Abstract
Cdc42 GTPase is required for polarization in eukaryotic cells, but its spatial regulation is poorly understood. In Schizosaccharomyces pombe, Cdc42p is activated by Scd1p and Gef1p, two guanine-nucleotide exchange factors. Two-hybrid screening identified Hob3p as a Gef1p binding partner. Hob3p is a BAR domain-containing protein ortholog of human Bin3. Hob3p also interacts directly with Cdc42p independently of Gef1p. Hob3p, Cdc42p and Gef1p form a complex, and Hob3p facilitates Gef1p–Cdc42p interaction and activation. Hob3p forms a ring in the division area, similar to that of Gef1p. This localization requires actin polymerization and Cdc15p but is independent of the septation initiation network. Hob3p is required for the concentration of Cdc42p to the division area. The actomyosin ring contraction is slower in hob3Δ than in wild-type cells, and this contributes to its cytokinesis defect. Moreover, this report extends previous evidence that human Bin3 suppresses the cytokinesis phenotype of hob3Δ cells, showing that Bin3 can partially recover the GTP-Cdc42p level and its localization. These results suggest that Hob3p is required to recruit and activate Cdc42p at the cell division site and that this function might be conserved in other eukaryotes.
Keywords: amphiphysin, Cdc42, cytokinesis, fission yeast, GEF, GTPases
Introduction
Schizosaccharomyces pombe is a highly polarized yeast growing by elongation of its ends and dividing by medial septation. Cytokinesis begins with the assembly of a contractile actomyosin medial ring attached to the cell membrane (Feierbach and Chang, 2001; Guertin et al, 2002). Most of the components of this ring are conserved among animal cells (Glotzer, 2005). The septation initiation network (SIN) triggers the contraction of the actin ring and the consecutive addition of new membranes and synthesis of the primary septum in coordination with the nuclear cycle (Balasubramanian et al, 2004; Krapp et al, 2004; Wolfe and Gould, 2005).
BAR (Bin–Amphiphysin–Rvs) domain-containing proteins are a well-conserved family that includes Rvs proteins in yeast, and amphiphysins and BIN proteins in mammals. BAR family members integrate signal pathways that regulate membrane dynamics, F-actin cytoskeleton, and nuclear processes (Peter et al, 2004; Zimmerberg and McLaughlin, 2004; Ren et al, 2006). There are three main functions associated with the BAR domain: (1) sensing and/or induction of membrane curvature at endocytic sites (Peter et al, 2004; Zimmerberg and McLaughlin, 2004), (2) binding to small GTPases (Habermann, 2004), and (3) transcriptional repression properties (Elliott et al, 1999; Miaczynska et al, 2004).
Saccharomyces cerevisiae RVS161 and RVS167 genes, coding for two BAR domain-containing proteins, were isolated in a screening for mutations causing reduced viability upon nutrient starvation. Rvs161p and Rvs167p regulate endocytosis, vesicle traffic, F-actin organization, and cell polarity (Ren et al, 2006). These two proteins form heterodimers through their respective BAR domains. Rvs167p contains other domains and interacts with many proteins that have well-characterized roles in the actin cytoskeleton organization, including Abp1p, Acf2p, Act1p, Las17p, Sla1p, Sla2p, and Srv2p (Ren et al, 2006). In addition, RVS167 shows synthetic lethal or synergistic negative growth interactions with MYO1, MYO2, and ACT1 as well as with SLT2 and KRE6 (Breton et al, 2001). Rvs161p is required for actin repolarization upon salt stress and is associated with lipid rafts (Balguerie et al, 2002).
S. pombe also has two BAR domain proteins, Hob1p, structurally similar to Rvs167/amphiphysin (Routhier et al, 2003), and Hob3p, similar to human BIN3 and Rvs161p (Routhier et al, 2001). Surprisingly, neither Hob1p nor Hob3p is required for endocytosis, unlike Rvs167p and Rvs161p. hob1+ deletion causes totally different phenotypes than those produced by the deletion of the budding yeast RVS167. hob1Δ cells are slightly elongated, fail to arrest upon nutrient starvation, and are hypersensitive to genotoxic stress. These defects were complemented by human BIN1 (Routhier et al, 2003). S. pombe cells lacking Hob3p are multiseptated and the F-actin patches are distributed randomly around the cell. Interestingly, the actin localization defects of hob3Δ mutants were rescued by human BIN3 and only partially rescued by RVS161 (Routhier et al, 2001). These findings suggest that hob3+ and BIN3 are orthologs, and have diverged from RVS161 during evolution.
Cdc42 GTPase is a key molecule in establishing cell polarity in eukaryotic cells. Like other GTPases, Cdc42p acts as a molecular switch, cycling between an inactive (GDP-bound) and an active (GTP-bound) state. The local activation of Cdc42p at the growth site is required for actin polymerization and growth establishment (Etienne-Manneville, 2004). Fission yeast Cdc42p is essential for cell proliferation and also for maintenance of the rod-like cell morphology (Miller and Johnson, 1994). Cdc42p is mainly localized to the medial region and growing tips of the cell. It also localizes to the periphery and internal membranes (Merla and Johnson, 2000). Cdc42p is activated by two GTPase nucleotide exchange factors (GEFs): Scd1p, required to maintain the apical growth (Chang et al, 1994); and Gef1p, which plays a role in cytokinesis and the switch to bipolar growth (Coll et al, 2003; Hirota et al, 2003). The scaffold protein Scd2p allows the interaction between Cdc42p, the exchange factor Scd1p, and the p21-activated kinase Shk1p that regulates apical growth (Chang et al, 1999). As mentioned, Cdc42p localizes to the medial region, but a possible role of Cdc42p in S. pombe cytokinesis has not been described. Additionally, it is not known if other scaffold proteins, different from Scd2p, would cause the local activation of Cdc42p in the division area. Here we describe the interaction of Gef1p and Cdc42p with the BAR domain-containing protein Hob3p. This protein concentrates Cdc42p to the medial region and it is also necessary for the Gef1p-mediated Cdc42p activation. We also show that overexpression of Bin3 can substitute for Hob3p, suggesting that they have a function conserved among eukaryotes.
Results
Hob3p interacts with Gef1p
It has been shown that Gef1p is a Cdc42-specific exchange factor that participates in the regulation of cytokinesis (Coll et al, 2003; Hirota et al, 2003). Gef1p contains a Dbl homology (DH) domain responsible for their exchange activity, but lacks a pleckstrin homology (PH) domain. Gef1p does not interact with any of the known proteins from the Cdc42p signaling pathway that interact with Scd1p, such as Ras1p, Scd2p, Shk1p, or Shk2p (Hirota et al, 2003; our unpublished results). In order to identify the possible proteins of the Gef1p-Cdc42p signaling pathway, we performed a two-hybrid screen using Gef1p as bait, and 12 positive clones were obtained. Six of them contained the sequence coding for Hob3p, a BAR domain-containing protein, ortholog of human Bin3 and S. cerevisiae Rvs161p (Routhier et al, 2001). To map the Gef1p interaction with Hob3p, different Gef1p coding fragments were used as bait with pAS2-hob3+. gef1 constructs lacking the sequence coding the C-terminal 243 amino-acid residues failed to associate with Hob3p, suggesting that this region is required for the interaction with Hob3p (Figure 1A).
Figure 1.
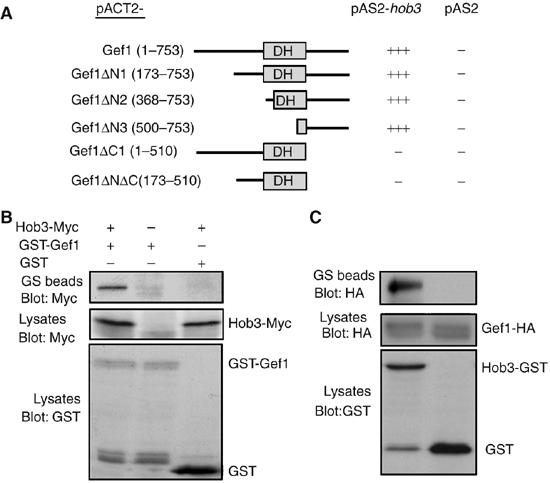
Gef1p interacts with Hob3p. (A) Two-hybrid analysis of the interactions between different fragments of Gef1p (pACT2) and Hob3p (pAS2). (B) Cells carrying endogenous hob3-myc were transformed with pREP1-GST-gef1+ and grown in the absence of thiamine for 12 h. Cell extracts were pulled down with GS beads and the precipitates were probed with anti-Myc antibody (top). Extracts were also analyzed by Western blot using anti-Myc (9E10) or anti-GST antibodies (bottom panels). (C) Extracts from cells carrying endogenous gef1-HA and hob3-GST were pulled down with GS beads and the precipitates were probed with anti-HA antibody. Extracts from gef1-HA cells transformed with the plasmid pREP-GST were used as negative control.
To verify the interaction between Gef1p and Hob3p, we performed co-immunoprecipitation experiments using cells transformed with the plasmid pREP-GST-Gef1p and we showed that GST-Gef1p co-precipitated with Myc-Hob3p expressed from the endogenous hob3+ promoter (Figure 1B). Additionally, we demonstrated that Hob3-GST and Gef1-HA, both expressed from the endogenous promoters, co-immunoprecipitated (Figure 1C). Together, these results indicate that Gef1p and Hob3p physically associate in S. pombe cells.
To see if Hob3p was required for Gef1p localization, we analyzed Gef1-GFP in hob3Δ cells, and observed that Gef1-GFP localized to the septation area, as in wild-type cells (data not shown). Therefore, Hob3p was not necessary for Gef1p localization. We also looked for any genetic interaction between gef1+ and hob3+. The absence of Gef1p causes an increase in septating cells (Coll et al, 2003), and aggravates the phenotype of other cytokinesis mutants (our unpublished results). Elimination of Hob3p causes an increase in septating cells and the appearance of some multiseptated cells. hob3Δ gef1Δ double mutant was viable at all temperatures and the percentage of cells with one or more septa was similar to hob3Δ (40% septating cells; 9% multiseptated cells; 7% elongated cells at 32°C, n=550, for hob3Δ cells and 42% septating cells; 9% multiseptated cells; 8% elongated cells at 32°C, n=720 for the hob3Δ gef1Δ cells). These results suggest that Hob3p and Gef1p are in the same signaling pathway.
Hob3p interacts with Cdc42p
Hob3p belongs to the BAR domain-containing protein family, and some members of this family can bind to small GTPases (Habermann, 2004). Hence, Hob3p could be an effector of Cdc42p, interacting with this GTPase when activated by Gef1p. To test this possibility, we analyzed whether Hob3p also binds to Cdc42p. Co-precipitation experiments were performed using extracts from cells expressing GST-hob3 and HA-cdc42 from their respective endogenous promoters. Interestingly, Hob3p co-precipitated with Cdc42p in both wild-type and gef1Δ cells (Figure 2A); therefore, Gef1p is not required for this interaction. Additionally, co-precipitation experiments in cells transformed with either the constitutively active cdc42G12V allele or the dominant-negative cdc42T17N allele showed that Hob3p interacted with Cdc42p independent of its activation state (Figure 2B). Taken together, these results suggest that Hob3p is not a Cdc42p effector but directly associates with this GTPase.
Figure 2.
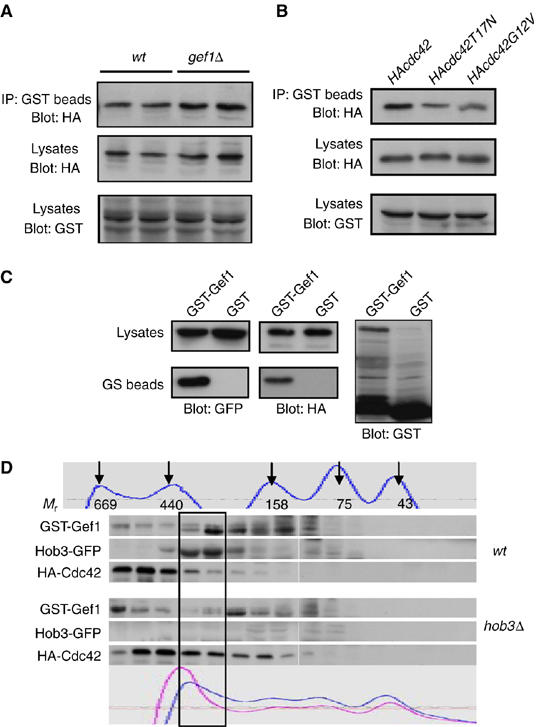
Hob3p interacts with Cdc42p and Gef1p. (A) Extracts from wild-type and gef1Δ cells carrying hob3-GST and HA-cdc42 expressed under their endogenous promoters were pulled down with GS beads and the precipitates were probed with anti-HA antibody (top). Extracts were assayed for expression of Hob3 and Cdc42 by Western blot analysis using anti-GST or anti-HA (12CA5) antibodies (bottom). (B) Cells expressing endogenous hob3-GST were transformed with pREP42-HA-Cdc42, pREP42-HA-Cdc42G12V, or pREP42-HA-Cdc42T17N and grown in the absence of thiamine for 12 h. Cell extracts were pulled down with GS beads and the precipitates were probed with anti-HA antibody. Hob3-GST and plasmid expression was analyzed by Western blot. (C) Gef1p, Cdc42p and Hob3p form an in vivo complex. hob3-GFP- and HA-cdc42-expressing cells were transformed with either pREP-GST-gef1+ or pREP-GST and grown in the absence of thiamine for 12 h. Cell extracts were immunoprecipitated with GS beads and blotted with anti-GFP antibody for the presence of Hob3-GFP (left), stripped and blotted with anti-HA antibody to detect HA-cdc42 (right). (D) hob3-GFP and hob3Δ cells expressing HA-cdc42 were transformed with pREP-GST-gef1+ and grown in the absence of thiamine for 12 h. Cell extracts were subjected to size-exclusion chromatography (Superdex 200) and fractions were analyzed by SDS–PAGE and blotted with the corresponding antibodies. Fractions 21–36 are shown. A portion of GST-Gef1p, Hob3-GFP, and HA-cdc42p co-migrated in 250–300 kDa molecular mass fractions (box). The standard molecular weight proteins and the UV absortion chromatograms of the extracts (wt cells darker and hob3Δ cells lighter) are shown in the top and bottom panels, respectively.
As Gef1p, Hob3p, and Cdc42p interact with each other, we considered the possibility that Hob3 might facilitate Gef1p–Cdc42p interaction. Therefore, we analyzed if the three proteins coexisted in a complex. Cells expressing hob3-GFP and HA-cdc42 from the respective endogenous promoters were transformed with the pREP-GST-gef1 plasmid. Moderate expression of the plasmid was induced and co-precipitation of the three proteins by pull-down with gluthation-Sepharose beads was successful (Figure 2C). These experiments suggest that a complex of Gef1p–Hob3p–Cdc42p may be present in vivo. To further investigate if the three proteins coexist in a complex, extracts from hob3-GFP HA-cdc42 and hob3Δ HA-cdc42 cells transformed with the pREP-GST-gef1 plasmid were fractionated by gel filtration (Figure 2D). HA-cdc42p has a predicted mass of 23 kDa; however, the elution position of HA-cdc42p from the column was that expected for a protein of much bigger size. Similarly, Hob3-GFP (predicted mass of 57 kDa) and GST-gef1p (predicted mass of 110 kDa) appear in fractions expected for proteins of bigger size. A small portion of the three proteins co-fractionated, suggesting that they might form part of a complex of 250–300 kDa. Most Cdc42p appears in higher molecular weight fractions, probably because in a logarithmic culture only 15–20% of the cells are septating and Cdc42p also forms complex with other signaling proteins, such as Scd1p and Scd2p, at the growing tips. In the absence of Hob3p, GST-Gef1p shifted toward lower molecular weight, and HA-cdc42p appears more spread throughout the column (fractions 21–28). The absence of Hob3p affects Cdc42p and Gef1p mobility, further supporting that they form part of the same complex.
Hob3p is necessary for the Gef1p-dependent activation of Cdc42p
We next analyzed whether Hob3p could be necessary for the Gef1p interaction with Cdc42p. Gluthation-Sepharose (GS) beads pull-down of cell extracts from wild-type and hob3Δ cells endogenously expressing HA-cdc42 and GST-gef1 were performed. HA-Cdc42p was present in both wild-type and hob3Δ cell pull-downs, but the level in hob3Δ cells was half that of the wild-type cells (Figure 3A). These results suggest that Hob3p facilitates the interaction of Gef1p with Cdc42p, although is not absolutely required for it. To further investigate whether Hob3p was necessary for Gef1p activation of Cdc42p, we analyzed the amount of active GTP-bound HA-Cdc42p in wild-type, hob3Δ, gef1Δ, and gef1Δhob3Δ double mutant cells. As expected, the levels of GTP-Cdc42p in gef1Δ cells were 30% that of wild-type cells. The lack of Hob3p also decreased the levels of GTP-Cdc42p to 45% that of wild-type cells (Figure 3B). Interestingly, hob3Δ gef1Δ cells had GTP-Cdc42p levels similar to gef1Δ cells (Figure 3B), suggesting that Hob3p and Gef1p are in the same Cdc42 activating pathway. Additionally, whereas the overexpression of Gef1p considerably increased GTP-Cdc42p, Hob3p overexpression did not increase the active Cdc42p levels (Figure 3C). These results suggest that Hob3p is not a Cdc42-GEF, but is necessary for Cdc42p activation. To test genetically whether Hob3p was necessary for the function of Gef1p, we made the hob3Δ scd1Δ double mutant strain. The double mutant gef1Δscd1Δ lacking both Cdc42p activators is lethal (Coll et al, 2003; Hirota et al, 2003). Therefore, if Hob3p were necessary for Gef1p function, hob3Δ scd1Δ would be lethal as well. scd1+ gene was replaced by KanMX6 in a hob3+/hob3∷ura4+ diploid strain and 44 tetrad spore analysis showed that the ura+ spores (hob3Δ) that germinated and proliferated were never resistant to kanamycin (scd1Δ). The spores Kanr ura+ germinated and underwent two or three cell divisions before arresting as small rounded cells (Figure 3D), similar to the spores of the double mutant gef1Δ scd1Δ (Coll et al, 2003). Taken together, these results indicate that Hob3p facilitates Gef1p–Cdc42p interaction and is necessary for Cdc42p activation mediated by Gef1p; consequently, Hob3p function is essential in the absence of Scd1p, the other Cdc42p activator.
Figure 3.
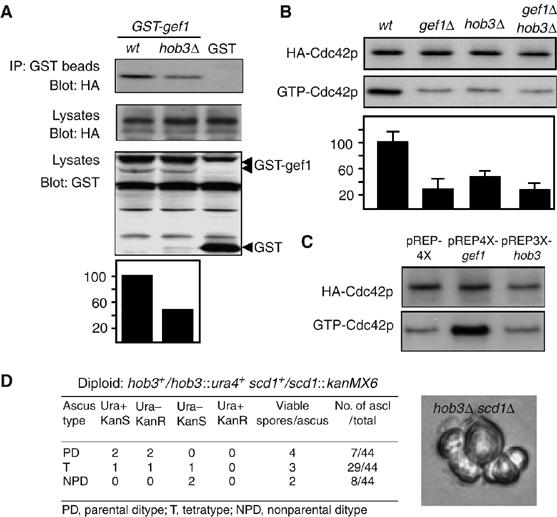
Hob3p is required for Cdc42p activation mediated by Gef1p. (A) Extracts from wild-type and hob3Δ cells carrying GST-gef1+ and HA-Cdc42 expressed under their own promoters were pulled down with GS beads and the precipitates were probed with anti-HA antibody. Extracts were assayed for expression of HA-Cdc42 and GST-gef1 by Western blot. Data were quantified and presented as percentage relative to the control extracts run in the same experiment. (B) HA-cdc42-expressing wild-type, gef1Δ, hob3Δ, and hob3Δ gef1Δ cells were analyzed for total HACdc42p by Western blot using 12CA5 anti-HA monoclonal antibody and for GTP-bound HA-Cdc42p by pulling down the cell extracts with GST-CRIB beads and blotting against 12CA5. Data were quantified and presented as percentage relative to the total HA-Cdc42 controls. (C) Wild-type cells transformed with pREP4X, pREP3X-hob3, or pREP4X-gef1+ were analyzed for total HA-Cdc42p and for GTP-bound HA-Cdc42p as in (B). (D) Tetrad analysis of the diploid strain hob3+/hob3∷ura4+ scd1+/scd1∷KanMX6 and a micrograph of hob3Δscd1Δ microcolonies formed after spore germination.
Hob3p forms a ring at the medial region and localizes to patches at the growing poles
To gain further insight into the Hob3p relationship with Cdc42p and Gef1p, we first examined the subcellular localization of Hob3-GFP. The genomic locus of hob3+ was tagged with the GFP gene fused in-frame to the 3′ end of hob3+ open reading frame (ORF). Hob3-GFP was observed in the medial region (Figure 4A, left). Confocal microscopy demonstrated that Hob3p formed a ring in that region (Figure 4A, right). To analyze Hob3p localization during septation, we constructed the double mutant cdc25-22 hob3-GFP and synchronized cells in G2 phase by arrest at 36.5°C for 4 h. Cells were returned to permissive temperature (25°C) and samples were taken at different time points to observe Hob3-GFP and calcofluor staining. Hob3-GFP appeared in the medial region before the septum was formed (60 min), remained during the ring contraction and septum formation, and disappeared by cell separation (Figure 4B).
Figure 4.
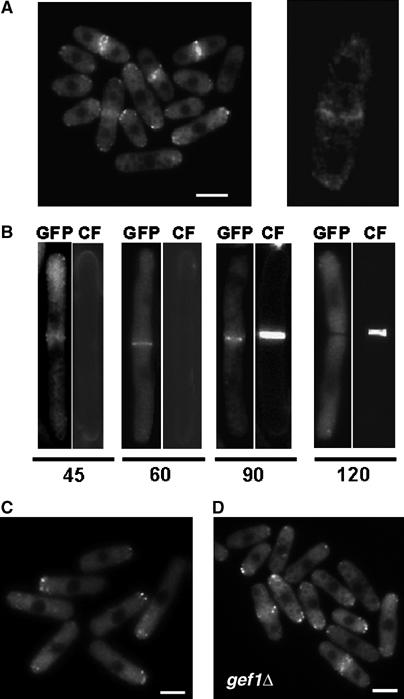
Hob3p localizes to the division area and patches at the growing poles. (A) Fluorescence microscopy of hob3-GFP cells grown to mid-log phase (left panel) and examined by confocal microscopy (right panel). (B) cdc25-22 hob3-GFP cells were grown at 25°C to log phase, then shifted to 36.5°C for 4 h to block cells in G2, and shifted back to 25°C. Cells were taken at different time points, stained with calcofluor, and examined for GFP and calcofluor fluorescence (CF). (C) cdc10-129 hob3-GFP cells were grown at 25°C to log phase, then shifted to 36.5°C and observed after 4 h. (D) gef1Δ hob3-GFP cells grown to mid-log phase. The bar corresponds to 5 μm.
Hob3p was also localized as patches at one or both poles in non-septating cells. cdc10-129 mutant cells expressing hob3-GFP were used to see if Hob3-GFP patches accumulate in the single growing pole. Cells were grown at 25°C and shifted to 36.5°C. At this temperature, cdc10-129 cells arrest in G1 and grow only through the old tip. In those cells, Hob3-GFP patches accumulate in the growing pole (Figure 4C). We also examined whether Gef1p was required for proper localization of Hob3p, but we did not observe any difference in Hob3-GFP localization in gef1Δ cells compared to wild-type cells (Figure 4D). Therefore, Hob3p and Gef1p do not depend on each other to localize correctly.
Hob3p localization is actin dependent
It has been described that the absence of Hob3p causes mislocalization of actin patches (Routhier et al, 2001). To see whether Hob3p patches contained actin, we examined rhodamine–phalloidin actin staining and Hob3-GFP simultaneously. Hob3-GFP patches overlapped some of those recognized by rhodamine–phalloidin, suggesting that Hob3p localizes to some actin patches at the growth poles (Figure 5A). Hob3-GFP ring also seemed to colocalize with the actin ring (Figure 5B). However, the overlap is not total. It has been described that Gef1p localizes to a contractile ring that resides immediately outside of the contractile actomyosin ring (Coll et al, 2003; Hirota et al, 2003). It is possible that Hob3p localizes to the same Gef1p ring. We constructed a strain carrying gef1-GFP and hob3-RFP and, although both proteins are difficult to visualize, the results showed that there was extensive overlapping between Hob3-RFP and Gef1-GFP in a contractile ring during cytokinesis (Figure 5C). Gef1p ring depends on the integrity of the contractile ring and it is dispersed by Latrunculin A treatment (Hirota et al, 2003). We examined whether Hob3p localization was also dependent on actin polymerization by promoting the disassembly of actin using 20 μM Latrunculin A. After 5 min with Latrunculin A, most actin was depolymerized, and Hob3-GFP, either from the middle region or from the growing pole patches, was dispersed to a diffuse cytoplasmic fluorescence (Figure 5D). Therefore, Hob3p localization is actin dependent.
Figure 5.
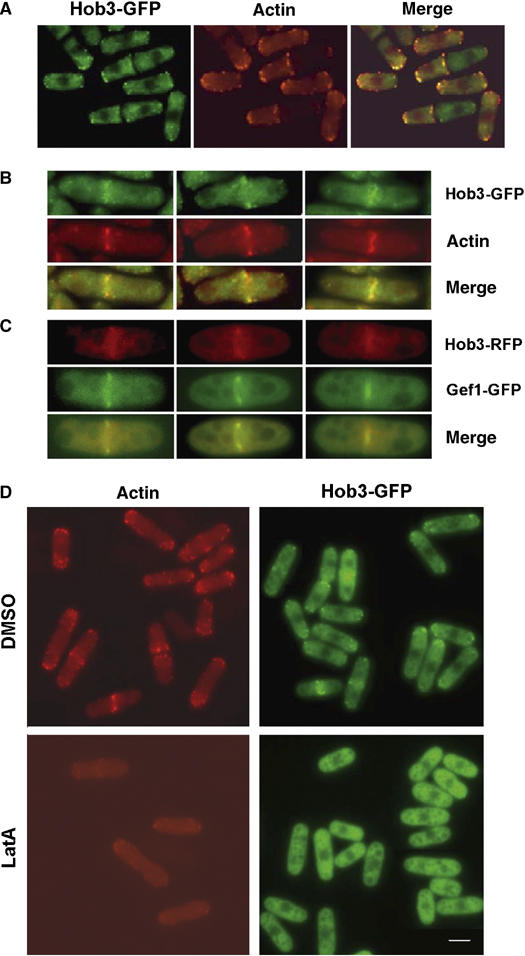
Hob3p localization depends on actin polimerization. (A) Colocalization of Hob3p and F-actin. hob3-GFP cells, grown to mid-log phase were stained with rhodamine–phalloidin and examined by fluorescence microscopy. (B) Three cells are shown in more detail to see the contractile rings. (C) Colocalization of Hob3-RFP and Gef1-GFP. Three cells are shown in detail to see the contractile rings. (D) Localization of Hob3-GFP depends on F-actin integrity. Fluorescence microscopy of cells grown to mid-log phase and incubated for 5 min in the presence of 1% dimethyl sulfoxide (DMSO) (top) or 20 μM Latrunculin A (LatA) bottom). Cells of the same culture were fixed and stained with rhodamine–phalloidin to observe actin localization. The bar corresponds to 5 μm.
Hob3p localization to the division area is dependent on Cdc15p but not on the SIN pathway
To further investigate how Hob3p localization is regulated, we analyzed Hob3-GFP in cdc15-140 mutant strain. Cdc15p is an FCH domain-containing protein necessary for the actomyosin ring formation (Fankhauser et al, 1995). cdc15-140 cells are impaired in the assembly of the actomyosin ring when grown at 36.5°C (Carnahan and Gould, 2003). Hob3-GFP was not recruited to the medial region in cdc15-140 cells grown at the restrictive temperature. By contrast, Hob3-GFP patches at the cell poles were still observed at 36.5°C (Figure 6A). When cdc15-140 cells were switched from 36.5°C to the permissive temperature, Hob3-GFP was observed in the medial region of all septating cells (Figure 6A). These results suggest that the actomyosin ring could serve as a spatial landmark to target Hob3p to the division site.
Figure 6.
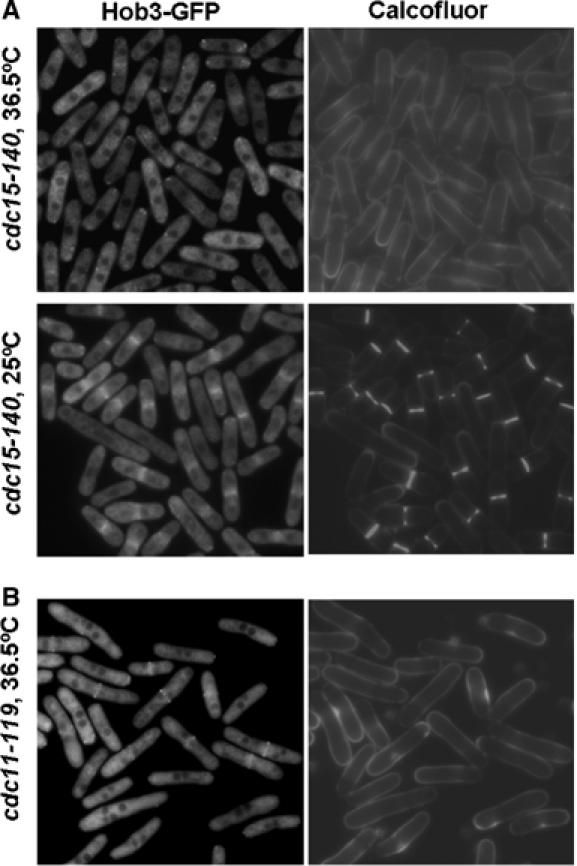
Hob3p localization to the division area depends on the actomyosin ring and is independent of the SIN pathway. (A) Fluorescence microscopy of cdc15-140 hob3-GFP. Cells were grown at 25°C to log phase, then shifted to 36.5°C for 4 h to block cells at cytokinesis (upper panel) and shifted back to 25°C (lower panel). (B) Fluorescence microscopy of cdc11-119 hob3-GFP cells grown at 25°C to log phase, then shifted to 36.5°C for 6 h. Cells were stained with calcofluor before observation.
We also analyzed Hob3-GFP in cdc11-119 mutant cells to see if Hob3p localization required the SIN, essential for the formation of the division septum after assembly of the actomyosin ring (Wolfe and Gould, 2005). Hob3-GFP localized properly in cdc11-119 cells grown at the restrictive temperature (Figure 6B). These observations indicate that Hob3p localization is not dependent on the SIN pathway.
Hob3p is required to localize Cdc42p to the division area
It has been speculated that Gef1p and Scd1p may play a key role not only in activating Cdc42p but also recruiting it to the septation site (Hirota et al, 2003). As Hob3p is necessary for Gef1p function, we considered the possibility that Hob3p was required to localize Cdc42p to the division area. We constructed a strain carrying endogenously GFP-tagged Cdc42p. Cells with GFP-Cdc42p were viable although slightly misshapen, indicating that GFP-Cdc42p was not completely functional. Endogenous Cdc42p localization was similar to that described using Cdc42p overexpressed from a plasmid (Merla and Johnson, 2000). It localizes to the periphery of the cell and to internal membranes, with a higher concentration at the growth poles and the division area, where it first forms a ring that closes to form a disc (Figure 7 and Supplementary movie S1). Similar localization was observed in cells lacking either Scd1p or Gef1p, although the GFP-Cdc42 signal in the latter was weaker and non-uniform (Figure 7). By contrast, in cells lacking Hob3p, we could not observe GFP-Cdc42p concentrated at the division area. Cdc42 was still mildly visible at the growth poles (Figure 7 and Supplementary movie S2). S. pombe contains another protein from the BAR family, Hob1p, ortholog of human Bin1 (Routhier et al, 2003). We examined GFP-Cdc42p localization in hob1Δ cells to see if the other protein containing a BAR domain could also regulate Cdc42p. However, GFP-Cdc42p was perfectly localized to the division site in hob1Δ cells (Figure 7 and Supplementary movie S3), suggesting that the effect on Cdc42p localization is specific to Hob3p. Western blot analysis of GFP-Cdc42p in total cell extracts showed that the lack of GFP-Cdc42p staining in the septum area of hob3Δ cells was not due to a decrease in the total amount of Cdc42p (data not shown). Taken together, these results indicate that Hob3p localizes to the actin ring and is specifically required to localize Cdc42p to the division site.
Figure 7.
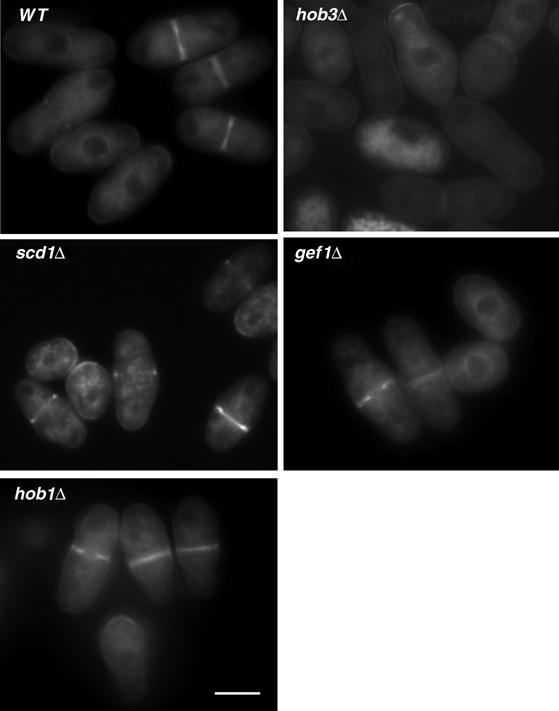
Hob3p is required for Cdc42p localization to the division area. GFP fluorescence micrographs of wild-type, hob3Δ, scd1Δ, gef1Δ, and hob1Δ cells with the endogenous cdc42 gene tagged with GFP. The bar corresponds to 5 μm.
Hob3p collaborates in the constriction of the actomyosin ring
The main morphologic defect in hob3Δ cells was an increase in septating cells and the appearance of some multiseptated cells (Routhier et al, 2001). These cytokinesis problems might be due to the mislocalization of Cdc42p. However, a particular role of this essential GTPase in S. pombe cytokinesis has not been demonstrated. We therefore asked whether other proteins required for cell separation were properly localized in hob3Δ mutant cells. In particular, we analyzed the following: Mid2p, a fission yeast anillin homolog that regulates septin organization (Berlin et al, 2003; Tasto et al, 2003); Rho4p, a GTPase that regulates septum degradation (Santos et al, 2003, 2005); Sec8p and Sec10p from the exocyst complex, involved in the targeting of enzymes responsible for septum cleavage (Wang et al, 2002); Eng1p and Agn1p, the glucanases responsible for the degradation of the primary septum and the lateral cell wall respectively (Martin-Cuadrado et al, 2003; Garcia et al, 2005); and Bgs1p and Bgs4p, identified as putative (1–3)β-D-glucan synthase catalytic subunits responsible for the biosynthesis of the primary and secondary septum, respectively (Cortes et al, 2002, 2005). All the proteins analyzed were perfectly localized to the septum area in hob3Δ cells (Supplementary Figure 1). Additionally, we analyzed the cell wall composition of cells lacking Hob3p and found no differences with respect to wild-type cells (data not shown). Therefore, we considered the possibility that the cytokinesis defect observed in hob3Δ cells could be caused by the absence of Cdc42p in the division area. In budding yeast, Cdc42p is necessary for the correct septin ring organization (Gladfelter et al, 2002). Although we did not observe any difference in the Mid2-GFP localization of hob3Δ cells, we analyzed localization of GFP-Spn1p in these cells. There was no difference in septin localization with respect to wild-type cells (see Supplementary Figure 2). Additionally, we quantified the phenotypes of hob3Δ, spn3Δ, and spn1Δ together with the double mutants hob3Δ spn3Δ and hob3Δ spn1Δ at 25 or 36°C. We observed additive effects in the double mutants lacking any of the septins and Hob3p (see Supplementary Figure 2). These results suggest that Hob3p plays an additional role to the septins in cell separation during cytokinesis.
Cdc42p causes actin rearrangements in animal cells and in S. cerevisiae (Etienne-Manneville, 2004). In budding yeast, Cdc42p recruits and activates the Arp2/3-activating complex formed by Bee1p/Las17p (the ortholog of mammalian WASp) and Vrp1p, which allows the local assembly of actin filaments. It has been proposed that actin polymerization is required for ring assembly and constriction (Pelham and Chang, 2002; Wu et al, 2006). We therefore studied if the separation defect of hob3Δ cells was due to a delay in ring assembly or constriction that could be caused by the lack of Cdc42p. The rates of ring formation and closure in wild-type and hob3Δ cells were analyzed by time lapse at 25°C using two GFP-tagged ring proteins, Cdc4p (myosin light chain) and Cdc15p. hob3Δ cells assembled the ring, as observed with GFP-cdc4p in cells stained with calcofluor. However, the initiation of ring constriction was delayed, and constriction lasted longer than in wild-type cells (Figure 8A). We also used confocal microscopy in cells carrying both Cdc15-GFP and GFP-tagged histone H3 (Hht2-GFP), to observe simultaneously the nuclear position and the ring constriction. As shown in Figure 8C, the ring closure rate in hob3Δ cells was slower than that in the wild-type strain. The constriction rate calculated at 25°C (n=10 cells of each type in three independent experiments) was slower in hob3Δ (70 ηm/min) than in wild-type cells (92 ηm/min) (Figure 8D). The total delay accumulated in ring constriction was, approximately, 15 min in each cycle. This delay accumulated during several cycles could cause the increase in septating cells observed in hob3Δ cell cultures. A similar phenotype is observed in the arp2-1 mutant, where septated cells accumulate when grown at the restrictive temperature (Morrell et al, 1999).
Figure 8.
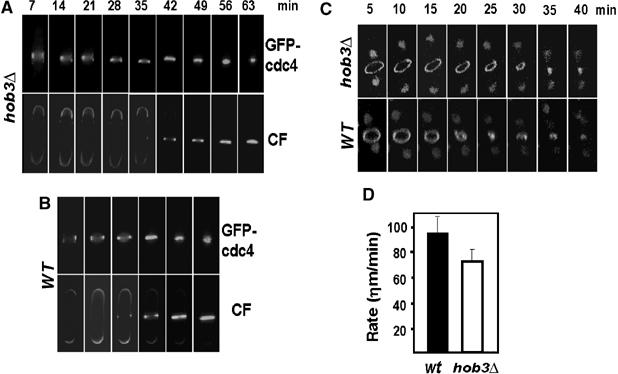
Ring constriction during cytokinesis is delayed in hob3Δ cells. Analysis of contractile ring closure at 25°C. Time-lapse fluorescence microscopy of GFP-cdc4 in (A) hob3Δ and (B) wild type cells stained with calcofluor. (C) Time-lapse images of Hht2-GFP and GFP-cdc15 in wild-type and hob3Δ cells examined by confocal microscopy. (D) Rate of ring constriction at 25°C of wild-type (n=10) and hob3Δ cells (n=10) calculated from the time-lapse confocal images in three independent experiments.
Human BIN3 can recover Cdc42 localization and activity in cells lacking Hob3p
It has been described that ectopic overexpression of human Bin3 suppresses cell morphology and actin patch polarization defects due to the absence of Hob3p (Routhier et al, 2001). We therefore tested if Bin3 can also complement the hob3Δ defects in localization and activation of Cdc42p that might in turn be the cause of the morphologic phenotype observed in these cells.
The localization of endogenous GFP-Cdc42p was examined in hob3Δ cells transformed with pREP41-HA-BIN3. As shown in Figure 9A, ectopic expression of human Bin3 partially restored the concentration of Cdc42p in the septum area that was lost in hob3Δ cells. More important, Bin3 was able to partially recover the levels of active GTP-bound Cdc42p, which were considerably lower in hob3Δ cells (Figure 9B). These results suggest that Hob3p function in Cdc42p localization and activation is also conserved in human Bin3.
Figure 9.
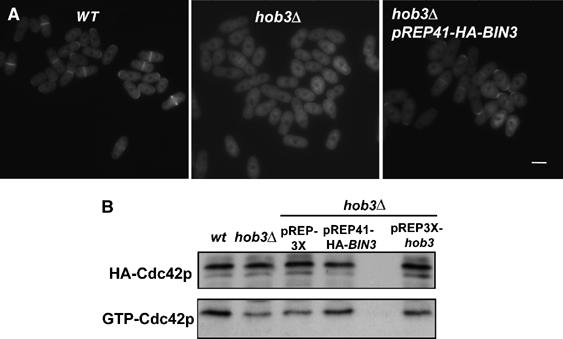
Bin3 can rescue the Cdc42p localization to the division area and activation in cells lacking Hob3p. (A) GFP fluorescence micrographs of wild-type, hob3Δ, and pREP41-HA-BIN3-transformed hob3Δ cells carrying the endogenous cdc42 gene tagged with GFP. The bar corresponds to 5 μm. (B) HA-cdc42 expressing wild-type, hob3Δ, and hob3Δ cells transformed with pREP3X, pREP41-HA-BIN3, or pREP3X-hob3 were analyzed for total HACdc42p by Western blot using 12CA5 anti-HA monoclonal antibody and for GTP-bound HACdc42p by pulling down the cell extracts with GST-CRIB beads and blotting against 12CA5.
Discussion
Most Rho GEFs contain a DH responsible for their exchange activity. These DH modules are located upstream of a PH domain, and the lipid-binding properties of this domain play a critical role in the localization of the protein and in the regulation of the DH domain activity (Hoffman and Cerione, 2002). In S. pombe, the Cdc42-GEF Scd1p contains the tandem DH–PH domains. In its signaling pathway, the SH3-containing protein, Scd2p, acts as a scaffold upon which Cdc42p, Scd1p, and the p21-activated kinase Shk1p assemble to regulate the apical growth and the mating response (Chang et al, 1999). Interestingly, Gef1p, the other fission yeast Cdc42-GEF, does not have a PH domain downstream of the DH domain, and does not interact with Scd2p (Hirota et al, 2003; our unpublished results). Instead, Gef1p interacts with Hob3p through the C-terminal region, downstream of the DH domain. Hob3p is structurally similar to S. cerevisiae Rvs161p and human Bin3 (Routhier et al, 2001). All of them are the simplest members of the BAR domain-containing protein family, containing a single BAR domain (Habermann, 2004). A possible function of this BAR protein could be to help in Gef1p localization, acting like a PH domain. However, Gef1p localization is not altered in hob3Δ cells. We therefore considered the possibility that Hob3p was required for the interaction of Gef1p and Cdc42p and analyzed whether Hob3p was interacting with Cdc42p, which was the case. Cdc42–Hob3 interaction was independent of Gef1p, and independent of the Cdc42p activation state, indicating that Hob3p was not a Cdc42p effector. We have also shown that the three molecules Gef1p, Hob3p, and Cdc42p form a complex that could be detected by co-immunoprecipitation.
Two possible roles for Hob3p, not mutually exclusive, arise from these results: (a) Hob3p is required to localize Cdc42 to the division area where it can be activated by Gef1p and (b) Hob3p is required for Gef1p–Cdc42p interaction and activation. The two roles are supported by several facts: (1) the level of Gef1p–Cdc42p co-immunoprecipitated decreased around 50% in the absence of Hob3p; (2) the level of active GTP-bound Cdc42p is considerably lower in hob3Δ cells, although Hob3p is not a GEF; (3) the decrease in GTP-bound Cdc42p caused by the simultaneous lack of Gef1p and Hob3p is similar to that caused by the lack of Gef1p alone; (4) a hob3Δscd1Δ double mutant strain, like a gef1Δscd1Δ strain, is not viable. Altogether, these results suggest that Hob3p is required for the activity of Gef1p. In mammalian cells, Tuba is a Cdc42-GEF with a DH domain upstream of a BAR domain that is required for the activation of Cdc42p (Salazar et al, 2003). This BAR may functionally replace the PH domain that typically follows a DH domain. Gef1p–Hob3p together could be similar to the protein Tuba in mammalian cells: Gef1p would bind and activate Cdc42p with the help of Hob3p. Interestingly, Scd2p acts as a Cdc42p adaptor but only binds activated Cdc42-GTP (Endo et al, 2003), suggesting that Scd2p might be important for the interaction of Cdc42p and some possible effectors but not for its activation. In fact, the double mutant strain gef1Δscd2Δ or hob3Δscd2Δ is not lethal (our unpublished results), corroborating that Scd2p is not required for the Scd1p-mediated Cdc42p activation.
The possible role of Hob3p as a protein required to localize Cdc42p to the division area would also imply that Hob3p is necessary for the function of Gef1p, as this GEF mainly localizes to the division area. It has been speculated that Gef1p and Scd1p may play a key role not only in activating Cdc42p but also in recruiting this GTPase to the septation site (Hirota et al, 2003). However, our results suggest that neither Gef1p nor Scd1p is required to localize Cdc42p to the septum, and that it is Hob3p that recruits this GTPase to the division area. In S. cerevisiae, the spatial control of Cdc42p activity is not only achieved through GEF recruitment but also by recruitment of itself to the site of polarized growth (Wedlich-Soldner et al, 2003). Localization of GFP-Cdc42p fusion proteins showed that, during cytokinesis, Cdc42p clusters at the division region before actomyosin ring contraction in S. cerevisiae (Richman et al, 2002). Although S. pombe Cdc42p mainly localizes to the division site, a particular Cdc42p role in cytokinesis has not been shown. Hob3p participates in cytokinesis, and our results suggest that it might act in this process by recruiting Cdc42p, thus providing a novel mechanism for the spatial regulation of Cdc42p signaling pathway. The fact that the cytokinesis defect of hob3Δ cells is more severe than that of gef1Δ cells suggests that Scd1p can substitute for Gef1p and might also activate Cdc42p during cytokinesis (Hirota et al, 2003), or that Hob3p is playing additional roles in cytokinesis besides recruiting Cdc42p. Other molecules participating in cell separation are perfectly localized in hob3Δ cells, and the cell wall has no differences in composition with respect to the wild type (data not shown). Therefore, one obvious hypothesis is that the absence of Cdc42p in hob3Δ cells causes the cytokinesis defect. Interestingly, Hob1p, the other S. pombe BAR domain-containing protein, localizes to actin patches at the cell ends during growth and to the medial site during cell division (Huang et al, 2005). However, hob1+ deletion failed to implicate Hob1p in cytokinesis (Routhier et al, 2003). Additionally, we have shown that Cdc42p localized to the medial region in cells lacking Hob1p. Therefore, the role of Hob3p regarding Cdc42p localization seems to be specific to this protein.
How does Cdc42p participate in cytokinesis? One possibility would be that Cdc42p organizes the septin cytoskeleton into a ring, as in S. cerevisiae (Gladfelter et al, 2002). S. pombe septin ring is required for cell separation. However, Mid2p or the septin Spn1p localization in hob3Δ cells is not altered. Moreover, the phenotype of mutants lacking any of the septins and Hob3p is more severe than that of the single mutants, suggesting that they play different roles in cytokinesis. Another possibility would be that Cdc42p participates in the actin polymerization needed for the assembly and/or closure of the contractile ring. Thus, the absence of Cdc42p could be causing the slower ring closure observed in cells lacking Hob3p, and the increase in the time cells spend in cytokinesis could raise the percentage of septating cells in hob3Δ cultures. In S. cerevisiae, Cdc42p regulates the proper spatio-temporal activation of the Arp2/3 complex, which regulates actin polymerization (Lechler et al, 2001). Cdc42p also activates Arp2/3 in animal cells via the Wiskott–Aldrich syndrome proteins (WASPs) (Pollard and Borisy, 2003). These targets of Cdc42p mediate most of its cytoskeletal effects (Bompard and Caron, 2004). In S. pombe, Wsp1p (a WASP homolog) has been implicated in the regulation of F-actin polymerization, and wsp1Δ cultures have an increased percentage of septating cells (Lee et al, 2000). arp2/3 mutant strains also have an increased percentage of septating cells at the restrictive temperature (Morrell et al, 1999). Previous works have shown evidence that Arp2/3 complex participates in the assembly of contractile ring filaments (Pelham and Chang 2002; Carnahan and Gould, 2003). However, more recent experiments show that the Arp2/3 complex and its activators do not contribute to the assembly of the contractile ring but participate in the initiation of ring constriction, the rate of ring constriction, the timing of septum formation, the rate of ring disassembly, and the timing of cell separation. All these processes were delayed slightly in strains lacking Myo1p or Wsp1p or in the cold-sensitive arp1-c1 mutant (Wu et al, 2006). The lack of Hob3p causes delay in the same processes, suggesting that Hob3p, and probably Cdc42p, participates in cytokinesis through the activation of Arp2/3 actin polymerization.
Human Bin3 is a widely expressed BAR protein of unknown function, structurally similar to Hob3p and S. cerevisiae Rvs161p. Interestingly, Bin3 but not Rsv161p expression completely complements the morphological defects of hob3Δ cells (Routhier et al, 2003), and we have shown here that Bin3 is able to partially relocalize Cdc42p to the division area and increase its activity in those cells, suggesting that the Hob3p function is fully conserved in eukaryotes. Does Bin3 function as a Cdc42p adaptor for cytokinesis in human cells? Clearly, future studies are required to characterize the possible role of Bin3 and Cdc42p in the mammalian cell cytokinesis.
Materials and methods
Fission yeast strains, media, and techniques
Standard S. pombe media and genetic manipulations were employed (Moreno et al, 1991). All the strains used were isogenic to wild-type strains 972 h− and 975 h+, and are described in Supplementary Table I. The strains were constructed by either random spore germination method or tetrad dissection. Cells were usually grown in rich medium (YES) or minimal medium (EMM) supplemented with the necessary requirements.
Plasmids and strain construction
The nmt promoter-containing vectors pREP3X, pREP4X, and pREP1-GST (Forsburg, 1993) were used for the overexpression of gef1+ or hob3+. For the two-hybrid analysis, the gef1+ ORF was cloned into the NcoI–BamHI sites of pAS2 and used as bait to screen an S. pombe cDNA library constructed in the pACT plasmid (generous gift from Dr SJ Elledge). S. cerevisiae Y190 (MATa gal4 gal80 his3 trp1-901 ura3-52 leu2-3,-112 URA3∷GAL--<lacZ, lys2∷GAL (UAS)--<HIS3 cyhr) cells were transformed and grown on plates without leucine, tryptophan, and histidine, and supplemented with 40 mM 3-aminotriazole. β-Galactosidase activity was analyzed in the transformant colonies as described (Coll et al, 2003). To map the interaction between Gef1p and Hob3p, different gef1+ fragments were cloned into the pACT2 plasmid.
To delete hob3+, hob1+, and scd1+ from the S. pombe genome, the whole ORF was replaced with the KanMX6 gene or the ura4+ gene by PCR-based gene targeting as described (Bähler et al, 1998). Stable transformants were selected and sporulated. Dissected tetrads were screened by PCR or Southern blot for the appropriate gene replacement. A genomic version of hob3+ with the GFP, the GST, the mRFP, or the Myc epitope coding sequences fused at the end of the ORF was generated as described (Bähler et al, 1998). A genomic version of cdc42+ with the GFP or the HA epitope coding sequences fused at the beginning of the ORF was generated by inserting in a Bluescript plasmid 500 bp of the 5′ cdc42 flanking sequences, the GFP or HA sequence, the cdc42 ORF, the KanMX6 gene, and 500 bp of the 3′ cdc42 flanking sequence. The insert was transformed into a homozygous leu1-32, ura4-D18 diploid strain and stable transformants were selected and screened by PCR for the appropriate gene replacement.
The entire gef1+ sequence was fused in-frame to the 3′-end of the GST sequence in the pREP-GST vector. Similarly, cdc42+, cdc42G12V, and cdc42T17N were fused in-frame to the 3′-end of HA epitope coding sequence in the pREP42-HA-N vector. Expression of the proteins was induced by growing the cells transformed with these plasmids in the absence of thiamine for 12 h. The entire gef1-GST and gef1-HA fusions were cloned under the control of the endogenous gef1+ promoter using the integrative plasmid pJK148 in a gef1Δ strain. The entire BIN3 sequence was amplified from a human cDNA library using the appropriate primers, sequenced, and cloned into pREP41-HA-N vector.
Immunoprecipitation
Extracts from 1 × 108 cells expressing the different tagged proteins were obtained as described (Arellano et al, 1997), using 200 μl of lysis buffer (20 mM Tris–HCl pH 8.2, 2 mM EDTA, 137 mM NaCl, 0.5% NP-40, 10% glycerol), containing 100 μM p-aminophenyl methanesulfonyl fluoride, leupeptin, and aprotinin). The cell extracts were incubated with glutathione beads for 2 h at 4°C. The beads were washed four times with lysis buffer and resuspended in sample buffer. Proteins were separated by SDS–PAGE, transferred to an Immobilon-P membrane (Millipore Corp.), and blotted to detect GST-, HA-, GFP-, or Myc-fused epitopes with the corresponding antibodies and the ECL detection kit (Amersham Corp). Total amounts of proteins were monitored in cell extract aliquots (30 μg of total protein) used directly for Western blot.
Gel filtration
Gel filtration was performed as described (Edwards et al, 1999) except that Superdex 200 GL 10/300 column was used. Extracts were prepared as described (Arellano et al, 1997) from 2 × 109 cells in 50 mM Na-phosphate buffer pH 7 carrying 150 mM NaCl, 0.1% NP-40 (Sigma), 10% glycerol, 0.5 mM dithiothreitol, 5 mM EGTA, 60 mM β-glycerophosphate, 0.1 mM NaF, 1 mM p-aminophenyl methanesulfonyl fluoride, 10 μg/ml leupeptin, and 10 μg/ml aprotinin). The column was equilibrated with two column volumes (CV=24 ml) of water and two volumes of 50 mM Na-phosphate buffer pH 7 carrying 150 mM NaCl, 0.1% NP-40, 60 mM β-glycerophosphate, and 0.1 mM NaF. The column was calibrated using the standard protein mixture HMW kit (Amersham Corp). A 1.5 mg portion of total protein in 100 μl volume was injected and the column flow rate was 0.4 ml/min. Fractions (0.5 ml) were collected and their UV absortion was recorded. Fractions 18–36 were TCA (20%) precipitated, washed with acetone, and analyzed by Western blot as described for immunoprecipitation.
In vivo analysis of GEF activity
The expression vector pGEX-CRIB (PAK-Cdc42 binding domain) (Manser et al, 1998) was used to transform Escherichia coli. The fusion proteins was produced according to the manufacturer's instructions and immobilized on glutathione-Sepharose 4B beads (Pharmacia).
The amount of GTP-bound Cdc42p was determined using a pull-down assay as described (Coll et al, 2003). Briefly, extracts from wild-type, gef1Δ, or hob3Δ strains carrying endogenously expressed HA-cdc42 were obtained as described (Arellano et al, 1997), using 500 μl of lysis buffer (50 mM Tris pH 7.5, 20 mM NaCl, 0.5% NP-40, 10% glycerol, 0.1 mM DTT, 1 mM NaF, 2 mM Cl2Mg, containing 100 μM p-aminophenyl methanesulfonyl fluoride, leupeptin, and aprotinin). A 2 mg portion of the cell extracts was incubated with 10 μg of GST-CRIB protein coupled to glutathione-Sepharose beads for 2 h, washed four times, and blotted as described with 12C5A mAb. Total HA-Cdc42p levels were monitored in whole-cell extracts (30 μg of total protein) that were used directly for Western blot.
Microscopy techniques
For calcofluor staining, exponentially growing S. pombe cells were harvested, washed, and resuspended in water with calcofluor at a final concentration of 0.1 mg/ml for 5 min at room temperature. After washing with water, cells were observed. Actin staining was performed with rhodamine–phalloidin. To disintegrate F-actin, Latrunculin A dissolved in DMSO at 20 mM was added to S. pombe cultures to a final concentration of 20 μM.
Cell samples were observed using a Leica DMRXA microscope equipped for Nomarski optics and epifluorescence, and photographed with a Photometrics Sensys CCD camera. Confocal microscopy was performed on a Leica TCS SL spectral confocal microscope and the images were analyzed with the Leica Confocal Software.
Supplementary Material
Supplementary movie S1
Supplementary movie S2
Supplementary movie S3
Supplementary Figure 1
Supplementary Figure 2
Supplementary Legends
Acknowledgments
We thank R Daga, JC Ribas, C Roncero, B Santos, H Valdivieso, and C Vazquez de Aldana for useful comments. We also thank D Posner for language revision. We are very grateful to M Balasubramanian, F Chang, K Gould, V Simanis, GC Prendergast, T Pollard , Y Sanchez, and JC Ribas for generous gifts of strains, and RY Tsien for kindly providing mRFP-containing plasmid. We specially thank C Duran and E Portales for technical help, and C Castro for the confocal microscopy. This work was supported by grant BIO2004-0834 from the Comision Interministerial de Ciencia y Tecnología, Spain.
References
- Arellano M, Duran A, Perez P (1997) Localization of the Schizosaccharomyces pombe Rho1 GTPase and its involvement in the organization of the actin cytoskeleton. J Cell Sci 110: 2547–2555 [DOI] [PubMed] [Google Scholar]
- Bähler J, Wu J-Q, Longtine MS, Shah NG, McKenzie A III, Steever AB, Wach A, Philippsen P, Pringle JR (1998) Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14: 943–951 [DOI] [PubMed] [Google Scholar]
- Balasubramanian MK, Bi E, Glotzer M (2004) Comparative analysis of cytokinesis in budding yeast, fission yeast and animal cells. Curr Biol 14: R806–R818 [DOI] [PubMed] [Google Scholar]
- Balguerie A, Bagnat M, Bonneu M, Aigle M, Breton AM (2002) Rvs161p and sphingolipids are required for actin repolarization following salt stress. Eukaryot Cell 1: 1021–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin A, Paoletti A, Chang F (2003) Mid2p stabilizes septin rings during cytokinesis in fission yeast. J Cell Biol 160: 1083–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bompard G, Caron E (2004) Regulation of WASP/WAVE proteins: making a long story short. J Cell Biol 166: 957–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton AM, Schaeffer J, Aigle M (2001) The yeast Rvs161 and Rvs167 proteins are involved in secretory vesicles targeting the plasma membrane and in cell integrity. Yeast 18: 1053–1068 [DOI] [PubMed] [Google Scholar]
- Carnahan RH, Gould KL (2003) The PCH family protein, Cdc15p, recruits two F-actin nucleation pathways to coordinate cytokinetic actin ring formation in Schizosaccharomyces pombe. J Cell Biol 162: 851–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang E, Bartholomeusz G, Pimental R, Che J, Lai H, Wang L, Yang P, Marcus S (1999) Direct binding and in vivo regulation of the fission yeast p21-activated kinase shk1 by the SH3 domain protein scd2. Mol Cell Biol 19: 8066–8074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang EC, Barr M, Wang Y, Jung V, Xu HP, Wigler MH (1994) Cooperative interaction of S. pombe proteins required for mating and morphogenesis. Cell 79: 131–141 [DOI] [PubMed] [Google Scholar]
- Coll PM, Trillo Y, Ametzazurra A, Perez P (2003) Gef1p, a new guanine nucleotide exchange factor for Cdc42p, regulates polarity in Schizosaccharomyces pombe. Mol Biol Cell 14: 313–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes JC, Carnero E, Ishiguro J, Sanchez Y, Duran A, Ribas JC (2005) The novel fission yeast (1,3)beta-D-glucan synthase catalytic subunit Bgs4p is essential during both cytokinesis and polarized growth. J Cell Sci 118: 157–174 [DOI] [PubMed] [Google Scholar]
- Cortes JC, Ishiguro J, Duran A, Ribas JC (2002) Localization of the (1,3)beta-D-glucan synthase catalytic subunit homologue Bgs1p/Cps1p from fission yeast suggests that it is involved in septation, polarized growth, mating, spore wall formation and spore germination. J Cell Sci 115: 4081–4096 [DOI] [PubMed] [Google Scholar]
- Edwards RJ, Bentley NJ, Carr AM (1999) A Rad3–Rad26 complex responds to DNA damage independently of other checkpoint proteins. Nat Cell Biol 1: 393–398 [DOI] [PubMed] [Google Scholar]
- Elliott K, Sakamuro D, Basu A, Du W, Wunner W, Staller P, Gaubatz S, Zhang H, Prochownik E, Eilers M, Prendergast GC (1999) Bin1 functionally interacts with Myc and inhibits cell proliferation via multiple mechanisms. Oncogene 18: 3564–3573 [DOI] [PubMed] [Google Scholar]
- Endo M, Shirouzu M, Yokoyama S (2003) The Cdc42 binding and scaffolding activities of the fission yeast adaptor protein Scd2. J Biol Chem 278: 843–852 [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S (2004) Cdc42—the centre of polarity. J Cell Sci 117: 1291–1300 [DOI] [PubMed] [Google Scholar]
- Fankhauser C, Reymond A, Cerutti L, Utzig S, Hofmann K, Simanis V (1995) The S. pombe cdc15 gene is a key element in the reorganization of F-actin at mitosis. Cell 82: 435–444 [DOI] [PubMed] [Google Scholar]
- Feierbach B, Chang F (2001) Cytokinesis and the contractile ring in fission yeast. Curr Opin Microbiol 4: 713–719 [DOI] [PubMed] [Google Scholar]
- Forsburg SL (1993) Comparison of Schizosaccharomyces pombe expression systems. Nucleic Acids Res 21: 2955–2956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia I, Jimenez D, Martin V, Duran A, Sanchez Y (2005) The alpha-glucanase Agn1p is required for cell separation in Schizosaccharomyces pombe. Biol Cell 97: 569–576 [DOI] [PubMed] [Google Scholar]
- Gladfelter AS, Bose I, Zyla TR, Bardes ES, Lew DJ (2002) Septin ring assembly involves cycles of GTP loading and hydrolysis by Cdc42p. J Cell Biol 156: 315–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glotzer M (2005) The molecular requirements for cytokinesis. Science 307: 1735–1739 [DOI] [PubMed] [Google Scholar]
- Guertin DA, Trautmann S, McCollum D (2002) Cytokinesis in eukaryotes. Microbiol Mol Biol Rev 66: 155–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermann B (2004) The BAR-domain family of proteins: a case of bending and binding? EMBO Rep 5: 250–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota K, Tanaka K, Ohta K, Yamamoto M (2003) Gef1p and Scd1p, the Two GDP-GTP exchange factors for Cdc42p, form a ring structure that shrinks during cytokinesis in Schizosaccharomyces pombe. Mol Biol Cell 14: 3617–3627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman GR, Cerione RA (2002) Signaling to the Rho GTPases: networking with the DH domain. FEBS Lett 513: 85–91 [DOI] [PubMed] [Google Scholar]
- Huang TY, Renaud-Young M, Young D (2005) Nak1 interacts with Hob1 and Wsp1 to regulate cell growth and polarity in Schizosaccharomyces pombe. J Cell Sci 118: 199–210 [DOI] [PubMed] [Google Scholar]
- Krapp A, Gulli MP, Simanis V (2004) SIN and the art of splitting the fission yeast cell. Curr Biol 14: R722–R730 [DOI] [PubMed] [Google Scholar]
- Lechler T, Jonsdottir GA, Klee SK, Pellman D, Li R (2001) A two-tiered mechanism by which Cdc42 controls the localization and activation of an Arp2/3-activating motor complex in yeast. J Cell Biol 155: 261–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WL, Bezanilla M, Pollard TD (2000) Fission yeast myosin-I, Myo1p, stimulates actin assembly by Arp2/3 complex and shares functions with WASp. J Cell Biol 151: 789–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manser E, Loo TH, Koh CG, Zhao ZS, Chen XQ, Tan L, Tan I, Leung T, Lim L (1998) PAK kinases are directly coupled to the PIX family of nucleotide exchange factors. Mol Cell 1: 183–192 [DOI] [PubMed] [Google Scholar]
- Martin-Cuadrado AB, Duenas E, Sipiczki M, Vazquez de Aldana CR, del Rey F (2003) The endo-beta-1,3-glucanase eng1p is required for dissolution of the primary septum during cell separation in Schizosaccharomyces pombe. J Cell Sci 116: 1689–1698 [DOI] [PubMed] [Google Scholar]
- Merla A, Johnson DI (2000) The Cdc42p GTPase is targeted to the site of cell división in the fisión yeast Schizosaccharomyces pombe. Int J Cell Biol 79: 469–477 [DOI] [PubMed] [Google Scholar]
- Miaczynska MS, Christoforidis S, Giner A, Shevchenko A, Uttenweiler JS, Habermann B, Wilm M, Parton RG, Zerial MM (2004) APPL proteins link Rab5 to nuclear signal transduction via an endosomal compartment. Cell 116: 445–456 [DOI] [PubMed] [Google Scholar]
- Miller PJ, Johnson DI (1994) Cdc42p GTPase is involved in controlling polarized growth in Schizosaccharomyces pombe. Mol Cell Biol 14: 1075–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S, Klar A, Nurse P (1991) Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol 194: 795–823 [DOI] [PubMed] [Google Scholar]
- Morrell JL, Morphew M, Gould KL (1999) A mutant of Arp2p causes partial disassembly of the Arp2/3 complex and loss of cortical actin function in fission yeast. Mol Biol Cell 10: 4201–4215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham RJ, Chang F (2002) Actin dynamics in the contractile ring during cytokinesis in fission yeast. Nature 419: 82–86 [DOI] [PubMed] [Google Scholar]
- Peter BJ, Kent HM, Mills IG, Vallis Y, Butler PJ, Evans PR, McMahon HT (2004) BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science 303: 495–499 [DOI] [PubMed] [Google Scholar]
- Pollard TD, Borisy GG (2003) Cellular motility driven by assembly and disassembly of actin filaments. Cell 112: 453–465 [DOI] [PubMed] [Google Scholar]
- Ren G, Vajjhala P, Lee JS, Winsor B, Munn AL (2006) The BAR domain proteins: molding membranes in fission, fusion, and phagy. Microbiol Mol Biol Rev 70: 37–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman TJ, Sawyer MM, Johnson DI (2002) Saccharomyces cerevisiae Cdc42p localizes to cellular membranes and clusters at sites of polarized growth. Eukaryot Cell 1: 458–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routhier EL, Burn TC, Abbaszade I, Summers M, Albright CF, Prendergast GC (2001) Human BIN3 complements the F-actin localization defects caused by loss of Hob3p, the fission yeast homolog of Rvs161p. J Biol Chem 276: 21670–21677 [DOI] [PubMed] [Google Scholar]
- Routhier EL, Donover PS, Prendergast GC (2003) hob1+, the fission yeast homolog of Bin1, is dispensable for endocytosis or actin organization, but required for the response to starvation or genotoxic stress. Oncogene 22: 637–648 [DOI] [PubMed] [Google Scholar]
- Salazar MA, Kwiatkowski AV, Pellegrini L, Cestra G, Butler MH, Rossman KL, Serna DM, Sondek J, Gertler FB, De Camilli P (2003) Tuba, a novel protein containing bin/amphiphysin/Rvs and Dbl homology domains, links dynamin to regulation of the actin cytoskeleton. J Biol Chem 278: 49031–49043 [DOI] [PubMed] [Google Scholar]
- Santos B, Gutierrez J, Calonge TM, Perez P (2003) Novel Rho GTPase involved in cytokinesis and cell wall integrity in the fission yeast Schizosaccharomyces pombe. Eukaryot Cell 2: 521–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos B, Martin-Cuadrado AB, Vazquez de Aldana CR, del Rey F, Perez P (2005) Rho4 GTPase is involved in the secretion of glucanases during fission yeast cytokinesis. Eukaryot Cell 5: 435–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasto JJ, Morrell JL, Gould KL (2003) An anillin homologue, Mid2p, acts during fission yeast cytokinesis to organize the septin ring and promote cell separation. J Cell Biol 160: 1093–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Tang X, Liu J, Trautmann S, Balasundaram D, McCollum D, Balasubramanian MK (2002) The multiprotein exocyst complex is essential for cell separation in Schizosaccharomyces pombe. Mol Biol Cell 13: 515–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedlich-Soldner R, Altschuler S, Wu L, Li R (2003) Spontaneous cell polarization through actomyosin-based delivery of the Cdc42 GTPase. Science 299: 1231–1235 [DOI] [PubMed] [Google Scholar]
- Wolfe BA, Gould KL (2005) Split decisions: coordinating cytokinesis in yeast. Trends Cell Biol 15: 10–18 [DOI] [PubMed] [Google Scholar]
- Wu JQ, Sirotkin V, Kovar DR, Lord M, Beltzner CC, Kuhn JR, Pollard TD (2006) Assembly of the cytokinetic contractile ring from a broad band of nodes in fission yeast. J Cell Biol 174: 391–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerberg J, McLaughlin S (2004) Membrane curvature: how BAR domains bend bilayers. Curr Biol 14: R250–R252 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary movie S1
Supplementary movie S2
Supplementary movie S3
Supplementary Figure 1
Supplementary Figure 2
Supplementary Legends


