betz H (1999b) Hydrophobic interactions mediate binding of the glycine receptor beta-subunit to gephyrin. J Neurochem 72: 1323–1326 [DOI] [PubMed] [Google Scholar]
- Langosch D, Hoch W, Betz H (1992) The 93 kDa protein gephyrin and tubulin associated with the inhibitory glycine receptor are phosphorylated by an endogenous protein kinase. FEBS Lett 298: 113–117 [DOI] [PubMed] [Google Scholar]
- Lim J, Lu KP (2005) Pinning down phosphorylated tau and tauopathies. Biochim Biophys Acta 1739: 311–322 [DOI] [PubMed] [Google Scholar]
- Liou YC, Sun A, Ryo A, Zhou XZ, Yu ZX, Huang HK, Uchida T, Bronson R, Bing G, Li X, Hunter T, Lu KP (2003) Role of the prolyl isomerase Pin1 in protecting against age-dependent neurodegeneration. Nature 424: 556–561 [DOI] [PubMed] [Google Scholar]
- Lu KP (2000) Phosphorylation-dependent prolyl isomerization: a novel cell cycle regulatory mechanism. Prog Cell Cycle Res 4: 83–96 [DOI] [PubMed] [Google Scholar]
- Lu KP (2004) Pinning down cell signaling, cancer and Alzheimer's disease. Trends Biochem Sci 29: 200–209 [DOI] [PubMed] [Google Scholar]
- Lu KP, Hanes SD, Hunter T (1996) A human peptidyl-prolyl isomerase essential for regulation of mitosis. Nature 380: 544–547 [DOI] [PubMed] [Google Scholar]
- Lu KP, Liou YC, Zhou XZ (2002) Pinning down proline-directed phosphorylation signaling. Trends Cell Biol 12: 164–172 [DOI] [PubMed] [Google Scholar]
- Lu PJ, Zhou XZ, Shen M, Lu KP (1999) Function of WW domains as phosphoserine- or phosphothreonine-binding modules. Science 283: 1325–1328 [DOI] [PubMed] [Google Scholar]
- Maas C, Tagnaouti N, Loebrich S, Behrend B, Lappe-Siefke C, Kneussel M (2006) Neuronal cotransport of glycine receptor and the scaffold protein gephyrin. J Cell Biol 172: 441–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammoto A, Sasaki T, Asakura T, Hotta I, Imamura H, Takahashi K, Matsuura Y, Shirao T, Takai Y (1998) Interactions of drebrin and gephyrin with profilin. Biochem Biophys Res Commun 243: 86–89 [DOI] [PubMed] [Google Scholar]
- Meier J, Grantyn R (2004) A gephyrin-related mechanism restraining glycine receptor anchoring at GABAergic synapses. J Neurosci 24: 1398–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier J, Vannier C, Serge A, Triller A, Choquet D (2001) Fast and reversible trapping of surface glycine receptors by gephyrin. Nat Neurosci 4: 253–260 [DOI] [PubMed] [Google Scholar]
- Meyer G, Kirsch J, Betz H, Langosch D (1995) Identification of a gephyrin binding motif on the glycine receptor beta subunit. Neuron 15: 563–572 [DOI] [PubMed] [Google Scholar]
- Morabito MA, Sheng M, Tsai LH (2004) Cyclin-dependent kinase 5 phosphorylates the N-terminal domain of the postsynaptic density protein PSD-95 in neurons. J Neurosci 24: 865–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastorino L, Sun A, Lu PJ, Zhou XZ, Balastik M, Finn G, Wulf G, Lim J, Li SH, Li X, Xia W, Nicholson LK, Lu KP (2006) The prolyl isomerase Pin1 regulates amyloid precursor protein processing and amyloid-beta production. Nature 440: 528–534 [DOI] [PubMed] [Google Scholar]
- Pribilla I, Takagi T, Langosch D, Bormann J, Betz H (1992) The atypical M2 segment of the β subunit confers picrotoxin resistance to inhibitory glycine receptor channels. EMBO J 11: 4305–4311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior P, Schmitt B, Grenningloh G, Pribilla I, Multhaup G, Beyreuther K, Maulet Y, Werner P, Langosch D, Kirsch J, Betz H (1992) Primary structure and alternative splice variants of gephyrin, a putative glycine receptor–tubulin linker protein. Neuron 8: 1161–1170 [DOI] [PubMed] [Google Scholar]
- Quandt K, Frech K, Karas H, Wingender E, Werner T (1995) MatInd and MatInspector: new fast and versatile tools for detection of consensus matches in nucleotide sequence data. Nucleic Acids Res 23: 4878–4884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan P, Dickson DW, Davies P (2003) Pin1 colocalization with phosphorylated tau in Alzheimer's disease and other tauopathies. Neurobiol Dis 14: 251–264 [DOI] [PubMed] [Google Scholar]
- Ramming M, Kins S, Werner N, Hermann A, Betz H, Kirsch J (2000) Diversity and phylogeny of gephyrin: tissue-specific splice variants, gene structure, and sequence similarities to molybdenum cofactor-synthesizing and cytoskeleton-associated proteins. Proc Natl Acad Sci USA 97: 10266–10271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan R, Lu KP, Hunter T, Noel JP (1997) Structural and functional analysis of the mitotic rotamase Pin1 suggests substrate recognition is phosphorylation dependent. Cell 89: 875–886 [DOI] [PubMed] [Google Scholar]
- Rees MI, Harvey K, Ward H, White JH, Evans L, Duguid IC, Hsu CC, Coleman SL, Miller J, Baer K, Waldvogel HJ, Gibbon F, Smart TG, Owen MJ, Harvey RJ, Snell RG (2003) Isoform heterogeneity of the human gephyrin gene (GPHN), binding domains to the glycine receptor, and mutation analysis in hyperekplexia. J Biol Chem 278: 24688–24696 [DOI] [PubMed] [Google Scholar]
- Schwarz G, Schrader N, Mendel RR, Hecht HJ, Schindelin H (2001) Crystal structures of human gephyrin and plant Cnx1 G domains: comparative analysis and functional implications. J Mol Biol 312: 405–418 [DOI] [PubMed] [Google Scholar]
- Shen M, Stukenberg PT, Kirschner MW, Lu KP (1998) The essential mitotic peptidyl-prolyl isomerase Pin1 binds and regulates mitosis-specific phosphoproteins. Genes Dev 12: 706–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sola M, Bavro VN, Timmins J, Franz T, Ricard-Blum S, Schoehn G, Ruigrok RW, Paarmann I, Saiyed T, O'Sullivan GA, Schmitt B, Betz H, Weissenhorn W (2004) Structural basis of dynamic glycine receptor clustering by gephyrin. EMBO J 23: 2510–2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sola M, Kneussel M, Heck IS, Betz H, Weissenhorn W (2001) X-ray crystal structure of the trimeric N-terminal domain of gephyrin. J Biol Chem 276: 25294–25301 [DOI] [PubMed] [Google Scholar]
- Thorpe JR, Mosaheb S, Hashemzadeh-Bonehi L, Cairns NJ, Kay JE, Morley SJ, Rulten SL (2004) Shortfalls in the peptidyl-prolyl cis–trans isomerase protein Pin1 in neurons are associated with frontotemporal dementias. Neurobiol Dis 17: 237–249 [DOI] [PubMed] [Google Scholar]
- Winkler KE, Swenson KI, Kornbluth S, Means AR (2000) Requirement of the prolyl isomerase Pin1 for the replication checkpoint. Science 287: 1644–1647 [DOI] [PubMed] [Google Scholar]
- Wulf G, Finn G, Suizu F, Lu KP (2005) Phosphorylation-specific prolyl isomerization: is there an underlying theme? Nat Cell Biol 7: 435–441 [DOI] [PubMed] [Google Scholar]
- Xiang S, Nichols J, Rajagopalan KV, Schindelin H (2001) The crystal structure of Escherichia coli MoeA and its relationship to the multifunctional protein gephyrin. Structure 9: 299–310 [DOI] [PubMed] [Google Scholar]
- Yaffe MB, Schutkowski M, Shen M, Zhou XZ, Stukenberg PT, Rahfeld JU, Xu J, Kuang J, Kirschner MW, Fischer G, Cantley LC, Lu KP (1997) Sequence-specific and phosphorylation-dependent proline isomerization: a potential mitotic regulatory mechanism. Science 278: 1957–1960 [DOI] [PubMed] [Google Scholar]
- Zacchi P, Gostissa M, Uchida T, Salvano C, Avorio F, Volinia S, Ronai Z, Blandino G, Schneider C, Del Sal G (2002) The prolyl isomerase Pin1 reveals a mechanism to control p53 functions after genotoxic insults. Nature 419: 853–857 [DOI] [PubMed] [Google Scholar]
- Zhou XZ, Kops O, Werner A, Lu PJ, Shen M, Stoller G, Kullertz G, Stark M, Fischer G, Lu KP (2000) Pin1-dependent prolyl isomerization regulates dephosphorylation of Cdc25C and tau proteins. Mol Cell 6: 873–883 [DOI] [PubMed] [Google Scholar]
- Zhou XZ, Lu PJ, Wulf G, Lu KP (1999) Phosphorylation-dependent prolyl isomerization: a novel signaling regulatory mechanism. Cell Mol Life Sci 56: 788–806 [DOI] [PMC free article] [PubMed] [Google Scholar]


